Abstract
Background: Of all central nervous systems tumors, 10–20% are located in the brainstem; diffuse intrinsic pontine glioma (DIPG) is diagnosed in 80% of them. With over five decades of clinical trial testing, there are no established therapeutic options for DIPG. This research article aims to collate recent clinical trial data and provide a landscape for the most promising therapies that have emerged in the past five years. Methods: PubMed/MEDLINE, Web of Science, Scopus, and Cochrane were systematically searched using the following keywords: Diffuse intrinsic pontine glioma, Pontine, Glioma, Treatment, Therapy, Therapeutics, curative, and/or Management. Both adult and pediatric patients with newly diagnosed or progressive DIPG were considered in the clinical trial setting. The risk of bias was assessed using the ROBINS-I tool. Results: A total of 22 trials were included reporting the efficacy and safety outcomes among patients. First, five trials reported outcomes of blood–brain barrier bypass via single or repeated-dose intra-arterial therapy or convection-enhanced delivery. Second, external beam radiation regimens were assessed for safety and efficacy in three trials. Third, four trials administered intravenous treatment without using chemotherapeutic regimens. Fourth, eight trials reported the combinations of one or more chemotherapeutic agents. Fifth, immunotherapy was reported in two trials in an adjuvant monotherapy in the post-radiotherapy setting. Conclusion: This research article captures a clinical picture of the last five years of the direction toward which DIPG research is heading. The article finds that re-irradiation may prolong survival in patients with progressive DIPG; it also instills that insofar palliative radiotherapy has been a key prognostic choice.
Keywords:
diffuse intrinsic pontine glioma; CNS; tumor; therapies; palliative; quality of life; advances 1. Introduction
Central nervous system (CNS) tumors have a higher mortality rate among all cancers in US children aged 1–19 years [,,]. Of all CNS tumors, 10–20% are located in the brainstem, with diffuse intrinsic pontine glioma (DIPG) diagnosed in 80% of them []. The prognosis is dismal with DIPG with the overall survival rate being lower than 10% at 2 years []. The median survival rates are less than 12 months and the 5-year survival rates are below 2% [,]. Radiation therapy is the current standard of treatment, yet it remains a palliative option as radiotherapy only temporarily relieves symptoms []. DIPG has been classified histopathologically as high-grade astrocytoma (HGA) with most cases being consistent with histological grade III or IV (anaplastic astrocytoma or glioblastoma, respectively) but certain less aggressive cases are histologically classified as grade II (diffuse astrocytoma) []. More recently, the World Health Organization (WHO) classified DIPG as diffuse midline gliomas with histone H3K27M mutation []. With over five decades of clinical trials exploring different chemotherapy and radiotherapy regimens, there are no promising therapeutic options in DIPG []. Several clinical trials of systematic therapies have been tested or are ongoing [].
Certain therapies have recently gained traction for their potential efficacy in DIPG []. Thereby, this research article aims to collate data from recent clinical trials and provide a landscape for the most promising therapeutic options that have emerged in the last five years.
2. Materials and Methods
2.1. Search Strategy
A comprehensive systematic search was conducted using the following databases, adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement 2020 guidelines: PubMed/MEDLINE, Web of Science, Scopus, and Cochrane. The search was conducted from 1 January 2017, until 16 October 2022, without any language limitations (non-English studies were translated to English using Google Translate). Applying the Boolean (and/or) logic, the following keywords were applied: Diffuse intrinsic pontine glioma, Pontine, Glioma, Treatment, Therapy, Therapeutics, curative, and/or Management. The titles and abstracts of shortlisted studies from the given databases were screened independently by two mid-career authors (Z.S., A.S.). In the screening phase, the reference lists were also reviewed, in line with the umbrella methodology to ensure no data were omitted. In the case of any disagreements, a third author (I.C.-O.) was present to resolve them and to reach a consensus. Cohen’s coefficient of the inter-reviewer agreement was computed using Statistical Package for Social Sciences (SPSS, v.25, IBM).
2.2. Inclusion and Exclusion Criteria
The inclusion criteria comprised clinical trials enrolling both adult and pediatric patients of any gender with newly diagnosed or progressive DIPG. The treatment could consist of any of the following: (i) blood–brain barrier bypass (intra-arterial therapy or convection enhanced delivery), (ii) external beam radiation, (iii) intravenous treatment regimens without administering chemotherapeutic agents, (iv) combination regimens with the use of one or more chemotherapeutic agents.
Studies intervening with only surgical procedures were not included. Further, cohorts (retrospective/prospective), case series, case reports, systematic reviews and meta-analyses, brief reports, and letters to editors were excluded.
2.3. Data Extraction (Selection and Coding)
Two mid-career authors (Z.S., A.S.) independently extracted the data from the included trials into a spreadsheet. The third author (I.C.-O.) was present for any disagreements. The pair identified the trials and treatments of the screened studies. Once the independent review of the studies was conducted, the third author (I.C.-O.) assessed the extracted domains from the spreadsheet and conducted the final review against the inclusion criteria.
The data were extracted as follows: Author, Year, Title, Journal, Country, Study design, Inclusion criteria, Intervention given, Method of administration; Number of patients with DIPG, Age at diagnosis (in years), Sex (percentage of males), Previous treatment, Outcome measures; Median OS (in months), Median EFS/PFS (in months), Radiological response (percentage), Clinical improvement (proportion), Tolerance and safety, and Steroid use discontinuation.
Individual study data were prepared in a presentable format during the inclusion phase and the concluding remarks were also added. EndNote X9 (Clarivate, London, UK) was the software used to omit duplicates during the study selection process. In addition, Mendeley (Elsevier, Amsterdam, The Netherlands) was used for bibliographic management.
2.4. Risk of Bias (Quality Assessment)
The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used to assess the risk of bias in the included trials. This tool comprised seven domains. (1) Bias due to confounding; (2) bias due to selection of participants; (3) bias in classification of interventions; (4) bias due to deviations from intended interventions; (5) bias due to missing data; (6) bias in the measurement of outcomes; (7) bias in the selection of the reported result.
Domain-level judgments about the risk of bias were classified as the following: (1) low risk; (2) moderate risk; and (3) serious risk. The traffic light plot of bias assessment and the weighted summary plot of the overall type of bias encountered is illustrated in Section 3.6.
