The Effect of Preoperative Use of High- vs. Low-PAP-Inducing-Potential FP Agonists on the Surgical Outcomes of Trabeculectomy and AGV Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patients and Subgroups
2.3. Outcome Measurements
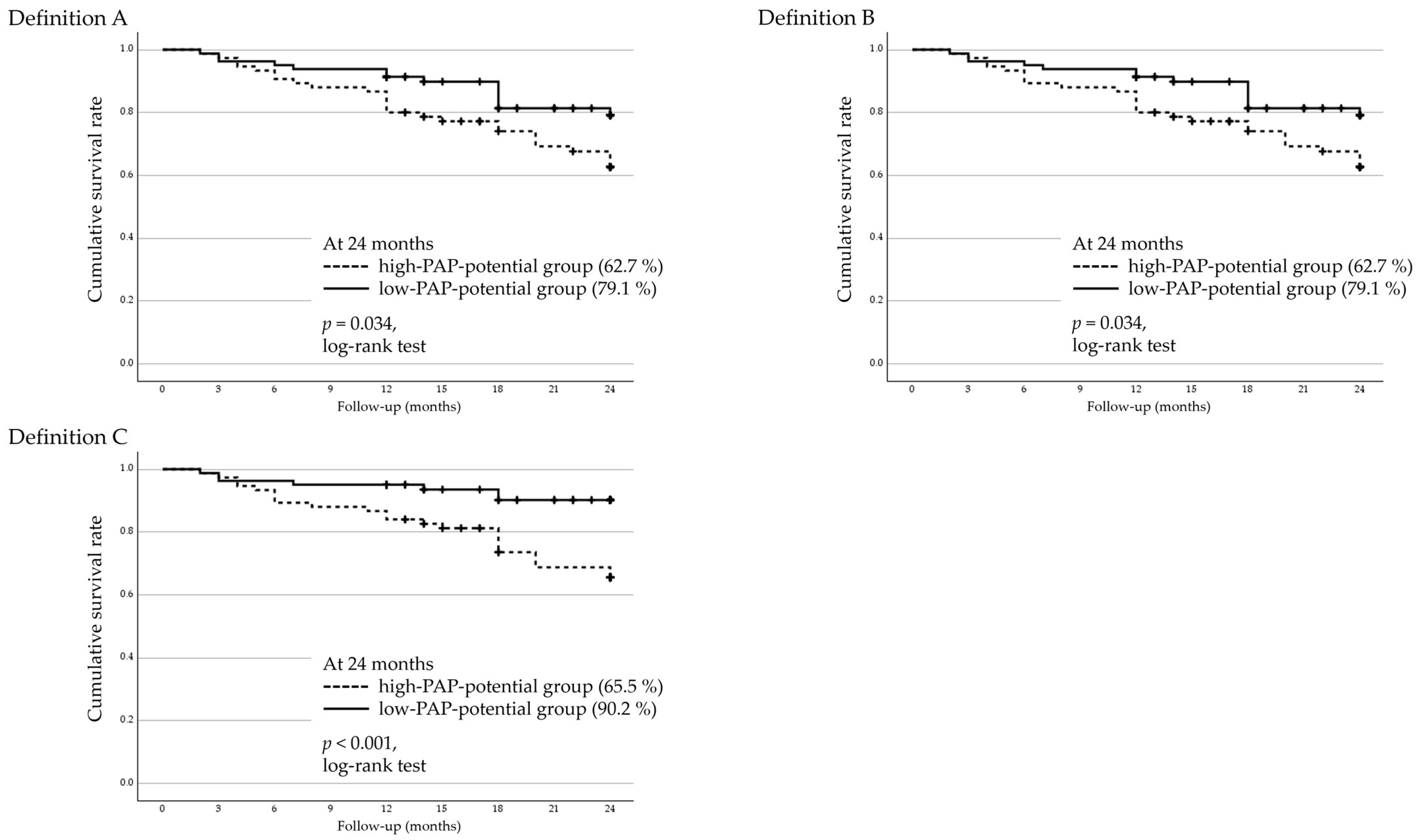
- Definition A: IOP > 21 mmHg or <20% reduction from baseline;
- Definition B: IOP > 17 mmHg or <20% reduction from baseline;
- Definition C: IOP > 14 mmHg.
