Abstract
This scoping review aims to systematically gather evidence from personalized cancer-screening studies across various cancers, summarize key components and outcomes, and provide implications for a future personalized melanoma-screening strategy. Peer-reviewed articles and clinical trial databases were searched for, with restrictions on language and publication date. Sixteen distinct studies were identified and included in this review. The studies’ results were synthesized according to key components, including risk assessment, risk thresholds, screening pathways, and primary outcomes of interest. Studies most frequently reported about breast cancers (n = 7), followed by colorectal (n = 5), prostate (n = 2), lung (n = 1), and ovarian cancers (n = 1). The identified screening programs were evaluated predominately in Europe (n = 6) and North America (n = 4). The studies employed multiple different risk assessment tools, screening schedules, and outcome measurements, with few consistent approaches identified across the studies. The benefit–harm assessment of each proposed personalized screening program indicated that the majority were feasible and effective. The establishment of a personalized screening program is complex, but results of the reviewed studies indicate that it is feasible, can improve participation rates, and screening outcomes. While the review primarily examines screening programs for cancers other than melanoma, the insights can be used to inform the development of a personalized melanoma screening strategy.
1. Introduction
Cancer was responsible for nearly one-sixth of global mortality, with an estimated 10 million deaths occurring in 2020 [1]. Early cancer detection through population-based screening programs has already resulted in significant improvements in survival rates for several cancers, including breast, cervical, and colorectal [2]. Although there are many benefits for reducing cancer incidence and mortality, population-based cancer-screening programs are also resource-intensive and may lead to overdiagnosis. This phenomenon pertains to individuals who, if left untreated, would not have been impacted by the detected cancer [3]. Overtreatment has also been reported as a result of false positive screening examinations [4,5]. Currently, most organized cancer-screening programs are based on fixed eligibility requirements such as age and sex. In a shift away from the ‘one size fits all’ approach, interest in personalized screening is growing [6,7].
Personalized screening approaches take into account individuals’ risk-factors to assess eligibility and determine screening frequency, such as genetic risk, family history and lifestyle factors [8,9]. One recent example is a lung-cancer-screening trial conducted in the United States, which was introduced only for people who have a strong smoking history [10]. Risk assessment at population level is thought to enable the identification and targeting of groups at elevated risk who stand to benefit the most from screening and may reduce or not offer screening for those who will benefit the least and for whom the benefit/risk ratio of screening is therefore worse [7]. Personalized screening is potentially more cost-effective and may require less healthcare resources than current population-based approaches [8,9,11]. As it would increase the prevalence of true disease in the screened group, it could minimize the risk of overdiagnosis and unnecessary treatments [5,9,12].
Many countries have well-established, organized, population-based screening programs in place for the early detection of breast [13], cervical [14], and colorectal cancers [15]. Although melanoma is less common than other types of skin cancer, it is the most serious. While some European countries, such as Germany and France [16,17], have implemented national guidance on melanoma screening by physicians in a legal framework, these guidelines do not qualify as personalized screening due to a lack of risk assessment for screening eligibility and/or the absence of tailored screening frequencies based on individual risk [18]. Consequently, there is a lack of evidence of effectiveness and cost-effectiveness in support of personalized melanoma screening [19]. Early detection of melanoma, along with keratinocyte cancers such as basal cell carcinoma and squamous cell carcinoma, is therefore incidental and usually initiated by the patient or a health professional [20]. The limitations of opportunistic early detection are reflected in inequitable melanoma outcomes among the community, with better outcomes for higher socioeconomic groups and those living near metropolitan centers [21,22,23]. In Australia, treatment for melanoma and other keratinocyte cancers combined already confer the highest healthcare cost burden compared to other cancers [24]. The burden from melanoma and other skin cancers is projected to increase substantially over the next two decades due to aging of the population, and increasing ultraviolet radiation exposure due to climate change [25], which could further increase the risk of overdiagnosis and overtreatment [26].
International recommendations [19,27] and Australian clinical practice guidelines already support personalized approaches to melanoma early detection, recommending clinicians identify people at high risk and conduct a skin examination, but there is limited evidence on how this should be implemented. A randomized, controlled trial of a personalized melanoma-screening program could help determine the benefits, costs, harms, and potential implementation strategy [28,29]. However, there are several complexities to establishing personalized screening programs that require decisions about the best approach to risk assessment, risk communication, risk thresholds, screening examination, and screening intervals.
To explore the feasibility and implementation strategies of personalized screening, we conducted a scoping review of relevant risk-based studies across various screen-detectable cancers. The World Health Organization’s principles and practice of screening for disease served as the foundational framework for this scoping review [30]. By leveraging insights and lessons learned from research in other cancer types, there is a significant opportunity to inform the design of a potential future personalized melanoma-screening program.
2. Methods
This review was conducted according to the PRISMA extension for scoping reviews and the Population/Concept/Context (PCC) Framework [31] and registered on the Open Science Framework (registration DOI: https://doi.org/10.17605/OSF.IO/FC8W4, accessed on 22 May 2024). The search process is displayed in Figure 1.
