Abstract
Background. The advantages of a laparoscopic approach for the treatment of gastric cancer have already been demonstrated in Eastern Countries. This review and meta-analysis aims to merge all the western studies comparing laparoscopic (LG) versus open gastrectomies (OG) to provide pooled results and higher levels of evidence. Methods. A systematic literature search was performed in MEDLINE(PubMed), Embase, WebOfScience and Scopus for studies comparing laparoscopic versus open gastrectomy in western centers from 1980 to 2021. Results. After screening 355 articles, 34 articles with a total of 24,098 patients undergoing LG (5445) or OG (18,653) in western centers were included. Compared to open gastrectomy, laparoscopic gastrectomy has a significantly longer operation time (WMD = 47.46 min; 95% CI = 31.83–63.09; p < 0.001), lower blood loss (WMD = −129.32 mL; 95% CI = −188.11 to −70.53; p < 0.0001), lower analgesic requirement (WMD = −1.824 days; 95% CI = −2.314 to −1.334; p < 0.0001), faster time to first oral intake (WMD = −1.501 days; 95% CI = −2.571 to −0.431; p = 0.0060), shorter hospital stay (WMD = −2.335; 95% CI = −3.061 to −1.609; p < 0.0001), lower mortality (logOR = −0.261; 95% the −0.446 to −0.076; p = 0.0056) and a better 3-year overall survival (logHR 0.245; 95% CI = 0.016–0.474; p = 0.0360). A slight significant difference in favor of laparoscopic gastrectomy was noted for the incidence of postoperative complications (logOR = −0.202; 95% CI = −0.403 to −0.000 the = 0.0499). No statistical difference was noted based on the number of harvested lymph nodes, the rate of major postoperative complication and 5-year overall survival. Conclusions. In Western centers, laparoscopic gastrectomy has better short-term and equivalent long-term outcomes compared with the open approach, but more high-quality studies on long-term outcomes are required.
1. Introduction
Gastric cancer is the fifth most common cancer in the world and the third leading cause of cancer-related death. Differently to the eastern countries, in Europe no screening programs are carried out (except for a limited amount of patients affected by atrophic gastritis [,]), and the diagnosis often occurs in an advanced stage with a 5-year survival of around 25% [,]. In patients with a resectable tumor (stage IB-III) the gold standard is radical gastrectomy with D2 lymphadenectomy [,,]. In Europe, for these patients, since the publication of the results of the “AIO-FLOT-4” trial, the gold standard of treatment is gastrectomy with D2 lymphadenectomy and perioperative chemotherapy [,].
D2 lymphadenectomy is mandatory and should be conducted by highly-experienced surgeons in high-volume centers, especially when a minimally invasive procedure is performed [,,]. To date, the laparotomic approach is still the most frequently performed kind of surgery.
The first laparoscopic distal gastrectomy was described by Kitano in 1994 [], and after that the technique gained popularity all over the world, especially in Eastern countries, where several randomized controlled trials (RCTs) on early gastric cancer (EGC) demonstrated better short-term results than open surgery, with comparable overall and disease specific survival rates [,,]. Laparoscopic subtotal gastrectomy (LSG) for stage I gastric cancer (T1N0M0, T1N1M0 or T2aN0M0) was first described in 2014 by the Japanese gastric cancer treatment guidelines as one treatment option in high-volume centers []. Nowadays, the indications for LSG are constantly increasing, including locally advanced gastric cancer, as demonstrated by the short-term results of the Eastern countries’ multicenter RCTs [,,].
Recently a non-inferiority, multicenter, international, randomized trial, performed in 13 hospitals in six European countries, showed that minimally invasive total gastrectomy after neoadjuvant therapy is not inferior regarding oncological quality of resection in comparison to open total gastrectomy in Western patients with resectable gastric cancer []. On the other hand. the Dutch LOGICA trial failed to demonstrate that laparoscopic gastrectomy leads to shorter hospital stay, but the oncological efficacy did not differ from that of the open gastrectomy group [].
The differences between East and West in the overall treatment of gastric cancer have been extensively documented over the last decade; different screening protocols and endoscopic, surgical and oncological approaches are currently used in the two situations, changing the final outcomes for this pathology [,,].
This systematic review and meta-analysis aims to merge all western studies comparing LG and OG available in the literature in an attempt to increase the statistical power and level of evidence supporting the use of laparoscopic gastrectomy for the treatment of gastric cancer.
2. Materials and Methods
2.1. Literature Search Strategy
A systematic review of the literature was accomplished according to the PRISMA statement [] in order to select articles comparing laparoscopic and open surgery in the treatment of gastric cancer. In this manuscript an electronic literature search was carried out through MEDLINE (PubMed), Embase, WebOfScience and Scopus from January 1980 to 31 December 2021. The search strategy is summarized in Supplemental File S1. A manual search using other search engine, such as Google Scholar, and reference to relevant articles was also conducted. English language terms were used to perform the search, but no restrictions were adopted to exclude any paper either by language or by study type. Records retrieved were managed by Mendeley Desktop version 1.19.4. (Elselvier, Amsterdam, The Netherlands) and Covidence (Veritas Health Innovation, Melbourne, Australia).
2.2. Inclusion and Exclusion Criteria
PICOS criteria (population, intervention, comparison, outcomes, and study design) were used to select studies []. In particular, only studies reporting a comparison between laparoscopic and open approach on adult patients undergoing gastrectomy for cancer were considered. At least one peri-operative outcome of interest should be reported including overall survival (OS) and/or disease-free survival (DFS). Studies including hybrid laparoscopic-robotic procedure or comparing robotic to laparoscopic gastrectomy were excluded. Other exclusion criteria were: (1) mixed cohort of patients from Western and Eastern countries, (2) limited D1 lymphadenectomy, and (3) merged benign and malignant diseases. Papers were also excluded from the quantitative analysis if it was not possible to quantify the number of patients or the outcomes of interest, as well as case series without control group, case reports, technical notes, papers related to video, or articles with a study period of more than fifteen years. Whenever the same group of authors had presented multiple papers through the years, all the papers were considered, but only the most informative or highest quality study was included.
The work has been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the Methodological quality of Systematic Reviews) Guidelines.
2.3. Data Extraction and Quality Assessment
According to the eligibility criteria and in order to minimize selection bias, two pairs of reviewers (GMG/GP and GGL/AL) independently reviewed each paper, assessed the quality of the studies by using the Newcastle-Ottawa Scale [] or Jadad’s scale for RCTs [], and even performed the data extraction. Any disagreements were discussed and resolved through a consensus meeting with a third pair of reviewers (GC/PM). The following demographic information were selected and collected if available: age, gender distribution, body mass index (BMI), ASA classification, and tumor size and/or staging. The following surgical outcomes were considered: operating time, blood loss, lymph node yield, intraoperative complications, conversion to open approach, length of hospital stay (LOS), time to first flatus, time to oral intake, duration of analgesic requirement, 30-days postoperative morbidity, and mortality, and long-term oncological outcomes (3 and 5-year OS). Whenever possible, we reported intraoperative and/or postoperative complications both as quantitative and qualitative.
