Abstract
Interleukin-33 (IL-33) is a cytokine belonging to the IL-1 family, playing a role in inflammatory, infectious and autoimmune diseases and expressed in the cellular nucleus in several tissues. High levels of IL-33 are expressed in epithelial barrier tissues and endothelial barriers. ST2 is a receptor for IL-33, expressed selectively on a subset of Th2 cells, mediating some of their functions. The IL-33/ST2 axis plays an important role in several acute and chronic inflammatory diseases, including asthma and rheumatoid arthritis. Different disorders are related to the activity of IL-33, ST2, or their axis, including cardiovascular disease or renal disturbances. Therefore, in the present work, a literature review was conducted, covering the period from 1 January 2000 to 30 November 2018, in PubMed, ScienceDirect, and Google Scholar database, to assess the involvement of the IL-33/ST2 axis in diabetic kidney disease. 6 articles directly dealing with the argument were identified, highlighting a clear link between IL-33/ST2 axis and diabetic kidney disease or related nephropathy. Overall, the involvement of ST2 seems to be more predictive than IL-33, especially in investigating the deterioration of kidney function; however, both compounds are pivotal in the field of renal diseases. Future studies are required to confirm the scientific evidences on larger and more heterogeneous cohorts.
1. Introduction
Interleukin-33 (IL-33) represents a recently discovered cytokine belonging to the IL-1 family, and plays a major role in inflammatory, infectious, and autoimmune diseases [1]. It is expressed as a nuclear alarmin in the nucleus of endothelial cells, fibroblasts, and epithelial cells in several tissues. Its role as an alarmin is pivotal in individuating damages in several inflammatory conditions, including atopic dermatitis and skin diseases [2,3]. It is released to the extracellular space in response to mechanical stress or cellular injury, and it elicits inflammatory responses. High levels of IL-33 are expressed in epithelial barrier tissues and endothelial barriers [4,5]. ST2 is a receptor for IL-33 and a member of the overall IL-1 receptor family. It is expressed selectively by many immune cells involved in type-2 immune response, including the group 2 innate lymphoid cells (ILC2s), mast cells, Th2 cells, eosinophils, basophils, as well as dendritic cells [6,7]. ILC2 recruitment is strongly related to the activity of IL-33, with their role being central in immunity against pathogens, type 2 inflammation, and tissue homeostasis and repair [8]. In this domain, IL-33 induces IL-5 and IL-13 release by ILC2, playing a key-role in the framework of allergic inflammation, including asthma and atopic dermatitis [9,10].
When it bonds with the ST2 receptor, IL-33 promotes the activation of nuclear factor (NF)-κB and mitogen-activated protein kinase, leading to an increased transcription of Th2 cytokines [11]. Therefore, it appears evident that the IL-33/ST2 axis mainly induces Th2 cytokines, differently from what occurs with other IL-1 family cytokines. Overall, this axis plays a key-role in several acute and chronic inflammatory diseases [12,13,14], including asthma [7] and rheumatoid arthritis [15].
In a human, IL-33 is expressed in human secondary lymphoid tissues, including the lymph nodes and appendix, and is spread along the vascular tree (large and small blood vessels from normal tissues, including the liver, skeletal muscle, kidney, and prostate, despite the micro-circulation of the brain and kidney glomeruli) [16]. Several literature works have highlighted the broad expression of IL-33 in normal, tumor, and chronically inflamed human tissues [16,17], confirming their significant expression in the kidneys [18,19].
Overall, apparently different disorders are related to the activity of IL-33, ST2 or their axis, including cardiovascular diseases [20], or renal disturbances, like chronic kidney disease (CKD), an immune inflammatory disease whose function is related to the presence of inflammatory biomarkers as much as the kidney function declines [21,22] (Figure 1).
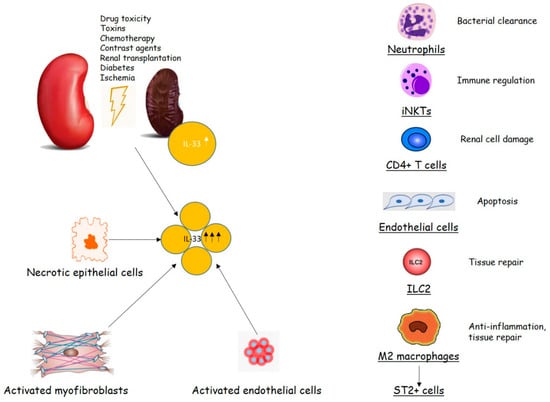
Figure 1.
