Simple Summary
Lipopolysaccharide (LPS) derived from intestinal bacteria is linked to long-lasting inflammation that contributes to the development of intestinal cancer. While much research has been performed on the interplay between LPS and intestinal immune cells, little is known about how LPS influences intestinal epithelial cell structure and function. In this study, we investigated the effects of LPS on the proliferation and function of genes in intestinal organoids derived from dogs with gastrointestinal diseases, including inflammatory bowel disease (IBD) and intestinal mast cell tumor. The goal of this study was to evaluate how LPS affects signaling pathways in intestinal epithelial cells to influence development of a pro-tumor-like environment. Using an ex vivo model system, LPS incubation of organoids activated cancer-causing genes and accelerated the formation of IBD organoids derived from the small and large intestines. In brief, the crosstalk that occurs between the LPS/TLR4 signal transduction pathway and several different metabolic pathways, including primary bile acid biosynthesis and secretion, peroxisome, renin-angiotensin system, glutathione metabolism, and arachidonic acid pathways, may play a prominent role in the development of chronic intestinal inflammation and intestinal cancer.
Abstract
Lipopolysaccharide (LPS) is associated with chronic intestinal inflammation and promotes intestinal cancer progression in the gut. While the interplay between LPS and intestinal immune cells has been well-characterized, little is known about LPS and the intestinal epithelium interactions. In this study, we explored the differential effects of LPS on proliferation and the transcriptome in 3D enteroids/colonoids obtained from dogs with naturally occurring gastrointestinal (GI) diseases including inflammatory bowel disease (IBD) and intestinal mast cell tumor. The study objective was to analyze the LPS-induced modulation of signaling pathways involving the intestinal epithelia and contributing to colorectal cancer development in the context of an inflammatory (IBD) or a tumor microenvironment. While LPS incubation resulted in a pro-cancer gene expression pattern and stimulated proliferation of IBD enteroids and colonoids, downregulation of several cancer-associated genes such as Gpatch4, SLC7A1, ATP13A2, and TEX45 was also observed in tumor enteroids. Genes participating in porphyrin metabolism (CP), nucleocytoplasmic transport (EEF1A1), arachidonic acid, and glutathione metabolism (GPX1) exhibited a similar pattern of altered expression between IBD enteroids and IBD colonoids following LPS stimulation. In contrast, genes involved in anion transport, transcription and translation, apoptotic processes, and regulation of adaptive immune responses showed the opposite expression patterns between IBD enteroids and colonoids following LPS treatment. In brief, the crosstalk between LPS/TLR4 signal transduction pathway and several metabolic pathways such as primary bile acid biosynthesis and secretion, peroxisome, renin–angiotensin system, glutathione metabolism, and arachidonic acid pathways may be important in driving chronic intestinal inflammation and intestinal carcinogenesis.
1. Introduction
Lipopolysaccharide (LPS) is the most effective cell wall-derived inflammatory toxin of Gram-negative bacteria with its inner component, lipid A, accountable for most of the toxin’s inflammatory effects []. The intestinal lumen, a habitat for many trillions of commensal bacteria, is the primary LPS reservoir in the body []. During normal circumstances, the intestinal epithelium retains a proper barrier function by promoting the transcellular movement of nutrients, water, and ions while reducing the paracellular transport of bacteria or its products/toxins (including LPS) into the systemic circulation []. In intestinal permeability disorders such as infection by pathogenic bacteria and faulty detoxification of LPS, the defective tight junction (TJ) barrier will permit the enhanced paracellular flux of LPS and other phlogistic luminal antigens []. Interestingly, apical but not basolateral exposure to LPS, induces epithelial apoptosis through caspase-3 activation and stimulates the disruption of tight junctional ZO-1, thus increasing epithelial permeability []. While transported in blood, LPS binds to either the LPS binding protein (LBP) or plasma lipoproteins and induces systemic inflammation []. Basically, LPS-induced intestinal inflammation occurs through the stimulation of Toll-like receptor 4 (TLR4), which subsequently leads to the recruitment of intracellular nuclear transcription factor-κB (NF-κB), followed by the release of chemokines and inflammatory cytokines including necrosis factor-alpha (TNF) [,]. TNF is accountable for various signaling events within cells, leading to necrosis or apoptosis, therefore playing a pivotal role in the resistance to infection and cancers. Activation of the TLR-4 dependent FAK-MyD88-IRAK4 signaling pathway controls LPS-induced intestinal inflammation and tight junction permeability [,].
The removal of circulating LPS (via amelioration of dysbiosis) facilitates the clinical recovery of inflammatory bowel disease (IBD), demonstrating its significant role in mediating chronic intestinal inflammation in IBD []. The biological role of LPS has been extensively characterized, particularly in the context of immune cell activation, where it causes pleiotropic inflammatory cytokine and chemokine secretion [] as well as its interactions with intestinal macrophages, dendritic cells, and T cells [,]. The pathogenesis of canine IBD is multifactorial and involves a loss of tolerance to diet and microbial components that cause an aberrant immune response in genetically susceptible hosts. Intestinal dysbiosis characterized by increased abundance of Gamma-Proteobacteria (e.g., Enterobacteriaceae) [] and defects in innate immune sensing are pivotal factors that initiate and drive chronic intestinal inflammation.
Mast cells (MCs) play an essential immunoregulatory role, especially at the mucosal barrier between the body and the environment. In intestinal lesions, MCs can be seen infiltrating the inflammatory microenvironment and regulating the synthesis of several pro-inflammatory cytokines and mediators of inflammatory cell production, thus promoting tumor growth and proliferation []. Numerous animal and human studies indicate the presence of an abundance of MCs surrounding the tumor cells or inflammation lesions [,,,]. For instance, high MC density is correlated with advanced stage and tumor progression in colorectal cancer (CRC) []. Similarly, intratumoral MC density correlates with tumor size, and peritumoral MC density correlates with the malignancy grade []. MCs also play a crucial part in IBD, and research has shown that the number of MCs increases in inflammatory bowel lesions in patients with IBD []. As a trigger of inflammatory responses, LPS has been associated with cancer pathogenesis including the development of gastrointestinal (GI) mast cell tumors and CRC [,,]. Different microbial products and TLR4 agonists have demonstrated the crucial role of TLR4 signaling in regulating tumor growth, survival, and progression in colonic, pancreatic, liver, and breast cancers []. The TLR4 signaling pathway has been shown to drive tumorigenesis and exhibit some antitumor effect on TLR4 activation [,]. TLR4 serves as a key bridge molecule linking oncogenic infection to colonic inflammatory and malignant processes by inducing miR-155 expression via transcriptional and post-transcriptional mechanisms []. Conversely, miR-155 can also augment TLR4 signaling by targeting negative regulators SOCS1 and SHIP1 []. Regardless of the significance of an impaired intestinal barrier indicated by elevated circulating LPS levels, the role of LPS in regulating signaling pathways involved in the development of intestinal inflammation in IBD remains mostly unknown. Gene expression profiling using microarray technology has been applied to identify physiologically and clinically significant subgroups of TNF-responsive tumors, elucidate the combinatorial and complex nature of cancers, [,] and advance our mechanistic understanding of oncogenesis.
The dog genome and its organization have been studied extensively in the last ten years. To understand the biology of human diseases, dogs have emerged as a primary large animal model [,,,,]. Of the large animal models used in translational GI research, the dog is particularly relevant since it presents similar gut physiology, dietary habits, and intestinal microbiota to humans [,]. Moreover, dogs spontaneously acquire severe chronic intestinal diseases such as IBD and CRC, which make them ideal animal models for translational GI research []. Our laboratory successfully developed and characterized a canine 3D enteroids/colonoids model system to study intestinal biology during health and disease and bridge the translational knowledge gap between mice and humans [,]. Adult stem cells can differentiate into different intestinal epithelial cells, so that the epithelial cell types normally present in vivo are also identified in cultured intestinal organoids. Adult stem cell-derived intestinal organoids have previously been shown to retain their genetic and epigenetic phenotype in vitro after numerous passages []. We, therefore, hypothesized that a stromal mast cell tumor in the small intestine of a dog could influence the phenotypic expression of the overlying epithelium, which would be retained in the organoid culture. Mast cell tumors are known to secret histamine, proteases, prostaglandin D2, leukotrienes, and heparin as well as a variety of pro-inflammatory cytokines [], which could change the expression profile of the epithelium overlying the tumor and sensitize it to the effects of LPS. The current study aimed to broaden our understanding of the LPS-induced regulation of signaling pathways in the intestinal epithelium and identify novel genes involved in inflammatory disease and colorectal cancer development.
2. Materials and Methods
2.1. Ethical Animal Use
The collection and analysis of intestinal biopsy samples from dogs with IBD and mast cell tumors were previously approved by the Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC-19-102; PI: Albert E. Jergens). All methods were performed in accordance with the relevant guidelines and regulations of IACUC as required by U.S. federal regulations []. The study is reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org, accessed on 29 November 2018).
2.2. Crypt Cell Isolation and Enrichment for Enteroid and Colonoid Culture
Ten to fifteen endoscopic mucosal biopsies (using 2.8/3.2 mm forceps cup) of the ileum and colon were obtained from two dogs, one diagnosed with IBD (to derive colonoids) and another one with a mast cell tumor (to derive enteroids/colonoids). Epithelial crypts were isolated and enriched from intestinal biopsy samples, as reported previously []. Briefly, ileal and colonic biopsies were cut into small pieces (1–2 mm thickness) with a scalpel and were washed six times using the complete chelating solution (1X CCS) (Table 1). Pre-wetted pipette and conical tubes with 1% bovine serum albumin (BSA) were used throughout the procedure to prevent the adherence of the crypt epithelia in the tubes and pipette, thereby minimizing the loss of cryptal units []. Then, tissues were incubated with 1X CCS containing EDTA (20 mM–30 mM) for 45 min for colonic biopsies and 75 min for ileal biopsies at 4 °C on a 20-degree, 24 rpm mixer/rocker (ThermoFisher Scientific, Rochester, NY, USA). After EDTA chelation, the cryptal epithelia release was augmented by trituration and/or mild shaking with a vortex. Additional trituration and/or mild shaking was performed after adding 2 mL of fetal bovine serum (FBS; Atlanta Biologicals, Flower Branch, Georgia, USA) to maximize the number of isolated crypts. After tissue fragments settled to the bottom of the tube, the crypt suspension containing the supernatant was transferred to a new conical tube and then centrifuged at 150g, 4 °C for 10 min. After centrifugation, the supernatant was removed, and the cell pellet was washed with 10 mL of incomplete media without an intestinal stem cell (ISC) growth factor (IMGF-) medium (Table 1) by repeating the centrifugation and decantation of the supernatant. Following crypt pellet washing, the cell pellet was re-suspended in 2 mL IMGF- medium, and the approximate number of crypt units isolated was enumerated using a hemocytometer [].

Table 1.
The composition of complete chelating solution (CCS) and organoid culture media.
An estimated 100 crypts were seeded per well in 30 μL of Matrigel (Corning® Matrigel® Growth Factor Reduced [GFR] Basement Membrane Matrix) into a 24-well plate format and incubated at 37 °C for 10 min []. Each well received 0.5 mL of complete media with ISC growth factors (CMGF+) supplemented with the rho-associated kinase ROCK-I inhibitor Y-27632 (StemGent) and glycogen synthase kinase 3β inhibitor CHIR99021 (StemGent) (Table 1) and was then placed in a tissue culture incubator. The CMGF + medium with ROCK-I inhibitor and glycogen synthase kinase 3β inhibitor was used for the first two days of ISC culture to enhance ISC survival and prevent apoptosis. After two days, the enteroid and colonoid cultures were replenished with CMGF+ medium without the ROCK-I inhibitor and glycogen synthase kinase 3β inhibitor every two days for 6–7 days. These freshly isolated 3D enteroid and colonoid cultures were termed Passage-0 (P0).
For subsequent passaging, enteroids and colonoids in Matrigel media were mechanically disrupted using a 1 mL syringe with a 23 g ¾” needle (trituration), collected into a 15 mL conical tube, and washed with CMGF- medium, then pelleted by centrifugation at 4 °C 100× g for 10 min. The pelleted enteroids and colonoids were cultured as previously described and designated as P1. This process was repeated for additional cell culture passages.
2.3. LPS Stimulation of Canine Enteroids and Colonoids
After four passages, colonoids from a dog with IBD and enteroids and colonoids from a dog diagnosed with an intestinal mast cell tumor were grown on 18 wells of a 24-well culture plate. Among the 18 wells, nine wells served as control cultures while nine wells served as LPS-treated cultures for these experiments. The LPS from Escherichia coli 026:B6 in an aqueous solution obtained from Thermo Fisher Scientific (Waltham, USA) was used for the study. To investigate the influence of a tumor microenvironment on the intestinal epithelium, we utilized enteroids from a canine intestinal mast cell tumor. In the control enteroid/colonoid cultures, the culture wells were treated with only the CMGF+ medium, whereas the LPS treatment group received the CMGF+ medium added with LPS at a concentration of 5 µg/mL. A high dose of LPS (5 µg/mL) was used to mimic the high concentration of bacterial-derived luminal LPS []. As small molecules are absorbed rapidly, LPS reaches the apical and basolateral sides of the organoids. After 48 h [], photographs of the culture wells containing the organoids were obtained using a phase-contrast microscope, and the culture media were collected and stored. All enteroids and colonoids from the separate nine well groups were pooled and washed with PBS and then homogenized in TRIzol (Invitrogen™:TRIzol™ Reagent #15596026) for the microarray and quantitative real-time PCR (qPCR) analyses. Because previous work by Yin et al. (2019) [] already provided intestinal transcriptome studies on exposure to LPS, we did not include this component in our current investigation. Instead, we utilized IBD enteroids, colonoids, and tumor enteroids as controls for the organoid groups treated with LPS. These samples were labeled separately (1–6) to avoid bias in interpretation and are described in Table 2.

