Abstract
Aim. To assess the efficacy of belimumab in joint and skin manifestations in a nationwide cohort of patients with SLE. Methods. All patients with skin and joint involvement enrolled in the BeRLiSS cohort were considered. Belimumab (intravenous, 10 mg/kg) effectiveness in joint and skin manifestations was assessed by DAS28 and CLASI, respectively. Attainment and predictors of DAS28 remission (<2.6) and LDA (≥2.6, ≤3.2), CLASI = 0, 1, and improvement in DAS28 and CLASI indices ≥20%, ≥50%, and ≥70% were evaluated at 6, 12, 24, and 36 months. Results. DAS28 < 2.6 was achieved by 46%, 57%, and 71% of patients at 6, 12, and 24 months, respectively. CLASI = 0 was achieved by 36%, 48%, and 62% of patients at 6, 12, and 24 months, respectively. Belimumab showed a glucocorticoid-sparing effect, being glucocorticoid-free at 8.5%, 15.4%, 25.6%, and 31.6% of patients at 6, 12, 24, and 36 months, respectively. Patients achieving DAS-LDA and CLASI-50 at 6 months had a higher probability of remission at 12 months compared with those who did not (p = 0.034 and p = 0.028, respectively). Conclusions. Belimumab led to clinical improvement in a significant proportion of patients with joint or skin involvement in a real-life setting and was associated with a glucocorticoid-sparing effect. A significant proportion of patients with a partial response at 6 months achieved remission later on during follow-up.
1. Introduction
Over ten years of experience in clinical practice has confirmed the efficacy and safety of belimumab in the treatment of systemic lupus erythematosus (SLE) [,,,,,,,]. This allowed the inclusion of belimumab in the 2019 updated European League Against Rheumatism (EULAR) recommendations for SLE management as an approved biological drug to be used in patients refractory to standard of care, which includes glucocorticoids and hydroxychloroquine, with or without concomitant or previous immunosuppressive therapy [].
The BLISS-LN study [] provided additional evidence of the efficacy of belimumab in patients with lupus nephritis, which was also confirmed in real-world studies [].
BeRLiSS (Belimumab in Real Life Setting Study) is the largest European nationwide cohort aimed at investigating belimumab effects on disease activity, damage progression, attainment of remission, and low disease activity (LDA) and assessing predictors of treatment response in SLE patients across Italian references lupus Centers for the treatment of SLE [,,,,].
Data from the BeRLiSS cohort showed that a consistent proportion of patients could experience clinical improvement and achieve LDA and remission []. In addition, BeRLiSS provided evidence that patients in the early phase of the disease, who initiated belimumab before accruing damage, have the highest chance of having a prompt clinical benefit. On the other hand, BeRLiSS showed that a proportion of patients not achieving a response to belimumab after 6 months of treatment could still attain a response later [], suggesting that a longer duration of treatment should be granted before considering the drug as ineffective [].
A pooled analysis from randomized controlled trials BLISS-52 and BLISS-76 [,] suggested that belimumab is effective in patients with musculoskeletal and skin involvement []. However, firm evidence in clinical practice and quantification through organ-specific indexes is still lacking.
Therefore, we considered all patients with joint and skin involvement enrolled in the BeRLiSS cohort (BeRLiSS-JS) and performed a sub-analysis intending to investigate the efficacy of belimumab in these patients.
Moreover, we also analyzed predictors of long-term response to belimumab in patients with early partial response.
2. Patients and Methods
2.1. Inclusion Criteria
Data from patients enrolled in the BeRLiSS cohort were retrospectively analyzed. BeRLiSS cohort has already been outlined elsewhere [].
Patients with joint or skin involvement requiring therapy with belimumab, according to physician judgment with available Disease Activity Score 28 (DAS28) and/or Cutaneous LE Area and Severity Index (CLASI) score [], were enrolled in this study. Baseline active joint involvement was defined according to SLEDAI-2K (Systemic Lupus Erythematosus Disease Activity Index 2000) specific item (arthritis) and skin involvement according to CLASI ≥ 1.
The study was approved by the University of Padova Ethics Committee (3806/AO/16) and carried out according to Helsinki Declaration. Informed consent regarding personal data treatment was obtained from patients.
2.2. Data Collection and Management
Patients were prospectively followed up according to EULAR recommendations [,], and participating in this study did not interfere with the daily clinical practice. Anonymized patient data were collected in an ad hoc database since belimumab initiation and regularly updated. DAS28, CLASI activity score, and daily prednisone intake were evaluated at baseline, at 6 and 12 months, and every 12 months thereafter.
All collected data were systematically and regularly evaluated. In case of inconsistencies or missing information, centers were required to amend the data. Patients not fulfilling inclusion criteria and data outside qualitative control were excluded.
