Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes
Abstract
1. Introduction
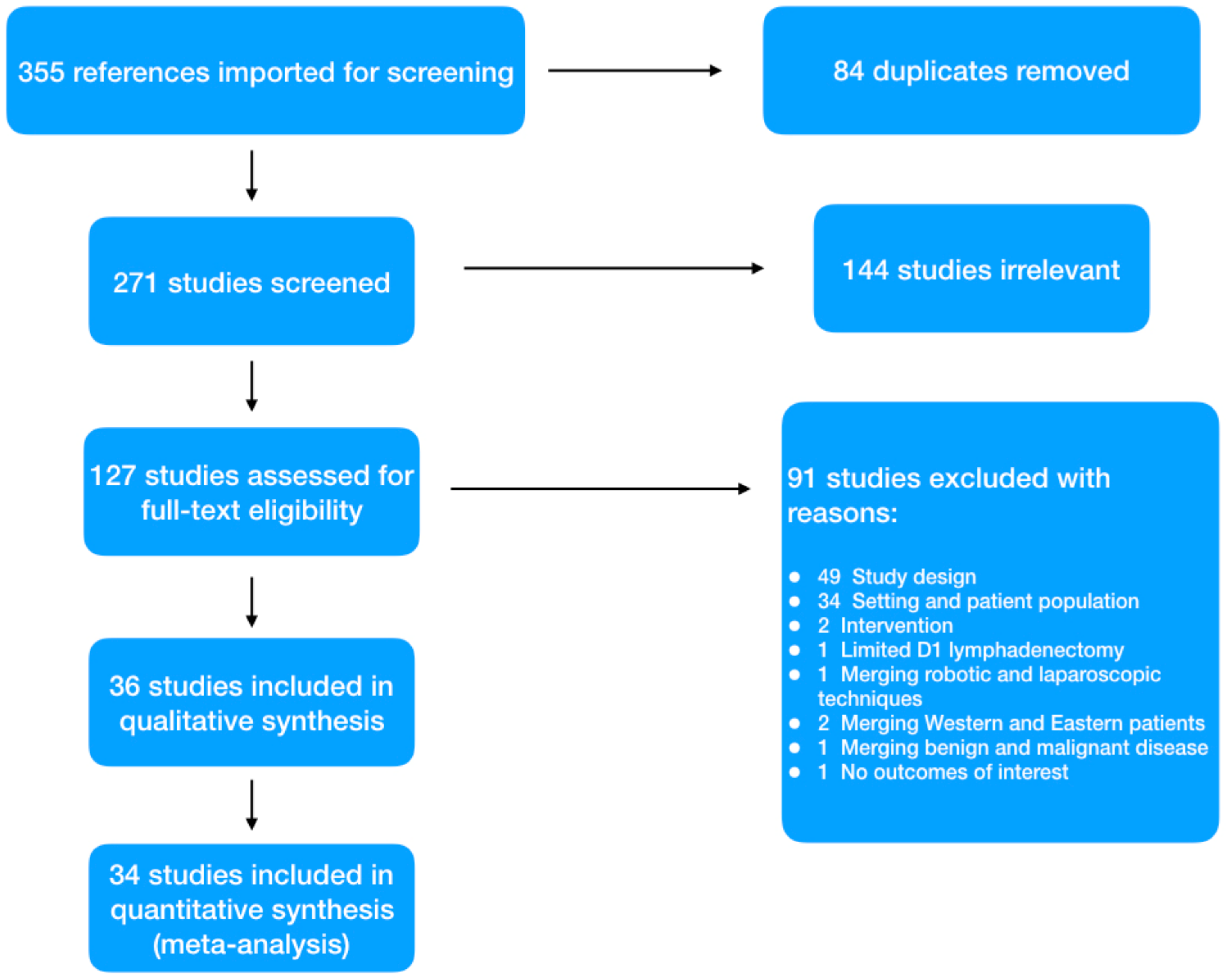
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
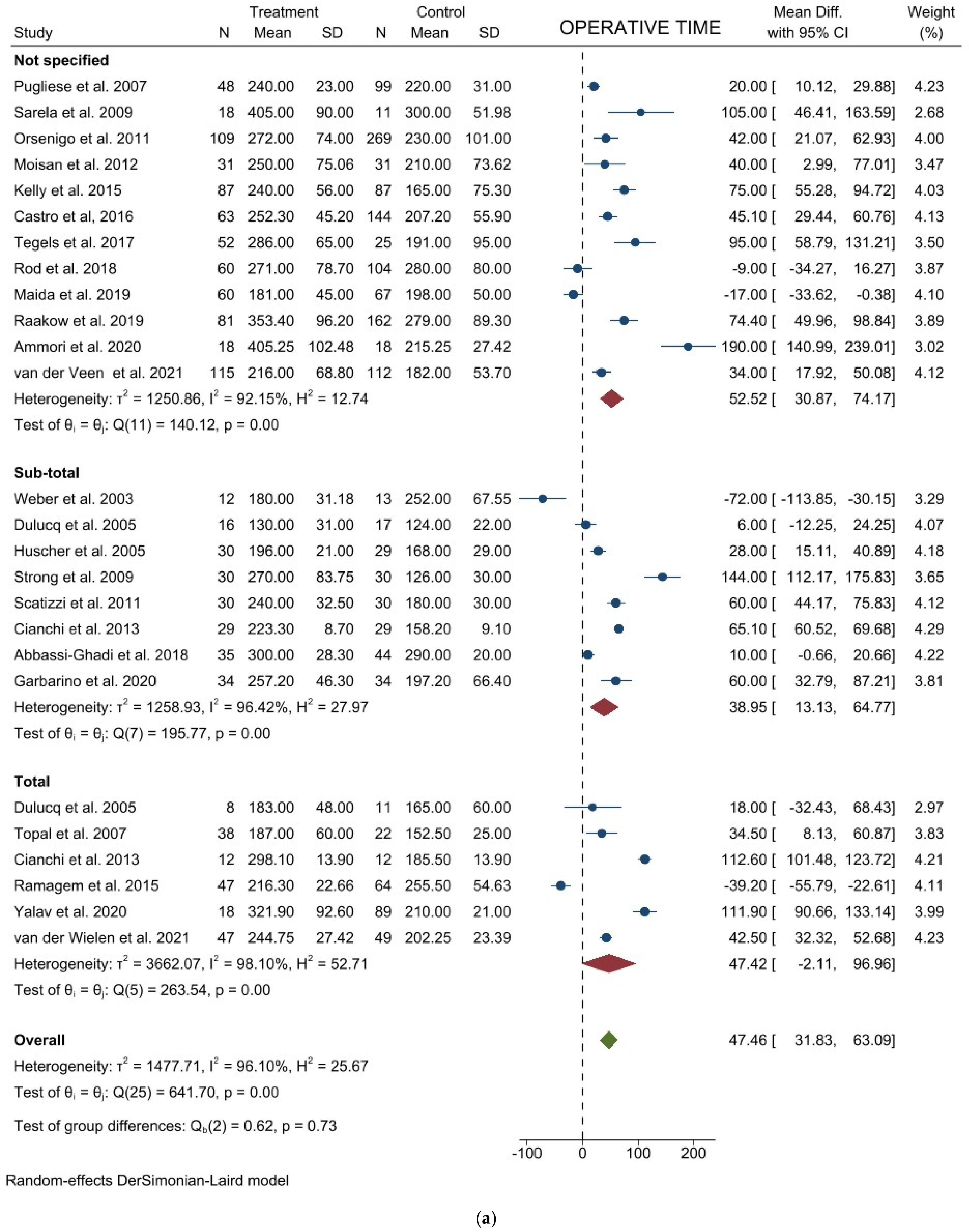
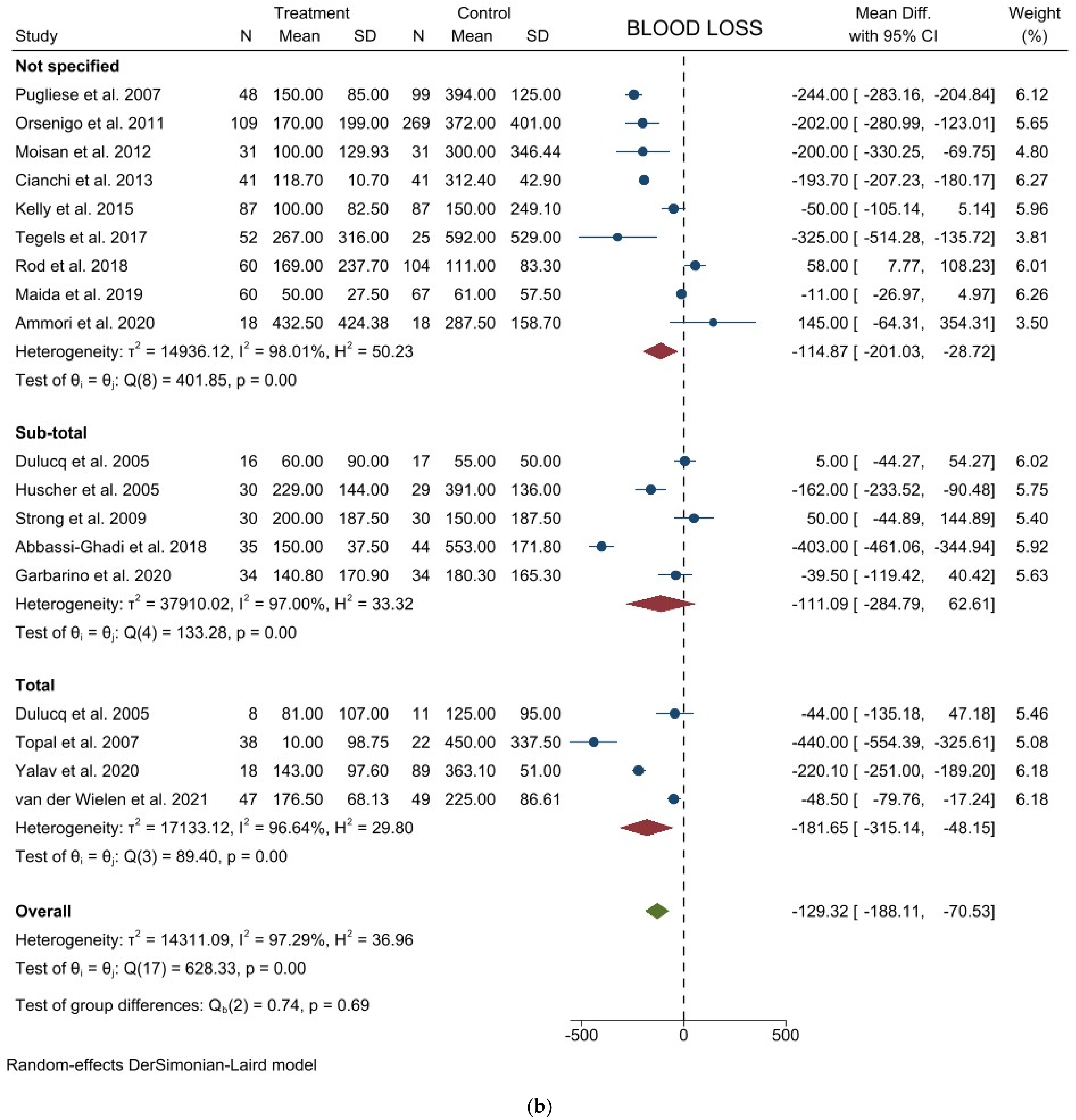
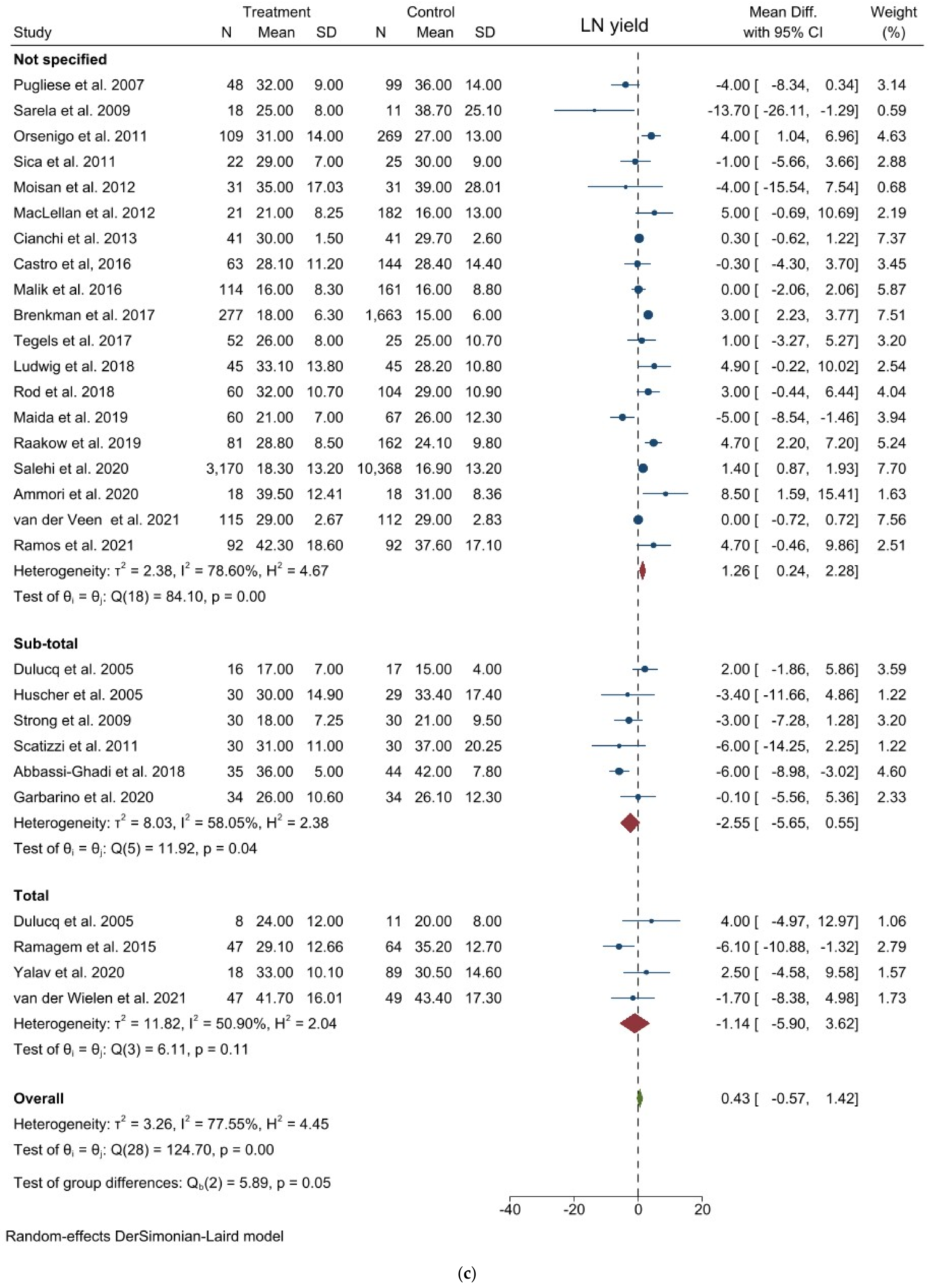
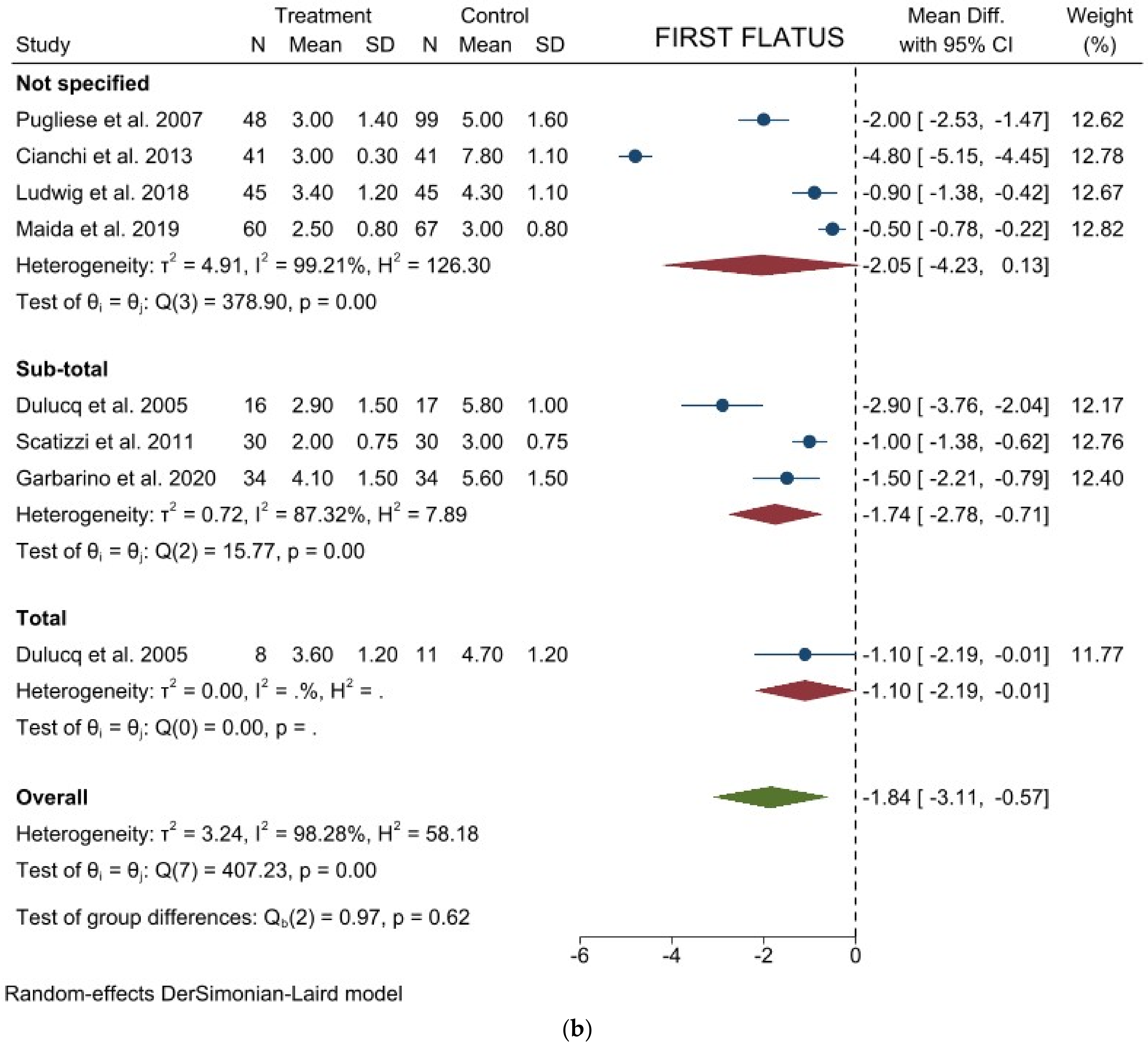
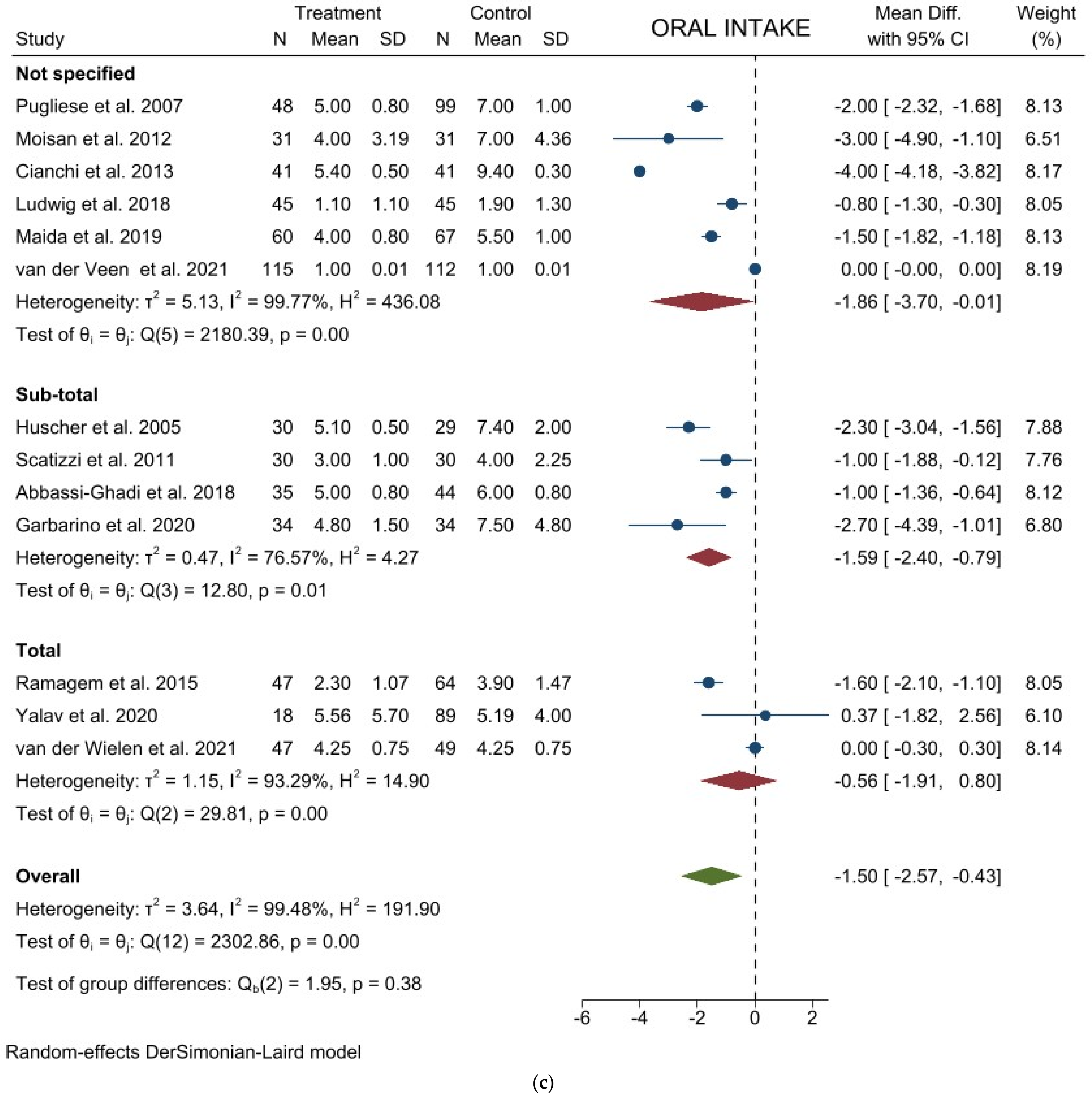
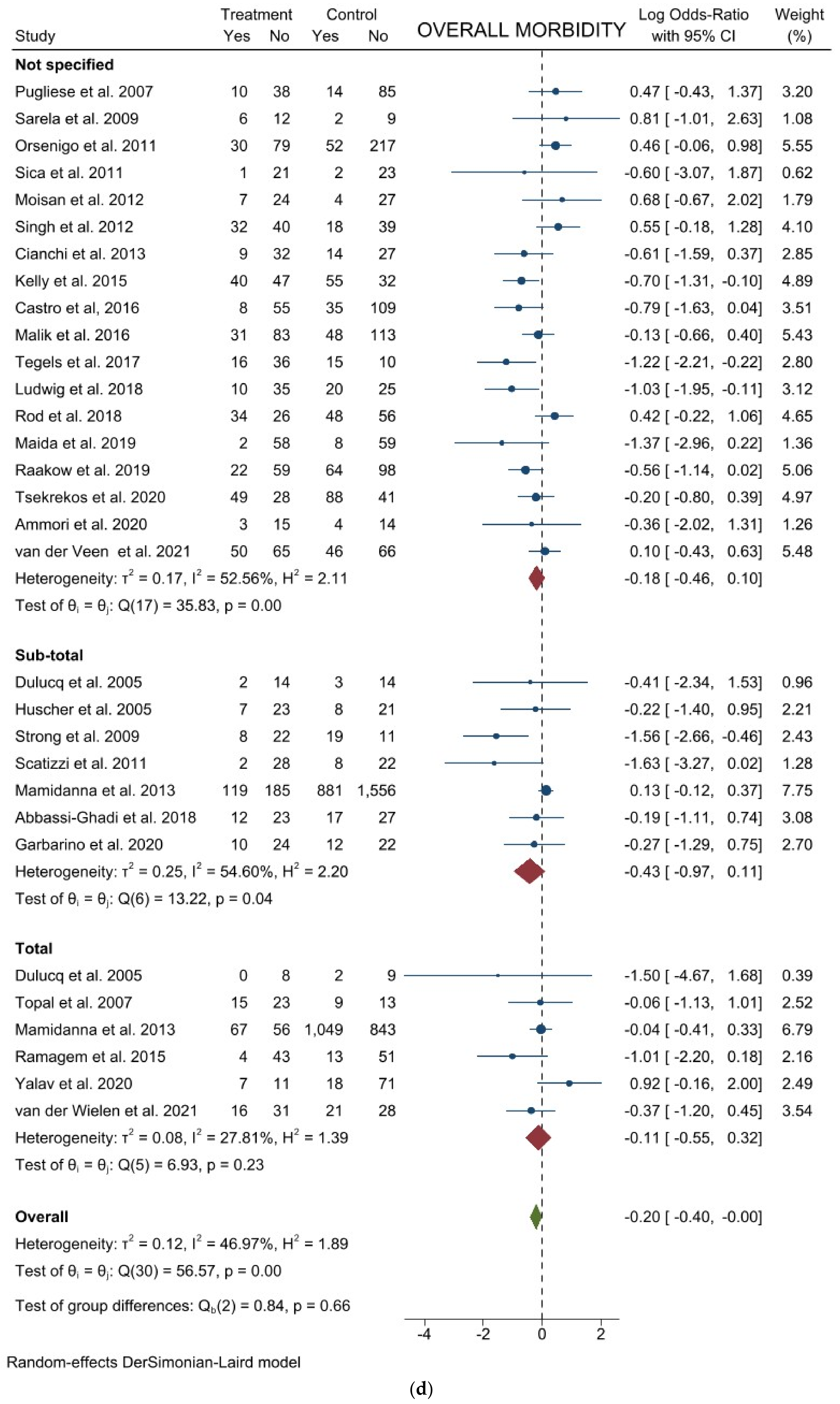
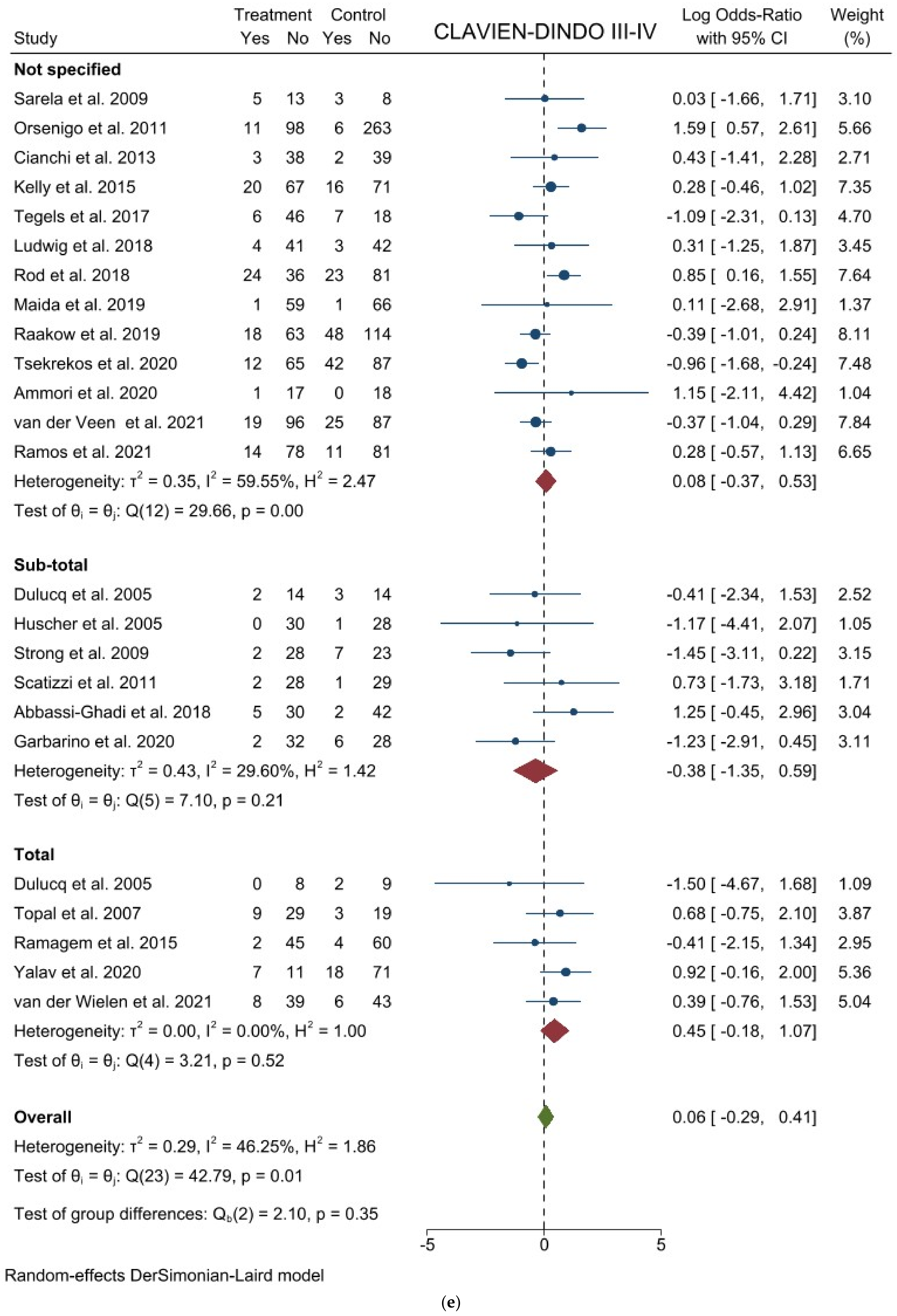
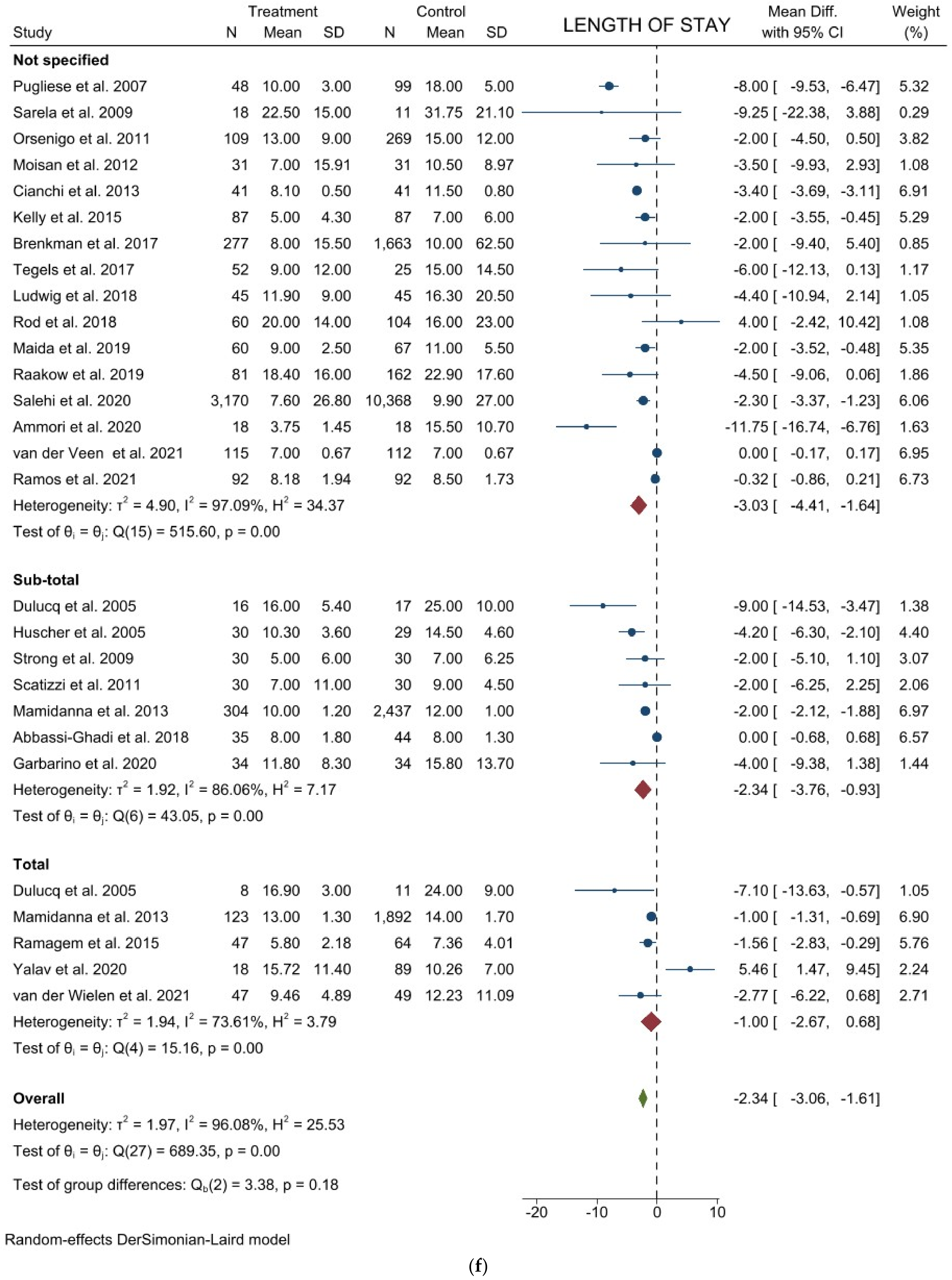
3.1. Comparison of Operative and Pathological Outcomes
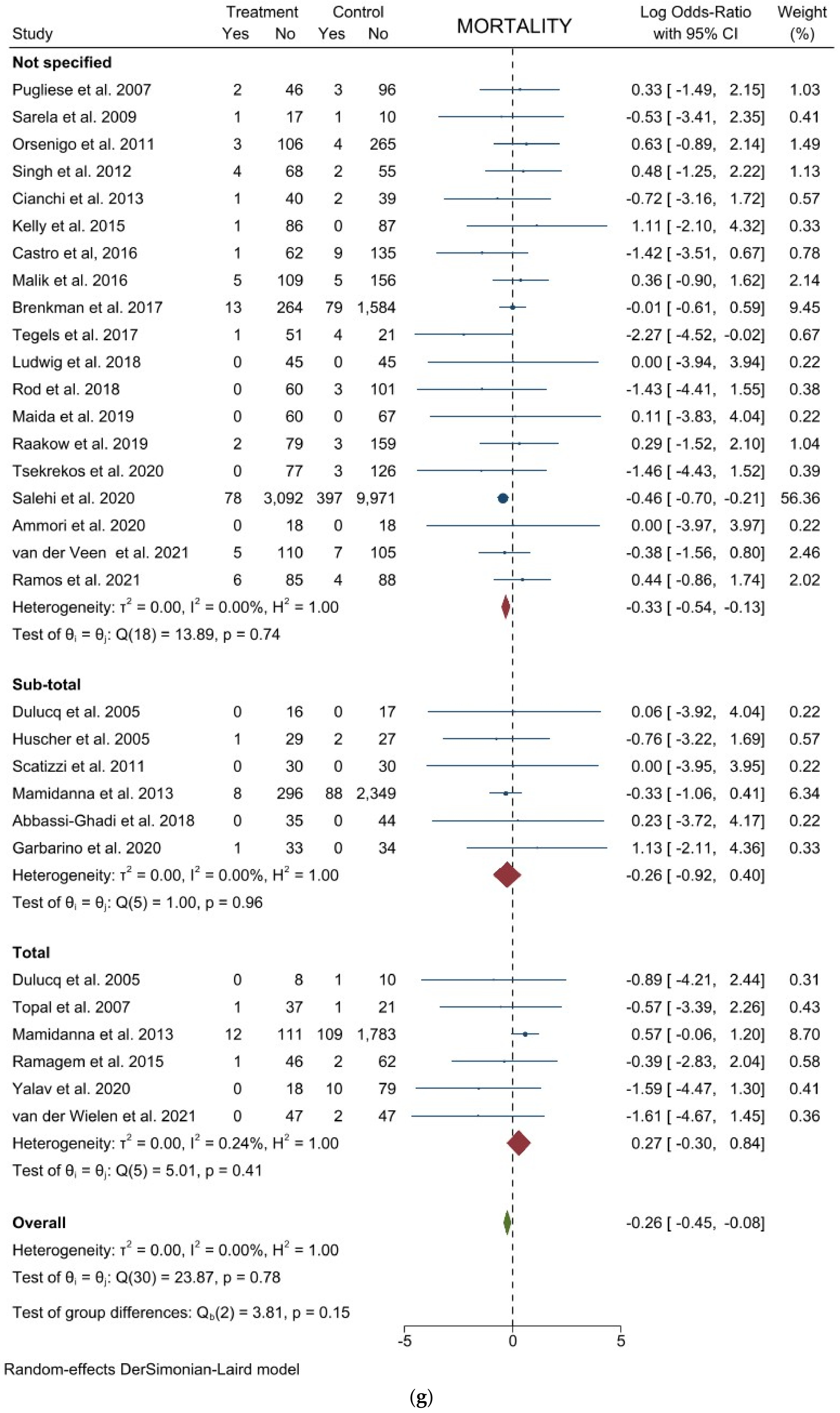
3.2. Comparison of Postoperative Outcomes
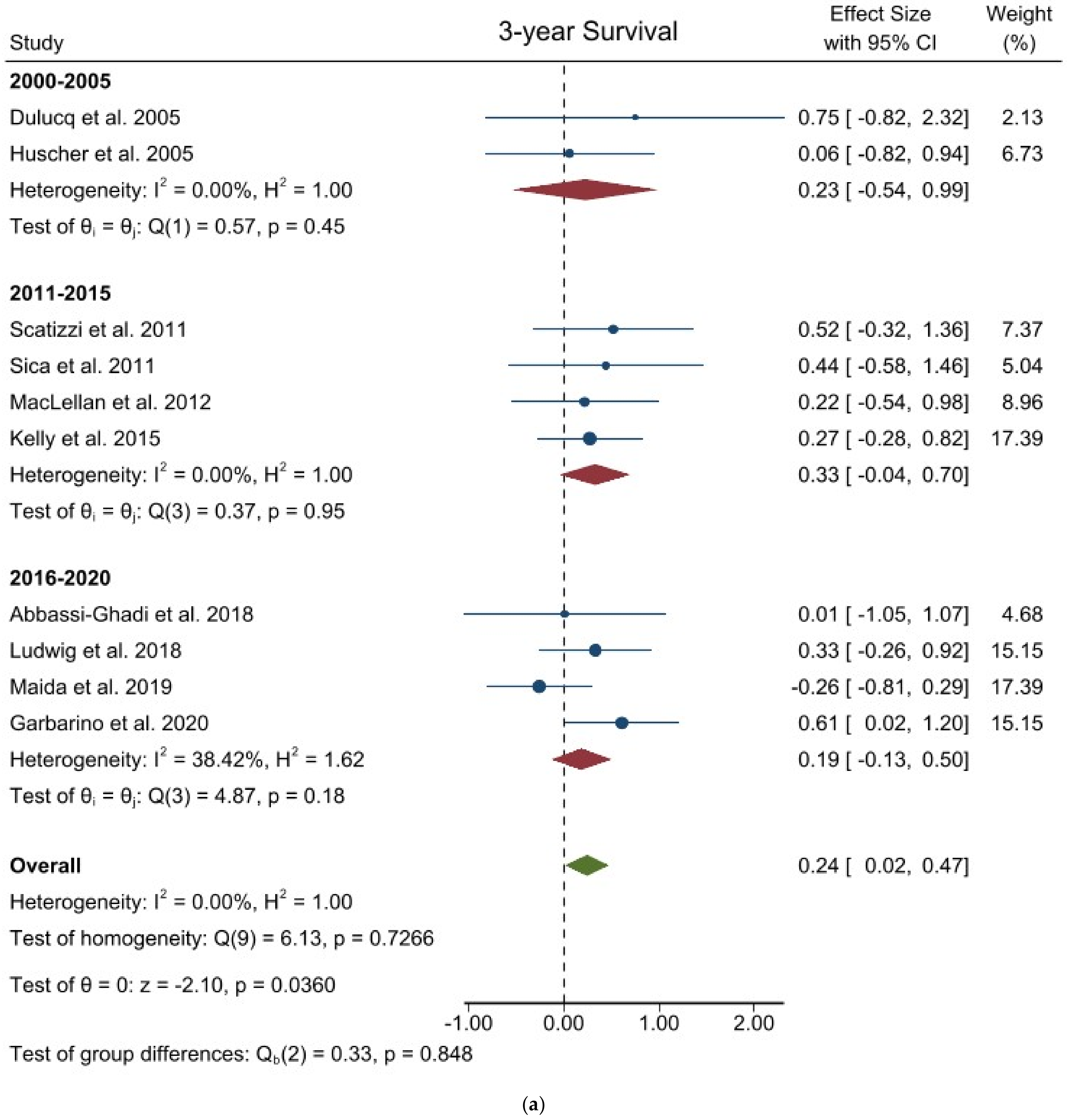
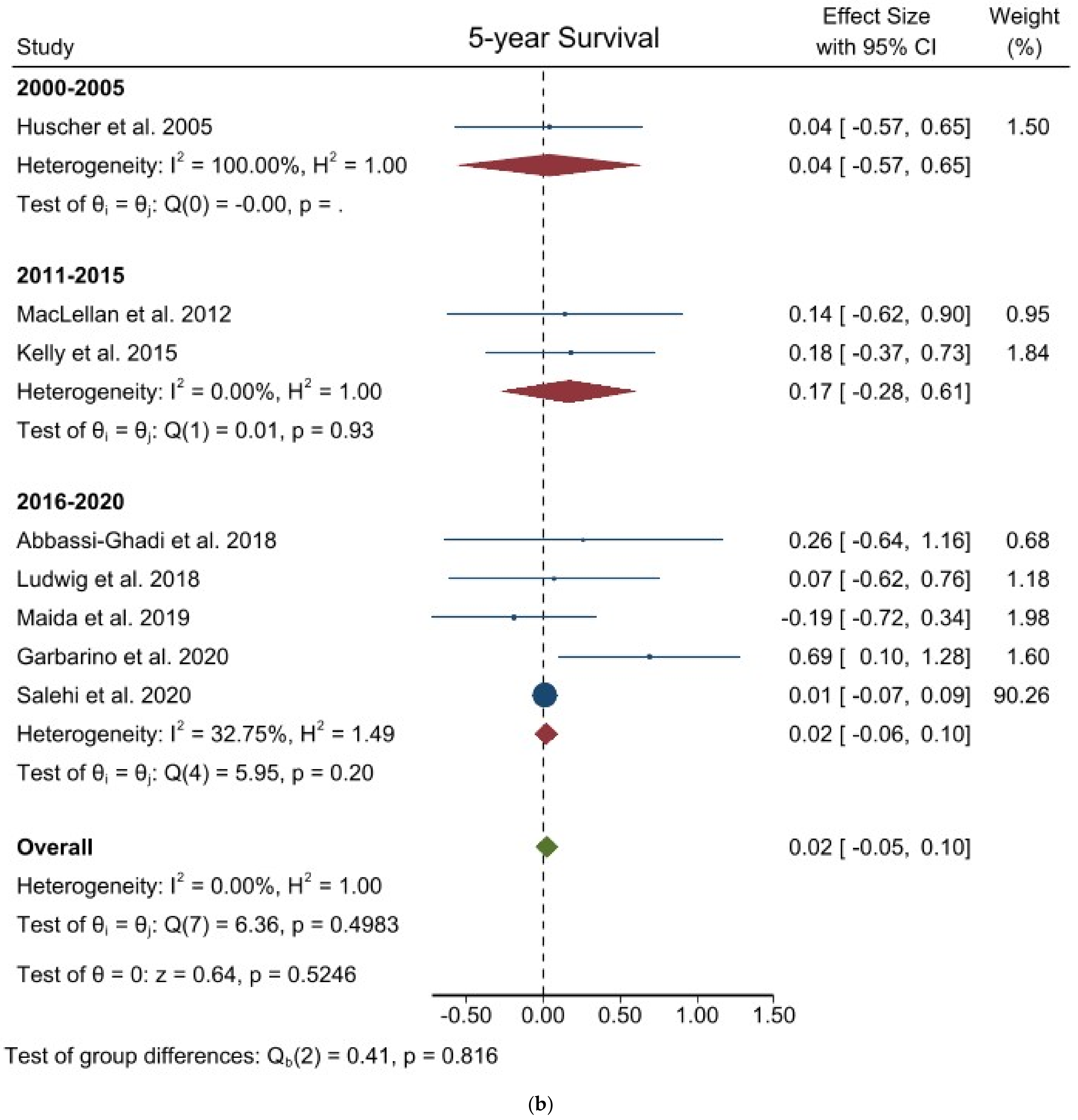
3.3. Comparison of Long-Term Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, G.; Dilaghi, E.; Cazzato, M.; Pilozzi, E.; Conti, L.; Carabotti, M.; di Giulio, E.; Annibale, B.; Lahner, E. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig. Liver Dis. 2020, 53, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Vannella, L.; Lahner, E.; Annibale, B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: A critical reappraisal. World J. Gastroenterol. 2012, 18, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer. 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 38–49. [Google Scholar] [CrossRef]
- McMillian, N.; Pluchino, M.A.; Ajani, J.A.; Chair, V.; Bentrem, D.J.; Chao, J.; Enzler, T.; Fanta, P.; Gerdes, H.; Gibson, M.; et al. NCCN Guidelines for Gastric Cancer, Version 4; National Comprehensive Cancer Network: Plymouth, PA, USA, 2020. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4). Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.K.; Sasako, M.; van de Velde, C.J.H. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Wu, C.W.; Hsiung, C.A.; Lo, S.S.; Hsieh, M.C.; Chen, J.H.; Li, A.F.Y.; Lui, W.Y.; Whang-Peng, J. Nodal dissection for patients with gastric cancer: A randomised controlled trial. Lancet Oncol. 2006, 7, 309–315. [Google Scholar] [CrossRef]
- Mocellin, S.; Mcculloch, P.; Kazi, H.; Gama-Rodrigues, J.J.; Yuan, Y.; Nitti, D. Extent of Lymph Node Dissection for Adenocarcinoma of the Stomach. Cochrane Database Syst. Rev. 2015, 2015, CD001964. [Google Scholar] [CrossRef]
- Uyama, I.; Ogiwara, H.; Takahara, T.; Kato, Y.; Kikuchi, K.; Iida, S. Laparoscopic and Minilaparotomy Billroth I Gastrectomy for Gastric Ulcer Using an Abdominal Wall-Lifting Method. J. Laparoendosc. Surg. 1994, 4, 441–445. [Google Scholar] [CrossRef]
- Nakamura, K.; Katai, H.; Mizusawa, J.; Yoshikawa, T.; Ando, M.; Terashima, M.; Ito, S.; Takagi, M.; Takagane, A.; Ninomiya, M.; et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912). Jpn. J. Clin. Oncol. 2013, 43, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.K.; Chan, Y.; Yang, H.K.; Park, D.J. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival among Patients with Stage i Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J. Korean Surg. Soc. 2013, 84, 123–130. [Google Scholar] [CrossRef]
- Yu, J.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Wang, K.; Suo, J.; Tao, K.; He, X.; et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients with Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 321, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hyung, W.J.; Yang, H.K.; Han, S.U.; Park, Y.K.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann. Surg. 2019, 270, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, N.; Straatman, J.; Daams, F.; Rosati, R.; Parise, P.; Weitz, J.; Reissfelder, C.; Diez del Val, I.; Loureiro, C.; Parada-Gonzalez, P.; et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: Results of a European randomized trial. Gastric Cancer 2021, 24, 258–271. [Google Scholar] [CrossRef]