Table 2.
A detailed description of the samples.
2.4. RNA Extraction and Affymetrix Canine Genome 2.0 Array Microarray Processing
Total RNA including microRNA was isolated from these six samples using the miRNeasy Mini Kit (QIAGEN #217004) following the manufacturers’ instructions. Extracted RNA was quantitated, and the purity was evaluated using a Synergy H1 Hybrid Multi-Mode Reader (BioTek). The high-quality RNA samples were then analyzed by the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center in Cleveland, OH. The samples were further purified using Qiagen miniprep RNA clean up (Qiagen) as per the Affymetrix protocols for array preparation. Purified RNA samples were quantified and assessed for quality using a Bioanalyzer 2100 instrument (Agilent Technologies, Palo Alto, CA, USA). A total of 0.1 µg of purified RNA was reverse transcribed and used to make cRNA. According to the manufacturer’s instructions, samples were hybridized to Canine Genome v.2.0 Affymetrix oligonucleotide arrays (Affymetrix, Santa Clara, CA, USA).
2.5. Assessment of Proliferation
As per the manufacturer’s instructions, samples containing 0.5 µg of total RNA were used to synthesize cDNA using the iScript™ Advanced cDNA Synthesis Kit (Bio-Rad, Life Science, Hercules, CA, USA). Real-time PCR for Ki-67 expression was carried out using the synthesized cDNA using PowerUp SYBR Green Master Mix following the manufacturer’s protocol. Thermocycling conditions were as follows: 50 °C for 2 min and then 95 °C for 2 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 1 min. The expression of Ki-67 (sense primer: CTCCCAGGTGCTATGTTCTATC; antisense primer: ACCTACTGGCCTGAGTAAGA; Amplicon size: 100 bp) in the cell cultures was normalized using GAPDH (sense primer: GATGCTGGTGCTGAGTATGT; antisense primer: GTCACCCTTCTCCACCTTTATG; Amplicon size: 102 bp) and quantified using the delta-delta Ct method []. The surface area and [] diameter of the organoids as well as the crypt length and the number of crypts per organoid were measured to determine the growth rate and proliferation of organoids under LPS stimulation. The organoid surface area, diameter, and crypt length as well as the total number of crypts present in an organoid were measured using Image J software []. An unpaired two-tailed Student’s t-test was used for statistical analysis.
2.6. Analysis of Microarray Data
Microarray CEL files were processed using the R library affylmGUI. Contrasts were computed as given below:
- Sample 2 vs. Sample 1
- Sample 4 vs. Sample 3
- Sample 6 vs. Sample 5
- Sample 5 vs. Sample 3
- Sample 3 vs. Sample 1
- Sample 6 vs. Sample 4
- Sample 4 vs. Sample 2
AffylmGUI [] was used to analyze the microarray data. The quality of the microarray chips was determined by NUSE and RNA degradation plots. Probe level data were converted into probe set expression data and normalized using GCRMA. Contrasts between the treatment and control were made using the built-in linear model of affylmGUI. Since pooled data were used without replication, there were no replicates, and empirical Bayes statistics could not be computed. MA plots were generated in R, where M is the log2 ratio of the probe intensity, and A is the average probe intensity in the contrast (Supplementary Table S1; Probe IDs and their associated gene information are available at https://www.ebi.ac.uk/arrayexpress/files/A-AFFY-149/A-AFFY-149.adf.txt, accessed on 5 May 2020). The MA plot shows a cone shape where log2 fold change M decreases with the average probe intensity A. Probes that were on the edge of this cone shape are of potential interest as they fall outside the bulk of the probes for a given intensity and are the most differentially expressed (DEG) given their average probe intensity. Therefore, to identify probes of interest, the hexbin library in R was used to identify probes that fell into low-density regions of the MA plot, which corresponded to the edges of the MA plot []. This method was chosen to select probes without bias and for reproducibility. One advantage of this approach is that the probes that are the most different in a comparison of samples will be identified. However, one point of caution is that regardless of the M value magnitude for all sample comparisons, there will still be a relatively small number of probes identified (~500). Given the lack of the replication of samples, we realize the limitations of these results and that follow-up experiments will be required to confirm these results. KEGG ids from these probes were explored using the GenomeNet KEGG pathway search [,,].
2.7. BLAST Search and KEGG Pathway Analyses
Blast2GO was used to determine the function and localization of differentially expressed genes (DEGs) []. It is a widely used annotation platform that uses homology searches to associate sequence with Gene Ontology (GO) terms and other functional annotations. Blast2GO generated Gene Ontology annotations were determined for the three sub-trees of GO, (a) biological process, (b) molecular function, (c) cellular component. The KEGG (Kyoto Encyclopedia of Genes and Genomes) [,,,,] pathway analyses were performed by Blast2GO.
3. Results
3.1. LPS Stimulates Higher Intestinal Epithelial Cell Proliferation
LPS at a concentration of 5 µg caused an increase in the size and number of canine enteroids and colonoids, which were apparent under phase-contrast microscopy (Figure 1a). Although all enteroids and colonoids showed increased proliferation following LPS stimulation, the magnitude of proliferation was higher in tumor enteroids, followed by IBD enteroids and IBD colonoids. To confirm the magnitude of increased proliferation of enteroids and colonoids after LPS stimulation, qPCR was used to evaluate the mRNA expression of Ki-67, a nuclear proliferation marker []. LPS stimulation caused an ~4-fold increase in the Ki-67 mRNA expression in both tumor enteroids and IBD enteroids, whereas LPS caused an ~1.5-fold increase in the IBD colonoids (Figure 1b). Genes associated with proliferation such as proliferating cell nuclear antigen (PCNA), prominin 1 (PROM1), HOP homeobox (HOPX), and olfactomedin 4 (OLFM4) were also upregulated in LPS treated IBD intestinal organoids and tumor enteroids (Figure 1c). Increased surface area and diameter of the organoids as well as the crypt length and the number of crypts per organoid were all detected after LPS treatment, indicating that LPS stimulation increased the growth rate of organoids (Figure 1d).
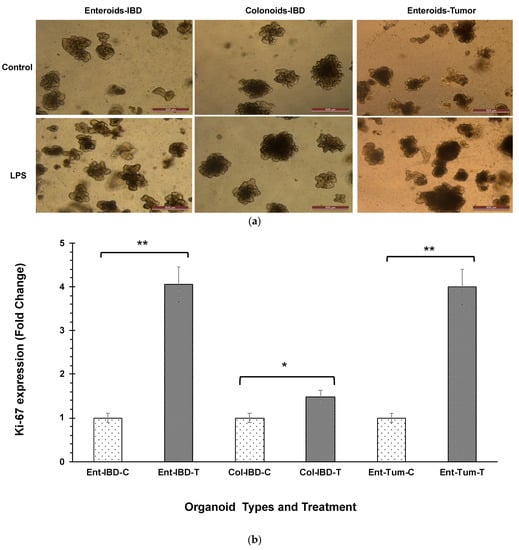
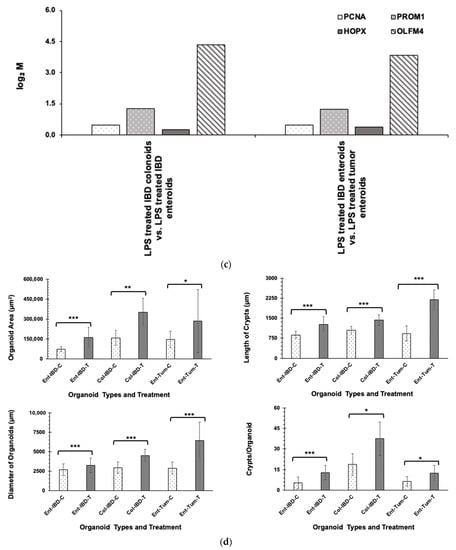
Figure 1.
LPS stimulates higher proliferation. (a) Enteroids and colonoids from dogs with IBD and intestinal mast cell tumor, 48 h after LPS stimulation. Representative phase-contrast images of enteroids and colonoids after LPS stimulation. The control group received complete growth medium, whereas the LPS group received LPS 5 µg/mL in complete growth medium. (b) Expression of Ki-67 in enteroids and colonoids from dogs with IBD and intestinal mast cell tumor, 48 h after LPS stimulation as measured by qPCR. The control group received complete growth medium, whereas the LPS group received LPS 5 µg/mL in complete growth medium. GAPDH was used to normalize. Ent-IBD-C: control IBD enteroids; Ent-IBD-T: IBD enteroids following LPS stimulation; Col-IBD-C: control IBD colonoids; Col-IBD-T: IBD colonoids following LPS stimulation; Ent-Tum-C: control tumor enteroids; Ent-Tum-T: tumor enteroids following LPS stimulation. Histograms represent the mean Ki-67 expression (fold change) ± SD of four technical replicates used in the qPCR experiment. Unpaired two-tailed Student’s t-test was used for statistical analysis. * p < 0.0005, ** p < 0.00001. (c) Histograms represent induced expression levels (log-ratio M values) of proliferating cell nuclear antigen (PCNA), prominin 1 (PROM1), HOP homeobox (HOPX), and olfactomedin 4 (OLFM4) in LPS treated IBD colonoids vs. LPS treated IBD enteroids (Contrast-6), and LPS treated IBD enteroids vs. LPS treated tumor enteroids (Contrast-7). The log-ratio M values represent log(R/G) (log fold change) []. (d) Growth rate of organoids by LPS stimulation was evaluated by surface area and diameter of the organoids, crypt length, and the number of crypts per organoid. Data are presented as mean ± SD. Ent-IBD-C: control IBD enteroids; Ent-IBD-T: IBD enteroids following LPS stimulation; Col-IBD-C: control IBD colonoids; Col-IBD-T: IBD colonoids following LPS stimulation; Ent-Tum-C: control tumor enteroids; Ent-Tum-T: tumor enteroids following LPS stimulation. Unpaired two-tailed Student’s t-test was used for statistical analysis. * p < 0.05, ** p < 0.005, *** p < 0.0005.
3.2. Microarray Analysis Reveals Differential Expression of Genes Stimulated by LPS in IBD Intestinal Organoids and Tumor Enteroids
In this study, we used the GeneChip® Canine Genome 2.0 Array that contains more than 42,800 Canis familiaris probe sets to evaluate gene expression []. These probe sets targeted more than 18,000 C. familiaris mRNA/EST-based transcripts and more than 20,000 non-redundant predicted genes for more comprehensive coverage []. We performed multiple comparisons between samples to determine the effect of LPS treatment, anatomical location, and the in vivo microenvironment (tumor/IBD) on the mRNA expression profiles of organoids following LPS stimulation. The highest number of highly differentially expressed mRNAs (684) was found in LPS-treated IBD colonoids vs. the control IBD colonoids (Table 3). Among this total number of highly differentially expressed mRNAs, 48% were upregulated, and 52% were downregulated (Table 3). Although similar numbers of differentially expressed mRNAs (677) were found between the LPS-treated tumor enteroids vs. the control tumor enteroids, 56% were upregulated, and 44% were downregulated.

Table 3.
The differentially expressed genes within the enteroid and colonoid groups.
The next highest number of differentially expressed mRNAs (639) was found between the LPS treated IBD enteroids vs. control untreated IBD enteroids (Table 3). Of the differentially expressed mRNAs, 49% were upregulated, and 51% were downregulated (Table 3). Interestingly, between the LPS treated IBD colonoids and enteroids, only 421 differentially expressed mRNAs were identified, of which 39% were upregulated and 61% were downregulated. LPS stimulation caused 314 differentially expressed mRNAs in the IBD enteroids vs. the LPS treated tumor enteroids. Among these differentially expressed mRNAs, 51% were upregulated, and 49% were downregulated. Furthermore, LPS stimulation resulted in a unique transcriptomic heat map for tumor enteroids, IBD enteroids, and colonoids (Supplementary Figure S1).
3.3. Gene Expression Profiles and Pathway Enrichment Analysis
3.3.1. The LPS Treated Tumor Enteroids vs. the Control Tumor Enteroids
Genes that demonstrated high levels of activity in tumor enteroids following LPS stimulation included KH domain-containing, RNA-binding, signal transduction-associated protein 3 (KHDRBS3), Flap endonuclease GEN homolog 1 (GEN1), krev interaction trapped-1 (KRIT1), Centromere protein F (CENPF), ciliogenesis and planar polarity effector complex subunit 1 (CPLANE1), and tyrosine-protein kinase (STYK1). However, expressions of quinone oxidoreductase-like protein 2/Crystallin Zeta Like 2 (LOC610994), G patch domain-containing protein 4 (Gpatch4), Solute Carrier Family 7 Member 1/high-affinity cationic amino acid transporter 1 (SLC7A1), cation-transporting ATPase 13A2 (ATP13A2), and testis expressed 45 (TEX45) genes were significantly repressed in LPS-treated tumor-adjacent enteroids (Figure 2 and Table 4).
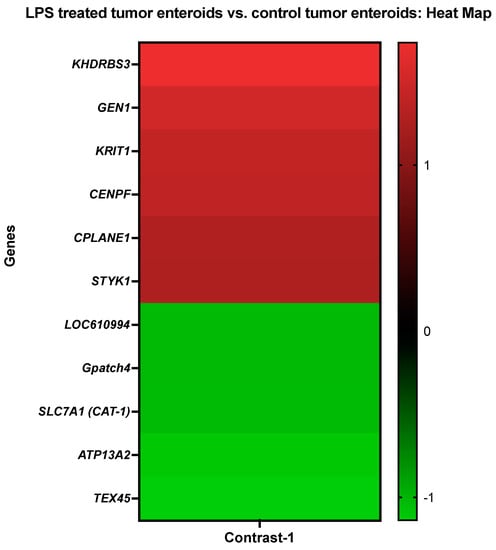
Figure 2.
A heat map representing the color-coded expression levels (log-ratio M values) of the top six upregulated and five downregulated DEGs in the LPS treated tumor enteroids vs. the control tumor enteroids (Contrast-1). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 4.

Table 4.
Gene Ontology (GO) analysis and details about the top six upregulated and five downregulated DEGs in the LPS treated tumor enteroids vs. the control tumor enteroids.
Using the Blast2GO functional group analysis [,] (Figure 2; Table 4), the highly differentially expressed genes (DEGs) stimulated by LPS treatment on tumor enteroids were found predominantly overrepresented in biological processes involved in cellular metabolic process and regulation, organic substance metabolic process/nitrogen compound metabolic process (18%), regulation of molecular function, cellular homeostasis/regulation of biological quality (7%), and cell cycle checkpoint/microtubule-based process (4%). In comparison, categories corresponding to identical protein binding (14%) and nucleic acid binding (14%) were significantly overrepresented in molecular function. However, in the cellular component category, the DEGs were overrepresented for intracellular organelles (17%), intracellular non-membrane-bounded organelles (10%), membrane-bounded organelles (10%), an integral component of membrane (10%), non-membrane-bounded organelles (10%), plasma membrane (7%), and centrosome (7%) (Figure 2; Table 4).
3.3.2. The LPS Treated IBD Enteroids vs. the Control IBD Enteroids
Genes that demonstrated high levels of activity in IBD enteroids following LPS stimulation included the DENN domain containing 5B (DENND5B), serine/threonine/tyrosine-interacting-like protein 1 (STYXL1), regenerating islet-derived protein 3-gamma-like (REG3G), and protein S100-A16 (S100A16). However, expressions of protein FAM122B (FAM122B), centrosomal protein of 70 kda (CEP70), beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase (MGAT3), and mucin 1 (MUC1) genes were significantly repressed in the LPS-treated IBD enteroids (Figure 3).
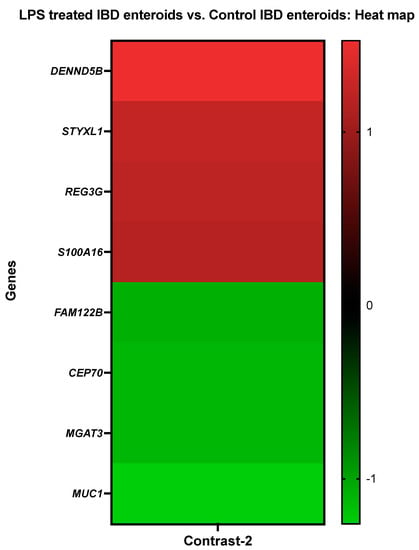
Figure 3.
A heat map depicting the color-coded expression levels (log-ratio M values) of the top four upregulated and four downregulated DEGs in the LPS treated IBD enteroids vs. the control IBD enteroids (Contrast-2). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 5.
The DEGs stimulated by LPS treatment on the IBD enteroids were found to be predominantly overrepresented in the biological process category for the organonitrogen compound metabolic process, microtubule cytoskeleton organization, regulation of microtubule-based process, cellular component biogenesis/organization and regulation, and the phosphorus/cellular macromolecule/protein metabolic process using Blast2GO functional group analysis [,] (Table 5). In comparison, the categories corresponding to phosphoprotein phosphatase activity, identical protein binding, cytoskeletal protein binding, hydrolase activity, acting on ester bonds, and cation binding were significantly overrepresented in molecular function. However, in the cellular component category, the DEGs were overrepresented for intracellular non-membrane-bounded organelles (14%) and the membrane/intrinsic component of membrane, cytoplasm (14%) (Table 5).

