Abstract
The n-butanol fraction (BF) obtained from the crude extract of the marine sponge Petromica citrina, the halistanol-enriched fraction (TSH fraction), and the isolated compounds halistanol sulfate (1) and halistanol sulfate C (2), were evaluated for their inhibitory effects on the replication of the Herpes Simplex Virus type 1 (HSV-1, KOS strain) by the viral plaque number reduction assay. The TSH fraction was the most effective against HSV-1 replication (SI = 15.33), whereas compounds 1 (SI = 2.46) and 2 (SI = 1.95) were less active. The most active fraction and these compounds were also assayed to determine the viral multiplication step(s) upon which they act as well as their potential synergistic effects. The anti-HSV-1 activity detected was mediated by the inhibition of virus attachment and by the penetration into Vero cells, the virucidal effect on virus particles, and by the impairment in levels of ICP27 and gD proteins of HSV-1. In summary, these results suggest that the anti-HSV-1 activity of TSH fraction detected is possibly related to the synergic effects of compounds 1 and 2.
1. Introduction
The drug of choice for the prophylaxis and treatment of Herpex Simplex Virus (HSV) infections is acyclovir (ACV), which selectively inhibits HSV DNA replication with low host-cell toxicity. However, the intensive use of antiviral drugs has led to the emergence of resistant viruses [1,2,3]. Recently, De Clercq [4] described the evolution of antiviral agents against some viral infections, including HSV, confirming that the search for new antiviral agents is still relevant.
Pharmaceutical interest in marine organisms has provided thousands of new and novel compounds that have shown important biological properties, such as anticancer, antiviral, antiprotozoal, and antibacterial activities [2,5,6,7,8]. In this context, marine sponges have been a prolific source of diverse secondary metabolites with complex and unique structures [2,9,10,11,12,13]. Some of them were used as lead compounds to obtain new drugs that are currently used in clinics, such as acyclovir, vidarabine, cytarabine, eribulin mesylate, and others, that are now in clinical stages of evaluation such hemiasterlin [14,15,16]. In addition, several highly active compounds from marine sponges have been reported as new biologically active structures [17,18,19,20,21,22,23,24].
Petromica citrina (Porifera, Demospongie) belongs to a marine sponge genus that occurs only on the Brazilian coast [25]. There are few studies with this species, and most of them describe the evaluation of different pharmacological properties such as antibacterial and antiviral activities for its aqueous extracts [26,27] and n-butanol fraction [28]. Moreover, a restricted number of chemical investigations and a few bioactive constituents have been reported, in particular, a sulfated steroidal compound, identified as halistanol sulfate [29,30].
Recently, our research group described the anti-herpes activity of the n-butanol fraction of P. citrina [28]. Thus, the aim of this investigation was to determine, through a bioguided study, the active compounds responsible for the anti-HSV-1 activity detected.
2. Results and Discussion
2.1. Bioguided Fractionation of the n-Butanol Fraction of P. citrina
In a previous screening of the anti-infective potential of marine invertebrates and seaweeds [28], we observed a promising activity for the n-butanol fraction (BF) obtained from the ethanolic crude extract of this sponge that led us to perform this study. Our goal was to isolate, through a bioguided study, the anti-herpes bioactive metabolites present in this fraction.
First, the BF fraction was submitted to several Sephadex LH-20 chromatography procedures yielding five fractions (Sep-1 to Sep-5), which were pooled based on thin-layer chromatography (TLC) similarity. Among these fractions, only fraction Sep-5 showed anti HSV-1 activity and was submitted to NMR analysis. The 1H NMR spectrum of Sep-5 displayed characteristic signals of the presence of halistanol sulfates as the major compounds. These major compounds were isolated by C18 column chromatography, yielding compounds 1 and 2 (Figure 1).
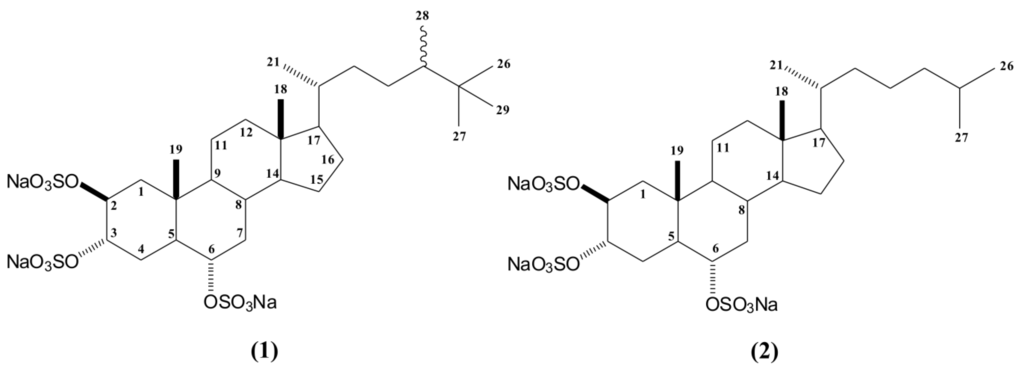
Figure 1.
Structures of halistanol sulfate (1) and halistanol sulfate C (2).
