Abstract
Background: Image-guided navigation has revolutionized precision cardiac interventions, yet current technologies face critical limitations in real-time guidance and procedural accuracy. Method: Here, we comprehensively evaluate state-of-the-art imaging modalities, from conventional fluoroscopy to emerging hybrid systems, analyzing their applications across coronary, structural, and electrophysiological interventions. Results: We demonstrate that novel approaches combining optical coherence tomography with near-infrared spectroscopy or fluorescence achieve unprecedented plaque characterization and procedural guidance through simultaneous structural and molecular imaging. Our analysis reveals key challenges, including imaging artifacts and resolution constraints, while highlighting recent technological solutions incorporating artificial intelligence and robotics. We show that non-imaging alternatives, such as fiber optic real-shape sensing and electromagnetic tracking, complement traditional techniques by providing real-time navigation without radiation exposure. This paper also discusses the integration of image-guided navigation techniques into augmented reality systems and patient-specific modeling, highlighting initial clinical studies that demonstrate their significant promise in reducing procedural times and improving accuracy. These findings establish a framework for next-generation cardiac interventions, emphasizing the critical role of multimodal imaging platforms enhanced by AI-driven decision support. Conclusions: We conclude that continued innovation in hybrid imaging systems, coupled with advances in automation, will be essential for optimizing procedural outcomes and expanding access to complex cardiac interventions.
1. Introduction
Advancements in cardiac interventions have revolutionized the treatment of coronary artery disease, structural heart disease, and arrhythmias, significantly improving patient outcomes [1,2]. However, these procedures demand unparalleled precision, particularly in minimally invasive techniques, where direct visualization is limited [3]. Accurate imaging and navigation are indispensable for planning, executing, and evaluating such interventions. Historically, imaging techniques such as fluoroscopy and echocardiography have served as the backbone of cardiac interventions, with advances being made using cardiac computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). However, as the complexity of cardiac diseases grows, there is an urgent need for innovative imaging solutions that provide real-time guidance, decision-making, and patient safety. Emerging image-guided navigation technologies are reshaping this landscape. Integrating multimodal imaging, artificial intelligence (AI), and extended reality (XR) enables unprecedented precision and efficiency in interventional cardiology. These advancements reduce radiation exposure, improve device placement accuracy, and expand treatment options for high-risk patients. Furthermore, they open the door to novel procedures previously deemed unfeasible due to anatomical or technical constraints. This review aims to explore the latest developments in image-guided navigation, highlighting their transformative impact on cardiac interventions. By identifying current challenges and examining future trends, this paper seeks to provide insights into the potential of these technologies to redefine interventional cardiology. While numerous imaging modalities have emerged for cardiovascular interventions, their relative advantages, limitations, and optimal clinical applications vary substantially. This review provides not only a description of these technologies but also a systematic comparison across key parameters, including spatial and temporal resolution, tissue penetration, radiation exposure, and procedural workflow integration. Additionally, we examine how AI is transforming these imaging platforms from passive visualization tools into interactive guidance systems that enhance clinical decision-making and procedural precision. By critically evaluating both technological innovations and implementation challenges, we aim to provide a comprehensive framework for understanding the evolving landscape of image-guided cardiovascular navigation.
1.1. Review Methodology
This scoping review was conducted following a structured and reproducible methodology to ensure a comprehensive and balanced evaluation of emerging image-guided navigation techniques for cardiovascular interventions. The reporting of this manuscript adheres to the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines. Given the rapid advancements in this interdisciplinary field, our approach prioritized the inclusion of recent, high-quality studies spanning clinical cardiology, biomedical engineering, and medical imaging domains.
1.1.1. Search Strategy and Databases
A systematic literature search was conducted across three major databases such as PubMed, Scopus, and IEEE Xplore to ensure broad coverage across biomedical, engineering, and computational research relevant to image-guided cardiac interventions. The search timeframe spanned publications from January 2013 to January 2025, with a deliberate emphasis on recent advances published in the last five years, aligning with the paper’s focus on emerging techniques. We employed a combination of controlled vocabulary (e.g., MeSH terms in PubMed) and keyword strategies tailored to each database. Search terms included but were not limited to the following: “image-guided cardiac intervention”, “catheter tracking fluoroscopy”, “multimodal cardiac imaging”, “AI in interventional cardiology”, “MRI-guided catheterization”, “OCT-guided PCI”, “intravascular ultrasound guidance”, “real-time navigation cardiovascular”, “robot-assisted cardiac imaging”, and “electromagnetic catheter tracking”. Boolean operators (AND, OR) and truncation techniques were employed to refine the search scope. Reference lists of the included studies were also reviewed to identify any additional relevant records and ensure completeness.
1.1.2. Inclusion and Exclusion Criteria
Articles were selected using predefined inclusion and exclusion criteria. Studies were eligible for inclusion if they satisfied the following:
- Were published in English in peer-reviewed journals or leading international conferences;
- Focused on imaging techniques, navigation systems, or image-guided interventions specifically applied to cardiovascular procedures;
- Demonstrated technical novelty, clinical validation, or translational relevance, such as evaluations of performance, workflow integration, or outcome improvements;
- Included clinical, preclinical, simulation-based, or computational studies with clearly defined methodologies.
Exclusion criteria included the following:
- Editorials, commentaries, abstracts, or opinion pieces without supporting data;
- Studies limited to non-cardiac applications or purely diagnostic modalities without interventional relevance;
- Studies focusing primarily on the development or evaluation of contrast media are excluded, as this review is centered on imaging techniques rather than contrast agent innovation.
1.1.3. Screening and Selection
The initial screening of titles and abstracts was independently carried out by two reviewers to minimize selection bias. Full texts of potentially relevant studies were then retrieved and assessed according to predefined inclusion and exclusion criteria. Any disagreements were resolved through consultation with a third senior reviewer to ensure consistency and objectivity. Through this structured review process, we identified over 180 publications, from which we selected a representative, high-quality, and thematically aligned subset for inclusion.
2. Advancements in Imaging Techniques for Cardiac Intervention
The evolution of navigation techniques in cardiac interventions has been fueled by advancements in imaging technologies, computational models, AI, and robotic systems [4,5,6]. These innovations have significantly enhanced procedural precision, safety, and efficacy, enabling clinicians to address complex cardiac conditions more confidently. This section explores the diverse approaches employed in navigation and control during interventional procedures. By reviewing the latest developments and their integration into clinical practice, this section highlights how these techniques are reshaping the landscape of cardiac interventions, opening up the way for more effective and minimally invasive solutions.
2.1. Fluoroscopy and X-Ray Imaging Techniques
Fluoroscopy and X-ray imaging are fundamental tools in interventional cardiology, offering the real-time visualization of catheters, guidewires, and devices during minimally invasive procedures. Despite their widespread use, these techniques face notable challenges that limit their effectiveness. High radiation exposure poses risks to both patients and clinicians, particularly during lengthy procedures [7]. Additionally, fluoroscopy provides limited soft tissue contrast, making it difficult to visualize certain anatomical structures without supplemental imaging. The precise tracking of devices such as catheters is another critical challenge, as motion artifacts and overlapping structures in fluoroscopic images can obscure visualization. Emerging strategies are being developed to reduce radiation exposure in fluoroscopy-based procedures. These include AI-driven image enhancement that allows for lower frame rates, fusion imaging with preoperative CT or MRI to minimize real-time fluoroscopy use, and robotic-assisted navigation systems that reduce the need for prolonged manual operation. These approaches aim to make fluoroscopy-guided interventions safer and more efficient. To address these limitations, researchers are developing advanced detection frameworks, navigation systems, and modeling techniques to enhance accuracy, reduce radiation exposure, and improve procedural outcomes.
Ma et al. developed an automatic detection framework to accurately identify catheters in fluoroscopic X-ray images [8]. Leveraging path reconstruction from image tensors derived from a multiscale vessel enhancement filter, their system traces smooth paths along eigendirection vectors to detect catheters and guidewires with high precision. Extensive testing on 7754 X-ray images demonstrated detection errors averaging 0.56 mm for catheters and 0.68 mm for guidewires. Clinical validation further showed its efficacy, with 2D target registration errors of 1.8 ± 0.9 mm for motion compensation and 2.3 ± 0.9 mm for co-registration between 2D X-ray images and 3D MRI models. To overcome navigation challenges in cardiac procedures, researchers developed CathPilot, an innovative cable-driven system that uses 3D cam mechanics to provide clinicians with precise control over medical devices, significantly improving upon traditional methods [9]. To improve catheter localization in endovascular and cardiac procedures, Chang et al. developed a novel system that combines deformable B-spline tube modeling with probabilistic algorithms, enabling the real-time tracking of complex catheter shapes and improving fluoroscopic guidance precision, as validated through phantom and in vivo studies [10]. Recent developments in deep learning have also enabled simultaneous force estimation [11] and catheter segmentation directly from biplane fluoroscopic images. For instance, Fekri et al. proposed Y-Net, a stereo-vision-based architecture that estimates 3D contact forces at the catheter tip using dual-view fluoroscopic images, eliminating the need for embedded sensors [12]. More recently, H-Net extended this concept by introducing an end-to-end multitask framework capable of both stereo segmentation and 3D force regression, demonstrating improved accuracy and computational efficiency [13].