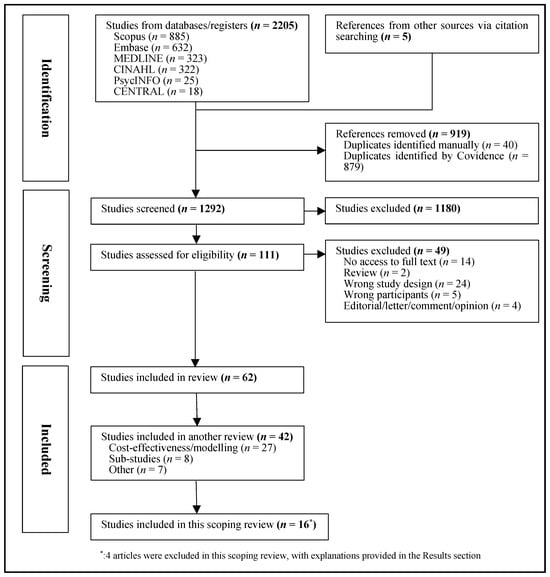
Figure 1.
Flowchart of selection of studies.
2.1. Data Source
Electronic databases, including Medline, Embase, Cochrane central, PsychINFO, CINAHL, Scopus, ANZCTR, and clinicaltrials.gov, were systematically searched. Eligible articles for this scoping review included those published in English from January 2013 to May 2023.
2.2. Study Selection
English language reports published in peer-reviewed sources or listed on clinical trials databases were included, in accordance with specific inclusion and exclusion criteria. The search strategy, terms, and exclusion/inclusion criteria were developed in consultation with an experienced librarian at the University of Sydney. The detailed search strings for each database are provided in the Supplementary Materials.
Studies were deemed eligible if they met all of the following criteria:
- Participants from the adult general population;
- Studies on personalized cancer-screening programs, where screening was tailored according to personal risk (beyond age or sex alone);
- Focus on cancer(s);
- January 2013 to May 2023, to focus on contemporary screening programs;
- Research in humans.
The studies were excluded if they met any of the following criteria:
- Written in a language other than English;
- General screening (not risk-based) studies;
- Reviews, letters, editorials;
- Evaluations of hypothetical personalized cancer screening.
2.3. Data Extraction
Data extraction was performed independently by two reviewers, and discrepancies were addressed through discussion. Key variables from each paper were compiled in a data extraction framework and included the following: author, year, country, study design, primary aim, cancer type, study period, outcomes and measures, risk assessment, risk thresholds, screening intervals, participants characteristics, outcomes measured, and results.
2.4. Quality Assessment
Quality assessment was carried out using the Cochrane Risk of Bias Tool for Randomized Controlled Trials [32] and the Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) [33] for non-randomized studies.
3. Results
A total of 2205 records were identified. Five additional papers were found through hand searching. After removing 919 duplicates, 1292 articles underwent title and abstract screening. Subsequently, 111 were selected for full-text review, with 49 being excluded (detailed reasons are provided in the Supplementary Materials). Reasons for exclusion were no access to full text (n = 14), review (n = 2), wrong study design (n = 24), wrong participants (n = 5), and editorials/ letter/ comments/ opinion (n = 4) (Figure 1).
This broader search retrieved 62 articles. Of these, 42 studies were focused on modeling or health economics (n = 27), reported on screening sub-studies (n = 8), or were categorized as ‘other’ (n = 7), and will be reported in a separate review. For this scoping review, 20 studies were selected, including 16 distinct personalized cancer-screening studies and 4 study protocols that focused on the evaluation of personalized cancer screening. Due to overlap with the identified results articles, the 4 protocol papers were excluded from this scoping review. Thus, the present review focuses on the 16 articles that reported evaluations of personalized cancer-screening implementation, including the key study components and outcomes (Figure 1). Overall, the studies included were of good quality. The overall risk of bias assessment for each study is provided in Supplementary Materials.
3.1. Key Characteristics of Eligible Studies
The key characteristics of the 16 studies are detailed in Table 1. Most studies were published from 2020 onwards. The majority of study designs were randomized controlled trials (n = 8) [11,34,35,36,37,38,39,40], followed by cohort studies (n = 2) [41,42]. Others included a non-randomized controlled trial [43], feasibility [44] and proof-of-concept [45], mixed-methods [46], pre–post implementation [47], and focus group [48] studies.

Table 1.
Key characteristics of Selected studies.
3.2. Cancer Types
The included studies mainly focused on breast cancer (n = 7) [38,41,42,43,45,46,48], colorectal cancer (n = 5) [11,34,35,39,40], prostate cancer (n = 2) [36,47], lung cancer (n = 1) [37], and ovarian cancer (n = 1) [44].
3.3. Study Locations
The study locations included six in European countries [36,37,43,44,45,48], four in North America [35,38,44,46], three in Australia [11,39,40], and three in Asian countries [34,41,42].
3.4. Participants
The study sample sizes varied greatly, spanning from over 1 million participants [42] to as few as slightly over a hundred [44]. Furthermore, studies varied widely in eligibility criteria, risk assessment tools, risk thresholds, and other key elements. For example, the cancer-screening programs ranged widely in terms of the age ranges considered for participation. In the context of breast cancer, most studies targeted unaffected women between 40 and 70 years [38,42,45,46,48], while Gareth Evans et al. specifically concentrated on women who were approaching the age of 60 years [43]. For colorectal cancer screening, adults 25 to 75 years were included [11,34,35,39,40]. Prostate cancer screening included males after reaching adulthood [36,47], while ovarian cancer screening recruited females once they turned 18 [44].
