Simple Summary
Cutaneous squamous cell carcinoma (cSCC) is one of the most common malignancies of the skin with poor survival outcomes in advanced stages of the disease. Recent clinical trials demonstrated the efficacy of checkpoint-inhibitors (CPI) therapy for advanced stage disease, but there is a lack of data from real-world cohorts and trial-ineligible patients. In this retrospective, real-world cohort study, we investigated the efficacy of first-line checkpoint-inhibitor treatment in 39 patients with advanced cSCC from eight different German cancer centers and stratified outcomes by the immune status of the patients. Our data demonstrate that patients receiving CPI achieved high response rates with durable remissions in about 20% of patients. CPI also evoked tumor responses in patients with active autoimmune diseases and lymphoproliferative disorders, although these responses were often short-lived, resulting in a significantly shorter overall survival. Notably, CPI therapy was safe with only 15% of patients discontinuing for toxicity.
Abstract
Cutaneous squamous cell carcinoma (cSCC) is a common malignancy of the skin and has an overall favorable outcome, except for patients with an advanced stage of the disease. The efficacy of checkpoint inhibitors (CPI) for advanced cSCC has been demonstrated in recent clinical studies, but data from real-world cohorts and trial-ineligible cSCC patients are limited. We retrospectively investigated patients with advanced cSCC who have been treated with CPI in a first-line setting at eight German skin cancer centers registered within the multicenter registry ADOReg. Clinical outcome parameters including response, progression-free (PFS) and overall survival (OS), time-to-next-treatment (TTNT), and toxicity were analyzed and have been stratified by the individual immune status. Among 39 evaluable patients, the tumor response rate (rwTRR) was 48.6%, the median PFS was 29.0 months, and the median OS was not reached. In addition, 9 patients showed an impaired immune status due to immunosuppressive medication or hematological diseases. Our data demonstrated that CPI also evoked tumor responses among immunocompromised patients (rwTRR: 48.1 vs. 50.0%), although these responses less often resulted in durable remissions. In line with this, the median PFS (11 vs. 40 months, p = 0.059), TTNT (12 months vs. NR, p = 0.016), and OS (29 months vs. NR, p < 0.001) were significantly shorter for this patient cohort. CPI therapy was well tolerated in both subcohorts with 15% discontinuing therapy due to toxicity. Our real-world data show that first-line CPI therapy produced strong and durable responses among patients with advanced cSCC. Immunocompromised patients were less likely to achieve long-term benefit from anti-PD1 treatment, despite similar tumor response rates.
1. Introduction
Cutaneous squamous cell carcinoma (cSCC) has the second highest incidence among skin cancers [1], with increasing rates in aging western populations [2]. While the majority of cSCCs are cured with surgery, approximately 5% of patients develop advanced disease that is defined either by locoregional or distant metastases or locally advanced cSCC not amenable to surgery or radiotherapy [3]. These patients have poor long-term outcomes as treatment options in the pre-checkpoint inhibitor (CPI) era were largely limited to palliative chemotherapy [4]. While these treatments have anti-tumor efficacy, responses are often short-lived with significant concomitant toxicity [3,5].
The initial success of CPI-blocking programmed cell death protein 1 (PD-1) or PD-1 ligand (PD-L1) to treat metastatic melanoma also gave an incentive for application in nonmelanoma skin cancers. As cSCC is a highly immunogenic tumor that features high somatic mutational burden, there was a strong rationale for treatment with CPI [3,6]. In accordance with this, data from phase I/ II clinical trials [7,8] demonstrated that advanced cSCC patients who were treated with the PD-1 inhibitors cemiplimab or pembrolizumab [9,10] achieved an objective response rate between 40 and 50% with >50% of these responses lasting longer than 6 months.
Despite the evidence reported by clinical trials, only limited data are thus far available about CPI activity in real-world cohorts [4,6,11,12,13] and several questions still need to be addressed, such as safety and efficacy in patients usually excluded from clinical trials and potential determinants of clinical benefit to CPI treatment [14]. In this regard, patients with chronic immune suppression (i.e., high doses of corticosteroids for autoimmune diseases or organ recipients) and concomitant immunocompromising hematological diseases are of particular interest.
In this study, we, therefore, investigated patients treated with first-line CPI for advanced cSCC outside of clinical trials at eight German skin cancer centers and stratified outcomes by the immune status of the patients.
2. Materials and Methods
2.1. Study Design and Data Source
In this retrospective, multicenter study, we used the data of eligible patients from the skin cancer registry of the ADOReg [15]. The ADOReg platform collects healthcare data on skin cancer patients from 59 skin cancer centers, eight of which contributed to the current study (Buxtehude, Erlangen, Regensburg, Neustadt, Mainz, Würzburg, Hamburg, and Gera). Details on treatment and outcome specifics were recorded in an unidentifiable, pseudonymized form at the patient level.