The complete structure of compound 1 was determined based on HSQC, HMBC, and COSY spectra, as well as by ESI mass spectrometry and by comparison with literature data [29,30,31,32,33]. The presence of three sulfate groups in the structure could be clearly defined by ESI mass spectrometry (m/z 731 [M − Na]−, m/z 611 [M − NaHSO4], m/z 491 [M − (NaHSO4)2] and m/z 354 [M − (NaHSO4)3]). These sulfate groups was also supported by the IR band (1230 cm−1).
In addition, the 1H NMR spectrum of compound 1 showed carbinol signals at δH 4.83 (sl), δH 4.76 (sl; J = 1.8 Hz), and δH 4.20 (dt; J = 11.0; 4.4 Hz), corresponding in the HSQC spectrum to the signals at δc 75.6 (CH-2 and CH-3), and δc 78.8 (CH-6), respectively. These data, together with characteristic signals of two methyl singlets at δ 0.70 (CH3-18) and δ 1.07 (CH3-19), suggested a sulfated sterol nucleus. The structure of the side chain of compound 1 was elucidated by analysis of 2D NMR data. The NMR spectra showed the presence of a side chain containing two secondary methyls at δ 0.95 (d; J = 6.4 Hz) and δ 0.84 (d; J = 6.8 Hz) attributed to positions C21 and C28, also based on HMBC data. The 1H NMR spectra revealed a singlet at δ 0.86 (9H), which was connected to carbon at δ 27.9, suggesting a t-butyl group on the side chain. HMBC correlations of carbons at δ 27.9 (C26, C27 and C29), δ 34.2 (C25), and δ 45.5 (C24) to the proton at δ 0.86 confirmed that C26, C27, and C29 were connected to C25. Therefore, compound 1 was identified as halistanol sulfate, a steroid previously reported for marine sponges such as Halichondria cf. [31], Epipolasis sp. [32], Petromica ciocalyptoides [29], Haliclona sp. [33], and Petromica citrina [30].
Halistanol sulfate (HS) was first reported in 1981 by Fusetani et al. [31] and, in that work, the authors only showed the 13C NMR data of HS. New compounds of the halistanol sulfate series (halistanol sulfates A to H) were isolated in the subsequent years [32,34], but the nomenclature and the chemical shift values in the 1H NMR spectra of the side chain are still not completely defined [29,32]. Therefore, it is important that the details of the structural elucidation of compounds 1 and 2 are also presented.
Compound 2 also showed the same halistanol steroidal nucleus signals, but with a shorter side chain, which was inferred by NMR data together with the information of the ESI mass spectrum. Moreover, the ESI/MS spectrum showed the presence of three sulfate groups (m/z 703 [M − Na]−; m/z 583[M − NaHSO4]; m/z 463 [M − (NaHSO4)2] and m/z 340 [M − (NaHSO4)3]) in the structure. As well as for compound 1 the presence of sulfate groups in the structure was also supported by the IR band (1226 cm−1). Although the 1H-NMR spectra of compound 1 displayed two methyl doublets at δ 0.95 and δ 0.84 on the side chain, corresponding to C21 and C28, respectively, the 1H NMR of compound 2 only one doublet signal at δ 0.94 (d; J = 6.6 Hz), corresponding to the C21 methyl group. In addition, the 1H NMR data did not show a t-butyl group at the end side of the chain. Furthermore, two new methyl signals at δ 0.87 (d; J = 6.6 Hz) and δ 0.89 (d; J = 6.6 Hz) were identified. Considering the J values of these protons, we could suggest the presence of an isopropyl on the side chain. Thus, based on the data obtained, compound 2 was identified as halistanol sulfate C, a steroid previously reported for Pseudoaxinissa digitata [34] and Epilopasis sp. [32]. As far as we are aware, this is the first report of halistanol sulfate C for Petromica citrina.
Sulfated sterols have been described from a wide variety of marine organisms, such as sponges and echinoderms. Several of these sterols have a great structural diversity and broad spectrum of biological activities [35,36,37,38,39].
The first reported compound of the halistanol family was halistanol sulfate, isolated from the marine sponge Halichondria cf. moorei Bergquist [31]. Important biological activities have been reported for this steroid sulfate, such as anti-HIV effects [38], cytotoxic activity against human hepatoma cells (QGY-7701), and chronic myelogenous leukemia cells (K562) [40]. Afterwards, the same compound was isolated from Petromica ciocalyptoides and Topsentia ophiraphidites, showing inhibitory activity of Leishmania tarentola [29] and a wide spectrum of activity against resistant bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Mycobacterium fortuitum, and Neisseria gonorrheae [30].
Thus far, eight sulfated sterols have been described with this fundamental nucleus and named as halistanol sulfates A to H (Figure 2). All of them are characterized by the same 2β, 3α, 6α-trisulfoxy functionalities, differing only in their side chains [32,34,35]. The most promising pharmacological activities described for these compounds were the anti-HIV-1 and anti-HIV-2 effects for halistanol sulfates F and G [32].
In addition, there are many other reports about different members of the halistanol series that have shown important pharmacological properties. One of the first reported members of this series was ibisterol sulfate, isolated from Topsentia sp., which showed anti-HIV activity [41]. Other examples of halistanol-type compounds with antiviral activity are weinbersterol disulfates A and B isolated from the sponge Petrosia weinbergi which exhibited activity against leukemia virus (FeLV), mouse influenza virus (PR8), and mouse coronavirus (A59) replication [42].
