Abstract
Modic changes (MCs) are believed to be potential pain generators in the lumbar and cervical spine, but it is currently unclear if their presence affects postsurgical outcomes. We performed a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All studies evaluating cervical or lumbar spine postsurgical outcomes in patients with documented preoperative MCs were included. A total of 29 studies and 6013 patients with 2688 of those patients having preoperative MCs were included. Eight included studies evaluated cervical spine surgery, eleven evaluated lumbar discectomies, nine studied lumbar fusion surgery, and three assessed lumbar disc replacements. The presence of cervical MCs did not impact the clinical outcomes in the cervical spine procedures. Moreover, most studies found that MCs did not significantly impact the clinical outcomes following lumbar fusion, lumbar discectomy, or lumbar disc replacement. A meta-analysis of the relevant data found no significant association between MCs and VAS back pain or ODI following lumbar discectomy. Similarly, there was no association between MCs and JOA or neck pain following ACDF procedures. Patients with MC experienced statistically significant improvements following lumbar or cervical spine surgery. The postoperative improvements were similar to patients without MCs in the cervical and lumbar spine.
1. Introduction
Vertebral bone marrow edema is a recognized clinical entity that is believed to be associated with degenerative disc disease and back pain [1]. The aberrant signal changes identified with magnetic resonance imaging (MRI) were characterized into specific types by Modic et al., and were thus termed Modic changes (MC). Each distinct signaling phenotype is characterized by their unique signal intensity in MRI. Type I changes are characterized by hypointense T1- and hyperintense T2-weighted images, type II changes are recognized as hyperintense signaling on T1- and isointense to slightly hyperintense signaling on T2-weighted imaging, while type III changes are predominantly hypointense on the T1 and T2-weighted images [1]. Histological analysis has uncovered that the MRI signal intensity corresponds to anatomical changes. For example, type I MCs demonstrate bone marrow replacement with fibrovascular stroma, type II MCs are associated with fatty infiltration of the bone marrow, and type III MCs are characterized by sclerotic endplate changes [2,3]. Longitudinal studies have suggested that these changes exist on a continuum with transformations reported between all types, but most commonly with the progression of type I to type II [2,4,5,6,7,8]. Degenerative disc disease is the most frequently identified disease accompanying MCs [1,9,10], but other injury patterns associated with MCs include autoimmune disease, low virulence bacterial infections, and microtrauma resulting in endplate abnormalities [11,12,13].
Given that MCs are associated with advanced spondylosis, studies have attempted to associate MCs with axial neck and back pain [14,15,16]. Some authors have even attempted to correlate type I MC with accelerated degeneration of the adjacent intervertebral disc [17]. However, while the severity of type I MCs have been linked to worse pain symptoms in observational studies, systematic reviews have not consistently substantiated this finding [18]. A meta-analysis in 2016 attempted to identify trends in outcomes based on pre-operative MCs and their effect on surgical outcomes. While they found a trend toward worse improvement in lumbar discectomy patients who had MCs, they concluded that it was unlikely that the difference met the minimal clinically important difference (MCID) threshold [19]. However, no previous study has performed a meta-analysis on patients undergoing cervical spine surgery, and additional literature has been published on patients with MCs undergoing lumbar discectomy since 2016 [20,21,22,23]. Therefore, our objective was to evaluate the impact of MCs on neck and back pain in patients undergoing cervical or lumbar spine surgery.
This research received no external funding. All data are contained within the article. The authors declare no conflict of interest.
2. Materials and Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Table A1). This study was IRB exempt since only published studies were incorporated. A systematic literature review was performed of the PubMed database from its inception until 1 May 2022. “Modic changes” or “endplate signal changes” coupled with “outcomes” were queried. To ensure the inclusion of all of the available evidence, the references of each study meeting the inclusion criteria were searched to identify additional studies that merited inclusion.
2.1. Study Eligibility
Studies were included if MCs, identified in preoperative MRI, were operatively treated in either the cervical or lumbar spine. Studies were excluded if (1) clinical follow-up time was not reported or it was less than one year; (2) a full text manuscript could not be obtained; (3) the article was not written in or translated to English; or (4) the article was a letter to editor or systematic review.
Two reviewers independently screened the identified articles and selected studies for full-text review after screening of the title and abstract. Articles were screened for inclusion based on the predetermined inclusion/exclusion criteria during the full-text review. A search of the references was performed of all articles meeting the inclusion criteria to identify any potentially missed manuscripts. For studies where article inclusion was unclear, a more senior author was consulted to resolve any discrepancies.
2.2. Data Collection Quality Assessment
The authors extracted all potentially relevant data from the identified studies with multiple self-designed tables. Data including the type and prevalence of Modic signal changes, clinical outcome measures, patient population, procedure type, and key study findings were reported based on the affected region of the spine. Studies were assessed for bias using the validated Newcastle–Ottawa Score with a high-quality score defined as a score greater than or equal to 6 [24].
2.3. Meta-Analysis
Due to significant data heterogeneity, variability in the clinical outcome measures assessed, and the different surgical procedures performed in the lumbar spine, only a limited meta-analysis could be performed. For meta-analysis inclusion, a minimum of four studies were required to report on the same patient reported outcome (PRO) with only four PROs meeting this criteria (Visual Analog Scale (VAS) Back, VAS Neck, Oswestry Disability Index (ODI), and Japanese Orthopedic Association (JOA) score). The weighted mean difference in values between patients with and without MCs was then assessed using forest plots and data heterogeneity was calculated as an I2 value. The meta-analysis data were generated using R Studio Version 4.0.2 (Boston, MA, USA).
3. Results
The initial PubMed search identified 259 articles, after which the title and abstract screening identified 36 potentially relevant articles. Two were excluded due to an inadequate follow-up of less than one year [25,26], another two were excluded because of the risk of potentially identical patients [27,28], and one article’s full-text could not be obtained [29]. Thirty-one articles were identified after a full-text review. Two studies were classified as a high risk of bias, so they were also excluded [30,31]. In total, 29 articles were included in the final analysis (Figure 1). The characteristics of the studies included in the systematic review are presented in Table 1 and Table 2. The included articles comprised a total of 1871 patients with cervical spine disease, 683 (36.5%) of whom displayed some type of Modic change and 4142 patients with lumbar disease, 2005 (48.4%) of whom displayed some type of Modic change.
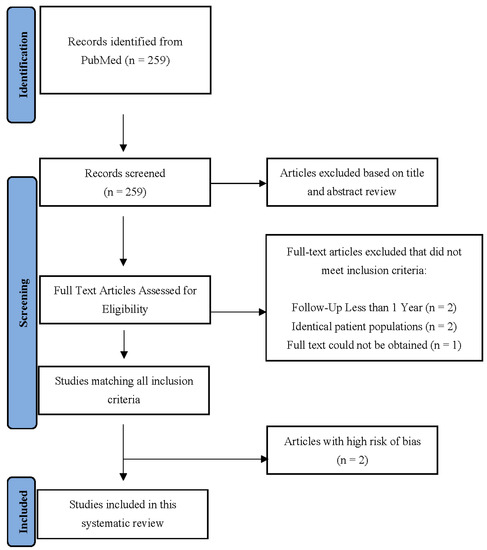
Figure 1.
