Abstract
This integrative literature review has been carried out with the aim of analyzing the scientific literature aimed at identifying and describing existing rehabilitation treatments/therapies for neonatal brachial plexus palsy (NBPP). NBPP is a frequent consequence of difficult birthing, and it impairs the function of the brachial plexus in newborns. This is why knowledge on rehabilitation strategies deserves special attention. The data collection was carried out in January 2019, in the EBSCOhost and BVS (Biblioteca Virtual em Saúde) platforms, in the CINAHL Complete, MEDLINE Complete, LILACS and PubMed databases. Thirteen articles were included in this integrative literature review, based on a literature search spanning title, abstract and full text, and considering the inclusion criteria. Two main treatments/therapies for NBPP rehabilitation were identified: conservative treatment and surgical treatment. Conservative treatment includes teamwork done by physiatrists, physiotherapists and occupational therapists. These professionals use rehabilitation techniques and resources in a complementary way, such as electrostimulation, botulinum toxin injection, immobilizing splints, and constraint induced movement therapy of the non-injured limb. Professionals and family members work jointly. Surgical treatment includes primary surgeries, indicated for children who do not present any type of spontaneous rehabilitation in the first three months of life; and secondary surgeries, recommended in children who after primary surgery have some limitation of injured limb function, or in children who have had some spontaneous recovery, yet still have significant functional deficits. Treatment options for NBPP are defined by clinical evaluation/type of injury, but regardless of the type of injury, it is unanimous that conservative treatment is always started as early as possible. It should be noted that there was no evidence in the literature of other types of rehabilitation and techniques used in clinical practice, such as preventive positioning of contractures and deformities, hydrotherapy/aquatic therapy, among others, so we consider there is a need for further studies at this level in this area.
1. Introduction
Neonatal Brachial Plexus Palsy (NBPP) is caused by traction of the brachial plexus during birth and can limit the function of the affected arm in various ways. It is the most common form of peripheral neuropathy, with an incidence rate of 0.5–2 cases per 1000 newborns in developed countries [1,2,3].
The causes associated with NBPP are macrosomia, breech/pelvic birth, diabetes in pregnancy, shoulder dystocia, small stature/cephalopelvic disproportion, primiparity, or a prolonged expulsion phase [4,5].
The clinical classification of NBPP is based on body structures, namely the complex of nerves that controls the affected finger, hand, arm, and shoulder muscles. In this sense, brachial plexus lesions are classified as “severing of the upper trunk” when the affected nerves are C5 and C6; “severing of the middle trunk” when the affected nerve is C7; and “severing of the lower trunk” when the affected nerves are C8 and T1. Finally, complete severing is considered when affecting C5–T1 nerves [6].
NBPP, in addition to being classified by considering the affected nerve roots, is also categorized according to the degree of lesion that affects the nerve and also to the function of the injured limb. According to the degree of lesion of the nerve, we can describe preganglionic and postganglionic avulsion injury (tearing near the dorsal root ganglion o near the spinal cord at an intraforaminal level, and tearing of the postganglionic nerve distant from the dorsal root ganglion and the spinal cord, respectively); neurotmesis lesion, also considered to be a severe injury of the nerve as there is a complete tearing of the axon and the connective tissue [7,8]; axonotmesis lesion, when there is an anatomic interruption of the axon but with no interruption or partial interruption of the connective tissue and the myelin; and, finally, stretching neuropraxic lesion without nerve rupture, implying a momentaneous physiological blockage of the nerve-axon connection, with spontaneous recovery. We must also consider neuroma lesion, which implies an interference of the injured nerve scar tissue that, when healing, does not allow the nervous impulse to the muscle.
Also termed Duchenne-Erb syndrome, upper brachial plexus palsy (C5–C6) is characterized by impaired abduction and external rotation of the shoulder and elbow flexion, while hand function is preserved. Also known as Dejerine-Klumpke syndrome, lower brachial plexus palsy (C7–T1) impairs hand and wrist function. In the case of a complete brachial plexus palsy (C5–T1), the function of the entire arm is impaired, presenting with a completely flaccid arm without sensitivity, and sometimes with ocular impairment. This combination of symptoms is known as Horner’s Syndrome [9,10,11,12,13].
With NBPP, prognosis and outcomes depend on the extent of the injury. The rehabilitation options depend on the type of injury and the regeneration evidenced by spontaneous recovery of the affected limb [12].
