Abstract
Objectives: To review the current knowledge regarding the involvement of human papilloma virus (HPV) infection and the immune system in the development of head and neck squamous cell carcinoma (HNSCC). Methods: An electronic literature search was conducted to identify articles published between 1990 and 2019 pertaining to tumor-infiltrating immune cells (TICs) in HNSCC using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Issues of clinical relevance, including tumor location, the number of tumor samples, the inclusion of additional specimens (dysplastic or normal mucosa), tumor size, methods used for HPV detection, relationship between antigen expression and patient characteristics (age, gender, smoking, alcohol consumption, etc.), and prognostic data (overall survival (OS) and recurrence-free survival (RFS)) were assessed by four blinded investigators. Results: The search identified 335 relevant studies, of which 41 met the inclusion criteria. Of these, 7 studies focused on the peripheral blood immune cell concentration in patients with HNSCC according to HPV status, and 36 studies investigated TICs in the intraepithelial and/or stromal compartment(s) according to HPV status. The immune cells studied were CD8+ T cells (N = 19), CD4+ T cells (N = 7), regulatory T cells (Tregs, N = 15), macrophages (N = 13), myeloid-derived suppressor cells (MDSCs, N = 4), and Langerhans cells (LCs, N = 2). Conclusions: Irrespective of tumor location, CD8+ and CD4+ T cells appear to play a key role in the development of HPV−related HNSCC, and their infiltration is likely associated with a significant impact on OS and RFS. To date, the roles and prognostic value of Tregs, macrophages, DCs and MDSCs remain unclear.
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in men and the eighth most common cancer in women, accounting for over 600,000 new cases per year worldwide [,]. The main risk factors for HNSCC are alcohol and tobacco consumption and persistent infection with human papillomavirus (HPV), which is associated with the rising incidence of oropharyngeal cancer in the United States and Europe [,]. HPV infection is predominantly attributable to subtypes HPV−16 and HPV−18, but geographical heterogeneity has been reported between continents []. Nondrinking and nonsmoking patients with HPV−induced HNSCC often present with advanced cancer, but they have a better prognosis than patients with HNSCC associated with smoking and drinking [,,,,,]. The better prognosis of patients with HPV−induced HNSCC could be due to the interactions between HPV antigens and the host-immune system and a better immune response against these tumors. In this respect, many studies have demonstrated significant differences in the composition of tumor-infiltrating immune cells (TICs) in HPV−induced and non-HPV−induced HNSCC over the past two decades [,]. Moreover, the results of some of these studies indicate that the overrepresentation of some immune cells should be considered a significant prognostic factor for HNSCC patients [,]. However, to date, there has been no systematic review summarizing the current knowledge of TICs and the tumor-host interaction in HPV−induced HNSCC.
The aim of this systematic review is to discuss the current knowledge of the involvement of HPV infection and the immune system in the development of HNSCC.
2. Materials and Methods
The criteria for considering studies for the systematic review were based on the population, intervention, comparison, and outcome (PICO) framework [].
Types of studies: Prospective or retrospective clinical trials published in peer-reviewed journals were included in this review. Studies were included if they explored TICs in HNSCC, including oropharyngeal, laryngeal, hypopharyngeal and oral squamous cell carcinoma. We included studies published in English and French.
Participants and inclusion/exclusion criteria: Papers were included in the analysis if they clearly reported TICs in excised HNSCC samples or pretherapeutic biopsies from patients who were treated with conventional treatment (i.e., surgery, chemoradiation, chemotherapy, or immunotherapy). Papers that examined the recruitment of immune cells through blood analyses were also included to improve the understanding of the tumor immune microenvironment. HPV detection was required to have been performed through DNA or p16 analysis of the tumor samples. Studies focused on non-HNSCC were excluded.
Outcomes: The first outcome was the study of TICs in the intraepithelial (or intratumoral) and stromal compartments in HNSCC according to HPV status. To better understand the involvement of the immune system in the development of HPV−induced HNSCC, investigations into the expression of both cytokines and checkpoint proteins involved in tumor development were performed. Table 1 summarizes the immune cells included in this systematic review [,,,,,,,,]. The second outcome was the study of tumor characteristics (tumor size, node, metastasis, and histopathology) and/or prognostic data (overall survival (OS) and recurrence-free survival (RFS)) in relation to TICs and HPV status.

Table 1.
Immune cell roles.
Intervention and comparison: In the case of the study of the prognostic value of TIC, the authors were required to have treated their patients with conventional surgical or conservative treatments.
2.1. Search Strategy
Four authors (JRL, IS, TV, and QM) conducted searches in PubMed, Cochrane, and Scopus databases to identify articles published between January 1990 and June 2019 pertaining to the relationship between HPV infection and TICs in the development of HNSCC. Clinical studies were screened if they had database abstracts, available full texts or titles that referred to these conditions. The following keywords were used: ‘HPV’, ‘cancer’, ‘carcinoma’, ‘head’, ‘neck’, ‘immune’, and ‘cell’. Final article selection was determined by the four authors, who provided a critical analysis of the publication content. The review was conducted according to the PRISMA checklist []. Institutional review board approval was not required.