2.2. Ultrasound-Based Navigation and Control
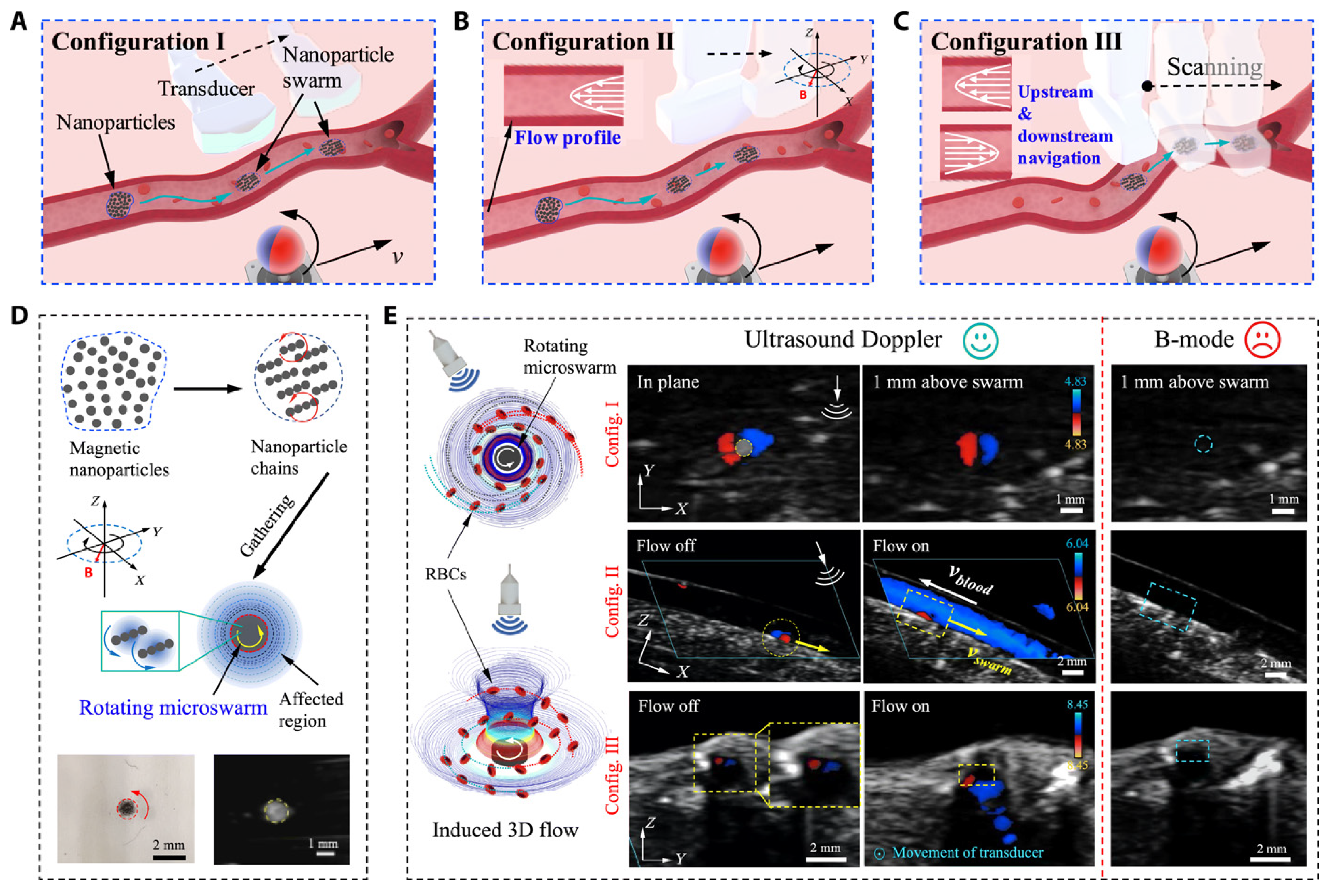
Ultrasound imaging is a cornerstone of cardiac interventions, providing the real-time visualization of anatomical structures without ionizing radiation. Despite its advantages, several challenges limit its effectiveness in complex procedures. Operator dependency remains a significant issue, as the accuracy and consistency of ultrasound imaging are highly dependent on the clinician’s expertise. Dynamic cardiac environments, such as the motion of heart structures during beating heart surgeries, further complicate imaging by introducing motion artifacts and reducing image clarity. In addition, maintaining stable probe positioning in minimally invasive procedures can be challenging, particularly in transesophageal echocardiogram (TEE) and intracardiac echocardiography (ICE). Despite these challenges, advancements in 3D ultrasound imaging have enhanced anatomical visualization in structural heart interventions, enabling dynamic 3D assessments of heart valve motion, particularly in procedures such as transcatheter edge-to-edge repair (TEER) and transcatheter aortic valve replacement (TAVR). To further enhance ultrasound’s precision and consistency, researchers have developed robotic systems, automated tracking tools, and innovative testing platforms to enhance ultrasound’s precision, consistency, and versatility. Agricola et al. highlighted the pivotal role of TEE in guiding transcatheter structural cardiac interventions, including valve repairs and left atrial appendage closures [14]. The study also emphasized the benefits of integrating echocardiography with other imaging modalities to enhance outcomes and mitigate limitations. However, challenges such as needing specialized imaging expertise and minimizing radiation exposure persist, necessitating further advancements in procedural strategies. Wang et al. proposed a robotic TEE system that automates ultrasound image acquisition along pre-planned paths, guided by electromagnetic tracking and image-based registration [15]. This system significantly reduces operator dependency and improves imaging stability. Phantom experiments validated its performance, achieving a mean positioning error of 10.44 mm in regions of clinical relevance. By integrating robotics with precise navigation, this approach enhances both the reliability and safety of TEE-based procedures. Recent advancements have combined magnetic actuation and ultrasound imaging to enhance guidewire navigation in cardiovascular interventions. Yang et al. developed a magnetically steerable guidewire controlled by mobile electromagnets and guided by ultrasound imaging [16]. Their system uses a computational model to predict guidewire deformation and enables precise, autonomous delivery to target regions, validated in a vascular phantom. This radiation-free alternative to fluoroscopy highlights the potential of ultrasound-based magnetic control to improve precision and safety in minimally invasive procedures. Wang et al. introduced a real-time navigation system for magnetic micro-swarms guided by ultrasound Doppler imaging [17] (see Figure 1). This innovative approach allowed precise swarm tracking and navigation near vessel boundaries, minimizing drag in dynamic blood flow conditions. Validated in porcine coronary arteries ex vivo, the system demonstrates the potential for localized therapeutic delivery in challenging vascular environments, advancing the capabilities of ultrasound-guided interventional techniques.
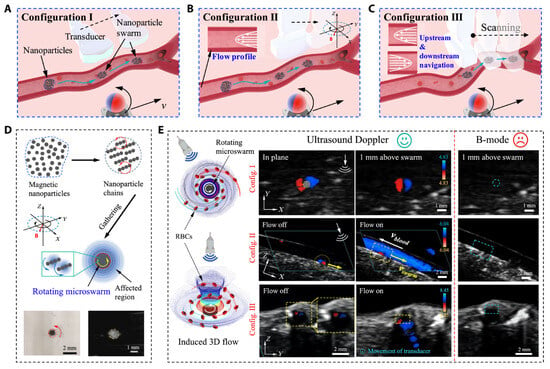
Figure 1.
Ultrasound Doppler imaging-guided swarm formation and navigation in blood vessels. (A–C) Three different configurations for magnetic nanoparticle swarm control and manipulation within vasculature, showing how field orientation affects navigation strategy. (D) Formation of nanoparticle chains through magnetic assembly and their targeted delivery capabilities. (E) Comprehensive visualization of swarm behavior under different blood flow conditions using B-mode and Doppler imaging for real-time tracking. This multimodal approach enables precise control of therapeutic delivery while providing continuous feedback on swarm positions relative to vessel boundaries, demonstrating superior navigation in complex vascular environments compared to conventional fluoroscopic guidance. Image reproduced with permission from [17].
2.3. MRI-Based Navigation and Control
MRI stands as a fundamental tool in diagnostic medicine, combining exceptional soft-tissue contrast with detailed anatomical imaging while eliminating radiation exposure risks. Current research is focused on leveraging the unique features of MRI, such as real-time volumetric imaging and compatibility with advanced robotic systems, to enable precise intra-operative guidance. The ultimate goal is to transform MRI from a diagnostic tool into a fully interactive platform capable of accurately guiding complex interventions. However, challenges such as the high cost of MRI systems, spatial constraints within MRI scanners, and the need for MRI-compatible devices remain barriers to widespread adoption. Several innovative studies have showcased the potential of MRI-based navigation. For instance, Padhan et al. introduced a computational system that integrates real-time image processing with virtual fixtures, dynamically updating visualizations and providing force feedback during procedures [18]. This approach reduced procedural times in simulated transapical aortic valve implantation, illustrating its potential to streamline surgical workflows. Velasco Forte et al. explored the feasibility of MRI-guided catheterization for right and left heart procedures in patients with congenital heart disease. The study demonstrated high procedural success and precise hemodynamic measurements using passive tracking techniques and advanced visualization [19]. Dong et al. tackled real-time catheter tracking under MRI by combining fiber Bragg grating (FBG) sensors with tracking coils [20]. Their system enabled precise shape and positional tracking, demonstrating minimal deviation during autonomous targeting tasks. Similarly, Kensicher et al. explored the use of magnetic field gradients in MRI scanners to control ferromagnetic agents, or MRbots, within blood vessels [21]. This approach represents a novel application of MRI’s capabilities, enabling highly controlled, minimally invasive interventions. Robotic-assisted interventions have also benefited from MRI integration. Miller et al. developed an MRI-compatible robotic arm and valve delivery system for TAVR [22]. The system provided real-time imaging and 3D reconstructions, significantly enhancing accuracy and success rates in preclinical swine models. In dynamic surgical environments, Yeniaras et al. demonstrated the potential of Cine MRI to guide robotic movements during beating heart surgeries, combining preoperative planning with real-time volumetric imaging for enhanced precision [23].