Rehabilitation treatment for neonatal brachial plexus palsy includes conservative treatment, started as soon as possible with passive movements, sensory stimuli and guidance to the child’s relatives, instead of surgical treatment, which implies surgical techniques, and is performed only after spontaneous recovery, usually at 3 months of age [14].
Thus, the aim of this integrative literature review is to analyze the scientific production so as to identify and describe the available treatments/therapies for the rehabilitation of neonatal brachial plexus palsy.
2. Experimental Section
This article is an integrative literature review. This type of review can cover both experimental and non-experimental research, providing a broader view of a given phenomenon [15].
In this review, we analyze and combine the data gathered through systematic searches, toward a further understanding of the subject of study: state of the art treatments for rehabilitating children with neonatal brachial plexus palsy.
This integrative literature review was structured based on a research theme and a research question, defined according to the PICo concept (Population (P); Interest Area (I), Context (Co)), on criteria for including and excluding articles, the choice of databases to search, and the selection and validation of descriptors on the DeCS (Descriptors in Health Sciences) and MeSH (Medical Subject Headings) websites. A Boolean conjunction was later used to search the databases, collect data, analyze the included studies, discuss and interpret the results and, finally, to draw a conclusion based on the main results. The research question was posed with resort to the PICo strategy: What are the available rehabilitation treatments/therapies for neonatal brachial plexus palsy?
The article search for this integrative literature review was performed during January 2019, using the EBSCOhost and BVS (Biblioteca Virtual em Saúde) platforms, and the CINAHL Complete, MEDLINE Complete, LILACS and PubMed databases. The following descriptors were eventually used: neonatal/obstetric brachial plexus palsy AND rehabilitation.
The inclusion criteria were availability (full-text articles), publication language (English, French, Spanish, and Portuguese), publication date (last five years), and all types of articles related to neonatal brachial plexus palsy. The references within the included articles were also considered. The exclusion criteria considered were articles that address brachial plexus palsy in adults (excluding articles on populations of 19 years of age or over this age), articles that address brachial plexus palsy non congenital and articles with costs of obtaining. Articles were selected by reading their title and abstract, and, whenever this proved insufficient, the full text of the article was read.
3. Results
Of the 49 initially identified articles, 13 were found to meet the inclusion criteria [4,16,17,18,19,20,21,22,23,24,25,26,27] after reading the title, abstract, and the full article. For a better understanding of the search strategy and selection of articles that constituted the sample for our integrative literature review, a PRISMA flow chart was built (Figure 1). Thus, 49 articles were then identified (24 in the FBSCOHost platform and 25 in the BVS platform). However, 1 article was excluded for finding a duplicate and 31 articles were also excluded after title search, resulting in 16 validated articles. After reading full texts and taking into account the inclusion criteria, 13 articles were selected as eligible and this is why our sample is made up of 13 articles.
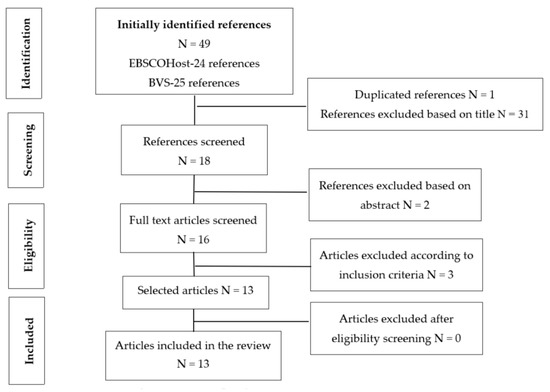
Figure 1.
PRISMA flowchart.
Two of these articles were published in 2018 [19,22], one in 2017 [26], two in 2016 [16,25], three in 2015 [4,20,24], and five in 2014 [17,18,21,24,27].
The countries of origin were Argentina [16], the United States of America [19,22,23], Spain [26], the Netherlands [17], Canada [18], India [4], Cuba [21], Saudi Arabia [24,27], and Brazil [20,25].
The articles presented rehabilitation treatments/therapies for neonatal brachial plexus palsy, including (i) multidisciplinary conservative treatment [4,19,20,21,22] using complementary means or techniques such as electrostimulation, botulinum toxin injection, immobilizing splints and constraint induced movement therapy [4,19,20,22,23,26], and (ii) surgical treatment, which includes primary and secondary surgeries [16,20,22], where nerve transfer surgeries can use grafts of the median nerve, phrenic nerve, and ulnar nerve [24,25,27]. The criteria that define the choice for conservative or surgical treatment are debated by several authors [17,18,21,22,27] (Table 1).