2.4. Statistical Analysis
We analyzed continuous variables through the weighted mean difference (WMD) and 95% confidence interval (CI). For categorical variables, analysis was performed by using the odds ratio (OR) and 95% CI. Variables were converted to mean and standard deviation (SD) if reported otherwise, according to Hozo []. Hazard ratios (HRs) were used to analyze time to event outcomes (OS and DFS). When the HRs and 95% CI were not provided in the studies, two authors (AC and EMM), following well-established methodologies, extracted data from Kaplan-Meier (KM) curves with GraphClick software 3.0 for Mac (Arizona-Software, Phoenix, AZ, USA) and estimated the HRs using an on-line calculator (https://www.gigacalculator.com/calculators/hazard-ratio-calculator.php, accessed on 15 April 2022). The method was validated with a blind approach by correlating the data extracted from our previously published KM curves [,,] with the original data or by comparing the HR of the same study reported in other meta-analyses []. The HR was converted to logHR and SE with variance. A positive logHR value (reference laparoscopic approach) indicated a survival benefit favoring laparoscopy over open surgery. Subgroup analyses were performed considering either the type of resection or 5-year periods. The degrees of heterogeneity between the studies were assessed by the I2 value. We considered an I2 value of 40% or lower as trivial or not important heterogeneity, and an I2 value of 75% or higher as considerable heterogeneity. When I2 value was higher than 50%, pooled estimates were obtained using a random effects model. As regards p value of Q index (chi-square test of heterogeneity), a p < 0.10 was considered significant, otherwise a conventional level of p < 0.05 was accepted as statistically significant. Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s test for continuous outcomes and with Harbord’s and Peters’ test for binary outcome [,,]. Statistical analysis was carried out using StataCorp2019 STATA Statistical Software: release 16 (College Station, TX, USA: StataCorp LLC).
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, and the experimental conclusions that can be drawn.
Using the described search strategy, 355 items were identified. After removing duplicates and screening titles and abstract, 127 full text papers were evaluated. Ninety-one papers were further eliminated with reasons; thus 36 studies were considered eligible (Figure 1). Two studies were included only in the qualitative analysis. One retrospective case-matched study conducted in Slovenia between 1992 and 2019 has been excluded because the time study period of 27 years was considered too long to compare a technically evolving surgical approach such as laparoscopy []. The second, from the group of Norero et al., has been excluded because a previous case-matched study from the same authors, with a higher quality assessment score, was included []. Finally, 34 relevant studies were selected for the meta-analysis [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
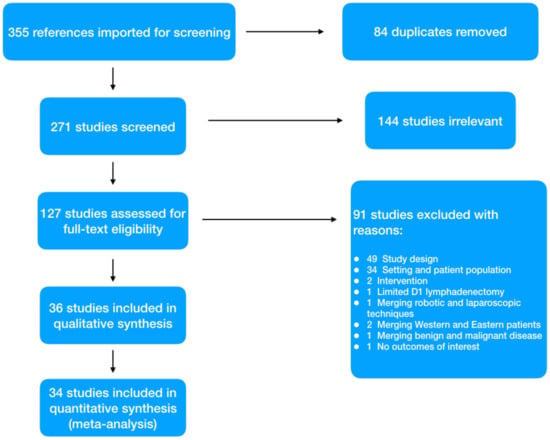
Figure 1.
PRISMA flowchart.
With regard to the retrieved studies, eight of these were conducted in Italy, five in the United Kingdom, four in the USA and in the Netherlands, two in France, in Germany and in Brazil, and one in Belgium, Portugal, Canada, Sweden, Turkey, Jordan, and Chile. The vast majority (17) were retrospective comparative analyses, 14 matched (eight retrospective and six prospective) and three randomized trials. All studies recruited patients between 1997 and 2019, and papers were published between 2003 and 2021. The overall quality of studies was deemed as acceptable (Newcastle-Ottawa Scale for cohort studies mean 7.7 (range 6–9) and Jadad scale for RCT mean score 3.3 (range 2–4)).
The total number of patients included in our meta-analysis was 24,098 (Open Group = 18,653; Laparoscopic Group = 5445). Baseline characteristics of the included studies are reported in Table 1.

Table 1.
Baseline characteristics of studies included in the meta-analysis.
The age was reported in all the studies except six [,,,,,] and the mean age in LG and OG group was 69.03 ± 4.38 years and 67.96 ± 4.09 years, respectively.
3.1. Comparison of Operative and Pathological Outcomes
In the laparoscopic groups conversion was reported in 20 studies [,,,,,,,,,,,,,,,,,,,] with a total of 79 conversions from laparoscopy to open surgery.
Operative time: Twenty-four studies with 2730 patients reported the operative time. The pooled analysis demonstrated a difference in favor of the open surgery group (WMD = 47.46 min; 95% CI = 31.83–63.09; p < 0.001). Heterogeneity among the studies was very considerable (I2 = 96.10%; p < 0.0001), thus a random-effect model was used. No difference was noted in the subgroup analysis (Figure 2a). Egger’s test for funnel plot asymmetry showed Y Intercept at 1.46 (p = 0.1441) (Figure 3a).
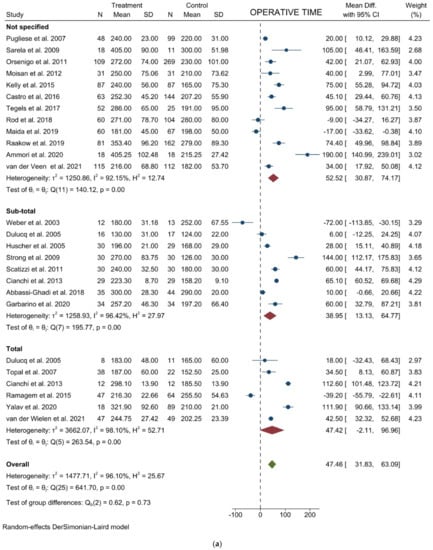
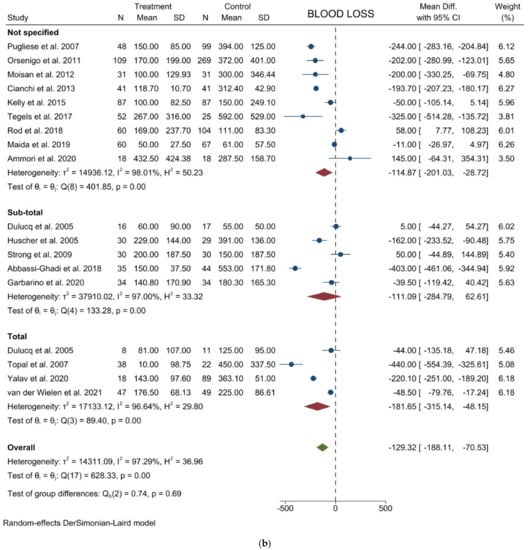
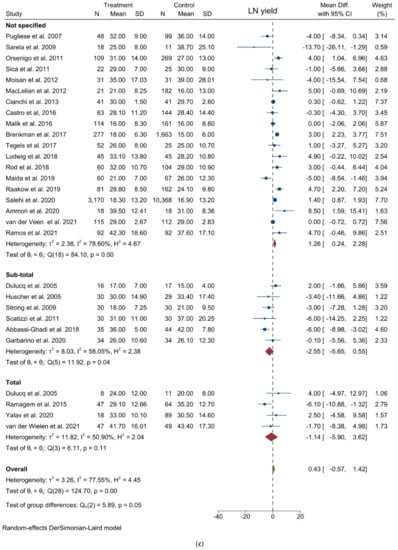
Figure 2.