2.4. Optical Coherence Tomography (OCT) Technique
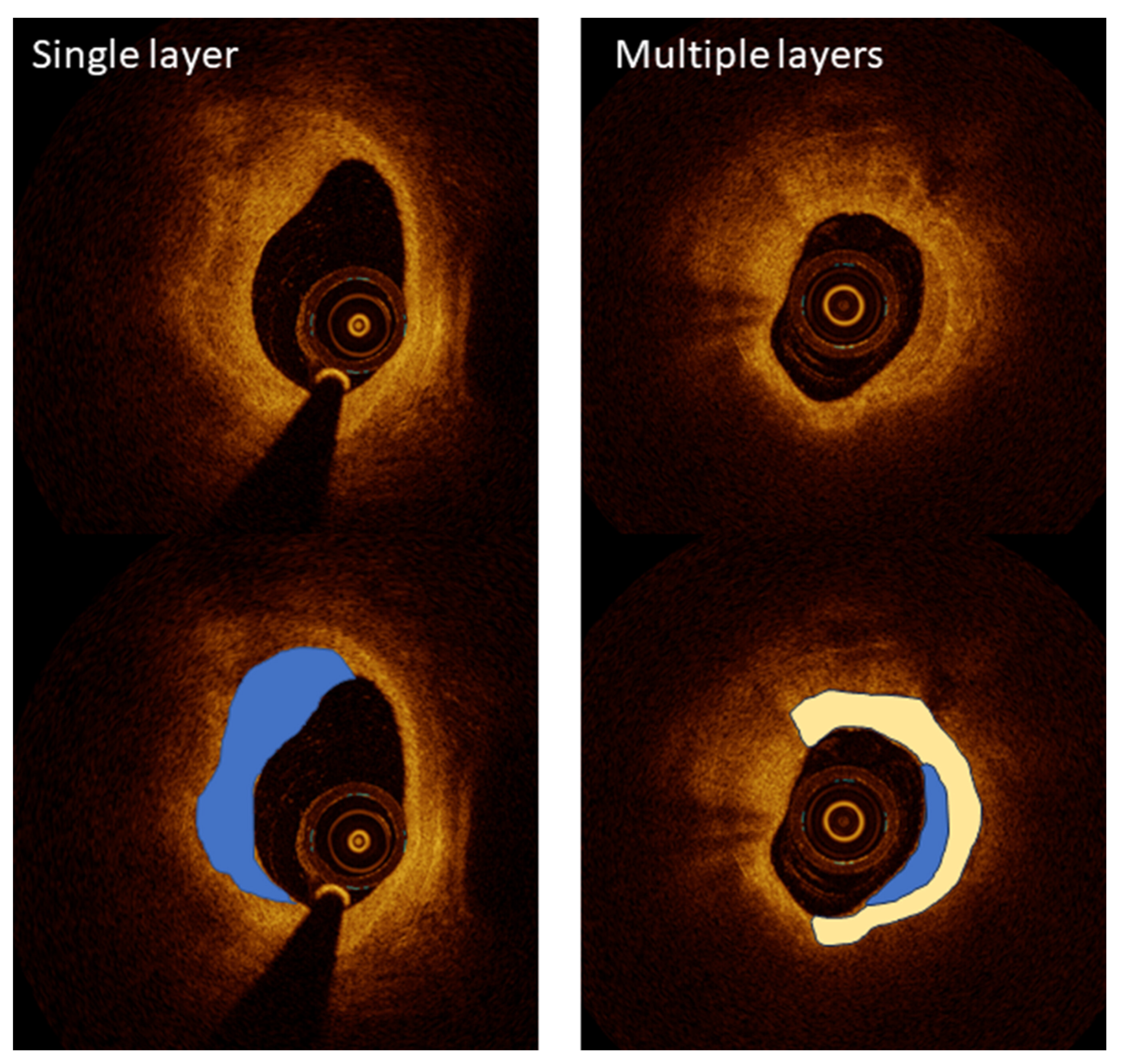
OCT has become a critical imaging modality in interventional cardiology, providing ultra-high-resolution images that enable the detailed visualization of coronary artery microstructures. Despite its transformative potential, OCT faces several challenges that must be addressed for broader clinical adoption. One challenge is its limited penetration depth, which restricts its use in imaging larger vessels. Another is the requirement for contrast injection during imaging, which adds complexity and risk, particularly in patients with renal impairment. To overcome these challenges, researchers have leveraged OCT’s unparalleled resolution for specific applications, including plaque characterization, stent optimization, and procedural guidance, ensuring its role as a vital tool in advanced coronary interventions. Kurogi et al. reviewed the clinical applications of OCT-guided percutaneous coronary intervention (PCI), emphasizing its high-resolution imaging capabilities for precise lesion assessment and the optimization of stent implantation [24]. OCT’s ability to characterize lipid-rich plaques and calcium thickness has proven valuable in pre-stent planning, while 3D reconstruction enables the improved management of bifurcation lesions. Although limitations such as high-contrast-volume requirements persist, ongoing advancements offer solutions to expand its clinical utility, underscoring OCT’s transformative impact on PCI outcomes [24]. The LEMON study by Amabile et al. explored the feasibility and safety of using OCT to guide PCI in the left main coronary artery [25]. The results showed that OCT provides superior visualization for optimizing stent apposition and expansion in the mid and distal left main interventions. This pilot study underscores the potential of OCT to enhance outcomes in complex coronary procedures, especially where detailed imaging is critical for procedural success. Yonetsu and Jang demonstrated OCT’s ability to characterize atherosclerotic plaques, differentiating between fibrous caps and lipid cores, a critical factor in assessing the risk of plaque rupture [26] (Figure 2). This high-resolution imaging capability surpasses intravascular ultrasound (IVUS), making OCT indispensable for evaluating vulnerable plaques.
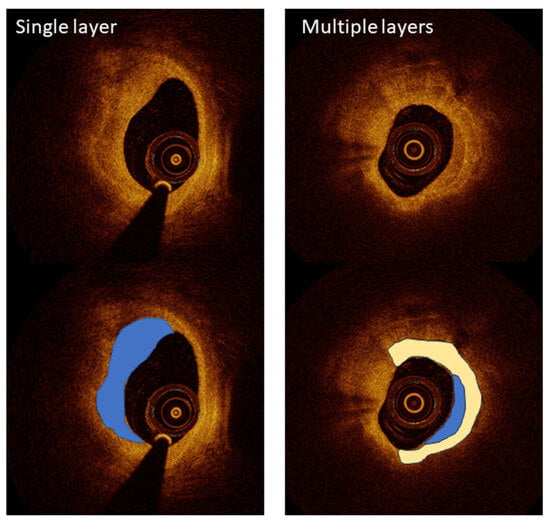
Figure 2.
A high-resolution optical coherence tomography (OCT) visualization of atherosclerotic plaque morphology. Upper images show raw OCT cross-sections, while lower images include color-coded interpretations of key plaque features. Left panels demonstrate a single-layered plaque structure (blue overlay) with a crescent-shaped morphology characteristic of healed coronary lesions, providing detailed visualization at 10–20 µm resolution. The right panels illustrate a more complex multi-layered plaque (blue and white overlays), indicating previous rupture and healing cycles that increase vulnerability to future events. This level of microstructural detail significantly exceeds the capabilities of other intravascular imaging modalities, enabling the precise assessment of fibrous cap thickness, a critical determinant of plaque stability. Image reproduced with permission from [26].
Additionally, OCT’s detailed visualization enables the precise assessment of stent deployment. The technology facilitates evaluating stent apposition and expansion and identifying complications such as tissue prolapse between stent struts, improving procedural outcomes and minimizing risks. A comprehensive analysis of the OCT/IVUS trial conducted by Kang et al. compared the effectiveness of OCT-guided and IVUS-guided PCI in complex coronary lesions [27]. The study, involving 2008 patients, found that both modalities delivered similar outcomes for major endpoints like cardiac death and myocardial infarction. However, OCT-guided PCI significantly reduced major procedural complications (1.7% vs. 3.4%, p = 0.03), highlighting its safety benefits in challenging cases. Similarly, Jones et al. analyzed the Pan-London PCI registry, encompassing over 87,000 patients, and found that OCT-guided PCI was associated with improved procedural success and reduced in-hospital major adverse cardiac event (MACE) rates [28]. Long-term mortality was also significantly lower in the OCT-guided group compared to IVUS-guided and angiography-guided groups, underscoring OCT’s contribution to both short- and long-term patient outcomes. In the same way, the iSIGHT randomized trial conducted by Chamie et al. compared OCT, IVUS, and angiography in guiding PCI. The study demonstrated that OCT-guided PCI achieved stent expansion outcomes that were non-inferior to IVUS and significantly better than angiography, with a stent expansion rate of 98.01% [29]. Using an external elastic membrane-based protocol for stent sizing, OCT showed superior precision in optimizing stent deployment, highlighting its potential to enhance procedural outcomes and reduce complications in PCI. To address variability in clinical use, IJsselmuiden et al. undertook a structured evaluation of OCT’s appropriate use [30]. Their findings emphasized OCT’s critical role in specific scenarios, such as assessing stent thrombosis and deploying bioresorbable vascular scaffolds. However, the study also highlighted limitations in its application, suggesting that OCT’s benefits are most pronounced in carefully selected cases, such as addressing unexplained angiographic abnormalities or managing high-risk stent scenarios. Table 1 summarizes the key features of the above imaging modalities, providing a baseline for currently available state-of-the-art cardiac imaging. When comparing conventional imaging techniques, several key performance metrics must be considered in the context of specific interventional requirements. Fluoroscopy offers excellent temporal resolutions (up to 30 frames per second) and a wide field of view, making it ideal for catheter tracking and device deployment, but its limited soft tissue contrast necessitates complementary imaging for detailed anatomical assessment. In contrast, TEE provides superior tissue characterization and the real-time monitoring of functional parameters without radiation exposure, though its invasive nature and operator dependency limit extended use. IVUS bridges some of these gaps, with intravascular views at 100–200 µm resolution that enable precise vessel wall assessment, yet it cannot match the 10–20 µm resolution of OCT for detailed plaque characterization and stent apposition analysis. The clinical decision regarding which imaging modality to employ often depends on the specific procedural phase and anatomical target. For example, while fluoroscopy remains the backbone for catheter navigation through vascular systems, OCT offers superior guidance for coronary stent optimization, and real-time 3D TEE provides crucial monitoring during structural interventions such as TAVR and TEER. This complementary relationship underscores the need for multimodal approaches that leverage the strengths of each technique while mitigating their individual limitations.

Table 1.
Key features and limitations of conventional image-guided navigation methods in cardiology.
To facilitate direct comparisons between imaging modalities, Table 2 provides a systematic evaluation of both conventional and emerging navigation techniques across key parameters, including spatial resolution, temporal resolution, radiation exposure, and demonstrated clinical outcomes, highlighting their relative strengths and limitations for specific cardiovascular interventions.

Table 2.
Comparison of image-guided navigation techniques for cardiovascular interventions.
3. Emerging Imaging Techniques for Next-Generation Cardiac Interventions
3.1. Near-Infrared Fluorescence (NIRF) and Near-Infrared Spectroscopy (NIRS) in Intravascular Imaging
Near-infrared spectroscopy (NIRS) and near-infrared fluorescence (NIRF) have emerged as powerful tools for intravascular imaging, offering valuable insights into coronary artery disease by enabling the molecular-level detection of lipid-rich plaques, inflammation, and vascular integrity. NIRS has been widely adopted in clinical cardiology, primarily for assessing lipid core burden and guiding PCI. NIRF remains in the preclinical stage, demonstrating the potential for the high-resolution molecular imaging of vascular disease and identifying inflammatory markers within plaques. Chowdhury et al. explored intravascular NIRF imaging as a molecular imaging technique for detecting and characterizing atherosclerotic plaques [31]. By leveraging deeper tissue penetration and reduced autofluorescence, NIRF enhances the detection of molecular markers within plaques, offering greater sensitivity than traditional imaging modalities. The first human intravascular NIRF study validated its potential for clinical coronary imaging, paving the way for targeted applications in diagnosing and managing atherosclerosis. Similarly, Hogue et al. demonstrated the clinical utility of NIRS in monitoring cerebral oxygenation during cardiovascular surgeries [32]. By tracking superficial cerebral cortex oxygenation and integrating oximetry signals with arterial pressure data, NIRS facilitates the real-time monitoring of ischemic thresholds, aiding in personalized patient management and reducing complications in high-risk cardiac procedures. Recent advancements have explored novel NIRF probes and imaging systems to improve visualization and guidance during interventions. Liu et al. introduced a lysosomal viscosity-activatable NIR-II fluorescent probe (NP-V) for intravascular imaging, enabling the real-time detection of oxidative stress and vascular inflammation in ischemic injury models [33]. These findings highlight the potential of NIR-II fluorescence in guiding cardiovascular interventions, particularly for identifying vulnerable plaques and monitoring post-procedural vascular healing.