- Van der Veen, A.; Brenkman, H.J.F.; Seesing, M.F.J.; Haverkamp, L.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; Stoot, J.H.M.B.; Tegels, J.J.W.; Wiknhoven, B.P.L.; Lagarde, S.M.; et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 978–989. [Google Scholar] [CrossRef]
- Russo, A.; Li, P.; Strong, V.E. Differences in the multimodal treatment of gastric cancer: East versus west. J. Surg. Oncol. 2017, 115, 603–614. [Google Scholar] [CrossRef]
- Russo, A.E.; Strong, V.E. Gastric cancer etiology and management in Asia and the west. Annu. Rev. Med. 2019, 70, 353–367. [Google Scholar] [CrossRef]
- Markar, S.R.; Karthikesalingam, A.; Jackson, D.; Hanna, G.B. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: Comparison between west and east. Ann. Surg. Oncol. 2013, 20, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Form for Cohort Studies; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014; pp. 2–4. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Costa, G.; Laracca, G.G.; Castagnola, G.; Mercantini, P.; di Paola, M.; Vita, S.; Masoni, L. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer in middle–low-volume centers in Western countries: A propensity score matching analysis. Langenbeck’s Arch. Surg. 2020, 405, 797–807. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Canali, G.; Tarantino, G.; Costa, G.; Ferri, M.; Balducci, G.; Pilozzi, E.; Berardi, G.; Mercantini, P. Laparoscopic versus open rectal resection: A 1, 2 propensity score–matched analysis of oncological adequateness, short- and long-term outcomes. Int. J. Colorectal. Dis. 2021, 36, 801–810. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Costa, G.; Frezza, B.; Biancafarina, A.; Balducci, G.; Mercantini, P.; de Prizio, M.; Laracca, G.G.; Ceccarelli, G. Robotic versus open oncological gastric surgery in the elderly: A propensity score-matched analysis. J. Robot. Surg. 2020, 15, 741–749. [Google Scholar] [CrossRef]
- Sica, G.S.; Iaculli, E.; Biancone, L.; di Carlo, S.; Scaramuzzo, R.; Fiorani, C.; Gentileschi, P.; Gaspari, A.L. Comparative study of laparoscopic vs. open gastrectomy in gastric cancer management. World J. Gastroenterol. 2011, 17, 4602–4606. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. J. Am. Med. Assoc. 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Jagric, T. East meets West: The initial results of laparoscopic gastric cancer resections with Eastern principles in a single Western centre—A propensity score-matched study. Langenbeck’s Arch. Surg. 2021, 406, 2699–2708. [Google Scholar] [CrossRef]
- Norero, E.; Vargas, C.; Achurra, P.; Ceroni, M.; Mejia, R.; Martinez, C.; Munoz, R.; Gonzalez, P.; Calvo, A.; Diaz, A. Survival and perioperative morbidity of totally laparoscopic versus open gastrectomy for early gastric cancer: Analysis from a single latin american centre. Arq. Bras. Cir. Dig. 2019, 32, e1413. [Google Scholar] [CrossRef]
- Mamidanna, R.; Almoudaris, A.M.; Bottle, A.; Aylin, P.; Faiz, O.; Hanna, G.B. National outcomes and uptake of laparoscopic gastrectomy for cancer in England. Surg. Endosc. 2013, 27, 3348–3358. [Google Scholar] [CrossRef]
- Kelly, K.J.; Selby, L.; Chou, J.F.; Dukleska, K.; Capanu, M.; Coit, D.G.; Strong, V.E. Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann. Surg. Oncol. 2015, 22, 3590–3596. [Google Scholar] [CrossRef]
- Castro, B.; Aral, M.; Fareleira, A.; Costa-Maia, J.; Santos-Sousa, H. Outcomes of laparoscopic and open gastrectomy for gastric cancer: A comparative analysis. Ann. Oncol. 2016, 27 (Suppl. S2), ii75. [Google Scholar] [CrossRef][Green Version]