The PRISMA flow diagram describing article selection for inclusion.

Table 1.
The patient characteristics of studies evaluating the cervical spine.
Table 1.
The patient characteristics of studies evaluating the cervical spine.
| Authors | Patient Population | Modic Subtypes | Age | Gender | Patients | Any MC (%) | MC-I (%) | MC-II (%) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [32] (2019) | Patients with cervical radiculopathy due to single-level disc herniation | I, II | 45.2 (7.3) | 131 | 223 | 41 (18.4%) | 10 (4.5%) | 29 (13%) | 7 |
| Baker et al. [33] (2020) | Patients with symptomatic degenerative pathology refractory to conservative management | I, II, III | NR | NR | 861 | 356 (41.3%) | 70 (8.1%) | 218 (25.3%) | 9 |
| Huang et al. [34] (2020) | Patients who underwent single-level ACDF with MC-II | II | 50.4 (1.6) | 58 | 116 | 24 (20.7%) | 0 | 24 (20.7%) | 8 |
| Li et al. [35] (2015) | Patients who underwent single-level ACDF with MC-II | II | 47.0 (7.2) | 134 | 248 | 35 (14.1%) | 0 | 35 (14.1%) | 7 |
| Li et al. [36] (2017) | Patients with chronic axial symptoms resulting from single-level radiculopathy or myelopathy | II | 56.1 (6.1) | 36 | 76 | 76 (100%) | 0 | 76 (100%) | 7 |
| Zhou et al. [37] (2018) | Patients with cervical spondylotic myelopathy | NR | 56.1 (7.3) | 56 | 117 | 28 (23.9%) | NR | NR | 8 |
| Li et al. [38] (2022) | Patients with MCs cervical spondylotic myelopathy with hernia behind the vertebrae or OPLL | I, II | 55.0 (22.2) | 67 | 124 | 61 (49.2%) | 20 (16.1%) | 41 (33.1%) | 6 |
| Li et al. [39] (2015) | Patients with chronic axial symptoms resulting from single-level cervical disk degeneration nonresponsive to appropriate nonsurgical treatment for at least 6 months | I, II | 55.8 (6.5) | 49 | 106 | 62 (58.5%) | 23 (21.7%) | 39 (36.8%) | 7 |
MC—Modic changes; MC-I—type I Modic change; MC-II—type II Modic change; ACDF—anterior cervical discectomy and fusion; OPLL—ossification of the posterior longitudinal ligament; NR—not reported.

Table 2.
The patient characteristics of studies evaluating the lumbar spine.
Table 2.
The patient characteristics of studies evaluating the lumbar spine.
| Authors | Patient Population | MC Subtypes | Age | Gender | Patients | Any MC (%) | MC-I (%) | MC-II (%) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Kumarasamy et al. [21] (2021) | Patients with LBP and single level lumbar disc herniation | I, II | 42.5 (12.6) | 107 | 309 | 86 (27.8%) | 6 (1.9%) | 68 (22%) | 7 |
| Jiao et al. [40] (2021) | Patients with LBP and either LDH, spinal stenosis, or spondylolisthesis who underwent single-segment TLIF with a PEEK cage | I, II | 56.7 (8.9) | 49 | 89 | 51 (57.3%) | 20 (22.5%) | 31 (60.8%) | 6 |
| el Barzouhi et al. [41] (2014) | Patients with sciatica | I, II | 43.2 (10.1) | 140 | 263 | 112 (42.6%) | 4 (1.5%) | 106 (40.3%) | 8 |
| Ulrich et al. [42] (2020) | Patients with claudication and lumbar stenosis | I, II | 66.8 (6.3) | 96 | 205 | 143 (69.8%) | 22 (15.4%) | 93 (65.0%) | 8 |
| Chung et al. [43] (2021) | Patients with lumbar DDD | I, II, III | 64.7 (9.1) | 54 | 86 | NR | NR | NR | 7 |
| MacLean et al. [23] (2021) | Patients with single level LDH | I, II, III | 53 (13) | 101 | 179 | 110 (61.5%) | 28 (15.6%) | 63 (35.2%) | 7 |
| Udby et al. [44] (2020) | Patients with bilateral or unilateral radiculopathy | I, II | 50.5 | 310 | 620 | 290 (46.8%) | 73 (11.8%) | 217 (35%) | 7 |
| Sørlie et al. [45] (2012) | Patients with one-level lumbar disc herniation | I, II | 41.2 (12.1) | 66 | 178 | 104 (58.4%) | 36 (20.2%) | 68 (38.2%) | 8 |
| Gornet et al. [46] (2014) | Patients with back pain due to DDD with pre-op ODI ≥ 30 | I, II | NR | NR | 89 | NR | NR | NR | 8 |
| Ohtori et al. [47] (2010) | Patients with LBP and leg pain due to lumbar spinal canal stenosis | I, II | 65.4 | 16 | 33 | 33 (100%) | 21 (63.6%) | 12 (36.4%) | 6 |
| Cao at al [48] (2014) | Patients with one-level LDH and MCs | I, II | NR | NR | 91 | 91 (100%) | 42 (46.2%) | 60 (65.9%) | 7 |
| Lurie et al. [49] (2013) | Patients with radicular pain due to intervertebral disc herniation | I, II | 41.7 (11.4) | 522 | 307 | 80 (26.1%) | 27 (8.8%) | 53 (17.3%) | 7 |
| Xu et al. [50] (2019) | Patients with unilateral radicular pain due to one-level intracanal disc herniation | I, II | 40.0 (12.5) | 104 | 276 | 94 (34.1%) | 44 (15.9%) | 50 (18.1%) | 6 |
| Djurasovic et al. [51] (2012) | Patients with “disc pathology” listed as primary surgical indication | I, II, III | 47 | 23 | 51 | NR | NR | NR | 7 |
| Masala et al. [52] (2014) | Patients with LBP without radicular symptoms unresponsive to conservative therapy for 6 months with type I MC | I | 40.3 (8.2) | 133 | 218 | 218 (100%) | 218 (100%) | 0 | 6 |
| Ohtori et al. [53] (2010) | Patients with one-level LDH | I | 35.5 | 19 | 45 | 23 (51.1%) | 23 (51.1%) | 0 | 6 |
| Rahme et al. [54] (2010) | Patients with one-level LDH | I, II, III | 54 | 14 | 41 | 32 (78%) | 6 (14.6%) | 26 (63.4%) | 7 |
| Blondel et al. [55] (2011) | Patients with chronic LBP due to single-level DDD | I, II | 42.1 | 101 | 221 | 114 (51.6%) | 65 (29.4%) | 49 (22.2%) | 8 |
| Gautschi et al. [56] (2016) | Patients with LBP due to disc herniation, spinal stenosis or DDD requiring lumbar fusion | I, II, III | 58.6 (15.5) | 144 | 338 | 202 (59.8%) | NR | NR | 7 |
| Hellum et al. [57] (2012) | Patients with LBP due to LDD with an ODI ≥ 30% | I, II | 41.2 (7.0) | 81 | 152 | 131 (85%) | 48 (31.6%) | 55 (36.2%) | 9 |
| Kwon et al. [58] (2009) | Patients who underwent PLIF | I, II, III | 47.4 | 232 | 351 | 92 (26.2%) | 26 (7.4%) | 55 (15.7%) | 7 |
MC—Modic changes; MC-I—type I Modic changes; MC-II—type II Modic change; LBP—low back pain; LDH—lumbar disc herniation; TLIF—transforaminal lumber interbody fusion; PEEK—polyetheretherketone; DDD—degenerative disc disease; ODI—Oswestry Disability Index; LDD—lumbar disc disease; PLIF—posterior lumbar interbody fusion; NR—not reported.