Interleukin-33 (IL-33)/ST2 signaling in renal injury (modified from [5]).
So far, it is well known that diabetes is closely related to kidney disorders, being the most common cause of end-stage renal disease [23], with hyperglycemia-induced hemodynamic and metabolic pathways as the mediators of kidney injury [24].
Given the widespread diffusion of renal diseases and diabetes worldwide and, consequently, the high impact such diseases have on society and on healthcare systems, we performed a literature review about the IL-33/ST2 axis involvement in diabetic kidney disease or related nephropathy. This review is mainly focused on the pathophysiological outcome of the mentioned axis in the relevant disorders.
2. Studies on the IL-33/ST2 Axis Involvement in Diabetic Kidney Disease or Related Nephropathy
This literature review has retrieved a handful of articles directly dealing with the IL-33/ST2 axis involvement in diabetic kidney disease or related nephropathy. The articles taken into account are displayed in Table 1.

Table 1.
Study selection.
2.1. Results Achieved by the Studies on CKD
One of the first studies on the argument was published in 2012 by Bao and colleagues [25]. Their focus was the investigation of the serum levels of IL-33 and sST2 in patients with CKD, a sub-group of which were diabetic, and their association with disease severity. Based on the eGFR, the patients were classified into five categories of disease severity, plus a cohort of healthy individuals. sST2 was seen to be higher among CKD patients with respect to controls (p = 0.003), whereas no difference was seen for IL-33 (p = 0.656). By investigating the relationship between sST2 (and IL-33) and disease stage, the authors found a significant correlation between the serum level of sST2 and disease severity (r = 0.586; p < 0.001).
A larger cohort (238 patients with CKD, of which 60 had diabetes) was enrolled in an observational study conducted in Turkey some years later [28]. Here, serum IL-33/ST2 levels were investigated together with their association with cardiovascular problems. In this regard, Flow-mediated dilatation (FMD) was employed as a marker for endothelial function (see [31] for details). IL-33 and ST2 levels were increased as eGFR decreased, whereas the FMD was seen to be lower in more severe CKD patients. Overall, both IL-33 and ST2 were inversely associated with FMD, with ST2 being being a reliable predictor. Finally, both IL-33 and ST2 were associated with the risk of cardiovascular events, with higher cumulative survival in patients with lower IL-33 and ST2, compared with patients with higher values of such compounds. Such risks were also seen to be associated with Diabetes Mellitus, smoking, and proteinuria.
2.2. Results Achieved by the Studies on Kidney Diseases-Related Diabetes
As widely known, one of the most important co-morbidities of renal diseases is diabetes. In fact, for example, Type-2 diabetic nephropathy (DN) is considered the most common cause of end-stage renal disease among patients undergoing renal dialysis [32,33]. Some of the works retrieved in the present review have analyzed diabetes related to renal diseases, starting from the research by Caner and colleagues [26].
In that study, it was investigated whether IL-33 can be used to show the early stage of kidney injury in diabetic patients. To accomplish this aim, 42 patients with DM with normal renal function, 32 patients with DM + MA, and 26 healthy controls were enrolled and assessed in terms of IL-33 levels. The IL-33 levels were higher overall in DM + MA, followed by DM and controls. The differences between DM + MA and DM were not significant; however, a statistically significant difference was seen for both groups with respect to controls.
Later on, Shruthi and colleagues [27] investigated the serum levels of several cytokines, including IL-33 in 125 T1DM subjects, 96 of which featured microvascular complications (retinopathy and nephropathy in particular) and 29 without such concurrent problems, as well as in 76 controls. All the cytokines studied, including IL-33, were higher in T1DM subjects, but without displaying any differences between subjects with, and without, microvascular complications (MVC).
Samuelsson et al. [29], in a prospective cohort study, tried to investigate the plasma levels of sST2 and to determine whether sST2 could be a reliable early predictor for diabetic complications (including nephropathy and retinopathy) in young (15–34 years old) patients with DM. For this study, 220 patients were enrolled and, after a 10-year follow-up period, 112 of them developed diabetes-related complications, including retinopathy (n = 91), nephropathy (n = 12) or both of them (n = 9). At the time of diagnosis, plasma levels of sST2 were significantly higher in patients who later developed nephropathy with respect to the others.