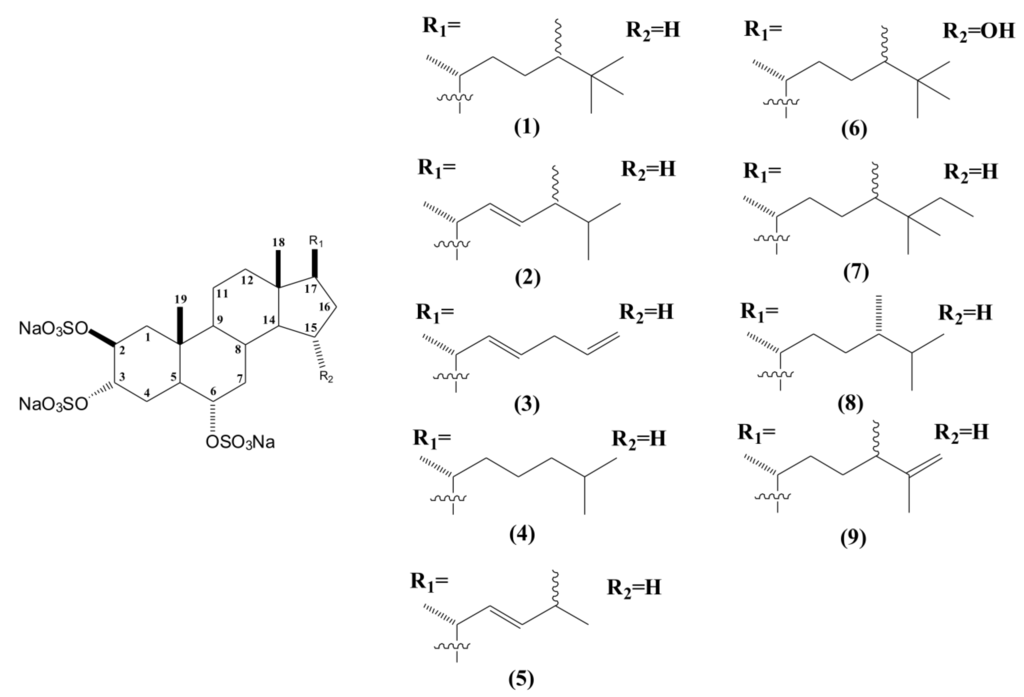
Figure 2.
Structures of halistanol sulfate (1) and derivatives halistanol sulfates A to H (2–9).
As compounds with sulfated groups are described to have antiviral properties [34,38,43,44,45,46], and due to the anti-herpetic activity shown by the BF fraction, we decided to verify the anti-HSV-1 activity of compounds 1 and 2 and the TSH fraction and to elucidate their mode of action.
3. Experimental Section
3.1. General Experimental Procedures
General 1D and 2D NMR experiments were performed on a Bruker Avance 2 (500 MHz) instrument at 500 MHz for 1H and 125 MHz for 13C. All spectra were recorded in CD3OD using the signals of residual non-deuterated solvent as internal reference. Mass spectrometric analyses were performed using a Bruker micrOTOF-Q II mass spectrometer (Bruker® Daltonics, Billerica, MA, USA), equipped with ESI. Multi-point mass calibration was carried out using a mixture of sodium formate from m/z 50 to 900. Data acquisition and processing were carried out using the Bruker Compass Data Analysis version 4.0 software supplied with the instrument. All the analytical solutions (0.5 mg/mL) were prepared using methanol LCMS grade. Compounds were infused into the source using a KDS 100 syringe pump (KD Scientific, Holliston, MA, USA) at a flow rate of 180 mL/min. General MS conditions: Capillary 3.5 kV (negative ion mode), dry heater 180 °C, nebulizer 0.4 bar, dry gas (N2), 4 L/min (UMYMFOR/UBA). Silica gel 60 (70–230 mesh) Merck®, RP18 (Fluka®, Buchs, Switzerland), Sephadex LH-20 (GE healthcare®, Chalfont St Giles, UK), TLC analysis was performed on Silica gel F254 and RP18 plates (Sigma-Aldrich®, St. Louis, MO, USA).
3.2. Sponge Collection
Petromica citrina was collected from January to July 2010 at Xavier Island (27°36′39′′ S; 48°23′32′′ W), Santa Catarina State, Brazil, at a depth of 9–17 m, and immediately frozen. The material was identified by Dr. João Luís Carraro and voucher specimens were deposited in the Porifera collection of the Museu de Ciências Naturais da Fundação Zoobotânica do Rio Grande do Sul, Brazil (MCNPOR 8777, 8778, 8779, 8780, 8781).
3.3. Extraction and Isolation of Compounds 1 and 2
The frozen sponge (1700 g, wet) was exhaustively extracted with ethanol for three days at room temperature. The crude ethanolic extract (CHE) was filtered, the ethanol was eliminated under reduced pressure, and the gummy residue was suspended in H2O before being extracted successively with ethyl acetate (EtOAc) and n-butanol (n-BuOH) (3 × 500 mL) yielding three fractions: EtOAc (EAF), n-BuOH (BF) and aqueous residue (AR), respectively. Next, the BF fraction (2.0 g) was subjected to Sephadex LH-20 column chromatography (790 mm × 25 mm) using methanol (MeOH) as eluent. A total of 180 tubes (20 mL) were collected and combined into five fractions (Sep-1, 650 mg; Sep-2, 450 mg; Sep-3, 350 mg; Sep-4, 250 mg; and Sep-5, 300 mg) based on Silica gel thin-layer chromatography (TLC) similarity. Because the fraction Sep-5 (named TSH fraction) showed only one spot by TLC analysis, this fraction was forwarded to 1HNMR analysis and proved to be a mixture of halistanol sulfate (Compound 1) and halistanol sulfate C (Compound 2) as the major compounds.