2.2. Epidemiological Characteristics and Outcomes
An analysis of the locations of tumors, the number of tumor samples, the inclusion of additional specimens (i.e., dysplastic tissues or normal mucosa), tumor size, the relationship between antigen expression and patient characteristics (age, gender, smoking, and alcohol consumption), and prognostic data (OS and RFS) was performed. The method used for HPV detection was carefully analyzed to provide a methodologically critical analysis of the included studies.
3. Results
3.1. Study Characteristics
The initial screening identified 335 studies, of which 41 met our inclusion criteria (Figure 1). A total of 7 studies investigated the peripheral blood immune cell concentration in patients with HNSCC according to HPV status (Table 2) [,,,,,,]. Three studies focused precisely on the evolution of the peripheral blood immune cell concentration between pre- and posttreatment (surgery or chemoradiation) [,,].
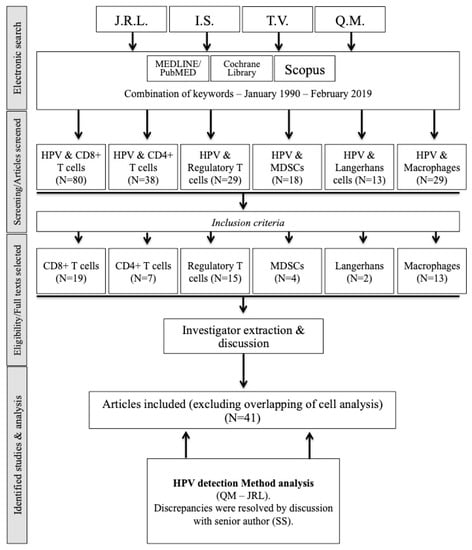
Figure 1.
Flow chart. Abbreviations: HPV = human papilloma virus; MDSC = myeloid-derived suppressor cells.

Table 2.
Peripheral blood immune cell concentration of patients with head and neck squamous cell carcinoma according to HPV status.
A total of 36 studies examined TICs in the intraepithelial and/or stromal compartment(s) according to HPV status (Figure 1). Of the papers that investigated several different immune cell categories, some focused on oropharyngeal SCCs, whereas others focused on HNSCCs, including oropharyngeal and other anatomical localizations. The studies are shown in Table 3, Table 4, Table 5 and Table 6 [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].

Table 3.
CD8+ and CD4+ infiltration in HPV+ and HPV− Head & Neck Squamous Cell Carcinoma.

Table 4.
Foxp3 T regulatory cell infiltration in HPV+ and HPV− head & neck squamous cell carcinoma.

Table 5.
Macrophage infiltration in HNSCC according to HPV status.

Table 6.
Infiltration of Langerhans cells and myeloid-derived suppressor cells in HNSCC according to HPV status.
3.2. Oropharyngeal SCCs
3.3. HNSCCs
3.4. HPV and the Peripheral Blood Concentrations of Immune Cells
Because some immune cells are derived from bone marrow and are transported to tissues through the circulation, the peripheral blood number of immune cells (PBNI) was explored in 7 studies. Overall, it seems that patients with HNSCC have a higher PBNI than healthy individuals [,,,]. The role of HPV status is still unclear because only one study reported significant differences between the PBNI in patients with HPV+ and HPV− HNSCC []. Two authors found a high concentration of circulating CD8+ T cells against the E6 and E7 proteins in the majority of HPV+ patients with oropharyngeal SCC [,]. However, these observations were not supported by Heusinkveld et al., who included patients with oropharyngeal and non-oropharyngeal SCC []. According to Lukesova et al., natural killer cells are another type of cell that can be increased in patients with HPV+ oral or oropharyngeal SCC []. The other types of immune cells were less well studied, and it is difficult to pursue some lines of investigation.
According to two studies [,], the treatment type could have an impact on the PBNI, but the differences between these two studies (in terms of tumor location, types of treatment, and HPV detection methods) limited our comparison. The relationship between PBNI and OS was addressed in two studies [,]. Masterson et al. found that a high blood concentration of CD8+ T cells enhanced immunoreactivity to antigen E7, which was associated with improved OS in patients with oropharyngeal SCC []. In the same vein, Lukesova et al. demonstrated that patients with oral or oropharyngeal HPV+ SCC and a high Treg blood concentration had improved OS and RFS [].
3.5. HPV and CD8+/CD4+ T Cell Tumor Infiltration
A total of 19 studies addressed CD8+ T cell infiltration (Table 3). Among the papers focusing on oropharyngeal SCC, the majority reported higher stromal or intraepithelial CD8+ T cell infiltration in HPV+ than in HPV− SCC [,,,,,,,,]. Only Wansom et al. did not find significant differences between these two types []. These results are relatively similar to those of studies that investigated HNSCC. Russel et al. and Badoual et al. observed increased CD8+ T cell infiltration in the intraepithelial compartment in HPV+ HNSCC [,], whereas when investigating laryngeal, oral, hypo- and oropharyngeal tumor samples, Balermpas et al. did not find significant differences between HPV+ and HPV− SCC []. In a second study, the same authors reported the occurrence of different patterns of CD8+ T cell infiltration according to the tumor location; CD8+ T cell infiltration was higher in HPV+ than in HPV− tumor samples only in oropharyngeal SCC []. Other authors reported an increase in infiltration in HPV+ HNSCC irrespective of the compartment [,,,].