3.5. Risk Assessments
Tools used for risk assessment in each study are presented in Table 2. Most studies used multiple data sources to predict an individual’s risk of developing cancer. Among the combinations with different approaches found in the 16 studies, eight incorporated self-reported questionnaires, blood, and/or saliva tests, and validated risk prediction model [37,38,41,43,45,46,48]. Three studies combined questionnaires with blood/saliva test results [36,40,47]. One study combined questionnaire data with polygenic risk score and a risk prediction model [44]. Five studies relied solely on self-reported questionnaire data for risk estimation [11,34,35,39,42].

Table 2.
A summary of the risk assessment approaches employed in the selected studies (by cancer type).
3.6. Risk Thresholds and Proposed Pathways
The risk thresholds and screening intervals are summarized for each cancer type in Table 3. There was no consistency in the risk thresholds or number of groupings used in the included studies. The majority categorized potential screening participants into two (high and low) risk groups [11,34,35,37,38,41,42,47] or three groups [36,38,39,40,44,45,46], while two studies incorporated four risk groups [43,48]. Several variations were also found with regard to the proposed screening intervals. Most screening intervals were based on a combination of participant’s age, family history, and risk level, resulting in a range of two to eight proposed screening pathways. Four studies did not propose pathway recommendations [35,36,44].

Table 3.
Summary of risk thresholds and screening pathways (by cancer type).
In a mixed-methods study from Brooks et al. [46], three risk categories were defined based on 10-year/remaining lifetime risk, namely less than 15% as average risk, 15–25% as higher than average, and 25% and above as high risk. The corresponding screening pathways were determined based on both participants age and their estimated risk levels. For those categorized as higher than average risk, apart from age and risk thresholds, screening pathways were also determined according to their residence location. For instance, participants in the Province of Ontario underwent an annual mammogram, while those residing in the Province of Québec followed a mammogram screening schedule of everyone to two years, with the possibility of incorporating ultrasound assessments if breast density exceeded 75%. In a separate study conducted in the United Kingdom [48], risk thresholds were classified based on 10-year risk, including low risk (≤1.5%), average risk (1.5–4.99%), moderate risk (5–7.99%), and high risk (≥8%). In contrast to the approach of Brooks et al. [46], the screening pathways in this study were solely dependent upon the assigned risk categories. For participants categorized as low to moderate risk, their screening frequency was every 3 years, while those in the high-risk group should follow an 18-month screening schedule. Detailed information specific to each study is available in Table 3.
3.7. Key Components of Eligible Studies
The key components of the reviewed studies extracted include their primary outcomes, measurement methodologies, and main findings. Out of 16 studies, the majority directed their focus towards the practical consideration inherent in the newly proposed personalized screening approach. Two studies were not completed at the time of conducting this scoping review [38,46], and two papers only presented study protocols without any released results [40,41]. The primary outcomes in the selected studies are summarized in line with the World Health Organization (WHO) Wilson Framework (condition, test, treatment, and screening program) [30] and displayed in Table 4.

Table 4.
Key components of screening studies (by cancer type).
3.7.1. Screening Process and Evaluation
Four studies conducted an analysis of recruitment outcomes by assessing the acceptability and/or feasibility of the suggested screening approach [37,41,44,45]. Six studies evaluated screening by reporting the participation rate of the targeted population who underwent screening over subsequent follow-up examinations [11,36,39,40,43,47]. Three studies reported the percentage of the screened population that required further assessment [34,38,42]. Another three studies focused on longer term outcome measurements, including morbidity [38] and mortality [38,42]. Two studies addressed other aspects within the screening process; one of them compared concordance between participants’ preferences and ordered screening test [35], while the other explored the adoption of healthy behaviors following participation in a screening program [48].
3.7.2. Main Outcomes of Screening
The primary outcomes of this scoping review include the potential benefits and harms associated with each screening program, categorized by cancer type and summarized in Table 5. The majority of studies concluded that the evaluated screening strategies were feasible and cost-effective. Potential benefits included improved cancer knowledge [36], positive attitudes and screening examination uptake [11,35,39,43,44,45,47,48], a higher cancer detection rate at an early stage [37], reduction in cancer-related deaths [42], and cost-effectiveness [34,37].

Table 5.
Benefits–harm aummary for screening studies (by cancer type).
The most frequently identified harms were false positive screening results and overdiagnosis [37,42,45,47,48]. Other potential harms included higher costs [34,39] and failure to align with participants’ preferences in a clinical setting [35].
Potential benefts and harms were unassessable for the studies still underway [38,46] or were presented in protocol format only [40,41].
3.7.3. Results by Cancer Types
In the context of breast cancer, uptake of the personalized screening program was acceptable [43], with positive feedback received from the participants [45,48], and the proposed personalized screening approach resulted in an increased cancer detection rate among all groups [42].
For colorectal cancer, the implementation of the suggested personalized screening approaches resulted in a higher participation rate for all screening groups [11,39] or high-risk groups only [39]. Chen et al. found that the proposed risk-based screening led to an increased cancer detection rate while requiring a reduced number of colonoscopies to be performed [34].