The TSH fraction (200 mg) was then dissolved in methanol and submitted to a reversed-phase column chromatography (300 mm × 20 mm) packed with RP18 as stationary phase and MeOH:H2O (1:1 v/v) as mobile phase. This procedure resulted in two isolated compounds: halistanol sulfate (30 mg, compound 1) and halistanol sulfate C (12 mg, compound 2). Figure 4 shows the steps of purification.
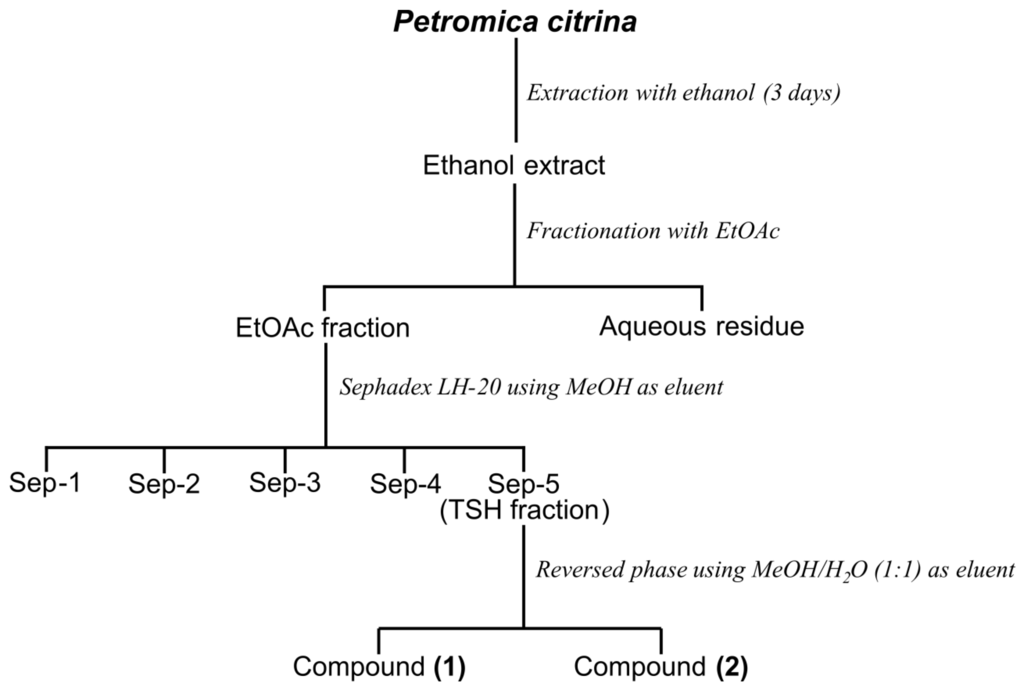
Figure 4.
Overview of the strategy used for the purification of sulfate halistanol (compound 1) and sulfate halistanol C (compound 2) from the butanol fraction of Petromica citrina.
Halistanol sulfate (1): White amorphous powder; IR (KBr)νmax 3442, 2953, 1643, 1392, 1230, 1068 cm−1; 1H NMR (CD3OD, 500 MHz) δ 4.83 (1H, sl, H-2), 4.76 (1H, sl, J = 1.8 Hz, H-3), 4.20 (1H, dt, J = 4.4, 10.9 Hz, H-6), 2.37 (1H, dl, J = 10.2 Hz, H-7a), 2.30 (1H, dl, J = 14.5 Hz; H-4a), 2.11 (1H, dl, J = 14.3 Hz, H-1a), 2.01 (1H, dl, J = 12.4 Hz, H-12a), 1.86 (1H, m, H-16a), 1.81 (1H, tl, J = 14.4 Hz, H-4b), 1.69 (1H, m, H-16b), 1.63 (1H, m, H-5), 1.64 (1H, m, H-15a), 1.58 (1H, m, H-22a), 1.55 (1H, m, H-11a), 1.54 (1H, m, H-8), 1.49 (1H, dd, J = 4.0, 14.3 Hz, H-1b), 1.38 (1H, m, H-20), 1.33 (1H, m, H-11b), 1.29 (1H, m, H-12b), 1.15 (1H, m, H-14), 1.12 (1H, m, H-15b), 1.10 (1H, m, H-17), 1.07 (3H, s, H-19), 1.02 (1H, m, H-7b), 0.99 (2H, m, H-24), 0.95 (3H, d, J = 7.4 Hz, H-21), 0.90 (1H, m, H-22b), 0.78 (2H, m, H-23), 0.86 (9H, sl, H-26, 27, 29), 0.84 (3H, d, H-28) 0.78 (1H, m, H-9), 0.70 (3H, sl, H-18); 13C NMR (CD3OD, 125 MHz): δ 78.8 (CH, C-6), 75.6 (CH, C-3), 75.6 (CH, C-2), 57.7 (CH, C-17), 57.6 (CH, C-14), 55.8 (CH, C-9), 45.5 (CH, C-24), 45.4 (CH, C-5), 43.8 (C, C-13), 41.3 (CH2, C-12), 40.1 (CH2, C-7), 39.2 (CH2, C-1), 37.7 (CH2, C-22), 37.7 (CH, C-20), 37.7 (C, C-10), 35.2 (CH, C-8), 34.1 (C, C-25), 29.2 (CH2, C-16), 27.9, (CH3, C-26), 27.9 (CH3, C-27), 27.9 (CH3, C-29), 25.2 (CH2, C-15), 25.1 (CH2, C-4), 21.9 (CH2, C-11), 22.03 (CH2, C-23), 19.6 (CH3, C-21), 15.3 (CH3, C-19), 15.0 (CH3, C-28), 12.5 (CH3, C-18). ESI-MS m/z 731.2198 [M − Na]− (calcd for C29H49Na3O12, 754.2100).
