Abstract
The prevalence of dementia is increasing and the care needs of people living with dementia are rising. Family carers of people living with dementia are a high-risk group for psychological and physical health comorbidities. Mindfulness-based interventions such as mindfulness-based cognitive therapy show potential for reducing stress experienced by family carers of people living with dementia. This study aims to systematically assess the efficacy of mindfulness-based cognitive therapy in reducing stress experienced by family carers of people living with dementia. Electronic databases including MEDLINE, APA PsycINFO, EMBASE, CINAHL, Scopus, Web of Science, Cochrane Library, AMED, ICTRP, and ALOIS were searched for relevant studies up to August 2020. All types of intervention studies were included. Quantitative findings were explored. Seven studies were eligible for inclusion. The analysis showed that there was a statistically significant reduction in self-rated carer stress in four studies for the mindfulness-based cognitive therapy group compared to controls. One study that was adequately powered also showed reductions in carer burden, depression, and anxiety compared to control. Mindfulness-based cognitive therapy appears to be a potentially effective intervention for family carers of people living with dementia, but large, high-quality randomized controlled trials in ethnically diverse populations are required to evaluate its effectiveness.
1. Introduction
As the world’s population grows and ages, the prevalence of dementia is rising rapidly [1]. Dementia is associated with a decline in cognitive function and the inability to perform activities of daily living, which results in substantial ongoing care needs for people with dementia as the disease progresses.
Family carers provide the majority of the care involved for people living with dementia (PLWD) [2] in a largely unpaid manner; therefore, saving society considerable costs of this care. It is well known that being a dementia carer is a risk factor for psychological stress [3] and poor physical health [4]. Approximately 40% of these carers experience clinical depression or anxiety [5]. Of particular concern are findings from a UK survey of 566 dementia carers which showed that 16% were suicidal [6].
1.1. Psychological Factors Related to Carers of PLWD
The experience of carer stress is significantly increased by the presence of behavioural and psychological symptoms of dementia (BPSD) in PLWD [7,8] such as agitation, apathy, wandering, and psychosis. Higher levels of stress may be experienced by carers looking after older adults with physical disabilities [9]. Carer stress is also affected by factors such as the severity of the cognitive decline in PLWD [10], the duration of caregiving [11], being older, female, and living with PLWD [7,8].
The quality of dyadic relationships between family carers and PLWD is important because closer relationships are predictive of positive outcomes for both the carers [12] and PLWD [13,14]. Without intervention, carer stress could increase the likelihood of premature entry into aged residential care [15] and elder abuse [16]. Therefore, there is an urgent clinical need and increasingly economic argument to provide dementia carers with cost-effective and sustainable stress-reduction interventions.
1.2. Traditional Stress-Reduction Interventions
Many psychosocial stress-reduction interventions such as respite, educational workshops, skills training, and support groups are offered to carers of PLWD. However, systematic reviews have shown that the effect on stress reduction from these interventions is not significant [17]. The evidence supporting psychological interventions for stress reduction in family carers is inconsistent and weak [10], transient when present [18], and lacking in specificity [3]. Interventions that require active participation are associated with greatest effect [19]. A recent meta-analysis of high quality but significantly heterogenous study designs showed that psychosocial interventions have a small to moderate effect on dementia carer burden, depression, and general health [20], but not an overall effect on quality of life (QOL).
Cognitive Behavioural Therapy (CBT) is the most widely studied psychotherapy that is used for depressed dementia carers [21]. However, despite a substantial evidence base for depression, concerns are increasing about the effect sizes of CBT being relatively small, with sizes in the range of 0.10–0.36 for carers [21] and effects not enduring over time [22,23]. Therefore, there is a need to explore other cost-effective interventions of more enduring benefit.
1.3. Mindfulness-Based Interventions
Mind-body interventions such as mindfulness are increasing in popularity, and there is some evidence for their use with family dementia carers. The most well-known mindfulness-based interventions (MBI) are the Mindfulness-Based Stress Reduction (MBSR) program [24] and Mindfulness-Based Cognitive Therapy (MBCT) [25]. MBSR was originally developed for patients with chronic pain. MBCT was based on MBSR, but with cognitive behavioural techniques added to the MBSR-style practices [25]. MBCT’s original indication was for recurrent depression, where it was shown to be effective in the prevention of relapse in a meta-analysis of six RCTs in various countries involving 593 participants [26]. Importantly, MBCT has been shown to be as effective as antidepressant medication treatment for the prevention of relapse into depression, and may be more effective than medication in those with histories of severe childhood abuse [27]. MBCT is recommended for recurrent depression in clinical practice guidelines both in the UK since 2004 [28] as well as Australia and New Zealand since 2015 [29]. Since its original use for depression, it has been applied to many other indications with good effect [30].
MBIs have been used with family carers of other chronic diseases such as cancer [31] and developmental disabilities [32]. Preliminary evidence from a recent systematic review suggests MBIs (mostly MBSR) are effective for stress reduction in family dementia carers [33]. A meta-analysis was performed with three (144 participants) of the five pilot studies included in this systematic review [34,35,36] and showed a significant reduction in stress levels after the MBI, with a moderate aggregated effect size of 0.57 (95% CI [0.23–0.92]).
1.4. Research Gap
A systematic review looking specifically at the efficacy of MBCT on outcomes in dementia carers has not been conducted to date, and there appear to be a number of trials in this area that need to be summarized. MBCT has a central principle of encouraging approach toward negative experiences rather than reacting with aversion. This principle shows particular promise in this population of carers because of the evidence from a systematic review that denial, avoidance, and wishful thinking as coping strategies are associated with poor outcomes for dementia carers [3]. Additionally, given that MBCT targets depressive rumination specifically [25] it holds more potential over other MBIs in the carer populations where there are high rates of clinical depression and anxiety. This justifies the need to conduct a systematic review looking specifically at its efficacy in this population separate to other MBIs.