3. Results
During the phase I, the identification phase, a total of 1249 studies were identified. Of these, 234 duplicates were removed. In phase II, the screening phase, 1015 study titles and abstracts were screened, of which 897 were omitted as they did not warrant inclusion against the inclusion criteria. Subsequently, 118 full-text studies were reviewed and assessed for eligibility. Of these, 96 studies were excluded as 28 were non-human studies, 43 of them were model/experimental papers, and 25 were not clinical trials. In phase III, the inclusion phase, a total of 22 trials were included (Figure 1). Kappa’s score was calculated to be 0.91.
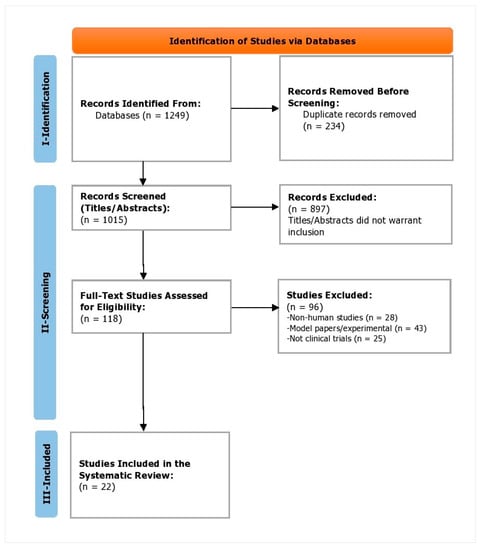
Figure 1.
PRISMA flowchart depicting the study selection process.
A total of 22 trials were found that reported efficacy and safety outcomes among patients with newly diagnosed or progressive DIPG (Table 1, Table 2 and Table 3). First, trials that reported bypassing of the blood–brain barrier (BBB) are reported. In five phase I trials, authors evaluated outcomes of blood–brain barrier (BBB) bypass via single or repeated-dose intra-arterial therapy [] or convection-enhanced delivery (CED) [,,,].

Table 1.
Baseline characteristics of the trial and dosing regimens.

Table 2.
Patient characteristics and outcome measures.

Table 3.
Efficacy and safety outcomes of the trials.
Second, trials that explored different external beam radiation regimens are elaborated. In two randomized controlled trials [,], the authors explored the efficacy and safety of different radiotherapy regimens including conventionally fractionated and hypofractionated radiotherapy in newly diagnosed DIPG. Re-irradiation at three dose levels among patients who had received initial radiotherapy ≥10 months ago was compared with patients with progressive DIPG in a phase I/II trial [].
Third, trials are listed that administered intravenous treatment regimens without administering chemotherapeutic agents. In a phase III trial, authors evaluated the outcomes of epidermal growth factor receptor (EGFR) inhibitors, nimoztuzumab with radiotherapy in newly diagnosed DIPG patients []. In a phase I/II trial, the authors evaluated the outcomes of vorinostat given concomitantly and as adjuvant to radiotherapy in newly diagnosed DIPG []. Adavosertib, a Wee 1 kinase inhibitor, was given with cranial radiation therapy (CRT) in newly diagnosed DIPG in a phase I trial []. A phase II trial administered EBT and valproic acid (VPA), an anti-convulsant, followed by bevacizumab, an anti-vascular endothelial growth factor, and VPA in newly diagnosed DIPG patients [].
Fourth, trials that evaluated combination regimens with the use of one or more chemotherapeutic agents are listed. One phase II trial with newly diagnosed DIPG combined radiotherapy and cefuximab, an epidermal growth factor receptor (EGFR) inhibitor, followed by cefuximab and irinotecan, a topoisomerase I inhibitor []. Another phase I/II trial combined erlotinib, an EGFR inhibitor, with bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, and irinocetan among patients with progressive DIPG []. Two trials explored outcomes of ribociclib (kinase inhibitor) concomitantly with radiotherapy and everolimus (kinase inhibitor) (phase I trial []) and as adjuvant monotherapy (in phase II []) in newly diagnosed patients with DIPG. One phase I/II trial administered concomitant EBT and veliparib, a PARP inhibitor, followed by veliparib and temozolomide, an alkylating agent []. Capecitabine, an alkylating agent, was given in combination with EBT and as adjuvant monotherapy in newly diagnosed DIPG patients in a phase II trial []. Gemcitabine, a nucleoside metabolic inhibitor, was combined with initial EBT among newly diagnosed DIPG patients in a phase I/II trial []. One trial explored the maximum tolerated dose (MTD) and efficacy of cabazitaxel, a microtubule inhibitor, as adjuvant monotherapy in progressive DIPG patients in a phase I/II dose-escalating trial [].
Fifth, trials that administered immunotherapeutic agents as adjuvant monotherapy after radiotherapy are reported. One phase II trial administered pomalidomide, an immunomodulatory drug, among patients with progressive DIPG. One phase I trial administered pelareorep, an immunomodulatory oncolytic virus, combined with sargramostin, a recombinant human granulocyte-macrophage colony-stimulating factor, in patients with progressive DIPG.
3.1. Bypassing the Blood–Brain Barrier
3.1.1. Intra-Arterial Therapy
McCrea et al. explored the tolerability and efficacy of super selective intraarterial cerebral infusion (SIACI) among 10 patients with DIPG who had all previously received radiotherapy, as well as other systemic therapies []. Mannitol (12.5 mL of 20%) was administered to disrupt the blood–brain barrier (BBB), followed by bevacizumab (15 mg/kg), a vascular endothelial growth factor A (VEGF-A) inhibitor, and cetuximab (200 mg/m2), an epidermal growth factor receptor (EGFR) inhibitor, respectively []. The treatment and technique were well-tolerated with no dose-limiting toxicities. In terms of efficacy, the median OS was 17.3 months, which is higher than that for historical controls despite DIPG patients being heavily treated []. This method of delivery warrants further investigation to establish efficacy in DIPG patients [].
3.1.2. Convection-Enhanced Delivery
Heiss et al. evaluated the outcomes including the safety and tolerability of single-dose IL13-PE38QQR, a recombinant cytotoxic chimera of human interleukin 13 (IL-13), and the enzymatic portion of pseudomonas exotoxin A, infused via single-catheter CED (0.125 μg/mL) into 5 patients with progressive DIPG []. The intervention was safe and well-tolerated which occurred due to infusion-related brainstem edema []. There was a temporary improvement in clinical and radiological status in 2 patients (40%) and their dose was escalated to 0.25 μg/mL. CED-supported delivery of IL13-PE did not reach optimal volumes in the tumor and only temporary anti-tumoral effects were observed in 2 patients [].