Table 5.
The Gene Ontology (GO) analysis and details about the top four upregulated and four downregulated DEGs in the LPS treated IBD enteroids vs. the control IBD enteroids. The DEGs annotated for the biological process, molecular function, and cellular component categories are shown. The percentages represent the number of genes in each functional category.
3.3.3. The LPS Treated IBD Colonoids vs. the Control IBD Colonoids
Genes such as collagenase 3 (MMP13), dual oxidase maturation factor 2 (DUOXA2), ceruloplasmin (CP), UMP-CMP kinase 2, mitochondrial (CMPK2), thrombospondin-4 (THBS4), and interleukin-8 (CXCL8) showed elevated levels of activity in the IBD colonoids after LPS treatment while the colonoids exposed to LPS had decreased expressions of abl interactor 2 (ABI2), ubiquitin-conjugating enzyme E2 E3 (UBE2E3), echinoderm microtubule-associated protein-like 2 (EML2), extracellular sulfatase Sulf-2-like (SULF2), and ectonucleotide pyrophosphatase/phosphodiesterase family member 6 (ENPP6) genes (Figure 4).
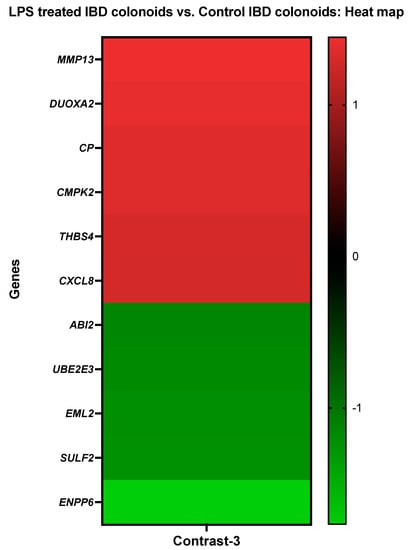
Figure 4.
A heat map depicting the color-coded expression levels (log-ratio M values) of the top six upregulated and five downregulated DEGs in the LPS treated IBD colonoids vs. the control IBD colonoids (Contrast-3). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 6.
Using the Blast2GO functional group analysis, it was noticed that the DEGs stimulated by LPS treatment on the IBD cololoids were predominantly overrepresented in the biological process category for the primary metabolic process, organic substance/nitrogen compound metabolic process (14%), establishment of localization (9%), regulation of biological quality, cellular homeostasis, oxidation–reduction process (5%), and cell communication, signal transduction, response to chemical/external stimulus, cellular response to stimulus, immune response (5%) (Table 6). When it comes to molecular function, the categories that correspond to metal ion binding (19%), cytokine receptor binding (6%), and oxidoreductase activity, oxidizing metal ions, oxygen as acceptors (6%) were significantly overrepresented. However, in the category of cellular components, the DEGs were overrepresented in the extracellular region (28%) and membrane (17%) (Table 6).

Table 6.
The Gene Ontology (GO) analysis and details of the DEGs in the LPS treated IBD colonoids vs. the control IBD colonoids annotated for the biological process, molecular function, and cellular component categories. The percentages represent the number of genes in each functional category.
3.3.4. The Control IBD Colonoids vs. the Control IBD Enteroids
Carbonic anhydrase 1 (CA1), protein S100-A16 (S100A16), mucin-1 (MUC1), and KH domain-containing, RNA-binding, signal transduction-associated protein 3 (KHDRBS3) showed increased activity in the IBD colonoids compared to the IBD enteroids, while bile acid-CoA: amino acid N-acyltransferase-like (BAAT), annexin A13 (ANXA13), and aminopeptidase N (ANPEP) exhibited decreased expression levels (Figure 5).
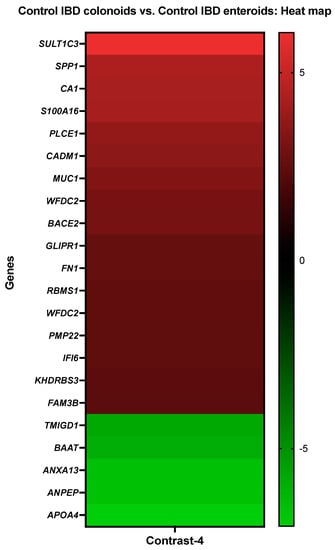
Figure 5.
A heat map depicting the color-coded expression levels (log-ratio M values) of the top 17 upregulated and six downregulated DEGs in the control IBD colonoids vs. the control IBD enteroids (Contrast 4). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 7.
With the help of the Blast2GO functional group analysis, it was observed that the top upregulated or downregulated DEGs in the IBD colonoids, compared to the IBD enteroids, were predominantly overrepresented in the biological process category, specifically in the cellular metabolic process (7%) and response to stress, regulation of metabolic process/molecular function/hydrolase activity (2%) (Table 7). In molecular function, the categories that correspond to metal ion binding (21%) and RNA binding (8%) were significantly overrepresented while in the cellular component categories, the DEGs were overrepresented in the membrane, cell membrane (14%), and cytoplasm and cytosol (12%) categories (Table 7).

Table 7.
The Gene Ontology (GO) analysis and details about the top 17 upregulated and six downregulated DEGs in the control IBD colonoids vs. the control IBD enteroids. The DEGs annotated for biological process, molecular function, and cellular component categories are presented. The number of DEGs that fell into each of these categories is shown in percentages.
3.3.5. The Control IBD Enteroids vs. the Control Tumor Enteroids
The activity of CA1, protein S100A16 (S100A16), beta-site APP-cleaving enzyme 2 (memapsin 1) (beta-secretase 2) (BACE2), and mucin-1 (MUC1) was increased in the IBD enteroids compared to the tumor enteroids, while the activity of bile acid-CoA:amino acid N acyltransferase-like (BAAT), annexin A13 (ANXA13), and aminopeptidase N (ANPEP) was decreased (Figure 6).
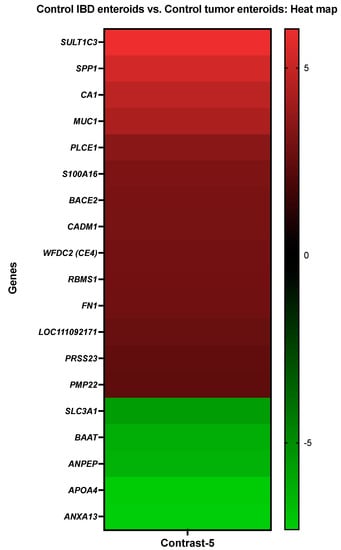
Figure 6.
A heat map depicting the color-coded expression levels (log-ratio M values) of the top 14 upregulated and five downregulated DEGs in the control IBD enteroids vs. the control tumor enteroids (Contrast-5). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 8.
With the help of the Blast2GO functional group analysis, we observed that the top upregulated or downregulated DEGs in the IBD enteroids, compared to tumor enteroids, were predominantly overrepresented in the biological process category, specifically in the primary metabolic process, organic substance metabolic process (10%), and cell communication, signal transduction, cellular response to stimulus (4%) (Table 8). In the molecular function, the categories that corresponded to ion binding (18%), hydrolase activity (14%), and enzyme regulator activity (11%) were significantly overrepresented, while in the cellular component categories, the DEGs were overrepresented in the cytoplasm (13%), membrane/plasma membrane (13%), organelle (11%), and extracellular region (10%) categories (Table 8).

Table 8.
The Gene Ontology (GO) analysis and details about the top 14 upregulated and five downregulated DEGs in the control IBD enteroids vs. the control tumor enteroids. The DEGs annotated for the biological process, molecular function, and cellular component categories are shown. The percentages represent the number of genes in each functional category.
3.3.6. The LPS Treated IBD Colonoids vs. the LPS Treated IBD Enteroids
The activities of CA1, S100A16, annexin A10 (ANXA10), and MUC1 were increased in the LPS treated IBD colonoids compared to the LPS treated tumor enteroids, while the activities of BAAT, long-chain-fatty-acid-CoA ligase 5 (ACSL5), ectonucleotide pyrophosphatase/phosphodiesterase family member 6 (ENPP6) were decreased (Figure 7).
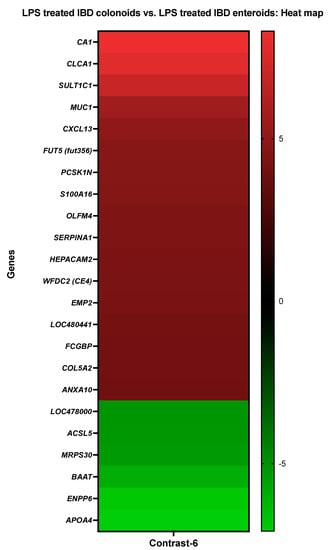
Figure 7.
A heat map depicting the color-coded expression levels (log-ratio M values) of the top 17 upregulated and six downregulated DEGs in the LPS treated IBD colonoids vs. the LPS treated IBD enteroids (Contrast-6). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 9.
With the help of the Blast2GO functional group analysis, it was noticed that the top upregulated or downregulated DEGs in the LPS treated IBD colonoids compared to the LPS treated IBD enteroids were predominantly overrepresented in the biological process category, specifically in the cellular metabolic process (7%) and signal transduction, cell communication, cellular response to stimulus (3%) categories (Table 9). In molecular function, the categories that corresponded to cation binding (17%), hydrolase activity, acting on ester bonds (10%), and signaling receptor binding (7%) were significantly overrepresented, while in the cellular component categories, the DEGs were overrepresented in the intracellular organelle (14%) and membrane-bounded organelle (14%) categories (Table 9).

Table 9.
The Gene Ontology (GO) analysis and details about the DEGs in the LPS treated IBD colonoids vs. the LPS treated IBD enteroids annotated for the biological process, molecular function, and cellular component categories. The number of genes that fell into each of these categories is shown in percentages.
3.3.7. The LPS Treated IBD Enteroids vs. the LPS Treated Tumor Enteroids
The activities of CA1, ANXA10, and MUC1 were increased in LPS treated IBD enteroids compared to the LPS treated tumor enteroids, while the activities of BAAT, ACSL5, and ENPP6 were decreased (Figure 8).
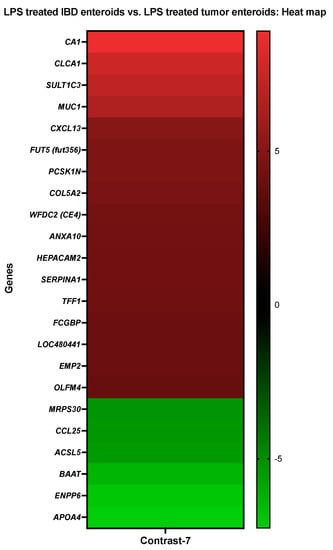
Figure 8.
A heat map depicting the color-coded expression levels (log-ratio M values) of the top 17 upregulated and six downregulated DEGs in the LPS treated IBD enteroids vs. the LPS treated tumor enteroids (Contrast-7). The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 10.
With the help of the Blast2GO functional group analysis, it was noticed that the top upregulated or downregulated DEGs in the LPS treated IBD enteroids compared to the LPS treated IBD tumor enteroids were predominantly overrepresented in the biological process category, specifically in the regulation of the cellular process (7%), cellular metabolic process (6%), regulation of molecular function (5%), and cell communication, signal transduction, cellular response to stimulus (4%) categories (Table 10). In the molecular function, the categories that corresponded to the receptor ligand activity (14%), metal ion binding (14%) and cytokine receptor binding, G protein-coupled receptor binding (9%) were significantly overrepresented, while it in the cellular component categories, the DEGs were overrepresented in the plasma membrane (23%), intracellular organelle, membrane-bounded organelle (15%), and integral component of the membrane (15%) (Table 10).

Table 10.
The Gene Ontology (GO) analysis and details about the top 17 upregulated and six downregulated DEGs in the LPS treated IBD enteroids vs. the LPS treated tumor enteroids. The DEGs annotated for the biological process, molecular function, and cellular component categories are shown. The percentages represent the number of genes in each functional category.
3.4. Genes Showing Similar Expression Patterns between IBD Enteroids and Colonoids Following LPS Stimulation
The pattern of gene expression was compared between the LPS-stimulated IBD enteroids and colonoids. We identified 25 genes across these two groups with similar expression patterns (Figure 9 and Table 11 and Table 12). Following LPS stimulation, 14 of these 25 genes were elevated in both the IBD enteroids and colonoids, whereas 11 were downregulated. These 25 genes may represent the intestinal epithelium signature LPS responsive gene network, regardless of the intestinal regions.
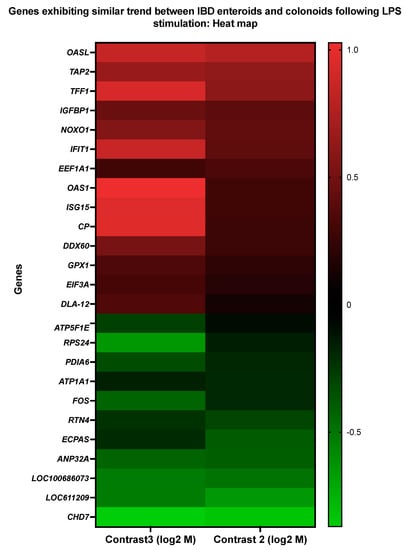
Figure 9.
A heat map representing the color-coded expression levels (log-ratio M values) of 25 DEGs exhibiting a similar pattern of expression between the IBD enteroids and colonoids following LPS stimulation. The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 11.