2.3. Results Achieved by the Studies on Other Renal Diseases
End-Sstage renal disease (ESRD) was investigated twice by the same group [30,34], with the second study making use of part of the data collected during the first one. In the study published in 2016, 55 patients with ESRD were assessed in terms of sST2, evaluated by ELISA, before and after Hemodiafiltration, showing that sST2 was unaffected by the treatment cited. In 2018, the cohort was increased up to 123 patients, who were prospectively followed up from the date of the sST2/NT-proBNP measurement until their death or maximally up to 829 days. Those patients were divided into low- (<35 ng/mL) and high- (≥35 ng/mL) sST2 level cohort, according to the concentration of sST2 measured at the start of the study. The survived patients (89 out of 123 patients) showed a lower sST2 level with respect to deceased subjects, with the latter showing a significantly lower survival rate (p < 0.01) according to the Kaplan-Meier survival analysis. The analysis performed demonstrated that sST2 alone, and in combination with NT-proBNP, can predict the causes of mortality, and is independent from both renal function and hemodiafiltration treatment.
3. IL-33/ST2 Axis Involvement in Diabetic Kidney Disease or Related Nephropathy
3.1. IL-33/ST2 in Diabetic Kidney Disease
IL-33 was first identified as a member of the IL-1 family in 2005 [7], and was seen as acting as a ligand for the ST2. As stated, the ST2, a receptor for IL-33, is selectively expressed by several immune cells involved in type-2 immune response, like ILC2 cells. The cells are recruited through the activity of IL-33, and are useful in the immunity against pathogens, type 2 inflammation, tissue homeostasis, and repair [8], and pivotal in conditions like asthma and atopic dermatitis [9,10].
According to the literature, sST2 serum levels were higher in CKD and associated with the severity of the disease [25], possibly having a role in the development of CKD or as a marker of disease severity. The role for sST2 appears to be stronger than the role of IL-33, as demonstrated by the lack of association between the increased IL-33 levels and kidney injury in diabetic nephropathy, with such increase possibly associated with diabetic disease [26]. Taken together with the evidences of other studies, which are not included in the detailed review as they do not directly deal with the conditions investigated in this work [35,36], such retrievals suggest that the serum levels of sST2 are more relevant in investigating the deterioration of kidney function with respect to the serum levels of IL-33.
As stated, diabetic nephropathy (DN) is probably the most prevalent etiology for CKD, featuring increases in excretion of urinary albumin, elevated blood pressure coupled with glomerular lesions, and functional loss of glomerular filtration leading to renal failure [37]. During its progression, several evidences for inflammatory responses have been shown, therefore the involvement of inflammatory cytokines in disease progression and severity is clear. The previously reported study by Caner and colleagues [26] showed that IL-33 levels were higher in DM patients with associated MA, followed by DM patients and controls. This demonstrated the up-regulation of IL-33 in diabetes, but failed to reliably show the usefulness of IL-33 for early diagnosis of DN. Interestingly, IL-33 was also significantly elevated in T1DM subjects, but without significant differences between those with, and without, microvascular complications [27]. On the other hand, the involvement of the ST2 expression was clear in all studies published to date, making it a reliable factor alone, or taken together, with IL-33, for the involvement in diabetic complications [29,38,39].
3.2. IL-33/ST2 in Other Renal Diseases
IL-33 and ST2 were also investigated in renal cell carcinoma (RCC) by Wu et al. [40]. A significant correlation between IL-33 expression and metastasis stage was found, and obviously this was also related to the overall prognosis of the patients. Indeed, the more advanced the metastasis stage, the worse the prognosis, and the higher the expression of IL-33. It was then hypothesized that IL-33 promotes RCC cell proliferation and chemotherapy resistance via its receptor ST2 and the JNK signaling activation in tumor cells. Therefore, targeting IL-33/ST2 and JNK signaling may have potential value in the treatment of RCC. However, the results obtained should be further confirmed in future studies on larger cohorts.