The high CD8+ T cell infiltration in HPV+ tumors was associated with the high expression of checkpoint proteins, including PD-1 [,,], LAG-3 [], and Tim-3 [], in HNSCC. The expression of PD-1 by HPV+ tumors was reported in the study by Kansy et al., who found a significant association between the CD8+ antigens CD8A and CD8B and the expression of PD-1 []. Partlova et al. also found that HPV+ tumors had higher expression of PD-1 mRNA than did HPV− HNSCC tumors []. The activity of CD8+ T cells is mediated by many cytokines, leading some authors to study the expression profile of TICs. Partlova et al. found that TICs in HPV+ SCC comprised IFN-g+ and IL-17+ CD8+ T cells [], supporting the key role of these cytokines in the inflammatory reaction related to HPV. Overall, a high number of TICs (including CD8+ T cells) in HPV+ tumors was associated with the increased secretion of the following proinflammatory cytokines: CCL-17, CCL-21, IL-2, IL-4, IL-8, IL-10, IL-12, IL-17, IL-21, TNF-a, and IFN-g [,]. However, neither of these two studies demonstrated that cytokine expression was directly related to the level of CD8+ T cells.
A high level of CD8+ T cell infiltration was associated with improved OS and RFS in all studies focusing on HPV+ oropharyngeal SCC [,,,,,]. Moreover, Ward et al. found that patients with HPV+ oropharyngeal SCC with low levels of tumor-infiltrating lymphocytes had the same prognosis as those with HPV− oropharyngeal SCC []. Similar overall findings were observed in HNSCC for OS [,,,] and RFS [,,]. One study reported that low CD8+ infiltration was associated with a high risk of metastases [].
3.6. HPV and CD4+ T Cell Tumor Infiltration
Seven publications have reported findings regarding CD4+ T cell infiltration in HPV−induced SCC (Table 3). Overall, CD4+ T cell stromal infiltration was reported to be higher in HPV+ than HPV− oropharyngeal SCC [,,,]. In HNSCC, Balermpas et al. reported similar levels of CD4+ T cell infiltration in HPV+ and HPV− HNSCC []. Regarding the prognostic value of CD4+ T cell infiltration, Nordfors et al. did not find a significant association between the level of CD4+ infiltration and OS in patients with HPV+ and HPV− oropharyngeal SCC []. Similar results were found in the study by Balermpas et al., in which CD4+ expression was not associated with a good prognosis []. Regarding the subpopulation of CD4+ T cells, Krupar et al. studied the infiltration of Th17 cells in oropharyngeal SCCs. They found that there was a significantly lower percentage of Th17+ T cells in the intratumoral compartment of HPV+ patients.
3.7. Regulatory T Cell Infiltration
Fifteen studies have examined the tumor infiltration of Foxp3 Tregs according to HPV status, including 7 that were focused on oropharyngeal SCC (Figure 1, Table 4). Overall, a few studies reported that the degree of Foxp3 Treg infiltration did not differ according to HPV status in oropharyngeal SCC [,], but the majority of the authors observed increased infiltration in HPV+ oropharyngeal SCC, especially in the intratumoral compartment [,,,,]. When analyzing HNSCC studies, Kindt et al. demonstrated that Foxp3 Treg infiltration increases with tumor progression; this increase is more important in HPV+ patients [].
Four studies reported increased infiltration of Foxp3 T cells in the intratumoral [,,] or stromal [] compartment in HPV+ compared with HPV− HNSCC. Additionally, a study by Partlova et al. reported a slightly lower proportion of Treg cells in HPV+ HNSCC. These authors also found higher levels of cytokines and chemokines in HPV+ patients than in HPV− patients, but they did not report which cells produced these cytokines []. In 3 studies, the authors found that the infiltration of Foxp3 T cells was similar in HPV+ and HPV− SCC [,,]. Two studies aimed to study the association between Treg infiltration and checkpoint protein expression [,]. Irrespective of HPV status, Badoual et al. and Lechner et al. found that the infiltration of Tregs was associated with higher levels of checkpoint protein expression (i.e., PD-1) in HNSCCs [,].
Regarding the prognostic value of Treg infiltration, the high infiltration of Foxp3 Tregs was associated with improved OS or RFS in both oropharyngeal cancer [,,,] and HNSCC [,,,]. Moreover, the results of the study by Kindt et al. suggested that the number of stromal Tregs is a strong prognostic factor that is independent of other risk factors, including tobacco and alcohol consumption and HPV status []. To date, only Balermpas et al. did not find a significant association between the degree of Treg infiltration and OS [].
3.8. Macrophage Infiltration
From our literature search, we identified 5 and 8 publications that investigated macrophage infiltration in oropharyngeal and HNSCC according to HPV status, respectively (Figure 1 and Table 5). In 3 studies, CD68+ macrophages showed increased infiltration in HPV+ compared to HPV− oropharyngeal SCC [,,,]; only one publication did not corroborate this finding []. In both oropharyngeal and nonoropharyngeal SCC, CD68+ macrophage infiltration increases between dysplastic tissues and carcinoma; the macrophage infiltration density is higher in the advanced stages [,]. Yu et al. focused on the CD68+/CD163+ macrophage (M2) subpopulation in oral SCCs, and they did not find a significant difference between HPV+ and HPV− oral SCC according to tumor progression and HPV status []. However, the relationship between CD68+ macrophage infiltration and HPV status is more controversial in HNSCC. Indeed, 5 authors did not find a significant difference between HPV+ and HPV− HNSCC regarding CD68+ macrophage infiltration [,,,,], while macrophage infiltration increased in the intratumoral compartment in HPV+ HNSCC in 3 studies [,,]. Among these studies, Ou et al. reported a tendency toward a higher proportion of M2 macrophages in HPV− HNSCC [].