Pérez-Larraya et al. determined the safety and efficacy outcomes of single-dose CED infusion of DNX-2401, an oncolytic adenovirus that only replicates in tumor cells, through the cerebellar peduncle, followed by radiotherapy in 12 newly diagnosed DIPG patients []. The median OS was favorable at 17.8 months; 11 of the 12 patients had a reduction or stabilization of tumor size but certain adverse events (hemiparesis in 1 patient and tetraparesis in 1 patient) were reported [].
Bander et al. evaluated the efficacy and safety of ≥2 doses of CED, via the supratentorial trajectory with intraprocedural stereotactic placement using MRI guidance, infusing I-8H9 monoclonal antibody among 7 DIPG patients who previously received radiotherapy []. Sequential CED infusions were well-tolerated and the second infusion significantly reduced radial error and absolute tip error compared to the first infusion []. This was a phase I trial whereby Bander et al. lay support for repeated CED in DIPG patients for further evaluation of improved survival rates [].
Majzner et al. similarly delivered two doses of disialoganglioside GD2-directed chimeric antigen receptor (CAR) T cell; the first dose administered intravenously and the second dose delivered intracerebroventricularly to 3 patients with K27M mutation in genes encoding histone H3 (H3K27M) at any stage. All patients had received radiotherapy ≥ 6 months before enrollment. Of the three patients, two had improvement radiologically and one had tumor progression []. All patients had infusion-related toxicity which was reversible with intensive support []. With the necessary management algorithm for tumor inflammation-associated neurotoxicity (TIAN), delivery of CAR T-cell therapy via CED is a promising treatment option that may be explored further in trial settings [].
3.2. Different Radiotherapy Regimens
3.2.1. Hypofractionated Radiation Therapy
Zagloul et al. confirmed the noninferiority of hypofractionated (HF) radiation therapy with three arms, arm 1 received HF therapy of 39 Gy in 13 fractions, arm 2 received HF therapy of 45 Gy in 15 fractions, and arm 3 received conventional fractionation (CF) of 54 Gy in 30 fractions []. The median OS was the highest in low-dose HF across arm 1 (9.6 months) followed by CF in arm 3 (8.7 months) and high-dose HF in arm 2 (8.2 months). Younger age (2–5 years) had a higher prognosis with lower HF dose given in arm 1 which was not found in arm 2 recipients [].
Izzuddeen et al. compared the efficacy and tolerability of CF radiotherapy and low-dose HF radiotherapy (39 Gy in 13 fractions) with concurrent and adjuvant temozolomide, an alkylating agent []. The group that received HF radiotherapy and temozolomide did not show any improved survival rates (12 months vs. 11 months) and there was an increase in grade 3/4 hematological toxicity (n = 5, 28%) [].
3.2.2. Re-Irradiation
Amsbaugh et al. found clinical improvement and improved quality of life with conventionally fractionated re-irradiation among patients who had ≥10 months from the end of initial radiotherapy []. The lowest dose arm of 24 Gy in 12 fractions had the highest utility and is considered safe and effective for re-irradiation in DIPG patients [].
3.3. Non-Chemotherapeutic Agent Regimens
Fleischhack et al. assessed the safety and efficacy of intravenous nimotuzumab, an anti-EGFR humanized monoclonal antibody, combined with external beam radiotherapy (EBT) among treatment-naïve patients with DIPG diagnosed in the last 3 months. The intervention was well-tolerated and no adverse events were observed, such as those produced by other EGFR-targeting agents e.g., severe acneiform rash, hypokalemia, or hypomagnesemia []. Nimotuzumab administered concomitantly and continued after EBT had comparable efficacy to intensive chemotherapy and EBT among newly diagnosed DIPG e.g., median OS of 9.4 months while offering the benefit of being administered in outpatient settings and associated with reduced hospitalization stays [].
Su et al. reported the efficacy and tolerability of Vorinostat, an oral histone deacetylase inhibitor, given with initial radiotherapy and as monotherapy afterward []. While it was well tolerated, there were no survival benefits in patients with newly diagnosed DIPG [].
Mueller et al. determined the tolerability of Adavosertib, an orally administered blood–brain barrier penetrant, Wee 1 kinase inhibitor when combined with cranial radiation therapy (CRT) among patients with newly diagnosed DIPG []. While the treatment was well-tolerated, there was no survival benefit with a median OS of 11.1 months [].
Su et al. explored the tolerability and efficacy of valproic acid (VPA), an anti-convulsant, and radiation, followed by VPA and bevacizumab, an anti-VEGF humanized monoclonal antibody, among patients with newly diagnosed DIPG in a phase II trial []. While the concomitant use of VPA and bevacizumab was well-tolerated, there was no improvement in survival outcomes with a median OS of 10.3 months [].
3.4. Chemotherapeutic Agent Regimens
Macy et al. evaluated the efficacy and safety of cetuximab, anti-EGFR humanized with concurrent radiation therapy followed by cetuximab and irinotecan, a topoisomerase I inhibitor, among patients with newly diagnosed DIPG []. While there was some improvement in PFS, the median OS was 12.1 months and the therapy regimen does not warrant further investigation [].
El-Khouly et al. conducted a phase I/II trial to determine the safety, tolerability, and efficacy of bevacizumab, a VEGF inhibitor, irinotecan, a topoisomerase I inhibitor, and Erlotinib, an EGFR inhibitor, among 9 patients with progressive DIPG. The median OS was 13.8 months which was higher than that of historical controls for this subset of patients and the regimen was well-tolerated [].
DeWire et al. conducted a phase I trial to determine the tolerability and efficacy of Ribociclib (CDK4/6-inhibitor) and Everolimus (kinase inhibitor) for patients newly diagnosed with DIPG within 30 days of receiving a 10% standard dose of radiotherapy []. The treatment was well-tolerated and apparently improved median OS to 13.9 months; however, when two patients who were less than 3 years at diagnosis e.g., associated with better prognosis were removed, the median OS decreased to 10.8 months which suggests no additional efficacy of treatment [].