Halistanol sulfate C (2): White amorphous solid; IR (KBr)νmax 3442, 2949, 1625, 1384, 1226, 1070 cm−1; 1H NMR (CD3OD, 500 MHz) δ 4.83(1H, m, H-2), 4.76 (1H, q, J = 2.7 Hz, H-3), 4H-2), 4.20 (1H, td, J = 11.1, 4.4 Hz, H-6), 2.37 (1H, dt, J = 12.3, 4.4 Hz, H-7a), 2.30 (1H, dt, J = 15.0, 2.8, 1.1 Hz; H-4a), 2.11 (1H, dd, J = 14.7, 1.7 Hz, H-1a), 2.01 (1H, dl, J = 12.7, 3.5 Hz, H-12a), 1.86 (1H, m, H-16a), 1.81 (1H, ddd, J = 15.0, 13.2, 2.8 Hz, H-4b), 1.63 (1H, m, H-5), 1.61 (1H, m, H-15a), 1.53 (1H, m, H-8), 1.52 (2H, m, H-8, H-25), 1.48 (1H, dd, J = 14.7, 3.9 Hz, H-1b), 1.38 (1H, m, H-20), 1.31 (1H, qd, J = 13.1, 3.5 Hz, H-11), 1.29 (1H, m, H-16b), 1.14 (1H, m, H-12b), 1.12 (3H, m, H-14, H-15a, H-17), 1.07 (3H, sl, H-19), 1.02 (1H, m, H-7b), 0.94 (3H, d, J = 6.5 Hz, H-21), 0.9–1.4 (6H, m, H2-22, H2-23, H2-24), 0.87 (3H, d, J = 6.6 Hz, H-26), 0.89(3H, d, J = 6.6 Hz, H-27), 0.78 (1H, m, H-9), 0.69 (3H, sl, H-18). 13C NMR (CD3OD, 125 MHz): δ 78.7 (CH, C-6), 75.5 (CH, C-2), 75.5 (CH, C-3), 57.6 (CH, C-17), 57.5 (CH, C-14), 55.8 (CH, C-9), 45.3 (CH, C-5), 43.8 (C, C-13), 41.2 (CH2, C-12), 40.8 (CH2, C-24), 40.0 (CH2, C-7), 40.0 (CH2, C-7), 39.2 (CH2, C-1), 37.6 (C, C-10), 37.3 (CH2, C-22), 37.0 (CH, C-20), 35.1 (CH, C-8), 29.2 (CH2, C-16), 29.1 (CH, C-25), 25.1 (CH2, C-4), 25.0 (CH2, C-23), 24.9 (CH2, C-15), 23.1 (CH3, C-26), 22.9 (CH3, C-27), 21.8 (CH2, C-11), 19.1 (CH3, C-21), 15.2 (CH3, C-19), 12.5 (CH3, C-18). ESI-MS m/z 703.2032 [M − Na]− (calcd for C27H45Na3O12S3, 726.1800).
3.4. Anti-HSV-1 Activity
3.4.1. Virus and Cell Line
HSV-1 (KOS strain, Faculty of Pharmacy, University of Rennes, France) was propagated in Vero cells. Viral stocks were stored at −80 °C and titrated based on plaque forming units (PFU) counted by plaque assay as previously described [53].
Vero (ATCC: CCL 81) cells were grown in Eagle’s minimum essential medium (MEM; Cultilab®, Campinas, Brazil) supplemented with 10% fetal bovine serum (FBS; Gibco®, Carlsbad, CA, USA), 100 U/mL penicillin G, 100 µg/mL streptomycin, and 25 µg/mL amphotericin B (Cultilab®), and maintained at 37 °C in humidified 5% CO2.
3.4.2. Cytotoxicity Assay
Vero cell viability was measured by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide—Sigma-Aldrich®, St. Louis, MO, USA) [54]. Briefly, confluent Vero cells were exposed to different concentrations of samples for 72 h, and after incubation, the 50% cytotoxic concentration (CC50) of each one was calculated as the concentration that reduces cell viability by 50%, when compared to untreated controls.
