Abstract
Laser treatments have become popular in Dermatology. In parallel to technologic development enabling the availability of different laser wavelengths, non-invasive skin imaging techniques, such as reflectance confocal microscopy (RCM), have been used to explore morphologic and qualitative skin characteristics. Specifically, RCM can be applied to cosmetically sensitive skin areas such as the face, without the need for skin biopsies. For these reasons, apart from its current use in skin cancer diagnosis, our systematic review reveals how RCM can be employed in the field of laser treatment monitoring, being particularly suitable for the evaluation of variations in epidermis and dermis, and pigmentary and vascular characteristics of the skin. This systematic review article aims to provide an overview on current applications of RCM laser treatment monitoring, while describing RCM features identified for different applications. Studies on human subjects treated with laser treatments, monitored with RCM, were included in the current systematic review. Five groups of treatments were identified and described: skin rejuvenation, scar tissue, pigmentary disorders, vascular disorders and other. Interestingly, RCM can assist treatments with lasers targeting all chromophores in the skin and exploiting laser induced optical breakdown. Treatment monitoring encompasses assessment at baseline and examination of changes after treatment, therefore revealing details in morphologic alterations underlying different skin conditions and mechanisms of actions of laser therapy, as well as objectify results after treatment.
Keywords:
laser; reflectance confocal microscopy; laser monitoring; rejuvenation; scar; pigmentation 1. Introduction
Over the past few decades, non-invasive treatments are increasingly requested in Dermatology [1,2,3,4]. Among these, laser therapy became popular due to technologic development leading both to the availability of different light wavelengths targeting different chromophores and to protocols reducing the downtime of treatment, according to patients’ requests [3,4,5,6].
In this scenario, in vivo reflectance confocal microscopy (RCM) has emerged as a non-invasive technique enabling horizontal visualization at different layers of the skin with good contrast and high resolution, providing cytologic and architectural details [7,8,9].
It has been proven as an excellent add-on tool for diagnostic purposes in Dermatology as well as for the analysis of healthy skin, since RCM provides an optical ‘‘histological’’ biopsy of the living tissue in a totally non-invasive manner, therefore avoiding scars, which is pivotal for aesthetic areas such as the face [10]. Specifically, RCM has been mainly employed in Cosmetic Dermatology to examine presence of regular/irregular keratinocytes at epidermis, collagen morphology and eventual elastosis at dermal level and pigmentary characteristics of the skin [8,10,11]. Importantly, these parameters have been recently standardized according to semi-quantitative and qualitative scales in order to improve recognition, reliability and reproducibility of evaluations [10]. Another advantage of non-invasive RCM is its ability to enable repeated examination of a given skin area, facilitating dynamic evaluation of skin changes, such as those that occur during treatment monitoring. RCM has been used to assess the effectiveness of various laser therapies in treating a variety of skin conditions, including acne, rosacea, and post-inflammatory hyperpigmentation. By offering non-invasive, real-time evaluation of skin changes, RCM can assist clinicians in customizing laser treatments to meet the individual needs of patients, optimizing treatment outcomes [10,12].
To summarize, RCM has become an essential tool in the field of dermatology, revolutionizing the approach of clinicians towards non-invasive skin treatments. In addition to its diagnostic and treatment monitoring applications, RCM has also been used to assess the efficacy of laser therapy in various skin conditions, including skin rejuventation, scars, pigmentatry and vascular disorders. By providing non-invasive, real-time evaluation of skin changes, RCM can assist clinicians in tailoring laser treatments to meet the unique needs of individual patients, resulting in optimal treatment outcomes.
Currently, an overview about RCM in laser treatment monitoring is lacking. Therefore, we systematically review literature on the topic in order to summarize fields of application and to identify pre- and post-treatment RCM features that can be assessed in laser treatment monitoring.
2. Materials and Methods
Studies conducted on human subjects involving laser treatments for skin conditions monitored with RCM were screened.
Electronic databases were systematically searched and included MEDLINE (PubMed), Web of Science and Cochrane library databases. Search strategy adopted was similar across the databases and developed using the following keyword: “laser” AND “reflectance confocal microscopy”. Our search included studies from inception to October 2022.