2.4. Statistical Analysis
2.5. AI Tools Statement
3. Results
3.1. Baseline Characteristics
3.2. Baseline Characteristics Stratified by the Type of FP Agonist Used: The High-PAP-Potential Group and the Low-PAP-Potential Group
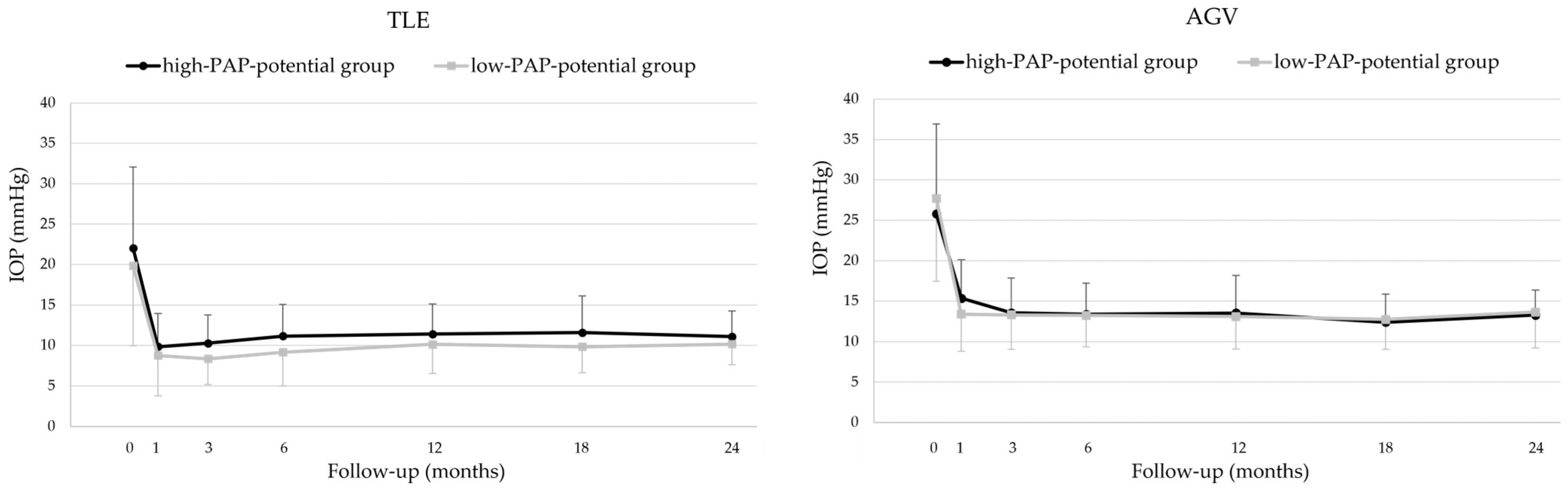
3.3. Intraocular Pressure
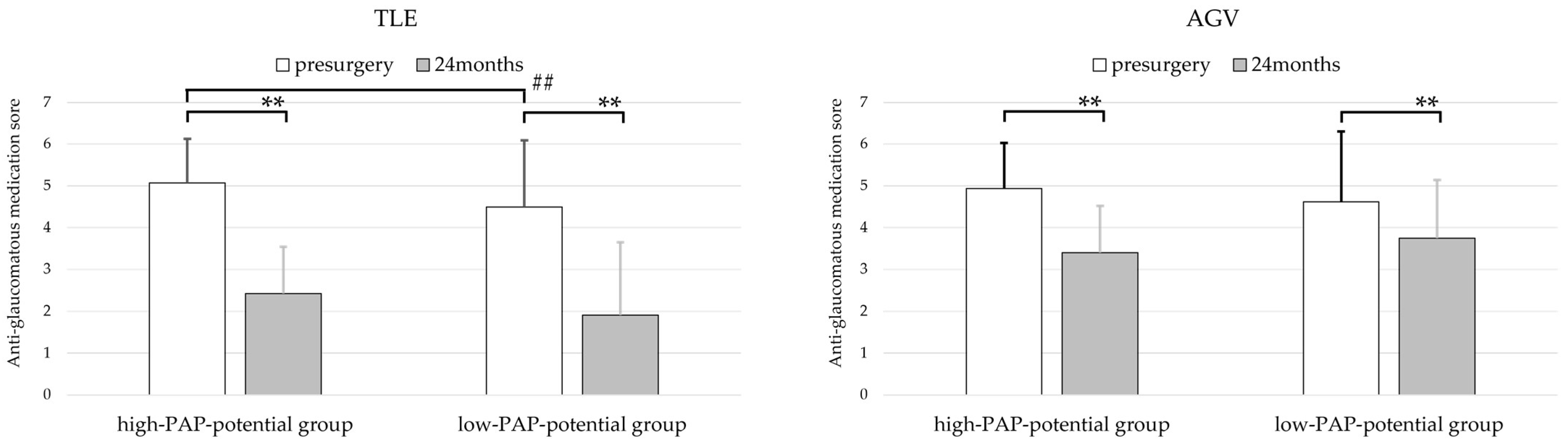
3.4. Anti-Glaucomatous Medication Scores
3.5. Two-Year Cumulative Survival Rates and Factors Associated with Surgical Failure in Regard to Trabeculectomy
3.6. Two-Year Cumulative Survival Rates and Factors Associated with Surgical Failure in AGV Implantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGV | Ahmed glaucoma valve |
| ANOVA | analysis of variance |
| BCVA | best-corrected visual acuity |
| BGI | Baerveldt glaucoma implant |
| CI | confidence interval |
| CPC | cyclophotocoagulation |
| dB | decibels |
| DUES | deepening of upper-eyelid sulcus |
| GEE | Generalized estimating equation |
| IOL | intraocular lens |
| IOP | intraocular pressure |
| MAR | minimum angle of resolution |
| MD | mean deviation |
| MP-CPC | micropulse laser cyclophotocoagulation |
| NTG | normal tension glaucoma |
| NVG | neovascular glaucoma |
| PAP | prostaglandin-associated periorbitopathy |
| PXG | pseudoexfoliation glaucoma |
| POAG | primary open-angle glaucoma |
| PTVT | Primary Tube Versus Trabeculectomy |
| SLT | selective laser trabeculoplasty |
| SU | Shimane University |
| TLE | trabeculectomy |
| TLO | trabeculotomy |
| TVT | Tube Versus Trabeculectomy |
References
- Alm, A.; Grierson, I.; Shields, M.B. Side effects associated with prostaglandin analog therapy. Surv. Ophthalmol. 2008, 53 (Suppl. 1), S93–S105. [Google Scholar] [CrossRef]
- Taketani, Y.; Yamagishi, R.; Fujishiro, T.; Igarashi, M.; Sakata, R.; Aihara, M. Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1269–1276. [Google Scholar] [CrossRef]
- Itoh, K.; Ida, Y.; Ohguro, H.; Hikage, F. Prostaglandin F2α agonists induced enhancement in collagen1 expression is involved in the pathogenesis of the deepening of upper eyelid sulcus. Sci. Rep. 2021, 11, 9002. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Shirato, S.; Sakata, R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn. J. Ophthalmol. 2011, 55, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Shiokawa, M.; Wakakura, M.; Tomita, G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J. Glaucoma 2013, 22, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Kucukevcilioglu, M.; Bayer, A.; Uysal, Y.; Altinsoy, H.I. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin. Exp. Ophthalmol. 2014, 42, 126–131. [Google Scholar] [CrossRef]