The relationship between CD68+ macrophage infiltration and checkpoint protein expression has been examined in two studies [,]. On the one hand, Lyfor-Pike et al. reported that CD68+ macrophages expressed high levels of PD-1 []. On the other hand, Oguejiofor et al. found that HPV− oropharyngeal SCC had an increase in CD68+ PD-L1+ macrophages compared to HPV+ tumors; these tumors were associated with improved OS compared to HPV− oropharyngeal SCC with low CD68+ PD-L1+ infiltration []. Lee et al. found that the high infiltration of CD68+ macrophages was associated with poor OS and DSS [], while Seminerio et al. demonstrated that the high intratumoral infiltration of CD68+ macrophages was linked with shorter OS in patients with HNSCC []. Finally, Welters et al. found that the increase in dendritic cell-like macrophage infiltration in HPV+ oropharyngeal SCC was correlated with improved OS and a low risk of lymph node metastases [].
3.9. Dendritic Cell Infiltration
3.9.1. Myeloid-Derived Suppressor Cells
A total of 4 included studies evaluated the relationship between MDSC infiltration and HPV status in HNSCCs using different antigens, including CD11b, Arg-1, INOS, and STAT3 (Figure 1, Table 6). MDSC infiltration increased throughout tumor progression [,], and overall, no significant difference was found between HPV+ and HPV− HNSCC [,,]. Moreover, MDSC infiltration seemed to be higher in advanced-stage than in early-stage HNSCC [].
Regarding the expression of checkpoint proteins, Yu et al. found a positive correlation between the infiltration of MDSCs and PD-L1 expression []. For CD8+ T cells, Partlova et al. reported high proinflammatory cytokine expression in tumor samples, characterized by a high infiltration of MDSCs, but they did not demonstrate a potential association []. To date, no study has investigated the prognostic value of MDSC infiltration according to HPV status.
3.9.2. Langerhans Cells
Only 2 studies assessed LC infiltration according to HPV status (Table 6). In a cohort of 27 patients with oral SCC, Perreira et al. did not find a significant difference between HPV+ and HPV− oral SCC []. More recently, Kindt et al. observed that LC infiltration increased in HNSCC throughout tumor progression but decreased in the presence of HPV infection. Moreover, there was a significant association between the LC infiltration level and cT and tumor node status []. The LC infiltration level was positively associated with improved OS and RFS in HPV− but not HPV+ HNSCC.
3.10. SCC Location and the Detection of HPV
The majority of studies (N = 17) were exclusively performed on oropharyngeal SCC [,,,,,,,,,,,,,,,]; 2 focused on oral SCC [,], 1 focused on nodes with unknown primary SCC [], and the rest included upper aerodigestive tract SCC in different locations. TICs were compared with dysplastic and normal mucosa samples in 10 studies [,,,,,,,,,].
To detect HPV infection in tumor samples, 4 authors exclusively assessed p16 expression in samples [,,,], while 36 authors performed DNA detection using different forms of PCR (in situ hybridization, E7 RT-PCR, E6/E7 multiplex qPCR, etc.). In one study, the detection method was not specified [].
4. Discussion and Perspectives
This systematic review emphasizes many lines of evidence and reveals uncertainties regarding the role of immune cells in the development of HPV−induced HNSCC. These findings are summarized in Table 7. Our analysis indicates that in both oropharyngeal and nonoropharyngeal SCC, HPV infection is associated with increased CD8+ T cell infiltration and PD-1 expression by CD8+ T cells and improved OS. CD8+ T cells interact with their environment through multiple cytokines, especially IFN-g and IL-2, -4, -8, -12 and -17 [,]. The increased expression of IL-17 in HPV+ HNSCC cells is associated with the infiltration of Th17 lymphocytes, resulting from the differentiation of CD4+ T cells. Thus, Krupar et al. reported an increased number of Th17 lymphocytes in TICs in HPV+ oropharyngeal SCC []. This observation makes particular sense according to studies that demonstrated increased CD4+ T cell infiltration in HPV+ SCC [,,,]. Because recent studies have suggested that lymphocyte plasticity can occur during HNSCC development, which is characterized by Th1 phenotype expression in Th17 cells [,], these findings could support a potential relationship between CD8+ and CD4+ T cells in HPV−induced SCC. CD4+ T cells could be converted into Th17 cells, potentiating the cytotoxic effects of CD8+ T cells against HPV−induced SCC antigens. According to several studies, the development of TICs, including CD8+ T cells, is associated with an increase in the detectable PBNI [,,,]. In this respect, and considering the data of Parikh et al., it is probable that the detection of CD8+ T cells against E6 or E7 HPV proteins could be used in future studies to better characterize tumor immunogenicity and, as recently suggested, the response to some treatments (i.e., immunotherapy and chemoradiation) [,]. However, scholars should remain prudent because the usual method of assessing the PBNI (blood sampling) is performed at a single time point and does not usually consider the variation in the blood concentrations of immune cells due to other factors related to the circadian rhythm or other external causes.

