Abstract
Background: Outcome prediction in patients undergoing lung transplantation (LUTX) for end-stage pulmonary disease can be challenging. We examined the prognostic value for mortality of respiratory system compliance (CRS) and mechanical power of ventilation (MP) at end of surgery in patients undergoing LUTX for end-stage pulmonary disease. Methods: In this single-center retrospective study, we included 755 patients undergoing LUTX between 2014 and 2023. The primary endpoint of this study was 1-year mortality, with 30-day mortality serving as a secondary endpoint. We conducted both univariate and multivariate analyses and constructed Receiver Operating Characteristic curves. Results: Of 755 patients, 1.9% and 12.2% patients died within 30 days and 1 year after LUTX. Fifteen-point four percent of all patients required extracorporeal membrane oxygenation (ECMO) prolongation into the early postoperative period. CRS, but not MP was higher in 1-year survivors compared to non-survivors [median 25.8 mL/cmH2O (20.1, 32.1) and 22.5 mL/cmH2O (15.2, 28.4); p < 0.001] and [median 10.0 J/min (7.8, 12.0) and 9.3 J/min (6.2, 13.1); p = 0.329]. Moreover, low CRS < 25.1 mL/cmH2O remained an independent factor for increased 1-year mortality after LUTX. Additionally, increased MP and CRS were predictive for 30-day survival with an acceptable area under the curve of 0.758 (95% CI: 0.6–0.8; p < 0.001) and 0.735 (95% CI: 0.5–0.9; p = 0.003), and a sensitivity and specificity of 51% and 75.5% for MP and 50% and 85% for CRS, respectively. Conclusions: Postoperative CRS serves as a significant independent predictor for short and long-term outcome in patients undergoing LUTX with and without ECMO prolongation into the early postoperative period.
1. Introduction
In the context of lung transplantation (LUTX), early prognostication of short-term and long-term outcomes is critical. Currently, prognostication remains complex and necessitates the integration of various clinical markers []. An ideal prognostic marker for both short- and long-term outcomes following LUTX should be easily determinable, robust, devoid of confounding factors, and exhibit a strong correlation with actual overall short- and long-term outcomes after transplantation [,].
Currently, primary graft dysfunction (PGD) assessment serves as a prognostic tool in order to predict early and late postoperative outcomes, including the development of chronic lung allograft dysfunction (CLAD) and long-term survival [,]. The International Society for Heart and Lung Transplantation (ISHLT) introduced a PGD scoring system, aligning it with the Berlin criteria for acute respiratory distress syndrome (ARDS) [].
However, the use of the PGD score has several limitations: The radiographic scoring of PGD varies substantially between observers [,]. Furthermore, the PaO2/FiO2 ratio cannot reliably be assessed in patients requiring postoperative extracorporeal membrane oxygenation (ECMO) due to the dual source of blood oxygenation [,,]. Consequently, there is a need for reliable and straightforward early prognostic parameters.
In ARDS patients, respiratory system compliance (CRS) has been shown to hold prognostic value, both in patients with and without ECMO support [,]. Studies have consistently demonstrated its utility in assessing lung function and guiding ventilatory management strategies []. CRS is determined by a simple calculation of immediately available parameters during mechanical ventilation such as driving pressure (ΔP) and tidal volume (VT). In addition to CRS, the mechanical power of ventilation (MP) has recently been introduced as a prognostic parameter for outcomes after ARDS as well [,].
Therefore, we tested the hypothesis that CRS and MP at the end of surgery have prognostic value in LUTX patients regarding 30-day and 1-year survival. For this purpose, we retrospectively analyzed a cohort of 755 consecutive patients who underwent LUTX for end-stage pulmonary disease at a high-volume lung transplantation center.
2. Methods
2.1. Design
This is a single center cohort study in patients who have been undergoing LUTX at the General Hospital of Vienna between January 2014 and April 2023. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the study protocol was approved by the Ethics Committee of the Medical University of Vienna (1501/2023). The need for individual informed consent was waived due to the observational and retrospective nature of this study.