Two authors independently screened the abstracts for inclusion and exclusion criteria (SG and SA). In case of doubt or discordance, a third opinion was obtained (CL).
Studies were excluded based on the following criteria:
- language other than English
- in vitro or animal studies
- not involving laser treatments
- concerning tumors and not used to understand underlying mechanisms of laser therapies
- studies without specific RCM features described before and after treatment
- studies involving less than 8 patients
From each of the included studies, the following data were extracted: first author, year of publication, indication, number of patients and numbers of female patients, age, skin type/ethnicity, study type, laser type, RCM criteria at baseline and variations post-treatment, follow up timing, efficacy and safety.
3. Results
3.1. Study Selection
A total of 142 records were screened after duplicate and preliminary screening removal. Based on title/abstract screening, 101 studies were excluded. Forty-one full texts were thus assessed for eligibility and 20 studies were included in the qualitative synthesis, Figure 1.
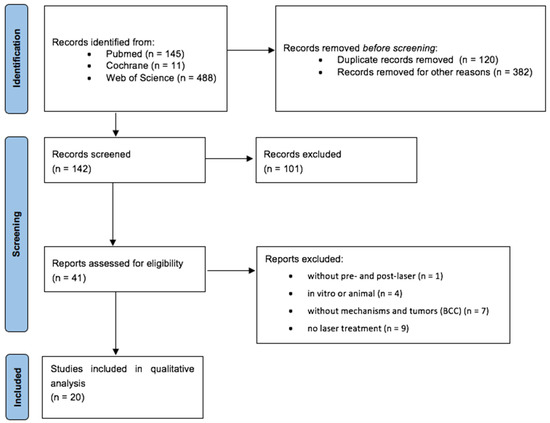
Figure 1.
PRISMA diagram.
Based on skin disease, 5 types of applications were identified: skin rejuvenation (n = 5), scar tissue (n = 6), pigmentary disorders (n = 5), vascular disorders (n = 2) and other (n = 2) [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Based on laser source employed in included studies, different laser sources were distinguished, those targeting water in the skin such as fractional C02 laser (n = 5) and erbium (n = 3), both ablative and non-ablative, those absorbed by hemoglobin, such as Nd:YAG nm (n = 1) and pulsed dye laser (n = 2). Additionally, laser wavelengths targeting pigment were also employed, including Q-switched laser (n = 5) and picosecond laser (PSL) (n = 4). Interestingly, PSL induce intraepidermal and dermal vacuole formation through laser-induced optical breakdown (LIOB), therefore resulting in multiphoton ionization due to high temperature and pressure created by high-energy irradiation with extremely short pulse durations, leading to additional applications.
A summary of RCM features is presented in Table 1; an overview of studies included is reported in Table 2.

Table 1.
Description of RCM features employed in laser treatment monitoring, classified according to skin layer.

Table 2.
Summary of characteristics of included studies.
3.2. Skin Rejuvenation
A total of 5 studies explored the role of RCM in laser monitoring for skin rejuvenation [13,14,15,16,17], Table 2. Most of the studies reported the use of lasers targeting water into the skin, including fractional CO2 laser and non-ablative fractional erbium laser while one study encompassed 1064 nm fractional PSL use, exploiting laser induced optical breakdown (LIOB). A total of 35 patients were treated for skin rejuvenation of the face while 18 for the neck. Mean follow up of patients was 1 to 4 months. Interestingly, RCM treatment monitoring enabled the visualization of variations supporting clinical improvement observed after laser treatments.
Early RCM parameters after laser treatment included the onset of micro-holes or microcolumns within the honeycombed pattern, corresponding to the micro-ablation induced by the fractional lasers, and dendritic cells at epidermal level, related to post-treatment inflammation, 1 to 6 weeks after treatment [14,15]. About 1 to 4 months after treatment, a clinical reduction of hyperpigmentation associated to aging (photoaging) was found to correspond to the reduction of both epidermal and dermo-epidermal junction (DEJ) RCM features of pigmentation, represented by mottled pigmentation or polycyclic papillary contours, respectively. Importantly, an increased number of dermal papillae and appearance of long straight fibers presenting a parallel alignment, or reticular collagen, was observed [13,14,15,16,17]. Interestingly, this last RCM feature corresponded to a clinical improvement of wrinkles/rhytids [16,17].