2.3. Outcome Measures
Set outcomes encompassed achievement of SLEDAI responder index (SRI)-4, clinical remission defined as c-SLEDAI = 0 and prednisone (PDN) ≤ 5 mg/day [], and LDA defined as SLEDAI ≤ 4, prednisone (PDN) ≤ 7.5 mg/day, and no major organ involvement []. Joint response was quantified by DAS28: DAS28 < 2.6 (remission), DAS ≥ 2.6 and <3.2 (LDA); DAS28-20, DAS28-50, and DAS28-70 defined as a decrease of ≥20, ≥50%, and ≥70% from baseline values, respectively. Cutaneous response was quantified through the CLASI, and four main outcomes were considered: CLASI = 0 (remission of skin manifestation), CLASI = 1 (LDA), CLASI-20, CLASI-50, and CLASI-70, defined as a decrease of ≥20, ≥50%, and ≥70% from baseline values, respectively. Achievement of all these outcomes at 6, 12, 24, and 36 months was recorded. Early response was set at 6 months, and late response at 12 and 24 months. Patients not achieving CLASI = 0 or DAS28 remission at month 6 were then considered in the analysis of predictors of late response to belimumab, with CLASI = 0 and DAS28 remission as outcomes.
Patients who discontinued belimumab throughout the follow-up due to inefficacy were counted among the non-responders at the subsequent timepoint after discontinuation. This was done to avoid a bias related to the selection of patients responding to belimumab.
2.4. Statistical Analysis
Parametric and non-parametric tests were used according to the types of variables. Comparisons of continuous data with parametric distribution were performed using t-test, t-test for paired data, and one-way analysis of covariance (ANCOVA) with Bonferroni’s post hoc analysis. Continuous data with non-parametric distribution were analyzed using Wilcoxon’s rank sum test and Wilcoxon’s test for paired data. Comparisons of categorical data were performed using χ2 test (Pearson test if indicated). p-values less than 0.05 were considered significant.
The following variables collected at baseline (belimumab initiation) and at 6 months were included in the univariate analyses: disease activity pattern (chronic active vs. relapsing-remitting pattern) []; concomitant immunosuppressive therapy (yes/no) and/or antimalarials (yes/no), and/or GC therapy (yes/no); prednisone-equivalent dose (categorized into ≤5 mg/day, >5 mg/day, and ≥7.5 mg/day); SLEDAI-2K score, anti-dsDNA antibodies, low complement levels (C3 and C4), smoking status, SDI (categorized into >0, >1, >2); CLASI activity score (categorized as CLASI-20, CLASI-50, CLASI-70, CLASI = 1, and CLASI = 0), and CLASI damage score in patients with skin involvement; DAS28 (categorized as DAS28-20, DAS28-50, DAS28-70, DAS28 remission, DAS28 LDA) in patients with joint involvement. Variables with a p-value < 0.2 at univariate analysis were included in the multivariate models.
Two multivariate analyses were performed: the analysis of baseline predictors of response to belimumab at different timepoints (6–12–24 months) and the analysis of predictors of late response to belimumab in patients not achieving DAS28 or CLASI remission at month 6. In the latter analysis, data at 6 months were analyzed as possible predictors of response at 12 and 24 months.
Backward stepwise logistic regression was employed to identify predictors of response at 6, 12, and 24 months, with CLASI = 0, CLASI = 1, DAS28 remission, and DAS28 LDA as dichotomous dependent variables, with significance set at 5%.
Statistical analyses were performed using the SPSS (version 28.0) software (Chicago, IL, USA).
3. Results
3.1. BeRLiSS Cohorts for Joint and Skin Involvement
Demographic, clinical, and serological variables and concomitant treatment at baseline are reported in Table 1.

Table 1.
Baseline demographic, clinical, and serological variables in SLE patients with joint and skin involvement at baseline treated with belimumab.
3.2. Follow-Up Data
A significant decrease in DAS28 and CLASI activity scores, as well as in prednisone intake, was observed during follow-up (Figure 1).
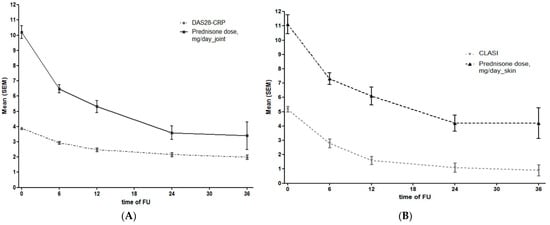
Figure 1.
(A) DAS28 and glucocorticoid intake during the follow-up in patients with joint involvement at different timepoints. (B) CLASI and glucocorticoid intake during the follow-up in patients with skin involvement at different timepoints.
3.3. Joint Involvement
According to the inclusion criteria, 328 patients with joint involvement were considered: 15 patients discontinued the drug before achieving a 6-month follow-up (5 cases due to inefficacy, 7 to adverse events, and 3 to loss of follow-up); 11 patients had incomplete data which prevented further analysis, and 22 patients did not complete 6 months at the time of data extraction. Thus, 277 patients were included in the final cohort to evaluate the drug efficacy at 6 months. The number of patients with joint involvement achieving the different timepoints and the number of patients discontinuing the drug due to inefficacy in the 6 months before achieving the timepoint is summarized in Table 2. The mean follow-up period of patients with joint involvement was 23.7 ± 14.3 months.

Table 2.