Forest plots of (a) Operative Time; (b) Blood Loss; (c) Lymph Node Yield.
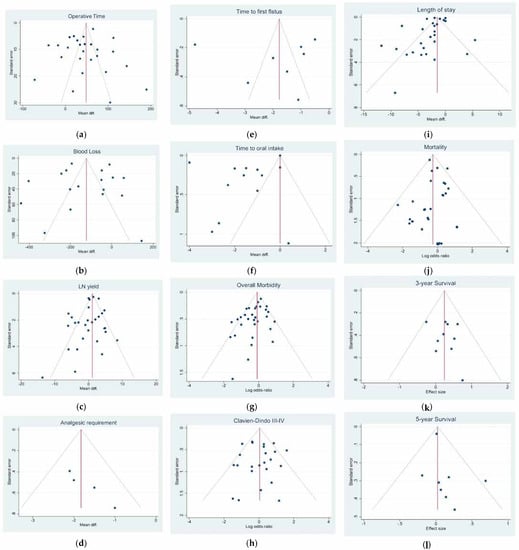
Figure 3.
Funnel plots of (a) Operative time; (b) Blood Loss; (c) Lymph Node Yield; (d) Analgesic Requirement; (e) Time to First Flatus; (f) Time to First Oral Intake; (g) Overall Morbidity; (h) Major Complications; (i) Length of Stay; (j) Mortality; (k) 3-year Overall Survival; (l) 5-year Overall Survival.
Blood loss: Seventeen studies with 1828 patients compared the blood loss. The results showed that the blood loss amount was lower in the laparoscopic approach (WMD = −129.32 mL; 95% CI = −188.11 to −70.53; p < 0.0001). Heterogeneity among the studies was very considerable (I2 = 97.29%; p < 0.0001); a random-effect model was used. No difference was noted in the subgroup analysis (Figure 2b). Egger’s test for funnel plot asymmetry showed Y Intercept at −0.19 (p = 0.8478) (Figure 3b).
LN yield: Twenty-eight studies reported the number of harvested nodes allowing a pooled analysis of 18748 patients. The results showed that the total LNH between the two groups was similar (WMD = 0.426; 95% CI = −0.566 to 1.419; p = 0.3998). Heterogeneity among the studies was substantial (I2 = 77.55%; p < 0.0001), thus a random-effect model was used. A slight difference was noted in the subgroup analysis (p = 0.053) (Figure 2c). Egger’s test for funnel plot asymmetry showed Y Intercept at −1.37 (p = 0.1701) (Figure 3c).
3.2. Comparison of Postoperative Outcomes
Quantitative description of postoperative complications is reported in Table 2. The 30-days mortality was reported in all the studies except four [,,,] with a total mortality of 1233, 140 in the LG group and 1093 in the OG group respectively.

Table 2.
Table of complications of studies included in the meta-analysis.
Analgesic requirement: Four studies with 441 patients reported this item. The results showed a significant lower mean time of analgesic administration in laparoscopic group (WMD = −1.824 days; 95% CI = −2.314 to −1.334; p < 0.0001). No Heterogeneity among the studies was detected (I2 = 0.00; p = 0.5301). No difference was noted in the subgroup analysis. (Figure 4a). Egger’s test for funnel plot asymmetry showed Y Intercept at 1.43 (p = 0.1518) (Figure 3d).
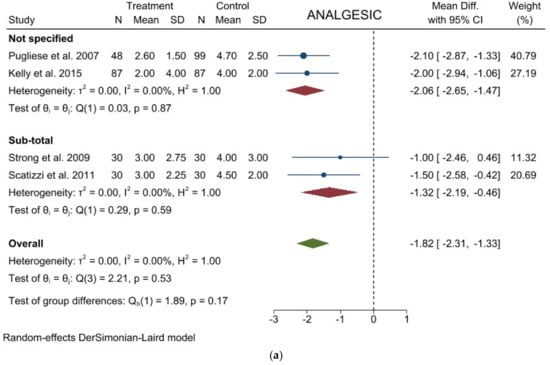
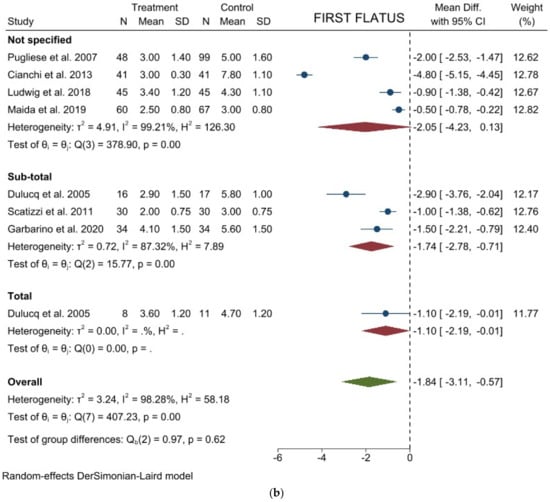
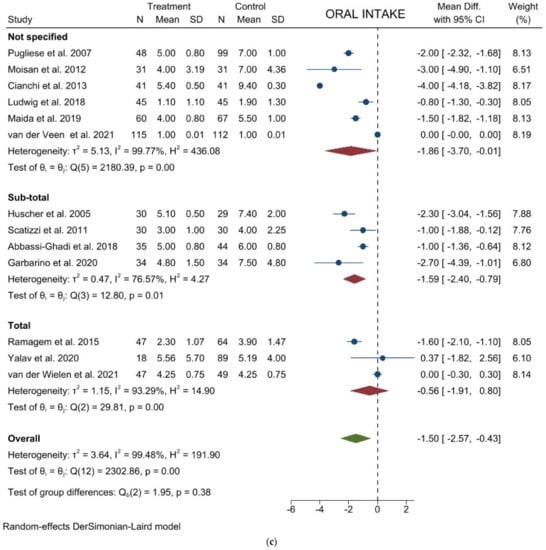
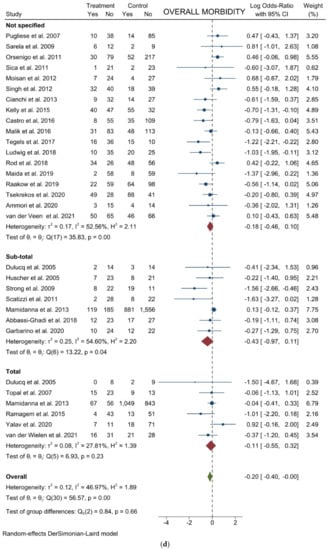
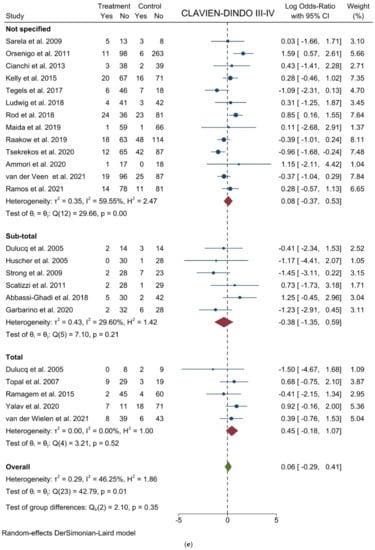
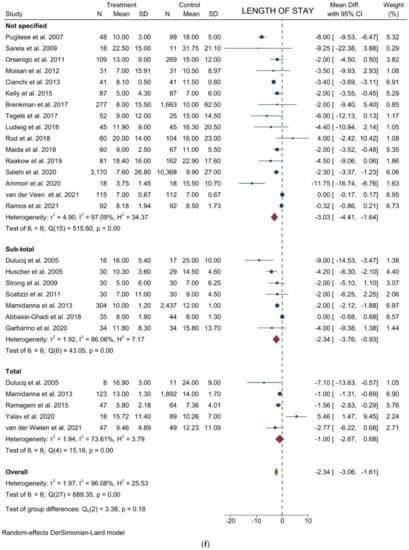
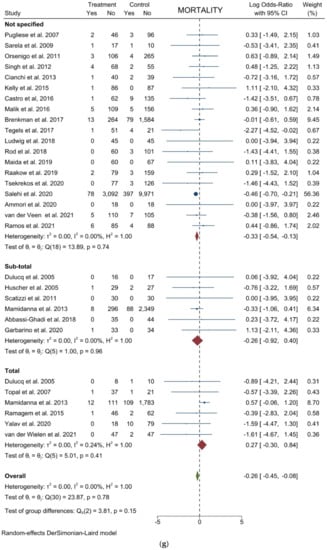
Figure 4.
Forest plots of (a) Analgesic Requirement; (b) Time to First Flatus; (c) Time to First Oral Intake; (d) Overall Morbidity; (e) Major Complications; (f) Length of Stay; (g) Mortality.
Time to first flatus: Seven studies with 626 patients focused on this item. The results showed a significant lower mean time to first flatus in laparoscopic group (WMD = −1.840 days; 95% CI = −3.107 to −0.573; p = 0.0044). Heterogeneity among the studies was very considerable (I2 = 98.28%; p < 0.001). No difference was noted in the subgroup analysis (Figure 4b). Egger’s test for funnel plot asymmetry showed Y Intercept at 0.09 (p = 0.9272) (Figure 3e).