Another role for IL-33 was demonstrated by Akcay and colleagues [41], finding that IL-33 promotes acute kidney injury (AKI), through CD4 T cell-mediated production of the pro-inflammatory chemokine CXCL1. The inhibition of either IL-33, or CXCL1, might have a significant therapeutic potential in AKI. Therefore, studies dealing with the targeting of IL-33 or IL-33/ST2 axis should be carried out in order to verify their effective usefulness in decreasing the burden of Diabetic kidney disease (DKD) and other renal diseases, where a role for this axis was hypothesized, as already occurring in other conditions, including inflammatory diseases [42,43].
Finally, IL-33 could be a factor in renal transplantation, since a serum up-regulation of this compound would contribute to the pathogenesis of the chronic allograft dysfunction (CAD), endangering the long-term allograft survival in in kidney transplant recipients (KDR) [44]. In such patients, higher circulating IL-33 is associated with diminished glomerular filtration rate, age, diabetes, serum phosphorus and microalbuminuria, as well as with higher score for major adverse cardiovascular events, supporting a role for IL-33 in the cardiovascular burden for KDR [45]. End-stage renal disease was studied in two consecutive studies conducted by Homsak and Ekart [30,34]. In light of their findings, sST2 was demonstrated to have a high prognostic value, independent of renal function and of hemodiafiltration treatment. It was demonstrated to be a useful prognostic marker for stratifying ESRD patients on HDF, at a high risk for life-threatening events, hospitalization, and death, especially in combination with NT-proBNP. Furthermore, sST2 levels were seen to be correlated with disease activity in systemic lupus erythematosus (SLE), thereby suggesting a potential role for this compound as a marker for disease activity [46].
4. Conclusions
Despite the relatively low number of studies published to date, the link between IL-33/ST2 axis and diabetic kidney disease, appears to be more clear, with significant correlations with the disease stage. The ST2 involvement appears to be more predictive than IL-33, especially in investigating the deterioration of kidney function; however, both compounds are pivotal in this topic, with future studies confirming the scientific evidences on larger and more heterogeneous cohorts.
Author Contributions
Literature search, A.T., P.Q.; conceptualization, A.T., S.G.; bias assessment, A.T., P.Q.; study selection, A.T., P.Q., S.G.; manuscript drafting, A.T., P.Q., S.G.; critical revision and approval of the manuscript, all authors.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar]
- Gangemi, S.; Franchina, T.; Minciullo, P.L.; Profita, M.; Zanghì, M.; David, A.; Kennez, I.; Adamo, V. IL-33/IL-31 axis: A new pathological mechanisms for EGFR tyrosine kinase inhibitors-associated skin toxicity. J. Cell Biochem. 2013, 114, 2673–2676. [Google Scholar] [CrossRef]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediators Inflamm. 2018, 2018, 3858032. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef]
- Chen, W.Y.; Li, L.C.; Yang, J.L. Emerging Roles of IL-33/ST2 Axis in Renal Diseases. Int. J. Mol. Sci. 2017, 18, 783. [Google Scholar] [CrossRef]
- Lu, J.; Kang, J.; Zhang, C.; Zhang, X. The role of IL-33/ST2L signals in the immune cells. Immunol. Lett. 2015, 164, 11–17. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Niu, Z.; Wang, C.; Wang, R.; Zhang, Z.; Chen, T.; Wang, X.M.; Li, Q.; Lee, V.W.S.; et al. Potentiating Tissue-Resident Type 2 Innate Lymphoid Cells by IL-33 to Prevent Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2018, 29, 961–976. [Google Scholar] [CrossRef]
- Zhu, J. Mysterious ILC2 tissue adaptation. Nat. Immunol. 2018, 19, 1042–1044. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Saito, H.; Peebles, R.S. Advances in mechanisms of allergic disease in 2016. J. Allergy Clin. Immunol. 2017, 140, 1622–1631. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef]