1.5. Aims
The primary aim of this systematic review was to examine the evidence for MBCT to reduce carer stress in family carers of PLWD when compared with treatment as usual, waitlist, or no control. Secondary aims are to review the evidence for MBCT to reduce carer burden, depression, and to increase QOL, resilience, and wellbeing. Other secondary aims are to review the evidence of MBCT to improve BPSD in people being cared for, and whether there are any reports of harms associated with the use of MBCT in this population. We will also review whether MBCT changes measures of trait mindfulness in carers.
2. Materials and Methods
This systematic review was registered on PROSPERO (CRD42020186414) on 5 May 2020 and the PRISMA 2009 [37] reporting checklist was used.
2.1. Search Strategy
Systematic searches were conducted between 1 June 2020 and 1 August 2020 using the following electronic databases: MEDLINE (via OVID), APA PsycINFO (via OVID), EMBASE (via OVID), CINAHL (via EBSCOhost), Scopus (via ELSEVIER), Web of Science, Cochrane Library (Wiley Interface), AMED, ICTRP, and ALOIS. Unpublished literature was also searched in ProQuest Dissertations and Theses Global, Google Scholar and MedNar. Search alerts were enabled in all databases to ensure ongoing retrieval of relevant studies. A hand search of reference lists of all relevant articles identified and of the Mindfulness journal was performed. Experts were contacted to ensure saturation of literature (authors of four identified studies were emailed to ask about other studies that they were aware of). Keywords used included “Mindful*”, “MBCT”, “Dementia”, “Alzheimer”, “Cognit*”, “neurocognit*”, “care*”, “caregive*” and other relevant subject headings of each database. A full search strategy for MEDLINE (via OVID) is available in the Supplementary Materials.
2.2. Eligibility Criteria
2.2.1. Study Types
Any experimental study design was included, such as randomized controlled trials (RCTs), quasi-experimental, prospective, or retrospective cohort studies that evaluated the efficacy of MBCT in family carers of PLWD. Studies with any type of control (treatment as usual, active, or inactive controls) were included. Studies of all languages were attempted to be included as long as translation resources were available. There was no restriction on setting and study duration. Studies were included from unpublished sources if data were available.
2.2.2. Participant Types
Studies involving family carers of any age were included. Family carers were defined as spouses, children, grandchildren, siblings, other relatives of a PLWD or person with significant cognitive impairment. Carers did not have to be blood relatives. Staff and paid caregivers were excluded.
2.2.3. Intervention Types
MBCT or adaptations of MBCT were included. Both online and in-person group formats of MBCT were included.
2.2.4. Outcome Measurement Types
The primary outcome of this systematic review was carers’ perceived stress levels. This was chosen as this appears to be the main way to assess efficacy of MBCT interventions in a manner that is relevant to carers. Secondary outcomes were carer burden, depression, QOL, resilience, wellbeing, trait mindfulness, BPSD in the PLWD, and potential adverse effects. The secondary outcomes were chosen because mindfulness interventions can improve a raft of other health outcomes [24], with potential for benefit on dyadic interactions involving PLWD. Trait mindfulness was also chosen because it is the process indicator that explains the change in other outcomes.
2.3. Data Extraction
Literature search results were transferred to reference management software (RefWorks). COVIDENCE, a systematic review software for screening and data extraction, was used. There was a first pass extraction using titles and abstracts from studies retrieved using the search strategy. This was conducted independently by two blinded review authors (EC, NA) to identify studies that potentially met the inclusion criteria outlined above. The second pass extraction involved retrieving the full text of the studies and independent assessment for eligibility by two blinded review authors (EC, NA). Disagreement between reviewers was resolved through a third review author (GC) through discussion. A log of excluded studies was kept with reasons at the full text screening stage. Two blinded reviewers extracted data independently (EC, BL) and discrepancies were identified and resolved through discussion with a third author (GC) where necessary.
Missing data were requested from study authors. Extracted information included setting, year, design, sociodemographic characteristics of the carers, intervention and control group details, outcomes, and suggested improvements.
2.4. Risk of Bias
We used Cochrane Collaboration’s revised risk of bias tool (RoB 2) to assess the risk of bias in the RCTs [38]. RoB 2 was used by two blinded review authors (EC, YB) who independently assessed the risk of bias in all RCTs. RoB 2 is structured into five domains to assess biases in the following areas: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Assessment results were discussed between the two blinded review authors and taken to a third author (GC) for a final decision made by consensus. The aim of this assessment was to determine the quality of the evidence presented by the studies, but all studies were still included regardless of their risk of bias.
2.5. Data Analysis
It was not anticipated that meta-analyses would be conducted due to heterogeneity of studies. Instead, a systematic review approach was planned with information presented in text and tables to summarize and explain the characteristics and outcomes of included studies. Findings both within and between included studies were explored. Findings were presented in order of main and additional outcomes.
3. Results
The search strategy including unpublished grey literature resulted in 595 articles (Figure 1). After duplicate removal there were 318 results. The first pass screening using titles and abstracts removed protocols, studies of non-MBCT interventions, and non-dementia carer populations. The remaining 18 results were screened using full texts. Two studies were not in English and translators screened these. A total of 11 results were excluded for the following reasons: five wrong interventions, one wrong patient population, three duplicates, one was not an intervention study, and one study could not be retrieved despite extensive searches using inter-library services and contacting the author, journal, and publisher. Ultimately, seven studies with a total of 291 subjects that fulfilled selection criteria were included for analysis.