4. Conclusions
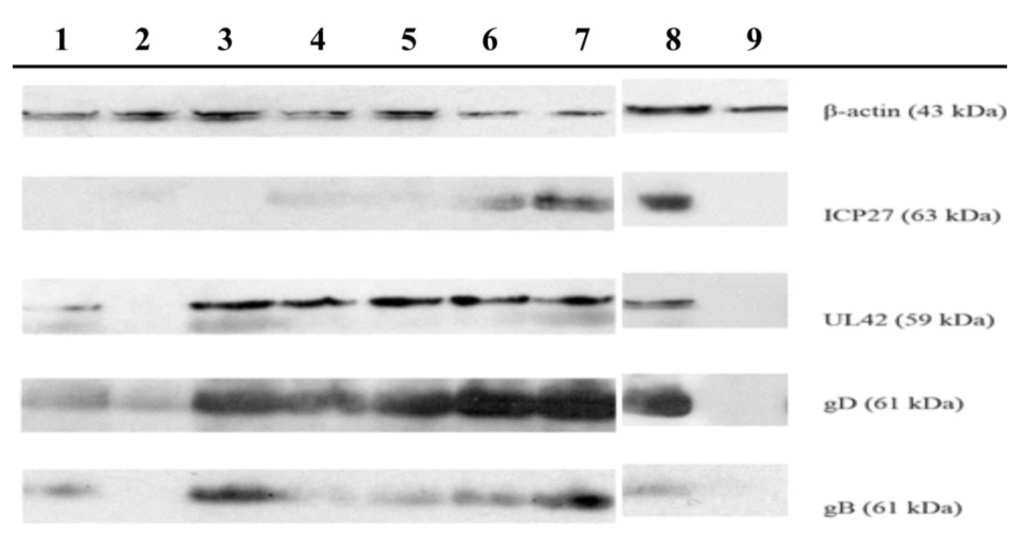
In summary, these results suggest that the TSH fraction and compounds 1 and 2 present antiherpes activity through the reduction of viral infectivity, inhibition of virus entry into the cells, and by the impairment of levels of ICP27 and gD proteins of HSV-1.
The relevant selectivity index of 15.33 of TSH fraction and its content (compounds 1 and 2) as well as the strong synergism effects observed suggest that the detected anti-HSV activity could be explained by the synergic effects of these major compounds present in the TSH fraction.
Acknowledgments
We would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq, MCTI (Grants 151561/2008-7, 306917/2009-2 and 471307/2011-4)], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [PNPD, CAPES, MEC (Grant 2207/2009)], Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC/BIODIVERSIDADE, Grant 14170/2010) for their financial support. The authors are also grateful to CNPq and CAPES for their research fellowships.
Abbreviations
| CMC | Carboxymethylcellulose |
| COSY | Correlation Spectroscopy |
| ESI | Electrospray ionization |
| HMBC | Heteronuclear Multiple Bond Correlation |
| HSQC | Heteronuclear Single Quantum Correlation |
| HSV | Herpes Simplex Virus |
| MEM | Minimal Essential Medium |
| NMR | Nuclear Magnetic Resonance |
| PFU | Plaque Forming Units |
| TSH | Halistanol-Enriched Fraction |
Conflict of Interest
The authors declare no conflict of interest.
References
- Nakakawa, E.; Reynold, S.J. The management of herpes simplex virus infections in HIV infected patients: Current issues and the role of cidofovir. Virus Adapt. Treat. 2011, 3, 35–43. [Google Scholar] [Green Version]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Snoeck, R. Antiviral therapy of herpes simplex. Int. J. Antimicrob. Agents 2000, 16, 157–159. [Google Scholar] [CrossRef]
- De Clercq, E. Antivirals: Past, present and future. Biochem. Pharmacol. 2013, 85, 727–744. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [CrossRef]
- Moura, L.A.; Ortiz-Ramirez, F.; Cavalcanti, D.N.; Ribeiro, S.M.; Muricy, G.; Teixeira, V.L.; Fuly, A.L. Evaluation of marine brown algae and sponges from Brazil as anticoagulant and antiplatelet products. Mar. Drugs 2011, 9, 1346–1358. [Google Scholar] [CrossRef]
- Sepčić, K.; Kauferstein, S.; Mebs, D.; Tur, T. Biological activities of aqueous and organic extracts from tropical marine sponges. Mar. Drugs 2010, 8, 1550–1566. [Google Scholar] [CrossRef]
- Uzair, B.; Mahmmod, Z.; Tabassum, S. Antiviral activity of natural products extracted from marine organisms. BioImpacts 2011, 1, 203–211. [Google Scholar]
- Abbas, S.; Kelly, M.; Bowling, J.; Sims, J.; Waters, A.; Hamann, M. Advancement into the arctic region for bioactive sponge secondary metabolites. Mar. Drugs 2011, 9, 2423–2437. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine pharmacology. Nat. Prod. Rep. 2000, 77, 135–140. [Google Scholar]
- Gordaliza, M. Cytotoxic terpene quinones from marine sponges. Mar. Drugs 2010, 8, 2849–2870. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Ivanchina, N.V.; Krasokhin, V.B.; Makarieva, T.N.; Stonik, V.A. Glycosides from marine sponges (Porifera, Demospongiae): Structures, taxonomical distribution, biological activities and biological roles. Mar. Drugs 2012, 10, 1671–1710. [Google Scholar] [CrossRef]
- Noro, J.C.; Kalaitzis, J.A.; Neilan, B.A. Papua new guinea marine sponges. Chem. Biodivers. 2012, 9, 2077–2095. [Google Scholar]
- Donia, M.; Hamann, M.T. Marine natural products and their potential applications as anti-infective agents. Lancet Infect. Dis. 2003, 3, 338–348. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug. Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Hadju, E.; Rocha, R.M.; Oliveira, J.H.H.L.; Hernandez, I.L.C.; Seleghim, M.H.R.; Granato, A.C.; Almeida, E.V.R.; Nunez, C.V.; Muricy, G.; et al. Challenges and rewards of research in marine natural products chemistry in Brazil. J. Nat. Prod. 2004, 67, 510–522. [Google Scholar] [CrossRef]