Table 11.
The Gene Ontology (GO) analysis and details about the DEGs exhibiting a similar pattern of expression between the IBD enteroids and colonoids following LPS stimulation annotated for the biological process, molecular function, and cellular component categories. The number of genes that fell into each of these categories is shown in percentages.

Table 12.
A list of genes in the common/same direction between the IBD enteroids, colonoids, and tumor-adjacent organoids following LPS stimulation. The log-ratio M values represent the log(R/G) (log fold change) [].
Following LPS stimulation of the IBD enteroids and colonoids, significant upregulation of the genes involved in stress response (OAS1, OASL, IFIT1, GPX1, and ISG15) was observed, even though several of these genes (OAS1, OASL, and ISG15) and EEF1A1 were previously implicated in primary metabolic processes and their regulation [,,,]. Additional upregulated genes include those involved in the cellular response to stimulus (TFF1, IFIT1, ISG15, GPX1, IGFBP1), signal transduction (TFF1, IGFBP1, ISG15), response to biotic stimulus, immune effector process, immune system regulation, immune system development/cytokine production (OAS1, OASL, IFIT1, ISG15), antigen processing and presentation (TAP2, DLA class I), oxidation–reduction process and cellular detoxification (CP, GPX1), regulation of molecular function (OASL, IFIT1, NOXO1), transmembrane transport (TAP2), and positive regulation of the viral process (IFIT1) (Figure 9 and Table 11). On the other hand, considerable downregulation of the RPS24 and ATP1A1 genes, involved in primary metabolic processes and transmembrane transport, respectively, was observed (Figure 9 and Table 11).
3.5. Genes Upregulated in IBD Enteroids and Downregulated in IBD Colonoids (and Vice Versa) Following LPS Stimulation
Interestingly, 20 genes were found to be altered in the opposite direction (Figure 10 and Table 13 and Table 14). These 18 genes were elevated in the LPS-treated IBD enteroids; however, these same 20 genes were downregulated in the LPS-treated colonoids. These findings imply that a distinct gene signature might be utilized to differentiate the intestinal region in response to LPS stimulation.
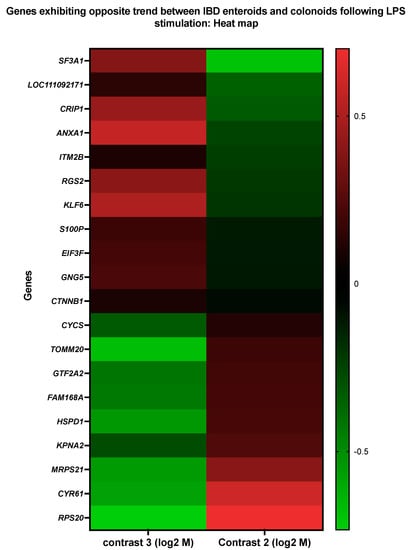
Figure 10.
A heat map representing the color-coded expression levels (log-ratio M values) of DEGs upregulated in the IBD enteroids and downregulated in the IBD colonoids and vice versa between the IBD enteroids and colonoids following LPS stimulation. The log-ratio M values represent the log(R/G) (log fold change) []. The detailed information on genes is presented in Table 12.

Table 13.
The Gene Ontology (GO) analysis and the details about the 20 DEGs exhibiting an opposite trend (upregulated in IBD enteroids and downregulated in the IBD colonoids and vice versa) between the IBD enteroids and colonoids following LPS stimulation annotated for the biological process, molecular function, and cellular component categories. The percentages represent the number of genes in each functional category.

Table 14.
The list of genes in the same or the opposite direction (upregulated in IBD enteriods/colonoids and downregulated in tumor-adjacent organoids and vice versa) between the IBD enteroids/colonoids and tumor-adjacent organoids following LPS stimulation. The log-ratio M values represent the log(R/G) (log fold change) [].
The LPS-stimulated genes that exhibited the opposite expression trends between the IBD enteroids and colonoids were involved in anion transport (TOMM20, ANXA1, KPNA2), translation (RPS20, EIF3F, MRPS21), protein import (TOMM20, KPNA2), protein-containing complex assembly (TOMM20, SF3A1), regulation of proteolysis (LOC111092171, HSPD1), RNA metabolic process (GTF2A2, SF3A1), positive regulation of leukocyte cell–cell adhesion, T-cell activation, apoptotic process, and the regulation of adaptive immune response (ANXA1, HSPD1), as shown by the Blast2GO analyses [,] (Figure 10 and Table 13 and Table 14). In the IBD colonoids, LPS treatment increased the expression of the EIF3F, ANXA1, SF3A1, and LOC111092171 genes, whereas it decreased their expression in the IBD enteroids (Figure 10). In contrast, the expression of RPS20, MRPS21, TOMM20, KPNA2, HSPD1, and GTF2A2 genes was elevated and reduced in the LPS treated IBD enteroids and colonoids, respectively (Figure 10). These unique gene expression signatures in the IBD enteroids and colonoids treated with LPS may provide critical insights into the intestinal physiology and novel inflammatory pathways that may serve as future drug discovery targets.
3.6. KEGG Pathway Analyses
The KEGG [,,,] pathway analysis (https://www.kegg.jp/kegg/kegg1.html, accessed on 5 May 2020) displayed that the most up- or downregulated DEGs in the IBD intestinal organoids and tumor enteroids were involved in metabolic pathways related to nitrogen metabolism (CA1), glutathione metabolism, renin–angiotensin system, hematopoietic cell lineage (ANPEP/LOC112653425), primary bile acid biosynthesis, taurine and hypotaurine metabolism, biosynthesis of unsaturated fatty acids, peroxisome, and bile secretion (BAAT). BACE2, which is involved in Alzheimer’s disease, was found to be one of the most prevalent DEGs in the IBD enteroids compared to the tumor enteroids. However, under LPS stimulation, in addition to CA1 and BAAT, a gene involved in fatty acid biosynthesis and degradation, the PPAR signaling pathway, peroxisome, ferroptosis, thermogenesis, and adipocytokine signaling pathway (ACSL5) as well as the ENPP6 gene involved in ether lipid metabolism, were among the top up- or downregulated DEGs in the IBD intestinal organoids and tumor enteroids (Figure 11 and Table 15).
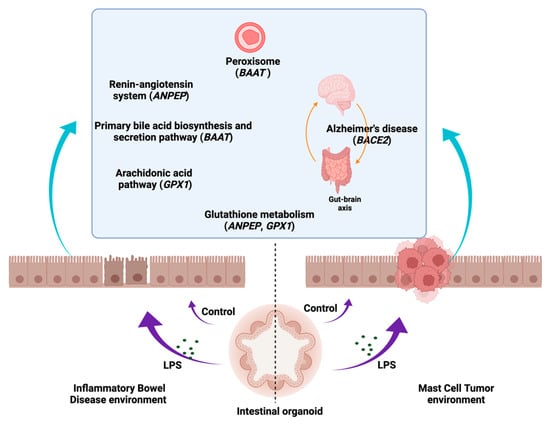
Figure 11.
The association of several metabolic pathway genes in the IBD intestinal organoids and tumor enteroids. The figure was created with BioRender.com.

