Abstract
Tetrahydroquinoline skeletons can be formed by a CuCl2-catalyzed one-pot reaction of N-methyl-N-alkylanilines and vinyl ethers in the presence of t-butyl-hydroperoxide.
Introduction
Tetrahydroquinolines are an important structural subunit of natural products and many tetrahydroquinoline derivatives exhibit interesting biological and pharmaceutical activities [1]. Consequently, synthetic methodologies for preparing tetrahydroquinoline derivatives have attracted considerable interest and several methods offering good results have been reported [2,3,4]. Nonetheless, there are still some features requiring improvement in these methods, e.g. the catalysts used are expensive, the systems require special atmospheres and organic solvents are always needed. Thus, a procedure involving a low-cost catalyst and simple and eco-friendly conditions might be very useful.
Copper is one of the oldest transition metals used in organic synthesis and copper salts are still broadly employed nowadays [5,6]. Among these copper salts, CuCl2, as a mild Lewis acid, is especially favored by chemists due to its inexpensiveness, lack of toxicity and easy-handling. Herein, we wish to report a one-pot synthesis of tetrahydroquinoline derivatives based on a CuCl2-catalyzed room temperature reaction of N-methyl-N-alkylanilines and vinyl ethers in the presence of t-butylhydroperoxide (TBHP).
Results and Discussion
Initailly, N,N-dimethylaniline (1a, 1 mmol) was reacted with n-butyl vinyl ether (2a, 1.0 mmol) in the presence of TBHP (1 mmol) at room temperature. No product was observed even after 2 days. On the other hand, when a catalytic amount of CuCl2 (5 mol%) was added to the reaction mixture, a smooth reaction occurred and tetrahydroquinoline derivative 3a was isolated in 40% yield. To optimize the CuCl2-based system, different conditions were tested on N,N-dimethylaniline (1a), and a 1:1.2:2 ratio of 1a/2a/TBHP was found to work best. Several other catalysts (CuBr, FeCl2, CuCl, InCl3 and SbCl3) were examined under the optimized conditions (Table 1). CuBr, FeCl2, and CuCl were found to be effective in the reaction, but gave much lower yields. It is noteworthy that when CuCl was used as the catalyst, the N-demethylation product 4a was found to be the main product [4c]. As for InCl3 and SbCl3, none of the target product was detected.

Table 1.
One-pot sythesis of tetrahedroquinonline catalyzed by Lewis acid. 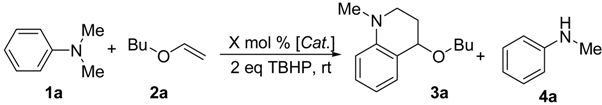

 |
a Isolated yield.
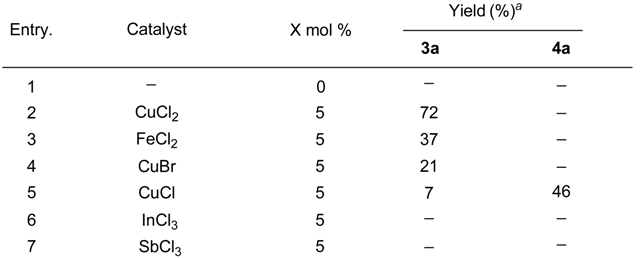
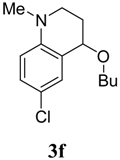
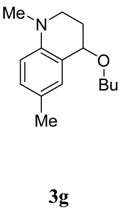
The transformation was found to be general for various aniline derivatives under the optimal conditions. Representative examples are listed in Table 2. In most cases, moderate to good yields of the corresponding tetrahydroquinoline derivatives were obtained. The scope of the reaction could also be extended to substituted N-methyl-N-alkylanilines 1b-h. When para-chloro-N,N-dimethylaniline (1f) was used, the desired product 3f was formed in good yield (Table 2, entry 8). The reaction of para-methyl substituted substrate 1g also afforded the desired product 3g, however, the yield diminished to 35% (Table 2, entry 9). When N,N-3-trimethylbenzenamine (1h) was used, a 1:1 mixture of two isomers with the methyl group on either the 5- or 8-positions was observed (entry 10). When ethyl vinyl ether (2b) was employed as the olefin and reacted with the N-methyl-N-alkylanilines, the desired products were formed in lower yields. Under the optimal condition, when styrene and 1-octene were used as the olefin reacted with the N-methyl-N-alkylanilines, no target products were observed.

Table 2.
Reaction of N-methyl-N-alkylanilines with vinyl ether catalyzed by CuCl2.