- Malik, H.T.; Parker, A.; Melhado, R.; Chaparala, R.; Formela, L.; Vickers, J.; Senapati, S.; Akhtar, K. Non randomised long term comparative analysis of totally laparoscopic with open gastrectomy in a western population. Surg. Endosc. Other Interv. Tech. 2016, 30, S24. [Google Scholar]
- Brenkman, J.P.; Ruurda, H.J.F.; Hillegersberg, R.H.A.; van Verhoeven, R. Safety and feasibility of minimally invasive gastrectomy during the early introduction in the Netherlands: Short-term oncological outcomes comparable to open gastrectomy. Gastric Cancer 2017, 20, 853–860. [Google Scholar] [CrossRef][Green Version]
- Tegels, J.J.; Silvius, C.E.; Spauwen, F.E.; Hulsewe, K.W.; Hoofwijk, A.G.; Stoot, J.H. Introduction of laparoscopic gastrectomy for gastric cancer in a Western tertiary referral centre: A prospective cost analysis during the learning curve. World J. Gastrointest. Oncol. 2017, 9, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Schneider-Koriath, S.; Scharlau, U.; Steffen, H.; Möller, D.; Bernhardt, J. Totally laparoscopic versus open gastrectomy for gastric cancer: A matched pair analysis. Zent. Chir. Z. Allg. Visz. Thorax Gefäßchir. 2018, 143, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Rod, X.; Fuks, D.; Macovei, R.; Levard, H.; Ferraz, J.M.; Denet, C.; Tubbax, C.; Gayet, B.; Perniceni, T. Comparison between open and laparoscopic gastrectomy for gastric cancer: A monocentric retrospective study from a western country. J. Visc. Surg. 2018, 155, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Maida, P.; Marte, G.; Spedicato, G.A.; Ferronetti, A.; Mauriello, C.; Canfora, A.; Ciorra, G.; Barra, L.; di Maio, V. Laparoscopic versus open gastrectomy with extended lymph node dissection for gastric carcinoma in a Western series: A Propensity Score Matching analysis. Minerva Chir. 2019, 16, 74. [Google Scholar] [CrossRef]
- Abbassi-Ghadi, N.; Durakovic, S.; Piessen, G.; Gatenby, P.; Sultan, J.; Preston, S.R. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma of the stomach in a Western population: Peri-operative and 5-year oncological outcomes. Surg. Endosc. 2020, 34, 3818–3826. [Google Scholar] [CrossRef]
- Raakow, J.; Denecke, C.; Chopra, S.; Fritz, J.; Hofmann, T.; Andreou, A.; Thuss-Patience, P.; Pratschke, J.; Biebl, M. Laparoscopic versus open gastrectomy for advanced gastric cancer: Operative and postoperative results. Chir. Z. Alle Geb. Oper. Medizen 2020, 91, 252–261. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Klevebro, F.; Hayami, M.; Kamiya, S.; Lindblad, M.; Nilsson, M.; Lundell, L.; Rouvelas, I. Laparoscopic Versus Open Gastrectomy for Cancer: A Western Center Cohort Study. J. Surg. Res. 2020, 247, 372–379. [Google Scholar] [CrossRef]
- Salehi, O.; Vega, E.A.; Kutlu, O.C.; James, D.; Alarcon, S.V.; Herrick, B.; Kozyreva, O.; Conrad, C. Western population-based study of oncologic surgical quality and outcomes of laparoscopic versus open gastrectomy for gastric adenocarcinoma. Surg. Endosc. 2020, 35, 4786–4793. [Google Scholar] [CrossRef]
- Ammori, B.J.; Asmer, H.; Al-Najjar, H.; Al-Bakri, H.; Dabous, A.; Daoud, F.; Almasri, M. Laparoscopic Versus Open D2 Gastrectomy for Gastric Cancer: A Case-Matched Comparative Study. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 777–782. [Google Scholar] [CrossRef]
- Yalav, O.; Topal, U.; Gümüş, S.; Ünal, A.G.; Rencüzoğulları, A. Laparoscopic versus open total radical gastrectomy for advanced gastric cancer: Surgical outcomes. Ann. Ital. Chir. 2020, 9, S2239253X20032673. [Google Scholar]