Table 15.
The KEGG enrichment analysis results by Blast2GO []. The details of each KEGG pathway [,,] are available at https://www.kegg.jp/kegg/kegg1.html. * Top upregulated and downregulated genes were studied by Blast2GO [].
As a result of LPS stimulation, genes that were expressed similarly in both the IBD enteroids and colonoids were shown to be involved in the metabolism of porphyrin and ferroptosis (CP), ABC transporters, phagosome, antigen processing and presentation, human cytomegalovirus infection, Herpes simplex virus 1 infection, Epstein–Barr virus infection, human immunodeficiency virus 1 infection, primary immunodeficiency (TAP2/ABCB3), arachidonic acid, glutathione metabolism (GPX1), and nucleocytoplasmic transport, legionellosis, and leishmaniasis (EEF1A1) (Table 15).
4. Discussion
In this study, we generated enteroids and colonoids from dogs diagnosed with IBD or intestinal mast cell tumor. The goal of these experiments was to better understand how intestinal epithelial cells respond to LPS stimulation under different pathological conditions. We further investigated the differences between the organoids derived from various intestinal compartments (small intestine vs. colon) in IBD dogs following LPS stimulation. IBD patients have long-lasting epithelial abnormalities that may be acquired during disease development, which contributes to the progression of the pathology. Genetic mutations associated with human IBD are involved in colonic epithelium architectural organization, resulting in the establishment of a permanent epithelial phenotype in organoid cultures []. However, recently published results on inflammatory bowel disease (IBD) indicate that patient-derived organoids may lose their inflammatory signature after 5–6 passages [,], and hence we decided to examine an earlier time point. However, it has been reported that for at least 17 passages, intestinal organoids express most gene orthologs that are particularly expressed in the human gut, suggesting that adult stem cells preserve intestine-associated gene expression across long-term culture conditions [,,]. Transcriptomic analyses using microarrays [] were used to identify differentially expressed genes in enteroids and colonoids derived from diseased dogs. These data were the first to characterize regional specific transcriptomic changes in the intestinal epithelial cells of dogs with IBD and intestinal cancer in response to ex vivo LPS stimulation.
An increase in proliferation in tumor enteroids and both IBD enteroids and colonoids was observed following LPS stimulation in the present study, as indicated by Ki-67 estimates, stem cell markers as well as an increase in the surface area and diameter of the organoids, and an increase in crypt length and the number of crypts per organoid. Adult stem cells expressing the leucine-rich repeat-containing G-protein coupled receptor (LGR5) could be identified close to the base of the intestinal crypts [] that were employed for organoid proliferation. Using canine-specific LGR5 probes, we previously detected ISCs within the intestine organoid crypts [], demonstrating that proliferation is restricted to crypt-like budding structures []. Ki67 has been used as a proliferation marker as its expression is strongly associated with proliferation and growth [,]. Other intestinal stem cell markers and genes associated with proliferation, notably proliferating cell nuclear antigen (PCNA), prominin 1 (PROM1), HOP homeobox (HOPX), and olfactomedin 4 (OLFM4), were also increased in LPS-treated IBD intestinal organoids and tumor enteroids. PCNA is widely accepted as a proliferation marker [], while PROM1 [] is a marker for stem cells and early progenitors in the mouse small intestine. Likewise, OLFM4 is a highly specific marker reported in murine and human intestinal stem cells and its expression is regulated by the NF-κB, Notch, and Wnt signaling pathways in digestive diseases []. The role of OLFM4 in pathogenesis relates to anti-inflammation, apoptosis, cell adhesion, and also proliferation, as it regulates the cell cycle and promotes the S phase transition [,]. While HOPX-expressing cells give rise to crypt base columnar (CBC) cells and all mature intestinal epithelial lineages, CBCs can give rise to +4 HOPX positive cells. It is also possible that LPS-induced enhanced proliferation in tumor enteroids was caused by the increased expression of genes involved in the cell cycle process such as centromere protein F (CENPF) and flap endonuclease GEN homolog 1 (GEN1) as well as a receptor protein tyrosine kinase (STYK1). CENP-F is a centromere-kinetochore complex-associated protein, and its expression is increased in tumors [] and it serves as a marker for cell proliferation in human malignancies []. CRISPR-Cas9 silencing of CENPF in human prostate cancer cells resulted in decreased cell proliferation [,]. CENPF regulates cancer metabolism by modulating the phosphorylation signaling of pyruvate kinase M2 []. Similarly, GEN1 expression was induced in tumor enteroids. GEN1, similar to other members of the Rad2/XPG family as FEN1, is a monomeric 59-flap endonuclease, but it can dimerize on Holliday junctions (HJs), providing the two symmetrically aligned active sites required for HJ resolution []. Members of the Rad2/XPG family such as FEN1 are significantly expressed in proliferating cell populations, consistent with their role in DNA replication []. For instance, its expression is associated with the proliferation of mammary epithelial cells []. FEN1 expression is increased in metastatic prostate cancer cells, gastric cancer cells, pancreatic cancer cells, and lung cancer cell lines, and with tumor progression []. Additionally, the receptor STYK1 is required for cell proliferation []. Oncogenic potential of STYK1 has been studied widely in gallbladder cancer (GBC) and was reported to be largely dependent on the PI3K/AKT pathway. The tumor-stimulating activity of STYK1 was abolished by the AKT-specific inhibitor MK2206 as well as by STYK1 gene silencing []. A crucial NF-κB regulator, Sam68 (KHDRBS3), was also observed to be elevated in LPS-treated tumor enteroids, along with the genes GEN1, KRIT1, CENPF, and STYK1. Sam68 (KHDRBS3) exhibited a prognostic significance in various malignancies and was elevated in cancer cell lines [,,]. Sam68 (KHDRBS1), a homolog of KHDRBS3, was found to be co-expressed with cancer-related genes in the genome-wide analysis [,]. Our results suggest the possible roles of GEN1, KRIT1, CENPF, STYK1, and Sam68/KHDRBS3 in promoting cancer cell proliferation, and these findings may provide a potential therapeutic target to control mast cell malignancy.
Even though LPS treatment failed to diminish the expression of genes involved in proliferation and malignancy such as GEN1, KRIT1, CENPF, STYK1, and Sam68/KHDRBS3, it did reduce the expression of numerous genes implicated in tumor growth. LPS treatment, for example, reduced the expression of the LOC610994 (zeta crystallin), Gpatch4, SLC7A1, ATP13A2, and TEX45 genes. Both TEX45 [] and zeta crystallin (CryZ) [] are expressed in several human cancer types including colorectal cancer. While CryZ may protect cancer cells from oxidative stress and help them maintain an optimal DNA complement, it can fuel the cancer requirement for energy by activating glutaminolysis. Furthermore, increased expression and/or activation of CryZ following acidification of the tumor microenvironment may contribute to cancer cell survival and drug resistance by increasing the anti-apoptotic gene expression in cancer cells []. GPATCH4 was identified in melanoma patient sera and was revealed to be increased in hepatocellular carcinoma []. Similarly, the SLC7A1/CAT1 arginine transporter plays a critical function in colorectal cancer by increasing arginine metabolism []. After LPS treatment, another important gene, ATP13A2, was downregulated in tumor enteroids. ATP13A2 regulates autophagy, as demonstrated by ATP13A2 knockdown, decreasing cellular autophagy levels, reversing ATP13A2-induced stemness in colon cancer cells with the autophagy inhibitor bafilomycin A1 [], and the reduction in the volume of colon cancer xenografts in mice treated with ATP13A2 siRNA []. While all of these pieces of evidence point to ATP13A2 and autophagy, studies have also revealed a link between the level of ATP13A2 expression and the survival rate of colon cancer patients. Colon cancer patients with elevated ATP13A2 expression displayed shorter overall survival than those with low ATP13A2 [].
Interestingly, while LPS treatment increased the proliferation and expression of the GEN1, KRIT1, CENPF, CPLANE1, STYK1, and KHDRBS3 genes, it decreased the expression of the cancer associated LOC610994, Gpatch4, SLC7A1, ATP13A2, and TEX45 genes [,,,,] in tumor enteroids. This could imply that LPS has some anticancer activity. LPS treatment has been shown to have potent anticancer effects [,] against adoptively transferred tumors (TA3/Ha murine mammary carcinoma) in mice [,]. LPS and the other two potent TLR4 agonists have been proven to be effective in treating a variety of carcinomas [,]. In addition, research on mice with lung tumors has demonstrated that a modest dose of LPS promotes tumor development, whereas a large amount induces tumor regression []. Plasmacytoid dendritic cells are required for LPS to exert its dose-dependent effects [].
The addition of LPS increased the production of proteins involved in cytokine signaling, most notably interferon and interleukin signaling []. After LPS treatment, we observed the upregulation of genes involved in biotic stimuli and immune effector processes (OAS1, OASL, IFIT1, ISG15) as well as antigen processing and presentation (TAP2, DLA class I) and signal transduction (TFF1, IGFBP1, ISG15). Emerging evidence suggests that the increased activity of these genes contributes to the inflammatory response induced by LPS. For example, LPS and interferons highly activate the ubiquitin-like protein ISG15 and 2′-5′-oligoadenylate synthetase-like (OASL) [,]. OASL is necessary for the antiviral signaling pathway mediated by IFNs [] as it regulates pro-inflammatory mediators such as cytokines and chemokines []. Likewise, OAS1 has a strong antiviral impact both in vivo and in vitro [] and protects cells from viral infection []. When exogenous recombinant OAS1 was added to the cultured cells, it was internalized and exerted a potent antiviral effect []. LPS has also been shown to induce OAS in marine sponges []. TAP2 and trefoil factor 1 (TFF1) upregulation in IBD enteroids and colonoids following LPS treatment could result from their ability to modulate TLR4 signaling or their anti-inflammatory properties [,,]. TAP2 has been shown to inhibit TLR4 signaling and diminish the systemic cytokine response induced by LPS []. TFF1 exhibits anti-inflammatory properties, and its involvement as a tumor suppressor gene has been shown through studies using the Tff1-knockout (Tff1-KO) mouse model [,]. While the Tff1-KO mice developed gastric malignancies via the activation of NF-κB and chronic inflammatory pathways [], treatment with exogenous TFFs alleviated the gastrointestinal inflammation [,]. Insulin-like growth factor-binding protein 1 (IGFBP1) is a member of the GFBPs family of proteins that interact with insulin-like growth factors (IGFs) to regulate their anabolic activity []. While LPS injection reduces the circulating IGF-I levels, it increases the IGFBP-1 levels [,]. LPS has also been shown to induce IGFBP-1 in the liver, muscle, and kidney tissues []. Similarly, NADPH oxidase 1 (NOX1) activity induced by LPS was detected in dendritic cells [], corroborating our current findings. NOX1 requires two additional proteins, NOXO1 and NOXA1, and it interacts with p22phox in a complex []. NOX1 is triggered by the GTPase Rac, which binds directly to it or via the NOXA1 TPR domain []. Genes such as CP and GPX1, involved in the oxidation–reduction process and cellular detoxification, were expressed more abundantly in the IBD intestine organoids following LPS treatment. The generation of reactive oxygen species (ROS) during the innate immune response to LPS is one of the anti-pathogen responses that can cause oxidative damage, and GPx-1, an antioxidant enzyme [,], can protect the intestine from such damage. Gpx-1 facilitates the generation of pro-inflammatory cytokines in response to LPS exposure []. GPx1 has been shown to regulate LPS-induced adhesion molecule expression in endothelial cells through modulating CD14 expression. Suppression of GPx-1 promotes the expression of the CD14 gene in human microvascular endothelial cells []. GPx-1 deficiency increases LPS-induced intracellular ROS and CD14 and intercellular adhesion molecule-1 (ICAM-1) expression []. Studies have shown a connection between LPS stimulation and NO production and the Ceruloplasmin (CP) activity []. Without changing the iNOS expression levels, CP enhances the LPS-activated iNOS activity. An unknown Cp receptor activates this intracellular signaling that cross-talks with the response stimulated by LPS []. Members of the S100 family, S100P and S100A16, were also induced by LPS stimulation and IBD; a similar induction of S100 protein members and their role in the regulation of inflammation have been reported in previous studies [,]. Furthermore, our current research shows the associations between the S100 proteins and IBD, with LPS and IBD both stimulating S100A16 activity. Our present study, along with earlier reports [], points to the potential use of S100 markers for diagnostic purposes, specifically S100A16 in IBD. The use of S100 A proteins in canine feces as biomarkers of inflammatory activity has previously been reported []. The transmembrane ion pump Na+/K+-ATPase (ATP1A1) has been linked to nuclear factor kappa B (NFκB) signaling [], a signal associated with the LPS induced immune response. While α2Na+/K+-ATPase haploinsufficiency was reported to regulate LPS-induced immune responses negatively, we observed that ATP1A1 was suppressed in both the IBD enteroids and colonoids treated with LPS in the present study. Nonetheless, our demonstration of the inhibited expression of ATP1A1 in IBD intestinal organoids identifies it as a promising candidate for further analysis.
The GI tract microbial ecology varies according to its microbial diversity and anatomic location. Oxygen tension is a primary determinate of microbial numbers and complexity []. The upper GI tract, stomach, and small intestine have a lower pH, shorter transit time, and reduced bacterial population. However, the colon harbors the greatest diversity of bacteria as it has a low cell turnover rate, a low redox potential, and a longer transit time []. As a result of the LPS reaction, the gene expression profile of enteroids and colonoids from the IBD dogs also altered. Twenty DEGs were identified as exhibiting an opposite expression trend (i.e., upregulated in IBD enteroids and downregulated in IBD colonoids and vice versa) following LPS stimulation. These 20 unique signature genes might be used to differentiate the intestinal regions in response to LPS stimulation.
The differential expression of TOMM20 and eIF3F between the IBD enteroids and colonoids may be a reason why IBD enteroids with a higher TOMM20 expression and lower eIF3F expression proliferated more than the colonoids (as indicated by Ki-67 expression and number of crypts/organoid). Mitochondrial protein, translocase of the outer mitochondrial membrane complex subunit 20 (TOMM20), promotes the proliferation and resistance to apoptosis and serves as a marker of mitophagy activity []. Reduced TOMM20 expression in response to LPS treatment implies that LPS activates mitophagy [], resulting in decreased proliferation in the IBD colonoids (as observed in the current study). In the tumor cells, enhanced eIF3F expression inhibits translation, cell growth, and cell proliferation and induces apoptosis, whereas knockdown of eIF3f inhibits apoptosis, showing the role of eIF3f as an essential negative regulator of cell growth and proliferation [,]. Furthermore, the expression of FAM168A(TCRP1) in IBD enteroids implies that IBD enteroids are more protected than colonoids against LPS stimulation. FAM168A operates via the PI3K/AKT/NFKB signaling pathway and has previously been shown to protect cells against apoptosis [].