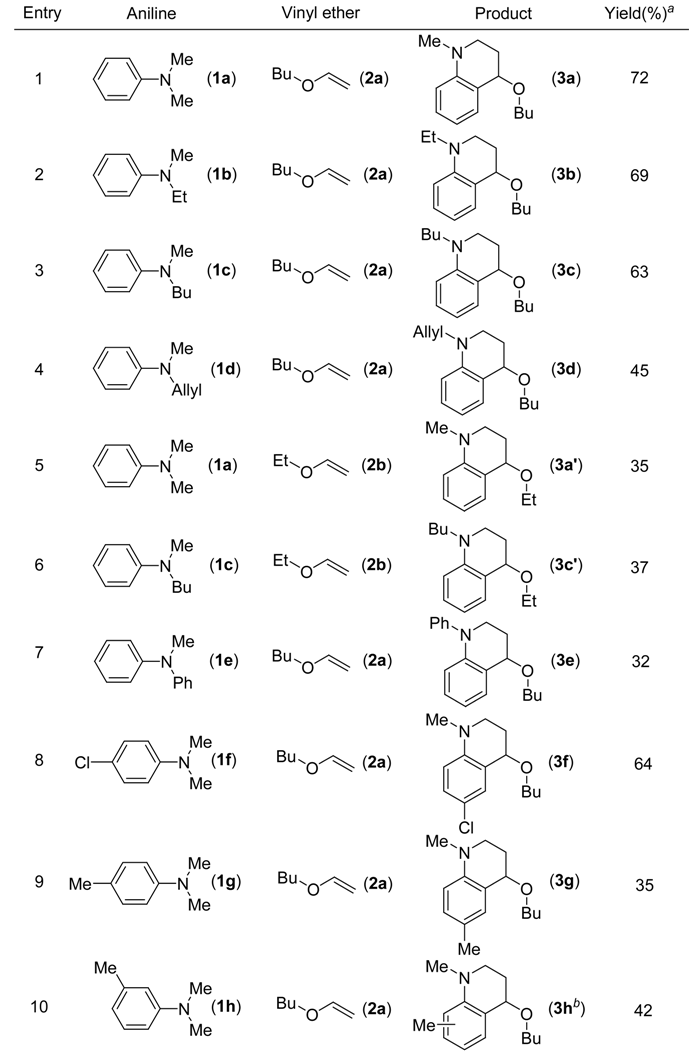 |
aIsolated yield be based on the substrate;bMixture of the two isomers, the ratio is one to one.
It is known that tertiary N-methylanilines can be converted chemoselectively into the corresponding N-(t-butyl-dioxymethyl)anilines 5 efficiently by the ruthenium-catalyzed oxidation with TBHP [4b-c].However, such products were not obtained in this work, in which N-phenylformamide 7 was obtained after hydrolysis. To clarify whether the final product was formed from N-(t-butyldioxymethyl)aniline 5, the compound 5a was prepared according to literature [4b-c]. The reaction of 5a with n-butyl vinyl ether (2a) in the presence of a catalytic amount of CuCl2 at room temperature was carried out and the tetrahydroquinonline 3a was obtained in 58 % yield (Scheme 1). This result indicated that CuCl2 acted as an effective Lewis acid in this reaction. It also explains why no formation of compounds 5 was observed in the presence of CuCl2.
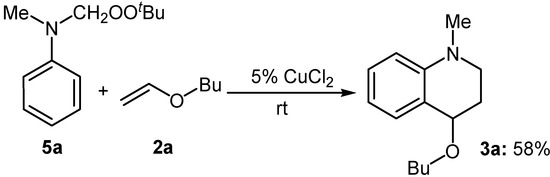
Scheme 1.
Scheme 1.