3.3. Scar Tissue
A total of six studies concerning treatment monitoring with RCM after laser treatments of scar tissue were included. Two studies involved acne scars, other two atrophic and hypertrophic surgical scars and the remnant two striae distensae. Similarly to skin rejuvenation, laser sources included CO2 fractional laser and non-ablative resurfacing lasers as well as PSL. RCM features included honeycombed or cobblestone pattern in epidermis, epidermal thickness, dermal papillae at DEJ and thin reticulated fibers or coarse collagen at dermal level [15,18,19,20,21,22], Table 2.
In detail, immediately after fractional CO2 laser or PSL to treat acne or surgical scars, black micro-holes surrounded by well-defined or fringed borders within the surrounding tissue could be observed [15,18,19,20,21,22]. Following the first week after treatment, a progressive repair of skin layers was observed. Three to 6 months post-treatment, thin reticulated collagen fibers arranged to form a net, with variable brightness of the collagen fibers, were observed [15,18,19,20]. Interestingly, this arrangement has been related to collagen remodeling [14].
Interestingly, both surgical scars and striae distensae showed parallel collagen fibers, mainly orthogonal to major axis of scar tissue. These parallel collagen fibers were significantly reduced after CO2 laser and PSL for striae distensae [21,22]. Interestingly, a new confocal feature, the “neat wall”, was described at baseline in striae distensae [21], Figure 2. This parameter corresponds to a distortion of the normal DEJ, visible only in RCM mosaic images; it resembles a well-demarcated wall separating regular areas of DEJ, which are formed by round papillae and fibrillar or reticular collagen (definition tab RCM). This parameter was reduced in patients receiving more than 4 CO2 laser sessions, as compared to those receiving ≤4 sessions at 4-week post-treatment [21].
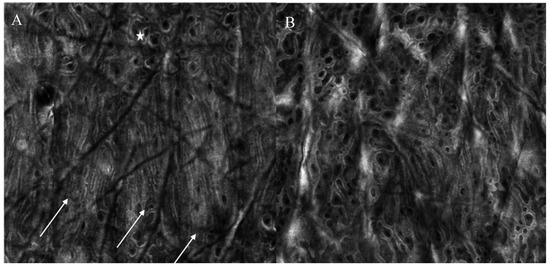
Figure 2.
Reflectance confocal microscopy images of a patient with striae distensae at baseline and one month after 5 CO2 laser sessions. (A) At baseline, neat wall feature can be observed (white arrows), with hyper-reflective compact collagen and elongated papillae as well as the presence of an area of regular architecture (white star) (B) After treatment, neat-wall is not detectable and the architecture is predominantly composed of roundish papillae.
3.4. Pigmentary Disorders
Due to the ability of RCM to visualize pigmentation at different layers, RCM has been used as a treatment monitoring tool in different pigmentary disorders: solar lentigines, café au lait macules (CALMs), infraorbital dark circles, melasma [23,24,25,26,27], Table 2. Intuitively, the main laser source employed was Q-switched and a comparison with this laser source and PSL was available for one study.
In solar lentigines, edged dermal papillae and polycyclic papillary contours were observed at baseline. Immediately after Q-switched treatment, dark structureless areas could be observed throughout the epidermis and dermal papillae were hyporefractive. After 10 days, at the DEJ, non-edged dermal papillae were observed, in 9 out of 12 cases containing a few melanophages. Bright reflective rims surrounding the dermal papillae were no longer observed at the DEJ [23].
For CALMs, length and density of papillae were estimated. Interestingly, CALMs with irregular borders showed lower length and density of papillae as compared to those with smooth borders and better response to laser treatment [24].
Two studies were performed concerning the treatment of infraorbital dark circles in a total of 60 women of which half treated with Q-switched ruby and Q-switched Nd:YAG laser. At baseline, greater melanin deposition in the upper dermis of the dark circle area was observed, as compared to the cheekbone skin (control), but no significant difference in epidermal pigmentation. After 8 sessions, about 70% of subjects showed over 50% improvement of pigmentation, while the control area did not show any variations [25,26].