2.2. Patients
Patients were eligible if (1) aged > 18 years; and (2) if they underwent LUTX for end-stage pulmonary disease. We excluded patients with single-lung transplantation, as well as patients having multiorgan or re-transplantations.
2.3. Data Collection
Demographic and clinical data were retrieved from our intensive care data management system archives (IntelliSpace Critical Care and Anesthesia, Philips, Bollingen, Germany). Respiratory parameters for calculating MP and CRS were collected at two distinct time points: baseline values (CRS1 and MP1) within the first 15 min after surgical incision and during the final 15 min before the end of surgery (CRS2 and MP2), i.e., after chest closure. Muscle relaxation was administered routinely before the skin incision and again before the end of surgery for subsequent tube exchange and bronchoscopy. Thus, CRS and MP used for the statistical analysis were routinely determined during muscle relaxation. Ventilatory settings during LUTX adhered strictly to institutional standards, utilizing exclusively pressure control ventilation. Tidal volume (VT) targets ranged from 6 to 8 mL/predicted body weight (PBW), with a maximum inspiratory peak pressure (Pmax) < 30 cmH2O and a positive end-expiratory pressure [] maintained at a minimum of 7 cmH2O. Lung-protective ventilation strategies were applied whenever feasible [].
The following data were included for further analysis: (i) patient demographics and baseline characteristics including age, sex, body height, body weight, medical history, indications for LUTX, perioperative ECMO use, perioperative fluid balance, and perioperative transfusion of fresh frozen plasma (FFP) and packed red blood cells (PRBCs); (ii) perioperative ventilatory settings (i.e., tidal volume (VT), PEEP, Pmax, set and respiratory rate (RR) at the beginning and the end of the surgery, (iii) survival on day 30 and at 1 year post LUTX, duration of postoperative invasive ventilation, length of ICU and hospital stay; and (iv.) occurrence as well as degree of PGD.
PGD was graded as described elsewhere [,]. Time point T0 for PGD evaluation was 2 h after the patient’s arrival in the ICU, once their respiration and hemodynamics had stabilized after transfer from the operating room (OR). Subsequent time points T24, T48, and T72 were set 24, 48, and 72 h after admission to the ICU, respectively.
2.4. Perioperative Management
Anesthesia and surgery were performed according to our institutional standards as reported elsewhere []. LUTX was conducted either supported by intraoperative central veno-arterial-extracorporeal membrane oxygenation (VA-ECMO), the cardiopulmonary bypass, or without extracorporeal circulatory support [,,]. If feasible, the patient was weaned from central VA-ECMO after implant of both lungs and adequate hemostasis at stable and sufficient gas exchange and hemodynamics. Prolongation of VA-ECMO support was chosen in response to the following conditions: PaO2/FiO2 ratio < 100, mean pulmonary arterial pressure > 2/3 of mean systolic arterial pressure, pronounced deterioration of respiratory and/or hemodynamic function. Therefore, central VA-ECMO was switched to a peripheral femoro-femoral VA-ECMO configuration [].
2.5. Definitions and Calculations
VT was expressed in mL/kg PBW with PBW being calculated using the Devine’s equations for females (1) and males (2), respectively:
PBW = 45.5 + 0.90 × (height in cm − 152.4),
PBW = 50.0 + 0.90 × (height in cm − 152.4).
CRS, driving pressure (ΔP), and MP were calculated during pressure-controlled ventilation using the following Equations (3)–(5):
CRS = VT/ΔP,
ΔP = Pmax − PEEP,
MP = 0.098 × VT × RR × (Pmax − 0.5 × ΔP).
2.6. Study Endpoints
Endpoints of this analysis were short- (i.e., 30-day) and long-term (i.e., 1-year) survival.
2.7. Statistical Analysis
Normally distributed data are reported as means ± SD, non-normally distributed data as medians with interquartile range (IQR). Mann–Whitney U test was used to compare non-normally distributed data and t-tests to compare parametric data. Pearson’s correlation coefficient r was employed to estimate the strength of a linear relationship between respiratory parameters and clinical variables, such as the number of transfused PRBCs and FFPs, the highest arterial lactate level, the lowest hemoglobin level, length of invasive ventilation, and duration of ICU stay (Supplementary Materials Table S1).