Table 7.
Summary of evidence and findings.
Macrophages have also been extensively studied in relation to HPV status. However, only a few studies have performed coimmunostaining to identify the M1 and M2 phenotypes []. M2 macrophages are involved in the enhancement of immunosuppression through the stimulation of Tregs and the secretion of TGF-b, TNF-a, and IL-10, leading to the creation of a favorable tumor microenvironment. Recently, Ou et al. indicated that CD68+ macrophage infiltration in HPV−SCC may consist mostly of M2 macrophages []. In HPV−HNSCC, the increased proportion of M2 cells could be one factor underlying the improvement in OS.
Because Foxp3 Tregs inhibit the activity of CD8+ T cells, these cells have been examined in many studies, and their relationship with the tumor microenvironment in HPV−induced SCC remains ambiguous. Indeed, in contrast with expectations, most studies reported that an increase in Treg infiltration, especially in HPV+ SCC, was associated with improved OS [,,,,,,,]. Irrespective of HPV status, some authors have reported similar findings in HNSCC [,]. In fact, the high infiltration of Foxp3 Tregs may inhibit the protumoral effects of inflammatory immune cells and may function as favorable prognostic markers at some tumor sites, whereas at other tumor sites, Treg infiltration may be linked to poor OS due to their conventional regulatory function [,,]. The activation of Tregs may be associated with MDSC infiltration because DC subpopulations are known to stimulate Tregs in HNSCC []. However, we cannot advance any hypotheses about the potential role of DC in the development of HPV−induced SCC because of the low number of studies on this topic in the current literature. Future studies that aim to investigate the role of Foxp3 Tregs in HPV−induced HNSCC should examine other immune cell populations, such as MDSCs.
This systematic review permitted the identification of many factors that should be taken into consideration in the analysis of results, the comparison of studies and the future establishment of immune models.
First, many authors have combined patients with oropharyngeal and non-oropharyngeal SCC into a single group. However, HNSCC includes malignancies that arise from functionally and anatomically distinct tumor sites with different characteristics. The most blatant example concerns oropharyngeal histology, as the Waldeyer ring is composed of preexisting lymphoid tissue that is characterized by a higher sensitivity to the immune response []. In that respect, oropharyngeal SCC is usually heavily infiltrated by lymphocytes, in contrast to other types of HNSCC such as oral carcinoma [,]. Additionally, recent data have shown the heterogeneous molecular and immunological tumor profile of HNSCC at different anatomical locations []. Differences in the prognostic value of TIC might reflect these different biological factors, making it likely that TICs exhibit different properties depending on the tumor site and the histological and molecular subtype.
In addition to the impact of the anatomical location of tumors, the consumption of tobacco and alcohol may also have a critical impact on the significance of TICs. Indeed, because cohorts of HPV+ patients are usually small, most authors do not consider these factors to be important in the development of TICs. However, it has been suggested that tobacco and alcohol consumption are capable of stimulating the mucosal recruitment of LCs, impacting the local immune response during SCC development []. In the same vein, Geng et al. demonstrated that tobacco smoking and, in particular, nicotine, are known to impair the responsiveness of T cells to antigenic stimulation, while other authors found that smoking is associated with a lower number of CD8+ T cells in tissue [,,].
Second, the analysis of TICs could ideally consider both the stromal and intraepithelial compartments because the level of infiltration could vary substantially between these two compartments, leading to differences in prognostic value. Some authors did not specify the compartment used for analysis, which makes an analysis of the results difficult [,,].
A third important point regarding the results analysis is the method used to detect HPV infection. In clinical practice, HPV infection is detected through p16 immunostaining in many centers. Some authors have used this approach to compare data from HPV+ and HPV− SCC, while others have used PCR and other direct methods of DNA identification. However, it is well known that some tumors can be HPV+/p16− or HPV−/p16+, which can be related to the lack of specificity of p16 in identifying HPV infection. As demonstrated in two studies, the use of p16 immunostaining versus DNA detection may lead to differences in the results, biasing the conclusions of related studies [,].
This systematic review also showed that some immune cells are rarely studied, such as eosinophils or natural killer cells (NK cells). However, NK cells have been identified as immune cells that may directly kill both HPV+ and HPV− HNSCC tumor cells [,]. This anti-tumor activity of NK cells could be significantly enhanced by cetuximab or avelumab, particularly in cells with higher baseline EGFR or PD-L1 expression []. Clinically, irrespective of HPV status, the low number of tumor-infiltrating CD56+ NK cells is correlated with significantly decreased OS, distant metastasis-free survival and local progression-free survival [].
Finally, the studies that investigated the impact of HPV infection on OS included patients subject to different treatment methods, including surgery, chemoradiation, radiotherapy and immunotherapy. These different therapeutic methods may significantly impact the OS of patients irrespective of HPV status. This point must be considered in analyses of OS in future large cohort studies.