2.2. Patient Cohort
At data request (February 2022), 436 patients with cSCC were identified within the ADOReg database. Thereof, we analyzed 39 patients with locally advanced, regionally or distant metastatic, or inoperable cSCC who received at least one dose of CPI agents in a first-line setting between February 2018 and June 2022 with follow-up until data cut-off in July 2022 (Figure 1). CPI agents included cemiplimab, nivolumab, pembrolizumab, and avelumab.
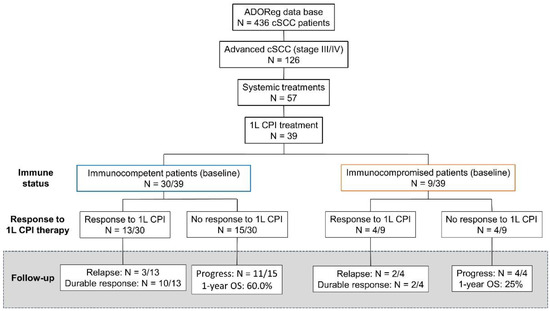
Figure 1.
Flow chart depicting the selection criteria for this retrospective, multicenter analysis. We analyzed the outcome of patients with advanced cutaneous squamous cell carcinoma (cSCC) who were treated with first-line (1L) immune checkpoint inhibitors (CPI) at stratified outcomes based on the immune status at baseline. Patients without significant immunosuppression generally showed more durable tumor responses compared to immunocompromised patients despite similar rates of initial tumor responses. In the case of 3 patients with ongoing CPI therapy (2 among immunocompetent patients and 1 patient with baseline immunosuppression), no tumor assessments were available at the time of data cut-off.
The collected data comprised core patient and tumor characteristics (i.e., age, gender, comorbidities, and immune status), sites of metastasis, LDH serum levels, as well as treatment specifics and survival outcomes. Immunocompromised patients either received immunosuppressive medication (chronic steroid use >10 mg prednisolone/day) or were diagnosed with hematological malignancies. The primary endpoint of this study was OS. Secondary endpoints included PFS, real-world tumor response (rwTR), and severe treatment-related adverse events (trAE) as defined in Table S1. Time-to-next treatment (TTNT) was included as an additional outcome parameter due to its role as a reliable surrogate for OS in real-world datasets [16].
2.3. Statistical Analysis
Descriptive statistics were used to analyze baseline characteristics. Treatment duration was calculated as the period between initial drug administration and treatment discontinuation. The chi-square test was used to assess the association between immune status and tumor response rates. For categorial variables, 95% confidence intervals (CIs) were calculated using the Clopper–Pearson method. Testing for equality between subgroups was performed using Student’s t-test and Fisher’s exact test.
We employed Kaplan–Meier survival plots to illustrate median OS and PFS probabilities and to explore associations between the immune status and survival outcomes. Survival curves were compared using the log-rank test. The median duration of follow-up was calculated using the reverse Kaplan–Meier method. Cox’s proportional hazards models were applied to identify predictors of patient survival by adjusting for baseline characteristics, treatment regimen, and immune status. Multivariable analysis was conducted for significant variables by the univariate test or by a priori selection for biological relevance to evaluate their conjoint, independent effects on PFS or OS. In all cases, two-tailed p-values were calculated and considered significant with values p < 0.05. SPSS version 27, RStudio (Version 1.3.1093), and GraphPad PRISM version 5 were used for all analyses.
3. Results
3.1. Baseline Patient Characteristics
We evaluated a total of 39 patients who received first-line CPI for advanced cSCC. The median follow-up upon CPI initiation was 27 months (Table 1). Patients were predominantly male and of advanced age (median: 78 years). Therapies prior to receiving CPI included surgery (n = 29/39; 74.3%) or radiotherapy (74.3%). Seven patients were considered inoperable due to extensive disease or field cancerization. Among all 39 patients, nine patients had a history of immunosuppression due to hematological malignancies (chronic lymphocytic leukemia, n = 4; polycythemia vera, n = 2) or immunosuppressive medications for autoimmune diseases (Crohn´s disease, n = 1; autoimmune hepatitis, n = 1; Lichen planus, n = 1; Table 2).

Table 1.
Baseline patient characteristics and treatment outcomes.

Table 2.
Baseline patient characteristics and treatment outcomes for patients diagnosed with autoimmune diseases.
