Simple Summary
Teclistamab belongs to a class of drugs known as bispecific antibodies. It is used to treat patients with relapsed or refractory multiple myeloma who have tried several other types of therapy. Teclistamab was initially studied in the MajesTEC-1 clinical trial and has been increasingly used in clinical practice since its approval in October 2022. In this study, we perform a systematic literature review to gather evidence on effectiveness, safety, healthcare resource utilization, and prescribing patterns associated with teclistamab in real-world observational studies. Results indicate that patients treated with teclistamab across health centers in the real-world have similar outcomes to patients treated in the clinical trial setting. As patients with relapsed or refractory multiple myeloma have a high disease burden and limited treatment options, results from this study will inform healthcare providers and health policy makers of the therapeutic benefits and safety profile of teclistamab in this patient population.
Abstract
Background: Teclistamab (TEC) is the first B-cell maturation antigen-directed bispecific antibody approved in 2022 by the European Medicines Agency and Food and Drug Administration for triple-class exposed relapsed/refractory multiple myeloma (RRMM). Objectives: As TEC is increasingly used in real-world (RW) settings, this study seeks to gather existing RW evidence on effectiveness, safety, healthcare resource utilization, and clinical practices associated with TEC. Methods: A systematic literature review was performed to identify RW observational studies of TEC-treated adults with RRMM from 2023 to June 2024. Results: Sixty-one records representing 41 unique studies were included; sample sizes ranged from 8 to 572 patients. Where reported, median follow-up ranged from 2.3 to 33.6 months, and >65% of the patients would have been ineligible for the pivotal trial of TEC (MajesTEC-1) in all but one study. In eight studies with ≥50 patients and ≥3 months follow-up, overall response rates were 59–66% and cytokine release syndrome (CRS) rates were 18–64%. Tocilizumab use for CRS management was reported in 14 studies, with two indicating CRS rates of 13% and 26% when used prophylactically. Survival and infection outcomes showed wide variability due to short follow-up in most studies. Conclusions: Overall, early RW effectiveness and safety outcomes of TEC were comparable to findings from MajesTEC-1.
1. Introduction
Multiple myeloma (MM), a plasma cell malignancy, is the second most common hematologic cancer in the United States (US), with an estimated 35,780 new cases (1.8% of all cancer diagnoses) and 12,540 deaths (2.0% of all cancer deaths) in 2024 []. While 5-year survival rates of MM have more than doubled since the 1970s to a rate of 61.1% [,], the disease is typically characterized by a series of responses and relapses meaning patients ultimately require multiple lines of therapy []. It imposes a substantial healthcare burden, as patients progress through successive lines of therapy; a recent retrospective database study found that all-cause healthcare costs averaged USD 35,760 per patient per month among patients with four or more prior lines of therapy []. After the three main classes of drugs for MM (proteasome inhibitors [PI], immunomodulatory imide drugs [IMiD], and anti-CD38 monoclonal antibodies [CD38 mAb]) have failed patients, few effective options remain for relapsed/refractory multiple myeloma (RRMM). Deciding on the next steps in treatment requires clinicians and patients to consider the pace of disease progression, patient comorbid conditions, potential clinically relevant toxicities, and the timing as well as financial implications of treatment [].
The treatment landscape for RRMM has evolved considerably with the introduction of innovative targeted therapies, including B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR) T-cell and bispecific therapies since 2020 []. Teclistamab-cqyv (TECVAYLI®, Janssen Biotech, Horsham, PA, USA) is the first-in-class BCMA-targeted bispecific antibody (BsAb) approved by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) in August and October 2022, respectively, for adult patients with RRMM who have previously received at least three prior lines of therapy in Europe or at least four prior lines of therapy in the US, including a PI, IMiD, and CD38 mAb.
Teclistamab (TEC) is administered subcutaneously, initially using a step-up dosing (SUD) schedule to lower the risk of cytokine release syndrome (CRS) and neurotoxicity, followed by weight-based weekly or every-other-week treatment doses; patients are recommended to be hospitalized for 48 h after SUD for monitoring. Although TEC SUD is recommended to be delivered in an inpatient setting, since its launch, clinicians have been exploring different care models, including outpatient administrations, with the goal of reducing healthcare resource utilization (HCRU) and improving treatment experiences while ensuring patient safety [,,].
MajesTEC-1, the initial phase I/II trial reporting on the efficacy and safety of TEC, demonstrated an overall response rate (ORR) of 63% [,], including 46% of patients achieving complete response (CR) or better []. CRS occurred in 72% of patients (most CRS events were grade 1 or 2 in severity while one patient [0.6%] experienced a grade 3 event in the setting of an ongoing infection), and 3% developed immune effector cell-associated neurotoxicity syndrome (ICANS) []. MajesTEC-1 utilized stringent eligibility criteria that excluded patients with significant disease burden, such as those with organ dysfunction, poor performance status, serious comorbid conditions, severe cytopenia, and/or prior BCMA-directed therapy exposure; however, these characteristics are common in real-world (RW) practice [,]. As a result, patients treated in MajesTEC-1 may not have been representative of all indicated patients in a RW setting []. Several consortia and institutions have disseminated reports on early RW outcomes and safety with TEC. As TEC is increasingly being used in RW settings with a growing amount of RW evidence being published, this systematic literature review (SLR) aims to identify and summarize the latest RW outcomes of TEC, including effectiveness, safety, healthcare provider practices, and associated HCRU.
2. Materials and Methods
This SLR was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines []. This review has not been registered. The full study protocol and checklist are provided in Table A1 and the Supplementary Materials for reference.
Study eligibility criteria were defined in terms of the population, intervention, comparator, outcome, and study design (PICOS) structure outlined in Table A1 which guided the identification and selection of studies for the SLR. The target population included adult patients (≥18 years) with MM. The only intervention of interest was TEC, but no restrictions were applied to comparators. No restrictions were applied to outcomes to ensure that all relevant evidence was captured. Observational RW studies (prospective and retrospective) were included, while clinical trials, pooled analyses of clinical trials, case reports, case series, and narrative reviews were excluded. Publications of SLRs were excluded, but the bibliographies of relevant SLRs were screened for any relevant citations not otherwise captured in the primary searches (i.e., these references served only as secondary sources to ensure that all studies meeting the eligibility criteria were identified). Only English-language publications of full-text articles and conference materials (i.e., abstracts, posters, or oral presentations) were included, and a time restriction from 2023 to 2024 was applied.
Relevant studies were identified by searching the following databases through the Ovid platform: Medical Literature Analysis and Retrieval System Online (MEDLINE) and Excerpta Medica database (Embase). The specific search algorithms included a combination of indexing and free-text terms (see search terms in Table A2 and Table A3). The population terms were adapted from existing reviews [,], and terms for the generic and brand names of TEC were incorporated.
The main database searches were augmented with searches of 15 specific clinical or managed care conference proceedings from 2023 and 2024 (Table A5). The Northern Light database was used to search for studies from conferences that were indexed in the database (see search terms in Table A4), while conference websites were hand searched for the remaining conferences. Database searches were conducted at the end of May 2024, and further hand searches were conducted through the end of June 2024 to ensure all relevant materials were captured through the first half of 2024.
One reviewer performed all citation screening and data extraction, with quality checks performed by a second reviewer; a third reviewer was included to reach a consensus on any remaining discrepancies, where necessary. Screening decisions and extracted data were stored and managed in Microsoft Excel (Microsoft, Redmond, WA, USA). The study identification and selection process was summarized with a PRISMA flow diagram []. Kaplan–Meier (KM) curves for progression-free survival (PFS) and overall survival (OS) were extracted using DigitizeIt (DigitizeIt, Braunschweig, Germany) and reconstructed using an algorithm published by Guyot et al., 2012 []. The Newcastle–Ottawa Scale (Ottawa Hospital Research Institute, Ottawa, ON, Canada) was used to assess the quality of observational studies where full-text publications were available (Table A6). The scale utilizes a ‘star system’ to judge (i) the selection of the study groups, (ii) the comparability of the groups, and (iii) the ascertainment of either the exposure or outcome of interest for case–control or cohort studies, respectively.
3. Results
3.1. Study Characteristics
Of the 156 records identified across all sources, a total of 61 publications (five full-texts [,,,,] and 56 conference abstracts, posters, or oral presentations) representing 41 unique studies evaluating TEC in a RW setting were identified (Figure 1). The included studies spanned seven countries in North America and Europe. Thirty-five of the 41 studies were retrospective in design. Fifteen studies (36.6%) reported results pooled from multiple centers, while 25 studies (61.0%) reported experience from a single center. Most studies (n = 34) were chart reviews, and seven reported data gathered from secondary databases. In 40 studies, sample sizes ranged from 8 to 572 patients. One study using the FDA Adverse Event Reporting System database reported 719 adverse event (AE) cases of TEC, rather than the event rates among patients [], and, therefore, was not included in the results summary. Median follow-up (mFU) duration was reported in 19 studies (46.3%) and ranged from 2.3 [] to 33.6 months []. This current review focused on eight studies with sample sizes ≥50 (range: 52 to 419) and mFU ≥3 (range: 3.1 to 9.5) months, as studies with larger sample sizes may be more generalizable to the wider population and those with longer follow-up duration may help to accurately evaluate AEs and outcomes (the median time to CR or better was 4.6 months [] in MajesTEC-1). Exceptions were made for seven studies in order to report on data from novel topics including prophylactic tocilizumab (TCZ) use, less frequent dosing, and step-up dosing in the outpatient setting that have not been extensively evaluated or reported in the real-world yet and where data are primarily limited to smaller sample sizes with shorter follow-up periods. Studies not reporting mFU were not included in this synthesis due to the limited interpretability of the outcomes.
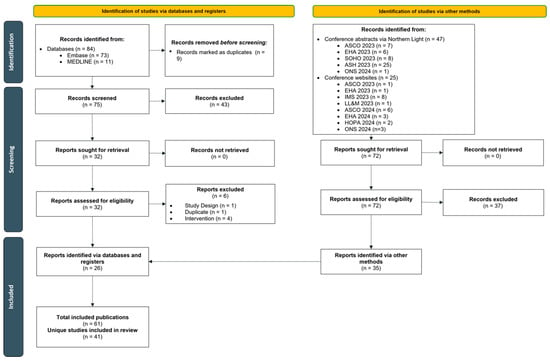
Figure 1.
PRISMA flow diagram of included studies. Abbreviations: ASCO, American Society of Clinical Oncology; ASH, American Society for Hematology; EHA, European Hematology Association; HOPA, Hematology/Oncology Pharmacy Association; IMS, International Myeloma Society; LL&M, Lymphoma, Leukemia & Myeloma Congress; ONS, Oncology Nursing Society; SOHO, Society of Hematologic Oncology.
Study characteristics of studies with ≥50 patients and ≥3 months mFU are presented in Table 1 (Table A7 summarizes all 41 included studies). ORR and CRS rates were the most frequently reported effectiveness and safety measures, respectively. AE management using TCZ, length of hospital stay during TEC SUD, ICANS rates, and infections were also frequently reported. Common subpopulations included patients with prior anti-BCMA exposure and those receiving outpatient SUD. Studies that included other interventions alongside TEC were included when TEC-only subpopulation data were available (labeled as “TEC treatment group” in subsequent tables). Based on the Newcastle–Ottawa scale, the five included full-text publications [,,,,] were high quality regarding study population selection and ascertainment of outcomes. However, all five studies had a non-comparative design and could not be compared directly.

Table 1.
Summary of study characteristics and outcomes evaluated in studies with ≥50 patients and ≥3 months median follow-up.
3.2. Population Characteristics
Population characteristics of studies with ≥50 patients and ≥3 months mFU are reported in Table 2 and Table 3. Summaries of all included studies are available in Table A8 and Table A9, with additional characteristics provided in Table A12 and Table A13. Within the overall populations, the median age ranged from 65 [] to 71 [] years, and 63.4% [] to 76.0% [] of the patients were White. High-risk cytogenetic abnormalities were reported in six studies and were present in 33% [] to 71% [] of the patients. Five studies consistently defined high-risk cytogenetics as having one of the following abnormalities: del17p, t(4;14), or t(14;16) [,,,,]; four of these studies included 1q gain/amp [,,,]; two studies included t(14;20) [,]; and one study included monosomy 17 []. The remaining study did not provide a definition [].

Table 2.
Summary of patient characteristics in studies with ≥50 patients and ≥3 months median follow-up.

Table 3.
Summary of MajesTEC-1 ineligibility in studies with ≥50 patients and ≥3 months median follow-up.
Extramedullary disease (EMD) was present in 19% [] to 44% [] of the patients in six chart review studies, where documented. One study utilizing data from a multi-center MM electronic medical record (EMR) database reported that EMD diagnosis codes were present in 5.7% of the patients []. Of these studies, two defined EMD as any plasmacytomas [,] and four defined EMD as plasmacytomas not associated with the bone [,,,]. Only one study did not provide a definition for EMD []. The median number of prior lines of therapy ranged from five [] to seven [], such therapies including patients with prior BCMA-directed therapy exposure. Other prior lines of therapy for all included studies can be found in Table A12.
MajesTEC-1 ineligibility was reported in three studies: ≥70% of patients were ineligible in two studies [,] and one study reported 39% of the patients as ineligible []. The most common reasons for ineligibility in these studies included prior BCMA-directed therapy (37% [] to 53% []), a poor performance status (Eastern Cooperative Oncology Group [ECOG] ≥2; 33% []), cytopenia(s) (31% []), and/or renal impairment/failure (12% [] to 13% []). Additionally, individual cytopenia ranges across studies with ≥50 patients and ≥3 months mFU included anemia (25% [] to 51% []), neutropenia (2% [] to 22% []), and thrombocytopenia (20% []).
3.3. Outcomes
The following sections summarize the most frequently reported outcomes of interest for the overall populations in the included studies with ≥50 patients and ≥3 months mFU, focusing on the latest timepoint in each study. Table A10 and Table A11 provide outcome summaries for all included studies.
3.3.1. Effectiveness Outcomes
Six of the eight studies with ≥50 patients and ≥3 months mFU reported on effectiveness outcomes (Table 4), and variability in response and survival rates was observed across these studies. Among these studies, ORR (partial response [PR] or better) ranged from 59% [] to 66% [] (n = 6 studies), very good partial response (VGPR) or better ranged from 38% [] to 51% [] (n = 6 studies), and CR or better ranged from 19% [] to 29% [] (n = 4 studies).

Table 4.
Summary of key effectiveness outcomes in studies with ≥50 patients and ≥3 months median follow-up.
Where reported, PFS and OS varied across studies with ≥50 patients and ≥3 months mFU, as shown in Table 4. Among the studies reporting the median PFS (n = 5 studies; mFU 3.1 [] to 5.5 [] months), the median PFS ranged from 5.4 [] to 13 [] months and was not reached in two studies [,]. The 6-month PFS rate was similar across studies, where reported (52% [] to 58% [] in three studies; mFU 3.5 [] to 9.5 [] months). Among the studies reporting OS, the median OS was not reached in three studies (mFU 3.5 [] to 5.5 [] months), while one reported a median of 15 months (mFU 5 months []). The 6-month OS rate ranged from 70% [] to 80% [] (n = 3 studies; mFU 3.5 [] to 5 [] months).
3.3.2. Safety Outcomes
Safety outcomes were reported in six of the eight studies (Table 5), with variability in the proportion of patients experiencing AEs observed across all studies. All six studies reported CRS rates, while three studies reported ICANS rates and four studies reported infection rates in the overall population. Among these studies, the proportion of patients who developed any grade of CRS ranged from 18% [] to 64% [] (n = 6 studies), and a small proportion of patients (0.5% [] to 4.5% []) experienced grade ≥3 CRS (n = 5 studies). When stratified by data source and care models, any grade CRS rates from chart reviews of patients with inpatient monitoring ranged from 52% [] to 64% [], one study reported a CRS rate of 36% [] from chart reviews of patients with outpatient monitoring, and one study reported a CRS rate of 18.4% [] from secondary databases (e.g., payer claims, EMRs), as identified by the International Classification of Diseases 10th Revision codes. Three studies reported any grade ICANS rates ranging from 4% [] to 14% [], and three studies reported grade ≥3 ICANS rates ranging from 0% [] to 4.5% []. Any grade infections were experienced by 31% [] to 60% [] of the patients (n = 4 studies), and approximately 26% of the patients had grade ≥3 infections (n = 2 studies) [,]. Two studies reported the impact on CRS rates when using prophylactic TCZ [,], as described in more detail below (Section 3.3.3).