3.1. Cervical Spine
Eight retrospective studies described the correlations between MC and surgical outcomes (Table 3). No prospective studies evaluated MC in the cervical spine. In general, studies did not report a difference in the PROs based on the presence of MC.

Table 3.
The key findings of surgery in patients with cervical Modic changes.
Huang et al. [34] reviewed 116 cases of single-level anterior cervical discectomy and fusions (ACDF) for the presence of MC and their association with fusion rates and PROs. Patients with type II MC experienced significantly delayed early fusion rates at 3- and 6-months, but the fusion rates were similar at one year. There were no significant differences between groups with regard to improvement in the VAS scores or JOA scores.
In a study by Li et al. [38] in 2022, 124 patients underwent single-level anterior cervical corpectomy and fusion for cervical myelopathy. They found that 41% of patients with MC had cage subsidence, defined as at least 1 mm, compared to only 15.9% of patients without MC (p = 0.003). Subsidence did not vary between the Modic subtypes. Patients with type I MC had a higher proportion of partial fusions, defined by incomplete bony remodeling (40% compared to 11% of controls). No cases of pseudarthrosis were observed and no other differences were identified with regard to the JOA, neck disability index (NDI), and VAS neck and arm pain scores.
Yang et al. [32] retrospectively reviewed patients who underwent one-level ACDFs or cervical disc arthroplasty. The presence of MCs did not result in different one-year VAS neck, NDI, or physical component summary (PCS-12) and mental component summary (MCS-12) scores from the 36-Item Short Form Health Survey (SF-36).
In a 2017 study by Li et al. [36], 72 patients were analyzed with type II MC and minimum five-year follow-up. Of those, 35 received cervical disc arthroplasty and 37 underwent ACDF for myelopathy or radiculopathy. All patients experienced postoperative improvement assessed by modified JOA (mJOA), NDI, and VAS, without differences between groups. The patients in the disc replacement group were noted to have improved range of motion at final follow-up.
In 2015, Li et al. [35] retrospectively compared 35 patients who had a single-level ACDF with type II MC at an adjacent level to 213 patients without type II MC. They observed no significant differences between groups with regard to the range of motion or disc height at adjacent levels, and no differences in improvement by the mJOA and NDI scores. The patients in the MC group had worse VAS neck pain at one-year follow-up (p < 0.05), although these differences resolved by 5-year follow-up.
A retrospective review of 117 patients with a single-level ACDF found that preoperative MCs at levels adjacent to surgery were associated with greater axial symptoms (p = 0.015) [37]. However, all patients demonstrated clinical improvement as assessed by the JOA without a significant difference between patients who did and did not have MCs.
In a separate 2015 study by Li et al. [39], 106 patients were retrospectively identified with one-level ACDFs for cervical spondylotic myelopathy. Preoperatively, 23 patients had type I MC, 28 had type II MC, and 44 had no MC. The patients in the type I MC group had significantly lower VAS neck pain at 24 months after surgery (1.5 ± 1.1) compared to patients without (MC 2.0 ± 1.5), although a specific p-value was not provided.
Baker et al. [33] retrospectively examined the records of 861 patients who underwent a one- to four-level ACDF for radiculopathy or myelopathy. Of those, 365 patients had MCs. No significant differences in postoperative NDI, VAS-neck, or VAS-arm were found between groups. However, after stratification by cervical level, they identified the presence of MC at C7–T1 predicted worse postoperative NDI (p < 0.001).
A limited number of studies collected PROs in a standardized manner, thus, a meta-analysis could only be performed for postoperative values. Four studies reported on neck pain and JOA following ACDF [34,35,38,39]. Random effects analysis of these studies demonstrated no significant association between MC and postoperative VAS neck scores (MD −0.17, 95% CI: −0.50–1.70) (Figure 2a) or the JOA scores (MD −0.07, 95% CI: −0.35–0.20) (Figure 2b). Among these studies, moderate heterogeneity in neck pain scores (I2 = 52%) and low heterogeneity in JOA scores (I2 = 0%) were observed.
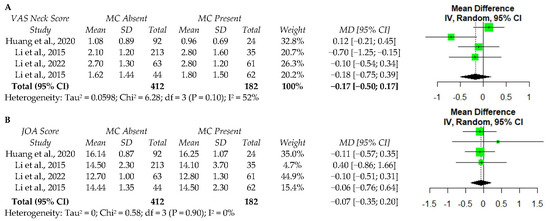
Figure 2.
The meta-analysis of one-year postoperative VAS neck score (A) and JOA score (B) following ACDF. VAS—visual analog scale; MC—Modic changes; SD—standard deviation; MD—Mean difference; CI—confidence interval; JOA—Japanese Orthopaedic Association score; df—degrees of freedom; IV—independent variable [34,35,38,39].
3.2. Lumbar—Discectomy/Microdiscectomy
There were a total of 11 articles discussing the outcomes after discectomy or microdiscectomy with three articles comparing those procedures to lumbar fusion (Table 4). Regardless of the presence of MC, patients experienced significant improvement following surgery. However, the studies delivered mixed results as to whether MCs modified the magnitude of benefit obtained from surgery.

Table 4.
The key findings of surgery in patients with lumbar Modic changes.
El Barzouhi et al. [41] conducted a multicenter, randomized trial of 283 patients with sciatica. They compared microdiscectomy to conservative care. Of the 283 patients, 41 percent of patients had vertebral endplate signal changes (VESCs). No significant differences in the amount of recovery or disabling back pain were identified between patients with and without VESCs at one year follow-up.