Several genes that participated in RNA metabolism, protein synthesis, import, protein complex assembly and proteolysis, anion transport, adaptive immune response, and apoptosis were also differentially expressed in the LPS-treated IBD colonoids and enteroids. For instance, LPS treatment enhanced the expression of SF3A1, S100P, CRIP1, ANXA1, and RGS2 in the IBD colonoids. The presence of SF3A and SF3B is required for a robust innate immune response to LPS and other TLR agonists []. SF3A1, a member of the SF3A complex, regulates LPS-induced IL-6 by primarily inhibiting its production []. Similarly, S100 proteins are known to be secreted in response to TLR-4 activation. S100 proteins also influence proliferation, differentiation, and apoptosis, in addition to inflammation []. Earlier reports and our recent observation of enhanced cysteine-rich intestinal protein 1 (CRIP1) expression in response to LPS suggest that CRIP may play a role in immune cell activation or differentiation []. While CRIP1 is abundant in the intestine [], it is abnormally expressed in certain types of tumor []. CRIP1 inhibits the expression of Fas and proteins involved in Fas-mediated apoptosis []. LPS activates the AnxA1 gene significantly, and in the absence of AnxA1, LPS induces a dysregulated cellular and cytokine response with a high degree of leukocyte adhesion. The protective role of AnxA1 was demonstrated in AnxA1-deficient mice. In AnxA1-deficient mice, LPS induced a toxic response manifested by organ injury and lethality, restored by the exogenous administration of AnxA1 [,]. In contrast, following LPS treatment, GTF2A2 expression was increased in the IBD enteroids. GTF2A2 is required for NF-kB signaling in the LPS-induced TNF responsive module []. Additionally, LPS promoted HSPD1 and Cyr61 expression in the IBD enteroids. Cyr61 is known to be activated by LPS and may have pleiotropic responses to LPS []. Human hsp60 directly promotes nitrite production and cytokine synthesis in macrophages. Human hsp60 was found to synergize with IFN-γ in its proinflammatory activity []. The inflammatory response to LPS was evaluated in RGS2−/− mice, which exhibited a higher expression of TNF and phosphorylated p38 levels in cardiomyocytes. This study demonstrates that RGS2 plays a function in cardioprotection and anti-inflammatory signaling via p38 []. RGS2 inhibits G protein-coupled receptor signaling by increasing the rate of G protein deactivation or by decreasing the G protein–effector interactions [].
The KEGG [,,,] pathway analysis showed the involvement of several metabolic pathway genes in the IBD intestinal organoids and tumor enteroids. For example, BAAT, which is involved in primary bile acid biosynthesis, taurine and hypotaurine metabolism, biosynthesis of unsaturated fatty acids, peroxisome, and bile secretion pathways, was identified. Bile acids (BAs) play key roles in intestinal metabolism and cell signaling and changes in BAs have also been demonstrated to influence gut homeostasis and contribute to the pathogenesis of IBD []. Several BA receptors have been reported to be involved in the development of colorectal cancer, cholangiocellular carcinoma, or their association with cancer cell lines []. The gene ANPEP/LOC112653425, involved in glutathione metabolism, the renin–angiotensin system, and hematopoietic cell lineage, was found to be one of the most prevalent DEGs in IBD enteroids and tumor enteroids. High levels of CD13/ANPEP expression have been observed in a variety of solid tumors []; additionally, CD13 has been shown to be associated with malignant activity in colon and prostate cancers []. Soluble CD13/ANPEP has several pro-inflammatory functions via its interaction with G protein-coupled receptors. CD13 is significant in the etiology of numerous inflammatory disorders by regulating the growth and activity of immune-related cells as well as functions of inflammatory mediators []. It has been suggested that IBD inflammation may be exacerbated by a disruption of the renin–angiotensin system []. Additionally, inflammation contributes significantly to the etiology of Alzheimer’s disease (AD) by activating C/EBPβ/δ-secretase and causing AD-associated disorders in the gut, which are then transferred to the brain via the vagus nerve []. BACE1 and BACE2 β-secretases have been widely investigated in the context of Alzheimer’s disease. Specifically, BACE2 is overexpressed in tumors. BACE2 increases tumor growth by hyperactivating the NF-κB pathway via a series of phosphorylation cascades of different members of this pathway []. We also identified that BACE2, participating in the Alzheimer’s disease pathway, was one of the top DEGs in both the IBD and tumor enteroids in the current study.
Additionally, LPS treatment altered the ACSL5, ENPP6, and CA1 expression in the intestine and tumor enteroids. ACSL5 is required for the de novo synthesis of lipids and fatty acid degradation, and its role in inflammation and cancer development has been reported. ACSL5 interacts with proapoptotic molecules and suppresses proliferation []. Following LPS treatment, genes involved in the glutathione metabolism pathway were significantly altered in serum, whereas genes involved in the bile acid biosynthesis pathway were significantly changed in numerous rat tissues []. LPS treatment also altered the expression of arachidonic acid, glutathione, and the nitrogen metabolic pathway genes in the IBD enteroids and colonoids. Earlier reports on the effects of LPS on nitrogen metabolism (CA1) in beef steers [] and altered expression of the ABCB transporters associated with IBD [] also corroborate the current study. The arachidonic acid (AA) pathway is implicated in a variety of inflammatory diseases [,]. The glutathione (GSH) pathway is a critical metabolic integrator in T-cell-mediated inflammatory responses []. The metabolism of AA results in the generation of reactive oxygen species (ROS) []. GSH and its metabolic enzymes protect tissues from oxidative damage [,,,,,,,,,,]. By interacting in both the AA and GSH pathways, GPX1 is a critical determinant of the AA effects []. Additionally, we identified changes in the expression of genes implicated in these metabolic pathways in the LPS-stimulated IBD intestinal organoids.
The peroxisomal metabolic gene, BAAT, was found to be among the top DEGs in both the IBD intestinal organoids and tumor enteroids. Peroxisomes have been implicated in the pathogenesis of inflammation and cancer. Peroxisomes are known to operate as immunometabolic hubs, producing and metabolizing ROS as well as unsaturated fatty acids such as docosahexaenoic acid (DHA), which can affect the inflammatory pathways [,]. Peroxisomal lipid production and redox balance can aid in the survival of cancer cells in the tumor microenvironment. The disruption of the interplay between peroxisomes and other cellular organelles such as the endoplasmic reticulum and the mitochondria has the potential to rewire the metabolism of cancer cells [].
Organoids are confined to mimic organ-specific or tissue-specific micro-physiology, a constraint that should be considered before joining this promising new field. The lack of inter-organ communication is a major weakness of organoid systems. On the other hand, initiatives are already underway to circumvent this constraint. For instance, multiple organoids have been linked together to explore the gastrointestinal tract–liver–pancreas connection []. Despite the lingering difficulties, organoids retain significant promise for clinical translational research []. The scope of organoid technology has extended to include genetic manipulation, diverse omics, and drug-screening studies and a diversified co-culture system involving viruses, bacteria, and parasites. In the near future, the combination of organoid technology with single-cell transcriptomics will have a significant impact on personalized medicine.
5. Conclusions
The current study provides new and comprehensive data describing how LPS induces differential gene expression in intestinal organoids derived from dogs with chronic intestinal inflammation and small intestinal cancer. The crosstalk between the LPS/TLR4 signal transduction pathway and other metabolic pathways such as primary bile acid biosynthesis and secretion, peroxisome, renin–angiotensin system, glutathione metabolism, arachidonic acid, and Alzheimer’s disease pathways demonstrates that LPS plays a key role in chronic inflammation and carcinogenesis. In contrast, we observed contradictory effects of LPS including the increased proliferation and expression of several tumor-associated genes and the decreased expression of other cancer-associated genes in tumor enteroids including LOC610994 (Zeta Crystallin), Gpatch4, SLC7A1, ATP13A2, and TEX45. In summary, this study may pave the way for the development of novel anti-inflammatory and anticancer therapeutics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14143525/s1, Figure S1. Heatmaps (a) Heatmap of the differentially expressed genes in IBD enteroids; (b) Heatmap of the differentially expressed genes in IBD colonoids; (c) Heatmap of the differentially expressed genes in tumor enteroids; Table S1. Microarray analysis results. (a) LPS treated tumor enteroids versus Control tumor enteroids; (b) LPS treated IBD enteroids versus Control IBD enteroids; (c) LPS treated IBD colonoids versus Control IBD colonoids; (d) Control IBD colonoids versus Control IBD enteroids; (e) Control IBD enteroids versus Control tumor enteroids; (f) LPS treated IBD colonoids versus LPS treated IBD enteroids; (g) LPS treated IBD enteroids versus LPS treated tumor enteroids. The log-ratio M values represent log(R/G) (log fold change).
Author Contributions
Conceptualization, D.K.S., A.E.J., M.M., K.A. and J.P.M.; methodology, D.K.S., D.C.B. and L.C.; software, A.J.S. and D.K.S.; validation, D.K.S., D.C.B., L.C. and T.A.; formal analysis, D.K.S., D.C.B. and L.C.; investigation, D.K.S., D.C.B., L.C., T.A., A.B.-M., N.M.E., E.S.; resources, D.K.S., D.C.B. and L.C.; data curation, D.K.S., D.C.B., L.C. and A.J.S.; writing—original draft preparation, D.K.S.; writing—review and editing, D.K.S., D.C.B., L.C., A.E.J., A.J.S., K.A. and J.P.M.; visualization, D.K.S., D.C.B. and L.C.; supervision, A.E.J., K.A. and J.P.M.; project administration, A.E.J., K.A. and J.P.M.; funding acquisition, K.A. and J.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Departmental Research Start-Up Grant 706-05-02, Iowa State University to KA, Miller Research Award, Office of the Vice-President for Research, Iowa State University to JPM, and the APC was funded by the Publication Subvention Grants Program –For Open Access Journal Articles, Office of the Vice-President for Research, Iowa State University.
Institutional Review Board Statement
The animal study protocol was approved by the Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC-19-102; PI: Albert E. Jergens).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Table and Figure.
Acknowledgments
The authors acknowledge the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center (P30 CA43703) for their support in the microarray experiment and the Department of Veterinary Clinical Sciences (VCS) Core Lab for GraphPad Prism 9 and BioRender program support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heinbockel, L.; Weindl, G.; Martinez-de-Tejada, G.; Correa, W.; Sanchez-Gomez, S.; Bárcena-Varela, S.; Goldmann, T.; Garidel, P.; Gutsmann, T.; Brandenburg, K. Inhibition of Lipopolysaccharide- and Lipoprotein-Induced Inflammation by Antitoxin Peptide Pep19-2.5. Front. Immunol. 2018, 9, 1704. [Google Scholar] [CrossRef] [PubMed]
- Im, E.; Riegler, F.M.; Pothoulakis, C.; Rhee, S.H. Elevated Lipopolysaccharide in the Colon Evokes Intestinal Inflammation, Aggravated in Immune Modulator-Impaired Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G490–G497. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Chin, A.C.; Flynn, A.N.; Fedwick, J.P.; Buret, A.G. The Role of Caspase-3 in Lipopolysaccharide-Mediated Disruption of Intestinal Epithelial Tight Junctions. Can. J. Physiol. Pharmacol. 2006, 84, 1043–1050. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Grimstad, O. Tumor Necrosis Factor and the Tenacious α. JAMA Dermatol. 2016, 152, 557. [Google Scholar] [CrossRef] [PubMed]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR-4 Signal Transduction Pathway Activation of FAK and MyD88. J. Immunol. 2015, 195, 4999. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-Induced Cytokine Production in Human Monocytes and Macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Ueda, Y.; Kayama, H.; Jeon, S.G.; Kusu, T.; Isaka, Y.; Rakugi, H.; Yamamoto, M.; Takeda, K. Commensal Microbiota Induce LPS Hyporesponsiveness in Colonic Macrophages via the Production of IL-10. Int. Immunol. 2010, 22, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Takeda, K. Functions of Innate Immune Cells and Commensal Bacteria in Gut Homeostasis. J. Biochem. 2016, 159, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, Y.D.; Xin, M.; ye Lim, J.; Lee, Y.M.; Kim, D.K. Mast Cell Modulates Tumorigenesis Caused by Repeated Bowel Inflammation Condition in Azoxymethane/Dextran Sodium Sulfate-Induced Colon Cancer Mouse Model. Biochem. Biophys. Rep. 2022, 30, 101253. [Google Scholar] [CrossRef]
- Crivellato, E.; Beltrami, C.A.; Mallardi, F.; Ribatti, D. Paul Ehrlich’s Doctoral Thesis: A Milestone in the Study of Mast Cells. Br. J. Haematol. 2003, 123, 19–21. [Google Scholar] [CrossRef]
- Sakalauskaitė, S.; Riškevičienė, V.; Šengaut, J.; Juodžiukynienė, N. Association of Mast Cell Density, Microvascular Density and Endothelial Area with Clinicopathological Parameters and Prognosis in Canine Mammary Gland Carcinomas. Acta Vet. Scand. 2022, 64, 14. [Google Scholar] [CrossRef]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; de Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, MicroRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]
- Hand, T.W.; Vujkovic-Cvijin, I.; Ridaura, V.K.; Belkaid, Y. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol. Metab. 2016, 27, 831–843. [Google Scholar] [CrossRef]
- Liu, W.-T.; Jing, Y.-Y.; Yan, F.; Han, Z.-P.; Lai, F.-B.; Zeng, J.-X.; Yu, G.-F.; Fan, Q.-M.; Li, R.; Zhao, Q.-D.; et al. LPS-Induced CXCR4-Dependent Migratory Properties and a Mesenchymal-like Phenotype of Colorectal Cancer Cells. Cell Adhes. Migr. 2017, 11, 13–23. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, Q.; Huang, Y.; Zheng, W.; Hua, J.; Yang, S.; Zhuang, J.; Wang, J.; Ye, J. Lipopolysaccharide Increases the Release of VEGF-C That Enhances Cell Motility and Promotes Lymphangiogenesis and Lymphatic Metastasis through the TLR4-NF-ΚB/JNK Pathways in Colorectal Cancer. Oncotarget 2016, 7, 73711–73724. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Tel, J.; Sliepen, K.H.E.W.J.; Benitez-Ribas, D.; Figdor, C.G.; Adema, G.J.; de Vries, I.J.M. Toll-like Receptor Expression and Function in Human Dendritic Cell Subsets: Implications for Dendritic Cell-Based Anti-Cancer Immunotherapy. Cancer Immunol. Immunother. 2010, 59, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B.; Vasquez-Dunddel, D.; Fu, J.; Albesiano, E.; Pardoll, D.; Kim, Y.J. Intratumoral Administration of TLR4 Agonist Absorbed into a Cellular Vector Improves Anti-Tumor Responses. Clin. Cancer Res. 2011, 17, 3984. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liao, M.; Wang, J. TLR4 Signaling in the Development of Colitis-Associated Cancer and Its Possible Interplay with MicroRNA-155. Cell Commun. Signal. 2021, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Narrandes, S.; Xu, W. Gene Expression Detection Assay for Cancer Clinical Use. J. Cancer 2018, 9, 2249. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Houlgatte, R.; Nguyen, C.; Viens, P.; Jordan, B.R.; Birnbaum, D. Gene Expression Profiling of Cancer by Use of DNA Arrays: How Far from the Clinic? Lancet Oncol. 2001, 2, 674–682. [Google Scholar] [CrossRef]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199. [Google Scholar] [CrossRef]