- Maruyama, K.; Shirato, S.; Tsuchisaka, A. Incidence of deepening of the upper eyelid sulcus after topical use of travoprost ophthalmic solution in Japanese. J. Glaucoma 2014, 23, 160–163. [Google Scholar] [CrossRef]
- Sakata, R.; Shirato, S.; Miyata, K.; Aihara, M. Incidence of deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy with a latanoprost ophthalmic solution. Eye 2014, 28, 1446–1451. [Google Scholar] [CrossRef]
- Nakakura, S.; Yamamoto, M.; Terao, E.; Nagatomi, N.; Matsuo, N.; Fujisawa, Y.; Fujio, Y.; Tabuchi, H.; Kiuchi, Y. Prostaglandin-associated periorbitopathy in latanoprost users. Clin. Ophthalmol. 2014, 30, 51–56. [Google Scholar] [CrossRef]
- Alm, A. Latanoprost in the treatment of glaucoma. Clin. Ophthalmol. 2014, 26, 1967–1985. [Google Scholar] [CrossRef]
- Maruyama, K.; Tsuchisaka, A.; Sakamoto, J.; Shirato, S.; Goto, H. Incidence of deepening of upper eyelid sulcus after topical use of tafluprost ophthalmic solution in Japanese patients. Clin. Ophthalmol. 2013, 7, 1441–1446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakata, R.; Shirato, S.; Miyata, K.; Aihara, M. Incidence of deepening of the upper eyelid sulcus on treatment with a tafluprost ophthalmic solution. Jpn. J. Ophthalmol. 2014, 58, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Naito, T.; Fujiwara, M.; Araki, R.; Kiyoi, R.; Shiode, Y.; Fujiwara, A.; Morizane, Y.; Shiraga, F. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS ONE 2017, 12, e0181550. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Miki, T.; Naito, T.; Ichioka, S.; Takayanagi, Y.; Tanito, M. Surgical Results of Trabeculectomy among Groups Stratified by Prostaglandin-Associated Periorbitopathy Severity. Ophthalmology 2023, 130, 297–303. [Google Scholar] [CrossRef]
- Kiuchi, Y.; Inoue, T.; Shoji, N.; Nakamura, M.; Tanito, M.; Glaucoma Guideline Preparation Committee, Japan Glaucoma Society. The Japan Glaucoma Society guidelines for glaucoma, 5th edition. Jpn. J. Ophthalmol. 2023, 67, 189–254. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef]