For the two prostate-cancer-screening studies, both studies observed an increased proportion of men adhering to the risk-appropriate recommended prostate-specific antigen (PSA) test schedules [36,47].
Regarding other cancer types, the use of a lung cancer risk prediction model was tested and found to be effective for identifying high-risk individuals who would be suitable for lung cancer screening [37], while a personalized ovarian cancer-screening program was appraised as acceptable and satisfactory by the participants, as indicated by validated questionnaires [44].
4. Discussion
There has been increasing interest in personalized cancer screening over the past decade, alongside accumulating evidence on its potential advantages compared to population-based cancer screening which may be unnecessary for those at low risk, and not intensive enough for those at high or very high risk. In this scoping review, we identified several key components and outcomes that may assist in the design and evaluation of future personalized screening studies and programs for implementation in melanoma settings. Clinical guidelines already recommend personalized approaches to early detection of melanoma, yet evidence on the benefits and harms of screening is currently insufficient. The targeted screening interventions reviewed 16 studies reported on personal risk-assessment design, tailored follow-up intervals for future check-ups, and primary outcomes of interest across multiple cancer types. While none specially targeted melanoma, these studies explored important aspects of the screening process that could be applicable to personalized melanoma screening. These findings are discussed within the framework of the WHO screening guidelines.
Prior investigations into cancer screening across various cancer types have been included into this review. While population-based screening programs for ovarian cancer have not been approved globally, this scoping review included the feasibility study for this cancer type, given the ongoing efforts in this area [49]. The screening studies included in this review demonstrated participation rates of over 50%, which were deemed acceptable [11,36,39,40,43,47]. The WHO guidelines highlight the importance of participant engagement to maximize recruitment to screening, as high participation rates form the foundation for effective screening programs. Several of the reviewed studies examined whether personalized approaches improved the acceptability and/or feasibility of cancer screening [37,41,44,45]. These studies showed positive attitudes towards the personalized cancer screening programs implemented, which further indicates that personalized screening programs may engage those that benefit the most from screening more effectively. Although none of the included studies specifically addressed melanoma screening, it is worthwhile to examine shared characteristics in the design of personalized screening programs, such as personalized cut-off scores for different demographic cohorts, proposed screening pathways, and suggested follow-up intervals. Such analysis would further enrich the groundwork for designing future melanoma screening pilot studies. We found that various risk assessment strategies were incorporated in the design of the personalized screening programs to enable more accurate risk assessment. The commonly self-reported risk factors included in the risk tools were age, gender, race, personal cancer history, family history of cancer, and lifestyle factors, which were also relevant for defining melanoma high-risk individuals. Moreover, some studies also included the assessment of genetic- or biomarkers from blood or saliva samples. Currently, in the context of melanoma, several self-reported risk factor online tools are already freely accessible to both health professionals and the public [50,51], which calculate personal melanoma risk based on general risk factors plus melanoma specific ones such as phenotypic characteristics (hair, skin, eye color), sun or sunbed exposure, and melanocytic nevus (moles) counts. Scientific literature has highlighted concerns regarding the lack of validation in current melanoma risk prediction models [52,53]. This issue must be addressed when designing personalized screening strategies, as a validated risk-prediction model is essential for ensuring effective and valid personalized melanoma screening. Genomic risk factors, such as polygenic scores, are also related to risk of many cancers, including melanoma, and are anticipated to be incorporated into risk prediction tools more commonly [54,55]. In addition, our search showed that ten studies included polygenic scores in their risk assessment process, suggesting a necessity to further explore whether the addition of genomic information improves risk assessment enough to be incorporated in personalized screening programs, as this is more complex and costly compared to self-reported risk only.
Two studies in this review reported an increased cancer detection rate [34,42]. While this can be seen as a benefit, it could also be a result of length–time bias and also raises concerns about potential overdiagnosis and overtreatment [37,42,45,47,48]. Despite the demonstrated benefits of nationwide mammographic screening programs in reducing breast cancer deaths reported in many countries [56,57,58,59,60,61], the overall effectiveness of screening is still debated due to evidence of potentially unnecessary treatments. Overdiagnosis is a major concern for melanoma screening, as incidence rates increased substantially over the past decade without concomitant decreases in mortality [62]. In Australia, 54% of all melanomas (including melanoma in situ) and 15% of invasive melanomas are estimated to be over-diagnosed [26] in the absence of a formal dedicated screening program [63].
The ultimate goal of cancer-screening programs is to reduce mortality and morbidity [29]. In the context of melanoma, no randomized controlled trial has evaluated the screening impact on melanoma mortality, although observational studies have suggested that having a screening examination during the past 3 years reduces the thickness of melanoma [19,22]. Based on the reviewed personalized screening programs in other cancers, morbidity and melanoma thickness might be a suitable primary parameter, given the low overall mortality risk from melanoma, particularly with advancements in targeted and immunotherapy treatments [64,65]. In addition, other considerations for implementing a personalized melanoma screening program in Australia should include medical intervention practices, healthcare accessibility across different regions, costs, and individual quality of life. Potential parameters in this context include the need for adjuvant or neoadjuvant treatment, individual’s quality of life, healthcare expenditure, or equitable access or outcomes. Given the variability in melanoma risk among individuals, influenced by factors such as age, gender, race, personal cancer history, family history of skin cancer, lifestyle factors, and phenotypic characteristics, the frequency of evaluations should be tailored to individual risk levels.