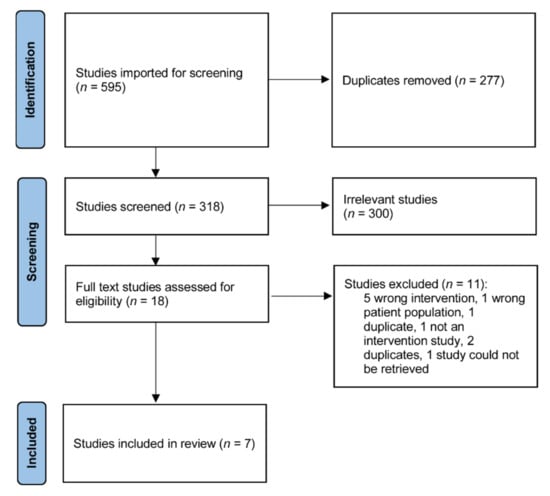
Figure 1.
PRISMA flow chart.
3.1. Study Characteristics
3.1.1. Study Design and Control Group Conditions
The study designs of all studies are presented in Table 1. The seven studies included were those by Cheung et al., (2020) [39]; Kor et al., (2019, 2020) [40,41]; Norouzi et al., (2015) [42]; Oken et al., (2010) [36]; Ozen (2013) [43]; and Zarei (2018) [44]. All included studies were randomized and controlled. Three studies were described as pilot RCTs [35,40,43]. The number of participants in the seven studies ranged from 12–113, with only two studies [39,41] recruiting over 50 participants.

Table 1.
Summary of study characteristics.
Oken et al., (2010) [35] was a three-armed RCT where participants were divided into an MBCT intervention group and two control groups (education group as active control and respite group as pragmatic control). Three other studies used active control groups [38,39,40]. Two studies [40,41] used brief education programs as the control that matched the MBCT group in terms of duration and number of sessions. These education sessions were also structured to provide a mix of didactic teaching and group sharing experiences which is similar to MBCT. The Cheung et al., (2020) study [39] compared MBCT with MBSR. MBCT and MBSR are similar in terms of duration and number of sessions. The other studies [42,43,44] used inactive control groups of usual care or waitlist. The Ozen (2013) [43] study included dyads of carers and PLWD, or carers alone, and was performed as an unblinded crossover RCT.
3.1.2. Family Carer Characteristics
The characteristics of participants in all studies are presented in Table 1. In all seven studies, most of the participants were women (ranging from 61–100%). The mean age ranged from 57.1–68.9 years. Whilst the studies were conducted in a range of countries (USA, Canada, Hong Kong, Iran), formal ethnicity data were only reported in two studies. Oken et al. (2010) [35] had mostly Caucasian participants (90.3%), with small numbers of African American (3.2%), and Asian (6.5%) participants. Participants in the Cheung et al. (2020) [38] study were all Chinese.
The carer’s relationship with PLWD was quite variable in the seven studies and included spouses (7.5–100%) as well as children. The mean duration of caregiving which was reported in four studies [39,40,41,44] ranged from 5.1–8.7 years.
3.1.3. Intervention
A summary of interventions used in all studies is presented in Table 1. In all studies, the experimental intervention was some form of MBCT. The original format of MBCT consists of eight, 2.5 h weekly sessions and a whole-day retreat. Almost all studies modified the MBCT protocol in some way from its original format. Only Norouzi et al. (2015) [42] did not provide details about any modifications to the MBCT protocol. Zarei (2018) [44] delivered the MBCT online as tele-MBCT, whilst all other studies used the original in-person group format. The Zarei (2018) study [44] also used the self-help book, The Mindful Way Workbook [45] to supplement the MBCT. The modifications were made by a panel of expert clinicians in three of the studies [39,40,41]. The other studies did not describe who made the modifications.
Adaptations were made to tailor the MCBT specifically for carers. In the Kor et al. (2020) [41] study, this included psychoeducation about stress and replacement of depression relapse content with dementia caregiving skills. Additionally, used were responding to negative moods associated with caregiving, and the identification of habitual emotional reactions to difficulties in caregiving. The Zarei (2018) [44] study also made adaptations to content to include issues of carer identity and ambiguous loss. This study modified the movement practices to enhance carer safety. The Oken et al. (2010) [35] study included a shared education session on dementia with the active control group. This [39] study described a focus on CBT concepts to help carers gain confidence early.
3.1.4. Outcome Measures
The primary outcome of this systematic review was the carers’ perceived stress level. Secondary outcomes were carer burden, depression, QOL, resilience, wellbeing, trait mindfulness, BPSD in PLWD, and potential side effects.
The perceived stress of carers in most studies was measured using the Perceived Stress Scale (PSS) [46]. This was measured at pre- and post-MBCT intervention, as well as at three months post intervention in Kor et al., (2019) [40], and six months post intervention in Kor et al., (2020) [41] to see if effects were sustained. The Oken et al., (2010) [35] study used the Revised Memory and Behavior Problems Checklist [47] as their primary outcome measure, which has the stress reaction of carers as one of two main components. Oken et al., (2010) [35] also measured salivary cortisol and inflammatory markers (IL-6, TNF-alpha, CRP) as additional and more objective measures of carer stress.
Carer burden was measured using the Zarit Burden Interview (ZBI) [48] in three studies ([39,40,41], its shortened version [49] in Ozen (2013) [43] or the Caregiver Burden Inventory (CBI) [50] in Norouzi et al., (2015) [42].
Depression was measured using the Center for Epidemiological Studies–Depression Scale (CES-D) [51] in most studies [36,39,40,41,44]. The Ozen (2013) [43] study used the 30-item Geriatric Depression Scale [52] and the Depression Anxiety Stress Scale [53]. The Hamilton Depression Rating Scale [54] was used in Norouzi et al., (2015) [42].
Resilience was measured using the Brief Resilience Scale (BRS) [55] in the Kor et al. (2019, 2020) [40,41] studies.
QOL for carers was measured using Short From 12 Physical Component Summary Score (SF-PCS) [56] and the Short Form 12 Mental Component Summary Score (SF12-MCS) [56] in both those [40,41] studies.
BPSD in PLWD was measured using the Apathy Evaluation Scale—Informant Version (AES) [57] in Oken et al., (2010) [35] and the Neuropsychiatric Inventory—Questionnaire (NPI-Q) [58] in Kor et al., (2020) [41].