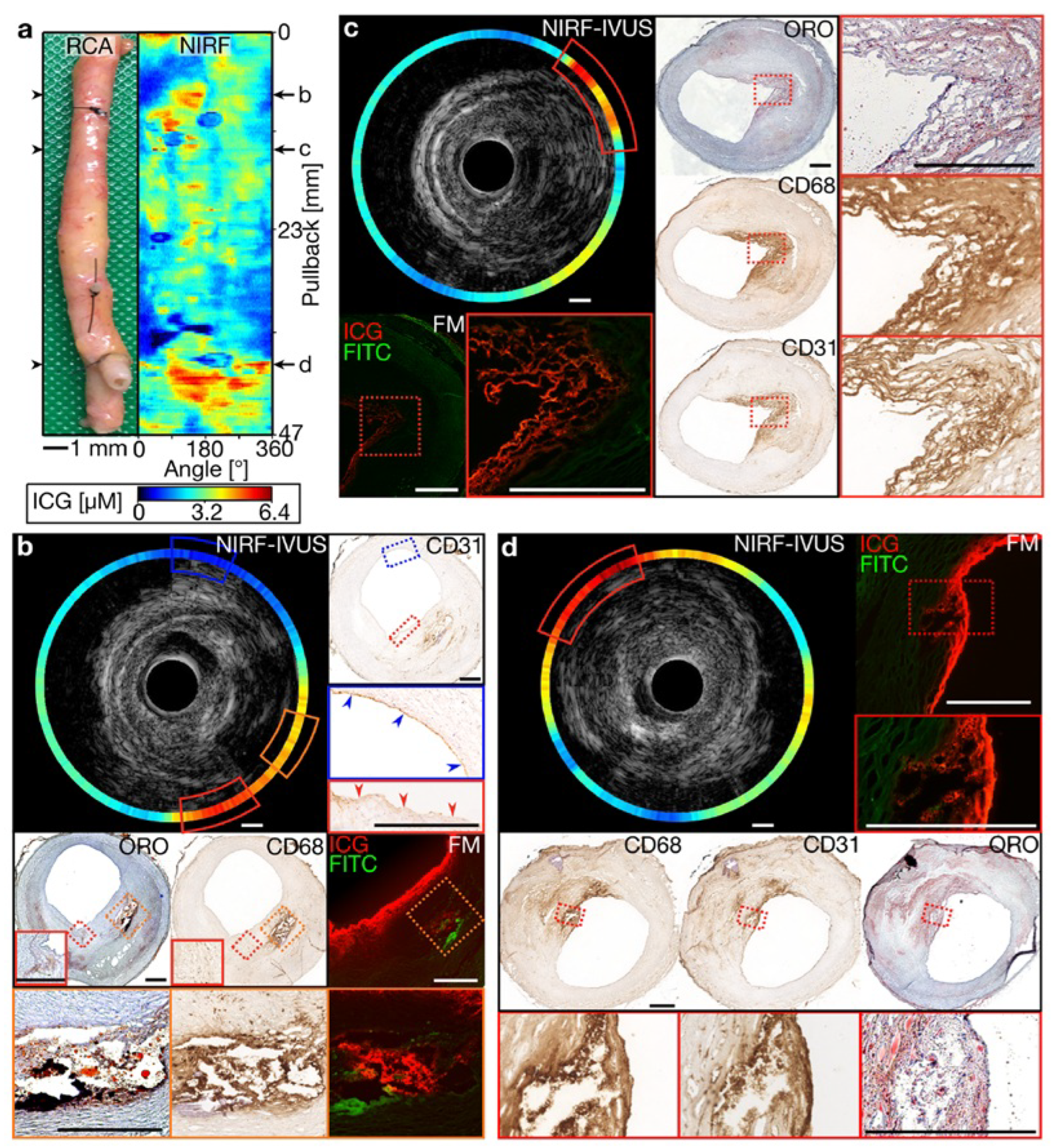
As shown in Figure 3, intravascular NIRF imaging combined with IVUS enables the real-time molecular characterization of atherosclerotic plaques, providing key insights into plaque vulnerability and lesion progression [34]. This multimodal approach enhances coronary imaging precision, offering a promising tool for both preclinical research and future clinical applications in interventional cardiology. Beyond coronary imaging, researchers have investigated dual-channel NIRF imaging for surgical applications. Bao et al. developed a dual-channel near-infrared fluorophore system for intraoperative imaging, addressing challenges such as nonspecific uptake and off-target signal detection [35]. Using renally clearable contrast agents, the system provided the precise visualization of tissue boundaries. Although this approach has been predominantly explored in oncological applications, it underscores the potential of dual-channel NIRF for enhanced visualization in complex cardiovascular procedures.
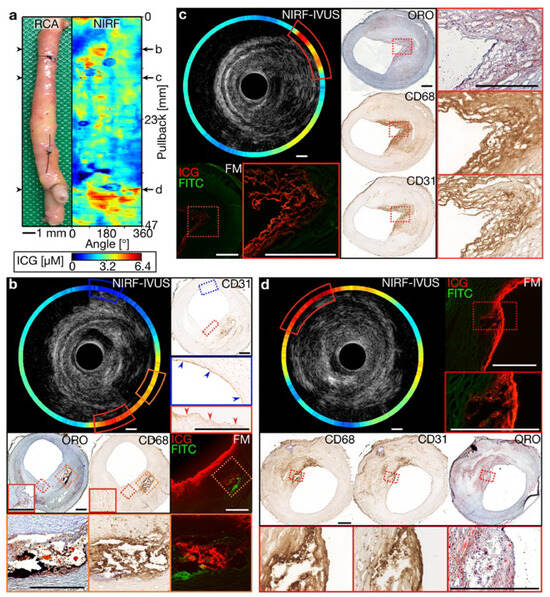
Figure 3.
Multimodal molecular and structural characterization of coronary atherosclerosis using integrated NIRF-IVUS imaging. (a) Longitudinal view of a human coronary artery with a corresponding NIRF signal map showing the heterogeneous distribution of inflammatory activity (high signal in yellow/red). (b–d) Cross-sectional NIRF-IVUS images at different arterial locations with corresponding histopathology, demonstrating the precise co-localization of NIRF signals with inflammatory markers (CD68 and CD31) and lipid-rich regions confirmed by histology (ORO staining). This hybrid imaging approach provides a simultaneous assessment of plaque structure (IVUS) and biological activity (NIRF) at a resolution unattainable by either modality alone, enabling more comprehensive risk stratification than conventional angiography or structural imaging. The integration of molecular and anatomical data in a single catheter platform represents a significant advancement for precision-guided coronary interventions. Image reproduced with permission from [34].
3.2. Nuclear Imaging
Nuclear imaging modalities, such as single-photon emission computed tomography (SPECT) and PET, serve as functional imaging techniques to provide crucial insights into myocardial physiology, perfusion, and molecular targets by focusing on physiological and biochemical processes. SPECT is the most widely used clinically in nuclear medicine and is being used to guide targeted drug delivery. SPECT uses gamma rays from the radiotracers accumulated in the specific molecular target. Several radioisotopes such as 99mTc, 123I, 67Ga, 111In, or 201Tl were used for clinical SPECT imaging. SPECT can be applied to a variety of molecular imaging targets and can also be used for multi-isotope multi-target imaging. Several radionuclides like 188Re, 166Ho, or 177Lu can also be used in image-guided radiotherapy and as probes for monitoring therapeutic efficacy [36]. There has been increasing interest in PET imaging with its higher sensitivity and resolution compared to SPECT. 18F-FDG PET/CT offered wide applications for detecting metabolically active diseases in a variety of disease sites and helped in planning radiation therapy [37]. The hybrid imaging between SPECT or PET and CT or MRI improved the detection of the target for biopsy or treatment, with a reduction in registration error and the capability of attenuation correction. Novel developments in SPECT or PET scanners are promising for providing higher spatial and temporal resolutions for the efficient and accurate detection of the target. The most common cardiac molecular targets are transthyretin-related cardiac amyloidosis, which can be detected by 99mTc-PYP SPECT, and cardiac sarcoidosis imaged by 18F-FDG PET. SPECT and PET can provide the quantitative disease staging and noninvasive monitoring of the disease [38,39]. 18F NaF PET is commonly used for detecting atherosclerotic plaques and active valvular calcification, which may help plan coronary and structural heart interventions [22,40,41]. Novel radiotracers targeting myocardial inflammation (matrix metalloproteinase, integrins, and fibroblast activation protein) may help guide drug delivery for myocardial infarction and evaluate remodeling [42].
3.3. Multimodalities Imaging Techniques
Multimodality imaging systems have emerged as a transformative approach in interventional cardiology, leveraging the complementary strengths of different imaging modalities to address the complexities of cardiac pathology. The emergence of hybrid imaging systems represents a significant advancement in addressing the inherent limitations of individual modalities. When comparing these integrated approaches, several key advantages become apparent. NIRS-IVUS systems combine the anatomical detail of ultrasound with molecular information on plaque composition, achieving a sensitivity of 89% and specificity of 78% for detecting lipid-rich plaques—significantly outperforming standalone IVUS (sensitivity of 65% and specificity of 64%). Similarly, OCT-NIRF integration enhances the microstructural visualization of OCT (10–20 µm resolution) with the molecular targeting of inflammatory biomarkers, enabling the identification of vulnerable plaques that would remain undetected by conventional angiography or ultrasound alone. The clinical impact of these hybrid systems extends beyond improved diagnostic accuracy to enhanced procedural guidance. In a head-to-head comparison, NIRS-IVUS-guided PCI demonstrated a 47% reduction in major adverse cardiac events at one year compared to angiography-guided interventions, primarily through improved stent sizing and placement. OCT-angiographic co-registration systems have shown similar benefits, reducing geographic miss by 38% and subsequent target lesion revascularization by 42% over conventional approaches. These outcome improvements highlight the transformative potential of multimodal imaging in complex interventional scenarios.