- Ahmad, T.; Wang, T.; O’Brien, E.C.; Samsky, M.D.; Pura, J.A.; Lokhnygina, Y.; Rogers, J.G.; Hernandez, A.F.; Craig, D.; Bowles, D.E.; et al. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail. 2015, 3, 30–39. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Pascual-Figal, D.; Daniels, L.B. ST2 testing for chronic heart failure therapy monitoring: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 70B–75B. [Google Scholar] [CrossRef]
- Ponce, D.M.; Hilden, P.; Mumaw, C.; Devlin, S.M.; Lubin, M.; Giralt, S.; Goldberg, J.D.; Hanash, A.; Hsu, K.; Jenq, R.; et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood 2015, 125, 199–205. [Google Scholar] [CrossRef]
- Palmer, G.; Talabot-Ayer, D.; Lamacchia, C.; Toy, D.; Seemayer, C.A.; Viatte, S.; Finckh, A.; Smith, D.E.; Gabay, C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009, 60, 738–749. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.-P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, P.; Duan, L.; Yang, L.; Wang, J. IL-33 and kidney disease (Review). Mol. Med. Rep. 2016, 13, 3–8. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, C.; Wang, Z.; Tao, J.; Han, Z.; Zhang, W.; Tan, R.; Gu, M. Interleukin-33 levels are elevated in chronic allograft dysfunction of kidney transplant recipients and promotes epithelial to mesenchymal transition of human kidney (HK-2) cells. Gene 2018, 644, 113–121. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Invest. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Hung, A.M.; Crawford, D.C.; Griffin, M.R.; Brown-Gentry, K.; Lipkowitz, M.S.; Siew, E.D.; Cavanaugh, K.; Lewis, J.B.; Ikizler, T.A.; AASK Study Group. CRP polymorphisms and progression of chronic kidney disease in African Americans. Clin. J. Am. Soc. Nephrol. 2010, 5, 24–33. [Google Scholar] [CrossRef]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Jhangri, G.S.; Curhan, G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005, 68, 237–245. [Google Scholar] [CrossRef]
- Satirapoj, B. Nephropathy in diabetes. Adv. Exp. Med. Biol. 2012, 771, 107–122. [Google Scholar]
- Satirapoj, B. Review on pathophysiology and treatment of diabetic kidney disease. J. Med. Assoc. Thai. 2010, 93, S228–S241. [Google Scholar]
- Bao, Y.S.; Na, S.P.; Zhang, P.; Jia, X.B.; Liu, R.C.; Yu, C.Y.; Mu, S.H.; Xie, R.J. Characterization of interleukin-33 and soluble ST2 in serum and their association with disease severity in patients with chronic kidney disease. J. Clin. Immunol. 2012, 32, 587–594. [Google Scholar] [CrossRef]
- Caner, S.; Usluoğulları, C.A.; Balkan, F.; Büyükcam, F.; Kaya, C.; Saçıkara, M.; Koca, C.; Ersoy, R.; Çakır, B. Is IL-33 useful to detect early stage of renal failure? Ren. Fail. 2014, 36, 78–80. [Google Scholar] [CrossRef]
- Shruthi, S.; Mohan, V.; Amutha, A.; Aravindhan, V. Increased serum levels of novel T cell cytokines IL-33, IL-9 and IL-17 in subjects with type-1 diabetes. Cytokine 2016, 86, 6–9. [Google Scholar] [CrossRef]
- Gungor, O.; Unal, H.U.; Guclu, A.; Gezer, M.; Eyileten, T.; Guzel, F.B.; Altunoren, O.; Erken, E.; Oguz, Y.; Kocyigit, I.; et al. IL-33 and ST2 levels in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS ONE 2017, 12, e0178939. [Google Scholar] [CrossRef]
- Samuelsson, M.; Dereke, J.; Svensson, M.K.; Landin-Olsson, M.; Hillman, M.; The DISS Study Group. Soluble plasma proteins ST2 and CD163 as early biomarkers of nephropathy in Swedish patients with diabetes, 15–34 years of age: A prospective cohort study. Diabetol. Metab. Syndr. 2017, 9, 41. [Google Scholar] [CrossRef]
- Homsak, E.; Ekart, R. ST2 as a novel prognostic marker in end-stage renal disease patients on hemodiafiltration. Clin. Chim. Acta 2018, 477, 105–112. [Google Scholar] [CrossRef]