- Gedde, S.J.; Feuer, W.J.; Lim, K.S.; Barton, K.; Goyal, S.; Ahmed, I.I.; Brandt, J.D.; Primary Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 5 Years of Follow-up. Ophthalmology 2022, 129, 1344–1356. [Google Scholar] [CrossRef]
- Harano, A.; Ichioka, S.; Murakami, K.; Iida, M.; Tanito, M. The Severity of Prostaglandin-Associated Periorbitopathy Did Not Affect the Surgical Effectiveness of the Ahmed Glaucoma Valve. J. Clin. Med. 2024, 14, 42. [Google Scholar] [CrossRef]
- Shen, J.; Moats, R.A.; Pollack, H.A.; Robinson, M.R.; Attar, M. Distribution of 14C-Latanoprost Following a Single Intracameral Administration Versus Repeated Topical Administration. Ophthalmol. Ther. 2020, 9, 929–940. [Google Scholar] [CrossRef]
- Pérez-Roca, F.; Rodrigo-Morales, E.; Garzón, I.; Oliveira, A.C.; Martín-Piedra, M.; Carriel, V.; Ortiz-Pérez, A.I.; Sánchez-Montesinos, I.; Campos, A.; Alaminos, M. Effects of Four Formulations of Prostaglandin Analogs on Eye Surface Cells. A Comparative Study. PLoS ONE 2015, 10, e0129419. [Google Scholar] [CrossRef]
- Esaki, Y.; Shimazaki, A.; Pellinen, P. Ocular Tolerability of Preservative-Free Tafluprost and Latanoprost: In vitro and in vivo Comparative Study. Open Ophthalmol. J. 2016, 10, 146–153. [Google Scholar] [CrossRef][Green Version]
- Ayyala, R.S.; Zurakowski, D.; Smith, J.A.; Monshizadeh, R.; Netland, P.A.; Richards, D.W.; Layden, W.E. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology 1998, 105, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Mahdavi, K.; Caprioli, J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am. J. Ophthalmol. 2003, 136, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Park, H.Y.L.; Park, C.K. Effects of Early Postoperative Intraocular Pressure after Ahmed Glaucoma Valve Implantation on Long-term Surgical Outcomes. Korean J. Ophthalmol. 2018, 32, 391–399. [Google Scholar] [CrossRef] [PubMed]

| TLE n = 162 Eyes | AGV n = 136 Eyes | p | ||
|---|---|---|---|---|
| Gender (n) | Male | 88 | 88 | 0.069 a |
| Female | 74 | 48 | ||
| Age (years) | 65.0 ± 12 (41~83) | 67.8 ± 12 (31~90) | 0.002 b | |
| Preoperative IOP (mmHg) | 21.0 ± 9.9 (9.0~58) | 27.1 ± 11 (10~70) | <0.001 b | |
| Preoperative anti-glaucomatous medication score | 4.70 ± 1.2 | 4.68 ± 1.3 | 0.823 b | |
| Preoperative prostanoid FP receptor agonist (eyes) | 0.214 a | |||
| bimatoprost | 62 | 56 | ||
| latanoprost | 42 | 29 | ||
| tafluprost | 39 | 23 | ||