Gautschi et al. [56] prospectively identified 338 patients with lumbar degenerative disc disease. Of the 338 patients, 175 of them had a microdiscectomy. Similar outcomes for VAS back and leg pain, ODI, Roland–Morris Disability Index, Timed Up and Go test, EuroQol-5D (EQ-5D), and PCS-12 were identified between patients in the MC and no-MC cohorts.
Kumarasamy et al. [21] prospectively followed 309 patients undergoing microdiscectomy, 86 of whom had MC. The patients in the MC group had worse postoperative numeric rating scale (NRS) back pain (1.6 to 1.1, p = 0.001), although the overall improvement in both groups was 4.3 points, indicating that there was no difference in the magnitude of overall improvement. Both groups experienced a significant improvement in disability, but the group without MC experienced statistically significant greater improvement, although this did not meet the MCID threshold. Patient satisfaction, as evaluated by the MacNab criteria, was noted to be significantly worse for patients with MCs.
Sørlie et al. [45] prospectively evaluated 178 patients undergoing microdiscectomy and found that 36 patients had type I MCs. Multivariate analysis demonstrated no statistical differences between groups for VAS back and leg pain, ODI, and EQ-5D. All patients had significant improvement after surgery, although patients who smoked and had type I MCs had less improvement.
Rahme et al. [54] retrospectively identified patients treated with lumbar microdiscectomy. They included 41 patients in the final analysis and identified 19 patients with preoperative MC at the level of the operation. At median follow-up of 41 months, 32 patients had MCs (since four of the 22 patients without MCs converted to type I MCs and another nine converted to type II MCs). Additionally, 60% of the type I MCs converted to type II MCs. The study concluded that the majority of patients after a lumbar discectomy will convert to type II MCs, but MCs do not results in worse outcomes, recurrent symptoms, or decreased satisfaction.
Ohtori et al. [53] prospectively identified 23 patients with type I MC undergoing lumbar discectomy and matched them to 22 patients without MC. All patients exhibited improvement in VAS back, JOA, and ODI with no significant differences between the groups at 12 or 24 months after surgery.
MacLean et al. [23] retrospectively analyzed 129 patients undergoing discectomy for lumbar radiculopathy with 77 patients having MCs. They had complete outcome data for 96 of these patients and concluded each group experienced statistically and clinically significant improvement as assessed by the PCS-12, ODI, and VAS leg pain scores. Most subgroups met the MCID and there were no significant differences in clinical improvement based on the presence or absence of MCs.
Lurie et al. [49] retrospectively reviewed 307 patients treated with discectomy for radicular pain and identified 81 patients with MC. The patients with type I MC improved significantly less in ODI compared to the type II or no MC patients. However, patients with type I MC did experience an average improvement in ODI of 26.4 points following discectomy.
Udby et al. [44] retrospectively reported on 620 patients undergoing lumbar discectomy for radiculopathy with 290 patients having MC. At two year follow-up, they concluded that both groups had significant improvement by ODI, EQ-5D, VAS back and leg pain, with no significant differences between groups.
Cao et al. [48] retrospectively reviewed 91 patients with lumbar disc herniation and MC who had a combination of low back and radicular leg pain. A total of 47 patients were treated with discectomy, while 44 underwent a posterior lumbar interbody fusion (PLIF). All patients had significant VAS back pain improvement; however, the patients in the PLIF group experienced greater VAS back improvement. Both groups had significant VAS leg pain improvement and there was no postoperative difference based on the type of surgery.
Xu et al. [50] retrospectively reviewed 276 patients undergoing percutaneous endoscopic lumbar discectomy for radiculopathy and identified 94 patients with MC. They found no differences in VAS leg pain through final follow-up of 30 months. At three months follow-up, all patients had similar improvement in ODI and VAS back pain, however, the patients with MCs were found to have worse trending ODI and VAS back pain at final follow-up.
Ulrich et al. [42] retrospectively identified 205 patients undergoing lumbar discectomy or lumbar fusion. They found no differences in the outcomes between patients with MCs versus those without MCs, regardless of the procedure type when assessing the Spinal Stenosis Measure, EQ-5D, and NRS back pain scores at the 36 month post-surgical visit. Over 70% of patients reached the postoperative MCID at 36 month follow-up. Thus, the study concluded that MCs do not significantly affect the postoperative clinical outcomes.
Five studies reported values for ODI [21,23,44,45,50], and four studies reported VAS back pain [21,44,45,50]. A meta-analysis was performed among these studies and identified no significant associations between MC and either postoperative VAS back pain or ODI (Figure 3). However, significant data heterogeneity was present for VAS and ODI (I2 = 88% and I2 = 96%, respectively), indicating that additional high quality studies are required to confirm the finding that MCs do not affect the improvement in VAS back or ODI scores postoperatively.
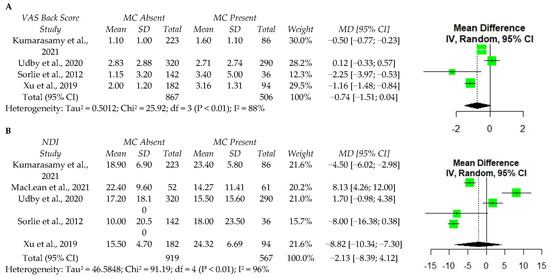
Figure 3.
A meta-analysis of one year postoperative VAS back score (A) and ODI (B) following discectomy. VAS—visual analog scale; MC—Modic changes; SD—standard deviation; MD—Mean difference; CI—confidence interval; NDI—Neck Disability Index; df—degrees of freedom; IV—independent variable [21,23,44,45,50].
3.3. Lumbar Fusion
Nine studies evaluating lumbar fusion surgery were identified. One study evaluated oblique lateral interbody fusions (OLIF) [43], two evaluated transforaminal lumbar interbody fusions (TLIFs) [23,40], two included multiple fusion techniques [51,56], two evaluated PLIF [42,47], and two evaluated posterolateral fusions [48,58]. In general, patients had substantial improvements in the clinical outcomes and fusion rates regardless of the presence of MCs, with the exception of one study, which found decreased fusion rates and worse outcomes in patients with type III MCs [58].
Chung et al. [43] retrospectively analyzed 86 patients with 125 operated levels by one- or two-level OLIF and identified MC at 72 of these levels. They found no association between the MC and fusion rate or cage subsidence at 28 months after surgery.
Jiao et al. [40] retrospectively identified 89 patients, 51 with MC, who underwent single-level TLIFs. The authors found no difference in the fusion rate or clinical outcomes based on MCs, but type I MCs were associated with significantly higher rates of cage subsidence (40% to 15.8%). All other outcome measures were similar between groups including disc height, segmental lordosis, and lumbar lordosis.