DeWire et al. also conducted a phase I/II trial to identify the safety, feasibility, and early efficacy of ribociclib (CDK4/6-inhibitor) in 9 newly diagnosed DIPG patients []. Ribociclib adjuvant monotherapy post-radiotherapy had improved median OS (16.1 months) []. Yet, its safety is not clear as there was increased tumor necrosis volume in 4 patients (40%) warranting further volumetric analyses of necrosed tumors [].
Baxter et al. conducted a phase I/II trial of 65 patients with newly diagnosed DIPG who received concomitant veliparib (PARP inhibitor) and radiation therapy followed by veliparib and temozolomide (alkylating agent) []. While the treatment was generally well-tolerated with limited DLTs, there were no survival benefits categorized as 1-year and 2-year survival rates of 37.2% and 5.3%, respectively [].
Kilburn et al. conducted a phase II trial with capecitabine (alkylating agent) given concomitantly with radiotherapy followed by adjuvant capecitabine among 44 patients with newly diagnosed DIPG []. There was no survival benefit (OS and PFS were comparable to historical controls) with the treatment regimen but it was well-tolerated [].
Zanten et al. conducted a phase I/II trial to determine the efficacy, safety, and tolerability of gemcitabine, a nucleoside metabolic inhibitor, during radiotherapy among 9 children with newly diagnosed DIPG []. The treatment was well-tolerated and a dose of up to 200 mg/m2/once weekly with radiotherapy was safe []. There were, however, no survival benefits with a median OS of 12.4 months and 8.7 months in intermediate- and high-risk patients [].
Manley et al. explored the maximum tolerated dose (MTD), safety, and efficacy of Cabazitaxel, a chemotherapeutic agent, in patients with progressive treatment-refractory DIPG in a phase I/II dose-escalating trial []. The maximum tolerated dose (MTD) was found to be 30 mg/m2 and was well-tolerated. There was no anti-tumor activity with the MTD and there was no improvement in survival outcomes [].
3.5. Immunotherapy
Fangusaro et al. conducted a phase II trial and determined the efficacy and safety of Pomalidomide, an immunomodulatory drug, among 9 patients with progressive DIPG []. There were no favorable survival outcomes of pomalidomide monotherapy with no objective response (OR) or long-term stable disease (LTSD) found in the patients and a median OS of 3.78 months [].
Schuelke et al. determined the safety of sargramostin, a recombinant human granulocyte-macrophage colony-stimulating factor, and pelareorep, an immunomodulatory oncolytic virus, among 2 patients with progressive DIPG in a phase I trial []. While the treatment was well-tolerated, the sample size was too small to evaluate survival rates; the treatment warrants further investigation for efficacy [].
3.6. Risk of Bias Synthesis
Overall, 19 studies (86.4%) had a low risk of bias, 2 (9.1%) had moderate risks and 1 (4.5%) had a serious risk of bias (Figure 2). On noting bias due to confounding, 17 studies (77.3%) had a low risk of bias, 4 (18.2%) had moderate risk, whereas 1 (4.5%) had a serious risk of bias. When assessing bias due to the selection of participants, a total of 14 studies (63.6%) had a low risk of bias, whereas 7 (31.8%) had a moderate risk of bias and 1 (4.5%) had a serious risk of bias. Noting the bias in the classification of interventions, 20 studies (90.9%) had low risks of bias while 2 studies (9.1%) had a moderate risk of bias. Bias due to deviations from intended interventions had low risk in 21 studies (95.5%) and moderate risk in 1 study (4.5%). On noting bias due to missing outcome data, 18 studies (81.8%) had a low risk of bias, and 2 studies each (9.1%) had moderate and serious risks of bias. Assessment of bias in the measurement of outcomes yielded 18 studies (81.8%) with a low risk of bias and 4 studies (18.2%) with a moderate risk of bias. The risk of bias in the selection of the reported result was low in 12 studies (54.4%), moderate in 9 studies (40.9%), and serious in 1 study (4.5%) (Figure 2).
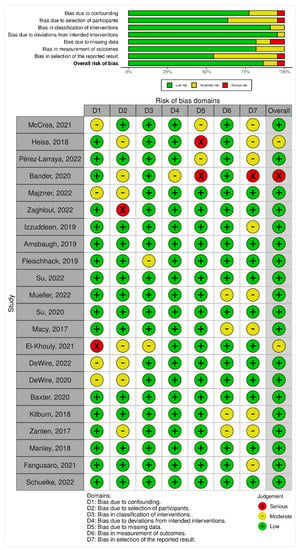
Figure 2.
Summary plot and traffic light plot depicting risk of bias among the included studies [,,,,,,,,,,,,,,,,,,,,,].
4. Discussion
This research article aimed to collate evidence from all trials conducted in the last five years to evaluate the efficacy and safety of different treatments for DIPG. We assessed 22 trials and five key therapeutic regimen themes emerged including blood–brain barrier (BBB) bypass (intra-arterial delivery or convection-enhanced delivery (CED)), radiotherapy regimens, non-chemotherapeutic agent regimens, chemotherapeutic agent regimens, and immunotherapy. The 22 trials included in this study are likely to constitute all available clinical trial evidence, due to the robust search strategy and rigorous screening process.
Of the 5 trials reporting BBB bypass techniques, 3 reported overall survival (OS), 4 reported radiological responses, 3 reported clinical improvement, all 5 reported tolerance and safety, and 3 reported steroid discontinuation. All 3 trials that explored radiotherapy regimens and doses did report OS, 2 trials reported progression-free survival (PFS), 1 reported radiological response, 1 reported clinical improvement, and all 3 reported tolerance and safety outcomes. Four trials that explored non-chemotherapeutic agents and OS were reported across all 4 trials, PFS in 1 trial, event-free survival (EFS) in 2 trials, radiological response in 3 trials, and safety/tolerance in all 4 trials. A total of 8 trials administered chemotherapeutic agents in combination with other therapeutics and all 8 trials reported OS, 5 trials reported PFS, all 8 trials reported radiological response, 3 trials reported clinical improvement, and all 8 reported tolerability/safety. Lastly, of the 2 trials that administered immunotherapy agents, both reported OS, 1 reported PFS, 2 reported radiological response, 1 reported clinical improvement, and both identified safety/tolerance. There were differences among studies, even within the same theme, with regard to outcome measures, specifically radiological response.
All therapeutic agents were initiated at different time points in the natural clinical course of DIPG. The most commonly observed subset of patients was newly diagnosed across 12 trials, followed by progressive DIPG in 8 trials. One trial enrolled patients at any stage and 1 trial did not specify the stage of DIPG. Patient demographics were similar across the 22 trials. Age at diagnosis was primarily mid-childhood. The gender ratio was somewhat well-balanced, ranging from 35–65% across the studies.