Table 1.
Description of the results.
4. Discussion
4.1. Multidisciplinary Conservative Treatment
Conservative treatment of neonatal brachial plexus palsy involves early diagnosis and follow-up, if possible, within two to three weeks after the child’s birth [21]. Conservative treatment should involve a multidisciplinary team, composed of physiatrists, clinical neurophysiologist, neurosurgeons, occupational therapists, and physiotherapists. The treatment administered by the physiotherapist and occupational therapist involves smooth joint movements and sensory stimulation, such as passive/active mobilization exercises, stretches, tactile stimulation with different textures, vibration and brushing techniques to promote sensory ability in the injured limb, and bimanual activities. Sensory stimulation is as important as motor stimulation and can consist in suckling any finger on the injured limb and stimulating the skin with different textures, temperatures, and vibrations [4,19,20,21,22]. Electrical stimulation/electrostimulation is a complementary means or technique used in conservative therapies for the rehabilitation of brachial plexus palsy [4,19], that promotes gaining muscle tone/strength on the affected muscles, and significant improvements in the mobility of the injured limb. These therapies aim to ensure the conditions needed for the functional recovery of the limb following nerve regeneration, which implies the prevention of muscle shrinkage, sagging, joint deformities, and muscle contractures [20,21]. Both therapies play a key role in the rehabilitation of neonatal brachial plexus palsy, but it is essential to involve parents in the rehabilitation program, so that professionals and family members work jointly. Therapy should be administered several times a week and, at home, as frequently as possible, for example at each meal or with every nappy change [20,21]. Most studies reveal that conservative treatment performed by therapists significantly reduces injuries, removing the need for surgical intervention [28,29].
There are different tools that are used as means and/or complementary techniques to the conservative/surgical treatment of neonatal brachial plexus palsy, such as electrostimulation, botulinum toxin injection, thermoplastic splints, posterior and anterior temporary splints (for physiological positioning, facilitating functional motor function, and preventing vicious postures. Anterior and posterior fist or hand splints control and prevent, at the fist level, extreme ulnar flexion and deviation. Anterior splints can simultaneously control thumb adduction, and posterior splints allow more freedom of the child’s palm), and constraint induced movement therapy [4,19,20,22,23,26].
Electrostimulation is commonly used to increase muscle strength, with the aim of promoting functional muscle recovery after nerve injury. It inhibits muscle atrophy during the reinnervation period, and accelerates nerve regeneration, resulting in improved muscle strength and range of motion in the injured limb [4,19,22]. The benefits of electrostimulation in the recovery of neonatal brachial plexus palsy as a complement to conservative/surgical treatment are evident [30].
Injection of botulinum toxin into healthy antagonist muscles has proven effective in the treatment of muscle imbalances, co-contractions, and muscle contractures in children with neonatal brachial plexus palsy. The aim of this complementary treatment option is to balance strength and to allow the affected muscles to develop, by adapting the movement pattern to the ongoing nerve recovery [26]. Studies on the benefits of using botulinum toxin for treating neonatal brachial plexus palsy demonstrate benefits for the elbow function, with improved flexion and supination [31].
The use of temporary immobilizing splints is indicated for children with impaired wrist function, which can help improve hand function and prevent wrist drop, thus promoting wrist extension. Some splints are used during sleep, and other more functional ones are used during awake time activities [20,21].
Constraint induced movement therapy demonstrates that performing activities at home for one hour a day can improve mobility, functional capacity, speed, range of motion, and hand manipulation ability [23]. Other studies reveal the effectiveness of movement therapy, leading to improvements in mobility, increased predisposition to use the injured limb, and frequency of use [32,33].
4.2. Surgical Treatment
Surgical nerve reconstruction may be necessary for rehabilitating patients with neonatal brachial plexus palsy, especially children who do not show spontaneous recovery during the first months of life [17]. However, conservative treatment should be favored whenever possible [16].
When surgical intervention is required, both primary and secondary microsurgeries are available. Primary microsurgery techniques include recession and reconstruction of the neuroma, neurolysis, and nerve transfer [16,20,22]. Studies reveal that, as a primary surgery for neonatal brachial plexus palsy, neurolysis combined with nerve transfer produces good results [34].