MacLean et al. [23] retrospectively reviewed 44 patients who underwent TLIF for radiculopathy and instability. They concluded that patients experienced significant improvement by PCS-12, ODI, and VAS leg pain regardless of the presence of MCs.
Two studies reported on multiple fusion techniques: TLIF, PLIF, PLF, extreme lateral interbody fusion, circumferential fusion, and anterior lumbar interbody fusion [51,56]. Between these studies, 121-disc levels were included in their analysis with 73 levels having MCs. No outcome measures were significantly affected by MCs including ODI, VAS back and leg, or SF-36.
Of the two studies reporting on decompression with PLIF, Ohtori et al. [47] prospectively evaluated 33 patients with MC while Ulrich et al. [42] retrospectively identified 57 patients undergoing lumbar fusion with 41 having MCs and 16 having no MCs. All patients in the study by Ohtori et al. [49] had solid arthrodesis at 24 months after surgery with average union at nine months. Neither study found differences in the VAS nor ODI based on the presence of MCs.
For patients undergoing posterolateral fusion, Kwon et al. [58] retrospectively reviewed 351 patients and identified MCs in 92 while Cao et al. [48] retrospectively identified 44 patients with MCs. Kwon et al. reported lower fusion rates for patients with type III MCs (54.5%) compared to those without MCs (96.5%) at a minimum three years follow-up. They also identified less improvement for those with type III MC when evaluated by Prolo’s scale and VAS scores. Cao et al. reported that all patients had significant improvement in the VAS back, VAS leg, and JOA.
3.4. Other Lumbar Surgeries
We identified four articles in our search that did not fit into the above categories. Three of these studies reported on disc arthroplasty while the other study reported on vertebroplasty. Masala et al. [52] prospectively identified 218 patients with type I MC who underwent vertebroplasty. Of those, 98% showed improvement in VAS and ODI, 1% showed no improvement, and 1% died for unrelated reasons. Three prospective studies evaluated lumbar disc arthroplasty [46,55,57]. They all reported improved ODI in patients with MC compared to those without. Gornet et al. [46] reported these findings for type II MC, Blondel et al. [55] reported these findings for type I MC, and Hellum et al. [57] reported these findings for both type I and II MCs.
4. Discussion
Radiographic evidence of intervertebral disc degeneration is reported to be present in greater than 90% of individuals by the time they reach 50 years of age [59]. Most patients with disc degeneration remain asymptomatic, but within the asymptomatic population, 21% of patients older than 60 have spinal stenosis, while 36% have a herniated intervertebral disc [60]. Therefore, identifying potential factors that may result in back or neck pain is prudent [61]. Modic changes are one potential etiology for symptomatic back and neck pain, thus an evaluation of their potential improvement after surgery is indicated. Although the heterogeneity in the literature precluded our team from performing a meta-analysis of all of the included studies, the majority of studies assessing the surgical outcomes after cervical and lumbar spine surgery have not detected clinically important differences between patients with and without Modic changes. Despite heterogeneity, our meta-analysis similarly identified no differences between postoperative VAS back pain or ODI in patients with MC undergoing lumbar discectomy. There was less data heterogeneity in patients undergoing ACDF, and our meta-analysis found no differences in postoperative VAS neck pain or JOA based on the presence of MCs.
Surgeons perform over 100,000 ACDFs each year [62] and MCs are present at rates surpassing 40% in patients greater than 50 years of age who undergo cervical spine MRI [63]. The results of our pooled analysis found that MCs were present in a similar 36.5% of patients who required cervical spine surgery. Thus, understanding the potential indicators of surgical success (e.g., presence or absence of MCs) is important for risk stratification and guiding the patient’s expectations on their potential postoperative clinical improvement. When evaluating the clinical outcomes, our pooled data suggest that MCs are not predictive of postoperative improvement in patients undergoing cervical spine surgery. While one study reported MCs at the level C7–T1 were associated with worse outcomes, this group comprised 1.2% of their overall patient population and was likely severely underpowered to detect a true difference [33]. In contrast, Li et al. [39] reported that patients with type I MC were more likely to have reduced back pain at 2.5 year follow-up. Given that the available data are based on retrospective studies, with approximately half the patients coming from one study [33], it is likely that additional well-designed studies are needed to definitively claim that MCs have no significant clinical effect on cervical spine postsurgical outcomes. However, the evaluated studies do pose a low risk of bias, and the current literature suggests that MCs do not significantly impact the clinical or surgical success of cervical spine surgery [34,38].
Seven studies evaluated the microdiscectomy or discectomy outcomes in relation to MC. While discectomy and microdiscectomy overall appear efficacious as a treatment for axial back pain reduction and PRO improvement for patients with MCs, those with type I MC may experience reduced improvements following discectomy. Only one study evaluating microdiscectomy identified worse improvement in patients with MCs, although this was not clinically significant [21]. Two other retrospective studies identified a relationship between MC and worse PRO improvement (ODI and VAS back) following discectomy. Although these studies identified discectomy as an effective treatment option for patients with MC, patients with type I MC experienced less improvement at long-term follow-up [49,50]. Type I MC likely represents inflammatory changes, which may be linked to exacerbations of low back pain. Despite the propensity of MC to transform to other types, they have also been shown to remain constant over time [64]. Although discectomy may alleviate discogenic pain, it may have little effect on the concomitant changes to the vertebral endplate. This may be one potential reason why patients with MC-I do not exhibit as robust of an improvement when compared to other patients. While the results of our meta-analysis indicated no differences in back pain or disability, significant heterogeneity and the limited number of studies prohibit strong conclusions, and more research is needed, especially comparing the improvement across MC types.
We identified eight retrospective studies and one prospective study reporting MC in lumbar fusion surgery. These studies demonstrated that MC do not influence patient-reported outcomes or fusion rates following lumbar fusion surgery regardless of approach. Jiao et al. [40] identified that patients with type I MC experienced greater improvement in PROs following TLIF. Kwon et al. [58] reported lower fusion rates and worse PROs in type III MC. They suggested that given the sclerotic nature of type III MC, additional fusion such as pedicle screw fixation may be required for patients with type III changes [58]. Although this sclerotic bone may interfere with fusion, resorption of the sclerotic bone has been observed over time [65]. Care should be taken when selecting patients for surgery with MC-III because this population remains understudied due to its low prevalence. While lumbar disc replacement was evaluated in three prospective analyses and all three demonstrated excellent results that are not affected by the presence of MC, further research is needed to analyze the impacts of disc arthroplasty on endplate disease.