Time to oral intake: Thirteen studies with 1315 patients focused on this item. The results showed a significant lower mean time to first flatus in laparoscopic group (WMD = −1.501 days; 95% CI = −2.571 to −0.431; p = 0.0060). Heterogeneity among the studies was very considerable (I2 = 99.48%; p < 0.0001). No difference was noted in the subgroup analysis (Figure 4c). Egger’s test for funnel plot asymmetry showed Y Intercept at −0.16 (p = 0.8727) (Figure 3f).
Overall morbidity: From 29 studies, 8208 participants were enrolled to assess postoperative complications between the two groups. The results showed a slight statistically significant difference in postoperative complications favoring laparoscopy (logOR = −0.202; 95% CI = −0.403 to −0.000 the = 0.0499). Heterogeneity existed among the studies (I2 =46.97%; p = 0.0023). No difference was noted in the subgroup analysis (Figure 4d). Harbord’s test for funnel plot asymmetry showed Y Intercept at −1.66 (p = 0.0964), while Peters’ z was −1.52 (p = 0.1274) (Figure 3g).
Major complications (Clavien-Dindo III-IV): From 23 studies, 2769 participants were enrolled to assess major postoperative complications. The results showed no difference between the two groups (logOR = 0.058; 95% CI = −0.292 to 0.408; p = 0.7451). Heterogeneity existed among the studies (I2 = 46.25%; p = 0.0073). No difference was noted in the subgroup analysis (Figure 4e). Harbord’s test for funnel plot asymmetry showed Y Intercept at −0.23 (p = 0.8155), while Peters’ z was −0.92 (p = 0.3553) (Figure 3h).
Length of stay: Twenty-six studies with 22946 patients were analyzed to compare postoperative hospital stay between laparoscopic and open groups. The results showed a statistically significant difference in LOS favoring laparoscopy (WMD = −2.335; 95% CI = −3.061 to −1.609; p < 0.0001). Heterogeneity among the studies was very considerable (I2 = 96.08%; p < 0.0001). No difference was noted in the subgroup analysis (Figure 4f). Egger’s test for funnel plot asymmetry showed Y Intercept at −2.38 (p = 0.0173) (Figure 3i).
Mortality: From 29 studies, 23,701 participants were enrolled to assess postoperative mortality. The results showed a statistically significant lower risk of death in the laparoscopic cohort (logOR = −0.261; 95% the −0.446 to −0.076; p = 0.0056). No heterogeneity existed among the studies (I2 = 0.00%; p = 0.7778). No difference was noted in the subgroup analysis (Figure 4g). Harbord’s test for funnel plot asymmetry showed Y Intercept at −0.62 (p = 0.5320), while Peters’ z was −0.30 (p = 0.7677) (Figure 3l).
3.3. Comparison of Long-Term Outcomes
Three-year overall survival: Ten studies involving 950 patients were identified to investigate the 3-year OS comparing laparoscopic versus open surgery. The pooled analysis of these studies showed that patients undergoing laparoscopic surgery had a slightly lower risk of death (logHR 0.245; 95% CI = 0.016–0.474; p = 0.0360) than patients in the open group which showed a cumulative mean HR of 1.106. No heterogeneity existed among the studies (I2 = 0.00%; p = 0.7266). No difference was noted in the subgroup analysis (Figure 5a). Egger’s test for funnel plot asymmetry showed Y Intercept at 0.58 (p = 0.5629) (Figure 3m).