4.1. Intra-Arterial Delivery
Superselective intraarterial cerebral infusion (SIACI) improved survival rates (median OS: 17.3 months) when offered to patients with progressive DIPG. SIACI offers an advantage over intravenous drug delivery through selective blood–brain barrier (BBB) opening. Our synthesis supports the intraarterial delivery of cetuximab and bevacizumab with the initial administration of mannitol to increase the absorption of the drugs. As we found support for safe and well-tolerated repeated CED infusions, further trials can consider expanding the number of patients and determining the efficacy of SIACI. A phase I trial (NCT05271240) is underway that is planning enrollment of 432 patients with glioblastoma multiforme (GBM) and comparing repeated mannitol-infusion followed by SIACI of bevacizumab with temozolomide and standard radiation to temozolomide and standard radiation only. As data are still emerging regarding the safety and tolerability of intra-arterial delivery, further trials can consider using a labeling agent to assess drug delivery distribution. Another consideration is to identify molecular targets with a biopsy to optimize the agent of choice. With evidence of safe bypassing of the BBB, it is of note to consider targeting tumor cells based on the biology e.g., EGFR and/or VEGF positive.
4.2. Convection-Enhanced Delivery
CED is an emerging therapy for DIPG due to its ability to bypass the BBB and deliver pertinent doses of treatment in relevant brain volumes. The agents tested in clinical trials via CED were 124I-8H9 [], IL13-Pseudomonas toxin [], DNX-2401 (an oncolytic virus) [], and GD2-CAR T cells []. Of the 4 trials evaluating the role of convection-enhanced delivery (CED) in the treatment of DIPG, there were somewhat favorable outcomes based on different techniques, agents used, and stages of DIPG. Intra-tumoral infusion with an oncolytic virus had the most favorable outcomes with a median OS of 17.8 months in newly diagnosed DIPG but there was an increased risk of adverse events related to infusion-related brainstem edema. Augmentation of CED-infused pharmacological agents gained support from observational studies such as Tsvankin et al. who found improvement in median OS with CED of dasatinib, a tyrosine kinase inhibitor, in a transgenic H3.3K27M mutant murine model []. It remains unclear which pharmacological agent offers the highest survival rates and it is reasonable to consider CED to have a plateau effect given its ability to target localized disease.
Repeated CED infusions were well-tolerated but neurological signs and symptoms are present. Hollingworth et al. [] measured infusion-related side effects of CED in DIPG with the Pontine Neurological Observation Score (PONScore), a standardized tool with a 57-point scale, to determine their frequency and recovery during infusion. As CED is gaining preliminary support from the literature for its efficacy in DIPG, there is a gap in standardized documentation of its side effects for which a scale such as PONScore [] can highlight the nature and timing of neurological injury during infusion. Further clinical trials must consider it imperative to consider meticulously documenting infusion-related side effects to address patient outcomes. As of now, CED is being tested in early phase clinical trials for treatment with DIPG which has the potential to control the regional disease. However, CED as a standalone may not be able to control spread to distant areas e.g., outside the brainstem [] or leptomeningeal involvement [], by nature of its delivery and it may be advantageous to consider combination approaches e.g., craniospinal radiation or intrathecal delivery [], to meaningfully improve survival rates in DIPG patients who already have a dismal prognosis.
4.3. Radiotherapy
The mainstay of treatment for DIPG is conventionally fractionated radiation therapy (RT), delivered across a 6-week period. However, such RT only transiently improves symptoms without prominent survival benefits. Hypofractionated RT regimens did not demonstrate survival benefits across two trials in our study but may provide temporary relief. In a trial, re-irradiation did improve survival outcomes and quality of life among DIPG patients who had received initial radiotherapy ≥ 10 months ago (strongest support for low-dose conventional RT at 24 Gy in 12 fractions). Gallitto et al. [] corroborate the findings of the trial and suggests re-irradiation at first progression as an effective palliative therapy with a mean increase of ~3 months to OS compared to controls. Combination immunotherapy agents (PD-1 inhibitor, nivolumab) [] with re-irradiation have also shown improved life spans in progressive DIPG patients. Overall, certain patients can tolerate re-irradiation, have reduced symptoms, and improve survival rates by a few months. Current trials have compared different radiation doses and fractionation for re-irradiation; support for conventional fractionation and low-dose radiation is found in clinical trials. Further clinical trials can address the frequency of re-irradiation, the gap between radiotherapy, and optimal radiation dose and fractionation for children with progressive DIPG [].
4.4. Other Regimens
Other trials explored the efficacy of radiotherapy with neoadjuvant non-chemotherapeutic interventions (nimotuzumab, bevacizumab, adavosertib, and vorinostat) with or without adjuvant therapy afterward in newly diagnosed DIPG patients and found no additional survival benefit compared to historical controls. Similarly, there was no significant improvement in survival outcomes with a neoadjuvant chemotherapeutic alkylating agent (temozolomide) or anti-metabolites (capecitabine, gemcitabine, cabazitaxel) in combination with radiotherapy, and other agents (cetuximab, an EGFR inhibitor, and veliparib, a PARP inhibitor). Moreover, immunotherapy regimens did not improve median OS with pomalidomide and pelareorep combined with sargramostim. There were, however, two trials by DeWire et al. [,] that found some improvement in median OS with combination regimens in newly diagnosed DIPG patients: median OS of 13.9 months with ribociclib and everolimus, both kinase inhibitors, within 30 days of receiving 10% standard radiation doses, and median OS of 16.1 months with ribociclib together with radiotherapy and adjuvant monotherapy. There is emerging support for ribociclib, a CDK4/6 inhibitor, together with targeted radiotherapy among treatment-naïve DIPG patients. However, in both these trials, there was a prominent frequency of ≥grade 3 events which may be due to the treatment. Another trial [] administered bevaziumab, a VEGF inhibitor, erlotinib, an EGFR inhibitor, and irinotecan, a topoisomerase I inhibitor among progressive DIPG patients. Of note, this trial indicated that anti-EGFR agents and anti-VEGF antibodies when combined with chemotherapy have some additive antitumor activity (median OS of 13.8 months) and are well-tolerated. These findings provide support for the collaboration of anti-VEGF and anti-EGFR for inhibiting tumor growth and angiogenesis in aggressive DIPG, combined with chemotherapy.