Strengths and Limitations
Evidence collected from this scoping review encompassed multiple cancer types, research methods, study locations, and provided up-to-date evidence. In addition, this review summarized a wide variety of outcomes assessed, ranging from knowledge and attitudes to mortality. Given our particular interest in melanoma screening, it was a limitation that no studies associated with melanoma or skin cancer were discovered in the search; however, insights for melanoma screening can be gained from personalized screening studies and programs for other cancer types. Indeed, numerous parallels existed in the design of cancer-screening initiatives, including the essential elements considered for high-risk groups, primary outcomes of interest, and strategies for participants recruitment. Second, because of the nature of a scoping review, we included studies with diverse methodologies, populations, and primary outcomes. This heterogeneity can make it challenging to identify patterns across different studies. Furthermore, the search was restricted to English peer-reviewed articles, which potentially prevented us from exploring non-English datasets or screening programs in regions where English is not the official language.
5. Conclusions
Given the limited evidence available on personalized melanoma screening, this scoping review summarizes the key components and outcomes that personalized screening studies have considered across different cancer types over the past decade. In addition to traditional screening outcomes such as cancer mortality and incidence, acceptability and feasibility to recruit were also identified as important aspects to ensure optimal screening-program effectiveness. In addition, measurement of potential harms such as overdiagnosis and overtreatment, and the balance of benefits and harms across different risk groups, are essential considerations for any screening program going forward, particularly in the context of melanoma. The sensitivity, specificity, and positive predictive value of the proposed screening method are vital factors to ensure the program’s effectiveness in reducing melanoma-related morbidity and mortality. Insights from this review can assist researchers, clinicians, and policymakers in designing new studies and programs for personalized melanoma screening.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm14080863/s1, Table S1: Search Terms per Database; Table S2: Quality Assessment; Table S3: Reasons for Exclusion.
Author Contributions
L.Z.: Data curation, formal analysis, writing—original draft, writing—review and editing, visualization. A.K.S.: conceptualization, methodology, software, investigation, data curation, formal analysis, writing—review and editing. A.E.C.: conceptualization, methodology, investigation, resources, writing—review and editing, supervision, funding acquisition. M.J.: conceptualization, methodology, investigation, resources, investigation, data curation, formal analysis, writing—review and editing, visualization, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Amelia K. Smit is supported by a NHMRC Synergy grant (#2009923). Anne E. Cust is supported by a NHMRC Investigator Grant (#2008454). Monika Janda is supported by NHMRC CRE grant (#2006551) and NHMRC Synergy grant (#2009923) Lejie Zheng is supported by RTP Scholarship from the University of Queensland.
Institutional Review Board Statement
Not applicable for scoping review.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the study findings are accessible from the corresponding author upon request.
Acknowledgments
The authors express special thanks to Linda Finch for her contribution to the screening process, including title/abstract review, full text evaluation, and data extraction. Additionally, the authors acknowledge the invaluable assistance and support provided by all reviewers.
Conflicts of Interest
All authors declare that they have no known competing interest that could have appeared to influence the work reported in this manuscript.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Learoyd, D.L. Estimating the magnitude of cancer overdiagnosis in Australia. Med. J. Aust. 2020, 213, 189. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.-B.; Feletto, E.; Wade, S.; Caruana, M.; Kang, Y.-J.; Nickson, C.; Simms, K.; Procopio, P.; Taylor, N.; Worthington, J.; et al. Benefits, harms and cost-effectiveness of cancer screening in Australia: An overview of modelling estimates. Public Health Res. Pract. 2019, 29, 29121913. [Google Scholar] [CrossRef]
- Marcus, P.M.; Prorok, P.C.; Miller, A.B.; DeVoto, E.J.; Kramer, B.S. Conceptualizing overdiagnosis in cancer screening. JNCI J. Natl. Cancer Inst. 2015, 107, djv014. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.C.; Hutchinson, A.; Law, K.; Shah, V.; Usher-Smith, J.A.; Dennison, R.A. Acceptability of risk stratification within population-based cancer screening from the perspective of the general public: A mixed-methods systematic review. Health Expect. 2023, 26, 989–1008. [Google Scholar] [CrossRef]
- Taylor, L.C.; Law, K.; Hutchinson, A.; Dennison, R.A.; Usher-Smith, J.A. Acceptability of risk stratification within population-based cancer screening from the perspective of healthcare professionals: A mixed methods systematic review and recommendations to support implementation. PLoS ONE 2023, 18, e0279201. [Google Scholar] [CrossRef]
- Clift, A.K.; Dodwell, D.; Lord, S.; Petrou, S.; Brady, S.M.; Collins, G.S.; Hippisley-Cox, J. The current status of risk-stratified breast screening. Br. J. Cancer 2022, 126, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Rees, C.J.; Sharp, L.; Koo, S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 773–780. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Emery, J.D.; A Jenkins, M.; Saya, S.; Chondros, P.; Oberoi, J.; Milton, S.; Novy, K.; Habgood, E.; Karnchanachari, N.; Pirotta, M.; et al. The Colorectal cancer RISk Prediction (CRISP) trial: A randomised controlled trial of a decision support tool for risk-stratified colorectal cancer screening. Br. J. Gen. Pract. 2023, 73, e556–e565. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Meester, R.; de Jonge, L.; Buron, A.; Haug, U.; Senore, C. Risk-stratified strategies in population screening for colorectal cancer. Int. J. Cancer 2022, 150, 397–405. [Google Scholar] [CrossRef]
- World Health Organization. Existence of National Screening Program for Breast Cancer. 2022. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/existence-of-national-screening-program-for-breast-cancer (accessed on 23 April 2024).