| travoprost | 13 | 18 | ||
| FP (-) | 6 | 10 | ||
| Preoperative BCVA (logMAR) | 0.174 ± 0.34 (−0.177~1.70) | 0.446 ± 0.52 (−0.176~2.00) | 0.001 b | |
| Preoperative MD (dB) | −15.5 ± 7.4 (−0.290~−32.7) (n = 129) | −13.7 ± 7.2 (−0.790~−32.9) (n = 77) | 0.074 b | |
| Lens status (eyes) | Phakic | 98 | 69 | <0.001 a |
| IOL | 64 | 67 | ||
| Glaucoma type (eyes) | POAG/NTG | 97 | 61 | <0.001 a |
| PXG | 21 | 16 | ||
| Uveitis | 29 | 15 | ||
| NVG | 3 | 14 | ||
| others | 12 | 30 | ||
| Prior glaucoma surgery (eyes) | 65 | 85 | <0.001 a | |
| TLE | AGV | |||||
| High-PAP- Potential n = 75 Eyes | Low-PAP- Potential n = 81 Eyes | p | High-PAP- Potential n = 74 Eyes | Low-PAP- Potential n = 52 Eyes | p | |
| Glaucoma type (eyes) | 0.788 a | 0.305 a | ||||
| POAG | 26 | 28 | 36 | 18 | ||
| NTG | 19 | 23 | 2 | 3 | ||
| PXG | 9 | 12 | 9 | 7 | ||
| Uveitis | 9 | 16 | 6 | 8 | ||
| NVG | 3 | 0 | 7 | 5 | ||
| Others | 9 | 2 | 14 | 11 | ||
| Preoperative IOP (mmHg) | 22.0 ± 10 | 19.8 ± 9.9 | 0.102 b | 25.8 ± 11 | 27.7 ± 10 | 0.105 b |
| Preoperative anti-glaucomatous medication score | 5.07 ± 1.1 | 4.49 ± 1.1 | <0.001 b | 4.93 ± 1.1 | 4.62 ± 1.1 | 0.079 b |
| Prior glaucoma surgery (eyes) * | 0.258 a | 0.695 a | ||||
| none | 39 | 55 | 30 | 21 | ||
| SLT | 20 | 15 | 26 | 14 | ||
| MP-CPC, CPC | 1 | 1 | 5 | 4 | ||
| TLO | 11 | 11 | 8 | 10 | ||
| TLE | 12 | 6 | 25 | 19 | ||
| Tube shunt | 2 | 2 | 6 | 2 | ||
| High-PAP-Potential Group | Low-PAP-Potential Group | p | ||
|---|---|---|---|---|
| TLE | Pre-surgery IOP (mmHg) | 22.0 ± 10 | 19.8 ± 9.9 | 0.102 |
| 24 months IOP (mmHg) | 11.1 ± 3.2 | 10.2 ± 2.5 | 0.197 | |
| AGV | Pre-surgery IOP (mmHg) | 25.8 ± 11 | 27.7 ± 10 | 0.105 |
| 24 months IOP (mmHg) | 13.3 ± 3.1 | 13.6 ± 4.4 | 0.985 |
| Definition A | Definition B | Definition C | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Glaucoma type | 1.09 (0.926–1.28) | 0.305 | 1.09 (0.926–1.28) | 0.307 | 1.04 (0.881–1.23) | 0.627 |
| Prior glaucoma surgery (Yes/No) | 0.588 (0.301–1.15) | 0.121 | 0.589 (0.301–1.15) | 0.121 | 0.495 (0.231–1.06) | 0.071 |
| Preoperative IOP (mmHg) | 0.989 (0.953–1.03) | 0.556 | 0.989 (0.953–1.03) | 0.559 | 1.04 (1.01–1.07) | 0.005 |
| Preoperative anti-glaucomatous medication score | 1.07 (0.802–1.41) | 0.664 | 1.06 (0.801–1.41) | 0.666 | 1.08 (0.782–1.50) | 0.632 |