Differently from what observed for dark circles, melasma patients can show pigmentation located at different layers. Jo et al. compared the effects of PSL and Q-switched laser on melasma. After treatment, either an increase of activated melanocytes at basal layer of epidermis or an increased amount of melanophages were observed at upper dermis 24 h after treatment [27]. Interestingly, the presence of dendritic-shaped cells after treatment has been associated with a relapse of melasma 3 months after multiple sessions of Q-switched laser treatment [28]. Figure 3 shows the presence of superficial pigmentation represented by mottled pigmentation at epidermal level at baseline and the disappearance of the pigmentation after Q-switched laser treatment.
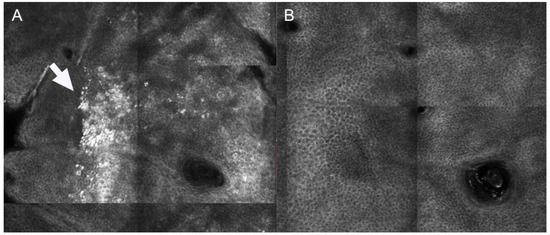
Figure 3.
Reflectance confocal microscopy images of patient showing superficial melasma at baseline and 3 months after Q-switched laser treatment. (A) At baseline, mottled pigmentation (white arrow) can be observed at epidermal level (B) After treatment, RCM shows disappearance of mottled pigmentation and recover of regular honeycombed pattern.
3.5. Vascular Disorders
Treatment monitoring of port-wine stains (PWS) with RCM has been reported in two papers [29,30], Table 2. PWS are a type of vascular malformation that affect the skin and can cause significant psychological distress. Treatment options include pulsed dye laser (PDL) therapy, which targets the abnormal blood vessels to reduce their appearance. Based on different clinical response and RCM analysis of blood flow, RCM features associated to resistance to treatment for facial PWS were identified, being represented by high blood flow, large diameter, deep location [30]. Interestingly, based on different depths of vessels, different pulse durations of laser are related to different reduction of diameter vessels while no influence has been observed for density [29].
Overall, RCM is a valuable tool for monitoring the vascular response to laser therapy and for characterizing the microvascular changes associated with various vascular disorders. Further research is needed to fully understand the potential applications of RCM in the field of vascular medicine.
3.6. Other Applications
Laser treatment monitoring with RCM has also been explored in other fields [31,32], Table 2. Wound healing has been studied after CO2 laser on healthy skin and results observed were in line with previous findings related to applications for skin resurfacing and scar tissue treatment [31].
Additionally, RCM has been employed to monitor sebaceous hyperplasia treated with dye laser. At baseline, lesions appeared like dilated sebaceous duct surrounded by dilated vessels [32].
4. Discussion
Nowadays, laser treatment monitoring can be performed with non-invasive skin imaging techniques, such as RCM [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. RCM is an advanced technique enabling the visualization of the skin at a quasi-histological resolution [7,9]. It has been mainly applied to the field of skin cancers [33,34] but has expanding applications in Cosmetic Dermatology [1,35]. As a matter of fact, the possibility to visualize pigmentation at different layers, collagen and vessels in a non-invasive manner, makes RCM particularly suitable to monitor laser treatments. Accordingly, when laser treatments are applied on cosmetically sensitive areas such as the face or are employed to treat aesthetic conditions, it would be preferable to avoid scarring as a consequence of skin biopsies [36,37].
Our results highlight that RCM can be applied to monitor skin rejuvenation treatments, scar tissue, pigmentation and vessels at different layers after laser therapy.
Specifically, RCM applied to skin rejuvenation and scar tissue treatment procedures revealed the advantage to monitor of skin healing process after laser therapy and to objectify variations of epidermis, DEJ and collagen or elastosis in the dermis. Additionally, RCM enables the visualization of micro-holes or microcolumns within the epidermal pattern, that have been associated to fractional laser treatment [14,15]. Similar findings were observed with histology [13].
Considering the high reflectivity of melanin, RCM is particularly useful to study pigmention distribution in different skin layers in different pigmentary disorders [38], overcoming the limitations related to other non-invasive tools, such as Wood’s lamp and dermoscopy which cannot locate the pigment precisely [39]. Interestingly, both pigment depth and the presence of dendritic-shaped cells in the epidermis have been associated with poor response to laser treatments, therefore highlighting the prognostic impact of RCM [27,28].