Trait mindfulness was measured using the Five-Facet Mindfulness Questionnaire (FFMQ) [59] in four studies [39,40,41,43]). The Oken et al., (2010) [35] study used the Mindful Attention Awareness Scale [60].
3.2. Risk of Bias Assessment of Included Studies
For all studies, the Cochrane Collaboration’s Risk of Bias 2 (RoB 2) tool [38] was used because all studies were randomized and controlled in design. The assessment of bias for all studies is reported in Figure 2.
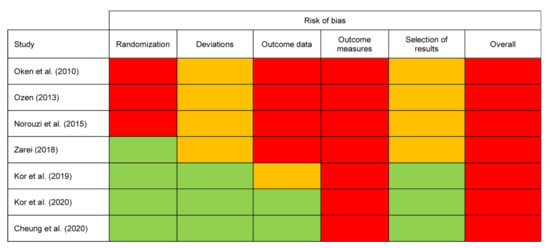
Figure 2.
Risk of bias assessment. Note. Green = low risk, Amber = some concerns, Red = high risk.
3.2.1. Randomization
The Ozen (2013) [43] and Norouzi et al., (2015) [42] studies scored a high risk of bias due to randomization processes. Neither study reported details of sequence generation, allocation concealment, and baseline characteristics. The Oken et al., (2010) [35] study also had the same high risk scores across those domains but did report an adequate randomization sequence process.
3.2.2. Deviations
Four studies [35,42,43,44] scored some concerns in the domain of bias due to deviations because of lack of reported blinding and intention to treat protocols. Participant blinding is challenging for mindfulness interventions such as MBCT and none of the included studies were able to do this. However, three studies [39,40,41] scored low risk in the domain where this was assessed (bias due to deviations from intended interventions) as a result of the algorithm allowing for this to be compensated by other more favourable aspects of risk such as intention to treat procedures.
3.2.3. Outcome Data
Four studies [35,42,43,44] scored high risk for missing outcome data. Three of these studies reported a significant attrition rate without reasons or appropriate statistical analysis to manage this, while the Norouzi et al., (2015) [42] study did not explicitly comment on attrition and was therefore also rated as high risk for this measure. The Kor et al., (2019) [40] study had some concerns in the domain of missing outcome data because two participants were lost to follow up with reasons that may have been significant in this small sample.
3.2.4. Outcome Measures
All studies were deemed high risk by virtue of having participant reported self-rated scores as their main outcome measure. According to the RoB 2 tool, the outcome assessors are study participants if measures are self-reported [38]. Self-report introduces social desirability bias as it is likely that the assessment of outcome is influenced by knowledge of the received intervention. This was the case for all included studies as no participants could be blinded to the intervention.
However, the Oken et al., (2010) [35] study also used objective physiological markers measured by blinded outcome assessors, and these outcomes would have had a low risk of bias in outcome assessment. Oken et al. [35] reported that participant “expectancies” had been assessed to be the same between groups, but no further details were available. However, since they also included subjective assessments, the overall risk remained high.
3.2.5. Selection of Results
Four studies [35,42,43,44] also scored some concerns in the domain of bias due to selection of results because there was no study protocol available.
3.2.6. Overall Risk of Bias
Setting the issue of outcome measurement aside, the studies then ranged in their risk of bias with some studies scoring well, with few other major concerns due to adequate reporting of quality procedures [39,41]. The studies with the highest risk of bias were Ozen (2013) [43] and Norouzi et al., (2015) [42]. These were small RCTs with biases across all domains. All studies are reported with combined outcomes because there was no clear difference in the validity or sensitivity of individual outcome measures used in each study.
3.3. Outcomes of MBCT Interventions
The main findings of the seven studies are summarized in Table 1. The between-group effect sizes of MBCT for outcomes were reported for three out of seven studies and are presented in Table 2.

Table 2.
Effect sizes of included studies.
3.3.1. Carer Stress
There was a statistically significant difference in self-rated carer stress in three studies using MBCT compared to active control groups [39,40,41]. The Kor et al., (2020) [41] study also showed a large significant reduction in BPSD related caregiver distress in the MBCT group compared to the control (Cohen’s d = 0.7) at six months and had the longest follow up period of 6 months. The mean PSS score at baseline of 31.8 reduced to 25.0 which is below the cut-off for high perceived stress. The Cheung et al., (2020) [39] study showed that the MBCT group had a significant improvement in stress from baseline to post intervention (PSS total score mean difference = 3.2, SE = 1.1, p = 0.03). Of significance, this [39] study compared MBCT to MBSR and showed that MBCT was better than MBSR for stress reduction in family carers (Cohen’s d = 0.6, p = 0.019). The MBCT intervention was shown to decrease self-rated carer stress compared to the pragmatic control group in the Oken et al. (2010) [35] study (but not compared to the active control). Pre–post results for stress were not significant in the tele-MBCT group of the Zarei (2018) [44] study.
The effect sizes for carer stress measured by the PSS ranged from Cohen’s d = 0.0 [35] to 0.4 [40] at post intervention, and increased up to 0.7 at six months in Kor et al., (2020) [41].
3.3.2. Carer Burden
Carer burden was significantly reduced in the Kor et al., (2019) [40] study in the MBCT group compared to the active control at three month follow up (ZBI mean difference = −2.7, p = 0.006, Cohen’s d = 1.0). The Cheung et al., (2020) [39] study also showed within group reductions in carer burden for the MBCT group between post intervention and at three months (ZBI mean difference = 5.2, SE = 1.7, p = 0.14). Carer burden was also reduced in the Norouzi et al., (2015) [42] study, but this was only a within-MBCT group finding at two month follow up.