4.5. Potential Molecular Targets
The lack of targeted therapies for DIPG is in part due to the lack of routine biopsies conducted in these tumors. Neurosurgeons have been reluctant to perform biopsies due to more risks than direct benefits to the patients. As molecular genetic techniques are expanding in oncology, many centers have begun conducting stereotactic biopsy to support ongoing research which requires molecular characterization and potentially druggable targets toward more individualized treatments [,]. About 80% of all DIPG cases have a specific point mutation that results in the substitution of lysine 27 on the amino-terminal tail with methionine (H3K27M) in histone isoforms H3.1 or H3.3, encoded by genes HIST1H3B and H3F3A respectively [,,]. There are subtle differences in prognosis and outcomes with both; H3.1 histone mutations have a better prognosis and this has been recognized by the World Health Organization of CNS tumors [,]. Along with these histone modifications, molecular profiling has enumerated numerous targets for therapeutic interventions. These include ACVR1 mutations (~30% of DIPG tumors) and co-occur with H3.1 and TP53 mutations (~22–40% of DIPG tumors) which co-occur with PDFR amplification [,,]. PDFR amplification (PDFRA) is common and present in nearly 1/3rd of high-grade gliomas; when co-segregated with H3.3 mutations (H3.3K27M), these tumors are clinically aggressive []. PDFRA combined with PIK3R1 and PIK3CA are drivers of the PI3K pathway which contributes to aggressive DIPG [,].
4.6. Strengths and Future Directions
The 22 studies in this study constitute the latest clinical trends for DIPG therapeutics with an inclusive search strategy and rigorous screening process. Two independent reviewers screened the full text and there was 91.6% agreement. As part of the search strategy, an umbrella review methodology was also applied which revealed four potential papers, though none were included. Another strength was the double-checking of data entry by the second reviewer. The conclusions made from this study are based on all the latest available evidence from clinical trials in the past 5 years. Therefore, the data included in this study are not observational, which means that the quality of included studies is not compromised. Lastly, the study was flexible in terms of eligibility criteria in relation to the nature of treatment which allowed for a comprehensive synthesis.
The risk of bias among the included trials was largely low with 86.4% depicting low concerns. However, two studies had moderate risks (El-Khouly, 2021; Heiss, 2018) and one had a serious risk of bias (Bander, 2020). Based on the biases we reported in this study, future trials in this discipline of research ought to ensure datasets reporting of patient outcomes including follow-up. Furthermore, in an effort to improve outcomes for patients with DIPG, confounding variables including multi-disciplinary therapies must be assessed in a manner that may quantify various approaches both singularly or combined for survival (OS, EFS, PFS) and associated outcomes.
4.7. Limitations
There are certain limitations of this study. This study included early phase clinical trials that had a small sample size. We omitted case series and case reports to ensure only high-tier evidence was included, which may have led to omission of specific cases in literature. As such, the findings must be explored in further clinical trials to prove the safety, characterization of adverse events, and clinical outcomes if given in a larger sample size. While certain trials have demonstrated efficacy with prolonged survival rates, the results cannot be generalized and must be corroborated in further multi-center, blinded, randomized controlled trials to prove its benefits.
5. Conclusions
DIPG is a pediatric brain tumor that has a dismal prognosis with a median survival rate of nine months. There is currently no effective treatment beyond palliative radiotherapy. Our analysis of all clinical trials in the last five years captures 22 studies that point toward the direction in which the ongoing research is headed. Importantly, we show that a promising therapeutic strategy is a blood–brain barrier (BBB) bypass. We also find support for chemotherapeutic agents combined with VEGF- and EGFR inhibitors. Finally, our synthesis suggests re-irradiation as another strategy that can prolong survival in progressive DIPG patients. We collate current evidence to guide future research and therapeutic candidates in DIPG.
Author Contributions
Conceptualization, S.F., S.H. and A.R.; methodology, Z.S., A.S., M.S. and K.R.-V.; software, Z.S., A.S. and M.S.; formal analysis, S.F., S.H. and A.R.; investigation, S.F., S.H., A.R., Z.S., A.S., M.S., K.R.-V., M.F. and I.C.-O.; resources, S.F., S.H., A.R., Z.S. and A.S.; data curation, S.F., S.H., A.R., Z.S. and A.S.; writing—original draft preparation, S.F., S.H., A.R., Z.S., A.S., M.S., K.R.-V., M.F. and I.C.-O.; writing—review and editing, S.F., S.H., A.R., Z.S., A.S., M.S., K.R.-V., M.F. and I.C.-O.; visualization, S.F., S.H., A.R. and A.S.; supervision, M.F. and I.C.-O.; project administration, M.F., A.S. and I.C.-O. are co-guarantors of this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data utilized for the purpose of this study are available publicly and online. Additional data may be requested by the corresponding author (A.S.) on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Linabery, A.M.; Ross, J.A. Trends in childhood cancer incidence in the US (1992–2004). Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 112, 416–432. [Google Scholar]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015, 16, x1–x36. [Google Scholar] [CrossRef]
- Li, G.; Mitra, S.S.; Monje, M.; Henrich, K.N.; Bangs, C.D.; Nitta, R.T.; Wong, A.J. Expression of epidermal growth factor variant III (EGFRvIII) in pediatric diffuse intrinsic pontine gliomas. J. Neurooncol. 2012, 108, 395–402. [Google Scholar] [CrossRef]