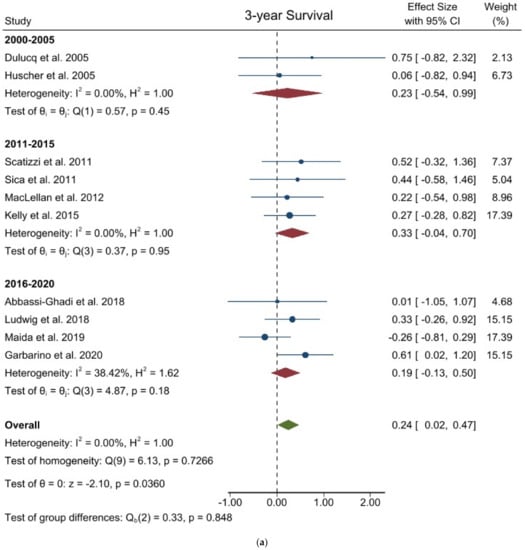
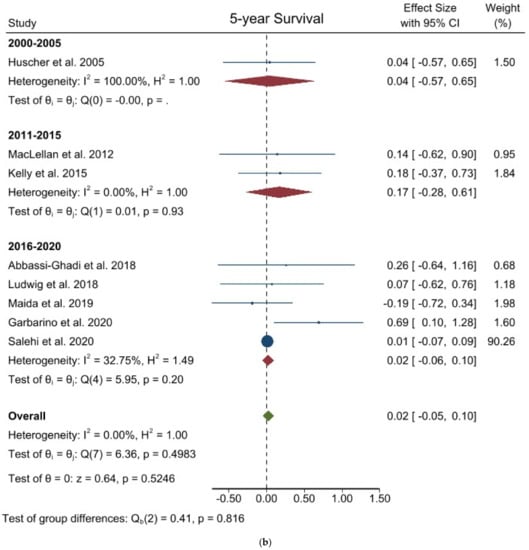
Figure 5.
Forest plots of (a) 3-year Overall Survival; (b) 5-year Overall Survival.
Five-year overall survival: Kaplan-Meier curves from eight studies involving 14,338 patients were identified to extract data for the 5-year OS. The pooled analysis of these studies showed there was no difference between the two groups (logHR 0.024; 95% CI = −0.050 to 0.099; p = 0.5246). Mean HR for open surgery was 1.012. No heterogeneity existed among the studies (I2 = 0.00%; p = 0.4983). No difference was noted in the subgroup analysis. (Figure 5b) Egger’s test for funnel plot asymmetry showed Y Intercept at 1.19 (p = 0.2360) (Figure 3n).
4. Discussion
Laparoscopic surgery for gastric cancer has gained tremendous popularity over open gastrectomy because of better short-term outcomes. Several meta-analyses, mainly focusing on early gastric cancer, have demonstrated that patients undergoing LG had better early postoperative and comparable long-term outcomes when compared with those undergoing OG [,,].
Moreover, results of eastern countries RCTs recently provided strong evidence in favor of laparoscopic gastrectomy concerning short-term outcomes even in the locally advanced setting [,,].
Due to the differences in the epidemiology, with lower incidence but more advanced tumors at the clinical presentation in western countries, few reports in a non-Asian population have been published. The present study aimed to merge all western studies comparing LG and OG available in the literature in the attempt to increase the statistical power and level of evidence supporting the use of laparoscopic gastrectomy for the treatment of gastric cancer even in western settings.
The main concerns regarding the laparoscopic approach for gastric cancer have always been the number of lymph nodes harvested during the surgery, and the long-term outcomes [,,,].
Concerning the lymph-node yield, the results of the present meta-analysis reflect those published by Beyer et al. in a meta-analysis of RCTs regarding open versus laparoscopic gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer []. This high-evidence study demonstrated the oncological equivalence of the laparoscopic approach for D2 lymphadenectomy compared to the open approach. Unfortunately, Beyer et al. in their meta-analysis of RCTs, concluded that the long-term oncological results could not be evaluated due to a lack of relevant data in four of the five included trials [].
However, in this regard, a recent meta-analysis of high-quality nonrandomized studies mainly performed in Eastern Asia, showed that 5-year overall survival rate (HR 0.95, 95% CI 0.86 to 1.05, p = 0.28), disease-free survival (DFS) rate (HR 0.93, 95% CI 0.81 to 1.06, p = 0.27) and recurrence rate (OR 0.87, 95% CI 0.72 to 1.04, p = 0.13) were comparable between LG and OG [].
Moreover, a recent retrospective multicenter analysis of Western centers focusing on the long-term outcomes following LG for advanced gastric cancer (stage II and III) showed the safety and feasibility of such a surgical approach [].
Interestingly, our present study revealed a 3-year slightly lower risk of death for patients undergoing laparoscopic surgery, though such data was not confirmed by the 5-year overall survival analysis.
This result could be explained by the better short-term outcomes of laparoscopic gastrectomy: the lower inflammatory response to surgery together with a faster return to routine activities could reduce the time to the beginning of postoperative chemotherapy. Nonetheless, because this difference was not relevant at the 5-year analysis, any other possible issue should be investigated.
Despite the higher operative time, as already widely demonstrated, even this meta-analysis of western series confirmed the better short-term outcomes of laparoscopic gastrectomy: lower blood loss, lower time to first oral intake, lower time to first flatus, lower analgesic requirement, and lower hospital stay. This result suggests that the laparoscopic approach for gastrectomy should also be encouraged in western countries.
Postoperative morbidity and mortality are the main indicators for assessing the safety and feasibility of a surgical procedure. It is widely accepted that laparoscopic surgery for gastric cancer is safe and could have fewer complications than open surgery [].
Our meta-analysis demonstrated an almost significant lower overall complication rate in LG versus OG group, whereas in the major complication (C.-D. III-IV) analysis, no differences emerged between groups.
Surprisingly, the mortality results showed a statistically significant lower risk of death in the laparoscopic cohort, without heterogeneity among the studies.
Whether for laparoscopy or open surgery, every patient diagnosed with gastric cancer needs to be discussed in a multi-disciplinary team meeting, which has been demonstrated to improve the outcomes for oncologic patients [,].
Non-oncological long-term outcomes, such as incisional hernia or adhesive bowel obstruction, were not reported by the majority of studies and therefore not included in our meta-analysis. These outcomes may be considered in favor of the laparoscopic approach when planning a gastrectomy.