We performed univariate and multivariate Cox regression analyses for 30-day and 1-year mortality. The median CRS and the median MP were used as a cutoff to create two groups. Variables with p < 0.05 in the univariate analysis were entered into the multivariate model. We did not include blood hemoglobin values (HB) because of co-linearity with the number of transfused PRBCs.
Consecutively, we constructed Receiver Operating Characteristic (ROC) curves to present additional continuous data and calculated the respective areas under the curve (AUROC) for CRS and MP to assess their predictive capability for short- and long-term survival. We plotted ROC curves for patients with and without ECMO prolongation separately. An AUROC between 0.5 and 0.7 was interpreted as poorly predictive for survival; 0.7 < AUROC < 0.8 as well; 0.8 < AUROC < 0.9 as very well, and 0.9 to 1.0 as excellently predictive. The median of CRS and MP were used to calculate the sensitivity (True positive (TP)/(TP + false negative (FN)), the specificity (true negative (TN)/(TN + false positive (FP)), the positive predictive value (TP/(TP + FP)) and the negative predictive value (TN/(TN + FN)) to assess the performance of these parameters.
Statistical analyses and the corresponding graphical depiction were performed using SPSS software (version 28; IBM SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA). A p < 0.05 was considered statistically significant.
3. Results
3.1. Clinical and Demographic Data
Demographic data and perioperative characteristics are given in Table 1. We included 755 patients who underwent primary double LUTX. Overall, 17 (1.9%) and 92 (12.2%) patients died within 30 days and 1 year after LUTX, respectively.

Table 1.
Clinical and demographic data. Clinical and patient characteristics of 1-year survivors and non-survivors.
At T0, 494 patients (65.4%) were classified as PGD grade 0, 30 patients (4.0%) as PGD grade 1, 40 patients (5.3%) as PGD grade 2, 91 patients (12.1%) as PGD grade 3, 84 patients (11.1%) were categorized as PGD ungradable and data from 16 patients (2.1%) was not available. The CONSORT statement of patients who underwent LUTX with and without ECMO prolongation is shown in Figure 1. In total, 118 patients required VA-ECMO support following LUTX. Among these patients, the 1-year mortality rate was 25% (n = 30). In contrast, 637 patients did not require postoperative ECMO support, with a 1-year mortality rate of 9% (n = 62).
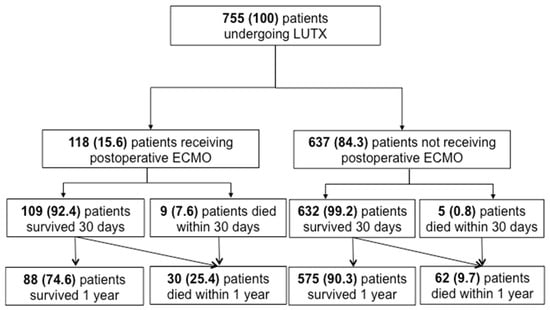
Figure 1.
CONSORT Statement of patients undergoing LUTX with and without ECMO prolongation n this study, we included 755 patients who underwent orthotopic primary double LUTX. 118 patients required ECMO support in the postoperative period, and 637 did not require prolonged ECMO support into the postoperative phase. Five and 9 patients with and without ECMO support died within 30 days after LUTX. Moreover, 30 and 62 patients died within 1 year after transplantation with and without ECMO support, respectively.
3.2. CRS and MP in Survivors and Non-Survivors
1-year survivors exhibited significantly better CRS, but not MP, post- LUTX in contrast to non-survivors, as illustrated in Table 1 and Figure 2A,C. This association retained statistical significance for CRS in patients with and without post-transplant VA-ECMO as delineated in Table 2. Furthermore, CRS was markedly decreased in ECMO-patients compared to those who did not need ECMO support post-transplant (Figure 2B), while MP was significantly reduced in postoperative ECMO patients relative to their non-ECMO counterparts (Figure 2C). The correlations between clinical and respiratory parameters at the end of surgery are outlined in the Supplementary Materials Table S1.