- Ramos, M.F.K.P.; Pereira, M.A.; Dias, A.R.; Ribeiro, U.; Zilberstein, B.; Nahas, S.C. Laparoscopic gastrectomy for early and advanced gastric cancer in a western center: A propensity score-matched analysis. Updates Surg. 2021, 73, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.J.; Reyes, C.D.; Gagner, M.; Divino, C.M. Comparison of laparoscopic and open gastrectomy for malignant disease. Surg. Endosc. Other Interv. Tech. 2003, 17, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Dulucq, J.L.; Wintringer, P.; Stabilini, C.; Solinas, L.; Perissat, J.; Mahajna, A. Laparoscopic and open gastric resections for malignant lesions: A prospective comparative study. Surg. Endosc. Other Interv. Tech. 2005, 19, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Huscher, C.G.S.S.; Mingoli, A.; Sgarzini, G.; Sansonetti, A.; di Paola, M.; Recher, A.; Ponzano, C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: Five-year results of a randomized prospective trial. Ann. Surg. 2005, 241, 232–237. [Google Scholar] [CrossRef]
- Pugliese, R.; Maggioni, D.; Sansonna, F.; Scandroglio, I.; Ferrari, G.C.; di Lernia, S.; Costanzi, A.; Pauna, J.; de Martini, P. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg. Endosc. Other Interv. Tech. 2007, 21, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Topal, B.; Leys, E.; Ectors, N.; Aerts, R.; Penninckx, F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg. Endosc. Other Interv. Tech. 2008, 22, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Sarela, A.I. Entirely laparoscopic radical gastrectomy for adenocarcinoma: Lymph node yield and resection margins. Surg. Endosc. Other Interv. Tech. 2009, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Moisan, F.; Norero, E.; Slako, M.; Varas, J.; Palominos, G.; Crovari, F.; Ibanez, L.; Pérez, G.; Pimentel, F.; Guzman, S.; et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: A matched cohort study. Surg. Endosc. 2011, 26, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Ramagem, C.A.G.; Linhares, M.; Lacerda, C.F.; Bertulucci, P.A.; Wonrath, D.; de Oliveira, A.T.T. Comparison of laparoscopic total gastrectomy and laparotomic total gastrectomy for gastric cancer. Arq. Bras. Cir. Dig. 2015, 28, 65. [Google Scholar] [CrossRef] [PubMed]
- Strong, V.E.; Devaud, N.; Allen, P.J.; Gonen, M.; Brennan, M.F.; Coit, D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: A case-control study. Ann. Surg. Oncol. 2009, 16, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Orsenigo, E.; di Palo, S.; Tamburini, A.; Staudacher, C. Laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: A monoinstitutional Western center experience. Surg. Endosc. 2011, 25, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Scatizzi, M.; Kröning, K.C.; Lenzi, E.; Moraldi, L.; Cantafio, S.; Feroci, F. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: A case-control study. Updates Surg. 2011, 63, 17–23. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, S.J.; MacKay, H.J.; Ringash, J.; Jacks, L.; Kassam, Z.; Conrad, T.; Khalili, I.; Okrainec, A. Laparoscopic gastrectomy for patients with advanced gastric cancer produces oncologic outcomes similar to those for open resection. Surg. Endosc. 2012, 26, 1813–1821. [Google Scholar] [CrossRef]
- Singh, S.; Ahmed, I.; Simmons, A.; Eltair, M.; George, R.; Senapati, S.; Akhtar, K. Non-randomized comparative study of laparoscopic and open gastrectomy. Surg. Endosc. Other Interv. Tech. 2012, 26, S131. [Google Scholar]