- Shearin, A.L.; Ostrander, E.A. Leading the Way: Canine Models of Genomics and Disease. Dis. Models Mech. 2010, 3, 27. [Google Scholar] [CrossRef]
- Kathrani, A.; Werling, D.; Allenspach, K. Canine Breeds at High Risk of Developing Inflammatory Bowel Disease in the South-Eastern UK. Vet. Rec. 2011, 169, 635. [Google Scholar] [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of Adult Canine Intestinal Organoids for Translational Research in Gastroenterology. BMC Biol. 2019, 17, 33. [Google Scholar] [CrossRef]
- Mochel, J.P.; Jergens, A.E.; Kingsbury, D.; Kim, H.J.; Martín, M.G.; Allenspach, K. Intestinal Stem Cells to Advance Drug Development, Precision, and Regenerative Medicine: A Paradigm Shift in Translational Research. AAPS J. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.; Young, J.; Moore, D.; Wang, C.; Hostetter, J.; Augustine, L.; Allenspach, K.; Schmitz, S.; Mosher, C. Bcl-2/Caspase 3 Mucosal Imbalance Favors T Cell Resistance to Apoptosis in Dogs with Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2014, 158, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kopper, J.; Iennarella-Servantez, C.; Jergens, A.E.; Sahoo, D.K.; Guillot, E.; Bourgois Mochel, A.; Martinez, M.; Allenspach, K.; Mochel, J.P. Harnessing the Biology of Canine Intestinal Organoids to Heighten Understanding of Inflammatory Bowel Disease Pathogenesis and Accelerate Drug Discovery: A One Health Approach. Front. Toxicol. 2021, 3, 773953. [Google Scholar] [CrossRef]
- Kraiczy, J.; Nayak, K.M.; Howell, K.J.; Ross, A.; Forbester, J.; Salvestrini, C.; Mustata, R.; Perkins, S.; Andersson-Rolf, A.; Leenen, E.; et al. DNA Methylation Defines Regional Identity of Human Intestinal Epithelial Organoids and Undergoes Dynamic Changes during Development. Gut 2019, 68, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Dean Befus, A.; Kulka, M. Mast Cell Mediators: Their Differential Release and the Secretory Pathways Involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef]
- Fish, R.E. How to Work with Your Institutional Animal Care and Use Committee (IACUC). 2004. Available online: https://ori.hhs.gov/education/products/ncstate/iacuc.htm (accessed on 29 November 2018).
- Blutt, S.E.; Crawford, S.E.; Ramani, S.; Zou, W.Y.; Estes, M.K. Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 241–251. [Google Scholar] [CrossRef]
- Chen, J.; Rao, J.N.; Zou, T.; Liu, L.; Marasa, B.S.; Xiao, L.; Zeng, X.; Turner, D.J.; Wang, J.-Y. Polyamines Are Required for Expression of Toll-like Receptor 2 Modulating Intestinal Epithelial Barrier Integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 568–576. [Google Scholar] [CrossRef]
- Yücel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.-H.; Cyganek, L.; Utikal, J.; et al. Lipopolysaccharides Induced Inflammatory Responses and Electrophysiological Dysfunctions in Human-Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 2935. [Google Scholar] [CrossRef]
- Yin, P.; Zou, W.; Li, J.; Jin, N.; Gao, Q.; Liu, F. Using High-Throughput Sequencing to Explore the Anti-Inflammatory Effects of α-Mangostin. Sci. Rep. 2019, 9, 15626. [Google Scholar] [CrossRef]
- Chandra, L.C.; Kumar, V.; Torben, W.; Van de Stouwe, C.; Winsauer, P.; Amedee, A.; Molina, P.E.; Mohan, M. Chronic Administration of Δ 9-Tetrahydrocannabinol Induces Intestinal Anti-Inflammatory MicroRNA Expression during Acute Simian Immunodeficiency Virus Infection of Rhesus Macaques. J. Virol. 2015, 89, 1168–1181. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wettenhall, J.M.; Simpson, K.M.; Satterley, K.; Smyth, G.K. AffylmGUI: A Graphical User Interface for Linear Modeling of Single Channel Microarray Data. Bioinformatics 2006, 22, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Draghici, S. Analysis of Microarray Experiments of Gene Expression Profiling. Am. J. Obstet. Gynecol. 2006, 195, 373. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward Understanding the Origin and Evolution of Cellular Organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A Universal Tool for Annotation, Visualization and Analysis in Functional Genomics Research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- BioBam Bioinformatics OmicsBox—Bioinformatics Made Easy 2019. 3 March 2019. Available online: https://www.biobam.com/omicsbox/?cn-reloaded=1 (accessed on 5 May 2020).
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Affymetrix GeneChip® Canine Genome Array. Available online: http://www.affymetrix.com/products_services/arrays/specific/canine.affx#1_2 (accessed on 5 May 2020).
- Jakobsson, M.E.; Ma-Lecki, J.; Nilges, B.S.; Moen, A.; Leidel, S.A.; Falnes, P. Methylation of Human Eukaryotic Elongation Factor Alpha (EEF1A) by a Member of a Novel Protein Lysine Methyltransferase Family Modulates mRNA Translation. Nucleic Acids Res. 2017, 45, 8239–8254. [Google Scholar] [CrossRef]
- Kespohl, M.; Bredow, C.; Klingel, K.; Voß, M.; Paeschke, A.; Zickler, M.; Poller, W.; Kaya, Z.; Eckstein, J.; Fechner, H.; et al. Protein Modification with ISG15 Blocks Coxsackievirus Pathology by Antiviral and Metabolic Reprogramming. Sci. Adv. 2020, 6, eaay1109. [Google Scholar] [CrossRef] [PubMed]
- Grebenjuk, V.A.; Kuusksalu, A.; Kelve, M.; Schütze, J.; Schröder, H.C.; Müller, W.E.G. Induction of (2′-5′)Oligoadenylate Synthetase in the Marine Sponges Suberites Domuncula and Geodia Cydonium by the Bacterial Endotoxin Lipopolysaccharide. Eur. J. Biochem. 2002, 269, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, F.; Chen, S.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Sun, K.; Yang, Q.; Wu, Y.; et al. Identification of 2′-5′-Oligoadenylate Synthetase-Like Gene in Goose: Gene Structure, Expression Patterns, and Antiviral Activity Against Newcastle Disease Virus. J. Interferon Cytokine Res. 2016, 36, 563–572. [Google Scholar] [CrossRef] [PubMed]
- d’Aldebert, E.; Quaranta, M.; Sébert, M.; Bonnet, D.; Kirzin, S.; Portier, G.; Duffas, J.P.; Chabot, S.; Lluel, P.; Allart, S.; et al. Characterization of Human Colon Organoids From Inflammatory Bowel Disease Patients. Front. Cell Dev. Biol. 2020, 8, 363. [Google Scholar] [CrossRef]
- Arnauts, K.; Verstockt, B.; Ramalho, A.S.; Vermeire, S.; Verfaillie, C.; Ferrante, M. Ex Vivo Mimicking of Inflammation in Organoids Derived from Patients with Ulcerative Colitis. Gastroenterology 2020, 159, 1564–1567. [Google Scholar] [CrossRef]
- Niklinska-Schirtz, B.J.; Venkateswaran, S.; Anbazhagan, M.; Kolachala, V.L.; Prince, J.; Dodd, A.; Chinnadurai, R.; Gibson, G.; Denson, L.A.; Cutler, D.J.; et al. Ileal Derived Organoids From Crohn’s Disease Patients Show Unique Transcriptomic and Secretomic Signatures. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1267. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A Single-Cell Survey of the Small Intestinal Epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Middendorp, S.; Schneeberger, K.; Wiegerinck, C.L.; Mokry, M.; Akkerman, R.D.L.; van Wijngaarden, S.; Clevers, H.; Nieuwenhuis, E.E.S. Adult Stem Cells in the Small Intestine are Intrinsically Programmed with Their Location-Specific Function. Stem Cells 2014, 32, 1083–1091. [Google Scholar] [CrossRef]
- van der Hee, B.; Madsen, O.; Vervoort, J.; Smidt, H.; Wells, J.M. Congruence of Transcription Programs in Adult Stem Cell-Derived Jejunum Organoids and Original Tissue during Long-Term Culture. Front. Cell Dev. Biol. 2020, 8, 375. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Sprangers, J.; Zaalberg, I.C.; Maurice, M.M. Organoid-Based Modeling of Intestinal Development, Regeneration, and Repair. Cell Death Differ. 2020, 28, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 Is a Promising Molecular Target in the Diagnosis of Cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Sándor, G.O.; Soós, A.Á.; Lörincz, P.; Rojkó, L.; Harkó, T.; Bogyó, L.; Tölgyes, T.; Bursics, A.; Buzás, E.I.; Moldvay, J.; et al. Wnt Activity and Cell Proliferation Are Coupled to Extracellular Vesicle Release in Multiple Organoid Models. Front. Cell Dev. Biol. 2021, 9, 1597. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L.; Lui, A.; Squerzanti, S.; Castaldelli, G.; Shinn, A.P.; Manera, M.; Lorenzoni, M. Proliferative Cell Nuclear Antigen (PCNA) Expression in the Intestine of Salmo Trutta Trutta Naturally Infected with an Acanthocephalan. Parasites Vectors 2012, 5, 198. [Google Scholar] [CrossRef] [PubMed]
- Karim, B.O.; Rhee, K.J.; Liu, G.; Yun, K.; Brant, S.R. Prom1 Function in Development, Intestinal Inflammation, and Intestinal Tumorigenesis. Front. Oncol. 2014, 4, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, S.H.; Zhang, Y.N.; Xu, C.F. Olfactomedin-4 in Digestive Diseases: A Mini-Review. World J. Gastroenterol. 2018, 24, 1881. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, L.; Chen, S. Olfactomedin 4, a Novel Marker for the Differentiation and Progression of Gastrointestinal Cancers. Neoplasma 2011, 58, 9–13. [Google Scholar] [CrossRef]
- Ueda, S.; Kondoh, N.; Tsuda, H.; Yamamoto, S.; Asakawa, H.; Fukatsu, K.; Kobayashi, T.; Yamamoto, J.; Tamura, K.; Ishida, J.; et al. Expression of Centromere Protein F (CENP-F) Associated with Higher FDG Uptake on PET/CT, Detected by CDNA Microarray, Predicts High-Risk Patients with Primary Breast Cancer. BMC Cancer 2008, 8, 384. [Google Scholar] [CrossRef]
- Landberg, G.; Erlanson, M.; Roos, G.; Tan, E.M.; Casiano, C.A. Nuclear Autoantigen p330d/CENP-F: A Marker for Cell Proliferation in Human Malignancies. Cytometry 1996, 25, 90–98. [Google Scholar] [CrossRef]
- Oono, K.; Takahashi, K.; Sukehara, S.; Kurosawa, H.; Matsumura, T.; Taniguchi, S.; Ohta, S. Inhibition of PC3 Human Prostate Cancer Cell Proliferation, Invasion and Migration by Eicosapentaenoic Acid and Docosahexaenoic Acid. Mol. Clin. Oncol. 2017, 7, 217. [Google Scholar] [CrossRef]
- Shahid, M.; Lee, M.Y.; Piplani, H.; Andres, A.M.; Zhou, B.; Yeon, A.; Kim, M.; Kim, H.L.; Kim, J. Centromere Protein F (CENPF), a Microtubule Binding Protein, Modulates Cancer Metabolism by Regulating Pyruvate Kinase M2 Phosphorylation Signaling. Cell Cycle 2018, 17, 2802–2818. [Google Scholar] [CrossRef] [PubMed]
- Rass, U.; Compton, S.A.; Matos, J.; Singleton, M.R.; Ip, S.C.Y.; Blanco, M.G.; Griffith, J.D.; West, S.C. Mechanism of Holliday Junction Resolution by the Human GEN1 Protein. Genes Dev. 2010, 24, 1559–1569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, L.; Zhang, Y.; Pan, Z.; Li, B.; Sun, M.; Zhang, X. Expression and Localization of GEN1 in Mouse Mammary Epithelial Cells. J. Biochem. Mol. Toxicol. 2014, 28, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ezzell, R.M.; Warren, H.S. Localization of Endotoxin in the Rat Intestinal Epithelium. J. Infect. Dis. 2000, 182, 873–881. [Google Scholar] [CrossRef]
- Hu, Y.P.; Wu, Z.B.; Jiang, L.; Jin, Y.P.; Li, H.F.; Zhang, Y.J.; Ma, Q.; Ye, Y.Y.; Wang, Z.; Liu, Y.C.; et al. STYK1 Promotes Cancer Cell Proliferation and Malignant Transformation by Activating PI3K-AKT Pathway in Gallbladder Carcinoma. Int. J. Biochem. Cell Biol. 2018, 97, 16–27. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Li, Y.; Xiong, H.; Wu, J.; Li, J.; Li, M. Sam68 Up-Regulation Correlates with, and Its down-Regulation Inhibits, Proliferation and Tumourigenicity of Breast Cancer Cells. J. Pathol. 2010, 222, 227–237. [Google Scholar] [CrossRef]
- Sumithra, B.; Saxena, U.; Das, A.B. A Comprehensive Study on Genome-Wide Coexpression Network of KHDRBS1/Sam68 Reveals Its Cancer and Patient-Specific Association. Sci. Rep. 2019, 9, 11083. [Google Scholar] [CrossRef]
- Ukai, S.; Sakamoto, N.; Taniyama, D.; Harada, K.; Honma, R.; Maruyama, R.; Naka, K.; Hinoi, T.; Takakura, Y.; Shimizu, W.; et al. KHDRBS3 Promotes Multi-Drug Resistance and Anchorage-Independent Growth in Colorectal Cancer. Cancer Sci. 2021, 112, 1196–1208. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Lulli, M.; Nencioni, D.; Papucci, L.; Schiavone, N. Zeta-Crystallin: A Moonlighting Player in Cancer. Cell. Mol. Life Sci. 2019, 77, 965–976. [Google Scholar] [CrossRef]
- Blotta, S.; Tassone, P.; Prabhala, R.H.; Tagliaferri, P.; Cervi, D.; Amin, S.; Jakubikova, J.; Tai, Y.T.; Podar, K.; Mitsiades, C.S.; et al. Identification of Novel Antigens with Induced Immune Response in Monoclonal Gammopathy of Undetermined Significance. Blood 2009, 114, 3276–3284. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, W.; Wang, J.; Yang, C.; Mao, H.; Fu, X.; Wu, Y.; Cai, J.; Han, J.; Xu, Z.; et al. Overexpression of Arginine Transporter CAT-1 Is Associated with Accumulation of L-Arginine and Cell Growth in Human Colorectal Cancer Tissue. PLoS ONE 2013, 8, e73866. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhong, L.; Zhou, C.; Feng, Y.; Liu, Q.-X.; Zhou, D.; Lu, X.; Du, G.-S.; Jian, D.; Luo, H.; et al. Knockdown of Parkinson’s Disease-Related Gene ATP13A2 Reduces Tumorigenesis via Blocking Autophagic Flux in Colon Cancer. Cell Biosci. 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Goto, M.; Hashimoto, K.; Imazawa, M.; Takahashi, M.; Oh-Iwa, I.; Shimozato, K.; Nagao, T.; Nomoto, S. Salivary CPLANE1 Levels as a Biomarker of Oral Squamous Cell Carcinoma. Anticancer Res. 2021, 41, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Schmid, P.; Mackensen, A.; Wehr, U.; Seiz, A.; Braun, M.; Galanos, C.; Mertelsmann, R.; Engelhardt, R. Phase II Trial of Intravenous Endotoxin in Patients with Colorectal and Non-Small Cell Lung Cancer. Eur. J. Cancer 1996, 32, 1712–1718. [Google Scholar] [CrossRef]
- Wiemann, B.; Starnes, C.O. Coley’s Toxins, Tumor Necrosis Factor and Cancer Research: A Historical Perspective. Pharmacol. Ther. 1994, 64, 529–564. [Google Scholar] [CrossRef]
- Rega, A.; Terlizzi, M.; Luciano, A.; Forte, G.; Crother, T.R.; Arra, C.; Arditi, M.; Pinto, A.; Sorrentino, R. Plasmacytoid Dendritic Cells Play a Key Role in Tumor Progression in Lipopolysaccharide-Stimulated Lung Tumor–Bearing Mice. J. Immunol. 2013, 190, 2391–2402. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Medzhitov, R. Toll-like Receptors and Cancer. Nat. Rev. Cancer 2009, 9, 57–63. [Google Scholar] [CrossRef]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR Agonists: Our Best Frenemy in Cancer Immunotherapy. J. Leukoc. Biol. 2013, 93, 847. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Arya, S.; Wiatrek-Moumoulidis, D.; Synowsky, S.A.; Shirran, S.L.; Botting, C.H.; Powis, S.J.; Stewart, A.J. Quantitative Proteomic Changes in LPS-Activated Monocyte-Derived Dendritic Cells: A SWATH-MS Study. Sci. Rep. 2019, 9, 4343. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, H.; Scherer, C.A.; McVean, M.; Iadonato, S.P.; Vends, S.; Thavachelvam, K.; Steffensen, T.B.; Horan, K.A.; Kuri, T.; Weber, F.; et al. Extracellular 2′-5′ Oligoadenylate Synthetase Stimulates RNase L-Independent Antiviral Activity: A Novel Mechanism of Virus-Induced Innate Immunity. J. Virol. 2010, 84, 11898. [Google Scholar] [CrossRef] [PubMed]
- Rysiecki, G.; Gewert, D.R.; Williams, B.R. Constitutive Expression of a 2′,5′-Oligoadenylate Synthetase CDNA Results in Increased Antiviral Activity and Growth Suppression. J. Interferon Res. 1989, 9, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, R.; Baron, J.; Dagtekin, G.; Jahnen-Dechent, W.; Zwadlo-Klarwasser, G. Differential Regulation of the Expression of Transporters Associated with Antigen Processing, TAP1 and TAP2, by Cytokines and Lipopolysaccharide in Primary Human Macrophages. Inflamm. Res. 2002, 51, 403–408. [Google Scholar] [CrossRef]