Table 5.
Summary of key safety outcomes in studies with ≥50 patients and ≥3 months median follow-up.
3.3.3. Healthcare Provider Practices and Resource Utilization
The most frequently reported outcome related to healthcare provider practices was TCZ use in CRS management, which was reported in 12 studies. Among the five studies with ≥50 patients and ≥3 months mFU, TCZ usage ranged from 15% [] to 41% [] in the overall population. Additionally, two studies with any sample size and any mFU reported on prophylactic TCZ use in the inpatient setting. Kowalski (2023) only included patients (n = 31) treated with prophylactic TCZ before the TEC treatment, and there was a CRS rate of 13% (all grade 1; 95% CI 4, 30; p < 0.01) []. Marin (2023) assessed patients treated with prophylactic TCZ prior to the second TEC SUD (n = 38) and compared them to a cohort of patients who received TEC without the prophylactic TCZ (n = 15) []. The cohort with the prophylactic TCZ had a 26% rate of any grade CRS, while the cohort without the prophylactic TCZ had a much higher rate of any grade CRS, at 73% []. Among the patients in the prophylactic TCZ cohort who experienced CRS events (n = 10), the majority of the events were grade 1 (n = 8), while only two patients had grade 2 or 3 events; CRS grades for the cohort without the prophylactic TCZ were not reported [].
The most frequently reported HCRU outcome was hospital length of stay (LOS) during TEC SUD for inpatient TEC administration or AE management if hospitalized after an outpatient SUD administration. Three studies with ≥50 patients and ≥3 months mFU reported an inpatient SUD administration model, with the median LOS ranging from 8 days [] to 9 days [,]. Two studies with any sample size and any mFU reported LOS data for 1-3-5 and 1-4-7 SUD schedules. Kawasaki (2024) reported a mean LOS of 7.6 days for the 1-3-5 schedule and 9.2 days for the 1-4-7 schedule. Graf (2024) reported a median LOS of 6 days for the 1-3-5 schedule and 9 days for the 1-4-7 schedule. Two studies reported that LOS for inpatient SUD decreased over time: Banerjee (2023a), using nationally representative payer claims data, reported a mean LOS of 11.4 (SD 9.0) days for patients who initiated TEC within the first four months of FDA approval (through February 2023), and a mean LOS of 7.0 (SD 1.4) days for patients who initiated TEC in the most recent month (July 2023 by the data cut-off) []. Similarly, Banerjee (2023b), using a multi-center MM EMR database, reported a median LOS of 11 days in December 2022 and a median LOS of 6.5 days in May 2023 []. Additionally, three studies (any sample size and any mFU) with patients undergoing outpatient SUD reported LOS for hospital admissions at any time due to AEs, with medians of 1.5 days [], 2 days [], and 4 days [] per admission, respectively.
Three recently published studies with ≥50 patients and ≥3 months mFU reported the proportion and timing of patients switching from weekly dosing to less frequent dosing. Tan (2024) reported that 30 patients (34.9%) at a single academic center switched from every week (QW) to every 2 weeks dosing (Q2W) and two patients (2.3%) switched from QW to every 4 weeks (Q4W), with a median time to switch of 3.3 months, for the primary reason of achieving ≥PR (n = 23) and/or safety (n = 14) []. Among these patients who switched the dosing frequency, the 6-month PFS rate post-switch was 90% after an mFU of 6.4 months after switching []. Two studies, using nationally representative secondary data, estimated the probability and timing of patients switching to less frequent dosing using KM analysis. The probability of switching to less frequent dosing (32 out of 39 switchers went from QW to Q2W []; 58 out of 78 switchers went from QW to Q2W []) after three months was 15.5% [] and 19.0% [], and this increased to 38.3% [] and 38.6% [], respectively, at six months. The median time to switch was 8.5 months in one study [] and it was not reached at an mFU of 4.2 months in the other [].
4. Discussion
This is the first SLR to consolidate RW studies of TEC, globally. Wide variance was observed in the effectiveness and safety of TEC across the entire evidence base, with ORR ranging from 44% to 87%, CRS rates ranging from 6% to 85% depending on data sources and care models, and ICANS being present in 0% to 23% of the patients. When restricting the review to larger studies that included ≥50 patients with an mFU of ≥3 months, the range of results narrowed: ORR ranged from 59% to 66%, CRS rates ranged from 18% to 64% depending on data sources and care models, and ICANS rates ranged from 4% to 14%. Wide variability in PFS and OS estimates and limited data on infection were observed given the short follow-up time in most studies, and this trend was still observed when only considering studies with larger sample sizes. Early RW use of TEC was characterized by administration to a diverse group of patients, including minorities, patients with significant comorbid conditions, high-risk features, and those with prior BCMA-directed therapy exposure.
The collation of these data provides early insights into how RW outcomes of TEC compare with the results of the MajesTEC-1 trial (Table A15), taking into account that most RW studies report that more than half of the patients would have been ineligible for the MajesTEC-1 trial. Patients in the RW differ from the trial populations, since they are not required to meet stringent eligibility criteria and may have more comorbidities and less hematopoietic or organ reserve [,]. Additionally, with TEC being a first-in-class BCMA BsAb and with these studies capturing data from the first year since TEC approval, the patients included in RW studies may have had advanced diseases and may have been more heavily pretreated. Despite this, the early effectiveness and safety profile of TEC in RW practice appeared comparable with MajesTEC-1.
The ORR in MajesTEC-1 was 63% []; studies evaluating ORR in this SLR also provided similar results, with RW evidence showing a range of 59% [] to 66% [] among the studies with ≥50 patients and ≥3 months mFU. Despite the similarities in ORR, the depth of response differed in the early RW patients. In MajesTEC-1, 46% of the patients achieved a response of CR or better [], whereas the CR rates ranged from 19% [] to 29% [] in the early RW setting. In RW practice, this may be a reflection of the hard-to-treat population which included heavily pretreated patients, high-risk features, significant comorbidities, and/or patients with prior exposure to BCMA-directed therapy, as well as the inability of the short follow-up period of the studies evaluated to assess CR or better. In RW practice, it may not be possible to undergo a bone marrow biopsy as frequently to confirm International Myeloma Working Group CR status, a fact which may result in the misclassification of responses as VGPR and result in an underestimated rate of CR or better. Furthermore, the median time to CR or better in MajesTEC-1 was 4.6 months [], but, in RW studies, the follow-up was short, with only eight studies having an mFU ≥3 months, with a range of 3.1 to 9.5 months. This short follow-up in the RW studies may have limited the ability to assess the deepening of responses due to the delayed clearance of paraprotein following tumor killing [], and thus may be unable to measure the best response, PFS, or OS effectively. Moreover, clarifying the duration between the patient’s prior BCMA-directed therapy and the first TEC dose in patients with prior exposure to BCMA-directed therapy will also be paramount to interpret their survival outcomes.
The rate of any grade CRS in the MajesTEC-1 clinical trial was 72% [], which was greater than the range reported in RW studies with ≥50 patients and ≥3 months mFU (18% [] to 64% []). However, the CRS rates in RW studies that used prophylactic TCZ (13% and 26%) [,] were similar to the subgroup in the MajesTEC-1 cohort that received prophylactic TCZ (26%) []. There was notable variation in CRS rates across the included studies, possibly due to differences in data sources, data collection methods, and the care models for CRS prophylaxis and monitoring. Patients found within secondary databases (e.g., payer claims, EMR) may have lower rates due to their reliance on diagnostic codes; for example, healthcare professionals may not code fever as CRS or code fever at all, and diagnosis codes specifically for CRS may not always be used. Chart reviews of patients with inpatient monitoring may represent a more rigorous definition of RW CRS rates with TEC, i.e., data are abstracted directly from physician’s notes, and CRS-relevant signs and symptoms are continuously monitored and recorded systematically in the hospital setting. However, it is important to note that these definitions may differ between settings and may not always be used. Data on patients who received outpatient monitoring tend to have lower rates compared to inpatient monitoring, as they may have different triggers and thresholds of CRS reporting and, sometimes, may rely on patient-reported symptoms. These differences in data sources and settings differ from the MajesTEC-1 trial as well, and, therefore, should be considered while interpreting results.
Studies included in the SLR also did not report if general neurotoxicity and ICANS were assessed together or if the reported value was for ICANS alone. Including neurotoxicity with ICANS may explain the higher rate of ICANS in the RW setting (4% [] to 14% [] among the studies with ≥50 patients and ≥3 months mFU) versus that observed in MajesTEC-1 (3%), as neurotoxicity symptoms cover a wide spectrum and definitions vary across practices.
Infection rates were sparsely reported across the RW studies, limiting the ability to analyze these data; among the larger studies with ≥3 months mFU, only four studies reported any grade infections (31% [] to 60% []) and only two studies reported grade ≥3 infections (26% [] and 27% []). MajesTEC-1 reported a higher rate of infections (79%), with 55% of the patients experiencing grade 3 or 4 infections [], though this was in the context of longer follow-ups. Unlike CRS and ICANS, both of which occur at the beginning of the treatment and are less dependent on follow-up times in RW studies, infection risk persists throughout the treatment; therefore, studies with shorter follow-up times may underreport infection rates. Additionally, the enrollment of patients for MajesTEC-1 occurred concurrently with the onset of the COVID-19 pandemic, before the widespread vaccine availability and use, a fact which may have resulted in higher infection rates among MajesTEC-1 patients compared to the RW infection rates reported in the studies considered here, all of which were conducted in the post-pandemic period. Guidelines agreed upon by expert consensus have since been published, allowing for optimal treatment of infections during TEC treatment [,]. With the adoption of infection management guideline recommendations for MM, other reasons for lower RW infection rates could be routine vaccination against COVID-19 and prophylactic intravenous immunoglobulin use for hypogammaglobulinemia and antibiotic use. Given the short follow-up durations, these results must be interpreted with caution, and data on long-term infection prophylaxis and management will need to be assessed in future work.
This review also provides initial insights into healthcare provider practices and HCRU associated with TEC in the RW setting. The use of TCZ during SUD and other medications often used for managing AEs associated with TEC were frequently reported in the studies included in the SLR. Although preliminary results showed the benefits of using prophylactic TCZ in Marin (2023) [] and Kowalski (2023) [], further studies with larger sample sizes are warranted. Based on studies published in 2023 and the first half of 2024, most patients received TEC SUD in an inpatient setting, with some institutions starting to implement outpatient SUD models [,,,,]. This is reflected in a recent study that conducted a panel interview of clinicians from 20 practices across 13 states who were among the first ones to start TEC in their practices after FDA approval. Within seven months of approval, 74% of these practices provided SUD exclusively in an inpatient setting, while 26% provided SUD in an outpatient or hybrid setting; all participating practices using inpatient SUD expressed desire of moving to outpatient SUD for BsAb in the future []. In two secondary database analyses that monitored inpatient LOS for SUD over time, a decreasing trend was observed [,]. This observation might be due to improved familiarity with TEC among providers over time, availability of well-established AE management protocols, and quality improvement models that institutions might have implemented to reduce HCRU. Furthermore, two studies using large databases of medical records suggest that patients were able to complete SUD without delay, with the two-day and three-day dosing schedules being the most common, and that the majority of the patients were taking pre-medications as recommended [,].
Recent studies with RW data available for patients who switched to a less frequent dosing schedule (most frequently switching to Q2W) have shown results in line with the MajesTEC-1 trial. In MajesTEC-1, patients were allowed to switch from weekly TEC doses to every-other-week if they achieved a PR or better after four or more cycles in phase 1 or a CR or better for six or more months in phase 2 [,]. With over two years of follow-up, TEC demonstrated deep and durable responses and reduced new onset grade ≥3 infections over time, aligning with the median time to switch to Q2W dosing (~11 month mFU), including in patients who switched to less frequent dosing []. The reduction in new onset grade ≥3 infections may also be impacted by the increasing usage of IVIG and prophylaxis []. Three RW studies identified in the SLR reported on less frequent TEC dosing, mainly with a Q2W schedule, among patients who switched [,,]; Q4W was rarely observed within these studies. Tan (2024) reported a 6-month PFS rate of 90% in patients who switched to less frequent dosing based on response or for safety management []; longer follow-ups are needed to understand the long-term impact on PFS and OS.
This SLR has some limitations. First, the recent approval of TEC resulted in the restriction of studies to the 2023 and 2024 calendar years, leading to an evidence base consisting of mostly conference materials with only five full texts. As conference abstracts may not include as detailed information as full-text publications, these results should be interpreted with caution. Due to the publication date restriction, most studies included patients who initiated TEC within 1 year of approval. This resulted in short follow-up periods and small sample sizes; longer follow-up time is needed for RW outcomes to mature. Many of these early patients were expected to be sicker and with a higher disease burden, contributing to the high percentage of reported MajesTEC-1 ineligibility. Despite this, the early effectiveness and safety outcomes remain consistent with the clinical trial. When sample sizes allow it, results may be stratified by MajesTEC-1 eligibility to provide a more balanced comparison with clinical trial results. Further, given the short follow-up periods, outcomes such as PFS, OS, and infection rate often need to be considered carefully, as current data may not reflect true estimates over time. Finally, numbers at risk over time or the total number of events are needed for reasonable accuracy during KM curve extraction and recreation [], but this information was not available in several studies. As a result, comparisons made through the KM curves are limited and need to be interpreted cautiously. These limitations are further affected by a wide variation in the data due to differences in sample sizes, differences in data sources, and differences in RW clinical practices across health centers. Due to these limitations, a meta-analysis with patient-level data would be an appropriate next step once the data mature.
5. Conclusions
In conclusion, this SLR summarizes the initial RW evidence available for TEC. Variation and immature data limit the interpretation of long-term outcomes; however, results from studies with larger sample sizes and ≥3 months mFU suggest that the early effectiveness and safety profiles for TEC in the RW are comparable to those from the pivotal trial, even for patients who were sicker, with a high disease burden, and which would not have met the eligibility criteria for MajesTEC-1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17071235/s1, Document S1: SLR Protocol; Document S2: PRISMA 2020 checklist. References [,,,,,,,,,,] are cited in the supplementary materials.
Author Contributions
Conception and design: B.D., F.R.W., D.L., B.W., M.F., J.F., A.P.-S., N.K., M.D., A.M., J.C. and S.K. Data collection: I.S., F.R.W., D.L., B.W. and N.K. Analysis and interpretation of results: All authors. Manuscript writing: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by Janssen Scientific Affairs, LLC.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Acknowledgments
Additional contributions by those who do not meet authorship requirements include Carole Lunny for data extraction and QC.
Conflicts of Interest
B. Derman is an employee of UChicago Medicine and declares consulting services for Janssen Scientific Affairs, Cota Healthcare, Guidepoint Global, Gerson Lehrman Group, Precision AQ, Sanofi, and Canopy, as well as honoraria from OncLive/MJH Life Sciences, Plexus, and Multiple Myeloma Research Foundation and research funding from GlaxoSmithKline and Amgen. C. Tan is an employee of the Memorial Sloan Kettering Cancer Center and declares consulting services for Janssen Medical Affairs and Sanofi, honoraria from MJH Life Sciences, and may hold stock and other ownership interests in Johnson & Johnson/Janssen, Moderna Therapeutics, ImmunityBio, Nektar, and Pfizer and research funding from Janssen and Takeda. I. Steinfield, F.R. Wilson, and S. Keeping are employees of Precision AQ. D. Lin, B. Wu, M. Fernandez, J. Fowler, A. Paner-Straseviciute, N. Kim, M. Doyle, A. Marshall, and J. Cheadle are employees of Johnson & Johnson Innovative Medicine and may hold stock or stock options of Johnson & Johnson. J.J. Liu is an employee of the Illinois Cancer Care.
Appendix A. PICOS Criteria