Number of SLE patients with joint or skin involvement considered in the analyses at different timepoints, including those in follow-up and those who discontinued the drug due to inefficacy in the 6 months before achieving the timepoint.
At baseline (belimumab initiation), 11 (4%) patients with joint involvement had DAS28 > 5.1, 180 (65%) DAS28 ≤ 5.1 and ≥3.2, 53 (19%) DAS28 LDA, and 33 (12%) DAS28 < 2.6. The latter group includes patients with active joint involvement not captured by the DAS28 cut-off for remission.
3.4. Efficacy of Belimumab in Patients with Joint Involvement
Among patients with joint involvement, SRI-4 response was achieved by 143 (51.6%), 147 (58.5%), 86 (62.3%), and 46 (64.8%) patients at 6, 12, 24, and 36 months, respectively. In Zen et al. [], remission was achieved by 62 (22.3%), 84 (33.4%), 41 (29.7%), and 25 (35.2%) patients at 6, 12, 24, and 36 months, respectively. LDA was observed in 103 (37.1%), 121 (48.2%), 77 (55.8%), and 61 (60.5%) patients at 6, 12, 24, and 36 months, respectively. Notably, 57.4% of patients were on PDN ≤ 5 mg/day, and 8.5% were PDN-free at 6 months; these proportions increased to 72.7% and 16.3% at 12 months, to 85.1% and 28.9% at 24 months, and 87.7% and 38.5% at 36 months, respectively.
The proportion of patients achieving DAS28-20, DAS28-50, DAS28-70, DAS28 remission, and DAS28 LDA at different timepoints is reported in Figure 2.
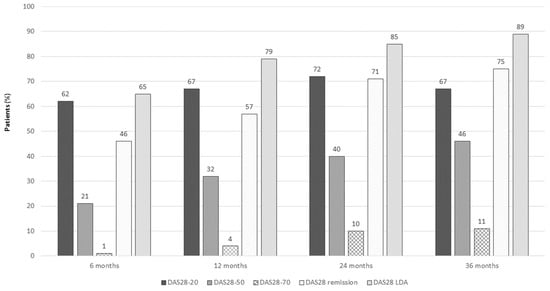
Figure 2.
DAS28-20, DAS28-50, DAS28-70, DAS28 remission, and DAS20 LDA in SLE patients on belimumab at different timepoints.
Among the 243 patients with DAS28 ≥ 2.6 at baseline, 109 patients (44.8%) achieved DAS28 remission at 6 months, 116 (50%) at 12 months, 81 (61.4%) at 24 months, and 45 (64.3%) at 36 months. In addition, patients with Boolean remission (meaning Swollen joint 0, Tender joint 0, VAS 1/10, PCR ≤ 1 mg/L) were 12 (4.9%), 26 (11.2%), 24 (18.2%), and 23 (32.8%) at 6, 12, 24, and 36 months, respectively.
3.5. Skin Involvement
According to the inclusion criteria, 172 patients with skin manifestations were considered: 9 discontinued the drug before achieving a 6-month follow-up (4 due to inefficacy and 5 due to adverse events), and 16 did not complete 6 months of follow-up at the time of data extraction. Thus, the cohort included 151 patients at baseline. The number of patients with skin involvement achieving the different timepoints and those discontinuing the drug due to inefficacy in the six months before achieving the timepoint is summarized in Table 2. The mean follow-up period in patients with skin involvement was 25.9 ± 15.7 months.
Among patients with skin manifestations at baseline, 17 patients (11.2%) had CLASI > 10, 59 (38.8%) CLASI ≤ 10 and >5, 68 (45,4%) CLASI ≤ 5 and >1, and 7 (4.6%) had CLASI = 1.
3.6. Efficacy of Belimumab in Patients with Skin Involvement
Among patients with skin manifestations, remission was achieved by 25 (16.5%), 36 (26.1%), 27 (33.7%), and 18 (36.7%) patients at 6, 12, 24, and 36 months, respectively; LDA was observed in 49 (32.4%), 57 (41.3%), 45 (56.2%), and 34 (69%) patients at 6, 12, 24, and 36 months, respectively. Fifty-four percent of patients were on PDN ≤ 5 mg/day, and 7.8% were PDN-free at 6 months. These percentages increased to 66.1% and 12.1% at 12 months, 81.7% and 19.4% at 24 months, and 85.7% and 26.5% at 36 months, respectively.
The proportion of patients achieving CLASI = 0, CLASI-20, CLASI-50, and CLASI-70 during follow-up is reported in Figure 3.
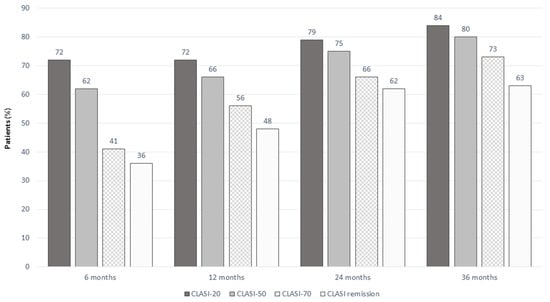
Figure 3.
