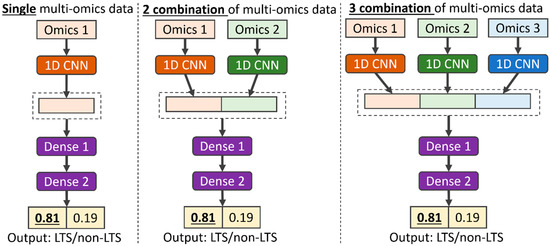
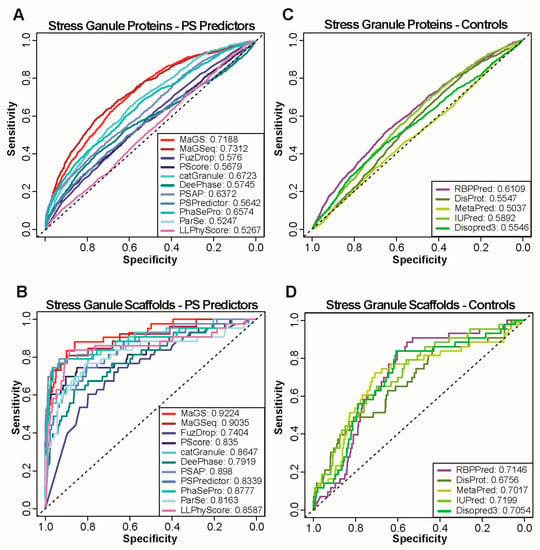
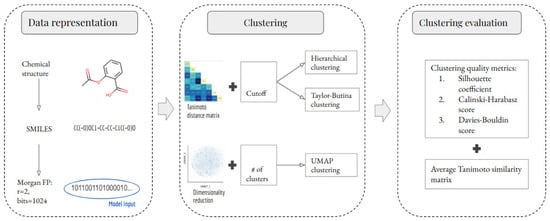
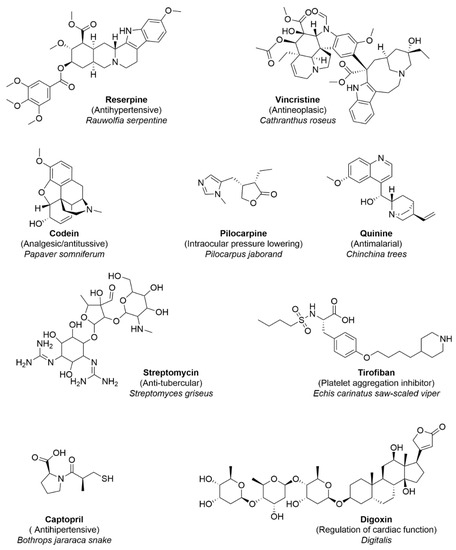
Feature Papers in Bioinformatics and Systems Biology Section
A topical collection in Biomolecules (ISSN 2218-273X). This collection belongs to the section "Bioinformatics and Systems Biology".
Viewed by 93845Editor
Interests: structural bioinformatics; intrinsically disordered proteins; protein function prediction; protein-ligand interactions; protein-nucleic acids interactions; structural genomics
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
This Topical Collection, “Feature Papers in Bioinformatics and Systems Biology”, collects high-quality research articles on outstanding new algorithms and databases in computational molecular biology and significant discoveries that have been developed through the specialized use of computational tools, methods, and databases. It also includes review articles that cover popular and emerging areas of research. With the goal to cover the frontiers of the research in bioinformatics and systems biology, the submissions that reflect the latest progress in their research field are limited to the the Editorial Board Members of Biomolecules and the senior authors they invite. These invited papers will be published online, free of charge, once accepted.
Topics covered in this Collection include, without being limited to, the following:
- Databases and ontologies;
- Function determination and prediction;
- Gene and protein expression analysis;
- Gene and protein networks;
- Genome and proteome analysis;
- Phylogenetics;
- Sequence analysis;
- Structural bioinformatics;
- Structure determination and prediction;
- Systems biology.
Dr. Lukasz Kurgan
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.