Table A1.
Eligibility criteria for the systematic literature review.
Table A1.
Eligibility criteria for the systematic literature review.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Adult (≥18 years) patients with multiple myeloma | Patients aged < 18 years |
| Interventions | Teclistamab | Interventions not listed |
| Comparators | No restrictions | -- |
| Outcomes | No restrictions | -- |
| Study design | Real-world observational studies (prospective, retrospective) | Clinical trials Case reports or case series Pooled analyses of trials Systematic literature reviews a Non-systematic/narrative reviews |
| Publication type | Full-text publications Conference abstracts/posters b | Letters to editors Editorials Commentary Expert opinion Guidelines |
| Language | Only studies published in English | Studies published in a language other than English (even if the abstract is in English) |
| Time | Published between 1 January 2023 and 30 June 2024 | Published before 2023 |
Notes: Double dashes (“—”) indicate data not reported. a Systematic reviews were excluded, but the bibliography of relevant systematic reviews was reviewed to capture relevant citations; b conference abstract/poster citations captured through a search of Embase were screened, and conferences of interest were also searched separately in the Northern Light database or published proceedings from target conferences.
Appendix B. Literature Search Strategies

Table A2.
Search strategy for Embase.
Table A2.
Search strategy for Embase.
| Database: Embase 1974 to 22 May 2024 Search Executed on 23 May 2024 | |||
|---|---|---|---|
| # | Criteria | Search Terms | Results |
| 1 | Population terms | exp multiple myeloma/ | 102,883 |
| 2 | exp plasmacytoma/ | 14,274 | |
| 3 | exp Paraproteinemias/ | 183,815 | |
| 4 | (myeloma or (multiple adj2 myeloma$) or plasmacytom$ or plasmocytom$ or mgus or (monoclonal adj2 gammopath$)).mp. | 149,247 | |
| 5 | or/1–4 | 221,071 | |
| 6 | Intervention terms | exp teclistamab/ | 406 |
| 7 | (teclistamab or Tecvayli or JNJ-64007957 or teclistamab-cqyv).mp. | 424 | |
| 8 | or/6–7 | 424 | |
| 9 | SIGN filters for observational studies | Clinical study/ | 166,793 |
| 10 | Case control study/ | 217,895 | |
| 11 | Family study/ | 25,824 | |
| 12 | Longitudinal study/ | 213,746 | |
| 13 | Retrospective study/ | 1,624,202 | |
| 14 | Prospective study/ | 919,580 | |
| 15 | Randomized controlled trials/ | 274,328 | |
| 16 | 14 not 15 | 908,367 | |
| 17 | Cohort analysis/ | 1,167,616 | |
| 18 | (Cohort adj (study or studies)).mp. | 517,217 | |
| 19 | (Case control adj (study or studies)).tw. | 176,157 | |
| 20 | (follow up adj (study or studies)).tw. | 76,223 | |
| 21 | (observational adj (study or studies)).tw. | 276,600 | |
| 22 | (epidemiologic$ adj (study or studies)).tw. | 125,861 | |
| 23 | (cross sectional adj (study or studies)).tw. | 373,627 | |
| 24 | (retrospective adj7 (study or studies or design or analysis or analyses or cohort or data or review)).ti,ab. | 1,254,000 | |
| 25 | or/9–13,16–24 | 4,466,453 | |
| 26 | Combined criteria | 5 and 8 and 25 | 96 |
| 27 | Language filter | limit 26 to english language | 96 |
| 28 | Date filter | limit 27 to yr=“2023-Current” | 72 |
Note: An Embase search was performed again at the end of June 2024 and no additional, relevant publications were identified.

Table A3.
Search strategy for MEDLINE.
Table A3.
Search strategy for MEDLINE.
| Database: Ovid MEDLINE(R) ALL <1946 to 22 May 2024> Search Executed on 23 May 2024 | |||
|---|---|---|---|
| # | Criteria | Search Terms | Results |
| 1 | Population terms | exp multiple myeloma/ | 49,135 |
| 2 | exp plasmacytoma/ | 8932 | |
| 3 | exp Paraproteinemias/ | 64,055 | |
| 4 | (myeloma or (multiple adj2 myeloma$) or plasmacytom$ or plasmocytom$ or mgus or (monoclonal adj2 gammopath$)).mp. | 84,636 | |
| 5 | or/1–4 | 96,987 | |
| 6 | Intervention terms | (teclistamab or Tecvayli or JNJ-64007957 or teclistamab-cqyv).mp. | 89 |
| 7 | SIGN filters for observational studies | Epidemiologic studies/ | 9543 |
| 8 | exp case control studies/ | 1,506,567 | |
| 9 | exp cohort studies/ | 2,607,741 | |
| 10 | Case control.tw. | 162,188 | |
| 11 | (cohort adj (study or studies)).tw. | 351,806 | |
| 12 | Cohort analy$.tw. | 13,035 | |
| 13 | (Follow up adj (study or studies)).tw. | 58,204 | |
| 14 | (observational adj (study or studies)).tw. | 178,369 | |
| 15 | Longitudinal.tw. | 345,820 | |
| 16 | Retrospective.tw. | 812,750 | |
| 17 | Cross sectional.tw. | 562,700 | |
| 18 | Cross-sectional studies/ | 502,656 | |
| 19 | or/7–18 | 4,003,471 | |
| 20 | Combined criteria | 5 and 6 and 19 | 11 |
| 21 | Language filter | limit 20 to english language | 11 |
| 22 | Date filter | limit 21 to yr=“2023-Current” | 11 |

Table A4.
Search strategy for Northern Light Life Sciences Conference Abstracts.
Table A4.
Search strategy for Northern Light Life Sciences Conference Abstracts.
| Database: Northern Light Life Sciences Conference Abstracts 2010–2024 Week 20 Search Executed on 23 May 2024 | |||
|---|---|---|---|
| # | Criteria | Search Terms | Results |
| 1 | Population terms | exp multiple myeloma/ | 27,388 |
| 2 | exp plasmacytoma/ | 1694 | |
| 3 | exp Paraproteinemias/ | 30,424 | |
| 4 | (myeloma or (multiple adj2 myeloma$) or plasmacytom$ or plasmocytom$ or mgus or (monoclonal adj2 gammopath$)).mp. | 30,055 | |
| 5 | or/1–4 | 32,556 | |
| 6 | Intervention terms | (teclistamab or Tecvayli or JNJ-64007957 or teclistamab-cqyv).mp. | 102 |
| 7 | Conference filter | Academy of Managed Care Pharmacy.cf. | 2749 |
| 8 | American Society of Clinical Oncology.cf. | 79,140 | |
| 9 | American Society of Hematology.cf. | 66,037 | |
| 10 | European Hematology Association.cf. | 31,727 | |
| 11 | European Society for Medical Oncology.cf. | 22,763 | |
| 12 | International Myeloma.cf. | 2497 | |
| 13 | Oncology Nursing Society.cf. | 1267 | |
| 14 | Society of Hematologic Oncology.cf. | 2052 | |
| 15 | or/7–14 | 208,232 | |
| 16 | Combined criteria | 5 and 6 and 15 | 82 |
| 17 | Date filter | limit 16 to yr=“2023-Current” | 47 |
Appendix C. List of Conferences Searched

Table A5.
Conferences searched in the systematic literature review.
Table A5.
Conferences searched in the systematic literature review.
| Conference | Dates Held | Search Method |
|---|---|---|
| Academy of Managed Care Pharmacy (AMCP) Meeting | 21–24 March 2023 15–18 April 2024 | Northern Light database via Ovid Hand search of website |
| AMCP Nexus | 16–19 October 2023 | Hand search of website |
| American Society for Transplantation and Cellular Therapy (ASTCT) and Center for International Blood and Marrow Transplant Research (CIBMTR) Tandem Meeting | 15–19 February 2023 21–24 February 2024 | Hand search of website |
| American Society of Clinical Oncology (ASCO) Annual Meeting | 2–6 June 2023 31 May–4 June 2024 | Northern Light database via Ovid Hand search of website |
| American Society of Hematology (ASH) Annual Meeting | 9–12 December 2023 | Hand search of website |
| European Hematology Association (EHA) Annual Congress | 8–11 June 2023 13–16 June 2024 | Northern Light database via Ovid Hand search of website |
| European Society for Medical Oncology (ESMO) | 20–24 October 2023 | Northern Light database via Ovid |
| European Myeloma Network (EMN) Meeting | 20–22 April 2023 18–20 April 2024 | Hand search of website |
| Hematology/Oncology Pharmacy Association (HOPA) Annual Conference | 29 March–1 April 2023 3–6 April 2024 | Hand search of website |
| International Conference on Oncology and Research Treatment (ORT) | 30 November–1 December 2023 | Hand search of website |
| International Myeloma Society (IMS) Annual Meeting | 27–30 September 2023 | Northern Light database via Ovid |
| Journal of the Advanced Practitioner in Oncology (JADPRO) Live | 9–12 November 2023 | Hand search of website |
| Lymphoma, Leukemia & Myeloma (LL&M) Congress | 18–21 October 2023 | Hand search of website |
| Oncology Nursing Society (ONS) Congress | 26–30 April 2023 24–28 April 2024 | Northern Light database via Ovid Hand search of website |
| Society of Hematologic Oncology (SOHO) Annual Meeting | 6–9 September 2023 | Northern Light database via Ovid |
Appendix D. Risk of Bias Assessment

Table A6.
Newcastle–Ottawa quality assessment scale—cohort studies.
Table A6.
Newcastle–Ottawa quality assessment scale—cohort studies.
| Domain | Response |
|---|---|
| Selection | |
| 1. Representativeness of the exposed cohort | Truly representative of the average _______________ (describe) in the community * Somewhat representative of the average ______________ in the community * Selected group of users (e.g., nurses, volunteers) No description of the derivation of the cohort |
| 2. Selection of the non-exposed cohort | Drawn from the same community as the exposed cohort * Drawn from a different source No description of the derivation of the non-exposed cohort |
| 3. Ascertainment of exposure | Secure record (e.g., surgical records) * Structured interview * Written self-report No description |
| 4. Demonstration that the outcome of interest was not present at the start of the study | Yes * No |
| Comparability | |
| 1. Comparability of cohorts on the basis of the design or analysis | Study controls for _____________ (select the most important factor) * Study controls for any additional factor (this criterion could be modified to indicate specific control for a second important factor) * |
| Outcomes | |
| 1. Assessment of outcome | Independent blind assessment * Record linkage * Self-report No description |
| 2. Was the follow-up long enough for the outcomes to occur? | Yes (select an adequate follow-up period for the outcome of interest) * No |
| 3. Adequacy of the follow-up of cohorts | Complete follow up—all subjects accounted for * Subjects lost to follow up unlikely to introduce bias—small number lost—>____% (select an adequate %) follow up, or description provided of those lost) * Follow up rate <____% (select an adequate %) and no description of those lost No statement |
Note: A study can be awarded a maximum of one star (*) for each numbered item within the selection and exposure categories. A maximum of two stars can be given for comparability.
Appendix E. Summary Tables of All the Included Studies