In conclusion, current knowledge regarding the immune environment of HPV−induced HNSCC is not sufficient for the establishment of a clear pathophysiological model. Although some evidence exists in terms of the roles of CD4+ and CD8+ T cells and their related impact on OS or RFS, many uncertainties persist regarding the role and prognostic value of Tregs, macrophages, DCs and other uninvestigated cells. The poor quality and the low number of available studies, the small number of HPV+ patients included in these studies and the lack of consideration of cofactors that can impact the TIC composition may explain the current inability to establish an immunological model that can better predict the prognosis of HPV−induced oropharyngeal SCC. Future studies are needed to understand the complex interaction between tumors and their immune environment. These studies should carefully consider a rigorous methodological approach for HPV detection and should include a large number of patients with well-defined tumor locations. A large panel of immune cells and the use of specific coimmunostaining should be considered in future work in order to establish a precise immunological overview of HPV+ and HPV− SCC.
Author Contributions
Study concept and design: J.R.L., S.S., D.D. Acquisition, analysis, or interpretation of data (literature): J.R.L., T.V., I.S., Q.M. Drafting of the manuscript: J.R.L., M.J., F.M., S.H. Critical revision of the manuscript for important intellectual content: S.H., G.D., M.J., F.M., S.S., F.J.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Filleul, O.; Preillon, J.; Crompot, E.; Lechien, J.; Saussez, S. Incidence of head and neck cancers in Belgium: Comparison with world wide and French data. Bull. Cancer 2011, 98, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.S.; Koldjaer Sølling, A.S.; Ovesen, T.; Rusan, M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int. J. Cancer 2014, 134, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Curado, M.P.; Boyle, P. Epidemiology of head and neck squamous cell carcinoma not related to tobacco or alcohol. Curr. Opin. Oncol. 2013, 25, 229–234. [Google Scholar] [CrossRef]
- Young, D.; Xiao, C.C.; Murphy, B.; Moore, M.; Fakhry, C.; Day, T.A. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol. 2015, 51, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. New Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- O’Rorke, M.; Ellison, M.; Murray, L.; Moran, M.; James, J.; Anderson, L.; Anderson, L. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef]
- Descamps, G.; Karaca, Y.; Lechien, J.R.; Kindt, N.; Decaestecker, C.; Remmelink, M.; Larsimont, D.; Andry, G.; Hassid, S.; Rodriguez, A.; et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 2185–2196. [Google Scholar] [CrossRef]
- Duray, A.; Descamps, G.; Decaestecker, C.; Sirtaine, N.; Gilles, A.; Khalife, M.; Chantrain, G.; Depuydt, C.E.; Delvenne, P.; Saussez, S. Human papillomavirus predicts the outcome following concomitant chemoradiotherapy in patients with head and neck squamous cell carcinomas. Oncol. Rep. 2013, 30, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Boil. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wang, S.-S.; Tang, Y.-J.; Chen, Y.; Zheng, M.; Tang, Y.-L.; Liang, X.-H. The Double-Edged Sword—How Human Papillomaviruses Interact With Immunity in Head and Neck Cancer. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journé, F.; Saussez, S. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, I.; Descamps, G.; Dupont, S.; de Marrez, L.; Laigle, J.A.; Lechien, J.R.; Kindt, N.; Journe, F.; Saussez, S. Infiltration of FoxP3+ Regulatory T Cells is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Systematic and literature Review Resources 2011. Available online: http://distillercer.com/resources (accessed on 1 July 2015).
- Stangl, S.; Tontcheva, N.; Sievert, W.; Shevtsov, M.; Niu, M.; Schmid, T.E.; Pigorsch, S.; Combs, S.E.; Haller, B.; Balermpas, P.; et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2018, 142, 1911–1925. [Google Scholar]
- Duray, A.; Demoulin, S.; Hubert, P.; Delvenne, P.; Saussez, S. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010, 2010. [Google Scholar] [CrossRef]
- Costa, N.L.; Gonçalves, A.S.; Martins, A.F.L.; Arantes, D.A.C.; Silva, T.A.; Batista, A.C. Characterization of dendritic cells in lip and oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2015, 45, 418–424. [Google Scholar] [CrossRef]
- Narayanan, B.; Narasimhan, M. Langerhans Cell Expression in Oral Submucous Fibrosis: An Immunohistochemical Analysis. J. Clin. Diagn. Res. 2015, 9, ZC39–ZC41. [Google Scholar] [CrossRef]
- Albuquerque, R.L.C.; Miguel, M.C.C.; Costa, A.L.L.; Souza, L.B. Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinoma. Int. J. Exp. Pathol. 2003, 84, 259–265. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Oluwasola, A.O.; Lasisi, O.A.; Akang, E.E. Association between langerhans cells population and histological grade of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2013, 17, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.; Patel, P.; Allen, C.T. How patients with an intact immune system develop head and neck cancer. Oral Oncol. 2019, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Delvenne, P.; Journé, F.; Saussez, S. High stromal Foxp3-positive T cell number combined to tumor stage improved prognosis in head and neck squamous cell carcinoma. Oral Oncol. 2017, 67, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Gao, D. Study on the interrelationship between human papilloma virus infection and Langerhans cell in carcinogenesis of esophagus. Zhonghua bing li xue za zhi = Chin. J. Pathol. 1996, 25, 83–85. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, M.; Goedemans, R.; Briet, R.J.; Gelderblom, H.; Nortier, J.W.; Gorter, A.; Smit, V.T.; Langeveld, A.P.; Jansen, J.C.; van der Burg, S.H. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neckcancer. Int J Cancer. 2012, 131, E74–E85. [Google Scholar] [CrossRef] [PubMed]