Our study reveals that specific terminology employed for healthy skin analysis can also be applied to define variations occurring after laser approach for skin rejuvenation, scar tissue and pigmentation [8,10,14,37].
Lastly, for vascular disorders, RCM has been employed to monitor the changes in blood vessels following laser treatments for port-wine stains. The ability to assess the depth, diameter, and density of blood vessels non-invasively may help optimize treatment parameters and improve overall treatment outcomes [29,30].
Currently, there are no studies comparing RCM with other techniques in laser treatment monitoring but some authors reported the alternative or complementary use of other techniques such as digital photography with automated features count and 3D imaging. Histologic studies, representing the reference for skin analysis, are mainly employed on ex vivo samples or in vivo in areas other than the face or on scars on the face in order to minimize visible scarring in aesthetic sites [40,41,42]. Therefore, many non-invasive techniques have been applied to the field of laser monitoring, apart from RCM, such as dermoscopy, digital photography with automatic features count (VISIA) and 3D assessment [3,43,44,45,46,47]. These techniques enable the visualization of pigment and vessels but, differently from RCM, they cannot be employed for the analysis of dermal features such as collagen characteristics and analyses per each skin layer.
In conclusion, RCM is a non-invasive technique that has shown promise in monitoring a variety of laser treatments in dermatology providing insights into the observation of variations associated with clinical improvement. RCM features identified in our systematic review can be applied to future studies to objectively evaluate changes in the skin and improve our understanding of the mechanisms of action of lasers. By facilitating non-invasive, real-time evaluation of skin changes, RCM can assist clinicians in tailoring laser treatments to meet the unique needs of individual patients, ultimately leading to optimal treatment outcomes.
Author Contributions
Conceptualization, validation, writing—review and editing: S.G. and C.L.; methodology, investigation, data curation S.G., S.A., A.M.R., M.M., S.C. and M.S.; writing—original draft preparation S.G. and S.A.; visualization, supervision S.P.N., S.R.M., F.R., N.Z. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rovatti, P.P.; Pellacani, G.; Guida, S. Hyperdiluted Calcium Hydroxylapatite 1: 2 for Mid and Lower Facial Skin Rejuvenation: Efficacy and Safety. Dermatol. Surg. 2020, 46, e112–e117. [Google Scholar] [CrossRef] [PubMed]
- De Melo, F.; Carrijo, A.; Hong, K.; Trumbic, B.; Vercesi, F.; Waldorf, H.A.; Zenker, S. Minimally Invasive Aesthetic Treatment of the Face and Neck Using Combinations of a PCL-Based Collagen Stimulator, PLLA/PLGA Suspension Sutures, and Cross-Linked Hyaluronic Acid. Clin. Cosmet. Investig. Dermatol. 2020, 13, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Nisticò, S.P.; Farnetani, F.; Del Duca, E.; De Carvalho, N.; Persechino, F.; Verdina, T.; Giannetti, L.; D’Alessandro, M.; Urtis, G.G.; et al. Resurfacing with Ablation of Periorbital Skin Technique: Indications, Efficacy, Safety, and 3D Assessment from a Pilot Study. Photomed. Laser Surg. 2018, 36, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Archer, K.A.; Carniol, P. Diode Laser and Fractional Laser Innovations. Facial Plast. Surg. 2019, 35, 248–255. [Google Scholar] [CrossRef]
- Wu, D.C.; Goldman, M.P.; Wat, H.; Chan, H.H.L. A Systematic Review of Picosecond Laser in Dermatology: Evidence and Recommendations. Lasers Surg. Med. 2021, 53, 9–49. [Google Scholar] [CrossRef]