CLASI-20, CLASI-50, CLASI-70, and CLASI remission in SLE patients treated with belimumab at different timepoints.
Patients achieving CLASI = 1 decreased over time due to the progressive achievement of remission (13.6%, 10.2%, 4.3%, and 0% at 6, 12, 24, and 36 months, respectively). The proportion of patients with CLASI > 10 significantly decreased from baseline (14.5%) to 4.8% at 6 months, 0% at 12 months, 1.4% at 24 months, and 0% at 36 months.
Patients with CLASI > 10 at baseline achieved CLASI remission in 1 (4.5%), 4 (19%), and 2 (20%) cases at 6, 12, and 24 months, respectively. CLASI-20 was achieved by 13 (59.1%), 4 (66.7%), and 8 (80%) patients at 6, 12, and 24 months, CLASI-50 by 8 (36.4%), 11 (52.4%), and 6 (60%) patients at 6, 12, and 24 months, and CLASI-70 by 5 (22.7%), 7 (33.3%), and 3 (30%) patients at 6, 12, and 24 months, respectively. This means that a great proportion of patients with high disease activity (CLASI > 10) not achieving remission still experienced a clinically significant improvement in their skin involvement during the follow-up.
3.7. Multivariate Analyses of Baseline Predictors of DAS28 Remission, DAS28 LDA and CLASI = 0 at 6, 12, and 24 Months
At multivariate analysis, the predictor of DAS28 remission at 6 months was a lower DAS28 at baseline, and predictors of DAS28 remission at 12 and 24 months were a shorter disease duration and a lower DAS28 at baseline. Similar findings were obtained when DAS28 LDA was used as the outcome measure (Table 3).

Table 3.
Baseline predictors of DAS28 remission (A) and LDA (B) at 6, 12, and 24 months (multivariate analysis).
A lower CLASI activity at baseline predicted CLASI remission at different timepoints (Table 4).

Table 4.
Baseline predictors of CLASI = 0 at 6, 12, and 24 months (multivariate analysis).
3.8. Patients with Partial Response to Belimumab at 6 Months
When considering patients who did not achieve DAS28 remission at 6 months, 37 out of 142 (26%) and 35 out of 84 (41.6%) achieved remission at 12 and 24 months, respectively. DAS28 LDA at month 6 was associated with remission in these patients at 12 months. Among patients in DAS28 LDA at 6 months, 59.4% achieved remission at 12 months compared to 24.3% of patients not in DAS28 LDA (p = 0.034). This result was confirmed in the multivariate model after correction for other variables, including disease duration, serological status, and PDN dose intake (Table 5).

Table 5.
Six-month predictors of long-term DAS28 remission in patients with partial response to belimumab.
Nevertheless, patients who were not in DAS28-LDA at 6 months could achieve remission later during the follow-up, as 20 out of 39 patients who were not in DAS28-LDA (51.3%) at 6 months were in DAS28 remission at 24 months. Furthermore, no other clinical and serological variables at 6 months were associated with the achievement of DAS28 remission in the long term.
Among patients who did not achieve CLASI = 0 at 6 months, a significant proportion could achieve complete CLASI remission thereafter: 24 out of 92 (26.1%) and 25 out of 54 (46.3%) at 12 and 24 months, respectively. Patients achieving CLASI-50 at 6 months were more likely to achieve CLASI = 0 at 12 (p = 0.028) and 24 months (p = 0.05). Conversely, CLASI-20 at 6 months was not associated with an increased chance of achieving CLASI = 0 in the following months. However, few patients with CLASI-20 at 6 months could become CLASI = 0 responders during the follow-up (1 out of 5 on average).
At multivariate analysis, a lower CLASI activity score at 6 months was the only predictor of CLASI = 0 at 12 months (Table 6).

Table 6.
Six-month predictors of long-term cutaneous remission in patients with partial response to belimumab.
4. Discussion
In this study, we confirmed in clinical practice data from randomized control trials showing the high effectiveness of belimumab in patients with joint and skin involvement [].
Belimumab is a human immunoglobulin G1k monoclonal antibody that binds the soluble BLyS, thus preventing the binding to its receptors on B cells. As a result, belimumab inhibits the survival of B cells, including autoreactive B cells, and reduces the differentiation of B cells into Ig-producing plasma cells []. In SLE, systemic over-expression of BLyS was reported in some studies [,]. In addition, local over-expression of BlyS and its receptor 3 (BR3; also known as BAFF-R) was demonstrated in different tissues, including lesional keratinocytes, kidney-derived cells, and infiltrating B cells in skin and kidney biopsies from lupus patients [,]. All this evidence supports the rationale for the use of belimumab in SLE.
In keeping with these observations, in our multicenter cohort, a third of refractory SLE patients achieved remission according to different disease activity indices, including SLEDAI-2K, DAS28, and CLASI. In addition, almost half of the patients could achieve LDA after 12 months of belimumab therapy.