- Chairman, K.; Jeyamala, M.; Sankar, S.; Murugan, A.; Ranjit Singh, A.J.A. Immunomodulating Properties of Bioactive Compounds Present in Aurora globostellata. Int. J. Mar. Sci. 2013, 3, 151–157. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Vagias, C.; Roussis, V. Natural products with anti-HIV activity from marineorganisms. Curr. Top. Med. Chem. 2003, 3, 1512–1535. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; Guo, X.; Yang, B.; Yang, X.W.; Liu, Y. Chemical constituents of the sponge Mycale species from south China sea. Rec. Nat. Prod. 2013, 7, 119–123. [Google Scholar]
- Munro, M.H.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.H.; Cerella, C.; Dicato, M.; Diederich, M. A Survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules 2013, 18, 3641–3673. [Google Scholar] [CrossRef]
- Sipkema, D.; Franssen, M.C.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar] [CrossRef]
- Muricy, G.; Hajdu, E.; Minervino, J.V.; Madeira, A.V.; Peixinho, S. Systematic revision of the genus Petromica Topsent (Demospongiae:Halichondrida), with a new species from the southwestern Atlantic. Hydrobiologia 2001, 443, 103–128. [Google Scholar] [CrossRef]
- Marinho, P.R.; Muricy, G.R.S.; Silva, M.F.L.; Marval, M.G.; Laport, M.S. Antibiotic-resistant bacteria inhibited b extracts and fractions from Brazilian marine sponges. Braz. J. Pharmacogn. 2010, 20, 267–275. [Google Scholar]
- Silva, A.C.; Kratz, J.M.; Farias, F.M.; Henriques, A.T.; Santos, J.; Leonel, R.M.; Lerner, C.; Mothes, B.; Barardi, C.R.M.; Simões, C.M.O. In vitro antiviral activity of marine sponges collected off Brazilian Coast. Biol. Pharm. Bull. 2006, 29, 135–140. [Google Scholar] [CrossRef]
- Bianco, E.M.; Oliveira, S.Q.; Rigotto, C.; Tonini, M.; Guimaraes, T.R.; Bittencourt, F.; Gouvêa, L.; Aresi, C.; Almeida, M.T.R.; Moritz, M.I.G.; et al. Anti-infective potential of marine invertebrates and seaweeds from the Brazilian coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef]
- Kossuga, M.H.; Lira, S.P.; Nascimento, A.P.; Gambardella, M.T.P.; Berlinck, G.S.; Torres, Y.R.; Nascimento, G.G.F.; Pimenta, E.F.; Silva, M.; Thiemann, O.H.; et al. Isolantion and biological activities of secondary metabolites from the sponges monanchora aff. arbuscula, aplysina sp. petromica ciocalyptoides and topsentia ophiraphidies, from the ascidian didemnum ligulum and from the octocoral carijoa riisei. Quim. Nova 2007, 5, 1194–1202. [Google Scholar]
- Marinho, P.R.; Simas, N.K.; Kuster, R.M.; Duarte, R.S.; Fracalanzza, S.E.L.; Ferreira, D.F.; Romanos, M.T.V.; Muricy, G.; Demarval, M.G.; Laport, M.S. Antibacterial activity and cytotoxicity analysis of halistanol trisulfate from marine sponge Petromica citrina. J. Antimicrob. Chemother. 2012, 67, 2396–2400. [Google Scholar] [CrossRef]
- Fusetani, N.; Matsunaga, S.; Konosu, S. Bioactive marine metabolites II. Halistanol sulfate, as antimicrobial novel steroid sulfate from the marine sponge Halichondria cf. moorei Bergquist. Tetrahedron Lett. 1981, 22, 1985–1988. [Google Scholar] [CrossRef]
- Kanazawa, S.; Fusetani, N.; Matsunaga, S. Halistanol sulfates A–E. New steroids sulfates, from a marine sponge, Epipolasis sp. Tetrahedron 1992, 48, 5467–5472. [Google Scholar] [CrossRef]
- Sperry, S.; Crews, P. Haliclostanone sulfate and halistanol sulfate from an indo-pacific Haliclona sponge. J. Nat. Prod. 1997, 60, 29–32. [Google Scholar] [CrossRef]
- Bifulco, I.B.; Minale, L.; Riccio, R. Novel HIV-inhibitory halistanol sulfates F–H from a marine sponge, Pseudoaxinissa digitata. J. Nat. Prod. 1994, 57, 164–167. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Menna, M. Steroids from sponges: Recent reports. Steroids 1999, 64, 687–714. [Google Scholar] [CrossRef]
- Kicha, A.A.; Ivanchina, N.V.; Kalinovskii, A.I.; Dmitrenok, P.S.; Sokolova, E.V.; Agafonova, I.G. Sulfated steroid glycosides from the viet namese starfish Linckia laevigata. Chem. Nat. Comp. 2007, 43, 76–80. [Google Scholar] [CrossRef]
- Levina, E.V.; Aminin, D.L.; Kovalchuk, S.N.; Kozhemyako, V.B.; Dyshlovoi, S.A.; Kalinovskii, A.I.; Dmitrenok, P.S. Polar steroids from Solaster endeca starfish and the physiological activity of polar steroids from three starfish species. Russ. J. Bioorg. Chem. 2010, 36, 233–239. [Google Scholar] [CrossRef]