3.3.3. Depression
There was a significant reduction in depression scores for the Kor et al. studies (2019, 2020) [40,41] in the MBCT group compared to active controls. There was a large effect size of Cohen’s d = 1.4 for depressive symptoms in Kor et al., (2020) [41] at six months. Within group findings for the MBCT group in the Cheung et al., [39] study also showed benefits in depressive symptoms at three months and in the Norouzi et al., (2015) [42] study at two month follow up.
The effect sizes for depression measured by the CES-D ranged from 0.04 [40] to 0.9 [41] post intervention, and increased up to 1.4 at six months [41].
3.3.4. Resilience
Resilience was measured only in the two [40,41] studies and no significant differences were noted between groups in those studies.
3.3.5. Quality of Life
Physical health related QOL did not change in Kor et al., (2020) [41]. However, mental health related QOL showed significant greater improvement at six months in this study with a medium effect size of Cohen’s d = 0.6 at six months.
3.3.6. Trait Mindfulness
A statistically significant increase in mindfulness as measured by the FFMQ was found in the MBCT group at three (mean difference = 18.5, p < 0.01) and six months (mean difference = 19.9, p = 0.4) in the Kor et al., (2020) study [41]. The Cheung et al. (2020) [39] study also showed a statistically significant increase in trait mindfulness in the MBCT group at both post intervention (Helmert’s contrast mean difference = 2.4, SE = 1.2) and at follow up at three months (Helmert’s contrast mean difference = 2.5, SE = 1.2). The level of mindfulness in Kor et al., (2020) [41] was significantly correlated with improvements in a number of psychological outcomes (stress, depression, anxiety).
3.3.7. BPSD in PLWD
BPSD in PLWD was not measured in most studies. The only positive result was small at three months in Kor et al., (2020) [41] (Cohen’s d = 0.2), but was not significant at six months.
3.3.8. Adverse Effects
Only two studies [39,41] looked for any potential adverse effects or evidence of harm, and none were found.
4. Discussion
This systematic review showed that MBCT had beneficial effects on stress and depression for family carers of PLWD in four out of seven studies. The key finding is the large effect size for carer stress and depression in two of the studies [35,41], with results maintained at six-month follow-up in one study [41]. Our findings are similar to a previous review on MBIs in general (which were mostly MBSRs) [33]; however, results were not maintained at longer term follow up elsewhere. We found quality was an issue for the majority of MBCT studies because they were mostly small pilot RCTs with likely limitations on funding in a range of countries. The risk of bias assessment highlights the need for some objective measures by blinded outcome assessors (for example, physiological markers that are sensitive to change or clinician assessed rating scales).
4.1. Carer Stress
Self-perceived stress was seen as a primary outcome measure in most studies and appears valid because it is most likely to be sensitive to a mind–body intervention [35]. Of note, a large effect size for stress reduction (Cohen’s d = 0.7) for MBCT at six months follow up was seen in Kor et al., (2020) [41]. This is larger than studies using mindfulness interventions without a CBT component [61]. The duration of follow-up shows the potential for significant enduring stress-reduction effects of MBCT for this population, long after the intervention has ended. The mean PSS score at baseline of 31.8 suggested that most participants were experiencing high levels of stress. The reduction to 25.0 is below the cut-off for high perceived stress, suggesting this is not just statistically significant, but also clinically significant.
4.2. Depression and Anxiety
The large effect sizes for depression and anxiety in the large study that was adequately powered [41] are not surprising given MBCT’s original indication for recurrent depression. These results are consistent with other recent studies [62,63] and reinforce studies that show MBCT’s equivalence to antidepressant medication [26,64]. This is of significant practical implication to family carers of PLWD who have high rates of depression and anxiety [5]. Sample size was an issue for all other studies in this review and therefore they were likely underpowered to detect results of interest.
4.3. BPSD in PLWD
Improvements in BPSD with MBCT was noted in the Kor et al., (2020) [41] study as an immediate post intervention effect. It has been hypothesized that the calmer interactions and improvements in carer energy and wellbeing may have indirectly been of benefit to PLWD [41]. Communication with PLWD is a key component of BPSD management and because the emphasis on non-judgmental acceptance of already existent BPSD, MBCT would be of benefit to care relationships. Thus, the benefits of MBCT extend indirectly but are of potentially great significance to QOL for PLWD. It is expected that improvements in carer symptoms will translate to improvements for PLWD, and therefore BPSD is an important outcome to measure.
4.4. Adaptations of the MBCT Protocol
We found almost all included studies adapted the MBCT protocol, which could be helpful to enhance adherence for time-poor family carers by shortening the duration and number of sessions, and tailoring the content for carer stress rather than depression. A recent systematic review on MBIs for family carers of PLWD recommended these adaptations due to concerns that studies using the original MBSR protocol (including a 7.5 h retreat day) were thought to be associated with higher attrition rates of 10–17% [33]. These modifications were specifically made to reduce attrition rate, whilst still resulting in significant increases in trait mindfulness in the Cheung et al., (2020) [39] study. This has also been noted in other research [65] and supports the adaptation of reducing session duration and total number by at least one without losing potential active ingredients. Adaptations of the MBCT protocol do, however, make it more challenging to compare studies as they varied and adaptations were not always described in detail.
4.5. Skill Maintenance
Home practice is considered an essential component of the MBCT program to reinforce learnt skills that can be used for ongoing management of negative experiences in participants’ lives [66]. In the Kor et al., (2020) [41] study, the duration of home practice significantly correlated with mindfulness levels. This has been noted in previous literature [67]. One of the mechanisms by which studies sought to increase their effects may have been through extension of the original program from 8–10 weeks (by spacing out the reduced number of sessions) which increased the total time for home practice to enhance longer term maintenance of skills [41]. The long-term maintenance of skills in a self-sustaining manner is what potentially sets apart mindfulness-based interventions such as MBCT. The Cheung et al., (2020) [39] study also spaced out their protocol even further, to monthly sessions for the last three, but they noted participant feedback suggested that monthly gaps were too long.