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; E Schoen, R.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Breitbart, E.W.; Waldmann, A.; Nolte, S.; Capellaro, M.; Greinert, R.; Volkmer, B.; Katalinic, A. Systematic skin cancer screening in Northern Germany. J. Am. Acad. Dermatol. 2012, 66, 201–211. [Google Scholar] [CrossRef]
- Rat, C.; Blachier, L.; Hild, S.; Molinie, F.; Gaultier, A.; Dreno, B.; Nguyen, J.-M. Targeted screening for melanoma after a 5-year follow-up: Comparison of melanoma incidence and lesion thickness at diagnosis in screened (versus unscreened) patients. La Presse Médicale Open 2021, 2, 100013. [Google Scholar] [CrossRef]
- Dunlop, K.L.A.; Singh, N.; Robbins, H.A.; Zahed, H.; Johansson, M.; Rankin, N.M.; Cust, A.E. Implementation considerations for risk-tailored cancer screening in the population: A scoping review. Prev. Med. 2024, 181, 107897. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Ivlev, I.; Blasi, P.R.; Nguyen, M.B.; Senger, C.A.; Perdue, L.A.; Lin, J.S. Skin Cancer Screening: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 329, 1296–1307. [Google Scholar] [CrossRef]
- Bhave, P.; Wong, J.; McInerney-Leo, A.; E Cust, A.; Lawn, C.; Janda, M.; Mar, V.J. Management of cutaneous melanoma in Australia: A narrative review. Med. J. Aust. 2023, 218, 426–431. [Google Scholar] [CrossRef]
- Reyes-Marcelino, G.; Tabbakh, T.; Espinoza, D.; Sinclair, C.; Kang, Y.-J.; McLoughlin, K.; Caruana, M.; Fernández-Peñas, P.; Guitera, P.; Aitken, J.F.; et al. Prevalence of skin examination behaviours among Australians over time. Cancer Epidemiol. 2021, 70, 101874. [Google Scholar] [CrossRef]
- Aitken, J.F.; Elwood, M.; Baade, P.D.; Youl, P.; English, D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int. J. Cancer 2010, 126, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.C.; Elwood, M.; Swetter, S.M.; Brooks, D.R.; Aitken, J.; Youl, P.H.; Demierre, M.; Baade, P.D. Factors related to the presentation of thin and thick nodular melanoma from a population-based cancer registry in Queensland Australia. Cancer 2009, 115, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Shih, S.; Watts, C.; Goldsbury, D.; Green, A.C. The economics of skin cancer prevention with implications for Australia and New Zealand: Where are we now? Public Health Res. Pract. 2022, 32, 31502119. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; O’connell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: A statistical modelling study. Lancet Public Health 2022, 7, e537–e548. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Lin, G.; Kerr, P.; Grant-Kels, J.M. Evidence concerning the accusation that melanoma is overdiagnosed. J. Am. Acad. Dermatol. 2021, 85, 841–846. [Google Scholar] [CrossRef]
- Watts, C.; Dieng, M.; Morton, R.; Mann, G.; Menzies, S.; Cust, A. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: A systematic review. Br. J. Dermatol. 2015, 172, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, O.A.; Dekio, I.; Layton, A.M.; Li, H.; Hughes, H.; Morris, T.; Zouboulis, C.C.; Patrick, S. Why we continue to use the name Propionibacterium acnes. Br. J. Dermatol. 2018, 179, 1227. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Cust, A.E.; Neale, R.E.; Aitken, J.F.; Baade, P.D.; Green, A.C.; Khosrotehrani, K.; Mar, V.; Soyer, H.P.; Whiteman, D.C. Early detection of melanoma: A consensus report from the Australian Skin and Skin Cancer Research Centre Melanoma Screening Summit. Aust. N. Z. J. Public Health 2020, 44, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.G.; Jungner, G.; World Health Organization. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Joanna Briggs Institute. Scoping Reviews. 2023. Available online: https://guides.library.unisa.edu.au/ScopingReviews (accessed on 23 May 2023).
- Cochrane Methods. Risk of Bias 2 (RoB 2) Tool. 2021. Available online: https://methods.cochrane.org/risk-bias-2 (accessed on 23 May 2023).
- Cochrane Methods. ROBINS-I Tool. 2017. Available online: https://methods.cochrane.org/robins-I (accessed on 23 May 2023).