Table A7.
Summary of study characteristics and outcomes evaluated.
Table A7.
Summary of study characteristics and outcomes evaluated.
| Study ID | Study Design | Data Source | Chart Review vs. Secondary Database | Setting | Study Timeframe | mFU (Months) | Sample Size | Relevant Outcomes Evaluated |
|---|---|---|---|---|---|---|---|---|
| Asoori 2023 [] | Retrospective | Patients treated at UCSF | Chart review | Academic, single-center | NR—July 2023 | 3 | 46 | ORR, OS, PFS, infection AEs, IVIG use in AEs |
| Banerjee 2023a [] | Retrospective | All-payer claims database (ARMMRD) | Secondary database | Multi-center | October 2022–July 2023 | -- | 182 | LOS, CRS |
| Banerjee 2023b [,] | Retrospective | Acentrus MM electronic medical records (EMRs) | Secondary database | Academic centers/community-based hospitals, multi-center | October 2022–November 2023 | 5.1 | 247 | LOS, CRS, ICANS, step-up dosing schedule, TTNT, or death |
| Bansal 2023 [,] | Retrospective | Patients treated at the Mayo Clinic, Rochester, MN | Chart review | Academic, single-center | October 2020–July 2023 | -- | 24 | LOS, CRS, remote monitoring, hospitalizations |
| Bansal 2024 [] | Retrospective | Patients treated at the Mayo Clinic Rochester, MN | Chart review | Academic, single-center | December 2022–January 2024 | -- | 48 | ORR |
| Bolton 2024 [] | Prospective | Patients treated at three Kaiser Permanente institutions | Chart review | Academic, multi-center | NR | -- | 9 | CRS, ICANS, discontinuations due to any cause |
| Catamero 2023 [] | Retrospective | Patients treated at the Mount Sinai Hospital | Chart review | Academic, single-center | December 2022–May 2023 | -- | 26 | CRS, TCZ use in AE management |
| Charkviani 2024 [] | Retrospective | Patients treated at the Mayo Clinic Healthcare System | Chart review | Academic, multi-center | December 2022–May 2023 | -- | 34 | Acute kidney injury (AKI) incidence and treatment |
| Dima 2023 [,,,,] | Retrospective | Patients treated at USMIRC centers | Chart review | Academic, multi-center | August 2022–August 2023 | 3.8 | 106 | CRS, ICANS, infections, ORR, DOR, OS, PFS, TCZ use in AE management, hospitalizations |
| Faiman 2023 [] | Retrospective | Patients treated at Cleveland Clinic | Chart review | Academic, single-center | December 2022–May 2023 | 2.5 | 26 | CRS, TCZ use in AE management, ICANS, infections, best response |
| Firestone 2023 [,,,] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | November 2022–July 2023 | 3.1 | 52 | Survival, PFS, ORR, safety |
| Ghamsari 2024 [] | Retrospective | Patients treated at the University of California San Diego | Chart review | Academic, single-center | January 2023–June 2023 | -- | 18 | CRS, ICANS, infections, ORR, DOR, OS, PFS, TCZ use in AE management |
| Glenn 2024 [] | Prospective | Patients treated at the Smilow Cancer Hospital at Yale New Haven Hospital | Chart review | Academic, single-center | NR | -- | 18 | CRS |
| Gong 2023 [] | Retrospective | FDA Adverse Event Reporting System database | Secondary database | Multi-center | 2019–2023 | -- | 719 a | Reporting OR, CRS, ICANS, infection, non-ICANS neurotoxicity, mortality, hospitalizations |
| Gordon 2023 [] | Retrospective | Patients treated at four New York City metro area centers | Chart review | Academic, multi-center | NR | -- | 58 | ORR, CRS, ICANS, tocilizumab use in AE management, discontinuations |
| Graf 2024 [] | Retrospective | Patients treated at the Medical University of South Carolina | Chart review | Academic, single-center | November 2022–August 2023 | -- | 25 | Hospitalization, LOS, proportion of fever at each dose of teclistamab, incidence, severity, and onset of CRS |
| Grajales-Cruz 2023 [,] | Retrospective | Patients treated at the Lee Moffitt Cancer Center; chart review | Chart review | Academic, single-center | December 2022–October 2023 | 4.2 | 36 | ORR, PFS, OS, CRS, TCZ use in AE management, infection, LOS, ICU admission |
| Hamadeh 2024 [,,] | Retrospective | Memorial Sloan Kettering Cancer Center’s institutional plasma cell disorders database | Chart review | Academic, single-center | November 2022–July 2023 | -- | 69 | CRS |
| Hebraud 2023 [] | Retrospective | Patients treated at Institut Universitaire du Cancer de Toulouse | Chart review | Academic, single-center | January 2021–July 2023 | -- | 8 | CRS, ORR, TCZ, and IVIG use in AE management |
| Howard 2023 [] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | NR | -- | 23 | LOS, unscheduled physician communications, hospitalizations |
| Kawasaki 2024 [] | Retrospective | Patients treated at the University of California Davis (UCD) medical center | Chart review | Academic, single-center | December 2022–December 2023 | -- | 27 | CRS, ICANS, LOS, hematological toxicities, infections, hepatotoxicity, diarrhea, and IVIG in AE management |
| Kowalski 2023 [] | Prospective | Patients treated at the University of Miami Hospital & Clinics, Sylvester Comprehensive Cancer Center | Chart review | Academic, single-center | October 2022–July 2023 | 3.4 b | 31 | Best response, ORR, CRS, ICANS, PFS |
| Kumar 2023 [] | Prospective | Patients treated at the University of Connecticut Health Center | Chart review | Academic, single-center | November 2022–February 2023 | -- | 9 | Best response, ORR, CRS, ICANS |
| Lachenal 2023 [] | Retrospective | Patients treated in the French AP program | Secondary database c | Multi-center | November 2022–October 2023 | 5.2 | 15 | CRS, TCZ use in AE management, ICANS, ORR, IVIG in AE management |
| Marin 2023 [] | Prospective | Patients treated at the Emory University hospital | Chart review | Academic, single-center | December 2022–August 2023 | -- | 53 | CRS, TCZ use in AE management, TCZ dosing, ICANS, readmissions, |
| Midha 2023 [] | Retrospective | Patients treated at the Dana–Farber Cancer Institute/Brigham and Women’s Hospital | Chart review | Academic, single-center | NR—August 2023 | 2.3 b | 56 | CRS, TCZ use in AE management, infections, ORR, PFS |
| Mohan 2024 [,] | Retrospective | Patients treated at five US academic centers | Chart review | Academic, multi-center | NR | 3.5 | 110 | CRS, ICANS, infections, ORR, best response, LOS, IVIG use in AE management, TCZ use in AE management |
| Mooney 2024 [] | Retrospective | Patients treated at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital | Chart review | Academic, single-center | January 2023—NR | -- | 19 | Hospital admission, LOS, CRS, and ICANS |
| Nader 2023 [] | Retrospective | Patients treated at the Indiana University School of Medicine d | Chart review | Academic, single-center d | 2000–2021 e | 33.6 f | 49 | PFS, OS, treatment response |
| Perrot 2023 [] | Retrospective | Patients treated in the French AP program | Secondary database c | Multi-center | October 2022–April 2023 | 2.9 | 572 | Discontinuations, AE-related mortality |
| Pianko 2024 [] | Retrospective | Komodo Healthcare MapTM | Secondary database | Multi-center | October 2022–December 2023 | 4.2 | 419 | Dosing schedule of teclistamab, time to less frequent dosing, TTNT |
| Rees 2024 [] | Retrospective | Patients treated at the Mayo Clinic centers | Chart review | Academic, multi-center | April 2018–June 2023 | 9 | 41 | PFS, DOR, OS |
| Riedhammer 2024 [,] | Retrospective | Patients treated at 18 German centers | Chart review | Multi-center | July 2022–October 2023 | 5.5 | 123 | Time to response, best response, ORR, PFS, infections |
| Sandahl 2023 [,] | Retrospective | Patients treated at three Mayo Clinic centers | Chart review | Academic, multi-center | October 2022–September 2023 | -- | 49 | CRS, ICANS, TCZ use in AE management, admissions, LOS, time between TEC administration and check-out |
| Schaefers 2023 [] | Prospective | Patients treated at the University Medical Center Hamburg-Eppendorf tertiary center | Chart review | Academic, single-center | July 2022–May 2023 | 3.4 b | 16 | CRS, ICANS, infections, neutropenia, ORR, OS, PFS, DOR |
| Tabbara 2024 [] | Retrospective | Patients treated at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center; Inpatient/Outpatient unit | Chart review | Academic, single-center | January 2023–December 2023 | -- | 25 | Neutropenia, infection, CRS, ICANS, patients receiving dexamethasone, hospital admission, death |
| Tan-Asoori 2023 [,,] | Retrospective | Patients treated at IMF-associated centers | Chart review | Academic, multi-center | NR—October 2023 | 5 | 204 | ORR, OS, PFS, CRS, ICANS, TCZ use in AE management, IVIG use in AE management, LOS |
| Tan 2023 [,] | Retrospective | Premier healthcare database | Secondary database | Multi-center | November 2022–March 2023 | -- | 113 | LOS, step-up dosing schedule, hospitalizations, CRS, TCZ use in AEs |
| Tan 2024 [,] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | November 2022–March 2024 | Overall population: 9.5 Patients switching to less-frequent dosing: 6.4 | 86 | ORR, DOR, PFS, time to response |
| Varshavsky-Yanovsky 2023 [,] | Retrospective | Patients treated at Fox Chase Cancer Center | Chart review | Academic, single-center | December 2022–May 2023 | -- | 18 | CRS, ICANS |
| Venkatesh 2023 [] | Retrospective | Patients treated at the University of Kansas | Chart review | Academic, single-center | NR—February 2023 | 3.1 | 22 | CRS, ICANS, ORR, mortality, best response |
Notes: a Number of adverse event cases; note that this differs from other studies which report the number of individual patients and which, therefore, were not included in the result summary; b converted from days to months; c the French AP program follows hospitals that request reimbursement for innovative medicines prior to and after market authorization, with data coming from these reimbursement claims; d not reported and assumed based on all but one authors’ affiliation being the Indiana University School of Medicine; e given the FDA approval of TEC in 2022, the year 2000 as the start date is assumed to be an error; f converted from years to months. Abbreviations: AE, adverse event; AKI, acute kidney injury; AP, Accès Précoce; ARMMRD, all-payer real-world multiple myeloma research-ready data; CRS, cytokine release syndrome; DOR, duration of response; EMR, electronic medical record; FDA, Food and Drug Administration; ICANS, immune effector cell-associated neurotoxicity syndrome; ICU, intense care unit; IMF, International Myeloma Foundation; IVIG, intravenous immunoglobulin; LOS, length of stay; mFU, median follow-up; MM, multiple myeloma; MN, Minnesota; NR, not recorded; PFS, progression-free survival; ORR, overall response rate; OS, overall survival; TCZ, tocilizumab; TEC, teclistamab; TTNT, time to next treatment; UCD, University of California Davis; UCSF, University of California San Francisco; US, United States; USMIRC, US Myeloma Innovations Research Collaborative.

Table A8.
Summary of patient characteristics.
Table A8.
Summary of patient characteristics.
| Study ID | Overall/Subgroup Details | Sample Size | Median Age (Range), Years | Female, n (%) | Race, n (%) | High Risk Cytogenetics, n (%) | Median Prior Lines of Therapy | Extramedullary Disease, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | Asian | ||||||||
| Asoori 2023 [] | Overall | 46 | 71 (50–91) | 23 (50.0) a | 23 (50.0) | 2 (4.3) | 14 (30.4) | 13 (38.2) | 7 | -- |
| Banerjee 2023a [] | Overall | 182 | 73 (39–85) | 91 (50.0) | 123 (67.6) | 20 (11.0) | 5 (2.7) | -- | -- | -- |
| Banerjee 2023b [,] | Overall | 247 | 69 (41–89) | 111 (44.9) | 138 (75.8) b | 23 (12.6) b | 21 (11.5) b | -- | -- | 14 (5.7) |
| Bansal 2023 [,] | Overall | 24 | 66 | 9 (39) a | -- | -- | -- | 9 (38) | 5 | -- |
| Bansal 2024 [] | TEC treatment group c | 48 | -- | -- | -- | -- | -- | -- | -- | -- |
| Bolton 2024 [] | Overall | 9 | -- | -- | -- | -- | -- | -- | -- | -- |
| Catamero 2023 [] | Overall | 26 | -- | -- | -- | -- | -- | -- | -- | -- |
| Charkviani 2024 [] | TEC treatment group | 34 | 66.4 (55.4–70.8) d | 11 (32.4) | 31 (91.2) | 1 (2.9) | 1 (2.9) | -- | -- | -- |
| Dima 2023 [,,,,] | Overall | 106 | 66.5 (35–87) | 55 (54) a | 72 (68) | 28 (26) | 2 (2) | 56 (59) | 6 | 45 (42) |
| >70 years old | 33 | 75 (71–87) | -- | -- | -- | -- | 19 (58) | 6 | 13 (39) | |
| Patients with EMD | 45 | 68 (43–83) | 10 (22.0) a | 35 (78.0) | -- | -- | 25 (55.5) | 6 | 45 (100.0) | |
| Faiman 2023 [] | Overall | 26 | 64 (45–75) | 14 e (54) | 19 e (73) | -- | -- | -- | 7 | -- |
| Firestone 2023 [,,,] | Overall | 52 | 70 (30–80) | -- | -- | -- | -- | 17 (33) | 7 | 18 (35) |
| Ghamsari 2024 [] | Overall | 18 | 67 (50–83) | -- | -- | -- | -- | 11 (84.6) f,g | 6.5 | 8 (44) |
| Glenn 2024 [] | Overall | 18 | -- | -- | -- | -- | -- | -- | -- | -- |
| Gong 2023 [] | Overall | 719 cases h | 65 (57–72) e | 229 (31.8) | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [] | Overall | 58 | 67 (45–88) | 31 (53.4) | 26 (45.6) | 15 (26.3) | 3 (5.2) | 21 (36.2) | 5 | 28 (48.3) |
| Graf 2024 [] | Overall | 25 | 66 (37–78) | 12 (48.0) | 14 (56.0) | -- | -- | 9 (36.0) | 5 | 13 (52.0) |
| Grajales-Cruz 2023 [,] | Overall | 36 | 67 (48–88) | 16 (44.4) a | -- | -- | -- | 15 (44.1) | 7 | 16 (57.0) |
| Hamadeh 2024 [,,] | Overall | 72 | (39–88) | 30 (41.7) i | 56 (77.8) i | 12 (16.7) i | -- | 36 (50.0) i | -- | 21 (29.2) i |
| Prior T-cell redirection therapies (TCRT) | 27 | 70 (51–88) | 10 (37) | 23 (85) | 2 (7) | -- | 19 (70) | 8 | 9 (33) | |
| No prior TCRT | 45 | 69 (39–88) | 20 (44) | 33 (73) | 10 (22) | -- | 17 (38) | 5 | 12 (27) | |
| Hebraud 2023 [] | Overall | 8 | 69 (43–81) | -- | -- | -- | -- | -- | 4 | -- |
| Howard 2023 [] | Overall | 23 | 67 (51–88) | -- | -- | -- | -- | 5 (22) | 6 | 6 (26) |
| Kawasaki 2024 [] | Overall | 27 | -- | 12 (44.4) i | 18 (66.7) i | -- | -- | -- | -- | 8 (29.6) i |
| Dosing schedule 1, 3, 5 (days) | 23 | 69 | 9 (39) f | 16 (69) | -- | -- | -- | -- | 6 (26) | |
| Dosing schedule 1, 4, 7 (days) | 4 | 64 | 3 (75) f | 2 (50) | -- | -- | -- | -- | 2 (50) | |
| Kowalski 2023 [] | Overall j | 31 | 71 (50–84) | 21 (68) | 22 (71) | -- | -- | 9 (29) | 5 | 24 (77) |
| Kumar 2023 [] | Overall | 9 | 75 (41–81) | -- | -- | -- | -- | -- | 6 | -- |
| Lachenal 2023 [] | Overall | 15 | 68 (58–83) | -- | -- | -- | -- | 7 (70.0) k | 4 | -- |
| Marin 2023 [] | Overall | 53 | 69 (43–83) | 15 (28.3) a,i | 25 (47.2) i | 24 (45.3) i | 1 (1.9) i | 21 (39.6) i | -- | -- |
| No prophylactic tocilizumab (TCZ) | 15 | 58 (47–73) | 2 (13.3) a | 7 (46.7) | 8 (53.3) | -- | 4 (26.7) | 6 | -- | |
| Prophylactic TCZ | 38 | 69 (43–83) | 13 (34.2) a | 18 (47.4) | 16 (42.1) | 1 (2.6) | 17 (44.7) | 5 | -- | |
| Midha 2023 [] | Overall | 56 | 69 (45–83) | 21 (37.5) a | -- | -- | -- | -- | 6 | 24 (42.9) |
| Mohan 2024 [,] | Overall | 110 | 68 (37–89) | 54 (49) | 67 (61) | 32 (29) | 2 (1.8) | 59 l (62) | 6 | 48 (44) |
| Mooney 2024 [] | Overall | 19 | -- | -- | -- | -- | -- | -- | -- | -- |
| Nader 2023 [] | Overall | 49 | 70 (59–75) d | 27 (55.0) | 41 (84.0) | 5 (10.0) | -- | -- | >4 | -- |
| Perrot 2023 [] | Overall | 572 | 71 (64–76) d | 241 (42.1) | -- | -- | -- | 124 (21.7) | 4 | 121 (21.2) |
| Pianko 2024 [] | Overall | 419 | 65 (58–73) d | 183 (43.7) | 185 (63.4) m | 91 (31.2) m | 16 (5.4) m | -- | 5 | -- |
| Rees 2024 [] | TEC treatment group c | 41 | -- | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [,] | Overall | 123 | 67 (35–87) | 53 e (43.1) | -- | -- | -- | 39 (36.8) n | 6 | 43 (36.1) |
| Sandahl 2023 [,] | Overall | 49 | 67.2 (38.7–84.2) | 18 (36.7) | 43 (87.8) | 3 (6.1) | 1 (2.0) | 31 (63.3) | -- | 3 (6.1) |
| Schaefers 2023 [] | Overall | 16 | 65.5 (51–86) | 5 (31) | -- | -- | -- | 8 (80) | 6 | -- |
| Tabbara 2024 [] | Overall | 25 | 70 (59–89) | 14 (56.0) | 19 (76.0) | 5 (20.0) | 0 (0.0) | -- | 6 | -- |
| Tan-Asoori 2023 [,,] | Overall | 204 | 66 (33–91) | 91 (45) | 143 (70) | 15 (7) | 19 (9) | 90 (44) | 6 | 38 (19) |
| Tan 2023 [,] | Overall | 113 | 65 (58–74) d | 44 (38.9) | 74 (65.5) | 24 (21.2) | 3 (2.7) | -- | -- | -- |
| Tan 2024 [,] | Overall | 86 | 71 (64–78) d | 44 (51) | 65 (76) | 14 (16) | -- | 56 (71) o | 6 | 30 (38) o |
| Patients switched to a less frequent dosing schedule | 32 | 70 (65–78) d | 20 (62) a | -- | 3 e (9) | -- | 17 (59) | 6 | 10 (34) p | |
| Varshavsky-Yanovsky 2023 [,] | Overall | 18 | 66 (46–81) | -- | -- | -- | -- | -- | -- | -- |
| Venkatesh 2023 [] | Overall | 22 | 70 (43–85) | 9 (41) g | -- | -- | -- | 9 (41) | 6 | 14 (64) |
Notes: Double dashes (“—”) indicate data not reported. a Calculated from the reported proportion of males; b evaluated population = 186; c overall population of combined multiple treatments, with TEC-only data limited; d IQR; e n hand calculated; f evaluable population n = 13; g % hand calculated; h number of individual cases; i calculated from stratified cohorts; j the overall population was treated with prophylactic tocilizumab; k n = 10 evaluable; l n = 95 evaluable; m evaluable population n = 292; n evaluable population n = 106; o evaluable population n = 79; p evaluable population n = 29. Abbreviations: EMD, extramedullary disease; IQR, interquartile range; TCRT, T-cell redirection therapies; TCZ, tocilizumab; TEC, teclistamab.