Nerve transfer surgeries reconnect nerves that have less important roles or are redundant with the target nerve, without innervation [22].
In situations where the lesion affects the suprascapular nerve, shoulder function is impaired (abduction and external rotation). Grafts extracted from the proximal C5 root stump or the accessory nerve are often used to reconstruct the suprascapular nerve. The use of the phrenic nerve has also been shown to provide a similar level of recovery to the use of the median nerve, increasing the number of graft options available to recover suprascapular nerve function [24].
When the C5–C6 nerve roots are affected, i.e., in Erb’s palsy, affecting shoulder abduction and external rotation, elbow flexion, and forearm supination, and when there is no evidence of spontaneous recovery, surgery is a valid treatment option. The Oberlin’s procedure involves the transfer of the ulnar nerve to the cutaneous nerve and is an effective way of recovering the elbow function, improving elbow flexion and leading to increased functional use of the affected limb [25]. Other studies corroborate the positive results in the recovery of the biceps function obtained with the Oberlin’s procedure [35,36].
Another alternative for treating Erb’s palsy is to extract a graft from the median nerve and use it to reconstruct the biceps nerve, which has been shown to improve elbow flexion [27]. The use of the ulnar or median nerves as grafts for treating the biceps are viable options, not only because of the evidence supporting the recovery of the biceps function, but also due to the proximity of the biceps nerve [37].
Secondary surgery involves tendon transfer, arthrodesis, or osteotomies, and may be an option for children who have partially recovered after primary surgery but still show some deficits deemed treatable, or for children who experience spontaneous recovery but still show some functional deficits. In summary, primary surgery is the initial treatment option for children who do not experience spontaneous recovery, and secondary surgery aims to promote the functional improvement of the limbs [16]. Other authors report that secondary surgery may include procedures to improve external shoulder rotation, such as the Hoffer’s procedure, and the Steindler flexorplasty to improve elbow flexion. Secondary surgery is also used to improve hand function, but relevant studies suggest that improving hand function is a great challenge, and the results of these surgeries are merely palliative in most cases [38,39,40].
In general, with regard to surgical treatment, the authors argue that primary surgery includes surgical procedures involving nerve transfer, and the ulnar, median and phrenic nerves are used as grafts/donors in this type of surgery. Secondary surgeries are used in patients who, after primary surgery, have reached a certain level of recovery but still show some deficits considered treatable, or in patients who have not undergone primary surgery and show some type of spontaneous recovery but still have deficits.
4.3. Criteria for Conservative/Surgical Treatment
Criteria for opting between conservative treatment or surgical treatment for neonatal brachial plexus palsy are not consensual. There is no scientific evidence favoring surgical treatment/nerve reconstruction over conservative treatment. Children who do not show spontaneous recovery during the first months of life are considered suitable recipients of reconstructive nerve surgery [17].
Some studies defend that primary surgery (neuroma excision or nerve graft) is indicated for children who do not have biceps function (elbow flexion against gravity) at the age of 3 months. If at this age there is evidence of nerve root avulsion, surgery is indicated. Some authors defend that the decision to operate can be postponed until 5–6 months if there is no biceps function at the age of 3 months, but some level of shoulder recovery is observed [18,21].
Some studies defend that if there is some level of recovery of the biceps function at the age of 3 months, the situation can be reassessed between the age of six and nine months to assess whether there is need for surgical intervention [22]. Other authors corroborate the influence of biceps recovery in opting for conservative/surgical treatment, stating that biceps recovery before the age of three months is a predictor of complete or near-complete shoulder recovery. The exact moment to decide on nerve reconstruction is difficult to identify, but a possible range is established between the child’s third and sixth month of life [41,42].
Neonatal brachial plexus palsy is complex, present with many different severity levels and prognoses, as well as reinnervation and recovery patterns, which are unpredictable factors that make it harder to define rigorous criteria for reconstructive surgery [18]. Several scientific studies reveal how difficult it is to opt for surgical treatment of neonatal brachial plexus palsy. For severe lesions, with avulsion and rupture of the nerve roots, the neurological prognosis without surgical intervention is poor, justifying surgical treatment. Another strong indicator for surgery is decreased hand function without spontaneous recovery. For children that present with partial lesions at the C5–C6 or C5–C6–C7 level, surgery is a "grey area", as different levels of spontaneous recovery can take place, leading to the decision for surgery at different stages during the child’s life, namely at 3 months of age, 5–6 months, or up to 9 months [43,44].