- Rashed, W.M.; Maher, E.; Adel, M.; Saber, O.; Zaghloul, M.S. Pediatric diffuse intrinsic pontine glioma: Where do we stand? Cancer Metastasis Rev. 2019, 38, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Janssens, G.O.; Gandola, L.; Bolle, S.; Mandeville, H.; Ramos-Albiac, M.; van Beek, K.; Benghiat, H.; Hoeben, B.; La Madrid, A.M.; Kortmann, R.-D. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur. J. Cancer 2017, 73, 38–47. [Google Scholar] [CrossRef]
- Wiese, M.; Hamdan, F.H.; Kubiak, K.; Diederichs, C.; Gielen, G.H.; Nussbaumer, G.; Carcaboso, A.M.; Hulleman, E.; Johnsen, S.A.; Kramm, C.M. Combined treatment with CBP and BET inhibitors reverses inadvertent activation of detrimental super enhancer programs in DIPG cells. Cell Death Dis. 2020, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Gallitto, M.; Lazarev, S.; Wasserman, I.; Stafford, J.M.; Wolden, S.L.; Terezakis, S.A.; Bindra, R.S.; Bakst, R.L. Role of radiation therapy in the management of diffuse intrinsic pontine glioma: A systematic review. Adv. Radiat. Oncol. 2019, 4, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Santa-Maria Lopez, V.; Hendrikse, N.H.; Kaspers, G.J.L.; Loizos, G.; Sumerauer, D.; Nysom, K.; Pruunsild, K.; Pentikainen, V. Diagnostics and treatment of diffuse intrinsic pontine glioma: Where do we stand? J. Neurooncol. 2019, 145, 177–184. [Google Scholar] [CrossRef]
- Long, W.; Yi, Y.; Chen, S.; Cao, Q.; Zhao, W.; Liu, Q. Potential new therapies for pediatric diffuse intrinsic pontine glioma. Front. Pharmacol. 2017, 8, 495. [Google Scholar] [CrossRef]
- Srikanthan, D.; Taccone, M.S.; Van Ommeren, R.; Ishida, J.; Krumholtz, S.L.; Rutka, J.T. Diffuse intrinsic pontine glioma: Current insights and future directions. Chin. Neurosurg. J. 2021, 7, 6. [Google Scholar] [CrossRef]
- McCrea, H.J.; Ivanidze, J.; O’Connor, A.; Hersh, E.H.; Boockvar, J.A.; Gobin, Y.P.; Knopman, J.; Greenfield, J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021, 28, 371–379. [Google Scholar] [CrossRef]
- Heiss, J.D.; Jamshidi, A.; Shah, S.; Martin, S.; Wolters, P.L.; Argersinger, D.P.; Warren, K.E.; Lonser, R.R. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2018, 23, 333–342. [Google Scholar] [CrossRef]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Bander, E.D.; Ramos, A.D.; Wembacher-Schroeder, E.; Ivasyk, I.; Thomson, R.; Morgenstern, P.F.; Souweidane, M.M. Repeat convection-enhanced delivery for diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2020, 26, 661–666. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Zaghloul, M.S.; Nasr, A.; Tolba, M.; Refaat, A.; Youssef, A.; Mosaab, A.; Enayet, A.; Arafa, O.; Maher, E.; Eldebawy, E. Hypofractionated Radiation Therapy For Diffuse Intrinsic Pontine Glioma: A Noninferiority Randomized Study Including 253 Children. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 360–368. [Google Scholar] [CrossRef]
- Izzuddeen, Y.; Gupta, S.; Haresh, K.P.; Sharma, D.; Giridhar, P.; Rath, G.K. Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: A randomized controlled trial. J. Neurooncol. 2020, 146, 91–95. [Google Scholar] [CrossRef]
- Amsbaugh, M.J.; Mahajan, A.; Thall, P.F.; McAleer, M.F.; Paulino, A.C.; Grosshans, D.; Khatua, S.; Ketonen, L.; Fontanilla, H.; McGovern, S.L. A phase 1/2 trial of reirradiation for diffuse intrinsic pontine glioma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 144–148. [Google Scholar] [CrossRef]
- Fleischhack, G.; Massimino, M.; Warmuth-Metz, M.; Khuhlaeva, E.; Janssen, G.; Graf, N.; Rutkowski, S.; Beilken, A.; Schmid, I.; Biassoni, V. Nimotuzumab and radiotherapy for treatment of newly diagnosed diffuse intrinsic pontine glioma (DIPG): A phase III clinical study. J. Neurooncol. 2019, 143, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Kilburn, L.B.; Mansur, D.B.; Krailo, M.; Buxton, A.; Adekunle, A.; Gajjar, A.; Adamson, P.C.; Weigel, B.; Fox, E. Phase I/II trial of vorinostat and radiation and maintenance vorinostat in children with diffuse intrinsic pontine glioma: A Children’s Oncology Group report. Neuro Oncol. 2022, 24, 655–664. [Google Scholar] [CrossRef]
- Mueller, S.; Cooney, T.; Yang, X.; Pal, S.; Ermoian, R.; Gajjar, A.; Liu, X.; Prem, K.; Minard, C.G.; Reid, J.M. Wee1 kinase inhibitor Adavosertib with radiation in newly diagnosed diffuse intrinsic pontine glioma: A Children’s Oncology Group phase 1 consortium study. Neuro-Oncol. Adv. 2022, 4, vdac073. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Murray, J.C.; McNall-Knapp, R.Y.; Bowers, D.C.; Shah, S.; Adesina, A.M.; Paulino, A.C.; Jo, E.; Mo, Q.; Baxter, P.A. A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr. Blood Cancer 2020, 67, e28283. [Google Scholar] [CrossRef]
- Macy, M.E.; Kieran, M.W.; Chi, S.N.; Cohen, K.J.; MacDonald, T.J.; Smith, A.A.; Etzl, M.M.; Kuei, M.C.; Donson, A.M.; Gore, L. A pediatric trial of radiation/cetuximab followed by irinotecan/cetuximab in newly diagnosed diffuse pontine gliomas and high-grade astrocytomas: A Pediatric Oncology Experimental Therapeutics Investigators’ Consortium study. Pediatr. Blood Cancer 2017, 64, e26621. [Google Scholar] [CrossRef] [PubMed]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Jansen, M.H.A.; Bakker, D.P.; Sanchez Aliaga, E.; Hendrikse, N.H.; Vandertop, W.P.; van Vuurden, D.G.; Kaspers, G.J.L. A phase I/II study of bevacizumab, irinotecan and erlotinib in children with progressive diffuse intrinsic pontine glioma. J. Neurooncol. 2021, 153, 263–271. [Google Scholar] [CrossRef]
- DeWire, M.; Lazow, M.; Campagne, O.; Leach, J.; Fuller, C.; Senthil Kumar, S.; Stanek, J.; de Blank, P.; Hummel, T.R.; Pillay-Smiley, N. Phase I study of ribociclib and everolimus in children with newly diagnosed DIPG and high-grade glioma: A CONNECT pediatric neuro-oncology consortium report. Neuro-Oncol. Adv. 2022, 4, vdac055. [Google Scholar] [CrossRef] [PubMed]