We also showed the glucocorticoid-sparing effect of belimumab. We observed a decrease in daily PDN dose intake and a consistent proportion of patients who could withdraw PDN during the follow-up. Interestingly, the proportion of glucocorticoid-free patients increased during the follow-up in both cohorts of patients with joint and skin involvement and was observed in about one-third of patients at 36 months (Figure 4). Notably, the decrease in the cumulative intake of glucocorticoids is one of the main goals in the modern management of SLE [] in order to prevent long-term damage accrual [,,,] and quality of life [,] not only in patients with joint and skin manifestations but also in patients with severe features of SLE [].
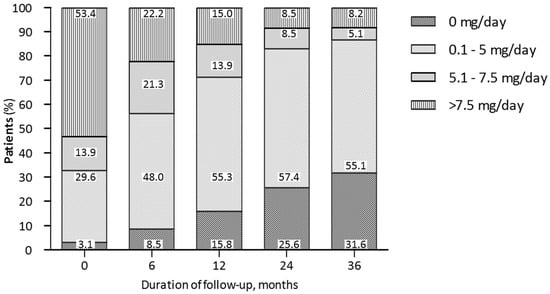
Figure 4.
Trends in daily dose PDN intake during belimumab treatment in patients with joint and skin manifestations at different timepoints.
In our multivariate models, a short disease duration and a lower DAS28 or CLASI at baseline predicted DAS28 and CLASI remission or LDA during the follow-up, suggesting that the earlier the use of belimumab, the better the outcome, regardless of the organ-specific activity score used to quantify organ-specific activity. In fact, this is in line with our previous studies, which demonstrated that the use of belimumab in the early stage of the disease was associated with a better SRI-4 response and damage prevention [,,].
Another critical aspect in the evaluation of drug effectiveness is the choice of outcome measures. A too-stringent response criteria might have contributed to several RCT failures [,,,]. In this paper, we considered not only organ-specific outcome measures but also the improvement in 20%, 50%, and 70% of DAS28 and CLASI as potential clinical targets. However, DAS28-20 and DAS28-70 do not seem appropriate outcomes being DAS28-20 too liberal and DAS28-70 too stringent. In addition, it has to be pointed out that patients with a low DAS28 at baseline can achieve remission without fulfilling 50% or 70% improvement in DAS28. Finally, it is worth noting that a non-negligible number of patients with active joint involvement had a DAS28 < 2.6 at baseline, meaning that DAS28 cannot always capture lupus joint manifestations, which can occur in ankles and feet or with tendinitis or bursitis.
On the other hand, in patients with skin involvement and high disease activity (CLASI > 10), where the achievement of remission is more difficult, CLASI-50 could be a reasonable and clinically meaningful outcome, especially in the short term [].
An open question in the belimumab treatment is when a patient can be considered a “non-responder” and, in turn, when belimumab should be discontinued []. We found that the response or a significant improvement in DAS28 or CLASI at 6 months predicted the response at 12 months. Still, even 24.3% of patients with joint and 26.1% of patients with skin manifestations who were non-responders at 6 months achieved full response at 12 months and 46.3% and 51.3% at 24 months, respectively. In patients with skin involvement, the lack of CLASI remission early during belimumab treatment did not prevent its achievement later during the follow-up, suggesting that 6 months may not be enough to fully evaluate belimumab efficacy [,]. According to our results, patients with mild improvement, such as those with CLASI-20 at 6 months, could benefit from belimumab treatment later during the follow-up. However, patients showing an earlier and higher improvement have a greater probability of achieving remission. Thus, our data suggest that one year might be the adequate time span of belimumab therapy needed to thoughtfully evaluate its efficacy. This observation is in keeping with other real-life clinical studies [] and clinical trials [,,]. Furthermore, it supports the rationale of continuing belimumab, even in patients who have not experienced a full response at 6 months.
Our study has both strengths and limitations. Limitations include the lack of a control group population, the exclusion of patients for whom data were unavailable at any given timepoints, and the absence of Patient-Reported Outcomes (PROs). These limitations are mainly connected to the retrospective nature of the study, which poses some objective restrictions to the amount of data that can be inferred. The greatest strengths of the study are the real-life setting, the large cohort of patients analyzed, and the long follow-up duration.
5. Conclusions
In conclusion, this study confirms the effectiveness of belimumab in SLE patients with joint and skin involvement and reinforces the previous findings suggesting that the early use of belimumab can maximize its efficacy.
A glucocorticoid-sparing effect of belimumab was also shown, with a decrease in daily dose and a consistent proportion of patients who become PDN-free during the follow-up, lowering the cumulative glucocorticoid intake.
Finally, patients with early partial improvement have a significant chance of achieving organ-specific remission later during the follow-up supporting the rationale for continuing belimumab, even in patients who have not experienced a full response at 6 months.