- Tonacci, A.; Bruno, R.M.; Ghiadoni, L.; Pratali, L.; Berardi, N.; Tognoni, G.; Cintoli, S.; Volpi, L.; Bonuccelli, U.; Sicari, R.; et al. Olfactory evaluation in Mild Cognitive Impairment: correlation with neurocognitive performance and endothelial function. Eur. J. Neurosci. 2017, 45, 1279–1288. [Google Scholar] [CrossRef]
- Striker, G.E.; Agodoa, L.L.; Held, P.; Doi, T.; Conti, F.; Striker, L.J. Kidney disease of diabetes mellitus (diabetic nephropathy): Perspectives in the United States. J. Diabet. Complications. 1991, 5, 51–52. [Google Scholar] [CrossRef]
- Anand, G.; Vasanthakumar, R.; Mohan, V.; Babu, S.; Aravindhan, V. Increased IL-12 and decreased IL-33 serum levels are associated with increased Th1 and suppressed Th2 cytokine profile in patients with diabetic nephropathy (CURES-134). Int. J. Clin. Exp. Pathol. 2014, 7, 8008–8015. [Google Scholar]
- Homsak, E.; Ekart, R. Hemodiafiltration affects NT-proBNP but not ST2 serum concentration in end-stage renal disease patients. Clin. Biochem. 2016, 49, 1159–1163. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Profita, M.; Alonci, A.; Saitta, S.; Russo, S.; Bonanno, A.; Innao, V.; Gangemi, S. Reduced IL-33 plasma levels in multiple myeloma patients are associated with more advanced stage of disease. Br. J. Haematol. 2013, 160, 709–710. [Google Scholar] [CrossRef]
- Duan, L.; Huang, Y.; Su, Q.; Lin, Q.; Liu, W.; Luo, J.; Yu, B.; He, Y.; Qian, H.; Liu, Y.; et al. Potential of IL-33 for preventing the kidney injury via regulating the lipid metabolism in gout patients. J. Diabetes Res. 2016, 2016, 1028401. [Google Scholar] [CrossRef]
- Lim, A.K.; Tesch, G.H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 2012, 146154. [Google Scholar] [CrossRef]
- Miller, A.M.; Purves, D.; McConnachie, A.; Asquith, D.L.; Batty, G.D.; Burns, H.; Cavanagh, J.; Ford, I.; McLean, J.S.; Packard, C.J.; et al. Soluble ST2 associates with diabetes but not established cardiovascular risk factors: A new inflammatory pathway of relevance to diabetes? PLoS ONE 2012, 7, e47830. [Google Scholar] [CrossRef]
- Ryba-Stanislawowska, M.; Werner, P.; Skrzypkowska, M.; Brandt, A.; Mysliwska, J. IL-33 effect on quantitative changes of CD4+CD25highFOXP3+ regulatory T cells in children with type 1 diabetes. Mediators Inflamm. 2016, 2016, 9429760. [Google Scholar] [CrossRef]
- Wu, C.W.; Wu, Y.G.; Cheng, C.; Hong, Z.D.; Shi, Z.M.; Lin, S.Q.; Li, J.; He, X.Y.; Zhu, A.Y. Interleukin-33 Predicts Poor Prognosis and Promotes Renal Cell Carcinoma Cell Growth Through its Receptor ST2 and the JNK Signaling Pathway. Cell. Physiol. Biochem. 2018, 47, 191–200. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; He, Z.; Turkmen, K.; Won Lee, D.; Hernando, A.A.; Altmann, C.; Toker, A.; Pacic, A.; Galesic Ljubanovic, D.; et al. IL-33 exacerbates acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 2057–2067. [Google Scholar] [CrossRef]
- Nabe, T. Interleukin (IL)-33: new therapeutic target for atopic diseases. J. Pharmacol. Sci. 2014, 126, 85–91. [Google Scholar] [CrossRef]
- Chen, W.Y.; Tsai, T.H.; Yang, J.L.; Li, L.C. Therapeutic Strategies for Targeting IL-33/ST2 Signalling for the Treatment of Inflammatory Diseases. Cell Physiol. Biochem. 2018, 49, 349–358. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Xu, Z.; Han, Z.; Tao, J.; Lu, P.; Huang, Z.; Zhou, W.; Zhao, C.; Tan, R.; et al. The Potential Role of IL-33 in Renal Transplant Recipients with Chronic Allograft Dysfunction. Ann Transplant 2016, 21, 611–618. [Google Scholar] [CrossRef]
- Mansell, H.; Soliman, M.; Elmoselhi, H.; Shoker, A. Elevated circulating Interleukin 33 levels in stable renal transplant recipients at high risk for cardiovascular events. PLoS ONE 2015, 10, e0142141. [Google Scholar] [CrossRef]
- Mok, M.Y.; Huang, F.P.; Ip, W.K.; Lo, Y.; Wong, F.Y.; Chan, E.Y.; Lam, K.F.; Xu, D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology 2010, 49, 520–527. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).