- Chen, H.; Shi, J.; Lu, M.; Li, Y.; Du, L.; Liao, X.; Wei, D.; Dong, D.; Gao, Y.; Zhu, C.; et al. Comparison of Colonoscopy, Fecal Immunochemical Test, and Risk-Adapted Approach in a Colorectal Cancer Screening Trial (TARGET-C). Clin. Gastroenterol. Hepatol. 2023, 21, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Schroy, P.C., 3rd; Duhovic, E.; Chen, C.A.; Heeren, T.C.; Lopez, W.; Apodaca, D.L.; Wong, J.B. Risk Stratification and Shared Decision Making for Colorectal Cancer Screening: A Randomized Controlled Trial. Med. Decis. Mak. 2016, 36, 526–535. [Google Scholar] [CrossRef]
- Fredsøe, J.; Koetsenruyter, J.; Vedsted, P.; Kirkegaard, P.; Væth, M.; Edwards, A.; Ørntoft, T.F.; Sørensen, K.D.; Bro, F. The effect of assessing genetic risk of prostate cancer on the use of PSA tests in primary care: A cluster randomized controlled trial. PLoS Med. 2020, 17, e1003033. [Google Scholar] [CrossRef]
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Whynes, D.K.; Devaraj, A.; Brain, K.E.; Eisen, T.; Gosney, J.; A Green, B.; A Holemans, J.; et al. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016, 71, 161–170. [Google Scholar] [CrossRef]
- Esserman, L.J.; Study, W.; Athena, I. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 2017, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Trevena, L.J.; Meiser, B.; Mills, L.; Dobbins, T.; Mazza, D.; Emery, J.D.; Kirk, J.; Goodwin, A.; Barlow-Stewart, K.; Naicker, S. Which Test Is Best? A Cluster-Randomized Controlled Trial of a Risk Calculator and Recommendations on Colorectal Cancer Screening Behaviour in General Practice. Public Health Genom. 2022, 25, 193–208. [Google Scholar] [CrossRef]
- Saya, S.; Boyd, L.; Chondros, P.; McNamara, M.; King, M.; Milton, S.; Lourenco, R.D.A.; Clark, M.; Fishman, G.; Marker, J.; et al. The SCRIPT trial: Study protocol for a randomised controlled trial of a polygenic risk score to tailor colorectal cancer screening in primary care. Trials 2022, 23, 810. [Google Scholar] [CrossRef]
- Liu, J.; Ho, P.J.; Tan, T.H.L.; Yeoh, Y.S.; Chew, Y.J.; Riza, N.K.M.; Khng, A.J.; Goh, S.-A.; Wang, Y.; Oh, H.B.; et al. BREAst screening Tailored for HEr (BREATHE)—A study protocol on personalised risk-based breast cancer screening programme. PLoS ONE 2022, 17, e0265965. [Google Scholar] [CrossRef]
- Yen, A.M.; Tsau, H.-S.; Fann, J.C.-Y.; Chen, S.L.-S.; Chiu, S.Y.-H.; Lee, Y.-C.; Pan, S.-L.; Chiu, H.-M.; Kuo, W.-H.; Chang, K.-J.; et al. Population-Based Breast Cancer Screening with Risk-Based and Universal Mammography Screening Compared with Clinical Breast Examination: A Propensity Score Analysis of 1 429 890 Taiwanese Women. JAMA Oncol. 2016, 2, 915–921. [Google Scholar] [CrossRef]
- Evans, D.G.; McWilliams, L.; Astley, S.; Brentnall, A.R.; Cuzick, J.; Dobrashian, R.; Duffy, S.W.; Gorman, L.S.; Harkness, E.F.; Harrison, F.; et al. Quantifying the effects of risk-stratified breast cancer screening when delivered in real time as routine practice versus usual screening: The BC-Predict non-randomised controlled study (NCT04359420). Br. J. Cancer 2023, 128, 2063–2071. [Google Scholar] [CrossRef]
- Gaba, F.; Blyuss, O.; Liu, X.; Goyal, S.; Lahoti, N.; Chandrasekaran, D.; Kurzer, M.; Kalsi, J.; Sanderson, S.; Lanceley, A.; et al. Population Study of Ovarian Cancer Risk Prediction for Targeted Screening and Prevention. Cancers 2020, 12, 1241. [Google Scholar] [CrossRef]
- Laza-Vásquez, C.; Martínez-Alonso, M.; Forné-Izquierdo, C.; Vilaplana-Mayoral, J.; Cruz-Esteve, I.; Sánchez-López, I.; Reñé-Reñé, M.; Cazorla-Sánchez, C.; Hernández-Andreu, M.; Galindo-Ortego, G.; et al. Feasibility and Acceptability of Personalized Breast Cancer Screening (DECIDO Study): A Single-Arm Proof-of-Concept Trial. Int. J. Environ. Res. Public Health 2022, 19, 10426. [Google Scholar] [CrossRef]
- Brooks, J.D.; Nabi, H.H.; Andrulis, I.L.; Antoniou, A.C.; Chiquette, J.; Després, P.; Devilee, P.; Dorval, M.; Droit, A.; Easton, D.F.; et al. Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). J. Pers. Med. 2021, 11, 511. [Google Scholar] [CrossRef]
- Shah, A.; Polascik, T.J.; George, D.J.; Anderson, J.; Hyslop, T.; Ellis, A.M.; Armstrong, A.J.; Ferrandino, M.; Preminger, G.M.; Gupta, R.T.; et al. Implementation and Impact of a Risk-Stratified Prostate Cancer Screening Algorithm as a Clinical Decision Support Tool in a Primary Care Network. J. Gen. Intern. Med. 2021, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rainey, L.; van der Waal, D.; Donnelly, L.S.; Southworth, J.; French, D.P.; Evans, D.G.; Broeders, M.J.M. Women’s health behaviour change after receiving breast cancer risk estimates with tailored screening and prevention recommendations. BMC Cancer 2022, 22, 69. [Google Scholar] [CrossRef]
- Nash, Z.; Menon, U. Ovarian cancer screening: Current status and future directions. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 32–45. [Google Scholar] [CrossRef]
- QIMR-Berghofer. QSkin2. 2023. Available online: https://publications.qimrberghofer.edu.au/Custom/QSkinMelanomaRisk (accessed on 30 August 2023).