Surgical treatment is not a consensual option among the different authors. Some authors defend the surgical intervention at the age of three months if there is no spontaneous recovery or if there is evidence of nerve root. Others defend the evaluation surgery at the age of 5–6 months, or at 6–9 months in children who, despite lacking biceps function at three months of age, experienced at this time some spontaneous recovery of the injured limb. An early rehabilitation treatment based on intensive multidisciplinary conservative treatment can lead to favorable functional outcomes in children whose biceps recover spontaneously between the ages of 3 and 6 months. When there is no spontaneous recovery or complete paralysis of the limb, the most widely prescribed treatment is the surgical treatment.
5. Conclusions
In the rehabilitation of NBPP there are two options of therapies or treatments: the conventional/conservative treatment and the surgical treatment. The conventional/conservative multidisciplinary treatment, which includes intensive physiotherapy/occupational therapy sessions, using in a complementary way, means and or techniques such as electrostimulation, immobilizing splints, constraint induced movement therapy, the work of joint action with families, and/or botulinum toxin injection; and the surgical treatment that includes primary and secondary surgeries, respectively the first ones are indicated for children who do not have any type of spontaneous rehabilitation in the first three months of life, and the later are an option for ex-post treatment for children who still present functional limitations or significant functional deficits of the injured limb, after spontaneous recovery or in children who were subjected initially to primary surgery. There is unanimous agreement that early conservative treatment is the main treatment option for the rehabilitation of neonatal brachial plexus palsy. Whatever the type of lesion, it is generally expected that clinical development/recovery will progressively help defining the diagnosis, thus facilitating decision making to maintain the conservative treatment or opt for surgical treatment, restarting the intensive conservative treatment after the surgeries.
Despite the important conclusions that this integrative literature revision allowed us to identify in relation to the rehabilitation treatment options of NBPP, we also noticed that there is no current scientific evidence on some rehabilitation means/techniques used in conservative treatments, that is, some positioning of the limbs (like external rotation of the shoulder and forearm to prevent contractures and deformities (glenohumeral dysplasia); the use of kinesiology tapes; the use of weight shift on the injured limb (at key stages of the child’s development), and hydrotherapy from the age of 6 months). Due to this multiplicity, we identified the need for further research on these types of means/techniques.
Author Contributions
Conceptualization, F.F.; Data curation, F.F. and F.F.-S.; Formal analysis, F.F., J.G.-S. and F.F.-S.; Investigation, J.G.-S. and L.J.; Methodology, J.G.-S., L.J. and F.F.-S.; Project administration, F.F.; Resources, F.F.-S.; Supervision, F.F.; Validation, F.F. and L.J.; Visualization, J.G.-S. and F.F.-S.; Writing–original draft, F.F. and L.J.; Writing–review & editing, J.G.-S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chauhan, S.P.; Blackwell, S.B.; Ananth, C.V. Neonatal brachial ple-xus palsy: Incidence, prevalence, and temporal trends. Semin Perinatol. 2014, 38, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Collado-Vázquez, S.; Jiménez-Antona, C.; Carrillo, J. Parálisis braquial obstétrica, una revisión histórica. Rev. Neurol. 2012, 55, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Abzug, J.M.; Chafetz, R.S.; Gaughan, J.P.; Ashworth, S.; Kozin, S.H. Shoulder function after medial approach and derotational humeral osteotomy in patients with brachial plexus birth palsy. J. Pediatr. Orthop. 2010, 30, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Jeyanthi, S. The effect of Nerve Branch Stimulation in Adjunct to convencional Treatment on C6-C7 Obstetric Brachial Plexus Injury: A Case Report. Indian J. Physiother Occup. Ther. 2015, 9, 150–155. [Google Scholar] [CrossRef]