5. Conclusions
Since the last review on this topic in 2016, several prospective and large retrospective studies have been published with a low risk of bias [19]. After summarizing all of the available data on surgical patients with MCs, quantitative and qualitative analyses suggest that patients with MCs have similar clinical improvements in PROs (ODI and VAS back/neck) and similar fusion rates when compared to patients without MCs. However, additional high-quality studies are needed to further elucidate changes in the fusion status, especially among patients with type III MC. Furthermore, long-term prospective studies evaluating the outcomes of patients with type I MC undergoing discectomy are merited given that some studies indicate that they have worse clinical improvement after surgery.
Author Contributions
All authors contributed significantly to the final production of this manuscript. The authors contributed in the following ways. Conceptualization: M.J.L., T.Z.I. and G.R.T.; Methodology: M.J.L. and T.Z.I.; Validation: M.J.L., P.B., T.Z.I., J.C.H., A.S. and M.M.S.; Formal analysis: M.J.L., P.B. and T.Z.I.; Investigation: M.J.L., P.B., T.Z.I., A.S. and M.M.S.; Data curation: M.J.L., P.B., T.Z.I., A.S. and M.M.S.; Original draft preparation: M.J.L., P.B., T.Z.I. and J.C.H.; Review and editing; M.J.L., T.Z.I., J.C.H., G.R.T., J.A.C., C.K.K. and A.R.V.; Visualization: M.J.L., J.A.C., C.K.K. and A.R.V.; Supervision: M.J.L., J.A.C., C.K.K. and A.R.V.; Project administration: M.J.L., J.A.C., C.K.K. and A.R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional review board approval was not obtained as this research did not involve human subjects.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is contained within the article or appendix.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
PRISMA Checklist.
Table A1.
PRISMA Checklist.
| Section/Topic | Number | Checklist Item | Reported on Page Number |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2,3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 2 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 2,3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 3 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 3 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 3 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 3 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | NA |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 3 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 3–16 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 3, Table 1 and Table 2 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 3–16, tables |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 9, 15 (Figure 2 and Figure 3) |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 3, Table 1 and Table 2 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., health care providers, users, and policy makers). | 16, 17 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 16, 17 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 16, 17 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 2 |
References
- De Roos, A.; Kressel, H.; Spritzer, C.; Dalinka, M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. Am. J. Roentgenol. 1987, 149, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef]
- Modic, M.T.; Masaryk, T.J.; Ross, J.; Carter, J.R. Imaging of degenerative disk disease. Radiology 1988, 168, 177–186. [Google Scholar] [CrossRef]
- Mitra, D.; Cassar-Pullicino, V.N.; Mccall, I.W. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur. Radiol. 2004, 14, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Teraguchi, M.; Hashizume, H.; Oka, H.; Cheung, J.P.; Samartzis, D.; Muraki, S.; Akune, T.; Kawaguchi, H.; Nakamura, K.; et al. A Prospective, 3-year Longitudinal Study of Modic Changes of the Lumbar Spine in a Population-based Cohort. Spine 2022, 47, 490–497. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Finnin, M.A.; Wang, Y.; Wluka, A.E.; Urquhart, D.M.; O’Sullivan, R.; Jones, G.; Cicuttini, F.M. The natural history of Modic changes in a community-based cohort. Jt. Bone Spine 2017, 84, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, J.; Karppinen, J.; Niinimäki, J.; Haapea, M.; Grönblad, M.; Luoma, K.; Rinne, E. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet. Disord. 2015, 16, 98. [Google Scholar] [CrossRef]
- Kuisma, M.; Karppinen, J.; Niinimäki, J.; Kurunlahti, M.; Haapea, M.; Vanharanta, H.; Tervonen, O. A Three-Year Follow-up of Lumbar Spine Endplate (Modic) Changes. Spine 2006, 31, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.B.; Manniche, C. Modic changes following lumbar disc herniation. Eur. Spine J. 2007, 16, 977–982. [Google Scholar] [CrossRef]
- Karchevsky, M.; Schweitzer, M.E.; Carrino, J.A.; Zoga, A.; Montgomery, D.; Parker, L. Reactive endplate marrow changes: A systematic morphologic and epidemiologic evaluation. Skelet. Radiol. 2005, 34, 125–129. [Google Scholar] [CrossRef]
- Brandt, J.; Haibel, H.; Cornely, D.; Golder, W.; Gonzalez, J.; Reddig, J.; Thriene, W.; Sieper, J.; Braun, J. Successful treatment of active ankylosing spondylitis with the anti–tumor necrosis factor α monoclonal antibody infliximab. Arthritis Care Res. 2000, 43, 1346–1352. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, X.; Ren, H.; Huang, B.; Chen, J.; Liu, J.; Shan, Z.; Zhu, Z.; Zhao, F. Low virulence bacterial infections in cervical intervertebral discs: A prospective case series. Eur. Spine J. 2018, 27, 2496–2505. [Google Scholar] [CrossRef]
- Crockett, M.T.; Kelly, B.S.; Van Baarsel, S.; Kavanagh, E.C. Modic Type 1 Vertebral Endplate Changes: Injury, Inflammation, or Infection? Am. J. Roentgenol. 2017, 209, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Fujiwara, H.; Nishiwaki, Y.; Daimon, K.; Okada, E.; Nojiri, K.; Watanabe, M.; Katoh, H.; Shimizu, K.; Ishihama, H.; et al. Modic changes in the cervical spine: Prospective 20-year follow-up study in asymptomatic subjects. J. Orthop. Sci. 2019, 24, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Maigne, J.-Y.; Genevay, S.; Marty, M.; Gandjbakhch, F.; Rozenberg, S.; Foltz, V. Inflammatory pain pattern and pain with lumbar extension associated with Modic 1 changes on MRI: A prospective case–control study of 120 patients. Eur. Spine J. 2013, 23, 493–497. [Google Scholar] [CrossRef]
- Luoma, K.; Vehmas, T.; Kerttula, L.; Grönblad, M.; Rinne, E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur. Spine J. 2016, 25, 2873–2881. [Google Scholar] [CrossRef]
- Kerttula, L.; Luoma, K.; Vehmas, T.; Grönblad, M.; Kääpä, E. Modic type I change may predict rapid progressive, deforming disc degeneration: A prospective 1-year follow-up study. Eur. Spine J. 2012, 21, 1135–1142. [Google Scholar] [CrossRef]
- Herlin, C.; Kjaer, P.; Espeland, A.; Skouen, J.S.; Leboeuf-Yde, C.; Karppinen, J.; Niinimäki, J.; Sørensen, J.S.; Storheim, K.; Jensen, T.S. Modic changes—Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS ONE 2018, 13, e0200677. [Google Scholar] [CrossRef]
- Laustsen, A.F.; Bech-Azeddine, R. Do Modic changes have an impact on clinical outcome in lumbar spine surgery? A systematic literature review. Eur. Spine J. 2016, 25, 3735–3745. [Google Scholar] [CrossRef]
- Djuric, N.; Yang, X.; Ostelo, R.W.J.G.; van Duinen, S.G.; Nijeholt, G.J.L.; van der Kallen, B.F.W.; Peul, W.C.; Vleggeert-Lankamp, C.L.A. Disc inflammation and Modic changes show an interaction effect on recovery after surgery for lumbar disc herniation. Eur. Spine J. 2019, 28, 2579–2587. [Google Scholar] [CrossRef]
- Kumarasamy, D.; Rajasekaran, S.; Anand, K.S.S.V.; Soundararajan, D.C.R.; Shetty, T.A.P.; Kanna, P.R.M.; Pushpa, B.T. Lumbar Disc Herniation and Preoperative Modic Changes: A Prospective Analysis of the Clinical Outcomes After Microdiscectomy. Glob. Spine J. 2021, 12, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Karis, D.S.; Vleggeert-Lankamp, C.L. Association between Modic changes, disc degeneration, and neck pain in the cervical spine: A systematic review of literature. Spine J. 2019, 20, 754–764. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.A.; Kureshi, N.; Shankar, J.; Stewart, S.A.; Christie, S.D. Modic Change and Clinical Assessment Scores in Patients Undergoing Lumbar Surgery for Disk Herniation. Clin. Spine Surgery A Spine Publ. 2020, 34, E205–E210. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 June 2022).