- Verner, I.; Prag Naveh, H.; Bertossi, D. Treatment of injection-induced ecchymoses with light/laser-assisted technology. Dermatol. Ther. 2019, 32, e12861. [Google Scholar] [CrossRef]
- Guida, S.; Arginelli, F.; Farnetani, F.; Ciardo, S.; Bertoni, L.; Manfredini, M.; Zerbinati, N.; Longo, C.; Pellacani, G. Clinical Applications of In Vivo and Ex Vivo Confocal Microscopy. Appl. Sci. 2021, 11, 1979. [Google Scholar] [CrossRef]
- Guida, S.; Ciardo, S.; De Pace, B.; De Carvalho, N.; Peccerillo, F.; Manfredini, M.; Farnetani, F.; Chester, J.; Kaleci, S.; Manganelli, M.; et al. The influence of MC1R on dermal morphological features of photo-exposed skin in women revealed by reflectance confocal microscopy and optical coherence tomography. Exp. Dermatol. 2019, 28, 1321–1327. [Google Scholar] [CrossRef]
- Guida, S.; Longhitano, S.; Ardigò, M.; Pampena, R.; Ciardo, S.; Bigi, L.; Mandel, V.D.; Vaschieri, C.; Manfredini, M.; Pezzini, C.; et al. Dermoscopy, confocal microscopy and optical coherence tomography features of main inflammatory and autoimmune skin diseases: A systematic review. Australas. J. Dermatol. 2022, 63, 15–26. [Google Scholar] [CrossRef]
- Ciardo, S.; Pezzini, C.; Guida, S.; Del Duca, E.; Ungar, J.; Guttman-Yassky, E.; Manfredini, M.; Farnetani, F.; Longo, C.; Pellacani, G. A plea for standardization of confocal microscopy and optical coherence tomography parameters to evaluate physiological and para-physiological skin conditions in cosmetic science. Exp. Dermatol. 2021, 30, 911–922. [Google Scholar] [CrossRef]
- Guida, S.; Pellacani, G.; Ciardo, S.; Longo, C. Reflectance Confocal Microscopy of Aging Skin and Skin Cancer. Dermatol. Pract. Concept. 2021, 11, e2021068. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Longo, C. Reflectance confocal microscopy: A crucial role for actinic keratosis treatment monitoring. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1055. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Kim, M.J.; Baek, J.H.; Yoo, M.A.; Koh, J.S.; Lee, S.J.; Lee, M.H. Analysis of the temporal change in biophysical parameters after fractional laser treatments using reflectance confocal microscopy. Skin Res. Technol. 2013, 19, e515–e520. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Galimberti, M.; De Pace, B.; Pellacani, G.; Bencini, P.L. Laser skin rejuvenation: Epidermal changes and collagen remodeling evaluated by in vivo confocal microscopy. Lasers Med. Sci. 2013, 28, 769–776. [Google Scholar] [CrossRef]
- Cameli, N.; Mariano, M.; Serio, M.; Ardigò, M. Preliminary comparison of fractional laser with fractional laser plus radiofrequency for the treatment of acne scars and photoaging. Dermatol. Surg. 2014, 40, 553–561. [Google Scholar] [CrossRef]
- Bencini, P.L.; Tourlaki, A.; Galimberti, M.; Pellacani, G. Non-ablative fractionated laser skin resurfacing for the treatment of aged neck skin. J. Dermatolog. Treat. 2015, 26, 252–256. [Google Scholar] [CrossRef]
- Guida, S.; Fusano, M.; Pellacani, G.; Bencini, P.L. Fractional 1064 nm picosecond laser and skin photoaging: In vivo evaluation of treatment effects with reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2021, 23, 92–96. [Google Scholar] [CrossRef]
- Bencini, P.L.; Tourlaki, A.; Galimberti, M.; Longo, C.; Pellacani, G.; De Giorgi, V.; Guerriero, G. Nonablative fractional photothermolysis for acne scars: Clinical and in vivo microscopic documentation of treatment efficacy. Dermatol. Ther. 2012, 25, 463–467. [Google Scholar] [CrossRef]
- Guida, S.; Bencini, P.L.; Pellacani, G. Picosecond laser for atrophic surgical scars treatment: In Vivo monitoring of results by means of reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e114–e116. [Google Scholar] [CrossRef]