Table A9.
Summary of MajesTEC-1 ineligibility.
Table A9.
Summary of MajesTEC-1 ineligibility.
| Study ID | Overall/Subgroup Details | Sample Size | MajesTEC-1 Ineligibility, n (%) | Prior BCMA Therapy, n (%) | ECOG PS ≥2, n (%) | Cytopenia, n (%) | Renal Impairment/Failure, n (%) | CrCl < 30 mL/min or 40 mL/min, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Anemia | Neutropenia | Thrombocytopenia | ||||||||
| Asoori 2023 [] | Overall | 46 | 31 (67.3) | 16 (34.8) | -- | -- | -- | -- | -- | -- | 15 (32.6) |
| Banerjee 2023a [] | Overall | 182 | -- | 15 (16.3) a | -- | -- | 108 (59.3) | 36 (19.8) | -- | 77 (42.3) | -- |
| Banerjee 2023b [,] | Overall | 247 | -- | 48 (19.4) | -- | -- | 126 (51.0) | 55 (22.3) | -- | 100 (40.5) | -- |
| Dima 2023 [,,,,] | Overall | 106 | 88 (83) | 56 (53) | 35 (33) | 33 (31) | 27 (25) | 2 (2) | 21 (20) | 14 (13) | 14 (13) |
| >70 years old | 33 | -- | -- | 15 (45.0) | -- | -- | -- | -- | -- | -- | |
| Patients with EMD | 45 | -- | 25 (55.5) | 16 (35.5) | -- | -- | -- | -- | -- | -- | |
| Faiman 2023 [] | Overall | 26 | -- | 9 (38) | -- | -- | -- | -- | -- | -- | -- |
| Firestone 2023 [,,,] | Overall | 52 | -- | 27 (52) | -- | -- | -- | -- | -- | -- | -- |
| Ghamsari 2024 [] | Overall | 18 | -- | 7 (39) | -- | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [] | Overall | 58 | 51 (87.9) | 23 (39.7) | 17 (29.8) | (48.3) | -- | -- | -- | 13 (22.4) | -- |
| Graf 2024 [] | Overall | 25 | -- | 11 (44) | -- | -- | -- | -- | -- | -- | -- |
| Grajales-Cruz 2023 [,] | Overall | 36 | 36 (100.0) | 36 (100.0) | -- | -- | 7 (22.6) | 2 (6.45) | 10 (32.3) | -- | 6 (18.8) |
| Howard 2023 [] | Overall | 23 | -- | 8 (35) | -- | -- | -- | -- | -- | -- | -- |
| Kowalski 2023 [] | Overall c | 31 | 26 (84) | 4 (13) | 16 (52) d | 15 (48) e | -- | -- | -- | -- | -- |
| Lachenal 2023 [] | Overall | 15 | -- | -- | -- | -- | -- | -- | -- | 15 (100) f | -- |
| Midha 2023 [] | Overall | 56 | 45 (80.0) | 20 (35.7) | 17 (30.4) | -- | -- | -- | -- | -- | 11 (19.6) |
| Mohan 2024 [,] | Overall | 110 | -- | 38 (35) | -- | -- | -- | -- | -- | -- | -- |
| Perrot 2023 [] | Overall | 572 | -- | 49 g (8.6) | 108 g (18.9) d | -- | -- | -- | -- | 102 g (17.8) | -- |
| Pianko 2024 [] | Overall | 419 | -- | 102 (24.3) | -- | -- | 164 (39.1) | 50 (11.9) | -- | 206 (49.2) | -- |
| Rees 2024 [] | TEC treatment group b | 41 | -- | 25 (61.0) | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [,] | Overall | 123 | 48 g (39) | 45 (37.4) | -- | -- | -- | -- | -- | -- | -- |
| Sandahl 2023 [,] | Overall | 49 | -- | 17 (34.7) | -- | -- | 27 (55.1) | 14 (28.6) | -- | 15 (30.6) | -- |
| Tan-Asoori 2023 [,,] | Overall | 204 | 122 (70) h | 91 (45.0) | -- | -- | -- | -- | -- | -- | 25 (12) |
| Tan 2023 [,] | Overall | 113 | -- | -- | -- | -- | 65 (57.5) | -- | -- | 32 (28.3) | -- |
| Tan 2024 [,] | Overall | 86 | -- | 32 (37) | 5 (10) i | -- | -- | -- | -- | -- | 9 (10) |
| Patients switched to less frequent dosing schedule | 32 | -- | 10 (31) | -- | -- | -- | -- | -- | -- | -- | |
Notes: Double dashes (“—”) indicate data not reported. a Based on the evaluated population (n = 92); b overall population of combined multiple treatments, with TEC-only data limited; c the overall population was treated with prophylactic tocilizumab; d % hand calculated; e reported as hematological ineligibility; f reported as renal disease; g n hand calculated; h based on the evaluated population (n = 175); i based on the evaluable population n = 50. Abbreviations: BCMA, B-cell maturation antigen; CrCl, creatinine clearance; ECOG, Eastern Cooperative Oncology Group performance status; EMD, extramedullary disease; TCRT, T-cell redirection therapy; TCZ, tocilizumab, TEC, teclistamab.

Table A10.
Summary of key effectiveness outcomes.
Table A10.
Summary of key effectiveness outcomes.
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | Overall Response Rate, n (%) | ||
|---|---|---|---|---|---|---|
| PR or Better | VGPR or Better | CR or Better | ||||
| Asoori 2023 [] | Overall | 46 | Median of 3 months | 32 b (70.0) | 25 (54.3) c,d | 6 (13.0) c,d |
| Bansal 2024 [] | TEC treatment group a | 48 | January 2024 cut-off | 29 b (61.0) | -- | -- |
| Dima 2023 [,,,,] | Overall | 104 | Median of 3.8 months | 70 (66) b | 49 (46)c | 31 (29) c |
| >70 years old | 34 | Median of 3.8 months e | 24 (71) | -- | 10 (30) f | |
| Faiman 2023 [] | Overall | 26 | Median of 2.5 months | 15 (60) c | 9 (36) c | 4 (16) c |
| Firestone 2023 [,,,] | Overall | 47 | Median of 3.1 months | 30 (64) | 18 b (38) | -- |
| Prior anti-BCMA exposure | 26 | Median of 3.1 months e | 13 (50.0) | -- | -- | |
| Faiman 2023 [] | Overall | 18 | June 2023 cut-off | 9 b (50) | 9 b (50) | -- |
| Gordon 2023 [] | Overall | 58 | -- | 29 b (50.0) | 14 b (24.1) | -- |
| Grajales-Cruz 2023 [,] | Overall | 36 | Median of 4.2 months | 19 b (52.8) | 16 b (44.5) c | 16 b (44.5) c |
| Hebraud 2023 [] | Overall | 8 | July 2023 cut-off | -- | 6 (75) d | -- |
| Kowalski 2023 [] | Patients with secretory disease g | 30 | Median of 3.4 months h | 15 (50) | -- | 9 (30) c |
| Kumar 2023 [] | Overall | 9 | 3-month evaluation | 6 (66.7) | 4 (44.4) c | 3 (33.3) c |
| Lachenal 2023 [] | Overall | 15 | Median of 5.2 months | 13 (86.7) d | 11 (73.3) c,e | 4 (26.7) c,d |
| Midha 2023 [] | Overall | 56 | Median of 2.3 months h | 30 b (53.6) | -- | -- |
| Mohan 2024 [,] | Overall | 98 | Median of 3.5 months | 61 (62) | 50 b (51) | 20 b (20) |
| Nader 2023 [] | Overall | 27 | Median of 33.6 months i | 19 (70) | -- | -- |
| Riedhammer 2024 [,] | Overall | 123 | Median of 5.5 months | 73 b (59.3) | 59 b (48.0) c | 27 b (22.0) c,j |
| Schaefers 2023 [] | Overall | 16 | Median of 3.4 months h | 7 b (44) | 5 b (31) | -- |
| Tan-Asoori 2023 [,,] | Overall | 180 | Median of 5 months | 115 b (64) | 90 b (50) c | 34 b (19) c |
| Tan 2024 [,] | Overall | 77 | Median of 9.5 months | 47 (61) | 33 (43) | -- |
| Prior BCMA-directed therapy | 32 | Median of 9.5 months e | 14 (43) | -- | -- | |
| Venkatesh 2023 [] | Overall | 22 | Median of 3.1 months | 11 b (50) | -- | -- |
Notes: Double dashes (“—”) indicate data not reported. a Overall population in which TEC was studied and other interventions in a combined cohort, the stratified subgroup data are presented here and are included in the overall ranges; b n hand calculated; c calculated from stratified best response; d based on July 2023 cut-off (n = 33); e the median follow-up was assumed to apply to the subgroup data, as both share the same data cut-off; f % hand calculated; g the population was treated with prophylactic tocilizumab; h converted from days to months; i converted from years to months; j reported as near complete or complete response. Abbreviations: BCMA, B-cell maturation antigen; CR, complete response; mFU, median follow-up; PR, partial response; TEC, teclistamab; VGPR, very good partial response.

Table A11.
Summary of key safety outcomes.
Table A11.
Summary of key safety outcomes.
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | CRS, n (%) | ICANS, n (%) | Infections, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 1 | Grade 2 | Grade 3+ | Any Grade | Grade 1 | Grade 2 | Grade 3+ | Any Grade | Grade 3+ | ||||
| Chart review—mixed inpatients and outpatient or not reported | |||||||||||||
| Asoori 2023 [] | Overall | 46 | Median of 3 months | -- | -- | -- | -- | -- | -- | -- | -- | 39 (84.8) | 14 (35.8) a |
| Bansal 2023 [,] | Overall b | 24 | July 2023 cut-off | 13 (54.2) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Catamero 2023 [] | Overall | 26 | May 2023 cut-off | 20 c (78) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| CRS patients | 20 | May 2023 cut-off | -- | 17 c (85) | 3 c (15) | 0 (0) | -- | -- | -- | -- | -- | -- | |
| Glenn 2024 [] | Overall | 18 | -- | 1 (5.6) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [] | Overall | 58 | -- | 30 c (52) | -- | -- | -- | 6 c (11) | -- | -- | -- | -- | -- |
| Grajales-Cruz 2023 [,] | Overall | 36 | Median of 4.2 months | 21 (58.3) | 15 (45.5) | 4 (12.1) | 0 (0) | -- | -- | -- | -- | 21 (58.3) | -- |
| Hamadeh 2024 [,,] | Overall | 72 | July 2023 cut-off | 46 c (63.9) d | 36 c (50.0) d | 10 c (13.9) d | -- | -- | -- | -- | -- | -- | -- |
| Prior TCRT | 27 | July 2023 cut-off | 10 c (37) | 8 c (30) | 2 c (7) | -- | -- | -- | -- | -- | -- | -- | |
| No prior TCRT | 45 | July 2023 cut-off | 36 c (80) | 28 c (62) | 8 c (18) | -- | -- | -- | -- | -- | -- | -- | |
| Kawasaki 2024 [] | Overall | 27 | -- | -- | 13 c (48.1) d,e | -- | 4 c (14.8) d | -- | -- | -- | -- | -- | |
| Dosing schedule 1, 3, 5 (days) | 23 | -- | -- | 11 (48) e | -- | 4 (17) | -- | -- | -- | -- | -- | ||
| Dosing schedule 1, 4, 7 (days) | 4 | -- | -- | 2 (50) e | -- | 0 (0) | -- | -- | -- | -- | -- | ||
| Kowalski 2023 [] | Prophylactic tocilizumab | 31 | Median of 3.4 months f | 4 c (13) | -- | -- | -- | 3 c (10) | -- | -- | -- | 8 (26) | -- |
| Kumar 2023 [] | Overall | 9 | 3-month evaluation | 7 (77.8) d | -- | -- | -- | 1 (11.1) d | -- | -- | -- | -- | -- |
| Midha 2023 [] | Overall | 56 | Median of 2.3 months f | 29 (51.8) | -- | -- | 1 (1.8) | -- | -- | -- | -- | 32 (57.1) | -- |
| Mohan 2024 [,] | Overall | 110 | Median of 3.5 months | 62 c (56) | 57 c (51.8) d,e | 5 (4.5) d | 12 (11) | -- | -- | 5 (4.5) | 44 (40) | 29 (26) | |
| Riedhammer 2024 [,] | Overall | 123 | Median of 5.5 months | 72 (58.5) | -- | -- | 2 (1.6) | -- | -- | -- | -- | 67 (54.5) | 33 (26.8) |
| Tabbara 2024 [] | Overall | 25 | December 2023 cut-off | -- | 15 (60) e | 0 (0) | 4 (16) | -- | 2 (8) | 0 (0) | 6 (24) | 3 (12) | |
| Tan-Asoori 2023 [,,] | Overall | 204 | Median of 5 months | 110 (53.9) c,d | 84 (41.2) c,d | 25 (12.3) c,d | 1 (0.5) c,d | -- | -- | -- | -- | 115 (60.0) | -- |
| Venkatesh 2023 [] | Overall | 22 | Median of 3.1 months | -- | 9 (41.0) d,e | -- | 5 (23) | -- | -- | 2 (9) | -- | -- | |
| Chart review—inpatient monitoring | |||||||||||||
| Bolton 2024 [] | Overall | 9 | -- | -- | 3 (33.3) | -- | -- | -- | 2 (22.2) | -- | -- | -- | -- |
| Dima 2023 [,,,,] | Overall | 106 | Median of 3.8 months | 68 (64) | 57 (54) | 10 (9) | 1 (1) | 15 (14) | 5 (5) | 7 (6) | 3 (3) | 33 (31) | -- |
| >70 years old | 33 | July 2023 cut-off | 22 (67) | -- | -- | 1 (3) | 7 (21) | -- | -- | 0 (0) | 11 (33) | -- | |
| Faiman 2023 [] | Overall | 26 | Median of 2.5 months | 22 (85) | -- | -- | -- | 5 (19) | -- | -- | -- | 7 (26.9) e | -- |
| Firestone 2023 [,,,] | Overall | 52 | Median of 3.1 months | 27 (52) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Ghamsari 2024 [] | Overall | 18 | June 2023 cut-off | 12 (67) | -- | 4 (22.2) d | 0 (0) | 2 (11) | -- | 1 (5.6) d | -- | 12 c (67) | -- |
| Graf 2024 [] | Overall | 25 | August 2023 cut-off | 13 (52) | 12 (48) | 1 (4) | -- | 1 (4) | -- | -- | -- | -- | -- |
| Marin 2023 [] | Overall | 53 | August 2023 cut-off | 21 c (39.6) g | -- | -- | -- | 5 (9.4) c | -- | -- | -- | -- | -- |
| Prophylactic TCZ | 38 | August 2023 cut-off | 10 c (26.3) | -- | -- | -- | 2 c (5.3) | -- | -- | -- | -- | -- | |
| No prophylactic TCZ | 15 | August 2023 cut-off | 11 c (73.3) | -- | -- | -- | 3 c (20.0) | -- | -- | -- | -- | -- | |
| Mooney 2024 [] | Patients admitted to hospital | 15 | -- | 13 (86.7) d,h | -- | -- | -- | 13 (86.7) d,h | -- | -- | -- | -- | -- |
| Schaefers 2023 [] | Overall | 16 | Median of 3.4 months f | 4 (25) | -- | -- | 0 (0) | 0 (0) | -- | -- | 0 (0) | 13 (81) | 9 (56) |
| Tan-Asoori 2023 [,,] | Inpatients | 160 | Median of 5 months | 94 (59) | 72 (45) | 22 (14) | 0 (0) | -- | -- | -- | -- | -- | -- |
| Chart review—outpatient monitoring | |||||||||||||
| Hebraud 2023 [] | Overall | 8 | July 2023 cut-off | 3 (37.5) d | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Sandahl 2023 [,] | Overall | 45 | September 2023 cut-off | 13 (28.9) | 10 (22.2) | 2 (4.4) | 1 (2.2) | 2 (4.4) | -- | -- | -- | -- | -- |
| Tan-Asoori 2023 [,,] | Outpatients | 44 | Median of 5 months | 16 (36) | 12 (27) | 3 (7) | 1 (2) | -- | -- | -- | -- | -- | -- |
| Varshavsky-Yanovsky 2023 [,] | Overall | 18 | 6-month evaluation | -- | 5 (27.8) | 1 (5.6) | -- | -- | -- | -- | -- | -- | -- |
| Secondary databases | |||||||||||||
| Banerjee 2023a [] | Overall | 131 | July 2023 cut-off | 54 (41.2) | 39 (29.8) | 9 (6.9) | 2 (1.5) | -- | -- | -- | -- | -- | -- |
| Banerjee 2023b [,] | Overall | 76 | Median of 5.1 months | 14 (18.4) | 8 (10.5) | 3 (3.9) | 1 (1.3) | 3 (3.9) | 2 (2.6) | 1 (1.3) | 0 (0) | -- | -- |
| Gong 2023 [] | Total count of AEs | 1317 events i | -- | 130 (9.5) events | -- | -- | -- | 34 (2.5) events | -- | -- | -- | 214 (15.7) events | -- |
| Lachenal 2023 [] | Overall | 15 | Median of 5.2 months | -- | 11 (73.3) d,e | -- | 1 (6.7) d | -- | -- | -- | 9 (60.0) d | -- | |
| Tan 2023 [,] | Overall | 58 | April 2023 cut-off | 15 (25.9) | 11 (19.0) | 4 (6.9) | -- | -- | -- | -- | -- | -- | -- |
Notes: Double dashes (“—”) indicate data not reported. Results are based on the most recent timepoint in each study. a Reported as grade ¾ infections; b the most recent data cut-off no longer reports TEC alone and is not included in the overall population summary ranges; c n hand calculated; d % hand calculated; e reported as grade 1 and 2; f converted from days to months; g only the no tocilizumab cohort of the overall population is included in the ranges; h reported as CRS and/or ICANS; i total number of events—note that this differs from other studies which report the total number of patients. Abbreviations: AE, adverse event; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; TCRT, T-cell redirection therapies; TCZ, tocilizumab.
Appendix F. Additional Patient Characteristics and Effectiveness Outcomes