- Al-Taei, S.; Banner, R.; Powell, N.; Evans, M.; Palaniappan, N.; Tabi, Z.; Man, S. Decreased HPV−specific T cell responses and accumulation of immunosuppressive influences in oropharyngeal cancer patients following radical therapy. Cancer Immunol. Immunother. 2013, 62, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Parikh, F.; Duluc, D.; Imai, N.; Clark, A.; Misiukiewicz, K.; Bonomi, M.; Gupta, V.; Patsias, A.; Parides, M.; Demicco, E.G.; et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV−related oropharyngeal cancer. Cancer Res. 2014, 74, 7205–7216. [Google Scholar] [CrossRef] [PubMed]
- Lukešová, E.; Boucek, J.; Rotnaglova, E.; Saláková, M.; Koslabova, E.; Grega, M.; Eckschlager, T.; Říhová, B.; Procházka, B.; Klozar, J.; et al. High Level of Tregs Is a Positive Prognostic Marker in Patients with HPV−Positive Oral and Oropharyngeal Squamous Cell Carcinomas. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Ma, X.; Sheng, S.; Wu, J.; Jiang, Y.; Gao, X.; Cen, X.; Wu, J.; Wang, S.; Tang, Y.; Tang, Y.; et al. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget 2017, 8, 42061–42075. [Google Scholar] [CrossRef]
- Lechner, A.; Schlößer, H.; Rothschild, S.I.; Thelen, M.; Reuter, S.; Zentis, P.; Shimabukuro-Vornhagen, A.; Theurich, S.; Wennhold, K.; Garcia-Marquez, M.; et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 44418–44433. [Google Scholar] [CrossRef] [PubMed]
- Wansom, D.; Light, E.; Thomas, D.; Worden, F.; Prince, M.; Urba, S.; Chepeha, D.; Kumar, B.; Cordell, K.; Eisbruch, A.; et al. Infiltrating lymphocytes and human papillomavirus-16--associated oropharyngeal cancer. Laryngoscope 2012, 122, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Tartour, E.; Roussel, H.; Bats, A.S.; Pavie, J.; Pernot, S.; Weiss, L.; Mohamed, A.S.; Thariat, J.; Hoffmann, C.; et al. HPV (Human Papilloma Virus) implication in other cancers than gynaecological. Rev. Med. Interne 2015, 36, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.C.; Guihard, S.; Krugell, S.; Ledrappier, S.; Brochot, A.; Dalstein, V.; Job, S.; de Reynies, A.; Noël, G.; Wasylyk, B.; et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int. J. Cancer. 2013, 132, E26–E36. [Google Scholar] [CrossRef] [PubMed]
- Rittà, M.; Landolfo, V.; Mazibrada, J.; De Andrea, M.; Dell’Oste, V.; Caneparo, V.; Peretti, A.; Giordano, C.; Pecorari, G.; Garzaro, M.; et al. Human papillomavirus tumor-infiltrating T-regulatory lymphocytes and P53 codon 72 polymorphisms correlate with clinical staging and prognosis of oropharyngeal cancer. New Microbiol. 2013, 36, 133–144. [Google Scholar] [PubMed]
- Russell, S.; Angell, T.; Lechner, M.; Liebertz, D.; Correa, A.; Sinha, U.; Kokot, N.; Epstein, A. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013, 5, 24–40. [Google Scholar] [PubMed]
- Nordfors, C.; Grün, N.; Tertipis, N.; Ährlund-Richter, A.; Haeggblom, L..; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer. 2013, 49, 2522–2530. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV−positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Weiss, C.; Rödel, C.; Fokas, E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. OncoImmunology 2014, 3. [Google Scholar] [CrossRef]
- Krupar, R.; Robold, K.; Gaag, D.; Spanier, G.; Kreutz, M.; Renner, K.; Hellerbrand, C.; Hofstaedter, F.; Bosserhoff, A.K. Immunologic and metabolic characteristics of HPV−negative and HPV−positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014, 465, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Partlová, S.; Bouček, J.; Kloudová, K.; Lukešová, E.; Zábrodský, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV−associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M.L. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV−positive oropharyngeal squamous carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Rödel, F.; Rödel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer. 2016, 138, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, P.M.; Noorlag, R.; Swartz, J.E.; Bovenschen, N.; Braunius, W.W.; Vermeulen, J.F.; Van Cann, E.M.; Grolman, W.; Willems, S.M. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol. Immunother. 2016, 65, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Ha, S.-J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef]
- Poropatich, K.; Hernandez, D.; Fontanarosa, J.; Brown, K.; Woloschak, G.; Paintal, A.; Raparia, K.; Samant, S. Peritumoral cuffing by T-cell tumor-infiltrating lymphocytes distinguishes HPV−related oropharyngeal squamous cell carcinoma from oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2017, 46, 972–978. [Google Scholar] [CrossRef]