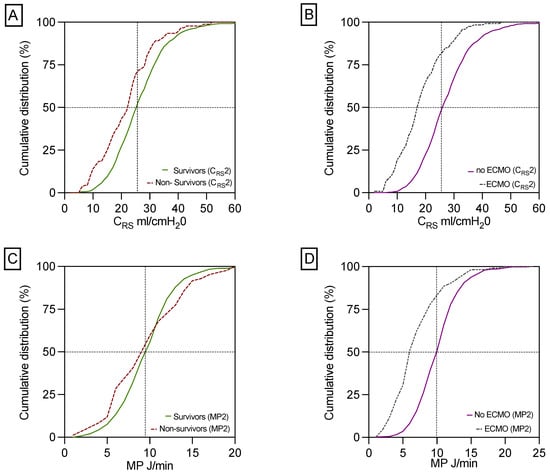
Figure 2.
Differences in CRS and MP between 1-year survivors and non-survivors and in patients with and without postoperative ECMO support. This panel shows the significant difference in CRS between 1-year survivors and non-survivors (A). CRS is decreased in patients with ECMO prolongation compared to patients without ECMO support (B). MP did not differ statistically significantly between 1-year survivors compared to 1-year non-survivors (C). In contrast, patients without ECMO prolongation had a significantly increased MP compared to patients on ECMO support (D). Respiratory parameters for calculating MP2 and CRS2 were collected during the final 15 min before the end of surgery, i.e., after chest closure.

Table 2.
Mechanical ventilation at the end of surgery.
3.3. Independent Predictors for Mortality in the Multivariate Analysis
CRS 25.1 mL/cmH2O was an independent risk factor for 1-year mortality. Younger age (i.e., <61 years) was associated with a lower risk for 30-day, but not for 1-year mortality. Serum creatinine (sCR) levels > 1 mg/dL was another independent risk factor for 30-day and 1-year mortality. CTEPH was independently related to 30-day mortality. Serum lactate levels exceeding 3 mmol/L were also associated with 1-year mortality. Furthermore, transfusion of >16 PRBCs remained an independent predictor of 30-day and 1-year mortality, as shown in Table 3.

Table 3.
Cox regression analysis of 30-day and 1-year mortality.
3.4. ROC Curves
In all patients, the AUROC of CRS for 30-day survival was 0.758 (95% CI: 0.6–0.8; p < 0.001). In patients without postoperative ECMO support it was 0.787 (95% CI: 0.6–0.9; p = 0.026) and in ECMO patients 0.587 (95% CI: 0.3–0.7; p = 0.386; Figure 3A).
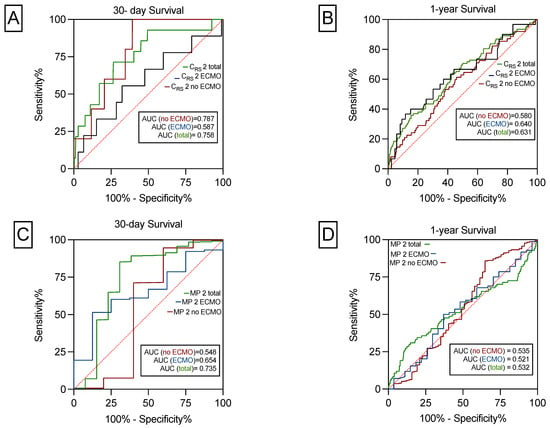
Figure 3.
The predictive power of CRS and MP on 30-day and 1-year survival. CRS significantly predicts 30-day survival for the total cohort and for patients with and without ECMO (A). CRS significantly predicts 1-year survival for the total cohort and for patients with, but not without ECMO (B). MP predicts 30-day survival for the total cohort, but not for patients with or without prolonged ECMO support (C). MP did not predict 1-year survival for the total cohort or for patients with and without postoperative ECMO use (D).
In the total cohort, the AUROC of CRS for 1-year survival was 0.631 (95% CI: 0.5–0.7; p < 0.001), in patients with postoperative ECMO it was 0.640 (95% CI: 0.5–0.7; p = 0.017), and in no-ECMO patients, 0.580 (95% CI: 0.5–0.6; p = 0.037) as depicted in Figure 3B.