4.6. The Superiority of MBCT over MBSR in This Context
The Cheung et al., (2020) [39] study that compared MBCT to MBSR gives some definitive evidence of the superiority of MBCT specifically in this population for stress reduction. Whilst the two interventions share many commonalities including structure of program, and were clearly both feasible for the population, the specific use of CBT techniques could be the key difference. Even though the family carer population is considered non-clinical, the prevalence of depressive and anxiety symptoms is sufficiently high to make CBT techniques an important beneficial component of the MBCT intervention. The MBCT protocol (unlike the MBSR protocol) also focuses on depression-specific phenomena such as negative thinking, rumination, and the consequences of low mood, and these may also have been key mechanisms to explain the stress reduction difference between the two programs for family carers. MBCT has shown superiority over MBSR in another study that compared them both, in addition to an inactive control, for patients with cardiovascular disease and comorbid depression [68].
4.7. Adverse Effect Reporting
There was no report of significant adverse effects in included studies which is consistent with the view that MBIs are relatively safe. However, there are known reports about harm with mindfulness meditation [69] which make it important for prospective RCTs to continue to assess for this.
4.8. Future Research Implications
A number of areas for future research have been identified by the authors of the included studies, and from the process of reviewing the included studies. The need for larger studies is clear, given the majority of included studies being small and of feasibility level only.
There needs to be more men included in future studies and more ethnic diversity in samples. The largest number of participants in the studies reviewed were from Hong Kong, where authors thought that the traditional Chinese population would take easily to meditation [41].
The high degree of outcome measure bias can be mitigated by the use of more objective measures such as clinician rating scales and biomarkers for stress.
The only tele-MBCT study included in this review did not show a significant reduction in carer stress (Zarei, 2018) [44]. In a post-COVID-19 pandemic world, tele-MBCT is particularly appealing for a number of reasons, but the convenience needs to be weighed against the efficacy of this modality and the equity issues faced by family carers who may not have access to high-speed internet, digital devices, or skills to use such technology.
5. Conclusions
In conclusion, MBCT appears to be a potentially effective intervention to reduce carer stress and improve other outcomes for family carers of PLWD. The effects seem to be sustainable with potential to also benefit PLWD. It can be delivered at low cost in relatively large groups. This has potentially significant implications on easing the public health burden of dementia internationally. Modifications of the MBCT protocol seem potentially beneficial to improve attrition rates in studies. Methodological issues noted could be used to inform future intervention studies. Large, high quality RCTs in ethnically diverse populations are required to evaluate its effectiveness for countries that are multicultural. Cost-effective larger scale health delivery also needs to be explored.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph19010614/s1, Table S1. MEDLINE search strategy (OVID interface.
Author Contributions
Conceptualization E.C., G.C., Y.B., S.C. and F.S., methodology E.C., G.C., S.C. and F.S.; software E.C.; validation, E.C., Y.B., N.A., B.L. and G.C.; formal analysis E.C.; investigation E.C., Y.B., N.A., B.L. and G.C.; resources E.C. and G.C.; data curation E.C.; writing—original draft preparation E.C.; writing—review and editing, E.C., G.C., Y.B., S.C., N.A., B.L. and F.S.; visualization E.C. and B.L.; supervision S.C., F.S. and G.C.; project administration E.C.; funding acquisition E.C., Y.B., G.C., S.C. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Health Research Council of New Zealand Clinical Research Training Fellowship NZ (HRC 20/019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Prince, M.J.; Wimo, A.; Guerchet, M.M.; Ali, G.C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015; Available online: https://www.alzint.org/u/WorldAlzheimerReport2015.pdf (accessed on 1 April 2019).
- Prince, M.; Bryce, R.; Ferri, C. World Alzheimer Report 2011: The Benefits of Early Diagnosis and Intervention; Alzheimer’s Disease International: London, UK, 2018; Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2011ExecutiveSummary.pdf (accessed on 1 April 2019).
- Gilhooly, K.J.; Gilhooly, M.L.M.; Sullivan, M.P.; McIntyre, A.; Wilson, L.; Harding, E.; Woodbridge, R.; Crutch, S. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016, 16, 106. [Google Scholar] [CrossRef] [Green Version]
- Pinquart, M.; Sörensen, S. Correlates of physical health of informal caregivers: A meta-analysis. J. Gerontol. 2007, 62, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Ory, M.; Hoffman, R.; Yee, J.; Tennstedt, S.; Schulz, R. Prevalence and impact of caregiving: A detailed comparison between dementia and nondementia caregivers. Gerontologist 1999, 39, 177–185. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, S.T.; Moyle, W.; Zimmer-Gembeck, M.; De Leo, D. Suicidal ideation in family carers of people with dementia. Aging Ment. Health 2015, 20, 222–230. [Google Scholar] [CrossRef]
- Cooper, C.; Balamurali, T.B.S.; Livingston, G. A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. Int. Psychogeriatr. 2007, 19, 175–195. [Google Scholar] [CrossRef]
- Mahoney, R.; Regan, C.; Katona, C.; Livingston, G. Anxiety and depression in family caregivers of people with Alzheimer disease: The LASER-AD study. Am. J. Geriatr. Psychiatry 2005, 13, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Tremont, G. Family caregiving in dementia. Med. Health Rhode Isl. 2011, 94, 36–38. [Google Scholar]
- Adelman, R.D.; Tmanova, L.L.; Delgado, D.; Dion, S.; Lachs, M.S. Caregiver burden: A clinical review. J. Am. Med. Assoc. 2014, 311, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, J.; Arokiasamy, Z.T.; Poi, P.J. Caregiving burden is associated with short rather than long duration of care for older persons. Asia Pac. J. Public Health 2003, 15, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Spaid, W.M.; Barusch, A. Emotional closeness and caregiver burden in the marital relationship. J. Gerontol. Soc. Work 1994, 21, 197–212. [Google Scholar] [CrossRef]