Table A12.
Summary of prior lines of therapy.
Table A12.
Summary of prior lines of therapy.
| Study ID | Overall/Subgroup Details | n | Prior BCMA CAR T, n (%) | Prior BCMA ADC, n (%) | Prior BCMA BsAb, n (%) | Prior Non-BCMA, n (%) | Prior AlloSCT, n (%) | Prior AutoSCT, n (%) | Triple-Class, n (%) | Penta-Class, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAR-T | BsAb | Exposed | Refractory | Exposed | Refractory | ||||||||
| Asoori 2023 [] | Overall | 46 | 9 (19.6) a | 4 (8.7) a | 3 (6.5) a | -- | -- | -- | -- | -- | 41 (89.1) | -- | -- |
| Banerjee 2023a [] | Overall | 92 | 8 (8.7) | 9 (9.8) b | -- | -- | -- | 43 (46.7) | -- | -- | -- | -- | |
| Banerjee 2023b [,] | Overall | 247 | 27 (10.9) | 25 (10.1) | 1 (<1) | -- | -- | -- | -- | -- | -- | -- | -- |
| Bansal 2024 [] | TEC treatment group | 48 | 20 (71.4) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Dima 2023 [,,,,] | Overall | 106 | -- | -- | -- | -- | -- | 3 (3) | 61 (58) | -- | 97 (92) | -- | 68 (64) |
| >70 years old | 33 | -- | -- | -- | -- | -- | -- | 19 (58) | -- | 32 (97) | -- | 19 (58) | |
| Patients with EMD | 45 | -- | -- | -- | -- | -- | -- | 29 (64) | -- | 42 (93) | -- | 26 (58) | |
| Faiman 2023 [] | Overall | 26 | -- | 1 (3.8) a | -- | -- | -- | 10 (38) c | -- | 26 (100) | -- | (92) | |
| Firestone 2023 [,,,] | Overall | 52 | 19 (37) | 16 (31) | 2 (4) | 3 (6) | 5 (10) | 3 (6) | 40 (77) | 50 (96) | -- | -- | 35 (67) |
| Ghamsari 2024 [] | Overall | 18 | 5 (27.8) a | 2 (11.1) a | -- | -- | -- | -- | -- | -- | 18 (100.0) | -- | 17 (94.4) |
| Gordon 2023 [] | Overall | 58 | 12 (20.7) | 15 (25.9) b | -- | -- | -- | 2 (3.4) | 40 (69) | 58 (100.0) | -- | 42 (72.4) | -- |
| Graf 2024 [] | Overall | 25 | -- | -- | -- | --- | -- | -- | -- | -- | 20 (80) | -- | 12 (48) |
| Grajales-Cruz 2023 [,] | Overall | 36 | 26 (72.2) | 4 (11.1) | -- | -- | -- | -- | 25/33 (75.8) c | -- | 35 (97.2) | -- | 22 (61.1) |
| Hamadeh 2024 [,,] | Overall | 72 | 19 (27.5) d | -- | 3 (4.2) d | 2 (2.8) d,e | 7 (9.7) d,f | 5 (6.9) d | 48 (66.7) d | -- | -- | -- | -- |
| Prior TCRT | 27 | 19/21 (90) | -- | 3/10 (30) | 2/21 (10) e | 7/10 (70) f | 3 (11) | 19 (70) | -- | -- | -- | -- | |
| No prior TCRT | 45 | -- | -- | -- | -- | -- | 2 (1) | 29 (64) | -- | -- | -- | -- | |
| Howard 2023 [] | Overall | 23 | -- | -- | -- | -- | -- | 1 (4) | 16 (70) | -- | 20 (87) | -- | 10 (43) |
| Kawasaki 2024 [] | Overall | 27 | -- | -- | -- | -- | -- | -- | 18 (66.7) | -- | 27 (100.0) d | -- | -- |
| Dosing schedule 1, 3, 5 (days) | 23 | -- | -- | -- | -- | -- | -- | 15 (65) | -- | 23 (100) | -- | -- | |
| Dosing schedule 1, 4, 7 (days) | 4 | -- | -- | -- | -- | -- | -- | 3 (75) | -- | 4 (100) | -- | -- | |
| Kowalski 2023 [] | Overall g | 31 | -- | -- | -- | -- | -- | 12 (39) h | 31 (100) | 26 (84) | 21 (68) | 6 (19) | |
| Marin 2023 [] | Overall | 53 | -- | -- | -- | -- | -- | -- | -- | -- | 53 (100.0) d,i | -- | -- |
| No prophylactic TCZ | 15 | -- | -- | -- | -- | -- | -- | -- | -- | 15 (100.0) i | -- | -- | |
| Prophylactic TCZ | 38 | -- | -- | -- | -- | -- | -- | -- | -- | 38 (100.0) i | -- | -- | |
| Midha 2023 [] | Overall | 56 | 13 (23.2) | 12 (23.2) | -- | -- | -- | 33 (58.9) j | -- | -- | -- | -- | |
| Mohan 2024 [,] | Overall | 110 | -- | -- | -- | -- | -- | -- | 96 (87) | -- | 95 (86) | -- | 84 (76) |
| Perrot 2023 [] | Overall | 572 | -- | -- | -- | -- | -- | -- | -- | -- | 398 k (69.6) | -- | 183 k (32.0) |
| Pianko 2024 [] | Overall | 419 | 51 (12.2) | 71 (16.9) | 0 (0.0) | -- | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [,] | Overall | 123 | 21 (17.1) a,l | 23 (18.7) a | -- | -- | -- | -- | -- | -- | 114 k (92.6) | -- | 74 k (60.2) |
| Sandahl 2023 [,] | Overall | 49 | 10 (20.4) | 5 (10.2) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Schaefers 2023 [] | Overall | 16 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 16 (100) |
| Tabbara 2024 [] | Overall | 25 | 1 (4) | -- | -- | -- | -- | 13 (52) j | -- | -- | -- | -- | |
| Tan-Asoori 2023 [,,] | Overall | 204 | 40 (19.6) a | 25 (12.3) a | 5 (2.5) a | -- | -- | -- | -- | -- | 123/156 (79.0) | -- | 56/150 (37.0) |
| Tan 2024 [,] | Overall | 86 | 21/32 (66) | 19/32 (60) | 3/32 (9) | -- | -- | 3 (3) | 53 (62) | -- | -- | -- | -- |
| Venkatesh 2023 [] | Overall | 22 | -- | -- | -- | -- | -- | 17 (77) j | -- | 20 (91) | -- | 13 (59) | |
Notes: Double dashes (“—”) indicate data not reported. The table only includes studies where the relevant characteristics were reported. a % hand calculated; b reported as belantamab; c reported as HSCT; d calculated from stratified cohorts; e includes one (5%) GPRC5D and one (5%) CD38-targeted CAR-T therapy; f includes three (30%) GPRC5D and four (40%) FcRH5-targeted BsAB therapies; g the overall population was treated with prophylactic tocilizumab; h reported as SCT; i IMID, PI, and CD38 mAb refractory; j allogenic or autologous not distinguished; k n hand calculated; l reported as ide-cel. Abbreviations: ADC, antibody-drug conjugate; AlloSCT, allogenic stem cell transplant; AutoSCT, autologous stem cell transplant; BCMA, B-cell maturation antigen; BsAB, bispecific antibody; CAR-T, chimeric antigen receptor t-cell; CD-38, cluster of differentiation 38; EMD, extramedullary disease; GPRC5D, G protein-coupled receptor, class C, group 5, member D; IMID, immunomodulatory imide drugs; HSCT, hemopoietic stem cell therapy; mAb, monoclonal antibody; PI, proteasome inhibitors; SCT, stem cell therapy; TCRT, T-cell redirection; TCZ, tocilizumab; TEC, teclistamab.

Table A13.
Summary of comorbidities at baseline.
Table A13.
Summary of comorbidities at baseline.
| Study ID | Overall/Subgroup Details | Sample Size | Hemodialysis/dialysis, n (%) | Peripheral Neuropathy, n (%) | Hypogammaglobulinemia, n (%) | CNS Involvement, n (%) |
|---|---|---|---|---|---|---|
| Asoori 2023 [] | Overall | 46 | 2 (5.4) | -- | 32 (69.6) | -- |
| Banerjee 2023a [] | Overall | 124 | 9 (4.9) | 57 (31.3) | 52 (28.6) | -- |
| Banerjee 2023b [,] | Overall | 247 | -- | 89 (36) | 39 (15.8) | -- |
| Dima 2023 [,,,,] | Overall | 106 | -- | -- | -- | 4 (4) |
| Grajales-Cruz 2023 [,] | Overall | 33 | -- | -- | 17 (53.1) | -- |
| Kowalski 2023 [] | Overall b | 31 | -- | -- | -- | 2 (7) |
| Lachenal 2023 [] | Overall | 15 | 15 (100) c | -- | -- | -- |
| Midha 2023 [] | Overall | 56 | -- | -- | -- | 7 (12.5) |
| Pianko 2024 [] | Overall | 419 | -- | -- | 125 (29.8) | -- |
| Sandahl 2023 [,] | Overall | 49 | -- | 28 (57.1) | 20 (40.8) | -- |
| Tabbara 2024 [] | Overall | 25 | 1 (4.0) | -- | -- | -- |
| Tan-Asoori 2023 [,,] | Overall | 204 | 2 (1) a | -- | 105/153 (69) | -- |
| Tan 2023 [,] | Overall | 113 | -- | 41 (36.3) | -- | -- |
| Tan 2024 [,] | Overall | 86 | 2 (2) | 35 (41) | -- | -- |
Notes: Double dashes (“—”) indicate data not reported. The table only includes studies where the relevant characteristics were reported. a n hand calculated; b the overall population was treated with prophylactic tocilizumab; c assumed based on inclusion criteria. Abbreviations: CrCl, creatine clearance; CNS, central nervous system; TCRT, T-cell redirection therapy; TCZ, tocilizumab; TEC, teclistamab.