As shown in Figure 3C, the AUROC of MP for 30-day survival was 0.735 (95% CI: 0.5–0.9; p = 0.003). In patients with postoperative ECMO prolongation, it was 0.654 (95% CI: 0.4–0.8; p = 0.147) and in patients without postoperative ECMO support 0.548 (95% CI: 0.1–0.9, p = 0.711). The AUROC of MP for 1-year survival was not statistically significant (Figure 3D). Sensitivity, specificity, NPV, PPV was shown for 30-day and 1-year survival for CRS, MP and PGD 0 ad depicted in Table 4. For calculations we used the median of CRS of 25.1 mL/cmH2O and the median for MP of 9.9 J/min. CRS and MP had the highest PPV of 99% to predict 30-day survival. The highest NPV of 15% was found for CRS and no PGD to predict 1-year survival. Moreover, no PGD had the highest sensitivity of 67% to predict 30-day and 1-year survival. In contrast, the highest specificity of 85% was found for CRS to predict 30-day survival.

Table 4.
Applicability of CRS, MP and no PGD to predict 30-day and 1-year survival.
4. Discussion
In this large-scale single center cohort study, our results underscore the pivotal role of CRS at the end of surgery as a robust and easily available predictor not only for short-term (30-day) but also for long-term (1-year) survival following LUTX, regardless of postoperative ECMO support. Moreover, CRS was an independent marker for 1-year mortality in the cox regression analysis. Despite the unclear influence of lung-protective ventilation on CRS in ECMO patients, we, nevertheless, identified a similar association between CRS and survival within this specific subgroup.
The predictive power of increased CRS on 30-day survival appeared to be more pronounced in patients with lower disease severity, who did not require postoperative ECMO support. However, this distinction was no longer evident regarding prediction of 1-year survival. Conversely, reduced CRS emerged as an independent marker for 1-year mortality in the Cox regression analysis. In addition, PPV and specificity to predict 30-day and 1 year survival was higher for CRS as compared to PGD 0.
Significantly elevated CRS at the end of LUTX in 1-year survivors reflected a correspondingly lower driving pressure (ΔP). This aligns with the findings of Amato et al., where decreased ΔP was positively associated with a survival benefit in ARDS patients []. Nonetheless, in 2012, the ARDS Berlin taskforce considered CRS solely as an ancillary variable and did not include CRS in its final definition of ARDS due to limited evidence supporting its predictive validity at that time [].
Contemporary scoring systems designed to define both ARDS and PGD that ultimately predict outcome face challenges, particularly related to high inter-observer variations in the interpretation of chest images [,]. Moreover, interpretation of chest X-rays immediately post-LUTX is prone to various confounders such as bleeding and pleural effusion, which can mimic bilateral infiltrations []. Additionally, a key variable in PGD grading is PaO2/FiO2 ratio, which is substantially confounded in patients requiring postoperative ECMO treatment. These downsides of current PGD grading led to the introduction of the term “PGD ungradable” in ECMO-supported patients without diffuse infiltrates in their chest X-rays [].
Our study revealed intriguing findings regarding the role of MP in predicting survival following LUTX. Surprisingly, increased MP at the end of LUTX was associated with better short-term survival (30 days), while reduced MP predicted higher than 30-day and 1-year mortality. This unexpected outcome diverges from the conventional understanding whereby higher MP typically signifies elevated stress on the respiratory system, potentially leading to adverse outcomes []. However, our study included patients with prolonged postoperative ECMO support, a group known to have a generally poorer prognosis. In these ECMO patients, we observed a reduced MP. This inverse relationship between MP and mortality in ECMO patients may be attributed to the application of lung-protective ventilation in patients on ECMO. These strategies aim to mitigate further lung injury and are known to lower MP []. Therefore, relying solely on MP as a predictive marker may be less advantageous in cohorts that include transplant recipients on ECMO. In contrast, a study focusing exclusively on ECMO patients suggested that MP, when corrected by CRS, was associated with increased mortality [], emphasizing the importance of considering both factors in this particular subgroup.