- McKee, T.C.; Cardellina, J.H.; Riccio, R.; D’auria, M.V.; Iorizzi, M.; Minale, L.; Moran, R.A.; Gulakowski, R.J.; Mcmahon, J.B.; Buckheit, R.W.; et al. HIV-inhibitory natural products, 11. Comparative studies of sulfated sterols from marine invertebrates. J. Med. Chem. 1994, 37, 793–797. [Google Scholar] [CrossRef]
- Whitson, E.L.; Bugni, T.S.; Chockalingam, P.S.; Concepcion, G.P.; Harper, M.K.; He, M.; Hooper, J.N.A.; Mangalindan, G.C.; Ritacco, F.; Ireland, C.M. Spheciosterol sulfates, PKCζ inhibitors from a philippine sponge Spheciospongia sp. J. Nat. Prod. 2008, 71, 1213–1217. [Google Scholar] [CrossRef]
- Jin, Y.; Fotso, S.; Yongtang, Z.; Sevvana, M.; Laatsch, H.; Zhang, W. Halichondria sulfonic acid, a new HIV-1 inhibitory guanidino-sulfonic acid, and halistanol sulfate isolated from the marine sponge Halichondria rugosa Ridley & Dendy. Nat. Prod. Res. 2006, 20, 1129–1135. [Google Scholar] [CrossRef]
- McKee, T.C.; Cardellina, J.H.; Tischler, M.; Snader, K.M.; Boyd, M.R. Ibisterol sulfate, a novel HIV-inhibitory sulfated sterol from the deep water sponge Topsentia sp. Tetrahedron Lett. 1993, 34, 389–392. [Google Scholar] [CrossRef]
- Sun, H.H.; Cross, S.S.; Gunasekera, M.; Koehn, F.E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron 1991, 47, 1185–1190. [Google Scholar] [CrossRef]
- Bouhlal, R.; Haslin, C.; Chermann, J.C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Cardozo, F.T.G.S.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R.M.; Mendonça, M.M.; Simões, C.M.O. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef]
- Cardozo, F.T.G.S.; Camelini, C.M.; Larsen, I.; Carballo, E.; Jose, G.; Stern, R.; Brummel, R.; Rossi, M.; Simões, C.M.O.; Brandt, C. In vivo anti-HSV activity of a sulfated derivative of Agaricus brasiliensis mycelial polysaccharide. Antimicrob. Agents Chemother. 2013, 57, 2541–2549. [Google Scholar] [CrossRef]
- Zhu, W.; Chiu, L.C.M.; Ooi, V.E.C.; Chan, P.K.S.; Ang, P.O., Jr. Antiviral property and mode of action of a sulphated polysaccharide from Sargassum patens against herpes simplex virus type 2. Int. J. Antimicrob. Agents 2004, 24, 81–85. [Google Scholar]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karnakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Fontaine-Rodrigues, E.C.; Knipe, D.M. Herpes simplex virus ICP27 increases translation of a subset of viral late mRNAs. J. Virol. 2008, 28, 3538–3545. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Marcelletti, J.F. Synergistic inhibition of herpesvirus replication by docosanol and antiviral nucleoside analogs. Antivir. Res. 2002, 56, 153–166. [Google Scholar] [CrossRef]
- Chuanasa, T.; Phromjai, J.; Lipipun, V.; Likhitwitayawuid, K.; Suzuki, M.; Pramyothin, P.; Hattori, M.; Shiraki, K. Anti-Herpes Simplex Virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antivir. Res. 2008, 80, 62–70. [Google Scholar] [CrossRef]
- Burleson, F.G.; Chamberts, T.M.; Wiedbrauk, D.L. Virology: A Laboratory Manual; Academic Press: San Diego, CA, USA, 1992; p. 250. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Silva, I.T.; Costa, G.M.; Stoco, P.H.; Schenkel, E.P.; Reginatto, F.H.; Simões, C.M.O. In vitro antiherpes effects of a c-glycosylflavonoid enriched fraction of Cecropia glaziovii Sneth. Lett. Appl. Microbiol. 2010, 51, 143–148. [Google Scholar]
- Ekblad, M.; Bergstrom, T.; Banwell, M.G.; Bonnet, M.; Renner, J.; Ferro, V.; Trybala, E. Anti-herpes simplex virus activities of two novel disulphated cyclitols. Antivir. Chem. Chemother. 2006, 17, 97–106. [Google Scholar]
- Bettega, J.M.R.; Teixeira, H.; Bassani, V.L.; Barardi, C.R.M.; Simões, C.M.O. Evaluation of the antiherpetic activity of standardized extracts of Achyrocline satureioides. Phytother. Res. 2004, 18, 819–823. [Google Scholar] [CrossRef]
- Bertol, J.W.; Rigotto, C.; De Pádua, R.M.; Kreis, W.; Barardi, C.R.M.; Braga, F.C.; Simões, C.M.O. Antiherpes activity of glucoevatromonoside, a cardenolide isolated from a Brazilian cultivar of Digitalis lanata. Antivir. Res. 2011, 92, 73–80. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).