Concerning the cost analysis, it is widely known that the laparoscopic technique itself implies higher costs, depending on the hospital policies, suppliers’ contracts and laparoscopic volume, but this is balanced by the shorter hospital length of stay. Adachi et al. demonstrated in a small series of patients undergoing a Billroth I gastrectomy that the reduction of hospital stay justifies the higher costs of laparoscopy []. In a Western scenario Tegels et al. demonstrated how the laparoscopic approach brings the burden of higher operative costs, but total costs were not significantly different due to shorter length of stay and less Intensive Care admission and length of stay in the laparoscopic group [].
There are evident limitations in this meta-analysis. First, the majority of the included studies were retrospective, enrolling a small sample size of patients. It is well known that such papers may limit the conclusions on the efficacy of one technique over another. Consequently, the meta-analyses carried biases resulting from the nature of those studies. Second, publication bias is present, and a considerable degree of heterogeneity was observed in most of the outcomes. Although a random effect model was used, the results must be considered prudently. Third, the study period of the included articles was quite long for comparison of a technically evolving surgery such as laparoscopic gastrectomy. Finally, the survival analyses were carried out on a minority of papers because no sufficient western studies included data on those variables.
Despite those limitations, this study could offer a comprehensive view on outcomes of laparoscopic surgery in western gastric cancer patients.
In conclusion, laparoscopic gastrectomy is associated with longer operative time, but better short-term outcomes compared to the open approach.
Survival data of LG seemed comparable with those of open gastrectomies, but further prospective studies on long-term outcomes should be performed to confirm these results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11133590/s1, File S1: Search strategy.
Author Contributions
Study conception and design: G.C. (Gianluca Costa), G.M.G. and P.M. Literature search and data extraction: G.P., G.G.L., G.M.G., A.L., A.C. and G.C. (Giulia Canali). Management, analysis and interpretation of data: G.C. (Gianluca Costa), A.C. and G.M.G. Drafting of manuscript: G.M.G., E.M.M. and G.C. (Giulia Canali). Critical revision: G.C. (Gianluca Costa), P.M., G.T. and G.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The institutional review board approval was not required due to the retrospective design of the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest. Giovanni Maria Garbarino, Giovanni Guglielmo Laracca, Alessio Lucarini, Gianmarco Piccolino, Paolo Mercantini, Alessandro Costa, Giuseppe Tonini, Giulia Canali, Edoardo Maria Muttillo and Gianluca Costa, have no conflicts of interest or financial ties to disclose.
References
- Esposito, G.; Dilaghi, E.; Cazzato, M.; Pilozzi, E.; Conti, L.; Carabotti, M.; di Giulio, E.; Annibale, B.; Lahner, E. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig. Liver Dis. 2020, 53, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Vannella, L.; Lahner, E.; Annibale, B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: A critical reappraisal. World J. Gastroenterol. 2012, 18, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer. 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 38–49. [Google Scholar] [CrossRef]
- McMillian, N.; Pluchino, M.A.; Ajani, J.A.; Chair, V.; Bentrem, D.J.; Chao, J.; Enzler, T.; Fanta, P.; Gerdes, H.; Gibson, M.; et al. NCCN Guidelines for Gastric Cancer, Version 4; National Comprehensive Cancer Network: Plymouth, PA, USA, 2020. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4). Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.K.; Sasako, M.; van de Velde, C.J.H. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Wu, C.W.; Hsiung, C.A.; Lo, S.S.; Hsieh, M.C.; Chen, J.H.; Li, A.F.Y.; Lui, W.Y.; Whang-Peng, J. Nodal dissection for patients with gastric cancer: A randomised controlled trial. Lancet Oncol. 2006, 7, 309–315. [Google Scholar] [CrossRef]
- Mocellin, S.; Mcculloch, P.; Kazi, H.; Gama-Rodrigues, J.J.; Yuan, Y.; Nitti, D. Extent of Lymph Node Dissection for Adenocarcinoma of the Stomach. Cochrane Database Syst. Rev. 2015, 2015, CD001964. [Google Scholar] [CrossRef]
- Uyama, I.; Ogiwara, H.; Takahara, T.; Kato, Y.; Kikuchi, K.; Iida, S. Laparoscopic and Minilaparotomy Billroth I Gastrectomy for Gastric Ulcer Using an Abdominal Wall-Lifting Method. J. Laparoendosc. Surg. 1994, 4, 441–445. [Google Scholar] [CrossRef]
- Nakamura, K.; Katai, H.; Mizusawa, J.; Yoshikawa, T.; Ando, M.; Terashima, M.; Ito, S.; Takagi, M.; Takagane, A.; Ninomiya, M.; et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912). Jpn. J. Clin. Oncol. 2013, 43, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.K.; Chan, Y.; Yang, H.K.; Park, D.J. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival among Patients with Stage i Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J. Korean Surg. Soc. 2013, 84, 123–130. [Google Scholar] [CrossRef]
- Yu, J.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Wang, K.; Suo, J.; Tao, K.; He, X.; et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients with Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 321, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hyung, W.J.; Yang, H.K.; Han, S.U.; Park, Y.K.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann. Surg. 2019, 270, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, N.; Straatman, J.; Daams, F.; Rosati, R.; Parise, P.; Weitz, J.; Reissfelder, C.; Diez del Val, I.; Loureiro, C.; Parada-Gonzalez, P.; et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: Results of a European randomized trial. Gastric Cancer 2021, 24, 258–271. [Google Scholar] [CrossRef]
- Van der Veen, A.; Brenkman, H.J.F.; Seesing, M.F.J.; Haverkamp, L.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; Stoot, J.H.M.B.; Tegels, J.J.W.; Wiknhoven, B.P.L.; Lagarde, S.M.; et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 978–989. [Google Scholar] [CrossRef]
- Russo, A.; Li, P.; Strong, V.E. Differences in the multimodal treatment of gastric cancer: East versus west. J. Surg. Oncol. 2017, 115, 603–614. [Google Scholar] [CrossRef]
- Russo, A.E.; Strong, V.E. Gastric cancer etiology and management in Asia and the west. Annu. Rev. Med. 2019, 70, 353–367. [Google Scholar] [CrossRef]