- Shahmohammadi, M.R.; Behrouzian, S. Effect of preoperative modic change in the outcome of patients with low back pain following posterior spinal fusion or laminectomy. Asian J. Neurosurg. 2019, 14, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Fujita, N.; Hosogane, N.; Hikata, T.; Watanabe, K.; Tsuji, O.; Nagoshi, N.; Yagi, M.; Kaneko, S.; Fukui, Y.; et al. Presence of Modic type 1 change increases risk of postoperative pyogenic discitis following decompression surgery for lumbar canal stenosis. J. Orthop. Sci. 2017, 22, 988–993. [Google Scholar] [CrossRef]
- Ba, J.D.B.; Sayari, A.J.; Harada, G.K.; Tao, Y.; Louie, P.K.; Basques, B.A.; Galbusera, F.; Niemeyer, F.; Wilke, H.; An, H.S.; et al. The Modic-endplate-complex phenotype in cervical spine patients: Association with symptoms and outcomes. J. Orthop. Res. 2021, 40, 449–459. [Google Scholar] [CrossRef]
- Baker, J.D.; Sayari, A.J.; Tao, Y.; Louie, P.K.; Basques, B.A.; Galbusera, F.; Niemeyer, F.; Wilke, H.; An, H.S.; Samartzis, D. Endplate abnormalities, Modic changes and their relationship to alignment parameters and surgical outcomes in the cervical spine. J. Orthop. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Erinç, S.; Talmaç, M.A.; Kemah, B.; Özdemir, M.H. The effect of modic changes on the fusion rates of posterior interbody fusion surgery modic changes and posterior interbody fusion. J. Neurosurg. Sci. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Esposito, P.; Pinheiro-Franco, J.; Froelich, S.; Maitrot, D. Predictive value of MRI vertebral end-plate signal changes (MODIC) on outcome of surgically treated degenerative disc disease: Results of a cohort study including 60 patients. Neurochirurgie 2006, 52, 315–322. [Google Scholar] [CrossRef]
- Ghodsi, S.M.; Rouhani, R.; Abdollahzade, S.; Khadivi, M.; Jouibari, M.F. Frequency of Vertebral Endplate Modic Changes in Patients with Unstable Lumbar Spine and Its Effect on Surgical Outcome. Asian Spine J. 2015, 9, 737–740. [Google Scholar] [CrossRef]
- Yang, X.; Donk, R.; Arts, M.P.; Vleggeert-Lankamp, C.L. Are Modic Vertebral End-Plate Signal Changes Associated with Degeneration or Clinical Outcomes in the Cervical Spine? World Neurosurg. 2019, 129, e881–e889. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.D.; Harada, G.K.; Tao, Y.; Louie, P.K.; Basques, B.A.; Galbusera, F.; Niemeyer, F.; Wilke, H.-J.; An, H.S.; Samartzis, D. The Impact of Modic Changes on Preoperative Symptoms and Clinical Outcomes in Anterior Cervical Discectomy and Fusion Patients. Neurospine 2020, 17, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Hong, Y.; Liu, H.; Duan, Y.; Wang, B.; Chen, H.; Ding, C.; Rong, X.; Wu, T. Is the bone fusion affected by Modic-2 changes in single-level anterior cervical discectomy and fusion? Medicine 2020, 99, e18597. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lei, T.; Shen, Y. The Impact of Modic-2 changes on the clinical outcomes of single-level anterior cervical discectomy and fusion. Eur. Spine J. 2015, 24, 2936–2940. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Ding, W.; Zhang, Y.; Shen, Y. Comparison of Clinical Outcomes After Anterior Cervical Discectomy and Fusion Versus Cervical Total Disk Replacement in Patients with Modic-2 Changes on MRI. Clin. Spine Surgery A Spine Publ. 2017, 30, E1088–E1092. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Li, T.; Xue, Y. Preoperative Modic changes are related to axial symptoms after anterior cervical discectomy and fusion. J. Pain Res. 2018, 11, 2617–2623. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Tong, T.; Shen, Y. Influence of Modic Changes on Cage Subsidence and Intervertebral Fusion after Single-Level Anterior Cervical Corpectomy and Fusion. J. Investig. Surg. 2020, 35, 301–307. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wei, J.; Shen, Y. A study on the cervical spondylotic myelopathy treated by anterior cervical diskectomy and fusion in accordance with Modic changes with a 2-year minimum follow-up. J. Orthop. Surg. Res. 2015, 10, 11. [Google Scholar] [CrossRef][Green Version]
- Jiao, J.; Li, J.; Luo, Y.; Zhang, W. Clinical and radiographic outcomes of hybrid graft in patients with Modic changes undergoing transforaminal lumbar interbody fusion. J. Orthop. Surg. Res. 2021, 16, 486. [Google Scholar] [CrossRef]
- El Barzouhi, A.; Vleggeert-Lankamp, C.L.; van der Kallen, B.F.; Nijeholt, G.J.L.; Hout, W.B.V.D.; Koes, B.W.; Peul, W.C. Back pain’s association with vertebral end-plate signal changes in sciatica. Spine J. 2014, 14, 225–233. [Google Scholar] [CrossRef]
- Ulrich, N.H.; Burgstaller, J.M.; Gravestock, I.; Winklhofer, S.; Porchet, F.; Pichierri, G.; Wertli, M.M.; Steurer, J.; Farshad, M.; The LSOS Study Group. The influence of endplate (Modic) changes on clinical outcomes in lumbar spinal stenosis surgery: A Swiss prospective multicenter cohort study. Eur. Spine J. 2020, 29, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.-S.; Lee, H.-D.; Jeon, C.-H. The Impact of Vertebral End Plate Lesions on the Radiological Outcome in Oblique Lateral Interbody Fusion. Glob. Spine J. 2021, 11, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Udby, P.M.; Ohrt-Nissen, S.; Bendix, T.; Paulsen, R.; Støttrup, C.; Andresen, A.; Brorson, S.; Carreon, L.Y.; Andersen, M. Are Modic Changes Associated with Health-related Quality of Life After Discectomy. Spine 2020, 45, 1491–1497. [Google Scholar] [CrossRef]