| FP agent (latanoprost or tafluprost/ bimatoprost or travoprost) | 0.473 (0.242–0.923) | 0.028 | 0.473 (0.243–0.923) | 0.028 | 0.271 (0.115–0.636) | 0.003 |
| Definition A | Definition B | Definition C | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Glaucoma type | 1.02 (0.895–1.17) | 0.755 | 0.990 (0.877–1.12) | 0.869 | 1.01 (0.906–1.11) | 0.931 |
| Prior glaucoma surgery (Yes/No) | 0.592 (0.310–1.13) | 0.111 | 0.625 (0.346–1.13) | 0.120 | 0.729 (0.440–1.21) | 0.220 |
| Preoperative IOP (mmHg) | 1.00 (0.967–1.03) | 0.977 | 1.01 (0.983–1.04) | 0.443 | 1.03 (1.00–1.05) | 0.026 |
| Preoperative anti-glaucomatous medication score | 0.898 (0.686–1.18) | 0.433 | 0.834 (0.652–1.07) | 0.148 | 1.02 (0.815–1.27) | 0.887 |
| FP agent (latanoprost or tafluprost/ bimatoprost or travoprost) | 0.402 (0.189–0.856) | 0.018 | 0.597 (0.315–1.13) | 0.120 | 0.834 (0.500–1.392) | 0.487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, I.; Kimura, M.; Sakamoto, R.; Kawai, Y.; Tsukamura, T.; Morita, H.; Kato, A.; Ozeki, H.; Nozaki, M.; Yasukawa, T. The Effect of Preoperative Use of High- vs. Low-PAP-Inducing-Potential FP Agonists on the Surgical Outcomes of Trabeculectomy and AGV Implantation. J. Clin. Med. 2025, 14, 6940. https://doi.org/10.3390/jcm14196940
Yamazaki I, Kimura M, Sakamoto R, Kawai Y, Tsukamura T, Morita H, Kato A, Ozeki H, Nozaki M, Yasukawa T. The Effect of Preoperative Use of High- vs. Low-PAP-Inducing-Potential FP Agonists on the Surgical Outcomes of Trabeculectomy and AGV Implantation. Journal of Clinical Medicine. 2025; 14(19):6940. https://doi.org/10.3390/jcm14196940
Chicago/Turabian StyleYamazaki, Iyo, Masayo Kimura, Risaki Sakamoto, Yukiko Kawai, Tomomi Tsukamura, Hiroshi Morita, Aki Kato, Hironori Ozeki, Miho Nozaki, and Tsutomu Yasukawa. 2025. "The Effect of Preoperative Use of High- vs. Low-PAP-Inducing-Potential FP Agonists on the Surgical Outcomes of Trabeculectomy and AGV Implantation" Journal of Clinical Medicine 14, no. 19: 6940. https://doi.org/10.3390/jcm14196940
APA StyleYamazaki, I., Kimura, M., Sakamoto, R., Kawai, Y., Tsukamura, T., Morita, H., Kato, A., Ozeki, H., Nozaki, M., & Yasukawa, T. (2025). The Effect of Preoperative Use of High- vs. Low-PAP-Inducing-Potential FP Agonists on the Surgical Outcomes of Trabeculectomy and AGV Implantation. Journal of Clinical Medicine, 14(19), 6940. https://doi.org/10.3390/jcm14196940