- Markar, S.R.; Karthikesalingam, A.; Jackson, D.; Hanna, G.B. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: Comparison between west and east. Ann. Surg. Oncol. 2013, 20, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Form for Cohort Studies; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014; pp. 2–4. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Costa, G.; Laracca, G.G.; Castagnola, G.; Mercantini, P.; di Paola, M.; Vita, S.; Masoni, L. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer in middle–low-volume centers in Western countries: A propensity score matching analysis. Langenbeck’s Arch. Surg. 2020, 405, 797–807. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Canali, G.; Tarantino, G.; Costa, G.; Ferri, M.; Balducci, G.; Pilozzi, E.; Berardi, G.; Mercantini, P. Laparoscopic versus open rectal resection: A 1, 2 propensity score–matched analysis of oncological adequateness, short- and long-term outcomes. Int. J. Colorectal. Dis. 2021, 36, 801–810. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Costa, G.; Frezza, B.; Biancafarina, A.; Balducci, G.; Mercantini, P.; de Prizio, M.; Laracca, G.G.; Ceccarelli, G. Robotic versus open oncological gastric surgery in the elderly: A propensity score-matched analysis. J. Robot. Surg. 2020, 15, 741–749. [Google Scholar] [CrossRef]
- Sica, G.S.; Iaculli, E.; Biancone, L.; di Carlo, S.; Scaramuzzo, R.; Fiorani, C.; Gentileschi, P.; Gaspari, A.L. Comparative study of laparoscopic vs. open gastrectomy in gastric cancer management. World J. Gastroenterol. 2011, 17, 4602–4606. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. J. Am. Med. Assoc. 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Jagric, T. East meets West: The initial results of laparoscopic gastric cancer resections with Eastern principles in a single Western centre—A propensity score-matched study. Langenbeck’s Arch. Surg. 2021, 406, 2699–2708. [Google Scholar] [CrossRef]
- Norero, E.; Vargas, C.; Achurra, P.; Ceroni, M.; Mejia, R.; Martinez, C.; Munoz, R.; Gonzalez, P.; Calvo, A.; Diaz, A. Survival and perioperative morbidity of totally laparoscopic versus open gastrectomy for early gastric cancer: Analysis from a single latin american centre. Arq. Bras. Cir. Dig. 2019, 32, e1413. [Google Scholar] [CrossRef]
- Mamidanna, R.; Almoudaris, A.M.; Bottle, A.; Aylin, P.; Faiz, O.; Hanna, G.B. National outcomes and uptake of laparoscopic gastrectomy for cancer in England. Surg. Endosc. 2013, 27, 3348–3358. [Google Scholar] [CrossRef]
- Kelly, K.J.; Selby, L.; Chou, J.F.; Dukleska, K.; Capanu, M.; Coit, D.G.; Strong, V.E. Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann. Surg. Oncol. 2015, 22, 3590–3596. [Google Scholar] [CrossRef]
- Castro, B.; Aral, M.; Fareleira, A.; Costa-Maia, J.; Santos-Sousa, H. Outcomes of laparoscopic and open gastrectomy for gastric cancer: A comparative analysis. Ann. Oncol. 2016, 27 (Suppl. S2), ii75. [Google Scholar] [CrossRef][Green Version]
- Malik, H.T.; Parker, A.; Melhado, R.; Chaparala, R.; Formela, L.; Vickers, J.; Senapati, S.; Akhtar, K. Non randomised long term comparative analysis of totally laparoscopic with open gastrectomy in a western population. Surg. Endosc. Other Interv. Tech. 2016, 30, S24. [Google Scholar]
- Brenkman, J.P.; Ruurda, H.J.F.; Hillegersberg, R.H.A.; van Verhoeven, R. Safety and feasibility of minimally invasive gastrectomy during the early introduction in the Netherlands: Short-term oncological outcomes comparable to open gastrectomy. Gastric Cancer 2017, 20, 853–860. [Google Scholar] [CrossRef][Green Version]
- Tegels, J.J.; Silvius, C.E.; Spauwen, F.E.; Hulsewe, K.W.; Hoofwijk, A.G.; Stoot, J.H. Introduction of laparoscopic gastrectomy for gastric cancer in a Western tertiary referral centre: A prospective cost analysis during the learning curve. World J. Gastrointest. Oncol. 2017, 9, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Schneider-Koriath, S.; Scharlau, U.; Steffen, H.; Möller, D.; Bernhardt, J. Totally laparoscopic versus open gastrectomy for gastric cancer: A matched pair analysis. Zent. Chir. Z. Allg. Visz. Thorax Gefäßchir. 2018, 143, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Rod, X.; Fuks, D.; Macovei, R.; Levard, H.; Ferraz, J.M.; Denet, C.; Tubbax, C.; Gayet, B.; Perniceni, T. Comparison between open and laparoscopic gastrectomy for gastric cancer: A monocentric retrospective study from a western country. J. Visc. Surg. 2018, 155, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Maida, P.; Marte, G.; Spedicato, G.A.; Ferronetti, A.; Mauriello, C.; Canfora, A.; Ciorra, G.; Barra, L.; di Maio, V. Laparoscopic versus open gastrectomy with extended lymph node dissection for gastric carcinoma in a Western series: A Propensity Score Matching analysis. Minerva Chir. 2019, 16, 74. [Google Scholar] [CrossRef]
- Abbassi-Ghadi, N.; Durakovic, S.; Piessen, G.; Gatenby, P.; Sultan, J.; Preston, S.R. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma of the stomach in a Western population: Peri-operative and 5-year oncological outcomes. Surg. Endosc. 2020, 34, 3818–3826. [Google Scholar] [CrossRef]
- Raakow, J.; Denecke, C.; Chopra, S.; Fritz, J.; Hofmann, T.; Andreou, A.; Thuss-Patience, P.; Pratschke, J.; Biebl, M. Laparoscopic versus open gastrectomy for advanced gastric cancer: Operative and postoperative results. Chir. Z. Alle Geb. Oper. Medizen 2020, 91, 252–261. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Klevebro, F.; Hayami, M.; Kamiya, S.; Lindblad, M.; Nilsson, M.; Lundell, L.; Rouvelas, I. Laparoscopic Versus Open Gastrectomy for Cancer: A Western Center Cohort Study. J. Surg. Res. 2020, 247, 372–379. [Google Scholar] [CrossRef]
- Salehi, O.; Vega, E.A.; Kutlu, O.C.; James, D.; Alarcon, S.V.; Herrick, B.; Kozyreva, O.; Conrad, C. Western population-based study of oncologic surgical quality and outcomes of laparoscopic versus open gastrectomy for gastric adenocarcinoma. Surg. Endosc. 2020, 35, 4786–4793. [Google Scholar] [CrossRef]
- Ammori, B.J.; Asmer, H.; Al-Najjar, H.; Al-Bakri, H.; Dabous, A.; Daoud, F.; Almasri, M. Laparoscopic Versus Open D2 Gastrectomy for Gastric Cancer: A Case-Matched Comparative Study. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 777–782. [Google Scholar] [CrossRef]
- Yalav, O.; Topal, U.; Gümüş, S.; Ünal, A.G.; Rencüzoğulları, A. Laparoscopic versus open total radical gastrectomy for advanced gastric cancer: Surgical outcomes. Ann. Ital. Chir. 2020, 9, S2239253X20032673. [Google Scholar]