- Guida, S.; Pellacani, G.; Bencini, P.L. Picosecond laser treatment of atrophic and hypertrophic surgical scars: In Vivo monitoring of results by means of 3D imaging and reflectance confocal microscopy. Skin Res. Technol. 2019, 25, 896–902. [Google Scholar] [CrossRef]
- Guida, S.; Losi, A.; Greco, M.; Ciardo, S.; Pellacani, G.; Longo, C. Reflectance confocal microscopy for striae distansae treatment monitoring after CO2 fractional laser. Dermatol. Ther. 2020, 33, e14318. [Google Scholar] [CrossRef] [PubMed]
- Fusano, M.; Galimberti, M.G.; Bencini, M.; Guida, S.; Bencini, P.L. Picosecond Laser treatment of Striae Distensae: In vivo Evaluation of Results by 3D Analysis, Reflectance Confocal Microscopy, and Patient’s Satisfaction. Lasers Surg. Med. 2021, 53, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Richtig, E.; Hofmann-Wellenhof, R.; Kopera, D.; El-Shabrawi-Caelen, L.; Ahlgrimm-Siess, V. In vivo analysis of solar lentigines by reflectance confocal microscopy before and after Q-switched ruby laser treatment. Acta Derm. Venereol. 2011, 91, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Shen, L.; Yu, W.; Lin, X.; Sun, K.; Zhou, G. Use of Reflectance Confocal Microscopy to Predict Treatment Efficacy in Café Au Lait Macules. Dermatol. Surg. 2021, 47, e71–e74. [Google Scholar] [CrossRef]
- Xu, T.H.; Yang, Z.H.; Li, Y.H.; Chen, J.Z.; Guo, S.; Wu, Y.; Liu, W.; Gao, X.H.; He, C.D.; Geng, L.; et al. Treatment of infraorbital dark circles using a low-fluence Q-switched 1,064-nm laser. Dermatol. Surg. 2011, 37, 797–803. [Google Scholar] [CrossRef]
- Xu, T.H.; Li, Y.H.; Chen, J.Z.; Gao, X.H.; Chen, H.D. Treatment of infraorbital dark circles using 694-nm fractional Q-switched ruby laser. Lasers Med. Sci. 2016, 31, 1783–1787. [Google Scholar] [CrossRef]
- Jo, D.J.; Kang, I.H.; Baek, J.H.; Gwak, M.J.; Lee, S.J.; Shin, M.K. Using reflectance confocal microscopy to observe in vivo melanolysis after treatment with the picosecond alexandrite laser and Q-switched Nd:YAG laser in melasma. Lasers Surg. Med. 2018, 51, 423–429. [Google Scholar] [CrossRef]
- Longo, C.; Pellacani, G.; Tourlaki, A.; Galimberti, M.; Bencini, P.L. Melasma and low-energy Q-switched laser: Treatment assessment by means of in vivo confocal microscopy. Lasers Med. Sci. 2014, 29, 1159–1163. [Google Scholar] [CrossRef]
- Ren, J.; Qian, H.; Xiang, L.; Pan, Z.; Zhong, L.; Yan, S.; Gold, M.H. The assessment of pulsed dye laser treatment of port-wine stains with reflectance confocal microscopy. J. Cosmet. Laser Ther. 2014, 16, 21–25. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, J.; Xiang, Y.; Huang, J.; Tang, Z.; Chen, J.; Nelson, J.S.; Tan, W.; Lu, J. Characterization of Laser-Resistant Port Wine Stain Blood Vessels Using In Vivo Reflectance Confocal Microscopy. Lasers Surg. Med. 2019, 51, 841–849. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.J.; Ahn, H.J.; Baek, J.H.; Shin, M.K.; Koh, J.S. Dynamic Evaluation of Microwound Healing Induced by a Fractional CO2 Laser Using Reflectance Confocal Microscopy. J. Clin. Aesthet. Dermatol. 2022, 15, 25–29. [Google Scholar] [PubMed]
- Aghassi, D.; González, E.; Anderson, R.R.; Rajadhyaksha, M.; González, S. Elucidating the pulsed-dye laser treatment of sebaceous hyperplasia in vivo with real-time confocal scanning laser microscopy. J. Am. Acad. Dermatol. 2000, 43, 49–53. [Google Scholar] [CrossRef]
- Pellacani, G.; Farnetani, F.; Ciardo, S.; Chester, J.; Kaleci, S.; Mazzoni, L.; Bassoli, S.; Casari, A.; Pampena, R.; Mirra, M.; et al. Effect of Reflectance Confocal Microscopy for Suspect Lesions on Diagnostic Accuracy in Melanoma: A Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 754–761. [Google Scholar] [CrossRef]