Primary cSCC tumors were mainly located in the head/neck area (64.1%). Most patients initially presented with advanced stage III or IV disease (51.3%) or locally advanced tumors (vertical tumor thickness >6 mm, poorly differentiated histology, or horizontal diameter >2 cm). A total of 30 patients later developed regional (53.8%) or distant metastases (23.1%), while seven patients showed locally advanced tumors that required treatment with CPI.
All patients received at least one dose of CPI, which included cemiplimab (48.7%), nivolumab (25.6%), pembrolizumab (23.1%), or avelumab (2.4%). The median treatment duration was 5.0 months with nine patients continuing treatment at data cut-off. RwTRR was 48.6%, with 10 patients achieving PR and 7 showing CR. Response rates were similar regardless of CPI used (p = 0.768). Treatment-related AE were reported for 34.3% of patients with 15.4% of patients ceasing CPI therapy due to toxicity. Following CPI discontinuation due to progression or intolerance, 10 patients (25.6%) received subsequent systemic therapies, which included EGFR inhibitors (5.1%), chemotherapy (2.6%), or CPI re-challenge (20.5%).
The median PFS among the entire cohort was 29.0 months and the median TTNT as well as median OS were not reached at data cut-off in July 2022.
3.2. Clinical and Pathological Factors Associated with Response and Survival upon CPI Therapy
When analyzing the association between baseline clinical factors and survival outcomes, univariate Cox-regression analysis showed that longer CPI treatment duration, response to CPI therapy, and good performance status were associated with a longer PFS (Table S2). In line with this, patients with good performance status showed favorable response to CPI therapy (p = 0.008). In addition, the response to first-line CPI treatment and the absence of immunosuppressive medical conditions were correlated with OS (Table S3). While LDH serum levels correlated with response to CPI therapy (p = 0.03, Figure S2), we found no significant association with survival outcomes.
Given the number of target events and the biological rationale, we included the immune status, performance status, rwTR, and treatment duration in a multivariable Cox regression analysis model (Figure 2). In this multivariable analysis, the presence of immunosuppression (HR: 11.8; p = 0.053) and the best response to CPI (HR: 0.09; p = 0.064) were associated with OS, while only the best tumor response (HR: 0.23; p = 0.006) was significantly associated with PFS.
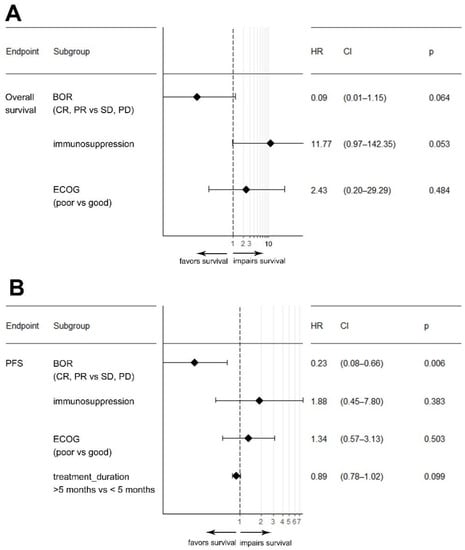
Figure 2.
Forest plot depicting results from a multivariable Cox-regression model for the variables real-world tumor response, immunosuppression, and ECOG performance status on overall survival (A) and progression-free survival (PFS) (B). Abbreviations: BOR: best overall response; CR: complete response; CI: confidence interval; ECOG: eastern cooperative oncology group; HR: hazard ratio; PR: partial response; PD: progressive disease; SD: stable disease.
Results from Kaplan–Meier analysis for the variables affecting PFS and OS are shown in Figure S1.
3.3. Response and Survival upon CPI Therapy in Immunocompromised Patients
We next investigated the impact of the immune status on response to first-line CPI therapy. It is well known that immunocompromised patients are at higher risk of developing locoregional or distant metastases and that immunosuppression is an adverse prognostic factor in advanced cSCC. However, our analysis unveiled no significant differences in tumor response of immunocompromised patients as compared to immunocompetent patients (p = 0.093). In addition, we found no significant differences for rwTRR (48.1% vs. 50.0%, p = 1.0) or rwTCR (85.2% vs. 62.5%, p = 0.321) (Table S4 and Figure S3).
Regarding survival outcomes, our analysis revealed that immunocompromised patients had a significantly shorter median OS (29 months vs. NR, p < 0.001) and TTNT (12 months vs. NR; p = 0.016) as compared to immunocompetent patients. Our data also showed that patients with immunosuppressive conditions presented with a shorter PFS (11 vs. 40 months, p = 0.059), although this association was below statistical significance (Figure 3).