- Doumouchtsis, S.K.; Arulkumaran, S. Are all brachial plexus injuries caused by shoulder dystocia? Obstet. Gynecol. Surv. 2009, 64, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Dijk, J.G.; Pondaag, W.; Malessy, M.J. Obstetric lesions of brachial plexus. Muscle Nerve. 2001, 24, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, A.A. Conceptos actuales en la parálisis braquial perinatal. Parte 1: Etapa temprana. Arch. Argent. Pediatr. 2011, 109, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.M. Update on management of pediatric brachial plexus palsy. J. Pediatr. Orthop. B. 2005, 25, 116–126. [Google Scholar]
- Birch, R. Surgical Disorders of the Peripheral Nerves, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Heise, C.O.; Martins, R.S.; Foroni, L.H.; Siqueira, M.G. Prognostic value of thumb pain sensation in birth brachial plexopathy. Arq. Neuropsiquiatr. 2012, 70, 590–592. [Google Scholar] [CrossRef][Green Version]
- Kay, S.P. Obstetrical brachial palsy. Br. J. Plast. Surg. 1998, 51, 43–50. [Google Scholar] [CrossRef]
- Alfonso, I.; Alfonso, D.T.; Papazian, O. Focal upper extremity neuropathy in neonates. Semin. Pediatr. Neurol. 2000, 7, 4–14. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Al-Khawashki, H. The “beggar’s” hand and the “unshakable” hand in children with total obstetric brachial plexus palsy. Plast. Reconstr. Surg. 2002, 109, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.P.S.; Watanabe, B.M.N.; Oliveira, T.C. A terapia ocupacional favorecendo o desenvolvimento neuropsicomotor, ao intervir precocemente, em crianças com paralisia braquial obstétrica. [Trabalho de Conclusão de Curso de Terapia Ocupacional]; Centro Universitário Católico Salesiano Auxilium: Lins, SP, Brazil, 2008. (In Portuguese) [Google Scholar]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Socolovsky, M.; Robla, C.J.; Dominguez, P.M.; Nizzo, G.; Valbuena, S.; Varone, E. Obstetric brachial plexus palsy: Reviewing the literature comparing the results of primary versus secondary surgery. Childs Nerv. Syst. 2016, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Pondaag, W.; Malessy, M. The Evidence for Nerve Repair in Obstetric Brachial Plexus Palsy Revisited. Biomed. Res. Int. 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bade, S.; Lin, J.; Curtis, C.; Clarke, H. Extending the Indications for Primary Nerve Surgery in Obstetrical Brachial Plexus Palsy. Biomed. Res. Int. 2014, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Justice, D.; Awori, J. Use of Neuromuscular Electrical Stimulation in the Treatment of Neonatal Brachial Plexus Palsy: A Literature Review. Open J. Occup. Ther. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Otto, H.C.; Martins, R.; Siqueira, M. Neonatal brachial plexus palsy: A permanente challenge. Arq. Neuropsiquiatr. 2015, 73, 803–808. [Google Scholar] [CrossRef]
- Yanes, S.V.L.; Sandobal, F.E.C.; Camero, A.D.; Ojeda, D.L. Obstetric Brachial Plexus Palsy in the Context of Early Physical Rehabilitation. MediSur 2014, 12, 635–649. [Google Scholar]
- Smith, B.; Daunter, A.; Yang, L.; Wilson, T. An Update on the Management of Neonatal Brachial Plexus Palsy-Replacing Old Paradigms A Review. JAMA Pediatr. 2018, 172, 585–591. [Google Scholar] [CrossRef]
- Brown, S.H.; Napier, R.; Nelson, V.S.; Yang, L.J. Home-based movement therapy in neonatal brachial plexus palsy: A case study. J. Hand Ther. 2015, 28, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Al-Qattan, M.M.; El-Sayed, A.A.F. The use of the Phrenic Nerve Communicating Branch to the Fifth Cervical Root for Nerve Transfer to the Suprascapular Nerve in Infants with Obstetric Brachial Plexus Palsy. Biomed. Res. Int. 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.M.; Grechi, G.; Gepp, R.A. Oberlin’s procedure in children with obstetric brachial plexus palsy. Childs Nerv. Syst. 2016, 32, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- García Ron, A.; Gallardo, R.; Huete Hermani, B. Utility of ultrasound-guided injection of botulinum toxin type A for muscle imbalance in children with obstetric brachial plexus palsy: Description of the procedure and action protocol. Neurologia 2017, 34, 1–9. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Al-Kharfy, T.M. Median Nerve to Biceps Nerve Transfer to Restore Elbow Flexion in Obstetric Brachial Plexus Palsy. Biomed. Res. Int. 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J. Neonatal brachial plexus palsy: Management andprognostic factors. Semin Perinatol. 2014, 38, 222–234. [Google Scholar] [CrossRef]