- Cianchi, F.; Qirici, E.; Trallori, G.; Macrì, G.; Indennitate, G.; Ortolani, M.; Paoli, B.; Biagini, M.R.; Galli, A.; Messerini, L.; et al. Totally laparoscopic versus open gastrectomy for gastric cancer: A matched cohort study. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 117–122. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Guo, T.K. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: A meta-analysis based on seven randomized controlled trials. Surg. Oncol. 2015, 34, 71–77. [Google Scholar] [CrossRef]
- Lu, W.; Gao, J.; Yang, J.; Zhang, Y.; Lv, W.; Mu, J.; Dong, P.; Liu, Y. Long-term clinical outcomes of laparoscopy-assisted distal gastrectomy versus open distal gastrectomy for early gastric cancer: A comprehensive systematic review and meta-analysis of randomized control trials. Medicine 2016, 95, e3986. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Wen, L.; Rui, Y.-Y.; Liu, C.-X.; Zhao, Q.-C.; Zhou, Z.-G.; Hu, J.-K. Long-term Survival Outcomes of Laparoscopic Versus Open Gastrectomy for Gastric Cancer. Medicine 2015, 94, e454. [Google Scholar] [CrossRef]
- Viñuela, E.F.; Gonen, M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Laparoscopic versus open distal gastrectomy for gastric cancer: A meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann. Surg. 2012, 255, 446–456. [Google Scholar] [CrossRef]
- Zhang, C.D.; Yamashita, H.; Zhang, S.; Seto, Y. Reevaluation of laparoscopic versus open distal gastrectomy for early gastric cancer in Asia: A meta-analysis of randomized controlled trials. Int. J. Surg. 2018, 56, 31–43. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Pan, Y.; Chen, K.; Gao, J.Q.; Cai, X.J. Comparison of Intracorporeal and Extracorporeal Esophagojejunostomy after Laparoscopic Total Gastrectomy for Gastric Cancer: A Meta-Analysis Based on Short-Term Outcomes. Chin. Med. J. 2018, 131, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Baukloh, A.K.; Kamphues, C.; Seeliger, H.; Heidecke, C.D.; Kreis, M.E.; Patrzyk, M. Laparoscopic versus open gastrectomy for locally advanced gastric cancer: A systematic review and meta-analysis of randomized controlled studies. World J. Surg. Oncol. 2019, 17, 68. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Lian, B.; Liu, Y.; Zhao, Q. Long-term oncological outcomes in laparoscopic versus open gastrectomy for advanced gastric cancer: A meta-analysis of high-quality nonrandomized studies. Am. J. Sur. 2019, 218, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bracale, U.; Merola, G.; Pignata, G.; Andreuccetti, J.; Dolce, P.; Boni, L.; Cassinotti, E.; Olmi, S.; Uccelli, M.; Gualtierotti, M.; et al. Laparoscopic gastrectomy for stage II and III advanced gastric cancer: Long-term follow-up data from a Western multicenter retrospective study. Surg. Endosc. 2022, 36, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Mercantini, P.; Lucarini, A.; Mazzuca, F.; Osti, M.F.; Laghi, A. How technology can help in oncologic patient management during COVID-19 outbreak. Eur. J. Surg. Oncol. 2020, 46, 1189–1191. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- Adachi, Y.; Shiraishi, N.; Ikebe, K.; Aramaki, M.; Bandoh, T.; Kitano, S. Evaluation of the cost for laparoscopic-assisted Billroth I gastrectomy. Surg. Endosc. 2001, 15, 932–936. [Google Scholar] [CrossRef]


| Author Year | Country | Study Period | Study Design | Tumor Stage | Extent of Resection | Sample Size | Age | M/F | Follow-Up (Months) | Newcastle-Ottawa Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LG | OG | LG | OG | LG | OG | LG | OG | |||||||
| Weber et al., 2003 [53] | USA | 1997–2000 | Retrospective comparative | EGC, AGC | SG | 12 | 13 | 67 | 67 | ns | ns | 18 | 18 | 7 |
| Dulucq et al., 2005 [54] | France | 1995–2004 | Prospective comparative | EGC, AGC | SG | 16 | 17 | 71 | 70 | 7/9 | 7/10 | ns | ns | 8 |