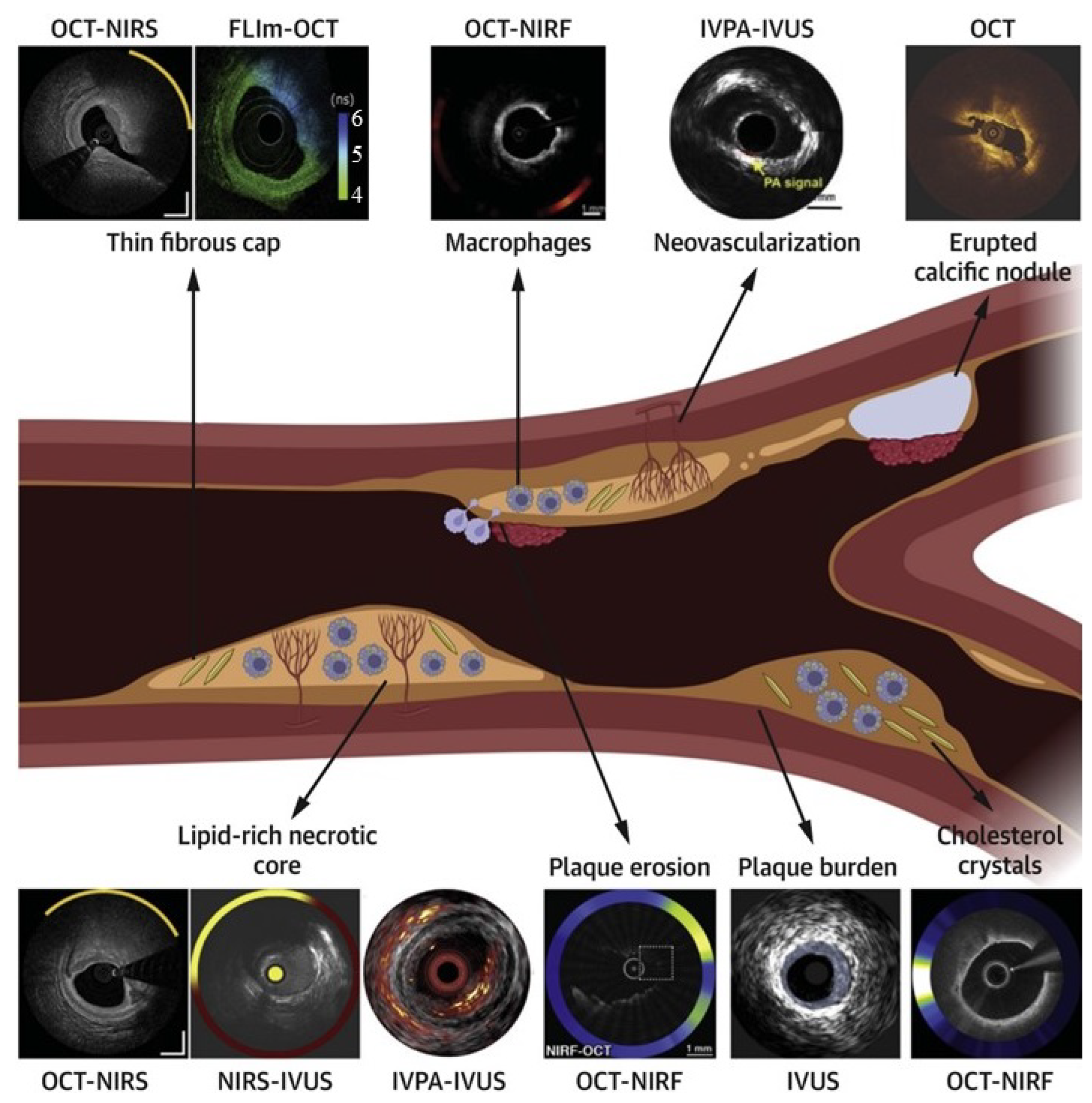
Integrating technologies such as IVUS, OCT, and angiography provides a more comprehensive understanding of coronary anatomy and disease. Despite their potential, multimodality systems must overcome challenges such as technological integration and increased procedural complexity. Nevertheless, recent advancements demonstrate their ability to enhance precision, reduce complications, and improve procedural outcomes. Tufaro et al. highlighted the advancements in hybrid intracoronary imaging technologies that integrate IVUS, OCT, and NIRS into multimodality systems [43] (Figure 4). These hybrid catheters provide complementary imaging capabilities, enabling enhanced plaque morphology assessment, high-risk plaque identification, and PCI guidance. Postmortem and clinical studies demonstrated the efficacy of multimodal imaging in improving procedural outcomes and supporting emerging pharmacotherapies targeting atherosclerosis. The NIRVUS trial, conducted by Noori et al., demonstrated the superiority of NIRS and IVUS-guided PCI over angiography-guided PCI in patients with acute myocardial infarction [44]. Imaging-guided PCI achieved significantly higher stent strut coverage (88.7% vs. 72.3%), larger minimal stent areas, and lower incomplete lesion coverage rates. These findings emphasize the importance of combining intravascular imaging modalities to optimize stent deployment and improve procedural outcomes in high-risk AMI cases. A notable example is the OPTICO-Integration II trial by Schneider et al., which investigated the risks associated with post-PCI complications, such as longitudinal geographic mismatch (LGM) and edge dissections [45]. These complications significantly increase the likelihood of adverse events post-PCI. The trial evaluated the efficacy of a novel system combining real-time OCT with angiographic co-registration (ACR). Among 84 patients randomized to ACR-guided PCI, OCT-guided PCI, and angiography-guided PCI, ACR-guided PCI showed a significant reduction in LGM and edge dissections. This superior performance underscores the transformative potential of integrating OCT and angiography for enhanced PCI planning and execution. Lim et al. demonstrated the prognostic value of the lipid core burden index (LCBi), measured using NIRS-IVUS, in predicting slow thrombolysis in myocardial infarction (TIMI) flow after PCI [46]. Their study found that a high LCBi (≥353 for 4 mm segments) was independently associated with reduced TIMI flow and worse clinical outcomes, such as myocardial infarction and stent thrombosis. These findings underscore the utility of NIRS-IVUS in identifying high-risk plaques, optimizing procedural planning, and reducing post-procedural complications.
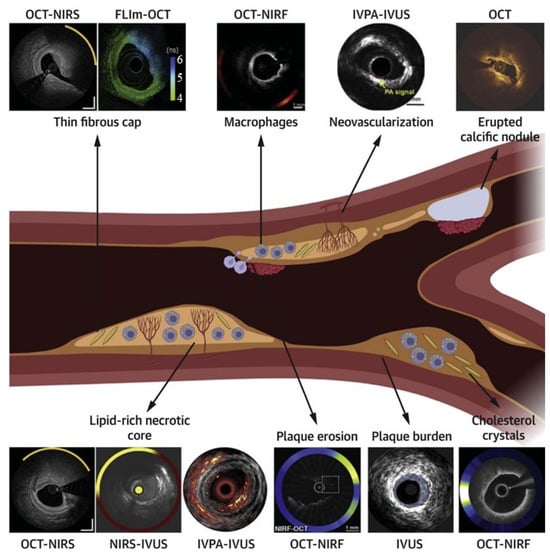
Figure 4.
Comprehensive assessment of plaque vulnerability using standalone and hybrid intracoronary imaging modalities. The central schematic illustrates a coronary artery with various plaque features, including a thin fibrous cap, macrophage infiltration, neovascularization, calcification, lipid core, and plaque erosion. Surrounding images demonstrate how specific imaging technologies visualize these features: OCT-NIRS and NIRS-IVUS for lipid detection and cap thickness assessment; OCT-NIRF for inflammatory activity visualization; IVPA-IVUS for neovascularization detection; OCT for calcification identification; and various multimodal approaches for comprehensive plaque characterization. This comparison highlights the complementary nature of different imaging techniques and demonstrates how hybrid approaches provide a more comprehensive assessment than single-modality imaging. Each technology’s unique capabilities address specific diagnostic challenges in vulnerable plaque identification, supporting tailored interventional strategies based on individualized risk assessment. Image reproduced with permission from [43].
Hybrid imaging systems that merge IVUS and OCT are another major advancement in multimodality imaging. Sheth et al. reported the first-in-human application of a hybrid imaging catheter capable of simultaneous IVUS and OCT imaging [47]. Featuring a 2.8 F imaging window and a 40 MHz center frequency for IVUS, this catheter was used to treat a 74-year-old male patient recovering from an inferior ST-segment elevation myocardial infarction. Over 90 mm of the right coronary artery was imaged, vividly demonstrating the complementary roles of IVUS and OCT in characterizing atherosclerotic plaque types. This innovation not only provides unparalleled precision in coronary imaging but also paves the way for hybrid systems to become standard tools in coronary interventions. Li et al. further advanced multimodality imaging by developing an ultrafast integrated IVUS-OCT system capable of simultaneous imaging at an unprecedented speed of 72 frames per second [48]. Validation studies using animal models and human cadaveric tissue confirmed the system’s ability to accurately characterize complex atherosclerotic plaques. These findings highlight the potential of hybrid systems to improve procedural guidance and diagnostic precision while reducing the need for multiple imaging setups. Muller and Madder presented a combined OCT and NIRS catheter system for enhanced coronary plaque assessment [49]. This multimodal approach leverages the high-resolution structural imaging of OCT and the lipid detection capabilities of NIRS, providing co-registered data to optimize stenting and identify high-risk plaques. The OCT-NIRS system significantly advances secondary prevention strategies and may pave the way for primary prevention through invasive imaging. Kassab et al. demonstrated the integration of OCT and NIRF imaging using the molecular imaging agent LUM015, which targets cathepsin protease activity (a marker of plaque inflammation) [50]. This multimodal approach leverages the high-resolution structural imaging of OCT and the functional capabilities of NIRF to provide a comprehensive assessment of atherosclerotic plaques. Validated in preclinical and human models, OCT-NIRF imaging highlights the inflammatory microenvironment of plaques, offering a promising tool for identifying high-risk lesions and guiding therapeutic strategies.