Previous literature has reported that higher BMI is associated with reduced CRS, primarily due to the mechanical effects of adiposity on lung expansion and elastic properties. In our study, 1-year non-survivors had a slightly higher BMI, though this difference was not statistically significant. Similarly, we found no significant difference in CRS between patients with high and low BMI when using the median as a cut-off. Since a BMI > 35 kg/m2 is the standard upper limit for lung transplant candidacy in Europe, our cohort did not include obese patients, which may explain the absence of significant differences in respiratory compliance.
Our study has several limitations due to its retrospective nature and the single-center analysis of prospectively and automatically collected data. While the single-center approach ensures homogeneous patient management, the impact of different management strategies in other transplant centers remains unclear. Another limitation concerns surgical access, as patients transplanted via clamshell or via thoracotomy have been included where the surgical access could potentially affect postoperative CRS. However, to minimize procedural variability, we excluded complex patient groups, as those who underwent multiorgan transplantations and patients scheduled for re-transplantation. Additionally, we did not include patients undergoing single-LUTX due to the uncertain extent of the impact on lung function by the residual lung []. Another limitation of our study concerns the use of serum creatinine as a proxy for renal function, as eGFR was not available in our dataset; while we adjusted for age and gender, which influence creatinine levels, the absence of eGFR may limit the precision of renal function assessment.
Furthermore, due to the lack of a clinically defined cut-off value for CRS that separates poor from acceptable compliance, we employed the median to dichotomize patients and to facilitate uni- and multivariate Cox regression analyses.
5. Conclusions
CRS serves as a significant independent predictor marker to predict 30-day and 1-year survival, irrespective of continued postoperative ECMO support. MP should be interpreted with caution in cohorts post-LUTX that include ECMO patients due to its inverse relationship seen in this subgroup.
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14196941/s1. Table S1: Correlation of respiratory and clinical parameters.
Author Contributions
C.V. contributed to the conception, design, data analysis, and writing. E.M.T., M.J.S., K.H., A.F. and M.D. contributed to the conception, design, supervision, writing, and critical revision. S.S., C.H. and B.M. contributed to patients’ recruitment and critical revision. S.S., C.H. and T.N. performed data collection and data analysis. J.G., P.W. and M.D. performed supervision and critical revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research work received no funding.
Informed Consent Statement
Patient consent was waived due to the retrospective design of our study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
All authors declare that they have no competing interests.
Abbreviations
| ARDS | Acute Respiratory Distress Syndrome |
| CRS | Respiratory System Compliance |
| CLAD | Chronic Lung Allograft Dysfunction |
| ECMO | Extracorporeal Membrane Oxygenation |
| FFP | Fresh Frozen Plasma |
| FN | False negative |
| HB | Hemoglobin values |
| ISHLT | International Society for Heart and Lung Transplantation |
| IQR | Interquartile Range |
| LUTX | Lung Transplantation |
| MP | Mechanical Power of Ventilation |
| OR | Operating Room |
| PBW | Predicted Body Weight |
| PGD | Primary Graft Dysfunction |
| ΔP | Driving Pressure |
| PRBC | Packed Red Blood Cells |
| TN | True Negative |
| TP | True Positive |
| VT | Tidal Volume |
| RR | Respiratory Rate |
| ROC | Receiver Operating Characteristic |
References
- Raskin, J.; Vanstapel, A.; Verbeken, E.K.; Beeckmans, H.; Vanaudenaerde, B.M.; Verleden, S.E.; Neyrinck, A.P.; Ceulemans, L.J.; Van Raemdonck, D.E.; Verleden, G.M.; et al. Mortality after lung transplantation: A single-centre cohort analysis. Transpl. Int. 2020, 33, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Kirschner, E.; Schwarz, S.; Jaksch, P.; Hoetzenecker, K.; Tschernko, E.; Dworschak, M.; Ankersmit, H.J.; Moser, B. Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO. Biology 2022, 11, 1475. [Google Scholar] [CrossRef]