- Vuong, K.; Armstrong, B.K.; Weiderpass, E.; Lund, E.; Adami, H.-O.; Veierod, M.B.; Barrett, J.H.; Davies, J.R.; Bishop, D.T.; Whiteman, D.C.; et al. Development and External Validation of a Melanoma Risk Prediction Model Based on Self-assessed Risk Factors. JAMA Dermatol. 2016, 152, 889–896. [Google Scholar] [CrossRef]
- Kaiser, I.; Pfahlberg, A.B.; Uter, W.; Heppt, M.V.; Veierød, M.B.; Gefeller, O. Risk Prediction Models for Melanoma: A Systematic Review on the Heterogeneity in Model Development and Validation. Int. J. Environ. Res. Public Health 2020, 17, 7919. [Google Scholar] [CrossRef] [PubMed]
- Usher-Smith, J.A.; Emery, J.; Kassianos, A.P.; Walter, F.M. Risk prediction models for melanoma: A systematic review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Kashani-Sabet, M.; Leachman, S.A.; Stein, J.A.; Arbiser, J.L.; Berry, E.G.; Celebi, J.T.; Curiel-Lewandrowski, C.; Ferris, L.K.; Grant-Kels, J.M.; Grossman, D.; et al. Early Detection and Prognostic Assessment of Cutaneous Melanoma: Consensus on Optimal Practice and the Role of Gene Expression Profile Testing. JAMA Dermatol. 2023, 159, 545–553. [Google Scholar] [CrossRef]
- Wong, C.K.; Dite, G.S.; Spaeth, E.; Murphy, N.M.; Allman, R. Melanoma risk prediction based on a polygenic risk score and clinical risk factors. Melanoma Res. 2023, 33, 293–299. [Google Scholar] [CrossRef]
- E Johns, L.; A Coleman, D.; Swerdlow, A.J.; Moss, S.M. Effect of population breast screening on breast cancer mortality up to 2005 in England and Wales: An individual-level cohort study. Br. J. Cancer 2016, 116, 246–252. [Google Scholar] [CrossRef]
- Katalinic, A.; Eisemann, N.; Kraywinkel, K.; Noftz, M.R.; Hübner, J. Breast cancer incidence and mortality before and after implementation of the German mammography screening program. Int. J. Cancer 2020, 147, 709–718. [Google Scholar] [CrossRef]
- Morrell, S.; Taylor, R.; Roder, D.; Robson, B.; Gregory, M.; Craig, K. Mammography service screening and breast cancer mortality in New Zealand: A National Cohort Study 1999–2011. Br. J. Cancer 2017, 116, 828–839. [Google Scholar] [CrossRef]
- Sankatsing, V.D.; van Ravesteyn, N.T.; Heijnsdijk, E.A.; Looman, C.W.; van Luijt, P.A.; Fracheboud, J.; Heeten, G.J.D.; Broeders, M.J.; de Koning, H.J. The effect of population-based mammography screening in Dutch municipalities on breast cancer mortality: 20 years of follow-up. Int. J. Cancer 2017, 141, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Van Ourti, T.; O’Donnell, O.; Koç, H.; Fracheboud, J.; de Koning, H.J. Effect of screening mammography on breast cancer mortality: Quasi-experimental evidence from rollout of the Dutch population-based program with 17-year follow-up of a cohort. Int. J. Cancer 2020, 146, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, R.; Duggan, C.; Anderson, B.O. Demographic changes in breast cancer incidence, stage at diagnosis and age associated with population-based mammographic screening. J. Surg. Oncol. 2017, 115, 517–522. [Google Scholar]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 341, 72–79. [Google Scholar] [CrossRef]
- Adamson, A.S.; Naik, G.; A Jones, M.; Bell, K.J. Ecological study estimating melanoma overdiagnosis in the USA using the lifetime risk method. BMJ Evid.-Based Med. 2024, 29, 156–161. [Google Scholar] [CrossRef]
- Cust, A.E.; Aitken, J.F.; Baade, P.D.; Whiteman, D.C.; Soyer, H.P.; Janda, M. Why a randomized melanoma screening trial may be a good idea. Br. J. Dermatol. 2018, 179, 1227. [Google Scholar] [CrossRef]
- Halvorsen, J.; Løberg, M.; Gjersvik, P.; Roscher, I.; Veierød, M.; Robsahm, T.; Nilsen, L.; Kalager, M.; Bretthauer, M. Why a randomized melanoma screening trial is not a good idea. Br. J. Dermatol. 2018, 179, 532–533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).