- Soutto, M.; Belkhiri, A.; Piazuelo, M.B.; Schneider, B.G.; Peng, D.F.; Jiang, A.; Washington, M.K.; Kokoye, Y.; Crowe, S.E.; Zaika, A.; et al. Loss of TFF1 Is Associated with Activation of NF-ΚB-Mediated Inflammation and Gastric Neoplasia in Mice and Humans. J. Clin. Investig. 2011, 121, 1753–1767. [Google Scholar] [CrossRef]
- Eletto, D.; Vllahu, M.; Mentucci, F.; Del Gaudio, P.; Petrella, A.; Porta, A.; Tosco, A. TFF1 Induces Aggregation and Reduces Motility of Helicobacter Pylori. Int. J. Mol. Sci. 2021, 22, 1851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, Y. Transgenic and Gene Knockout Mice in Gastric Cancer Research. Oncotarget 2017, 8, 3696. [Google Scholar] [CrossRef]
- Obermayr, E.; Sanchez-Cabo, F.; Tea, M.K.M.; Singer, C.F.; Krainer, M.; Fischer, M.B.; Sehouli, J.; Reinthaller, A.; Horvat, R.; Heinze, G.; et al. Assessment of a Six Gene Panel for the Molecular Detection of Circulating Tumor Cells in the Blood of Female Cancer Patients. BMC Cancer 2010, 10, 666. [Google Scholar] [CrossRef]
- Tran, C.P.; Cook, G.A.; Yeomans, N.D.; Thim, L.; Giraud, A.S. Trefoil Peptide TFF2 (Spasmolytic Polypeptide) Potently Accelerates Healing and Reduces Inflammation in a Rat Model of Colitis. Gut 1999, 44, 636–642. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.P.; Kang, J.K.; Song, S.H.; Han, S.W.; Kim, T.Y. Identification of Diverse Adenosine-to-Inosine RNA Editing Subtypes in Colorectal Cancer. Cancer Res. Treat. 2017, 49, 1077–1087. [Google Scholar] [CrossRef]
- Soto, L.; Martín, A.I.; Millán, S.; Vara, E.; López-Calderón, A. Effects of Endotoxin Lipopolysaccharide Administration on the Somatotropic Axis. J. Endocrinol. 1998, 159, 239–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Molina, P.E.; Gelato, M.C.; Lang, C.H. Differential Tissue Regulation of Insulin-like Growth Factor-I Content and Binding Proteins after Endotoxin. Endocrinology 1994, 134, 1685–1692. [Google Scholar] [CrossRef]
- Matsumoto, M.; Liu, J.; Iwata, K.; Ibi, M.; Asaoka, N.; Zhang, X.; Katsuyama, M.; Matsuda, M.; Nabe, T.; Schröder, K.; et al. NOX1/NADPH Oxidase Is Involved in the LPS-Induced Exacerbation of Collagen-Induced Arthritis. J. Pharmacol. Sci. 2021, 146, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, S.; Petersen, B.-S.; Barann, M.; Piecyk, A.; Tran, F.; Mayr, G.; Jentzsch, M.; Aden, K.; Stengel, S.T.; Klostermeier, U.C.; et al. Missense Variants in NOX1 and P22phox in a Case of Very-Early-Onset Inflammatory Bowel Disease Are Functionally Linked to NOD2. Cold Spring Harb. Mol. Case Stud. 2019, 5, a002428. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and Oxidative Stress: An Overview. Free. Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Roy, A. Compromised Rat Testicular Antioxidant Defence System by Hypothyroidism before Puberty. Int. J. Endocrinol. 2012, 2012, 637825. [Google Scholar] [CrossRef]
- Bozinovski, S.; Seow, H.J.; Crack, P.J.; Anderson, G.P.; Vlahos, R. Glutathione Peroxidase-1 Primes Pro-Inflammatory Cytokine Production after LPS Challenge In Vivo. PLoS ONE 2012, 7, 33172. [Google Scholar] [CrossRef]
- Lubos, E.; Mahoney, C.E.; Leopold, J.A.; Zhang, Y.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 Modulates Lipopolysaccharideinduced Adhesion Molecule Expression in Endothelial Cells by Altering CD14 Expression. FASEB J. 2010, 24, 2525–2532. [Google Scholar] [CrossRef]
- Lazzaro, M.; Bettegazzi, B.; Barbariga, M.; Codazzi, F.; Zacchetti, D.; Alessio, M. Ceruloplasmin Potentiates Nitric Oxide Synthase Activity and Cytokine Secretion in Activated Microglia. J. Neuroinflamm. 2014, 11, 164. [Google Scholar] [CrossRef]
- Lee, K.H.; Yun, S.J.; Nam, K.N.; Gho, Y.S.; Lee, E.H. Activation of Microglial Cells by Ceruloplasmin. Brain Res 2007, 1171, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lan, S. Interference of S100A16 Suppresses Lipid Accumulation and Inflammation in High Glucose-Induced HK-2 Cells. Int. Urol. Nephrol. 2021, 53, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. S100: A Multigenic Family of Calcium-Modulated Proteins of the EF-Hand Type with Intracellular and Extracellular Functional Roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Manolakis, A.C.; Kapsoritakis, A.N.; Tiaka, E.K.; Potamianos, S.P. Calprotectin, Calgranulin C, and Other Members of the S100 Protein Family in Inflammatory Bowel Disease. Dig. Dis. Sci. 2011, 56, 1601–1611. [Google Scholar] [CrossRef]
- Hanifeh, M.; Sankari, S.; Rajamäki, M.M.; Syrjä, P.; Kilpinen, S.; Suchodolski, J.S.; Heilmann, R.M.; Guadiano, P.; Lidbury, J.; Steiner, J.M.; et al. S100A12 Concentrations and Myeloperoxidase Activities Are Increased in the Intestinal Mucosa of Dogs with Chronic Enteropathies. BMC Vet. Res. 2018, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Askari, A. Na+/K+-ATPase as a Signal Transducer. Eur. J. Biochem. 2002, 269, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.S.; Liechty, E.R.; Vogel, O.A.; Chang, E.B. Evolutionary and Ecological Forces That Shape the Bacterial Communities of the Human Gut. Mucosal Immunol. 2017, 10, 567–579. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300. [Google Scholar] [CrossRef]
- Swiader, A.; Nahapetyan, H.; Faccini, J.; D’Angelo, R.; Mucher, E.; Elbaz, M.; Boya, P.; Vindis, C. Mitophagy Acts as a Safeguard Mechanism against Human Vascular Smooth Muscle Cell Apoptosis Induced by Atherogenic Lipids. Oncotarget 2016, 7, 28821–28835. [Google Scholar] [CrossRef]
- Luo, X.; Cai, S.; Li, Y.; Li, G.; Cao, Y.; Ai, C.; Gao, Y.; Li, T. Drp-1 as Potential Therapeutic Target for Lipopolysaccharide-Induced Vascular Hyperpermeability. Oxid. Med. Cell. Longev. 2020, 2020, 5820245. [Google Scholar] [CrossRef]
- Marchione, R.; Leibovitch, S.A.; Lenormand, J.-L. The Translational Factor EIF3f: The Ambivalent EIF3 Subunit. Cell. Mol. Life Sci. 2013, 70, 3603. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Long, J.; Sun, Y.; Li, H.; Jiang, E.; Zeng, C.; Zhu, W. The Function and Clinical Significance of EIF3 in Cancer. Gene 2018, 673, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Gu, Y.; Xiong, Y.; Zheng, G.; He, Z. Microarray-Assisted Pathway Analysis Identifies MT1X & NFκB as Mediators of TCRP1-Associated Resistance to Cisplatin in Oral Squamous Cell Carcinoma. PLoS ONE 2012, 7, e51413. [Google Scholar] [CrossRef]
- De Arras, L.; Seng, A.; Lackford, B.; Keikhaee, M.R.; Bowerman, B.; Freedman, J.H.; Schwartz, D.A.; Alper, S. An Evolutionarily Conserved Innate Immunity Protein Interaction Network. J. Biol. Chem. 2013, 288, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, R.; Zhang, W.; Yao, X.; Li, W.; Xu, L.; Sun, X.; Zhao, L. Cysteine-Rich Intestinal Protein 1 Suppresses Apoptosis and Chemosensitivity to 5-Fluorouracil in Colorectal Cancer through Ubiquitin-Mediated Fas Degradation. J. Exp. Clin. Cancer Res. 2019, 38, 120. [Google Scholar] [CrossRef]
- Hallquist, N.A.; Khoo, C.; Cousins, R.J. Lipopolysaccharide Regulates Cysteine-Rich Intestinal Protein, a Zinc-Finger Protein, in Immune Cells and Plasma. J. Leukoc. Biol. 1996, 59, 172–177. [Google Scholar] [CrossRef]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-A1—A Blessing or a Curse in Cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef]
- Damazo, A.S.; Yona, S.; D’Acquisto, F.; Flower, R.J.; Oliani, S.M.; Perretti, M. Critical Protective Role for Annexin 1 Gene Expression in the Endotoxemic Murine Microcirculation. Am. J. Pathol. 2005, 166, 1607–1617. [Google Scholar] [CrossRef]
- Scicluna, B.P.; van ’t Veer, C.; Nieuwdorp, M.; Felsmann, K.; Wlotzka, B.; Stroes, E.S.G.; Poll, T. van der Role of Tumor Necrosis Factor-α in the Human Systemic Endotoxin-Induced Transcriptome. PLoS ONE 2013, 8, e79051. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, H.P.; Ifedigbo, E.; Lau, L.F.; Choi, A.M.K. Cyr61 Protects against Hyperoxia-Induced Cell Death via Akt Pathway in Pulmonary Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2005, 33, 297–302. [Google Scholar] [CrossRef]
- Chen, W.; Syldath, U.; Bellmann, K.; Burkart, V.; Kolb, H. Human 60-KDa Heat-Shock Protein: A Danger Signal to the Innate Immune System. J. Immunol. 1999, 162, 3212–3219. [Google Scholar] [PubMed]
- Linder, A.; Hagberg Thulin, M.; Damber, J.E.; Welén, K. Analysis of Regulator of G-Protein Signalling 2 (RGS2) Expression and Function during Prostate Cancer Progression. Sci. Rep. 2018, 8, 17259. [Google Scholar] [CrossRef] [PubMed]
- Panetta, R.; Guo, Y.; Magder, S.; Greenwood, M.T. Regulators of G-Protein Signaling (RGS) 1 and 16 Are Induced in Response to Bacterial Lipopolysaccharide and Stimulate c-Fos Promoter Expression. Biochem. Biophys. Res. Commun. 1999, 259, 550–556. [Google Scholar] [CrossRef]
- Kriaa, A.; Mariaule, V.; Jablaoui, A.; Rhimi, S.; Mkaouar, H.; Hernandez, J.; Korkmaz, B.; Lesner, A.; Maguin, E.; Aghdassi, A.; et al. Bile Acids: Key Players in Inflammatory Bowel Diseases? Cells 2022, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Shirokova, E.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Sinitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; et al. A Recent Ten-Year Perspective: Bile Acid Metabolism and Signaling. Molecules 2022, 27, 1983. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Wada, H.; Eguchi, H.; Ogawa, H.; Yamada, D.; Noda, T.; Asaoka, T.; Kawamoto, K.; Gotoh, K.; Umeshita, K.; et al. A CD13 Inhibitor, Ubenimex, Synergistically Enhances the Effects of Anticancer Drugs in Hepatocellular Carcinoma. Int. J. Oncol. 2016, 49, 89–98. [Google Scholar] [CrossRef]
- Hashida, H.; Takabayashi, A.; Kanai, M.; Adachi, M.; Kondo, K.; Kohno, N.; Yamaoka, Y.; Miyake, M. Aminopeptidase N Is Involved in Cell Motility and Angiogenesis: Its Clinical Significance in Human Colon Cancer. Gastroenterology 2002, 122, 376–386. [Google Scholar] [CrossRef]
- Lu, C.; Amin, M.A.; Fox, D.A. CD13/Aminopeptidase N Is a Potential Therapeutic Target for Inflammatory Disorders. J. Immunol. 2020, 204, 3–11. [Google Scholar] [CrossRef]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Batu, D.; Velkoska, E.; Burrell, L.M.; Patel, S.K.; Beswick, L.; Jackson, A.; et al. Imbalance of the Renin–Angiotensin System May Contribute to Inflammation and Fibrosis in IBD: A Novel Therapeutic Target? Gut 2020, 69, 841–851. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Wang, H.; Alam, A.; Kang, S.S.; Ahn, E.H.; Liu, X.; Jia, J.; Ye, K. Gut Inflammation Triggers C/EBPβ/δ-Secretase-Dependent Gut-to-Brain Propagation of Aβ and Tau Fibrils in Alzheimer’s Disease. EMBO J. 2021, 40, e106320. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Wang, S.; Gao, X.; Qian, M.; Qiu, W.; Zhang, Z.; Zhang, S.; Qi, Y.; Sun, X.; et al. TGFβ1-induced Beta-site APP-cleaving Enzyme 2 Upregulation Promotes Tumorigenesis through the NF-κB Signalling Pathway in Human Gliomas. Mol. Oncol. 2020, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Jeon, M.K.; Kaemmerer, E.; Gassler, N. Intestinal Acyl-CoA Synthetase 5: Activation of Long Chain Fatty Acids and Behind. World J. Gastroenterol. 2013, 19, 7369. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Guo, Y.; Wang, C.; Cui, C.; Han, W.; Liao, D.; Jiang, P. Comprehensive Evaluation of Lipopolysaccharide-Induced Changes in Rats Based on Metabolomics. J. Inflamm. Res. 2020, 13, 477–486. [Google Scholar] [CrossRef]
- Waggoner, J.W.; Löest, C.A.; Turner, J.L.; Mathis, C.P.; Hallford, D.M. Effects of Dietary Protein and Bacterial Lipopolysaccharide Infusion on Nitrogen Metabolism and Hormonal Responses of Growing Beef Steers. J. Anim. Sci. 2009, 87, 3656–3668. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Ahuja, V.; Paul, J. Profiling of ABC Transporters during Active Ulcerative Colitis and in Vitro Effect of Inflammatory Modulators. Dig. Dis. Sci. 2013, 58, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of Arachidonic Acid Metabolism: A Review. J. Adv. Res. 2018, 11, 23. [Google Scholar] [CrossRef]
- Mak, T.W.; Grusdat, M.; Duncan, G.S.; Dostert, C.; Nonnenmacher, Y.; Cox, M.; Binsfeld, C.; Hao, Z.; Brüstle, A.; Itsumi, M.; et al. Glutathione Primes T Cell Metabolism for Inflammation. Immunity 2017, 46, 675–689. [Google Scholar] [CrossRef]
- Modrick, M.L.; Didion, S.P.; Lynch, C.M.; Dayal, S.; Lentz, S.R.; Faraci, F.M. Role of Hydrogen Peroxide and the Impact of Glutathione Peroxidase-1 in Regulation of Cerebral Vascular Tone. J. Cereb. Blood Flow Metab. 2009, 29, 1130. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Chainy, G.B.N. Thyroid Dysfunction and Testicular Redox Status: An Intriguing Association. In Book Oxidants, Antioxidants, and Impact of the Oxidative Status in Male Reproduction, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128125014. [Google Scholar]
- Sahoo, D.K.; Roy, A.; Chainy, G.B.N. Protective Effects of Vitamin E and Curcumin on L-Thyroxine-Induced Rat Testicular Oxidative Stress. Chem. Biol. Interact. 2008, 176, 121–128. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sahoo, D.K.; Roy, A.; Samanta, L.; Chainy, G.B.N. Thiol Redox Status Critically Influences Mitochondrial Response to Thyroid Hormone-Induced Hepatic Oxidative Injury: A Temporal Analysis. Cell Biochem. Funct. 2010, 28, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Roy, A.; Chattopadhyay, S.; Chainy, G.B.N. Effect of T3 Treatment on Glutathione Redox Pool and Its Metabolizing Enzymes in Mitochondrial and Post-Mitochondrial Fractions of Adult Rat Testes. Indian J. Exp. Biol. 2007, 45, 338–346. [Google Scholar] [PubMed]
- Sahoo, D.K.; Chainy, G.B.N. Tissue Specific Response of Antioxidant Defence Systems of Rat to Experimentally-Induced Hyperthyroidism. Natl. Acad. Sci. Lett. 2007, 30, 247–250. [Google Scholar]
- Chattopadhyay, S.; Sahoo, D.K.; Subudhi, U.; Chainy, G.B.N. Differential Expression Profiles of Antioxidant Enzymes and Glutathione Redox Status in Hyperthyroid Rats: A Temporal Analysis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Roy, A.; Chainy, G.B.N. PTU-Induced Neonatal Hypothyroidism Modulates Antioxidative Status and Population of Rat Testicular Germ Cells. Natl. Acad. Sci. Lett. 2006, 29, 133–135. [Google Scholar]
- Sahoo, D.K.; Roy, A.; Chainy, G.B.N. Rat Testicular Mitochondrial Antioxidant Defence System and Its Modulation by Aging. Acta Biol. Hung. 2008, 59, 413–424. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Roy, A.; Bhanja, S.; Chainy, G.B.N. Hypothyroidism Impairs Antioxidant Defence System and Testicular Physiology during Development and Maturation. Gen. Comp. Endocrinol. 2008, 156, 63–70. [Google Scholar] [CrossRef]
- Tabbaa, M.; Golubic, M.; Roizen, M.F.; Bernstein, A.M. Docosahexaenoic Acid, Inflammation, and Bacterial Dysbiosis in Relation to Periodontal Disease, Inflammatory Bowel Disease, and the Metabolic Syndrome. Nutrients 2013, 5, 3299. [Google Scholar] [CrossRef] [PubMed]
- di Cara, F.; Andreoletti, P.; Trompier, D.; Vejux, A.; Bülow, M.H.; Sellin, J.; Lizard, G.; Cherkaoui-Malki, M.; Savary, S. Peroxisomes in Immune Response and Inflammation. Int. J. Mol. Sci. 2019, 20, 3877. [Google Scholar] [CrossRef]
- Kim, J.A. Peroxisome Metabolism in Cancer. Cells 2020, 9, 1692. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Giesbrecht, K.; Saiki, N.; Ferguson, A.; Kimura, M.; Thompson, W.L.; Wells, J.M.; et al. Modelling Human Hepato-Biliary-Pancreatic Organogenesis from the Foregut-Midgut Boundary. Nature 2019, 574, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Mosichuk, A.P.; Lucien, F.; Frank, I.; Cheville, J.C.; Allenspach, K.; Mochel, J.P. Abstract 3092: Urine-Derived Urinary Carcinoma Organoids: A Novel Tool for Providing New Insights into Human and Canine Bladder Cancer Treatment. Cancer Res. 2022, 82, 3092. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).