- Ramos, M.F.K.P.; Pereira, M.A.; Dias, A.R.; Ribeiro, U.; Zilberstein, B.; Nahas, S.C. Laparoscopic gastrectomy for early and advanced gastric cancer in a western center: A propensity score-matched analysis. Updates Surg. 2021, 73, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.J.; Reyes, C.D.; Gagner, M.; Divino, C.M. Comparison of laparoscopic and open gastrectomy for malignant disease. Surg. Endosc. Other Interv. Tech. 2003, 17, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Dulucq, J.L.; Wintringer, P.; Stabilini, C.; Solinas, L.; Perissat, J.; Mahajna, A. Laparoscopic and open gastric resections for malignant lesions: A prospective comparative study. Surg. Endosc. Other Interv. Tech. 2005, 19, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Huscher, C.G.S.S.; Mingoli, A.; Sgarzini, G.; Sansonetti, A.; di Paola, M.; Recher, A.; Ponzano, C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: Five-year results of a randomized prospective trial. Ann. Surg. 2005, 241, 232–237. [Google Scholar] [CrossRef]
- Pugliese, R.; Maggioni, D.; Sansonna, F.; Scandroglio, I.; Ferrari, G.C.; di Lernia, S.; Costanzi, A.; Pauna, J.; de Martini, P. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg. Endosc. Other Interv. Tech. 2007, 21, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Topal, B.; Leys, E.; Ectors, N.; Aerts, R.; Penninckx, F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg. Endosc. Other Interv. Tech. 2008, 22, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Sarela, A.I. Entirely laparoscopic radical gastrectomy for adenocarcinoma: Lymph node yield and resection margins. Surg. Endosc. Other Interv. Tech. 2009, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Moisan, F.; Norero, E.; Slako, M.; Varas, J.; Palominos, G.; Crovari, F.; Ibanez, L.; Pérez, G.; Pimentel, F.; Guzman, S.; et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: A matched cohort study. Surg. Endosc. 2011, 26, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Ramagem, C.A.G.; Linhares, M.; Lacerda, C.F.; Bertulucci, P.A.; Wonrath, D.; de Oliveira, A.T.T. Comparison of laparoscopic total gastrectomy and laparotomic total gastrectomy for gastric cancer. Arq. Bras. Cir. Dig. 2015, 28, 65. [Google Scholar] [CrossRef] [PubMed]
- Strong, V.E.; Devaud, N.; Allen, P.J.; Gonen, M.; Brennan, M.F.; Coit, D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: A case-control study. Ann. Surg. Oncol. 2009, 16, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Orsenigo, E.; di Palo, S.; Tamburini, A.; Staudacher, C. Laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: A monoinstitutional Western center experience. Surg. Endosc. 2011, 25, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Scatizzi, M.; Kröning, K.C.; Lenzi, E.; Moraldi, L.; Cantafio, S.; Feroci, F. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: A case-control study. Updates Surg. 2011, 63, 17–23. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, S.J.; MacKay, H.J.; Ringash, J.; Jacks, L.; Kassam, Z.; Conrad, T.; Khalili, I.; Okrainec, A. Laparoscopic gastrectomy for patients with advanced gastric cancer produces oncologic outcomes similar to those for open resection. Surg. Endosc. 2012, 26, 1813–1821. [Google Scholar] [CrossRef]
- Singh, S.; Ahmed, I.; Simmons, A.; Eltair, M.; George, R.; Senapati, S.; Akhtar, K. Non-randomized comparative study of laparoscopic and open gastrectomy. Surg. Endosc. Other Interv. Tech. 2012, 26, S131. [Google Scholar]
- Cianchi, F.; Qirici, E.; Trallori, G.; Macrì, G.; Indennitate, G.; Ortolani, M.; Paoli, B.; Biagini, M.R.; Galli, A.; Messerini, L.; et al. Totally laparoscopic versus open gastrectomy for gastric cancer: A matched cohort study. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 117–122. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Guo, T.K. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: A meta-analysis based on seven randomized controlled trials. Surg. Oncol. 2015, 34, 71–77. [Google Scholar] [CrossRef]
- Lu, W.; Gao, J.; Yang, J.; Zhang, Y.; Lv, W.; Mu, J.; Dong, P.; Liu, Y. Long-term clinical outcomes of laparoscopy-assisted distal gastrectomy versus open distal gastrectomy for early gastric cancer: A comprehensive systematic review and meta-analysis of randomized control trials. Medicine 2016, 95, e3986. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Wen, L.; Rui, Y.-Y.; Liu, C.-X.; Zhao, Q.-C.; Zhou, Z.-G.; Hu, J.-K. Long-term Survival Outcomes of Laparoscopic Versus Open Gastrectomy for Gastric Cancer. Medicine 2015, 94, e454. [Google Scholar] [CrossRef]
- Viñuela, E.F.; Gonen, M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Laparoscopic versus open distal gastrectomy for gastric cancer: A meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann. Surg. 2012, 255, 446–456. [Google Scholar] [CrossRef]
- Zhang, C.D.; Yamashita, H.; Zhang, S.; Seto, Y. Reevaluation of laparoscopic versus open distal gastrectomy for early gastric cancer in Asia: A meta-analysis of randomized controlled trials. Int. J. Surg. 2018, 56, 31–43. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Pan, Y.; Chen, K.; Gao, J.Q.; Cai, X.J. Comparison of Intracorporeal and Extracorporeal Esophagojejunostomy after Laparoscopic Total Gastrectomy for Gastric Cancer: A Meta-Analysis Based on Short-Term Outcomes. Chin. Med. J. 2018, 131, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Baukloh, A.K.; Kamphues, C.; Seeliger, H.; Heidecke, C.D.; Kreis, M.E.; Patrzyk, M. Laparoscopic versus open gastrectomy for locally advanced gastric cancer: A systematic review and meta-analysis of randomized controlled studies. World J. Surg. Oncol. 2019, 17, 68. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Lian, B.; Liu, Y.; Zhao, Q. Long-term oncological outcomes in laparoscopic versus open gastrectomy for advanced gastric cancer: A meta-analysis of high-quality nonrandomized studies. Am. J. Sur. 2019, 218, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bracale, U.; Merola, G.; Pignata, G.; Andreuccetti, J.; Dolce, P.; Boni, L.; Cassinotti, E.; Olmi, S.; Uccelli, M.; Gualtierotti, M.; et al. Laparoscopic gastrectomy for stage II and III advanced gastric cancer: Long-term follow-up data from a Western multicenter retrospective study. Surg. Endosc. 2022, 36, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Mercantini, P.; Lucarini, A.; Mazzuca, F.; Osti, M.F.; Laghi, A. How technology can help in oncologic patient management during COVID-19 outbreak. Eur. J. Surg. Oncol. 2020, 46, 1189–1191. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- Adachi, Y.; Shiraishi, N.; Ikebe, K.; Aramaki, M.; Bandoh, T.; Kitano, S. Evaluation of the cost for laparoscopic-assisted Billroth I gastrectomy. Surg. Endosc. 2001, 15, 932–936. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).