- Veraar, C.; Schwarz, S.; Thanner, J.; Direder, M.; Boehm, P.M.; Harnoncourt, L.; Ortmayr, J.; Veraar, C.; Mascherbauer, J.; Klepetko, W.; et al. Transient perioperative inflammation following lung transplantation and major thoracic surgery with elective extracorporeal support: A prospective observational study. Ann. Transl. Med. 2021, 9, 385. [Google Scholar] [CrossRef]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef]
- Schwarz, S.; Benazzo, A.; Dunkler, D.; Muckenhuber, M.; Del Sorbo, L.; Di Nardo, M.; Sinn, K.; Moser, B.; Matilla, J.R.; Lang, G.; et al. Ventilation parameters and early graft function in double lung transplantation. J. Heart Lung Transplant. 2021, 40, 4–11. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Taylor Thompson, B.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Sjoding, M.W.; Hofer, T.P.; Co, I.; Courey, A.; Cooke, C.R.; Iwashyna, T.J. Interobserver Reliability of the Berlin ARDS Definition and Strategies to Improve the Reliability of ARDS Diagnosis. Chest 2018, 153, 361–367. [Google Scholar] [CrossRef]
- Benazzo, A.; Schwarz, S.; Frommlet, F.; Schweiger, T.; Jaksch, P.; Schellongowski, P.; Staudinger, T.; Klepetko, W.; Lang, G.; Hoetzenecker, K.; et al. Twenty-year experience with extracorporeal life support as bridge to lung transplantation. J. Thorac. Cardiovasc. Surg. 2019, 157, 2515–2525.e10. [Google Scholar] [CrossRef]
- Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Beer, L.; Gittler, F.; Prosch, H.; Röhrich, S.; Milos, R.; Schweiger, T.; Jaksch, P.; et al. Interobserver variability impairs radiologic grading of primary graft dysfunction after lung transplantation. J. Thorac. Cardiovasc. Surg. 2019, 158, 955–962.e1. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A.; Avalli, L.; Rossi, F.; Bombino, M. Pressure-volume curve of total respiratory system in acute respiratory failure: Computed tomographic scan study. Am. Rev. Respir. Dis. 1987, 136, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A. The concept of “baby lung”. Intensive Care Med. 2005, 31, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.E.; Schiefer, J.; Maleczek, M.; Schwarz, S.; Benazzo, A.; Rath, A.; Kulu, A.; Hritcu, R.; Faybik, P.; Schaden, E.; et al. The Effect of Prone Positioning After Lung Transplantation. Ann. Thorac. Surg. 2023, 117, 1045–1051. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Nikolla, K.; et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Belliato, M.; Epis, F.; Cremascoli, L.; Ferrari, F.; Quattrone, M.G.; Fisser, C.; Malfertheiner, M.V.; Taccone, F.S.; Di Nardo, M.; Broman, L.M.; et al. Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study. Membranes 2021, 11, 30. [Google Scholar] [CrossRef]
- Grieco, D.L.; Maggiore, S.M.; Bellani, G.; Spadaro, S.; Spinelli, E.; Tonetti, T.; Menga, L.S.; Pozzi, M.; Battaglini, D.; Di Mussi, R.; et al. Individualized positive end-expiratory pressure guided by end-expiratory lung volume in early acute respiratory distress syndrome: Study protocol for the multicenter, randomized IPERPEEP trial. Trials 2022, 23, 63. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W.; Kifjak, D.; Baron, D.; Hager, H.; et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e1. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving Pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Paudel, R.; Trinkle, C.A.; Waters, C.M.; Robinson, L.E.; Cassity, E.; Sturgill, J.L.; Broaddus, R.; Morris, P.E. Mechanical Power: A New Concept in Mechanical Ventilation. Am. J. Med. Sci. 2021, 362, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.-C.; Lin, S.-W.; Chuang, L.-P.; Li, H.-H.; Liu, P.-H.; Tsai, F.-C.; Chang, C.-H.; Hung, C.-Y.; Lee, C.-S.; Leu, S.-W.; et al. Mechanical power during extracorporeal membrane oxygenation and hospital mortality in patients with acute respiratory distress syndrome. Crit. Care 2021, 25, 13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).