- Burgener, S.; Twigg, P. Relationships among caregiver factors and quality of life in care recipients with irreversible dementia. Alzheimer Dis. Assoc. Disord. 2002, 16, 88–102. [Google Scholar] [CrossRef]
- Edwards, H.B.; Ijaz, S.; Whiting, P.F.; Leach, V.; Richards, A.; Cullum, S.J.; Cheston, R.I.; Savović, J. Quality of family relationships and outcomes of dementia: A systematic review. BMJ Open 2018, 8, e015538. [Google Scholar] [CrossRef] [Green Version]
- Gaugler, J.E.; Kane, R.L.; Newcomer, R. Resilience and transitions from dementia caregiving. J. Gerontol. 2007, 62, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Selwood, A.; Blanchard, M.; Walker, Z.; Blizard, R.; Livingston, G. The determinants of family carers’ abusive behaviour to people with dementia: Results of the CARD study. J. Affect. Disord. 2010, 121, 136–142. [Google Scholar] [CrossRef]
- Sörensen, S.; Pinquart, M.; Duberstein, P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist 2002, 42, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Dam, A.E.H.; de Vugt, M.E.; Klinkenberg, I.P.M.; Verhey, F.R.J.; van Boxtel, M.P.J. A systematic review of social support interventions for caregivers of people with dementia: Are they doing what they promise? Maturitas 2016, 85, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Sörensen, S. Helping caregivers of persons with dementia: Which interventions work and how large are their effects? Int. Psychogeriatr. 2006, 18, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Teahan, Á.; Lafferty, A.; McAuliffe, E.; Phelan, A.; O’Sullivan, L.; O’Shea, D.; Nicholson, E.; Fealy, G. Psychosocial interventions for family carers of people with dementia: A systematic review and meta-analysis. J. Aging Health 2020, 32, 1198–1213. [Google Scholar] [CrossRef]
- Hopkinson, M.D.; Reavell, J.; Lane, D.A.; Mallikarjun, P. Cognitive behavioral therapy for depression, anxiety, and stress in caregivers of dementia patients: A systematic review and meta-analysis. Gerontologist 2019, 59, e343–e362. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Asnaani, A.; Vonk, I.J.J.; Sawyer, A.T.; Fang, A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cogn. Ther. Res. 2012, 36, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Lynch, D.; Laws, K.R.; McKenna, P.J. Cognitive behavioural therapy for major psychiatric disorder: Does it really work? A meta-analytical review of well-controlled trials. Psychol. Med. 2010, 40, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Delacorte Press: New York, NY, USA, 1990. [Google Scholar]
- Segal, Z.V.; Williams, J.M.G.; Teasdale, J.D. Mindfulness-Based Cognitive Therapy for Depression; Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- Piet, J.; Hougaard, E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: A systematic review and meta-analysis. Clin. Psychol. Rev. 2011, 31, 1032–1040. [Google Scholar] [CrossRef]
- Kuyken, W.; Hayes, R.; Barrett, B.; Byng, R.; Dalgleish, T.; Kessler, D.; Lewis, G.; Watkins, E.; Brejcha, C.; Cardy, J.; et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): A randomised controlled trial. Lancet 2015, 386, 63–73. [Google Scholar] [CrossRef]
- National Institute for Clinical Excellence. Depression: Management of Depression in Primary and Secondary Care (NICE Guideline CG23); National Institute for Clinical Excellence: London, UK, 2004; Available online: https://www.nice.org.uk/guidance/CG23 (accessed on 1 April 2019).
- Malhi, G.S.; Bassett, D.; Boyce, P.; Bryant, R.; Fitzgerald, P.B.; Fritz, K.; Hopwood, M.; Lyndon, B.; Mulder, R.; Murray, G.; et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust. N. Z. J. Psychiatry 2015, 49, 1087–1206. [Google Scholar] [CrossRef]
- Metcalf, C.A.; Dimidjian, S. Extensions and mechanisms of mindfulness-based cognitive therapy: A review of the evidence. Aust. Psychol. 2014, 49, 271–279. [Google Scholar] [CrossRef]
- Birnie, K.; Garland, S.N.; Carlson, L.E. Psychological benefits for cancer patients and their partners participating in mindfulness-based stress reduction (MBSR). Psycho-Oncol. 2010, 19, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, A.; Wolfe, C.; Zylowska, L.; Wang, S.; Schuster, E.; Barrett, C.; Lehrer, D. Mindfulness based stress reduction (MBSR) for parents and caregivers of individuals with developmental disabilities: A community-based approach. J. Child Fam. Stud. 2015, 24, 298–308. [Google Scholar] [CrossRef]
- Kor, P.P.K.; Wai, T.C.; Liu, J.Y.W.; Lai, C.K.Y. Mindfulness-based intervention for stress reduction of family caregivers of people with dementia: A systematic review and meta-analysis. Mindfulness 2018, 9, 7–22. [Google Scholar] [CrossRef]
- Epstein-Lubow, G.; Miller, I.W.; McBee, L. Mindfulness training for caregivers. Psychiatr. Serv. 2006, 57, 421. [Google Scholar] [CrossRef]
- Oken, B.S.; Fonareva, I.; Haas, M.; Wahbeh, H.; Lane, J.B.; Zajdel, D.; Amen, A. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J. Altern. Complement. Med. 2010, 16, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Whitebird, R.R.; Kreitzer, M.; Crain, A.L.; Lewis, B.A.; Hanson, L.R.; Enstad, C.J. Mindfulness-based stress reduction for family caregivers: A randomized controlled trial. Gerontologist 2013, 53, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, i4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, D.S.K.; Kor, P.P.K.; Jones, C.; Davies, N.; Moyle, W.; Chien, W.T.; Yip, A.L.K.; Chambers, S.; Yu, C.T.K.; Lai, C.K.Y. The use of modified mindfulness-based stress reduction and mindfulness-based cognitive therapy program for family caregivers of people living with dementia: A feasibility study. Asian Nurs. Res. 2020, 14, 221–230. [Google Scholar] [CrossRef]
- Kor, P.P.K.; Liu, J.Y.W.; Chien, W.T. Effects of a modified mindfulness-based cognitive therapy for family caregivers of people with dementia: A pilot randomized controlled trial. Int. J. Nurs. Stud. 2019, 98, 107–117. [Google Scholar] [CrossRef]
- Kor, P.P.K.; Liu, J.Y.W.; Chien, W.T. Effects of a modified mindfulness-based cognitive therapy for family caregivers of people with dementia: A randomized clinical trial. Gerontologist 2021, 61, 977–990. [Google Scholar] [CrossRef]
- Norouzi, M.; Golzari, M.; Faramarz, S. Effectiveness of mindfulness based cognitive therapy on the quality of life, depression and burden of demented women caregivers. Zahedan J. Res. Med. Sci. 2015, 16, 5–11. [Google Scholar] [CrossRef]
- Ozen, L.J. The Efficacy of Mindfulness-Based Cognitive Therapy (MBCT) to Improve Depression Symptoms and Quality of Life in Individuals with Dementia and Their Caregivers: A Pilot Study. Open Trials. 2013. Available online: https://www.openaire.eu/search/dataset?datasetId=opentrials__::10b9db3e55e93fa0bb24fb5db3ccf7dd (accessed on 7 May 2020).