| TG | 8 | 11 | 75 | 67 | 3/5 | 5/6 | ||||||||
| Huscher et al., 2005 [55] | Italy | 1992–1996 | Randomized trial | EGC, AGC | SG | 30 | 29 | 63.2 | 63.6 | 18/12 | 21/8 | 52.2 | 49.7 | 2 * |
| Pugliese et al., 2007 [56] | Italy | 2000–2005 | Retrospective comparative | EGC, AGC | SG, TG | 48 | 99 | ns | ns | 29/19 | ns | 1–60 | ns | 9 |
| Topal et al., 2007 [57] | Belgium | 2003–2006 | Retrospective comparative | EGC, AGC | TG | 38 | 22 | 68 | 69 | 23/15 | 17/5 | 12 | 12 | 7 |
| Sarela et al., 2009 [58] | UK | 2005–2007 | Retrospective comparative | EGC, AGC | SG | 18 | 11 | ns | ns | ns | ns | ns | Ns | 8 |
| Strong et al., 2009 [61] | USA | 2005–2008 | Retrospective case-matched | EGC, AGC | SG | 30 | 30 | 71 | 73 | 13/17 | 14/16 | 36 | 36 | 7 |
| Orsenigo et al., 2011 [62] | Italy | 2002–2008 | Retrospective comparative | EGC, AGC | SG | 109 | 269 | 66.57 | 66.73 | 56/53 | 169/100 | 33 | 33 | 8 |
| Scatizzi et al., 2011 [63] | Italy | 2006–2009 | Retrospective comparative | EGC, AGC | SG | 30 | 30 | 70 | 69 | 16/14 | 14/16 | 18 | 18 | 8 |
| Sica et al., 2011 [31] | Italy | 2000–2004 | Retrospective comparative | EGC, AGC | SG | 22 | 25 | 67 | 68 | 13/9 | 13/12 | 39 | 38 | 8 |
| Moisan et al., 2011 [59] | Chile | 2003–2010 | Retrospective case-matched | EGC, AGC | SG, TG | 31 | 31 | 67 | 67 | 21/10 | 20/11 | 28 | 40 | 8 |
| MacLellan et al., 2012 [64] | Canada | 2000–2009 | Retrospective comparative | EGC, AGC | SG, TG | 21 | 182 | 61 | 57 | 15/6 | 113/69 | 21.3 | ns | 7 |
| Singh et al., 2012 [65] | UK | 2003–2010 | Prospective comparative | ns | SG, TG | 72 | 57 | ns | ns | ns | ns | ns | ns | 6 |
| Cianchi et al., 2013 [66] | Italy | 2008–2012 | Prospective case-matched | EGC, AGC | SG, TG | 41 | 41 | 73 | 74 | 25/16 | 25/16 | ns | ns | 6 |
| Mamidanna et al., 2013 [37] | UK | 2000–2010 | Retrospective comparative | EGC, AGC | SG, TG | 427 | 4329 | ns | ns | 276/204 | 6781/ 3502 | ns | ns | 8 |
| Kelly et al., 2015 [38] | USA | 2005–2013 | Retrospective case-matched | EGC, AGC | SG, TG | 87 | 87 | 64 | 64 | 37/50 | 54/33 | 11 | 31.1 | 6 |
| Ramagem et al., 2015 [60] | Brazil | 2009–2013 | Retrospective comparative | EGC, AGC | TG | 47 | 64 | 58 | 60 | 34/13 | 43/21 | ns | ns | 8 |
| Castro et al., 2016 [39] | Portugal | 2010–2014 | Prospective comparative | ns | SG, TG | 63 | 144 | ns | ns | ns | ns | 29 | 29 | 7 |
| Malik et al., 2016 [40] | UK | 2003–2014 | Retrospective comparative | ns | SG, TG | 114 | 161 | 73 | 72 | 55/56 | 101/48 | 60 | 60 | 7 |
| Brenkman et al., 2017 [41] | Netherlands | 2010–2014 | Retrospective comparative | EGC, AGC | SG, TG | 277 | 1663 | 68.5 | 68.4 | 173/104 | 1035/ 628 | 12 | 12 | 8 |
| Tegels et al., 2017 [42] | Netherlands | 2013–2014 | Retrospective + prospective comparative | EGC, AGC | SG, TG | 52 | 25 | 68 | 70 | 32/20 | 17/8 | ns | ns | 7 |
| Abbassi-Ghadi et al., 2018 [46] | UK | 2006–2016 | Retrospective comparative | EGC, AGC | SG, TG | 35 | 44 | 77 | 71 | 21/14 | 35/9 | 60 | 60 | 8 |
| Ludwig et al., 2018 [43] | Germany | 2013–2016 | Prospective case-control | EGC, AGC | SG, TG | 45 | 45 | 61.1 | 64.8 | 26/19 | 26/19 | 31 | 31 | 8 |
| Rod et al., 2018 [44] | France | 2005–2015 | Retrospective comparative | EGC, AGC | SG, TG | 60 | 104 | 62 | 65 | 37/23 | 63/41 | ns | ns | 8 |
| Maida et al., 2019 [45] | Italy | 2009–2013 | Retrospective case-matched | EGC, AGC | SG, TG | 60 | 67 | 71 | 67 | 28/32 | 36/31 | ns | ns | 8 |
| Raakow et al., 2019 [47] | Germany | 2005–2017 | Retrospective case-matched | EGC, AGC | SG, TG | 81 | 162 | 64.7 | 64.2 | 58/23 | 116/46 | ns | ns | 9 |
| Garbarino et al., 2020 [28] | Italy | 2009–2014 | Retrospective case-matched | AGC | SG | 34 | 34 | 70.9 | 71.1 | 23/11 | 21/13 | 31 | 31 | 8 |