Author Contributions
M.Z.: conceptualization; data curation; investigation; methodology; formal analysis; visualization; writing—original draft. M.G. (Mariele Gatto): conceptualization; visualization; writing—review and editing. R.D.: data curation; visualization; writing—original draft; F.R.: data curation, writing—review and editing; M.F.: data curation, writing—review and editing. L.A.: data curation, writing—review and editing; F.F.: data curation, writing—review and editing; M.L.U.: data curation, writing—review and editing; G.E.: data curation, writing—review and editing. F.C. (Fulvia Ceccarelli): data curation, writing—review and editing; F.C. (Fabrizio Conti): data curation, writing—review and editing; A.B.: data curation, writing—review and editing; M.G. (Marcello Govoni): data curation, writing—review and editing; C.T.: data curation, writing—review and editing; M.M.: data curation, writing—review and editing; T.U.: data curation, writing—review and editing; M.G. (Maria Gerosa): data curation, writing—review and editing; E.P.B. (Enrica P. Bozzolo): data curation, writing—review and editing; V.C.: data curation, writing—review and editing; P.C.: data curation, writing—review and editing; A.G.: data curation, writing—review and editing. G.T.: data curation, writing—review and editing; E.G.: data curation, writing—review and editing. G.D.M.: data curation, writing—review and editing; S.D.V.: data curation, writing—review and editing. S.F.: data curation, writing—review and editing; F.C. (Francesco Ciccia): data curation, writing—review and editing; G.P.: data curation, writing—review and editing; C.S. (Carlo Salvarani): data curation, writing—review and editing. S.N.: data curation, writing—review and editing; A.D.M.: data curation, writing—review and editing; R.D.A.: data curation, writing—review and editing. G.O.: data curation, writing—review and editing; M.R.: data curation, writing—review and editing. P.F.: data curation, writing—review and editing; A.L.: data curation, writing—review and editing; M.P. (Matteo Piga): data curation, writing—review and editing. A.C. (Alberto Cauli): data curation, writing—review and editing. S.S.: data curation, writing—review and editing; F.W.R.: data curation, writing—review and editing; A.D.P.: data curation, writing—review and editing; E.B. (Enrico Brunetta): data curation, writing—review and editing; A.C. (Angela Ceribelli): data curation, writing—review and editing; C.S. (Carlo Selmi): data curation, writing—review and editing. M.P. (Marcella Prete): data curation, writing—review and editing; V.R.: data curation, writing—review and editing; A.V.: data curation, writing—review and editing. E.B. (Elena Bartoloni): data curation, writing—review, and editing. R.G.: data curation, writing—review and editing; E.Z.: data curation; writing—review and editing; M.L.: data curation; writing—review and editing; F.S.: data curation; writing—review and editing. A.D.: supervision; writing—review and editing; L.I.: conceptualization; supervision; investigation; methodology; writing—original draft; writing—review and editing; All authors approved the final version to be published. A.D. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Azienda Ospedale Università di Padova (protocol code 5592/AO/22, 10 November 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarzi-Puttini, P.; Atzeni, F.; Iaccarino, L.; Doria, A. Environment and systemic lupus erythematosus: An overview. Autoimmunity 2005, 38, 465–472. [Google Scholar] [CrossRef]
- Nikoloudaki, M.; Nikolopoulos, D.; Koutsoviti, S.; Flouri, I.; Kapsala, N.; Repa, A.; Katsimbri, P.; Theotikos, E.; Pitsigavdaki, S.; Pateromichelaki, K.; et al. Clinical response trajectories and drug persistence in systemic lupus erythematosus patients on belimumab treatment: A real-life, multicentre observational study. Front. Immunol. 2023, 13, 1074044. [Google Scholar] [CrossRef]
- Trentin, F.; Gatto, M.; Zen, M.; Maddalena, L.; Nalotto, L.; Saccon, F.; Zanatta, E.; Iaccarino, L.; Doria, A. Effectiveness, Tolerability, and Safety of Belimumab in Patients with Refractory SLE: A Review of Observational Clinical-Practice-Based Studies. Clin. Rev. Allergy Immunol. 2018, 54, 331–343. [Google Scholar] [CrossRef]
- Iaccarino, L.; Andreoli, L.; Bocci, E.B.; Bortoluzzi, A.; Ceccarelli, F.; Conti, F.; De Angelis, R.; De Marchi, G.; De Vita, S.; Di Matteo, A.; et al. Clinical predictors of response and discontinuation of belimumab in patients with systemic lupus erythematosus in real life setting. Results of a large, multicentric, nationwide study. J. Autoimmun. 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Iaccarino, L.; Bettio, S.; Reggia, R.; Zen, M.; Frassi, M.; Andreoli, L.; Gatto, M.; Piantoni, S.; Nalotto, L.; Franceschini, F.; et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. 2017, 69, 115–123. [Google Scholar] [CrossRef]