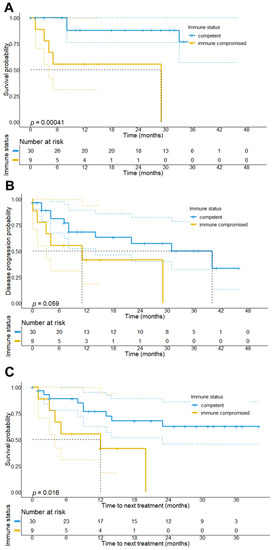
Figure 3.
Overall survival, progression-free survival, and time-to-next treatment (TTNT) stratified by the immune status of the patients. Results show that patients being immunocompromised at the start of CPI treatment have a significantly shorter overall survival (29 months vs. NR, p < 0.001) (A) and progression-free survival (11 months, 95% CI: 0–28.1 months vs. 40 months, 95% CI: 16.9–63.1 months, p = 0.059) (B), as well as TTNT (median TTNT: 12 months, 95% CI: 0–29.1 months vs. NR; p = 0.016) (C) as compared to immunocompetent patients.
3.4. Duration of CPI Treatment Response in Immunocompromised Patients
To explain the divergent survival outcomes for immunocompromised patients, we analyzed the treatment outcomes of responders to CPI therapy. The median follow-up time for this patient cohort was 31 months (95% CI: 23.4–38.6 months). During this follow-up period, we observed that 2/4 patients with baseline immunosuppression progressed at 3 and 11 months of follow-up, while among immunocompetent responders, 10/13 patients (76.9%) remained relapse-free. In line with this, immunocompromised patients who showed at least PR to CPI therapy had a significantly shorter median PFS (11 vs. 40 months, p = 0.0046) compared to immunocompetent responders, suggesting that tumor responses in this patient subgroup less frequently result in durable tumor remissions (Figure 4 and Figure 5). Among the 17 responders, 16 were alive at data collection.
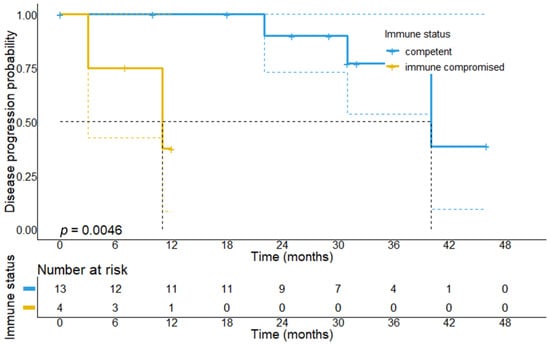
Figure 4.
Progression-free survival in patients who achieved response to first-line CPI treatment stratified by the immune status. It can be found that immunocompromised responders have a significantly shorter PFS (11 months, 95% CI: 0–23.0 months vs. 40 months, 95% CI: 27.1–52.9 months, p = 0.0046) as compared to immunocompetent patients, which suggests that responses in immunocompromised patients are short-lived, whereas immunocompetent patients achieve more durable responses.
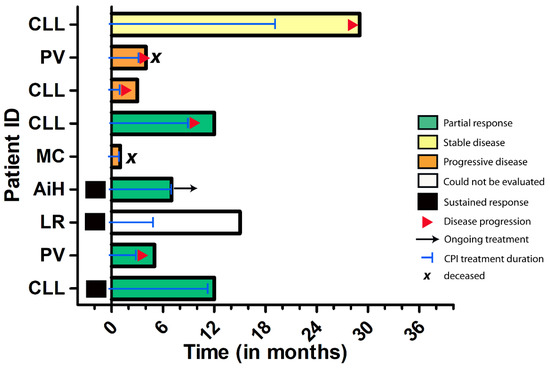
Figure 5.
Swimmer’s plot depicting the individual outcomes of immunocompromised patients in this study. Among those patients receiving first-line CPI treatment, three patients showed sustained tumor responses, whereas the majority of patients experienced tumor progression in the course of the disease. Abbreviations: CLL = chronic lymphocytic leukaemia, PV = polycythemia vera; MC = Crohn´s disease, AiH = autoimmune hepatitis, LR = lichen planus mucosae.
3.5. Durable Response upon CPI Cessation and Efficacy of CPI Re-Challenge
Thirteen patients discontinued CPI therapy during ongoing remission (7 PR, 6 CR, or no evidence of disease, NED) at a median of 9 months. Reasons for treatment cessation included CPI-associated toxicities (pneumonitis, grade 2; hepatitis, grade 3; fatigue), the explicit wish of the patient, or cessation-sustained tumor remission. Within the subsequent 22-month follow-up-period, three patients relapsed. Notably, among all patients who discontinued CPI in ongoing remission, three patients had an immunosuppressive condition and 2 of them relapsed shortly after treatment cessation (median: 1.5 months). Among these patients, one patient with concomitant B-CLL was re-challenged with cemiplimab after relapse of the primary tumor in the genital area and achieved stable disease thereafter.