Table A14.
Summary of key effectiveness outcomes stratified by best response.
Table A14.
Summary of key effectiveness outcomes stratified by best response.
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | Overall Response Rate, n (%) | Stratified Best Response, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR or Better | VGPR or Better | CR or Better | PD | SD | PR | VGPR | CR | sCR | ||||
| Asoori 2023 [] | Overall | 46 | Median of 3 months | 32 a (70.0) | -- | -- | -- | -- | 7 (15.2) b | 19 (41.3) b | 6 (13.0) b | |
| Dima 2023 [,,,,] | Overall | 104 | Median of 3.8 months | 70 (66) | -- | -- | 26 (24) | 10 (9.5) | 21 (20) | 18 (17) | 31 (29) | |
| >70 years old | 34 | Median of 3.8 months | 24 (71) | -- | 10 (30) c | -- | -- | -- | -- | -- | -- | |
| Faiman 2023 [] | Overall | 26 | Median of 2.5 months | -- | -- | -- | 6 (24) | 4 (16) | 6 (24) | 5 (20) | 4 (16) | -- |
| Firestone 2023 [,,,] | Overall | 47 | Median of 3.1 months | 30 (64) | 18 a (38) | -- | -- | -- | -- | -- | -- | -- |
| Ghamsari 2024 [] | Overall | 18 | June 2023 cut-off | 9 a (50) | 9 a (50) | -- | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [] | Overall | 58 | -- | 29 a (50.0) | 14 a (24.1) | -- | -- | 8 a (13.1) b | 15 a (25.9) | 14 a (24.1) | -- | -- |
| Grajales-Cruz 2023 [,] | Overall | 36 | Median of 4.2 months | 19 a (52.8) | -- | -- | -- | -- | 3 a (8.3) | 0 (0.0) | 16 a (44.5) | |
| Hebraud 2023 [] | Overall | 8 | July 2023 cut-off | -- | 6 (75) b | -- | -- | -- | -- | -- | -- | -- |
| Kowalski 2023 [] | Patients with secretory disease d | 30 | Median of 3.4 months e | 15 (50) | -- | -- | 4 (13) | 11 (37) | 4 (13) | -- | 9 (30) | -- |
| Kumar 2023 [] | Overall | 9 | 3 months | 6 (66.7) | -- | -- | 3 (33.3) b | -- | 2 (22.2) b | 1 (11.1) b | 1 (11.1) b | 2 (22.2) b |
| Lachenal 2023 [] | Overall | 15 | Median of 5.2 months | 13 (86.7) b | -- | -- | -- | -- | 2 (13.3) b | 7 (46.7) b | 2 (13.3) b | 2 (13.3) b |
| Midha 2023 [] | Overall | 56 | Median of 2.3 months e | 30 a (53.6) | -- | -- | -- | -- | -- | -- | -- | -- |
| Mohan 2024 [,] | Overall | 98 | Median of 3.5 months | 61 (62) | 50 a (51) | 20 a (20) | -- | -- | -- | -- | -- | -- |
| Nader 2023 [] | Overall | 27 | 33.6 months f | 19 (70) | -- | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [,] | Overall | 123 | Median of 5.5 months | 73 a (59.3) | -- | -- | -- | -- | 14 a (11.4) | 32 a (26.0) | 27 a (22.0) g | |
| Schaefers 2023 [] | Overall | 16 | 3.4 months e | 7 a (44) | 5 a (31) | -- | 7 a (44) | 2 a (13) | 2 a (13) | -- | -- | -- |
| Tan-Asoori 2023 [,,] | Overall | 180 | Median of 5 months | 115 a (64) | -- | -- | 40 a (22) | 25 a (14) | 25 a (14) | 56 a (31) | 34 a (19) | |
| Tan 2024 [,] | Overall | 86 | Median of 9.5 months | 47 (61.0) | 33 (43.0) | -- | -- | -- | -- | -- | -- | -- |
| Prior BCMA-directed therapy | 32 | Median of 9.5 months | 14 (43) | -- | -- | -- | -- | -- | -- | -- | -- | |
| Venkatesh 2023 [] | Overall | 22 | Median of 3.1 months | 11 a (50) | -- | -- | -- | -- | -- | -- | -- | |
Notes: Double dashes (“—”) indicate data not reported. The table only includes studies where the relevant outcomes were reported. a n hand calculated; b % hand calculated; c based on July 2023 cut-off (n = 33); d the population was treated with prophylactic tocilizumab; e converted from days to months; f converted from years to months; g reported as near complete or complete response. Abbreviations: BCMA, B-cell maturation antigen; CI, confidence interval; CR, complete response; NA, not available; NR, not reached; PD, progression of disease; PR, partial response; sCR, stringent complete response; SD, stable disease; TEC, teclistamab; VGPR, very good partial response.
Appendix G. Summary of MajesTEC-1 Trial Characteristics and Outcomes