- Wallace, D.J.; Ginzler, E.M.; Merrill, J.T.; Furie, R.A.; Stohl, W.; Chatham, W.W.; Weinstein, A.; Mckay, J.; McCune, W.J.; Petri, M.; et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2019, 71, 1125–1134. [Google Scholar] [CrossRef]
- Anjo, C.; Mascaró, J.-M., Jr.; Espinosa, G.; Cervera, R. Effectiveness and safety of belimumab in patients with systemic lupus erythematosus in a real-world setting. Scand. J. Rheumatol. 2019, 48, 469–473. [Google Scholar] [CrossRef]
- D’Cruz, D.; Eriksson, G.; Green, Y.; Hammer, A.; Ji, B.; Meizlik, P.; A Roth, D. Safety and efficacy of belimumab in older adults with SLE: Results of an integrated analysis of clinical trial data. Lupus Sci. Med. 2023, 10, e000830. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Gatto, M.; Saccon, F.; Andreoli, L.; Bartoloni, E.; Benvenuti, F.; Bortoluzzi, A.; Bozzolo, E.; Brunetta, E.; Canti, V.; Cardinaletti, P.; et al. Durable renal response and safety with add-on belimumab in patients with lupus nephritis in real-life setting (BeRLiSS-LN). Results from a large, nationwide, multicentric cohort. J. Autoimmun. 2021, 124, 102729. [Google Scholar] [CrossRef]
- Gatto, M.; Saccon, F.; Zen, M.; Regola, F.; Fredi, M.; Andreoli, L.; Tincani, A.; Urban, M.L.; Emmi, G.; Ceccarelli, F.; et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real-life setting. Arthritis Rheumatol. 2020, 72, 1314–1324. [Google Scholar] [CrossRef]
- Zeher, M.; Doria, A.; Lan, J.; Aroca, G.; Jayne, D.; Boletis, I.; Hiepe, F.; Prestele, H.; Bernhardt, P.; Amoura, Z. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus 2011, 20, 1484–1493. [Google Scholar] [CrossRef]
- Depascale, R.; Gatto, M.; Zen, M.; Saccon, F.; Larosa, M.; Zanatta, E.; Bindoli, S.; Doria, A.; Iaccarino, L. Belimumab: A step forward in the treatment of systemic lupus erythematosus. Expert Opin. Biol. Ther. 2021, 21, 563–573. [Google Scholar] [CrossRef]
- Navarra, S.V.; Guzmán, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.-M.; Thomas, M.; Kim, H.-Y.; León, M.G.; et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 721–731. [Google Scholar] [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3920. [Google Scholar] [CrossRef]
- Manzi, S.; Sánchez-Guerrero, J.; Merrill, J.T.; Furie, R.; Gladman, D.; Navarra, S.V.; Ginzler, E.M.; D’Cruz, D.P.; Doria, A.; Cooper, S.; et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: Combined results from two phase III trials. Ann. Rheum. Dis. 2012, 71, 1833–1838. [Google Scholar] [CrossRef]
- Albrecht, J.; Taylor, L.; Berlin, J.A.; Dulay, S.; Ang, G.; Fakharzadeh, S.; Kantor, J.; Kim, E.; Militello, G.; McGinnis, K.; et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): An outcome instrument for cutaneous lupus erythematosus. J. Investig. Dermatol. 2005, 125, 889–894. [Google Scholar] [CrossRef]
- Mosca, M.; Tani, C.; Aringer, M.; Bombardieri, S.; Boumpas, D.T.; Brey, R.L.; Cervera, R.; Doria, A.; Jayne, D.; A Khamashta, M.; et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann. Rheum. Dis. 2010, 69, 1269–1274. [Google Scholar] [CrossRef]
- Zen, M.; Iaccarino, L.; Gatto, M.; Bettio, S.; Saccon, F.; Ghirardello, A.; Punzi, L.; Doria, A. The effect of different durations of remission on damage accrual: Results from a prospective monocentric cohort of Caucasian patients. Ann. Rheum. Dis. 2017, 76, 562–565. [Google Scholar] [CrossRef]
- Ugarte-Gil, M.F.; Hanly, J.; Urowitz, M.; Gordon, C.; Bae, S.-C.; Romero-Diaz, J.; Sanchez-Guerrero, J.; Bernatsky, S.; Clarke, A.E.; Wallace, D.J.; et al. Remission and low disease activity (LDA) prevent damage accrual in patients with systemic lupus erythematosus: Results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann. Rheum. Dis. 2022, 81, 1541–1548. [Google Scholar] [CrossRef]
- Zen, M.; Bassi, N.; Nalotto, L.; Canova, M.; Bettio, S.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Disease activity patterns in a monocentric cohort of SLE patients: A seven-year follow-up study. Clin. Exp. Rheumatol. 2012, 30, 856–863. [Google Scholar]
- Baker, K.P.; Edwards, B.M.; Main, S.H.; Choi, G.H.; Wager, R.E.; Halpern, W.G.; Lappin, P.B.; Riccobene, T.; Abramian, D.; Sekut, L.; et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003, 48, 3253–3265. [Google Scholar] [CrossRef]