3.4. Alternative Navigation Methods
While image-guided techniques are predominant in cardiac interventions, several alternative methods provide effective navigation without relying exclusively on imaging. Electromagnetic navigation is one such method, which uses sensors and electromagnetic fields to track the position of catheters and other interventional tools within the heart. The system generates a magnetic field within which sensors detect the precise location and orientation of the catheter [51,52]. This method allows real-time tracking without ionizing radiation, making it a safer option. Another notable method is using catheters with electrode impedance mapping for tracking. This technique involves specialized electrophysiology (EP) catheters equipped with multiple electrodes that measure impedance at various points within the heart. These impedance measurements create detailed anatomical maps that reflect the heart’s structure and function, making it particularly useful for electrophysiological studies and ablation procedures. The real-time tracking and precise localization of the catheter tip provided by impedance mapping avoids the need for traditional imaging and the associated ionizing radiation. However, the quality of impedance data in specific anatomical regions, such as the left atrium or areas with dense scar tissue, can be limited. Magnetic navigation for sensing is another innovative approach that employs magnetic fields to steer and navigate catheters within the heart. Small magnets within the catheter respond to external magnetic fields generated by a navigation system, allowing the precise control of the catheter’s movement. Fiber optic realShape (FORS) technology is another emerging approach for navigation in cardiac interventions [53,54]. FORS technology utilizes fiber optic sensors embedded within catheters to provide real-time shape and position information during procedures. The fiber optic sensors measure curvature, torque, and tip deflection, allowing clinicians to precisely navigate the catheter within the heart. Unlike traditional electromagnetic or magnetic navigation systems, FORS technology does not rely on external magnetic fields or complex imaging systems, reducing the risk of interference and improving procedural efficiency. Additionally, FORS technology offers compatibility with existing catheterization equipment.
5. Challenges and Limitations
Despite the remarkable advancements in imaging techniques for cardiac interventions, significant challenges remain that hinder their widespread adoption and full potential. These obstacles span technical limitations, clinical integration, and regulatory hurdles, each requiring targeted solutions to enable the seamless integration of these technologies into routine practice. The operational complexity of advanced imaging systems often leads to increased procedural times, requiring highly precise calibration and setup. Clinically, several challenges affect both patients and healthcare providers. Radiation exposure remains a major concern, particularly with prolonged fluoroscopy use, which can pose long-term risks to patients and clinicians alike. Cost and accessibility further exacerbate these issues, as the high acquisition, maintenance, and operational expenses of advanced imaging systems limit their availability to well-resourced centers. This disparity creates a gap in equitable access to cutting-edge care, particularly in underserved regions. The integration of complex imaging systems into existing clinical workflows also presents difficulties.
These technologies often require significant workflow adjustments, increasing procedural times and sometimes introducing inefficiencies. In addition to technical and clinical challenges, regulatory and training barriers also play a critical role. The regulatory approval process for novel imaging systems, particularly those integrating artificial intelligence, can be slow and cumbersome, delaying their adoption. Meanwhile, the steep learning curve associated with advanced tools, such as robotic-guided ultrasound and hybrid imaging platforms, demands specialized training that many clinicians may lack the time or resources to pursue. Furthermore, the absence of standardized guidelines for the appropriate use of specific imaging modalities can lead to inconsistent application and suboptimal outcomes, highlighting the need for consensus-driven recommendations. Addressing these challenges will require a concerted effort from researchers, clinicians, and policymakers. Technological innovations that simplify system operation, improve image quality, and reduce radiation exposure are critical to overcoming technical barriers. Enhancing clinician training programs and streamlining regulatory pathways will be equally important to ensure that these advancements are both accessible and practical in clinical settings. Finally, fostering collaboration between stakeholders to establish standardized guidelines and equitable access can pave the way for a future where advanced imaging techniques become integral to safe, effective, and minimally invasive cardiac care.
Beyond technical performance, the widespread clinical adoption of advanced image-guided technologies faces several practical barriers. Regulatory approval processes, particularly for AI-driven or autonomous systems, are often lengthy and require extensive multicenter validation to ensure safety and efficacy across diverse patient populations. Cost is another major consideration, as the acquisition, integration, and maintenance of multimodal imaging platforms, robotic systems, and real-time processing tools may limit availability to well-resourced centers. Additionally, these technologies introduce a steep learning curve, requiring targeted physician training and institutional workflow adjustments. Addressing these barriers will be essential to translating innovation into routine clinical practice and ensuring equitable access to advanced cardiac interventions.
6. Conclusions and Future Directions
As cardiac imaging continues to evolve, future advancements are set to address current limitations while introducing transformative innovations in interventional cardiology. The next generation of imaging and navigation systems will prioritize enhanced precision, accessibility, and patient-centered care through technological breakthroughs, AI, robotics, wearable devices, and personalized medicine. Together, these developments promise to redefine the standard of care and expand the possibilities of minimally invasive cardiac procedures. Technological innovations are poised to drive the next phase of progress in imaging systems. Non-invasive approaches, such as high-resolution functional MRI and non-contrast-enhanced CT, are emerging as safer alternatives to current methods, reducing procedural risks while maintaining diagnostic accuracy. Simultaneously, augmented reality (AR) and virtual reality (VR) systems are being refined to provide interactive, real-time guidance during interventions [67]. By overlaying detailed anatomical visualizations onto the surgical field, AR/VR systems have the potential to improve precision in device placement and navigation within complex anatomical regions, revolutionizing procedural workflows.
In addition to advances in visualization and guidance technologies, innovations in contrast media are also improving the diagnostic power and safety profile of cardiovascular imaging. For example, carbon dioxide (CO2) is being used as an alternative contrast agent in angiography, particularly for patients with renal impairment, and has shown utility in detecting endoleaks during lower limb interventions [68,69]. Similarly, the development of novel agents for ultrasound and MRI enables enhanced imaging of tissue perfusion, inflammation, and fibrosis. These emerging contrast materials extend the capabilities of traditional imaging platforms and will likely contribute to more personalized and effective cardiovascular interventions in the future.
Integrating AI and robotics also represents a pivotal step in advancing the field [70]. Machine learning algorithms are already being developed to provide real-time decision support, helping clinicians assess lesion severity, optimize device selection, and refine procedural strategies based on patient-specific data [71]. Fully automated robotic navigation systems are also on the horizon, promising to reduce operator dependency and enhance procedural accuracy. These robotic systems are designed to autonomously manipulate catheters and devices, minimizing human error while ensuring consistent outcomes, particularly in high-stakes or technically demanding procedures. Wearable technologies and Internet of Things (IoT) devices are emerging as key tools for advancing patient monitoring and remote intervention capabilities [72,73,74,75,76]. Wearables that track cardiac activity and vitals in real time can provide immediate feedback during and after procedures, improving safety and post-operative care. In emergency scenarios, IoT-enabled systems could enable remote navigation, allowing experts to guide interventions from distant locations. This capability has the potential to revolutionize care delivery in underserved or remote regions, bridging gaps in access to specialized expertise and resources.
Looking ahead, the seamless integration of these emerging technologies into clinical workflows will be critical for their success. Innovations such as non-invasive imaging, AI-driven decision support, and wearable technologies must align with the practical needs of clinicians and patients alike. By addressing challenges such as cost, accessibility, and regulatory approval, the field of interventional cardiology can fully embrace these advancements, unlocking new possibilities for precision, safety, and patient-centered care. The future of cardiac imaging is bright, with these trends promising to set new standards for excellence and expand the boundaries of what is possible in minimally invasive interventions, including addressing challenges such as peri-device leaks in cardiac occlusion procedures [77].
Author Contributions