- Sørlie, A.; Moholdt, V.; Kvistad, K.A.; Nygaard, P.; Ingebrigtsen, T.; Iversen, T.; Kloster, R.; Solberg, T.K. Modic type I changes and recovery of back pain after lumbar microdiscectomy. Eur. Spine J. 2012, 21, 2252–2258. [Google Scholar] [CrossRef]
- Gornet, M.F.; Schranck, F.; Wharton, N.D.; Beall, D.P.; Jones, E.; Myers, M.E.; Hipp, J.A. Optimizing success with lumbar disc arthroplasty. Eur. Spine J. 2014, 23, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Koshi, T.; Suzuki, M.; Orita, S.; Eguchi, Y.; Ochiai, N.; Kishida, S.; et al. Change in Modic Type 1 and 2 Signals After Posterolateral Fusion Surgery. Spine 2010, 35, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Chen, Z.; Zheng, Y.; Wang, Y.; Jiang, L.; Yang, Y.; Zhuang, C.; Liang, Y.; Zheng, T.; Gong, Y.; et al. Comparison of simple discectomy and instrumented posterior lumbar interbody fusion for treatment of lumbar disc herniation combined with Modic endplate changes. Chin. Med. J. 2014, 127, 2789–2794. [Google Scholar] [PubMed]
- Lurie, J.D.; Moses, R.A.; Tosteson, A.N.A.; Tosteson, T.D.; Carragee, E.J.; Carrino, J.A.; Kaiser, J.A.; Herzog, R.J. Magnetic Resonance Imaging Predictors of Surgical Outcome in Patients with Lumbar Intervertebral Disc Herniation. Spine 2013, 38, 1216–1225. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, B.; Lv, G.-H.; Wu, P.; Dai, Y.; Jiang, B.; Zheng, Z.; Xiao, S. Percutaneous Endoscopic Lumbar Discectomy for Lumbar Disc Herniation with Modic Changes via a Transforaminal Approach: A Retrospective Study. Pain Physician 2019, 22, E601–E608. [Google Scholar]
- Djurasovic, M.; Carreon, L.Y.; Crawford III, C.H.C.; Zook, J.D.; Bratcher, K.R.; Glassman, S.D. The influence of preoperative MRI findings on lumbar fusion clinical outcomes. Eur. Spine J. 2012, 21, 1616–1623. [Google Scholar] [CrossRef][Green Version]
- Masala, S.; Anselmetti, G.C.; Marcia, S.; Nano, G.; Taglieri, A.; Calabria, E.; Chiocchi, M.; Simonetti, G. Treatment of painful Modic type I changes by vertebral augmentation with bioactive resorbable bone cement. Neuroradiology 2014, 56, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Koshi, T.; Suzuki, M.; Orita, S.; Eguchi, Y.; Ochiai, N.; Kishida, S.; et al. Low Back Pain After Lumbar Discectomy in Patients Showing Endplate Modic Type 1 Change. Spine 2010, 35, E596–E600. [Google Scholar] [CrossRef] [PubMed]
- Rahme, R.; Moussa, R.; Bou-Nassif, R.; Maarrawi, J.; Rizk, T.; Nohra, G.; Samaha, E.; Okais, N. What happens to Modic changes following lumbar discectomy? Analysis of a cohort of 41 patients with a 3- to 5-year follow-up period. J. Neurosurg. Spine 2010, 13, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Tropiano, P.; Gaudart, J.; Huang, R.C.; Marnay, T. Clinical Results of Lumbar Total Disc Arthroplasty in Accordance with Modic Signs, With a 2-Year-Minimum Follow-up. Spine 2011, 36, 2309–2315. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Stienen, M.N.; Joswig, H.; Smoll, N.; Schaller, K.; Corniola, M.V. The usefulness of radiological grading scales to predict pain intensity, functional impairment, and health-related quality of life after surgery for lumbar degenerative disc disease. Acta Neurochir. 2016, 159, 271–279. [Google Scholar] [CrossRef]
- Hellum, C.; Johnsen, L.G.; Gjertsen, Ø.; Berg, L.; Neckelmann, G.; Grundnes, O.; Rossvoll, I.; Skouen, J.S.; Brox, J.I.; The Norwegian Spine Study Group; et al. Predictors of outcome after surgery with disc prosthesis and rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up. Eur. Spine J. 2012, 21, 681–690. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Chin, D.-K.; Jin, B.-H.; Kim, K.-S.; Cho, Y.-E.; Kuh, S.-U. Long Term Efficacy of Posterior Lumbar Interbody Fusion with Standard Cages alone in Lumbar Disc Diseases Combined with Modic Changes. J. Korean Neurosurg. Soc. 2009, 46, 322–327. [Google Scholar] [CrossRef]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2013, 22, 104–110. [Google Scholar] [CrossRef]
- Boden, S.D.; Davis, D.O.; Dina, T.S.; Patronas, N.J.; Wiesel, S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Joint Surg. Am. 1990, 72, 403–408. [Google Scholar] [CrossRef]
- Lurie, J.D.; Tosteson, T.D.; Tosteson, A.N.A.; Zhao, W.; Morgan, T.S.; Abdu, W.A.; Herkowitz, H.; Weinstein, J.N. Surgical Versus Nonoperative Treatment for Lumbar Disc Herniation. Spine 2014, 39, 3–16. [Google Scholar] [CrossRef]
- Saifi, C.; Fein, A.W.; Cazzulino, A.; Lehman, R.A.; Phillips, F.M.; An, H.S.; Riew, K.D. Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. Spine J. 2018, 18, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Peterson, C.K.; Hodler, J. Degenerative Marrow (Modic) Changes on Cervical Spine Magnetic Resonance Imaging Scans. Spine 2011, 36, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Toyone, T.; Takahashi, K.; Kitahara, H.; Yamagata, M.; Murakami, M.; Moriya, H. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J. Bone Jt. Surg. Br. Vol. 1994, 76, 757–764. [Google Scholar] [CrossRef]
- Ha, K.-Y.; Molon, J.N.; Ahn, J.-H.; Kim, Y.-H. Fate of Osteophytes and Sclerosis in Fused Segments After Lumbar Fusion. Spine 2014, 39, E1110–E1115. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).