| Tsekrekos et al., 2020 [48] | Sweden | 2010–2018 | Retrospective comparative | EGC, AGC | SG, TG | 77 | 129 | 69 | 68 | 47/30 | 77/52 | ns | ns | 9 |
| Salehi et al., 2020 [49] | USA | 2010–2016 | Retrospective comparative | EGC, AGC | SG, TG | 3170 | 10368 | 67.9 | 68.1 | 2162/ 1008 | 6814/ 2554 | ns | ns | 9 |
| Yalav et al., 2020 [51] | Turkey | 2015–2018 | Retrospective comparative | EGC, AGC | TG | 18 | 89 | 57.3 | 59.4 | 12/6 | 58/31 | 25 | 15 | 8 |
| Ammori et al., 2020 [50] | Jordan | 2017–2019 | Retrospective case-matched | EGC, AGC | SG, TG | 18 | 18 | 60.5 | 57.5 | 12/6 | 13/5 | ns | ns | 8 |
| van der Veen et al., 2021 [19] | Netherlands | 2015–2018 | Randomized trial | EGC, AGC | SG, TG | 115 | 112 | 67.9 | 66.9 | 68/47 | 72/40 | 12 | 12 | 4 * |
| Ramos et al., 2021 [52] | Brazil | 2009–2019 | Retrospective case-matched | EGC, AGC | SG, TG | 92 | 92 | ns | ns | 50/42 | 49/43 | 31 | 31 | 8 |
| van der Wielen et al., 2021 [18] | Netherlands | 2015–2018 | Randomized trial | EGC, AGC | TG | 47 | 49 | 59.4 | 61.8 | 28/19 | 32/17 | 12 | 12 | 4 * |
| Author Year | Total | Complications | Grading of Complications | Mortality | Readmissions | Reoperations | Duodenal Stump Leak | Anastomotic Leak | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LG | OG | LG | OG | LG | OG | LG | OG | LG | OG | LG | OG | LG | OG | LG | OG | ||
| Weber et al., 2003 [53] | 12 | 13 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Dulucq et al., 2005 [54] | SG | 16 | 17 | 2 | 3 | C.D. I/II 1 C.D. III/IV 1 | C.D. I/II 3 C.D. III/IV 0 | 0 | 1 | ns | ns | 1 | 0 | 1 | 0 | 0 | 0 |
| TG | 8 | 11 | 0 | 1 | C.D. I/II 0 C.D. III/IV 0 | C.D. I/II 1 C.D. III/IV 0 | 0 | 0 | ns | ns | 0 | 0 | 0 | 0 | 0 | 0 | |
| Huscher et al., 2005 [55] | 30 | 29 | 7 | 8 | C.D. I/II 7 C.D. III/IV 0 | C.D. I/II 7 C.D. III/IV 1 | 1 | 2 | ns | ns | ns | ns | 0 | 1 | 0 | 0 | |
| Pugliese et al., 2007 [56] | 48 | 99 | 10 | 14 | ns | ns | 2 | 3 | ns | ns | 0 | 0 | 2 | 1 | 0 | 2 | |
| Topal et al., 2007 [57] | 38 | 22 | 15 | 9 | TOSGS I/II 6 TOSGS III/V 9 | TOSGS I/II 6 TOSGS III/V 3 | 1 | 1 | ns | ns | 6 | 0 | 0 | 0 | 2 | 0 | |
| Sarela et al., 2009 [58] | 18 | 11 | ns | ns | ns | ns | 1 | 1 | ns | ns | 3 | 2 | 3 | 1 | 1 | 0 | |
| Strong et al., 2009 [61] | 30 | 30 | 8 | 13 | C.D. I/II 6 C.D. III/IV 2 | C.D. I/II 6 C.D. III/IV 7 | ns | ns | ns | ns | ns | ns | 0 | 0 | 0 | 1 | |
| Orsenigo et al., 2011 [62] | 109 | 269 | 30 | 52 | ns | ns | 3 | 4 | ns | ns | 11 | 6 | 20 | 14 | ns | ns | |
| Scatizzi et al., 2011 [63] | 30 | 30 | 2 | 8 | TOSGS I/II 2 TOSGS III/V 0 | TOSGS I/II 6 TOSGS III/V 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | |
| Sica et al., 2011 [31] | 22 | 25 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Moisan et al., 2012 [59] | 31 | 31 | 8 | 6 | ns | ns | ns | ns | ns | ns | 4 | 4 | 2 | 0 | 2 | 2 | |
| MacLellan et al., 2012 [64] | 21 | 182 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Singh et al., 2012 [65] | 72 | 57 | 32 | 18 | ns | ns | 4 | 2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Cianchi et al., 2013 [66] | 41 | 41 | 9 | 14 | ns | ns | 1 | 2 | ns | ns | 3 | 2 | 2 | 2 | 0 | 2 | |
| Mamidanna et al., 2013 [37] | 480 | 10233 | 208 | 2661 | ns | ns | 23 | 571 | 58 | 1044 | 22 | 409 | ns | ns | ns | ns | |
| Kelly et al., 2015 [38] | 87 | 87 | 27 | 42 | C.D. I/II 11 C.D. III/IV 16 | C.D. I/II 26 C.D. III/IV 16 | 1 | 0 | ns | ns | ns | ns | 6 | 4 | 4 | 4 | |
| Ramagen et al., 2015 [60] | 47 | 64 | 4 | 13 | ns | ns | 1 | 2 | 2 | 3 | 3 | 4 | ns | ns | 1 | 3 | |