Overall, 10 patients received subsequent treatments upon disease progression after initial CPI treatment. Among these patients, 7 were re-challenged with CPI (1 CR; 2 PR; 3 SD; 1 not assessed, due to early discontinuation for severe AE), 1 was treated with taxol-based chemotherapy (SD), 1 received EGFR-inhibitor cetuximab (PR), and 1 patient received a combination of cemiplimab and cetuximab (not assessed at data cut-off).
3.6. Treatment-Related Adverse Events during CPI Therapy
Among the entire cohort, seven patients developed AE of CTCAE grade III or higher (17.9%), which included hepatitis (n = 2), colitis (n = 2), pancytopenia (n = 1), fatigue (n = 1), anaphylactic shock (n = 1), and pancreatitis (n = 1). Thereof, three patients permanently discontinued CPI therapy. Serious AE were more frequent among immunocompromised patients, although this association was below statistical significance (p = 0.319, Table 1). Among patients with serious AE, six achieved at least stable disease upon CPI therapy with three of these showing an ongoing tumor remission. Other documented side-effects of grade II included diarrhea, colitis, exanthema, increased liver enzymes, pyrexia, fatigue, thyroiditis, and pneumonitis. Upon CPI re-challenge, 3/7 patients developed trAE, including exanthema, elevated liver enzymes, pneumonitis, and colitis.
4. Discussion
In this study, we provided real-world data on the efficacy and safety in a well-defined cohort of advanced cSCC patients with extended follow-up times who received first-line CPI therapy, which was complemented by data on survival outcomes for immunocompromised patients. Our data confirmed efficacy results for first-line CPI therapy from previous clinical trials, transferring them into real-world cases. In particular, our data on rwTRR (48.5%) and rwTCR (76.4%) are consistent with response rates reported earlier [7,8,17]. Notably, we observed a higher rate of patients achieving CR to CPI treatment (20.0%), which might be attributed to the longer follow-up period of our trial, the fact that our patients were treated with CPI in a first-line setting, and a potential sampling bias due to the smaller sample size.
Further, and in line with previous reports analyzing the durability of CPI responses for melanoma, we observed that CPI evoked durable responses, particularly among immunocompetent patients that showed at least PR. These tumor responses were ongoing even after CPI-cessation. Of note, patients who restarted CPI after previously having achieved at least SD upon CPI therapy also showed at least SD upon CPI rechallenge. Next, we observed that an impaired performance status, weak response to CPI, and a shorter treatment duration were associated with shorter survival outcomes. Contrasting previous reports, we could not detect a higher probability of treatment response for patients with the primary site of the head-neck area [6,12]. Further, we observed a significant correlation between elevated serum LDH levels and a weaker response to CPI therapy, as previously described for melanoma [18] and more recently for advanced cSCC [6].
Most importantly, our real-world data allowed us to better define the efficacy and safety of CPI therapy in a subgroup of immunocompromised patients. These patients are at higher risk of developing cSCC and present with a more aggressive course of the disease [19]. As immunocompromised patients were largely excluded from clinical trials investigating CPI efficacy, evidence for these patients is limited to small observational studies with heterogeneous cohorts. Thus far, data indicated that patients with concomitant hematological diseases achieved lower response rates and shorter PFS-periods upon CPI therapy [20] and that CPI were associated with a higher risk of severe AE [12,21]. By contrast, our data demonstrate that patients with active autoimmune disease and lymphoproliferative disorders achieved comparable response rates as compared to immunocompetent patients, highlighting the feasibility and activity of CPI in patients with such immunosuppressive conditions, as reported in previous series [12,20]. However, further analysis also showed that these patients were less likely to achieve durable remissions and disease progression was frequently observed within our follow-up period, resulting in a substantially shorter PFS for this subcohort.
In our cohort of advanced cSCC patients, we found low rates of severe trAE, with less than 20% discontinuing CPI therapy due to toxicity, which is in line with previous reports for CPI safety in advanced melanoma [22,23]. Given the higher rate of severe AE in immunocompromised patients, which could be explained by the immunological imbalance inherent to the patients´ autoimmune disorder, and the potential development of life-threatening events, we propose a consistent monitoring of AE during and after CPI therapy.