- Sivars, L.; Landin, D.; GRÜN, N.; Vlastos, A.; Marklund, L.; Nordemar, S.; RAMQVIST, T.; Munck-Wikland, E.; NÄSMAN, A.; Dalianis, T.; et al. Validation of Human Papillomavirus as a Favourable Prognostic Marker and Analysis of CD8+ Tumour-infiltrating Lymphocytes and Other Biomarkers in Cancer of Unknown Primary in the Head and Neck Region. Anticancer Res. 2017, 37, 665–673. [Google Scholar] [CrossRef]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef]
- Park, K.; Cho, K.J.; Lee, M.; Yoon, D.H.; Kim, S.-B. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer. Res. 2013, 33, 5667–5673. [Google Scholar] [PubMed]
- Punt, S.; Dronkers, E.A.; Welters, M.J.; Goedemans, R.; Koljenović, S.; Bloemena, E.; Snijders, P.J.F.; Gorter, A.; van der Burg, S.H.; de Jong, R.J.B.; et al. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17(+) cell frequency. Cancer Immunol. Immunother. 2016, 65, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ritta’, M.; De Andrea, M.; Mondini, M.; Mazibrada, J.; Giordano, C.; Pecorari, G.; Garzaro, M.; Landolfo, V.; Schena, M.; Chiusa, L.; et al. Cell cycle and viral and immunologic profiles of head and neck squamous cell carcinoma as predictable variables of tumor progression. Head Neck 2009, 31, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV−associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-T.; Bu, L.-L.; Huang, C.-F.; Zhang, W.-F.; Chen, W.-J.; Gutkind, J.S.; Kulkarni, A.B.; Sun, Z.-J. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPα axis in HPV negative head and neck squamous cell carcinoma. Oncotarget 2015, 6, 42067–42080. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, J.Y.; Cho, K.J.; Kim, S.B.; Lee, S.W.; Choi, S.H.; Roh, J.L.; Nam, S.Y.; Kim, S.Y. Composition of inflammatory cells regulating the response to concurrent chemoradiation therapy for HPV (+) tonsil cancer. Oral Oncol. 2015, 51, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Galletta-Williams, H.; Dovedi, S.J.; Roberts, D.L.; Stern, P.L.; West, C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV− oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017, 8, 14416–14427. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; De Jong, A.; Eeden, S.J.F.V.D.; Van Der Hulst, J.M.; Kwappenberg, K.M.C.; Hassane, S.; Franken, K.L.M.C.; Drijfhout, J.W.; Fleuren, G.J.; Kenter, G.; et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003, 63, 636–641. [Google Scholar] [PubMed]
- Ou, D.; Adam, J.; Garberis, I.; Blanchard, P.; Nguyen, F.; Levy, A.; Casiraghi, O.; Gorphe, P.; Breuskin, I.; Janot, F.; et al. Influence of tumor-associated macrophages and HLA class I expression according to HPV status in head and neck cancer patients receiving chemo/bioradiotherapy. Radiother. Oncol. 2019, 130, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Marbaix, E.; Bouzin, C.; Hamoir, M.; Mahy, P.; Bol, V.; Grégoire, V. Immune cell infiltration in head and neck squamous cell carcinoma and patient outcome: A retrospective study. Acta Oncol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.M.A.; Soares, R.C.; Oliveira, M.C.; Pinto, L.P.; Costa, A.D.L.L. Immunohistochemical staining of Langerhans cells in HPV−positive and HPV−negative cases of oral squamous cells carcinoma. J. Appl. Oral Sci. 2011, 19, 378–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Pottier, C.; Larsimont, D.; Journé, F.; Delvenne, P.; Saussez, S. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral Oncol. 2016, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Guéry, L.; Hugues, S. Th17 Cell Plasticity and Functions in Cancer Immunity. Biomed Res Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay, N.E.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic value of tumor-infiltrating CD4 + T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006, 12, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bron, L.; Jandus, C.; Andrejevic-Blant, S.; Speiser, D.E.; Monnier, P.; Romero, P.; Rivals, J.P. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int. J. Cancer 2013, 132, 501–509. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Zwaenepoel, K.; Deron, P.; Duprez, F.; Rottey, S.; Ferdinande, L. Prognostic markers in oropharyngeal squamous cell carcinoma: Focus on CD70 and tumour infiltrating lymphocytes. Pathology 2017, 49, 397–404. [Google Scholar] [CrossRef]
- Pai, S.I.; Westra, W.H. Molecular Pathology of Head and Neck Cancer: Implications for Diagnosis, Prognosis, and Treatment. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 49–70. [Google Scholar] [CrossRef]
- Geng, Y.; Savage, S.M.; Razani-Boroujerdi, S.; Sopori, M. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J. Immunol. 1996, 156, 2384–2390. [Google Scholar]
- Cho, Y.-A.; Yoon, H.-J.; Lee, J.-I.; Hong, S.-P.; Hong, S.-D. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011, 47, 1148–1153. [Google Scholar] [CrossRef]
- Friedman, J.; Padget, M.; Lee, J.; Schlom, J.; Hodge, J.; Allen, C. Direct and antibody-dependent cell-mediated cytotoxicity of head and neck squamous cell carcinoma cells by high-affinity natural killer cells. Oral Oncol. 2019, 90, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Concha-Benavente, F.; Shayan, G.; Srivastava, R.M.; Gibson, S.P.; Wang, L.; Gooding, W.E.; Ferris, R.L. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV+ status in head and neck cancer. Oral Oncol. 2018, 78, 186–193. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).