- Zarei, S. Tele-Mindfulness for Dementia’s Family Caregivers: A Randomized Trial with a Usual Care Control Group. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2018. Available online: http://hdl.handle.net/1807/91463 (accessed on 7 May 2020).
- Teasdale, J.D.; Williams, J.M.G.; Segal, Z.V.; Kabat-Zinn, J. The Mindful Way Workbook; Guilford: New York, NY, USA, 2014. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Teri, L.; Truax, P.; Logsdon, R.; Uomoto, J.; Zarit, S.; Vitaliano, P.P. Assessment of behavioral problems in dementia. Psychol. Aging 1992, 7, 622–631. [Google Scholar] [CrossRef]
- Zarit, S.H.; Reever, K.E.; Bach-Peterson, J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 1980, 20, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Bedard, M.; Molloy, D.; Squire, L.; Dubois, S.; Lever, J.; O’Donnell, M. The Zarit Burden Interview: A new short version and screening version. Gerontologist 2001, 42, 652–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caserta, M.S.; Lund, D.A.; Wright, S.D. Exploring the caregiver burden inventory (CBI): Further evidence for a multidimensional view of burden. Int. J. Aging Hum. Dev. 1996, 43, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radloff, L.S. The CES-D scale. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety Stress Scales, 2nd ed.; Psychology Foundation: Sydney, NSW, Australia, 1995. [Google Scholar]
- Hamilton, M. Rating depressive patients. J. Clin. Psychiatry 1980, 41, 21–24. [Google Scholar]
- Lai, J.C.L.; Yue, X. Using the brief resilience scale to assess Chinese people’s ability to bounce back from stress. SAGE Open 2014, 4, 2158244014554386. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.; Kosinski, M.; Keller, S.D. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, R.S.; Biedrzycki, R.C.; Firinciogullari, S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991, 38, 143–162. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [Green Version]
- Bohlmeijer, E.; ten Klooster, P.M.; Fledderus, M.; Veehof, M.; Baer, R. Psychometric properties of the five facet mindfulness questionnaire in depressed adults and development of a short form. Assessment 2011, 18, 308–320. [Google Scholar] [CrossRef]
- Brown, K.W.; Ryan, R.M. The benefits of being present. J. Personal. Soc. Psychol. 2003, 84, 822–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.W.; Coogle, C.L.; Wegelin, J. A pilot randomized controlled trial of mindfulness-based stress reduction for caregivers of family members with dementia. Aging Ment. Health 2016, 20, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Godfrin, K.A.; van Heeringen, C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: A randomized controlled study. Behav. Res. Ther. 2010, 48, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G.; Gómez, A.F. Mindfulness-based interventions for anxiety and depression. Psychiatr. Clin. N. Am. 2017, 40, 739–749. [Google Scholar] [CrossRef]
- Chiesa, A.; Serretti, A. Mindfulness based cognitive therapy for psychiatric disorders: A systematic review and meta-analysis. Psychiatry Res. 2011, 187, 441–453. [Google Scholar] [CrossRef]
- Speca, M.; Carlson, L.E.; Goodey, E.; Angen, M. A randomized, wait-list controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom. Med. 2000, 62, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Hawley, L.L.; Schwartz, D.; Bieling, P.J.; Irving, J.; Corcoran, K.; Farb, N.A.S.; Anderson, A.K.; Segal, Z.V. Mindfulness practice, rumination and clinical outcome in mindfulness-based treatment. Cogn. Ther. Res. 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Del Re, A.C.; Hoyt, W.T.; Davis, J.M. The secret ingredient in mindfulness interventions? A case for practice quality over quantity. J. Couns. Psychol. 2014, 61, 491–497. [Google Scholar] [CrossRef]
- Alsubaie, M.; Dickens, C.; Dunn, B.D.; Gibson, A.; Ukoumunne, O.C.; Evans, A.; Vicary, R.; Gandhi, M.; Kuyken, W. Feasibility and acceptability of mindfulness-based cognitive therapy compared with mindfulness-based stress reduction and treatment as usual in people with depression and cardiovascular disorders: A three-arm randomised controlled trial. Mindfulness 2020, 11, 30–50. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, N.T.; van Vugt, M.K.; Vago, D.R.; Schmalzl, L.; Saron, C.D.; Olendzki, A.; Meissner, T.; Lazar, S.W.; Kerr, C.E.; Gorchov, J.; et al. Mind the hype: A critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspect. Psychol. Sci. 2018, 13, 36–61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).