- Wilkinson, C.; Henderson, R.B.; Jones-Leone, A.R.; Flint, S.M.; Lennon, M.; Levy, R.A.; Ji, B.; Bass, D.L.; Roth, D. The role of baseline BLyS levels and type 1 interferon-inducible gene signature status in determining belimumab response in systemic lupus erythematosus: A post hoc meta-analysis. Arthritis Res. Ther. 2020, 22, 102. [Google Scholar] [CrossRef]
- Petri, M.; Stohl, W.; Chatham, W.; Joseph McCune, W.; Chevrier, M.; Ryel, J.; Recta, V.; Zhong, J.; Freimuth, W. Association of BLyS™ with measures of disease activity in a prospective SLE observational study. Arthritis Rheum. 2004, 50, S603. [Google Scholar] [CrossRef]
- de Vos, L.; Guel, T.; Niebel, D.; Bald, S.; ter Steege, A.; Bieber, T.; Wenzel, J. Characterization of B cells in lupus erythematosus skin biopsies in the context of different immune cell infiltration patterns. Front. Med. 2022, 9, 1037408. [Google Scholar] [CrossRef]
- Itotagawa, E.; Tomofuji, Y.; Kato, Y.; Konaka, H.; Tsujimoto, K.; Park, J.; Nagira, D.; Hirayama, T.; Jo, T.; Hirano, T.; et al. SLE stratification based on BAFF and IFN-I bioactivity for biologics and implications of BAFF produced by glomeruli in lupus nephritis. Rheumatology 2022, keac528. [Google Scholar] [CrossRef]
- Sada, K.-E.; Katayama, Y.; Asano, Y.; Hayashi, K.; Miyawaki, Y.; Ohashi, K.; Katsuyama, E.; Katsuyama, T.; Takano-Narazaki, M.; Matsumoto, Y.; et al. Association of one-point glucocorticoid-free status with chronic damage and disease duration in systemic lupus erythematosus: A cross-sectional study. Lupus Sci. Med. 2022, 9, e000772. [Google Scholar] [CrossRef]
- Tselios, K.; Gladman, D.D.; Su, J.; Urowitz, M.B. Gradual Glucocorticosteroid Withdrawal Is Safe in Clinically Quiescent Systemic Lupus Erythematosus. ACR Open Rheumatol. 2021, 3, 550–557. [Google Scholar] [CrossRef]
- Urowitz, M.; Ohsfeldt, R.L.; Wielage, R.; Kelton, K.A.; Asukai, Y.; Ramachandran, S. A propensity scorematched study of organ damage in patients with systemic lupus erythematosus from the BLISS long-term extension trials versus the toronto lupus cohort: A post hoc longitudinal analysis [abstract no 2923]. Arthritis Rheumatol. 2017, 69 (Suppl. S10), 4217–4218. [Google Scholar]
- Rinaldi, S.; Doria, A.; Salaffi, F.; Ermani, M.; Iaccarino, L.; Ghirardello, A.; Zampieri, S.; Sarzi-Puttini, P.; Gambari, P.F.; Perini, G. Health-related quality of life in Italian patients with systemic lupus erythematosus. I. Relationship between physical and mental dimension and impact of age. Rheumatology 2004, 43, 1574–1579. [Google Scholar] [CrossRef]
- Emamikia, S.; Oon, S.; Gomez, A.; Lindblom, J.; Borg, A.; Enman, Y.; Morand, E.; Grannas, D.; van Vollenhoven, R.F.; Nikpour, M.; et al. Impact of remission and low disease activity on health-related quality of life in patients with systemic lupus erythematosus. Rheumatology 2022, 61, 4752–4762. [Google Scholar] [CrossRef]
- Briani, C.; Lucchetta, M.; Ghirardello, A.; Toffanin, E.; Zampieri, S.; Ruggero, S.; Scarlato, M.; Quattrini, A.; Bassi, N.; Ermani, M.; et al. Neurolupus is associated with anti-ribosomal P protein antibodies: An inception cohort study. J. Autoimmun. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Reddy, V.; Jayne, D.; Close, D.; Isenberg, D. B-cell depletion in SLE: Clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res. Ther. 2013, 15 (Suppl. S1), 1–16. [Google Scholar] [CrossRef]
- Petri, M.; Bruce, I.N.; Dörner, T.; Tanaka, Y.; Morand, E.F.; Kalunian, K.C.; Cardiel, M.H.; E Silk, M.; Dickson, C.L.; Meszaros, G.; et al. Baricitinib for systemic lupus erythematosus: A double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet 2023, 401, 1011–1019. [Google Scholar] [CrossRef]
- A Furie, R.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Kalunian, K.C.; Vital, E.M.; Ford, T.L.; Gupta, R.; Hiepe, F.; Santiago, M.; et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): A randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019, 1, e208–e219. [Google Scholar] [CrossRef]
- Zen, M.; Iaccarino, L.; Gatto, M.; Saccon, F.; LaRosa, M.; Ghirardello, A.; Punzi, L.; Doria, A. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian patients with SLE, but overlaps with remission. Ann. Rheum. Dis. 2017, 77, 104–110. [Google Scholar] [CrossRef]
- Levy, R.A.; Gonzalez-Rivera, T.; Khamashta, M.; Fox, N.L.; Jones-Leone, A.; Rubin, B.; Burriss, S.W.; Gairy, K.; van Maurik, A.; Roth, D.A. 10 Years of belimumab experience: What have we learnt? Lupus 2021, 30, 1705–1721. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Doria, A.; Mosca, M.; Gambari, P.F.; Bombardieri, S. Defining unclassifiable connective tissue diseases: Incomplete, undifferentiated, or both? J. Rheumatol. 2005, 32, 213–215. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).