| Castro et al., 2016 [39] | 63 | 144 | 8 | 35 | ns | ns | 1 | 9 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Malik et al., 2016 [40] | 114 | 161 | 31 | 48 | ns | ns | 5 | 5 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Brenkman et al., 2017 [41] | 277 | 1663 | ns | ns | ns | ns | 13 | 79 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Tegels et al., 2017 [42] | 52 | 25 | 16 | 15 | C.D. I/II 10 C.D. III/IV 6 | C.D. I/II 8 C.D. III/IV 7 | 1 | 1 | 6 | 4 | ns | ns | ns | ns | 2 | 10 | |
| Abbassi-Ghadi et al., 2018 [46] | 35 | 44 | 35 | 47 | C.D. I/II 30 C.D. III/IV. 5 | C.D. I/II 45 C.D. III/IV 2 | 0 | 0 | 3 | 1 | 3 | 1 | ns | ns | 0 | 1 | |
| Ludwig et al., 2018 [43] | 45 | 45 | 10 | 20 | C.D. I/II 6 C.D. III/IV 4 | C.D. I/II 17 C.D. III/IV 3 | 0 | 0 | ns | ns | 1 | 1 | 1 | 1 | 1 | 1 | |
| Rod et al., 2018 [44] | 60 | 104 | 34 | 48 | C.D. I/II 10 C.D. III/IV 24 | C.D. I/II 25 C.D. III/IV 23 | 0 | 3 | ns | ns | 16 | 6 | 8 | 10 | 10 | 10 | |
| Maida et al., 2019 [45] | 60 | 67 | 2 | 8 | C.D. I/II 1 C.D. III/IV 1 | C.D. I/II 7 C.D. III/IV 1 | 0 | 0 | ns | ns | 1 | 0 | 0 | 1 | ns | 0 | |
| Raakow et al., 2019 [47] | 81 | 162 | 22 | 64 | C.D. I/II 4 C.D. III/IV 18 | C.D. I/II 16 C.D. III/IV 48 | 2 | 3 | ns | ns | 5 | 22 | 1 | 1 | 4 | 9 | |
| Garbarino et al., 2020 [28] | 31 | 34 | 7 | 11 | C.D. I/II 5 C.D. III/IV 2 | C.D. I/II 5 C.D. III/IV 6 | 1 | 0 | ns | ns | 2 | 6 | 2 | 3 | 1 | 1 | |
| Tsekrekos et al., 2020 [48] | 77 | 129 | 49 | 88 | C.D. I/II 37 C.D. III/IV 12 | C.D. I/II 46 C.D. III/IV 42 | 0 | 3 | ns | ns | ns | ns | ns | ns | 1 | 18 | |
| Salehi et al., 2020 [49] | 3170 | 10368 | ns | ns | ns | ns | 78 | 397 | 205 | 791 | ns | ns | ns | ns | ns | ns | |
| Yalav et al., 2020 [51] | 18 | 89 | 7 | 18 | C.D. I/II ns C.D. III/IV 7 | C.D. I/II ns C.D. III/IV 12 | 0 | 10 | 3 | 18 | ns | ns | 2 | 3 | 2 | 4 | |
| Ammori et al., 2020 [50] | 18 | 18 | 3 | 4 | ns | ns | 0 | 0 | 0 | 1 | 1 | 0 | ns | ns | ns | ns | |
| Van der Veen et al., 2021 [19] | 115 | 112 | 50 | 46 | C.D. I/II 31 C.D. III/IV 14 | C.D. I/II 21 C.D. III/IV 17 | 12 | 10 | 11 | 10 | ns | ns | ns | ns | 10 | 11 | |
| Ramos et al., 2021 [52] | 92 | 92 | ns | ns | C.D. I/II ns C.D. III/IV 14 | C.D. I/II ns C.D. III/IV 11 | 6 | 4 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Van der Wielen et al., 2021 [18] | 47 | 49 | 16 | 21 | C.D. I/II 8 C.D. III/IV 8 | C.D. I/II 15 C.D. III/IV 4 | 0 | 2 | ns | ns | 1 | 2 | ns | ns | 4 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbarino, G.M.; Laracca, G.G.; Lucarini, A.; Piccolino, G.; Mercantini, P.; Costa, A.; Tonini, G.; Canali, G.; Muttillo, E.M.; Costa, G. Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes. J. Clin. Med. 2022, 11, 3590. https://doi.org/10.3390/jcm11133590
Garbarino GM, Laracca GG, Lucarini A, Piccolino G, Mercantini P, Costa A, Tonini G, Canali G, Muttillo EM, Costa G. Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes. Journal of Clinical Medicine. 2022; 11(13):3590. https://doi.org/10.3390/jcm11133590
Chicago/Turabian StyleGarbarino, Giovanni Maria, Giovanni Guglielmo Laracca, Alessio Lucarini, Gianmarco Piccolino, Paolo Mercantini, Alessandro Costa, Giuseppe Tonini, Giulia Canali, Edoardo Maria Muttillo, and Gianluca Costa. 2022. "Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes" Journal of Clinical Medicine 11, no. 13: 3590. https://doi.org/10.3390/jcm11133590
APA StyleGarbarino, G. M., Laracca, G. G., Lucarini, A., Piccolino, G., Mercantini, P., Costa, A., Tonini, G., Canali, G., Muttillo, E. M., & Costa, G. (2022). Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes. Journal of Clinical Medicine, 11(13), 3590. https://doi.org/10.3390/jcm11133590