- DeWire, M.; Fuller, C.; Hummel, T.R.; Chow, L.M.L.; Salloum, R.; de Blank, P.; Pater, L.; Lawson, S.; Zhu, X.; Dexheimer, P. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J. Neurooncol. 2020, 149, 511–522. [Google Scholar] [CrossRef]
- Baxter, P.A.; Su, J.M.; Onar-Thomas, A.; Billups, C.A.; Li, X.-N.; Poussaint, T.Y.; Smith, E.R.; Thompson, P.; Adesina, A.; Ansell, P. A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: A Pediatric Brain Tumor Consortium study. Neuro Oncol. 2020, 22, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, L.B.; Kocak, M.; Baxter, P.; Poussaint, T.Y.; Paulino, A.C.; McIntyre, C.; Lemenuel-Diot, A.; Lopez-Diaz, C.; Kun, L.; Chintagumpala, M. A pediatric brain tumor consortium phase II trial of capecitabine rapidly disintegrating tablets with concomitant radiation therapy in children with newly diagnosed diffuse intrinsic pontine gliomas. Pediatr. Blood Cancer 2018, 65, e26832. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen van Zanten, S.E.M.; El-Khouly, F.E.; Jansen, M.H.A.; Bakker, D.P.; Sanchez Aliaga, E.; Haasbeek, C.J.A.; Wolf, N.I.; Zwaan, C.M.; Vandertop, W.P.; van Vuurden, D.G. A phase I/II study of gemcitabine during radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma. J. Neurooncol. 2017, 135, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.E.; Trippett, T.; Smith, A.A.; Macy, M.E.; Leary, S.E.S.; Boklan, J.; Cohen, K.J.; Goldman, S.; Kilburn, L.B.; Dhall, G. A phase 1/2 dose-finding, safety, and activity study of cabazitaxel in pediatric patients with refractory solid tumors including tumors of the central nervous system. Pediatr. Blood Cancer 2018, 65, e27217. [Google Scholar] [CrossRef]
- Fangusaro, J.; Cefalo, M.G.; Garré, M.L.; Marshall, L.V.; Massimino, M.; Benettaib, B.; Biserna, N.; Poon, J.; Quan, J.; Conlin, E. Phase 2 Study of Pomalidomide (CC-4047) Monotherapy for Children and Young Adults With Recurrent or Progressive Primary Brain Tumors. Front. Oncol. 2021, 11, 660892. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.R.; Gundelach, J.H.; Coffey, M.; West, E.; Scott, K.; Johnson, D.R.; Samson, A.; Melcher, A.; Vile, R.G.; Bram, R.J. Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors. Neuro-Oncol. Adv. 2022, 4, vdac085. [Google Scholar] [CrossRef] [PubMed]
- Tsvankin, V.; Hashizume, R.; Katagi, H.; Herndon, J.E.; Lascola, C.; Venkatraman, T.N.; Picard, D.; Burrus, B.; Becher, O.J.; Thompson, E.M. ABC transporter inhibition plus dexamethasone enhances the efficacy of convection enhanced delivery in H3. 3K27M mutant diffuse intrinsic pontine glioma. Neurosurgery 2020, 86, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, M.; Zacharoulis, S. Infusion-related side-effects during convection enhanced delivery for brainstem-diffuse midline glioma/diffuse intrinsic pontine glioma. J. Neurooncol. 2022, 159, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Buczkowicz, P.; Hawkins, C. Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front. Oncol. 2015, 5, 147. [Google Scholar] [CrossRef]
- Sethi, R.; Allen, J.; Donahue, B.; Karajannis, M.; Gardner, S.; Wisoff, J.; Kunnakkat, S.; Mathew, J.; Zagzag, D.; Newman, K. Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. J. Neurooncol. 2011, 102, 121–127. [Google Scholar] [CrossRef]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165, 77–95. [Google Scholar] [CrossRef]
- Kline, C.; Liu, S.J.; Duriseti, S.; Banerjee, A.; Nicolaides, T.; Raber, S.; Gupta, N.; Haas-Kogan, D.; Braunstein, S.; Mueller, S. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: A single-institution experience. J. Neurooncol. 2018, 140, 629–638. [Google Scholar] [CrossRef]
- Cacciotti, C.; Liu, K.X.; Haas-Kogan, D.A.; Warren, K.E. Reirradiation practices for children with diffuse intrinsic pontine glioma. Neuro-Oncol. Pract. 2021, 8, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Puget, S.; Beccaria, K.; Blauwblomme, T.; Roujeau, T.; James, S.; Grill, J.; Zerah, M.; Varlet, P.; Sainte-Rose, C. Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Child’s Nerv. Syst. 2015, 31, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Hamisch, C.; Kickingereder, P.; Fischer, M.; Simon, T.; Ruge, M.I. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: A systematic review and meta-analysis of 735 cases. J. Neurosurg. Pediatr. 2017, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012, 44, 251–253. [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M. Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Jones, C.; Baker, S.J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer 2014, 14, 651–661. [Google Scholar] [CrossRef]
- Grill, J.; Puget, S.; Andreiuolo, F.; Philippe, C.; MacConaill, L.; Kieran, M.W. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr. Blood Cancer 2012, 58, 489–491. [Google Scholar] [CrossRef]
- Puget, S.; Philippe, C.; Bax, D.A.; Job, B.; Varlet, P.; Junier, M.-P.; Andreiuolo, F.; Carvalho, D.; Reis, R.; Guerrini-Rousseau, L. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS ONE 2012, 7, e30313. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Hoeman, C.; Rakopoulos, P.; Pajovic, S.; Letourneau, L.; Dzamba, M.; Morrison, A.; Lewis, P.; Bouffet, E.; Bartels, U. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014, 46, 451–456. [Google Scholar] [CrossRef]
- Paugh, B.S.; Zhu, X.; Qu, C.; Endersby, R.; Diaz, A.K.; Zhang, J.; Bax, D.A.; Carvalho, D.; Reis, R.M.; Onar-Thomas, A. Novel Oncogenic PDGFRA Mutations in Pediatric High-Grade GliomasPDGFRA Mutations in Gliomagenesis. Cancer Res. 2013, 73, 6219–6229. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; DeWire, M.; Ryall, S.; Buczkowicz, P.; Leach, J.; Miles, L.; Ramani, A.; Brudno, M.; Kumar, S.S.; Drissi, R. Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: Implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol. Commun. 2016, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).