We acknowledge the limitations of our study, including the retrospective nature, which adds a selection bias. When discussing safety data of this real-world population, the nonstandardized documentation of safety data should be considered, which might have affected the identification of AE, particularly those of lower grade, resulting in the underrepresentation of AE. Moreover, the heterogeneity in terms of disease status, autoimmune diseases, and medication might have affected our results. Most importantly, the small number of patients with autoimmune diseases and concomitant immunosuppressive treatments must be considered a relevant limitation.
In summary, we provide real-life data from a multicenter analysis that confirms the safety and efficacy of first-line CPI therapy in advanced cSCC patients. Among the entire cohort, 48.5% of patients achieved a tumor response to first-line CPI with a median PFS of 29.0 months. Although our study was exploratory in its nature and, therefore, does not allow for definitive conclusions, our data indicate that immunocompromised patients were able to achieve similar response rates without significantly increased toxicities. Therefore, CPI therapy may offer a promising treatment approach for these high-risk cSCC patients. Given that remissions are often short-lived in this patient cohort with substantially shorter PFS and that severe AE may occur at any time during treatment, an individual approach to therapy and consistent monitoring will be necessary for these patients.
5. Conclusions
Our retrospective, multicenter analysis demonstrates that first-line CPI therapy evokes strong and durable tumor responses in a real-world cohort of advanced cSCC patients. Importantly, our data provide evidence that patients with immunosuppressive conditions, such as active autoimmune diseases or lymphoproliferative disorders, show similar response rates but are less likely to achieve durable tumor remissions and frequently develop tumor recurrence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14225543/s1, Figure S1. Overall survival and progression-free survival stratified by ECOG performance status, real-world tumor response, and baseline serum LDH levels; Figure S2. Violin plots depicting the association between response to CPI therapy and LDH serum levels at baseline; Figure S3. Bar charts showing the real-world tumor response rate (rwTRR) and real-world tumor control rate (rwTCR) upon first-line CPI treatment in the overall patient cohort and stratified by the immune status; Table S1. Definition of real-world endpoints used in this study; Table S2. Univariate Cox proportional hazards model for overall survival; Table S3. Univariate Cox proportional hazards model for progression-free survival; Table S4. Response to immune checkpoint inhibitor therapy stratified by the class of the immune status of the patients.
Author Contributions
Writing—review and editing, H.S., M.H. (Maximilian Haist), A.T., M.S., C.L. and S.G.; methodology, software, validation, formal analysis, funding acquisition, illustration of tables and figures, M.H. (Maximilian Haist) and H.S.; conceptualization, H.S., M.H. (Markus Heppt) and C.L.; project administration, C.L.; supervision, S.G. and C.L.; data acquisition, M.H. (Maximilian Haist), H.S., B.M.L., A.T., J.-M.P., P.M., D.S., S.U., M.W., U.L., R.G., M.K., S.H., C.B., M.H. (Markus Heppt), B.T., P.S., C.G. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
H.S. and M.H. are supported by an intramural research funding of the UMC Mainz. M.H. is supported by a Walter-Benjamin Fellowship (project number: HA 9793/1-1).
Institutional Review Board Statement
The ADOREG registry was approved by the ethics committee of the University Duisburg-Essen (14-5921-BO) and provides real-world data from skin cancer patients of clinical centers of the DeCOG. The analysis of the anonymized retrospective data from the ADOReg registry was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Patient consent was obtained for inclusion in the registry, and institutional review board approval for the ADOReg database includes the use of data for research purposes. Informed consent was waived due to the anonymized collection of retrospective patient data within the ADOReg framework to which patients initially gave consent.
Data Availability Statement
All relevant data are within the manuscript and its supporting tables and figures. The retrospective data used for statistics have been collected within the framework of the ADOReg.