- Farnetani, F.; Scope, A.; Coco, V.; Guida, S.; Cesinaro, A.M.; Piana, S.; Peris, K.; Pellacani, G.; Longo, C. Paradigmatic cases of pigmented lesions: How to not miss melanoma. J. Dermatol. 2016, 43, 1433–1437. [Google Scholar] [CrossRef]
- Longo, C.; Casari, A.; De Pace, B.; Simonazzi, S.; Mazzaglia, G.; Pellacani, G. Proposal for an in vivo histopathologic scoring system for skin aging by means of confocal microscopy. Skin Res. Technol. 2013, 19, e167–e173. [Google Scholar] [CrossRef]
- Pezzini, C.; Ciardo, S.; Guida, S.; Kaleci, S.; Chester, J.; Casari, A.; Manfredini, M.; Longo, C.; Farnetani, F.; Brugués, A.O.; et al. Skin ageing: Clinical aspects and in vivo microscopic patterns observed with reflectance confocal microscopy and optical coherence tomography. Exp. Dermatol. 2023, 32, 348–358. [Google Scholar] [CrossRef]
- Guida, S.; Farnetani, F.; De Pace, B.; Kaleci, S.; Chester, J.; Stanganelli, I.; Ciardo, S.; De Carvalho, N.; Longo, C.; Pellacani, G. Flat-pigmented facial lesions without highly specific melanocytic dermoscopy features: The role of dermoscopic globules and dots in differential diagnosis with corresponding reflectance confocal microscopy substrates. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e153–e156. [Google Scholar] [CrossRef] [PubMed]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Bostan, E.; Cakir, A. The dermoscopic characteristics of melasma in relation to different skin phototypes, distribution patterns and wood lamp findings: A cross-sectional study of 236 melasma lesions. Arch. Dermatol. Res. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Tanghetti, E.; Jennings, J. A comparative study with a 755 nm picosecond Alexandrite laser with a diffractive lens array and a 532 nm/1064 nm Nd:YAG with a holographic optic. Lasers Surg. Med. 2018, 50, 37–44. [Google Scholar] [CrossRef]
- Chang, Y.S.; Yang, T.H.; Li, C.N. Histology changes of in vivo human skin after treatment with fractional 1064 nm Nd:YAG picosecond laser in different energy settings. Lasers Med. Sci. 2022, 37, 2087–2092. [Google Scholar] [CrossRef]
- Habbema, L.; Verhagen, R.; Van Hal, R.; Liu, Y.; Varghese, B. Minimally invasive non-thermal laser technology using laser-induced optical breakdown for skin rejuvenation. J. Biophotonics. 2012, 5, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Tawfic, S.O.; Abdel Hay, R.M.; Abouelazm, D.I.; Said, E.R. Tranexamic Acid Microinjection Alone Versus Its Combination With Fractional Carbon Dioxide Laser in Melasma Treatment: A Dermoscopic Evaluation. Dermatol. Surg. 2022, 48, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lin, E.T.; Chang, C.C.; Lin, B.S.; Chiang, H.M.; Huang, Y.H.; Lin, H.Y.; Wang, K.Y.; Chang, T.M. Efficacy and Safety Evaluation of Picosecond Alexandrite Laser with a Diffractive Lens Array for Treatment of Melasma in Asian Patients by VISIA Imaging System. Photobiomodul. Photomed. Laser Surg. 2019, 37, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, M.G.; Guida, S.; Pellacani, G.; Bencini, P.L. Hyaluronic acid filler for skin rejuvenation: The role of diet on outcomes. A pilot study. Dermatol. Ther. 2018, 31, e12646. [Google Scholar] [CrossRef]
- Zawodny, P.; Stój, E.; Kulig, P.; Skonieczna-Żydecka, K.; Sieńko, J. VISIA Skin Analysis System as a Tool to Evaluate the Reduction of Pigmented Skin and Vascular Lesions Using the 532 Nm Laser. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2187–2195. [Google Scholar] [CrossRef]
- Conforti, C.; Guida, S.; Dianzani, C.; Turco, P.; Cazzato, V.; Zalaudek, I.; Piccolo, D. Carbon Peeling Laser Treatment to Improve Skin Texture, Pores and Acne Lesions: A Retrospective Study. Medicina 2022, 58, 1668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).