Table A15.
Summary of MajesTEC-1 trial characteristics and outcomes.
Table A15.
Summary of MajesTEC-1 trial characteristics and outcomes.
| Characteristic | MajesTEC-1 (n = 165) |
|---|---|
| Study description | |
| Study design | Clinical trial |
| MajesTEC-1 ineligibility, % | 0 |
| Median follow-up, months | 23 |
| Median prior LOTs | 5 |
| Select efficacy outcomes | |
| ORR, % (overall) | 63 |
| CR or better, % | 45.5 |
| VGPR or better, % | 59.4 |
| Median PFS, months (95% CI) | 11.3 (8.8–16.4) |
| Median OS, months (95% CI) | 21.9 (15.1—NE) |
| Select safety outcomes | |
| CRS (any grade), % | 72 |
| ICANS (any grade), % | 3 |
| Infections (any grade), % | 80 |
Abbreviations: CR, complete response; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; LOT, line of therapy; NE, not estimated; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
References
- National Cancer Institute. SEER Cancer Stat Facts: Myeloma. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 2 August 2024).
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Hari, P.; Romanus, D.; Palumbo, A.; Luptakova, K.; Rifkin, R.M.; Tran, L.M.; Raju, A.; Farrelly, E.; Noga, S.J.; Blazer, M.; et al. Prolonged Duration of Therapy Is Associated with Improved Survival in Patients Treated for Relapsed/Refractory Multiple Myeloma in Routine Clinical Care in the United States. Clin. Lymphoma Myeloma Leuk 2018, 18, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Joseph, N.; He, J.; Crivera, C.; Fu, A.Z.; Garrett, A.; Shah, N. Healthcare Costs of Multiple Myeloma Patients with Four or More Prior Lines of Therapy, Including Triple-Class Exposure in the United States. Oncol. Ther. 2022, 10, 411–420. [Google Scholar] [CrossRef]
- Bazarbachi, A.H.; Al Hamed, R.; Malard, F.; Harousseau, J.L.; Mohty, M. Relapsed refractory multiple myeloma: A comprehensive overview. Leukemia 2019, 33, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rivas, J.A.; Rios-Tamayo, R.; Encinas, C.; Alonso, R.; Lahuerta, J.J. The changing landscape of relapsed and/or refractory multiple myeloma (MM): Fundamentals and controversies. Biomark. Res. 2022, 10, 1–23. [Google Scholar] [CrossRef]
- Sandahl, T.B.; Soefje, S.A.; Calay, E.S.; Lin, Y.; Fonseca, R.; Ailawadhi, S.; Parrondo, R.; Lin, D.; Wu, B.; Silvert, E.; et al. Real-World Treatment Outcomes of Teclistamab Under an Outpatient Model for Step-up Dosing Administration. Blood 2023, 142, 5154. [Google Scholar] [CrossRef]
- Derman, B.A.; Roach, M.; Lin, D.; Wu, B.; Murphy, R.; Kim, N.; Doyle, M.; Prood, N.; Fowler, J.; Marshall, A.; et al. Panel Interview of Oncology Practices with Emergent Experience of Teclistamab in the Real World: The Tec-Pioneer Study. Blood 2023, 142, 7249. [Google Scholar] [CrossRef]
- Varshavsky-Yanovsky, N.A.; Styler, M.; Khanal, R.; Abdelmessieh, P.; Fung, H. P940: An outpatient model for teclistamab step-up dosing administration—Initial experiences at fox chase cancer center BMT program. HemaSphere 2023, 7, e605007f. [Google Scholar]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Garfall, A.L.; Nooka, A.K.; van de Donk, N.W.C.J.; Moreau, P.; Bhutani, M.; Oriol, A.; Martin, T.G.; Rosiñol, L.; Mateos, M.-V.; Bahlis, N.J.; et al. Long-term follow-up from the phase 1/2 MajesTEC-1 trial of teclistamab in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, 7540. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.A.; Ahmed, N.; Jia, X.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; et al. Safety and Efficacy of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Real-World Experience. Transplant. Cell. Ther. 2023, 30, 308.e1–308.e13. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Kumar, A.; Loughran, T.; Alsina, M.; Durie, B.G.; Djulbegovic, B. Management of multiple myeloma: A systematic review and critical appraisal of published studies. Lancet Oncol. 2003, 4, 293–304. [Google Scholar] [CrossRef]
- Maiese, E.M.; Ainsworth, C.; Le Moine, J.G.; Ahdesmaki, O.; Bell, J.; Hawe, E. Comparative Efficacy of Treatments for Previously Treated Multiple Myeloma: A Systematic Literature Review and Network Meta-analysis. Clin. Ther. 2018, 40, 480–494.e423. [Google Scholar] [CrossRef] [PubMed]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Firestone, R.S.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.; Wang, A.; Zhu, M.; Patel, D.; Tan, C.R.; Hultcrantz, M.; et al. CD8 effector T cells enhance response in BCMA-exposed and -naïve multiple myeloma. Blood Adv. 2023, 8, 1600–1611. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.; Firestone, R.S.; Wang, A.X.; Korde, N.; Hultcrantz, M.L.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; et al. Patterns of CRS with teclistamab in relapsed/refractory multiple myeloma with or without prior T-cell redirection therapy. Blood Adv. 2024, 8, 3038–3044. [Google Scholar] [CrossRef]
- Mohan, M.; Monge, J.; Shah, N.; Luan, D.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in relapsed refractory multiple myeloma: Multi-institutional real-world study. Blood Cancer J. 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Riedhammer, C.; Bassermann, F.; Besemer, B.; Bewarder, M.; Brunner, F.; Carpinteiro, A.; Einsele, H.; Faltin, J.; Frenking, J.; Gezer, D.; et al. Real-world analysis of teclistamab in 123 RRMM patients from Germany. Leukemia 2024, 38, 365–371. [Google Scholar] [CrossRef]
- Gong, Z.; Umoru, G.; Monge, J.; Shah, N.; Mohyuddin, G.R.; Radhakrishnan, S.; Chakraborty, R.; Schinke, C.; D’Souza, A.; Mohan, M. Adverse Effects and Non-Relapse Mortality of BCMA-Directed Immunotherapies: An FDA Adverse Event Reporting System (FAERS) Database Study. Blood 2023, 142, 358. [Google Scholar] [CrossRef]
- Midha, S.; Laubach, J.; Mo, C.; Sperling, A.; Nadeem, O.; Bianchi, G.; Hartley-Brown, M.; Ramsdell, L.; Reyes, K.; Costello, P.; et al. Real world experience of patients treated with teclistamab: aBCMA-directed bispecific t-cell engaging therapy for multiple myeloma. In Proceedings of the Internation Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Nader, S.M.; Jines, S.T.; Collier, C.; Zimmers, T.; Abonour, R.; Farag, S.; Zaid, M.A.; Lee, K.P.; Suvannasankha, A. Characterization of Anthropometric Changes in Patients with Relapsed/Refractory Myeloma Treated with BCMA Bispecific Antibody Teclistamab. Blood 2023, 142, 6683. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Moreau, P.; Garfall, A.L.; Bhutani, M.; Oriol, A.; Nooka, A.K.; Martin, T.G.; Rosiñol, L.; Mateos, M.-V.; Bahlis, N.J.; et al. Long-term follow-up from MajesTEC-1 of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2023, 41, 8011. [Google Scholar] [CrossRef]
- Banerjee, R.; Chang, H.-Y.; Lin, D.; Fu, A.Z.; Hester, L.; Kim, N.; Fowler, J.; Walker, S.; Gifkins, D.; Hearty, C.; et al. Real-World Patterns of Step-Up Dosing Period and Early Safety Outcomes in US Patients Treated with Teclistamab for Multiple Myeloma. In Proceedings of the Lymphoma, Leukemia & Myeloma Congress, New York, NY, USA, 18–21 October 2023; pp. S3–S57. [Google Scholar]
- Banerjee, R.; Chang, H.-Y.; Lin, D.; Harper, J.S.; Fu, A.Z.; Kim, N.; Fowler, J.; Fernandez, M.; Doyle, M.; Min, E.E.; et al. Teclistamab (TEC) step-up dosing (SUD) and treatment dose schedule de-escalation in the real-world (RW) setting—An analysis of multicenter electronic medical records. In Proceedings of the European Hematology Association 2024, Barcelona, Spain, 15 August 2024. [Google Scholar]
- Dima, D.; Davis, J.A.; Ahmed, N.; Sannareddy, A.; Shaikh, H.; Mahmoudjafari, Z.; Khouri, J.; Kaur, G.; Strouse, C.; Valent, J.; et al. Real-World Safety and Efficacy of Teclistamab for Patients with Heavily Pretreated Relapsed-Refractory Multiple Myeloma. Blood 2023, 142, 91. [Google Scholar] [CrossRef]
- Dima, D.; Sannareddy, A.; Ahmed, N.; Davis, J.A.; Shaikh, H.; Mahmoudjafari, Z.; Duco, M.; Khouri, J.; Kaur, G.; Lochner, J.; et al. Toxicity and Efficacy Outcomes of Teclistamab in Patients with Relapsed-Refractory Multiple Myeloma (RRMM) Above the Age of 70 Years: A Multicenter Study. Blood 2023, 142, 3330. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.; Ahmed, N.; Jia, X.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; et al. Safety and Efficacy of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Real-World Experience from the US Myeloma Innovations Research Collaborative (USMIRC). Transplant. Cell. Ther. 2024, 30, S384. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.; Ahmed, N.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; Strouse, C.S.; et al. Outcomes of BCMA-Directed Chimeric Antigen Receptor T-Cell (CART) Therapy and Teclistamab in Patients with Relapse-Refractory Multiple Myeloma with Extramedullary Disease: A Real-World Experience of the US Myeloma Innovations Research Collaborative (USMIRC). Transplant. Cell. Ther. 2024, 30, S384–S385. [Google Scholar] [CrossRef]
- Firestone, R.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.S.; Wang, A.; Zhu, M.; Patel, D.; Tan, C.; Hultcrantz, M.; et al. Evaluating Tumor-Intrinsic and Patient-Specific Mechanisms of Resistance to Teclistamab in Anti-BCMA Exposed and Naïve Multiple Myeloma. Blood 2023, 142, 333. [Google Scholar] [CrossRef]
- Firestone, R.; Shekarkhand, T.; Patel, D.; Hultcrantz, M.; Lesokhin, A.; Mailankody, S.; Hassoun, H.; Tan, C.; Shah, U.; Korde, N.; et al. P962: Commercial Teclistamab in anti-BCMA therapy exposed relapsed refractory multiple myeloma patients: The MSKCC experience. HemaSphere 2023, 7, e81198bb. [Google Scholar]
- Firestone, R.; Shekarkhand, T.; Patel, D.; Tan, C.R.C.; Hultcrantz, M.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; Shah, U.A.; Korde, N.; et al. Evaluating the efficacy of commercial teclistamab in relapsed refractory multiple myeloma patients with prior exposure to anti-BCMA therapies. J. Clin. Oncol. 2023, 41, 8049. [Google Scholar] [CrossRef]
- Mohan, M.; Shah, N.; Luan, D.; Monge, J.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in Relapsed Refractory Multiple Myeloma: Multi-Institutional Real-World Study. Blood 2023, 142, 545. [Google Scholar] [CrossRef]
- Matthew, J.; Pianko, J.H.; Lin, D.; Chang, H.-Y.; Nina, K.; Harper, J.S.; Fowler, J.; Fernandez, M.; Doyle, M.; Hester, L.; et al. Real-world schedule de-escalation of teclistamab in patients with relapsed or refractory multiple myeloma—A US national healthcare claims analysis. In Proceedings of the European Hematology Association 2024, Barcelona, Spain, 15 August 2024. [Google Scholar]
- Riedhammer, C.; Bassermann, F.; Besemer, B.; Bewarder, M.; Brunner, F.; Carpinteiro, A.; Einsele, H.; Faltin, J.; Frenking, J.; Gezer, D.; et al. Real-World Analysis of Teclistamab in 115 RRMM Patients from Germany. Blood 2023, 142, 3329. [Google Scholar] [CrossRef]
- Maringanti, S.A.; Lin, Y.; Estritis, S.; Martinez-Lopez, J.; Bansal, R.; Fotiou, D.; Corona, M.; Chhabra, S.; Brunaldi, L.; Corraes, A.D.M.S.; et al. MM-527 Real World Evaluation of Teclistamab for the Treatment of Relapsed or Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2023, 23, S506–S507. [Google Scholar] [CrossRef]
- Yi, L.S.A.; Brunaldi, L.; Bansal, R.; De Menezes Silva Corraes, A.; Chhabra, S.; Parrondo, R.; Ailawadhi, S.; Kastritis, E.; Fotiou, D.; Popat, R.; et al. Real world evaluation of Teclistamab in patients with RRMM: Results from the IMF Immunotherapy Database Project. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Asoori, S.; Popat, R.; Martínez-Lopez, J.; Kastritis, E.; Brunaldi, L.; Bansal, R.; De Menezes Silva Corraes, A.; Yong, K.; Mactier, C.; Corona, M.; et al. Real-World Evaluation of Teclistamab for the Treatment of Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2023, 142, 3347. [Google Scholar] [CrossRef]
- Tan, C.R.C.; Derkach, A.; Maclachlan, K.; Hultcrantz, M.; Hassoun, H.; Mailankody, S.; Shah, U.A.; Rajeeve, S.; Shah, G.L.; Scordo, M.; et al. Real-world schedule de-escalation of teclistamab in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, 7536. [Google Scholar] [CrossRef]
- Tan, C.R.C.; Derkach, A.; Maclachlan, K.; Hultcrantz, M.; Hassoun, H.; Mailankody, S.; Shah, U.A.; Rajeeve, S.; Shah, G.L.; Scordo, M.; et al. Real-world schedule de-escalation of teclistamab in patients with relapsed/refractory multiple myeloma. In Proceedings of the European Hematology Association 2024, Barcelona, Spain, 15 August 2024. [Google Scholar]
- Kowalski, A.; Lykon, J.L.; Diamond, B.; Coffey, D.; Kaddoura, M.; Maura, F.; Hoffman, J.E.; Kazandjian, D.; Landgren, O. Tocilizumab Prophylaxis for Patients Treated with Teclistamab: A Single-Center Experience. Blood 2023, 142, 4709. [Google Scholar] [CrossRef]
- Marin, E.; Scott, S.; Maples, K.; Joseph, N.S.; Hofmeister, C.C.; Gupta, V.A.; Dhodapkar, M.V.; Kaufman, J.L.; Lonial, S.; Nooka, A.K. Prophylactic Tocilizumab to Prevent Cytokine Release Syndrome (CRS) with Teclistamab Administration. Blood 2023, 142, 2008. [Google Scholar] [CrossRef]
- Banerjee, R.; Kim, N.; Kohli, M.; Hester, L.; Achter, E.; Fowler, J.; Umeh, E.; Lin, D.; Aweh, G.; Gifkins, D.; et al. Evolving Real-World Characteristics and Step-up Dosing Among Early Initiators of Teclistamab for Multiple Myeloma—A National All-Payer Claims Database Study. Blood 2023, 142, 5087. [Google Scholar] [CrossRef]
- Bansal, R.; Paludo, J.; de Menezes Silva Corraes, A.; Megan, S.; Khurana, A.; Hampel, P.; Durani, U.; Dingli, D.; Hayman, S.; Kapoor, P.; et al. Outpatient practice utilization for CAR-T and T cell engager in patients with lymphoma and multiple myeloma. J. Clin. Oncol. 2023, 41, 1533. [Google Scholar] [CrossRef]
- Kathy Mooney, N.A.; Anderson, K.; Zukas, A. Taking a BiTE out of Hospital Admission Days Using a Team Approach to Managing Patients at Risk for Treatment Related Toxicities. In Proceedings of the Oncology Nursing Society 2024, Washington DC, USA, 24–28 April 2024. [Google Scholar]
- Howard, A.J.; Shekarkhand, T.; Hamadeh, I.S.; Wang, A.; Patel, D.; Tan, C.; Hultcrantz, M.; Mailankody, S.; Hassoun, H.; Shah, U.A.; et al. Identifying Causes of Unscheduled Healthcare Interactions and Changes to Patient Disposition in Individuals Receiving Outpatient Commercial Bispecific Antibody Therapy in Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2023, 142, 3707. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Raje, N.; Anderson, K.; Einsele, H.; Efebera, Y.; Gay, F.; Hammond, S.P.; Lesokhin, A.M.; Lonial, S.; Ludwig, H.; Moreau, P.; et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: Consensus recommendations from an expert panel. Blood Cancer J. 2023, 13, 116. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Usmani, S.; Cohen, A.D.; van de Donk, N.W.C.J.; Leleu, X.; Gállego Pérez-Larraya, J.; Manier, S.; Nooka, A.K.; Mateos, M.V.; Einsele, H.; et al. International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of T-cell-engaging bispecific antibodies in multiple myeloma. Lancet Oncol. 2024, 25, e205–e216. [Google Scholar] [CrossRef] [PubMed]
- Hebraud, B.; Granell, M.; Lapierre, L.; Mouchel, P.L.; Beziat, G.; Sicard, N.; Bonneau, M.; Higue, J.; Sougy, F.; Perriat, S.; et al. French Monocentric Experience of Outpatient Step-up Dosing of Teclistamab in Relapsed Refractory Multiple Myeloma. Blood 2023, 142, 4736. [Google Scholar] [CrossRef]
- Derman, B.A.; Roach, M.; Lin, D.; Wu, B.; Murphy, R.; Kim, N.; Doyle, M.; Prood, N.; Fowler, J.; Marshall, A.; et al. Panel Interview of ONcology practices with Emergent Experience of teclistamab in the Real world: The TecPIONEER Study. Curr. Med. Res. Opin. 2024, 40, 1053–1058. [Google Scholar] [CrossRef]
- Tan, C.R.; Chinaeke, E.; Kim, N.; Hester, L.; Fowler, J.; Gifkins, D.; Lin, D.; Walker, S.; Fu, A.Z.; Wu, B. MM-344 Real-World Patient Profile and Step-Up Dosing Process of Early Initiators of Teclistamab for Multiple Myeloma (MM) in a Hospital Setting in the US: Premier Healthcare Database Study. Clin. Lymphoma Myeloma Leuk. 2023, 23, S491. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network; Health Improvement Scotland. SIGN Search Filters. 2021. Available online: https://www.sign.ac.uk/what-we-do/methodology/search-filters/ (accessed on 23 May 2024).
- Wells, G.S.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 October 2016).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Asoori, S.; Martin, T.; Wolf, J.; Chung, A.; Arora, S. CT-630 Real World Evaluation of Teclistamab: A Focus on Infections in Patients with Relapsed Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2023, 23, S536–S537. [Google Scholar] [CrossRef]
- Bansal, R.; Paludo, J.; Corraes, A.; Spychalla, M.; Haugen, K.; Khurana, A.; Hampel, P.J.; Durani, U.; Dingli, D.; Hayman, S.R.; et al. Outpatient Management of CAR-T and Teclistamab for Patients with Lymphoma and Multiple Myeloma. Blood 2023, 142, 253. [Google Scholar] [CrossRef]
- Bansal, R.; De Menezes Silva Corraes, A.; Brunaldi, L.; Sandahl, T.B.; Rees, M.J.; Hayman, S.R.; Binder, M.; Abdallah, N.; Dingli, D.; Cook, J.; et al. Real world outcome of patients with multiple myeloma who received bispecific antibodies after CAR-T therapy. J. Clin. Oncol. 2024, 42, 7520. [Google Scholar] [CrossRef]
- Deborah Bolton, A.B.; Martin, J.; Monsale, S. Administration of Teclistamab Shortly after Food and Drug Administration Approval in a Community Hospital System. In Proceedings of the Oncology Nursing Society Congress 2024, Washington DC, USA, 24–28 April 2024. [Google Scholar]
- Catamero, D.D. Real world experience of teclistamab using a promptmanagement strategy for cytokine release syndrome. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Charkviani, M.; Vaughan, L.E.; Sandahl, T.B.; Lin, Y.; Leung, N.; Herrmann, S.M. Incidence of acute kidney injury in patients with relapsed and refractory multiple myeloma treated with teclistamab vs chimeric antigen receptor T-cell therapy. J. Clin. Oncol. 2024, 42, 7542. [Google Scholar] [CrossRef]
- Beth Faiman, D.D.; Duco, M.; Rice, M.; Rudoni, J.; Anwer, F.; Khouri, J.; Mazzoni, S.; Raza, S.; Samaras, C.; Williams, L.; et al. Initial Report of a Single Institution Experience with Teclistamabfor Relapsed or Refractory Multiple Myeloma Including Prior BCMA. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Ghamsari, F.; Trando, A.; Medley, K.; Martino, J.; Block, S.; Doan, T.; Wells, K.; Quach, A.C.; Cheng, R.G.; Costello, C.; et al. Real-world outcomes of teclistamab for the treatment of relapsed/refractory multiple myeloma at UC San Diego Health: A single-institution experience. J. Clin. Oncol. 2024, 42, e19504. [Google Scholar] [CrossRef]
- Melanie, R.; Glenn, K.G.; Bucknall, J.; Ricca, M. Teclistamab Early Discharge Program. In Proceedings of the Oncology Nursing Society Congress 2024, Washington DC, USA, 24–28 April 2024. [Google Scholar]
- Gordon, B.; Fogel, L.; Varma, G.; Mejia Saldarriaga, M.; Ahn, J.; Aleman, A.; Caro, J.; Chen, X.; Monge, J.; Parmar, H.; et al. Teclistamab Demonstrates Clinical Activity in Real-World Patients Ineligible for the Pivotal Majestec-1 Trial. Blood 2023, 142, 4741. [Google Scholar] [CrossRef]
- Graf, K.C.; Davis, J.A.; Cendagorta, A.M.; Granger, K.; Gaffney, K.; Green, K.; Hess, B.T.; Hashmi, H. ‘Fast but Not so Furious’: A Condensed but Safe and Cost-Effective Step-up Dosing Regimen of Teclistamab for Relapsed Refractory Multiple Myeloma. Transplant. Cell. Ther. 2024, 30, S381. [Google Scholar] [CrossRef]
- Grajales-Cruz, A.S.K.; Blue, B.; Hansen, D.; Puglianini, O.C.; Freeman, C.; De Avila, G.; Ochoa-Bayona, L.; Liu, H.; Nishihori, T.; Frederick; et al. Safety and Efficacy of Standard of Care (SOC) Teclistamab (TEC) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM), a single center experience. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Grajales-Cruz, A.F.; Castaneda, O.; Hansen, D.K.; Vazquez-Martinez, M.A.; Blue, B.; Khadka, S.; Liu, H.; Ochoa-Bayona, J.L.; Freeman, C.L.L.; Locke, F.L.; et al. Teclistamab Induces Favorable Responses in Patients with Relapsed and Refractory Multiple Myeloma after Prior BCMA-Directed Therapy. Blood 2023, 142, 3351. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.; Firestone, R.; Wang, A.; Korde, N.; Hulcrantz, M.; Lesokhin, A.; Mailankody, S.; Hassoun, H.; et al. Patterns of Cytokine Release Syndrome with Teclistamab in Relapsed/Refractory Multiple Myeloma with or without Prior T-Cell Redirection Therapy. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.; Firestone, R.; Wang, A.; Korde, N.; Hultcrantz, M.; Lesokhin, A.; Mailankody, S.; Hassoun, H.; et al. Patterns of Cytokine Release Syndrome with Teclistamab in Relapsed/Refractory Multiple Myeloma with or without Prior T-Cell Redirection Therapy. Blood 2023, 142, 1961. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Steele, A.P.; Rosenberg, A.; Guglielmo, J. Safety Outcomes of Teclistamab Accelerated Dose Escalation. In Proceedings of the Hematology/Oncology Pharmacy Association 2024, Tampa, FL, USA, 3–6 April 2024. [Google Scholar]
- Swarup Kumar, K.H.; Clark, J.; Soto, A.A.; Rounds, A. Early outcomes and therapy modification strategies in Multiple Myeloma patients treated with teclistamab, CD3XBCMA BITE: A singlecenter experience. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–3 September 2023. [Google Scholar]
- Lachenal, F.; Lebreton, P.; Bouillie, S.; Pica, G.M.; Aftisse, H.; Pascal, L.; Montes, L.; Macro, M.; Vignon, M.; Harel, S.; et al. Teclistamab in Relapsed Refractory Multiple Myeloma Patients on Dialysis: A French Experience. Blood 2023, 142, 4739. [Google Scholar]
- Harrell, S.; de Coella, J.-M.S.; Sonntag, C.; Cazaubiel, T. P-014 teclistamab in relapsed and refractory multiple myeloma: Patients characteristics from post marketing acces (acces precoce) in France. In Proceedings of the Internation Myeloma Society Annual Meeting and Exposition, Athens, Greece, 17–30 September 2023. [Google Scholar]
- Rees, M.J.; Mammadzadeh, A.; Bolarinwa, A.; Elhaj, M.E.; Bohra, A.; Ailawadhi, S.; Parrondo, R.; Chhabra, S.; Lin, Y.; Binder, M.; et al. Class comparison of BCMA-directed therapies in relapsed multiple myeloma. J. Clin. Oncol. 2024, 42, 7515. [Google Scholar] [CrossRef]
- Sandahl, T.B.; Soefje, S.A.; Calay, E.S.; Lin, Y.; Fonseca, R.; Ailawadhi, S.; Parrondo, R.; Lin, D.; Wu, B.; Silvert, E.; et al. Real-World Treatment Outcomes of Teclistamab Under an Outpatient Model for Step-Up Dosing Administration. In Proceedings of the Hematology/Oncology Pharmacy Association 2024, Tampa, FL, USA, 3–6 April 2024. [Google Scholar]
- Schaefers, C.T.; Alsdorf, W.; Schaefers, C.; Kosch, R.; Leypoldt, L.; Bokemeyer, C.; Weisel, K.K.A. BCMA x CD3 bispecifc antibody treatment with teclistamab in relapsed and/or refractory multiple myeloma: A real-world monocentric analysis. Oncol. Res. Treat. 2023, 46, 1–354. [Google Scholar] [CrossRef]
- Tabbara, N.; Singel, M.; Allen, N.; Mooney, K.; Shedeck, A.; Zukas, A.; Campion, K.; Sollenberger, C.; Gocke, C.B.; Ali, S.A.; et al. Ambulatory teclistamab administration in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, 11146. [Google Scholar] [CrossRef]
- Tan, C.R.; Kim, N.; Chinaeke, E.; Hester, L.; Fowler, J.; Gifkins, D.; Lin, D.; Walker, S.; Fu, A.Z.; Wu, B. Real-World Patient Profile and Step-up Dosing Process of Early Initiators of Teclistamab for Multiple Myeloma in US Hospitals—An Updated Analysis Using Premier Healthcare Database. Blood 2023, 142, 3792. [Google Scholar] [CrossRef]
- Yanovsky, A.V.; Styler, M.; Khanal, R.; Abdelmessieh, P.; Fung, H. MM-595 Feasibility and Safety of Outpatient Model for Teclistamab Step-Up Dosing Administration: A Single Center Experience. Clin. Lymphoma Myeloma Leuk. 2023, 23, S509. [Google Scholar] [CrossRef]
- Venkatesh, P.; Atrash, S.; Paul, B.; Alkharabsheh, O.; Afrough, A.; Mahmoudjafari, Z.; Mushtaq, M.U.; Hashmi, H.; Davis, J.A.; Abdallah, A.-O.A. Efficacy of teclistamab in patients (pts) with heavily pretreated, relapsed/refractory multiple myeloma (RRMM), including those refractory to penta RRMM and BCMA (B-cell maturation antigen) directed therapy (BDT). J. Clin. Oncol. 2023, 41, e20044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).