Conflicts of Interest
H.S., M.H., M.K., J.-M.P., and B.T. declare no conflict of interest. A.T. declares travel support from Novartis. B.M.L. declares honoraria and travel assistance from Novartis, Sanofi, and MSD. M.S. declares honoraria and/or travel grants from Abbvie, Bristol-Myers Squibb (BMS), Merck, Merck Sharp & Dohme (MSD), Novartis, Pfizer, and Sanofi. D.S. declares research support from Bristol-Myers Squibb, Novartis, and Amgen; speaker and advisory board honoraria from Array, Bristol-Myers Squibb, Helsinn, Merck Sharp & Dohme, Merck Serono, Immunocore, InFlarX, Nektar, Neracare, Novartis, OncoSec, Pfizer, Philogen, Pierre-Fabre, Replimune, Roche, Sandoz, Sanofi, and Sun Pharma; travel support from Bristol-Myers Squibb, Merck Sharp & Dohme, Merck-Serono, Nektar, Novartis, Pierre-Fabre, Roche, and Sanofi. S.U. declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Novartis, and Roche; travel support from Bristol Myers Squibb, Merck Sharp & Dohme, and Pierre Fabre, outside the submitted work. J.-M.P served as consultant and/or has received honoraria from Bristol-Myers Squibb and Novartis, and has received travel support from Bristol-Myers Squibb, Novartis and Therakos. P.M. declares research support from Bristol-Myers Squibb and Merck Sharp & Dohme; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Amgen, GlaxoSmithKline, Pierre Fabre, Merck, Sanofi, Beiersdorf, and Sun Pharma; travel support from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Amgen, and Roche; consulting or advisory role: Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Roche, GlaxoSmithKline, Merck, Pierre Fabre, Beiersdorf Immunocore, and Sanofi. M.W. declares speaker and advisory board honoraria from Merck, Bristol-Myers Squibb, Roche, Novartis, Sanofi, Pierre Fabre, Beiersdorf, Sun Pharma, and Takeda. S.H. declares research support from Bristol-Myers Squibb; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Pierre Fabre, and Sanofi. R.G. declares research support from Pfizer, Johnson&Johnson, Novartis, Amgen, Sanofi, Merck-Serono, and Kyowa-Kirin; honoraria for lectures from Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Merck-Serono, Bayer, 4SC, SUN, Pierre-Fabre, and Sanofi; advisory honoraria from Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Pierre-Fabre, Merck-Serono, 4SC, SUN, Merck-Serono, Sanofi, and Immunocore. P.S. declares honoraria from Bristol-Myers Squibb; an institutional research grant from Novartis; travel support from Bristol-Myers Squibb, Lilly, Sanofi-Aventis, Novartis, Pierre Fabre Pharmaceuticals, and Sun Pharmaceuticals. M.V.H. declares honoraria from MSD, BMS, Roche, Novartis, Sun Pharma, Sanofi, Almirall, Biofrontera, and Galderma. C.B. declares honoraria from Almirall, BMS, Immunocore, Leo Pharma, MSD, Novartis, Roche, Pierre Fabre, and Sanofi. C.G. is a member of the advisory board of, and has received honoraria and travel expenses by, Almirall, Amgen, Beiersdorf, BioNTech, Bristol-Myers Squibb, Immunocore, Janssen, MSD Sharp & Dohme, Novartis, Pierre-Fabre, Roche, Sanofi Genzyme, SUN Pharma, and Sysmex/Inostics. U.L declares research support from MSD; honoraria from Roche, Novartis, Sanofi, MSD, and Sun Pharma; advisory honoraria from Roche, Sanofi, MSD, Novartis, Sun Pharma, and Almirall Hermal. S.G. declares honoraria for advisory boards, oral presentations, and travel expenses from Roche, Novartis, MSD, and BMS outside the submitted work. C.L. declares speakers, advisory board honoraria, and travel support from Bristol Myers Squibb, Merck Sharp and Dohme, Merck Serono, Novartis, Roche, Pierre Fabre, Sun Pharma, Kiowa Kirin, Sanofi, Biontech, and Almirall Hermal outside the submitted work.
Abbreviations
| AE | Adverse events |
| AID | Autoimmune disease |
| AiH | Autoimmune hepatitis |
| CI | Confidence interval |
| CPI | Immune-checkpoint inhibitors |
| CR | Complete response |
| ECOG | Eastern Cooperative Oncology Group |
| 1 L | First-line |
| HR | Hazard ratio |
| trAE | Treatment-related adverse events |
| NED | No evidence of disease |
| OS | Overall survival |
| PD | Progressive disease |
| PD-1 | Programmed death protein 1 |
| PD-L1 | Programmed death protein ligand 1 |
| PFS | Progression-free survival |
| PR | Partial response |
| rwTCR | Real-world tumor control rate |
| rwTR | Real-world tumor response |
| rwTRR | Real-world tumor response rate |
| SD | Stable disease |
References