- Amador, E.V. Trauma del plexo braquial: Conceptos actuales en el diagnóstico y tratamiento quirúrgico. Rev. Col. Med. Fis. Rehab. 2013, 23, 160–169. [Google Scholar] [CrossRef]
- Okafor, U.A.; Akinbo, S.R.; Sokunbi, O.G.; Okanlawon, A.O.; Noronha, C.C. Comparison of electrical stimulation and conventional physiotherapy in functional rehabilitation in Erb’s palsy. Nig. Q. J. Hosp. Med. 2008, 18, 202–205. [Google Scholar] [CrossRef]
- Shin, Y.B.; Shin, M.J.; Chang, J.H.; Cha, Y.S.; Ko, H.Y. Effects of botulinum toxin on reducing the co-contraction of antagonists in birthbrachial plexus palsy. Ann. Rehabil. Med. 2014, 38, 127–131. [Google Scholar] [CrossRef]
- Buesch, F.E.; Schlaepfer, B.; de Bruin, E.D.; Wohlrab, G.; Ammann-Reiffer, C.; Meyer- Heim, A. Constraint-induced movement therapy for children with obstetric brachial plexus palsy: Two single-case series. Int. J. Rehabil. Res. 2010, 33, 187–192. [Google Scholar] [CrossRef]
- Murphy, K.M.; Rasmussen, L.; Hervey-Jumper, S.L.; Justice, D.; Nelson, V.S.; Yang, L.J.-S. An assessment of the compliance and utility of a home exercise DVD for caregivers of children and adolescents with brachial plexus palsy: A pilot study. PMR 2012, 4, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Schwentker-Colizza, A.; Curtis, C.G.; Clarke, H.M. Final results of grafting versus neurolysis in obstetrical brachial plexus palsy. Plast. Reconstr. Surg. 2009, 123, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Al-Qattan, M.M. Oberlin’s ulnar nerve transfer to the bíceps nerve in Erb’s birth palsy. Plast. Reconstr. Surg. 2002, 109, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, C.; Ameur, N.E.; Teboul, F.; Beaulieu, J.Y.; Vacher, C. Restoration of elbow flexion in brachial plexus injury by transfer of ulnar nerve fascicles to the nerve to the biceps muscle. Tech. Hand Upr. Extrem. Surg. 2002, 6, 86–90. [Google Scholar] [CrossRef]
- Sungpet, A.; Suphachatwong, C.; Kawinwonggowit, V. One fascicle median nerve transfer to biceps muscle in C5 and C6 root avulsions of brachial plexus injury. Microsurgery 2003, 23, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Murabit, A.; Gnarra, M.; O’Grady, K.; Morhart, M.; Olson, J. Functional outcome after the Hoffer procedure. Plast. Reconstr. Surg. 2013, 131, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.; Valbuena, S.; Posso, C. Obstetrical brachial plexus injuries: Late functional results of the Steindler procedure. J. Hand Surg. Eur. Vol. 2014, 39, 68–875. [Google Scholar] [CrossRef]
- Ruchelsman, D.E.; Ramos, L.E.; Price, A.E.; Grossman, L.A.; Valencia, H.; Grossman, J.A. Outcome after tendon transfers to restore wrist extension in children with brachial plexus birth injuries. J. Pediatr. Orthop. 2011, 31, 455–457. [Google Scholar] [CrossRef]
- Chin, K.F.; Misra, V.P.; Sicuri, G.M.; Fox, M.; Sinisi, M. Intra-operative neurophysiological prediction of upper trunk recovery in obstetric brachial plexus palsy with neuroma incontinuity. Bone Joint J. 2013, 95, 699–705. [Google Scholar] [CrossRef]
- Al-Qattan, M.M. The outcome of Erb’s palsy when the decision to operate is made at 4months of age. Plast. Reconstr. Surg. 2000, 106, 1461–1465. [Google Scholar] [CrossRef]
- Pondaag, W.; Malessy, M.J. Recovery of hand function following nerve grafting and transfer in obstetric brachial plexus lesions. J. Neurosurg. 2006, 105, 33–40. [Google Scholar] [CrossRef]
- Birch, R.; Ahad, N.; Kono, H.; Smith, S. Repair of obstetric brachial plexus palsy. Results in 100 children. J. Bone Jt. Surg. 2005, 87, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).