Conceptualization, M.R. and B.M.; investigation, M.R., M.S. and B.M.; resources, S.-J.J., A.J.S. and B.M.; data curation, M.R.; writing—original draft preparation, M.R. and M.S.; writing—review and editing, all authors; visualization, M.R.; supervision, B.M.; funding acquisition, S.-J.J. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the Dalio Institute of Cardiovascular Imaging for providing funds to publish this work. Sun-Joo Jang is also supported by the Yale T32 grant (T32HL098069).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ullah, A.; Kumar, M.; Sayyar, M.; Sapna, F.; John, C.; Memon, S.; Qureshi, K.; Agbo, E.C.; Ariri, H.I.; Chukwu, E.J.; et al. Revolutionizing cardiac care: A comprehensive narrative review of cardiac rehabilitation and the evolution of cardiovascular medicine. Cureus 2023, 15, e46469. [Google Scholar] [CrossRef]
- Vento, V.; Kuntz, S.; Lejay, A.; Chakfe, N. Evolutionary trends and innovations in cardiovascular intervention. Front. Med. Technol. 2024, 6, 1384008. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Campbell-Washburn, A.E.; Ramasawmy, R.; Yildirim, D.K.; Bruce, C.G.; Grant, L.P.; Stine, A.M.; Kolandaivelu, A.; Herzka, D.A.; Ratnayaka, K.; et al. Interventional cardiovascular magnetic resonance: State-of-the-art. J. Cardiovasc. Magn. Reson. 2023, 25, 48. [Google Scholar] [CrossRef]
- Sachdeva, R.; Armstrong, A.K.; Arnaout, R.; Grosse-Wortmann, L.; Han, B.K.; Mertens, L.; Moore, R.A.; Olivieri, L.J.; Parthiban, A.; Powell, A.J. Novel techniques in imaging congenital heart disease: JACC scientific statement. J. Am. Coll. Cardiol. 2024, 83, 63–81. [Google Scholar] [CrossRef]
- Sermesant, M.; Delingette, H.; Cochet, H.; Jaïs, P.; Ayache, N. Applications of artificial intelligence in cardiovascular imaging. Nat. Rev. Cardiol. 2021, 18, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.M.; Katsaggelos, A.K.; Hammond, K.J.; Hong, H.; Ahmad, F.S.; Ouyang, D.; Shah, S.J.; McCarthy, P.M.; Thomas, J.D. Deep learning for cardiovascular imaging: A review. JAMA Cardiol. 2023, 8, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, M.; Javid, R.N.; Shaghaghi, Z.; Farzipour, S.; Nosrati, S. An in-depth analysis of the adverse effects of ionizing radiation exposure on cardiac catheterization staffs. Curr. Radiopharm. 2024, 17, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, D.; Ye, L.; Housden, R.J.; Fazili, A.; Rhode, K.S. A tensor-based catheter and wire detection and tracking framework and its clinical applications. IEEE Trans. Biomed. Eng. 2021, 69, 635–644. [Google Scholar] [CrossRef]
- Zhou, J.J.; Quadri, A.; Sewani, A.; Alawneh, Y.; Gilliland-Rocque, R.; Magnin, C.; Dueck, A.; Wright, G.A.; Tavallaei, M.A. The CathPilot: A novel approach for accurate interventional device steering and tracking. IEEE/ASME Trans. Mechatron. 2022, 27, 5812–5823. [Google Scholar] [CrossRef]
- Chang, P.L.; Rolls, A.; De Praetere, H.; Vander Poorten, E.; Riga, C.V.; Bicknell, C.D.; Stoyanov, D. Robust catheter and guidewire tracking using b-spline tube model and pixel-wise posteriors. IEEE Robot. Autom. Lett. 2016, 1, 303–308. [Google Scholar] [CrossRef]
- Roshanfar, M.; Fekri, P.; Dargahi, J. A deep learning model for tip force estimation on steerable catheters via learning-from-simulation. In Proceedings of the Hamlyn Symposium on Medical Robotics, London, UK, 26–29 June 2023. [Google Scholar]
- Fekri, P.; Khodashenas, H.; Lachapelle, K.; Cecere, R.; Zadeh, M.; Dargahi, J. Y-net: A deep convolutional architecture for 3d estimation of contact forces in intracardiac catheters. IEEE Robot. Autom. Lett. 2022, 7, 3592–3599. [Google Scholar] [CrossRef]
- Fekri, P.; Zadeh, M.; Dargahi, J. H-Net: A Multitask Architecture for Simultaneous 3D Force Estimation and Stereo Semantic Segmentation in Intracardiac Catheters. IEEE Robot. Autom. Lett. 2024, 10, 844–851. [Google Scholar] [CrossRef]
- Agricola, E.; Meucci, F.; Ancona, F.; Sanz, A.P.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. Euro Interv. 2022, 17, 1205. [Google Scholar] [CrossRef]
- Wang, S.; Singh, D.; Lau, D.; Reddy, K.; Althoefer, K.; Rhode, K.; Housden, R.J. Probe tracking and its application in automatic acquisition using a trans-esophageal ultrasound robot. In Proceedings of the Computer-Assisted and Robotic Endoscopy: Third International Workshop, CARE 2016, Held in Conjunction with MICCAI 2016, Athens, Greece, 17 October 2016; Revised Selected Papers 3. Springer: Berlin/Heidelberg, Germany, 2017; pp. 14–23. [Google Scholar]
- Yang, Z.; Yang, L.; Zhang, M.; Wang, Q.; Yu, S.C.H.; Zhang, L. Magnetic control of a steerable guidewire under ultrasound guidance using mobile electromagnets. IEEE Robot. Autom. Lett. 2021, 6, 1280–1287. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, K.F.; Schweizer, K.; Du, X.; Jin, D.; Yu, S.C.H.; Nelson, B.J.; Zhang, L. Ultrasound Doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 2021, 7, eabe5914. [Google Scholar] [CrossRef] [PubMed]
- Padhan, J.; Tsekos, N.; Al-Ansari, A.; Abinahed, J.; Deng, Z.; Navkar, N.V. Dynamic Guidance Virtual Fixtures for Guiding Robotic Interventions: Intraoperative MRI-guided Transapical Cardiac Intervention Paradigm. In Proceedings of the 2022 IEEE 22nd International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 7–9 November 2022; IEEE: Piscataway, NY, USA, 2022; pp. 265–270. [Google Scholar]
- Velasco Forte, M.N.; Roujol, S.; Ruijsink, B.; Valverde, I.; Duong, P.; Byrne, N.; Krueger, S.; Weiss, S.; Arar, Y.; Reddy, S.R.V.; et al. MRI for guided right and left heart cardiac catheterization: A prospective study in congenital heart disease. J. Magn. Reson. Imaging 2021, 53, 1446–1457. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, X.; Fang, G.; He, Z.; Ho, J.D.L.; Cheung, C.L.; Tang, W.L.; Xie, X.; Liang, L.; Chang, H.C.; et al. Shape tracking and feedback control of cardiac catheter using MRI-guided robotic platform—Validation with pulmonary vein isolation simulator in MRI. IEEE Trans. Robot. 2022, 38, 2781–2798. [Google Scholar] [CrossRef]
- Kensicher, T.; Leclerc, J.; Biediger, D.; Shah, D.J.; Seimenis, I.; Becker, A.T.; Tsekos, N.V. Towards MRI-guided and actuated tetherless milli-robots: Preoperative planning and modeling of control. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, Canada, 24–28 September 2017; IEEE: Piscataway, NY, USA, 2017; pp. 6440–6447. [Google Scholar]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Yeniaras, E.; Lamaury, J.; Navkar, N.V.; Shah, D.J.; Chin, K.; Deng, Z.; Tsekos, N.V. Magnetic resonance based control of a robotic manipulator for interventions in the beating heart. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; IEEE: Piscataway, NY, USA, 2011; pp. 6270–6275. [Google Scholar]
- Kurogi, K.; Ishii, M.; Yamamoto, N.; Yamanaga, K.; Tsujita, K. Optical coherence tomography-guided percutaneous coronary intervention: A review of current clinical applications. Cardiovasc. Interv. Ther. 2021, 36, 169–177. [Google Scholar] [CrossRef]
- Amabile, N.; Range, G.; Souteyrand, G.; Godin, M.; Boussaada, M.; Meneveau, N.; Cayla, G.; Casassus, F.; Lefevre, T.; Hakim, R.; et al. Optical coherence tomography to guide percutaneous coronary intervention of the left main coronary artery: The LEMON study: OCT guidance in LM PCI. Euro Interv. 2021, 17, e124. [Google Scholar]
- Yonetsu, T.; Jang, I.K. Cardiac optical coherence tomography: History, current status, and perspective. JACC Asia 2024, 4, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Ahn, J.M.; Yun, S.C.; Hur, S.H.; Cho, Y.K.; Lee, C.H.; Hong, S.J.; Lim, S.; Kim, S.W.; Won, H.; et al. Guiding intervention for complex coronary lesions by optical coherence tomography or intravascular ultrasound. J. Am. Coll. Cardiol. 2024, 83, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Rathod, K.S.; Koganti, S.; Hamshere, S.; Astroulakis, Z.; Lim, P.; Sirker, A.; O’Mahony, C.; Jain, A.K.; Knight, C.J.; et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: Outcomes from the pan-London PCI cohort. JACC Cardiovasc. Interv. 2018, 11, 1313–1321. [Google Scholar] [CrossRef]
- Chamié, D.; Costa Jr, J.R.; Damiani, L.P.; Siqueira, D.; Braga, S.; Costa, R.; Seligman, H.; Brito, F.; Barreto, G.; Staico, R.; et al. Optical coherence tomography versus intravascular ultrasound and angiography to guide percutaneous coronary interventions: The iSIGHT randomized trial. Circ. Cardiovasc. Interv. 2021, 14, e009452. [Google Scholar] [CrossRef] [PubMed]
- IJsselmuiden, A.; Zwaan, E.; Oemrawsingh, R.; Bom, M.; Dankers, F.; de Boer, M.J.; Camaro, C.; van Geuns, R.; Daemen, J.; Van Der Heijden, D.J.; et al. Appropriate use criteria for optical coherence tomography guidance in percutaneous coronary interventions: Recommendations of the working group of interventional cardiology of the Netherlands Society of Cardiology. Neth. Heart J. 2018, 26, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.; Piao, Z.; Albaghdadi, M.S.; Coughlin, P.A.; Rudd, J.H.; Tearney, G.J.; Jaffer, F.A. Intravascular fluorescence molecular imaging of atherosclerosis. In Atherosclerosis: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 853–872. [Google Scholar]
- Hogue, C.W.; Levine, A.; Hudson, A.; Lewis, C. Clinical applications of near-infrared spectroscopy monitoring in cardiovascular surgery. Anesthesiology 2021, 134, 784. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhou, C.; Li, M.; Wang, X.; Zhang, W.; Liu, Z.; Wu, L.; James, T.D.; Li, P.; et al. Precision navigation of hepatic ischemia–reperfusion injury guided by lysosomal viscosity-activatable NIR-II fluorescence. J. Am. Chem. Soc. 2022, 144, 13586–13599. [Google Scholar] [CrossRef]
- Rauschendorfer, P.; Lenz, T.; Nicol, P.; Wild, L.; Beele, A.; Sabic, E.; Klosterman, G.; Laugwitz, K.L.; Jaffer, F.A.; Gorpas, D.; et al. Intravascular ICG-enhanced NIRF-IVUS imaging to assess progressive atherosclerotic lesions in excised human coronary arteries. npj Cardiovasc. Health 2024, 1, 14. [Google Scholar] [CrossRef]
- Bao, K.; Tully, M.; Cardenas, K.; Wang, H.; Srinivas, S.; Rho, J.; Jeon, O.H.; Dinh, J.; Yokomizo, S.; McDonnell, R.; et al. Ultralow Background Near-Infrared Fluorophores with Dual-Channel Intraoperative Imaging Capability. Adv. Healthc. Mater. 2023, 12, 2203134. [Google Scholar] [CrossRef]