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Eigentler, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Garbe, C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Investig. Dermatol. 2017, 137, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- In, G.K.; Vaidya, P.; Filkins, A.; Hermel, D.J.; King, K.G.; Ragab, O.; Tseng, W.W.; Swanson, M.; Kokot, N.; Lang, J.E.; et al. PD-1 inhibition therapy for advanced cutaneous squamous cell carcinoma: A retrospective analysis from the University of Southern California. J. Cancer Res. Clin. Oncol. 2021, 147, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Krause-Bergmann, A.; Schulze, H.J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns-Results of a non-interventional study of the DeCOG. Eur. J. Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Steeb, T.; Wessely, A.; Schatton, T.; Berking, C.; Heppt, M.V. Comparative efficacy analysis identifies immune checkpoint blockade as a new survival benchmark in advanced cutaneous squamous cell carcinoma. Eur. J. Cancer 2022, 170, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, M.; Leiter, U.; Loquai, C.; Zimmer, L.; Ugurel, S.; Gutzmer, R.; Thoms, K.M.; Enk, A.H.; Hassel, J.C. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: Real-world data of a retrospective, multicenter study. Eur. J. Cancer 2020, 138, 125–132. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, Å.; Gutzmer, R.; Roshdy, O.; González Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dréno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II Study of Pembrolizumab As First-Line, Single-Drug Therapy for Patients with Unresectable Cutaneous Squamous Cell Carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Amaral, T.; Osewold, M.; Presser, D.; Meiwes, A.; Garbe, C.; Leiter, U. Advanced cutaneous squamous cell carcinoma: Real world data of patient profiles and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S8), 44–51. [Google Scholar] [CrossRef] [PubMed]
- Baggi, A.; Quaglino, P.; Rubatto, M.; Depenni, R.; Guida, M.; Ascierto, P.A.; Trojaniello, C.; Queirolo, P.; Saponara, M.; Peris, K.; et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur. J. Cancer 2021, 157, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hober, C.; Fredeau, L.; Ledard, A.P.; Boubaya, M.; Herms, F.; Aubin, F.; Benetton, N.; Dinulescu, M.; Jannic, A.; Cesaire, L.; et al. 1086P Cemiplimab for advanced cutaneous squamous cell carcinoma: Real life experience. Ann. Oncol. 2020, 31, S737. [Google Scholar] [CrossRef]
- Migden, M.R.; Chandra, S.; Rabinowits, G.; Chen, C.I.; Desai, J.; Seluzhytsky, A.; Sasane, M.; Campanelli, B.; Chen, Z.; Freeman, M.L.; et al. CASE (CemiplimAb-rwlc Survivorship and Epidemiology) study in advanced cutaneous squamous cell carcinoma. Future Oncol. 2020, 16, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Weichenthal, M. ADOReg–wissenschaftliches Register der Arbeitsgemeinschaft Dermatologische Onkologie. JDDG J. Dtsch. Dermatol. Ges. 2014, 12, 1156–1157. [Google Scholar]
- Mohr, P.; Scherrer, E.; Assaf, C.; Bender, M.; Berking, C.; Chandwani, S.; Eigentler, T.; Grimmelmann, I.; Gutzmer, R.; Haferkamp, S.; et al. Real-World Therapy with Pembrolizumab: Outcomes and Surrogate Endpoints for Predicting Survival in Advanced Melanoma Patients in Germany. Cancers 2022, 14, 1804. [Google Scholar] [CrossRef]
- Rischin, D.; Khushalani, N.I.; Schmults, C.D.; Guminski, A.D.; Chang, A.L.S.; Lewis, K.D.; Lim, A.M.L.; Hernandez-Aya, L.F.; Hughes, B.G.M.; Schadendorf, D.; et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): Longer follow-up. J. Clin. Oncol. 2020, 38, 10018. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Li, F.Y.; Ko, C.J.; Colegio, O.R. Cutaneous Squamous Cell Carcinomas in Solid Organ Transplant Recipients Compared With Immunocompetent Patients. JAMA Dermatol. 2018, 154, 60–66. [Google Scholar] [CrossRef]
- Leiter, U.; Loquai, C.; Reinhardt, L.; Rafei-Shamsabadi, D.; Gutzmer, R.; Kaehler, K.; Heinzerling, L.; Hassel, J.C.; Glutsch, V.; Sirokay, J.; et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: A retrospective multicenter DeCOG study of 84 patients. J. Immunother. Cancer 2020, 8, e000897. [Google Scholar] [CrossRef]
- Fisher, J.; Zeitouni, N.; Fan, W.; Samie, F.H. Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review. J. Am. Acad. Dermatol. 2020, 82, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Koop, A.; Meier, F.; Hassel, J.C.; Terheyden, P.; Zimmer, L.; Heinzerling, L.; Ugurel, S.; Pfohler, C.; Gesierich, A.; et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur. J. Cancer 2017, 75, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kahler, K.C.; Eigentler, T.K.; Gesierich, A.; Heinzerling, L.; Loquai, C.; Meier, F.; Meiss, F.; Pfohler, C.; Schlaak, M.; Terheyden, P.; et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol. Immunother. 2018, 67, 825–834. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).