- Chakravarty, R. Development of Radionuclide Generators for Biomedical Applications. Ph.D. Thesis, Homi Bhabha National Institute, Mumbai, India, 2011. [Google Scholar]
- Ford, E.C.; Herman, J.; Yorke, E.; Wahl, R.L. 18F-FDG PET/CT for image-guided and intensity-modulated radiotherapy. J. Nucl. Med. 2009, 50, 1655–1665. [Google Scholar] [CrossRef]
- Bokhari, S.; Castaño, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ. Cardiovasc. Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; DaSilva, J.; Birnie, D.; et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Jones, C.; Joshi, N.V.; Fletcher, A.M.; Richardson, H.; White, A.; Marsden, M.; Pessotto, R.; Clark, J.C.; Wallace, W.A.; et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012, 125, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Cartlidge, T.R.; Doris, M.K.; Sellers, S.L.; Pawade, T.A.; White, A.C.; Pessotto, R.; Kwiecinski, J.; Fletcher, A.; Alcaide, C.; Lucatelli, C.; et al. Detection and prediction of bioprosthetic aortic valve degeneration. J. Am. Coll. Cardiol. 2019, 73, 1107–1119. [Google Scholar] [CrossRef]
- Thorn, S.L.; Shuman, J.A.; Stacy, M.R.; Purcell, B.P.; Doviak, H.; Burdick, J.A.; Spinale, F.G.; Sinusas, A.J. Matrix metalloproteinase-targeted SPECT/CT imaging for evaluation of therapeutic hydrogels for the early modulation of post-infarct myocardial remodeling. J. Cardiovasc. Transl. Res. 2023, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tufaro, V.; Jaffer, F.A.; Serruys, P.W.; Onuma, Y.; van der Steen, A.F.; Stone, G.W.; Muller, J.E.; Marcu, L.; Van Soest, G.; Courtney, B.K.; et al. Emerging hybrid intracoronary imaging technologies and their applications in clinical practice and research. Cardiovasc. Interv. 2024, 17, 1963–1979. [Google Scholar] [CrossRef]
- Noori, M.; Hougaard, M.; Maehara, A.; Trøan, J.; Hansen, K.; Ellert, J.; Veien, K.; Hansen, H.; Junker, A.; Lassen, J.; et al. TCT-53 Near-Infrared Spectroscopy and Intravascular Ultrasound-Guided vs Angiography-Guided Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction: The NIRVUS Trial. J. Am. Coll. Cardiol. 2024, 84, B174. [Google Scholar] [CrossRef]
- Schneider, V.S.; Böhm, F.; Blum, K.; Riedel, M.; Abdelwahed, Y.S.; Klotsche, J.; Steiner, J.K.; Heuberger, A.; Skurk, C.; Mochmann, H.C.; et al. Impact of real-time angiographic co-registered optical coherence tomography on percutaneous coronary intervention: The OPTICO-integration II trial. Clin. Res. Cardiol. 2021, 110, 249–257. [Google Scholar] [CrossRef]
- Lim, S.; Cha, J.J.; Joo, H.J.; Park, J.H.; Yu, C.W.; Ahn, T.H.; Lim, D.S.; Hong, S.J. The Role of Lipid Core Burden Index Measured by Near-Infrared Spectroscopy in Predicting Slow TIMI Flow After Coronary Intervention. Circulation 2021, 144, A12190. [Google Scholar] [CrossRef]
- Sheth, T.N.; Pinilla-Echeverri, N.; Mehta, S.R.; Courtney, B.K. First-in-human images of coronary atherosclerosis and coronary stents using a novel hybrid intravascular ultrasound and optical coherence tomographic catheter. JACC Cardiovasc. Interv. 2018, 11, 2427–2430. [Google Scholar] [CrossRef]
- Li, J. Development of an Ultrafast Integrated IVUS-OCT System and Catheter for In Vivo Applications. Ph.D. Thesis, UC Irvine, Irvine, CA, USA, 2015. [Google Scholar]
- Muller, J.; Madder, R. OCT-NIRS imaging for detection of coronary plaque structure and vulnerability. Front. Cardiovasc. Med. 2020, 7, 90. [Google Scholar] [CrossRef]
- Kassab, M.; Thrapp, A.; Gardecki, J.A.; Ahsen, O.O.; Kawamura, Y.; Mauskapf, A.; Spicer, G.; Gavgiotaki, E.; Kumar, A.; Modi, M.; et al. LUM015, A Translatable Molecular Imaging Agent, Enables OCT-NIRF Imaging of Inflammatory Protease Activity in Preclinical and Human Atherosclerosis. J. Am. Coll. Cardiol. 2022, 79, 1762. [Google Scholar] [CrossRef]
- Cochennec, F.; Riga, C.; Hamady, M.; Cheshire, N.; Bicknell, C. Improved catheter navigation with 3D electromagnetic guidance. J. Endovasc. Ther. 2013, 20, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Torabinia, M.; Dhrif, H.; Caprio, A.; Liu, J.; Wong, S.C.; Mosadegh, B. Development of a hybrid training simulator for structural heart disease interventions. Adv. Intell. Syst. 2020, 2, 2000109. [Google Scholar] [CrossRef]
- Finnesgard, E.J.; Simons, J.P.; Marecki, H.; Ofori, I.; Kölbel, T.; Schurink, G.W.H.; van Herwaarden, J.A.; Schanzer, A. Fiber Optic RealShape technology in endovascular surgery. Semin. Vasc. Surg. 2021, 34, 241–246. [Google Scholar] [CrossRef]
- van Herwaarden, J.A.; Jansen, M.M.; Vonken, E.j.P.; Bloemert-Tuin, T.; Bullens, R.W.; de Borst, G.J.; Hazenberg, C.E. First in human clinical feasibility study of endovascular navigation with Fiber Optic RealShape (FORS) technology. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, M.; Mitchell, H.R.; Buccola, J.; Pinilla-Echeverri, N. Impact of Artificial Intelligence-Enhanced Optical Coherence Tomography Software on Percutaneous Coronary Intervention Decisions. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Improving the diagnosis and treatment of congenital heart disease through the combination of three-dimensional echocardiography and image guided surgery. BMC Med. Imaging 2024, 24, 61. [Google Scholar] [CrossRef]
- Gandhi, S.; Mosleh, W.; Shen, J.; Chow, C.M. Automation, machine learning, and artificial intelligence in echocardiography: A brave new world. Echocardiography 2018, 35, 1402–1418. [Google Scholar] [CrossRef]
- Kweon, J.; Kim, K.; Lee, C.; Kwon, H.; Park, J.; Song, K.; Kim, Y.I.; Park, J.; Back, I.; Roh, J.H.; et al. Deep reinforcement learning for guidewire navigation in coronary artery phantom. IEEE Access 2021, 9, 166409–166422. [Google Scholar] [CrossRef]
- Ma, Y.; Gogin, N.; Cathier, P.; Housden, R.J.; Gijsbers, G.; Cooklin, M.; O’Neill, M.; Gill, J.; Rinaldi, C.A.; Razavi, R.; et al. Real-time x-ray fluoroscopy-based catheter detection and tracking for cardiac electrophysiology interventions. Med. Phys. 2013, 40, 071902. [Google Scholar] [CrossRef]
- Unberath, M.; Gao, C.; Hu, Y.; Judish, M.; Taylor, R.H.; Armand, M.; Grupp, R. The impact of machine learning on 2d/3d registration for image-guided interventions: A systematic review and perspective. Front. Robot. AI 2021, 8, 716007. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, A.; Xu, Y.; Xiong, H.; Meng, M.Q.H. Rl-tee: Autonomous probe guidance for transesophageal echocardiography based on attention-augmented deep reinforcement learning. IEEE Trans. Autom. Sci. Eng. 2023, 21, 1526–1538. [Google Scholar] [CrossRef]
- Kienzlen, A.; Jaensch, F.; Verl, A.; Cheng, L. Concept for a reinforcement learning approach to navigate catheters through blood vessels. In Proceedings of the 2022 28th International Conference on Mechatronics and Machine Vision in Practice (M2VIP), Nanjing, China, 16–18 November 2022; IEEE: Piscataway, NY, USA, 2022; pp. 1–4. [Google Scholar]
- Omisore, O.M.; Akinyemi, T.; Duan, W.; Du, W.; Wang, L. A novel sample-efficient deep reinforcement learning with episodic policy transfer for PID-based control in cardiac catheterization robots. arXiv 2021, arXiv:2110.14941. [Google Scholar]
- Bian, G.; Lipowicz, M.; Kruger, G.H. Self-learning of inverse kinematics for feedforward control of intracardiac robotic ablation catheters. In Proceedings of the 2015 Pattern Recognition Association of South Africa and Robotics and Mechatronics International Conference (PRASA-RobMech), Port Elizabeth, South Africa, 26–27 November 2015; IEEE: Piscataway, NY, USA, 2015; pp. 72–77. [Google Scholar]
- Liu, W.; Tian, T.; Xu, W.; Liang, B.; Lu, Q.; Pan, X.; Zhao, W.; Yang, H.; Su, R. Image-Guided Autonomous Guidewire Navigation in Robot-Assisted Endovascular Interventions using Reinforcement Learning. arXiv 2024, arXiv:2403.05748. [Google Scholar]
- Li, K.; Xu, Y.; Wang, J.; Ni, D.; Liu, L.; Meng, M.Q.H. Image-guided navigation of a robotic ultrasound probe for autonomous spinal sonography using a shadow-aware dual-agent framework. IEEE Trans. Med. Robot. Bionics 2021, 4, 130–144. [Google Scholar] [CrossRef]
- Annabestani, M.; Sriram, S.; Caprio, A.; Janghorbani, S.; Wong, S.C.; Sigaras, A.; Mosadegh, B. High-fidelity pose estimation for real-time extended reality (XR) visualization for cardiac catheterization. Sci. Rep. 2024, 14, 26962. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.; Marjan, A.E. Current status of carbon dioxide angiography. J. Vasc. Surg. 2017, 66, 618–637. [Google Scholar] [CrossRef]
- Ali, M.; Noureldin, M.; Kashef, O.E.; Zaghlol, H. Safety and effectiveness of carbon dioxide contrast medium in infra-inguinal endovascular interventions for patients with chronic threatening lower limb ischemia and renal impairment: A multicentric trial. J. Endovasc. Ther. 2024, 31, 772–783. [Google Scholar] [CrossRef]
- Roshanfar, M.; Salimi, M.; Kaboodrangi, A.H.; Jang, S.J.; Sinusas, A.J.; Wong, S.C.; Mosadegh, B. Advanced Robotics for the Next-Generation of Cardiac Interventions. Micromachines 2025, 16, 363. [Google Scholar] [CrossRef]
- Salimi, M.; Roshanfar, M.; Tabatabaei, N.; Mosadegh, B. Machine Learning-Assisted Short-Wave InfraRed (SWIR) Techniques for Biomedical Applications: Towards Personalized Medicine. J. Pers. Med. 2023, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Pevnick, J.M.; Birkeland, K.; Zimmer, R.; Elad, Y.; Kedan, I. Wearable technology for cardiology: An update and framework for the future. Trends Cardiovasc. Med. 2018, 28, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm. 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Cheung, C.C.; Krahn, A.D.; Andrade, J.G. The emerging role of wearable technologies in detection of arrhythmia. Can. J. Cardiol. 2018, 34, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Nimmi, K.; Wajid, M.A. Revolutionizing Healthcare with IoT in Cardiology. In Wellness Management Powered by AI Technologies; Scrivener: Beverly, MA, USA, 2025; pp. 231–273. [Google Scholar]
- Yashudas, A.; Gupta, D.; Prashant, G.; Dua, A.; AlQahtani, D.; Reddy, A.S.K. Deep-cardio: Recommendation system for cardiovascular disease prediction using iot network. IEEE Sens. J. 2024, 24, 14539–14547. [Google Scholar] [CrossRef]
- Roshanfar, M.; Jang, S.J.; Sinusas, A.; Wong, S.C.; Mosadegh, B. Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question. Surgeries 2025, 6, 15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).