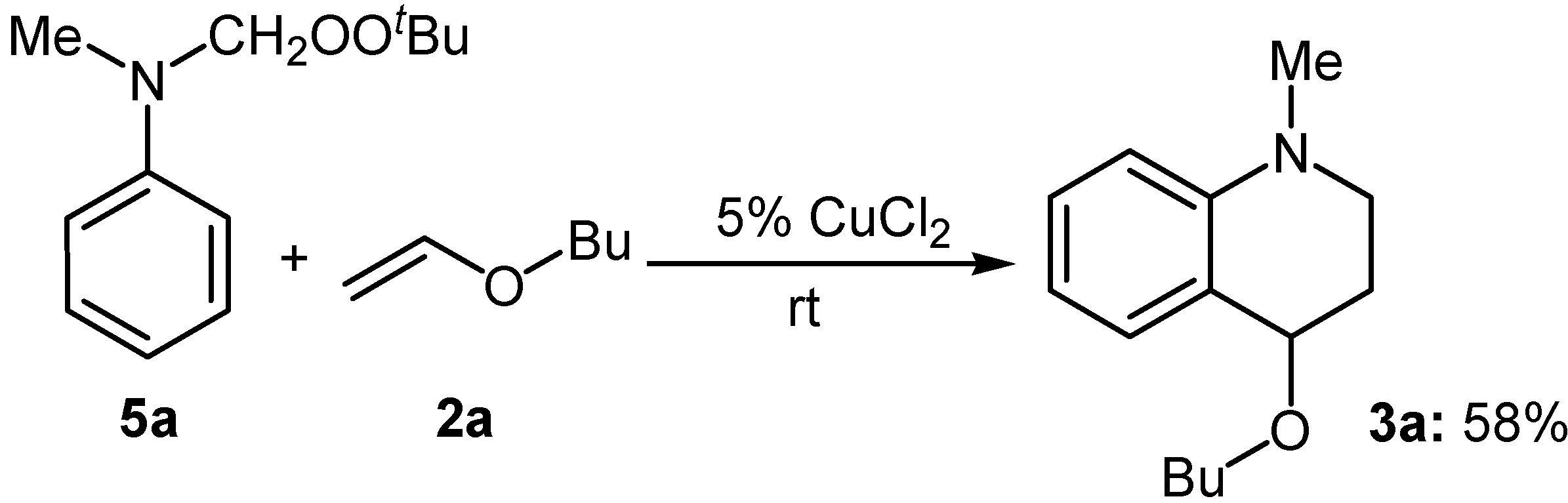
Although the mechanism of reaction presented here is not yet clear, based on the above mentioned results, one possible reaction pathway is shown in Scheme 2. First, N-methyl-N-alkylaniline 1 reacts with TBHP, catalyzed by CuCl2, to give compound 5. The latter transforms into the iminium ion intermediate 6 in the presence of the Lewis acid (CuCl2), which can form N-phenylformamide 7 after quenching by water. Compound 6 then reacts with vinyl ether 2 to give the cationic intermediate 8. Finally, electrophilic aromatic ring closure affords the 1,4-disubstituted tetrahydroquinoline derivative 3.
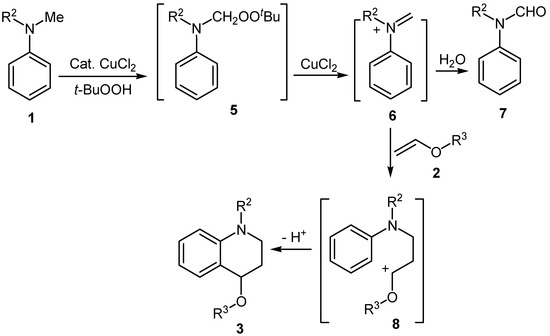
Scheme 2.
Proposed mechanism.
Scheme 2.
Proposed mechanism.
Conclusions
In summary, a one-pot reaction for the formation of tetrahydroquinolines based on the reaction of N-methyl-N-alkylanilines and vinyl ethers in the presence of t-butyl hydroperoxide using CuCl2 as the catalyst was developed. The scope and synthetic applications of this reaction are currently under investigation.
Experimental
General
1H- and 13C-NMR spectra were recorded at 300 and 75 MHz, respectively, using CDCl3 as solvent on a JEOL JNM-ECA300 spectrometer. ESI-MS were recorded on Bruker Esquire-LC/MSn instrument. Reagents such as N,N-dimethylaniline (1a), n-butyl vinyl ether (2a), ethyl vinyl ether (2b) and TBHP were purchased; others, like N-ethyl-N-methylaniline (1b), N-butyl-N-methylaniline (1c), N-allyl-N-methylaniline (1d), N-methyl-N-phenylaniline (1e), 4-chloro-N,N-dimethylaniline (1f), 4,N,N-trimethylaniline (1g) and 3,N,N-trimethylaniline (1h) were prepared according to the literature [7].
Representative procedure for the CuCl2-catalyzed one-pot formation of tetrahydroquinolines from N‑methyl-N-alkylanilines and vinyl ethers in the presence of t-butyl hydroperoxide: reaction of N,N-dimethylaniline (1a) and n-butyl vinyl ether (2a)
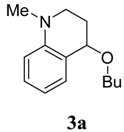
CuCl2 (14 mg, 0.1 mmol), N,N- dimethylaniline (1a, 252 µL, 2 mmol), and n-butyl vinyl ether (2a, 312 µL, 2.4 mmol) were placed together into an open glass vessel and stirred at room temperature. Then, t-butyl hydroperoxide (TBHP) (600 µL, 2.0 mmol) was slowly added dropwise. The reaction mixture was stirred at room temperature for 12 h before being quenched with aqueous K2CO3. The organic phase was extracted with CH2Cl2 (10 mL x 2), the solvent was evaporated under reduced pressure and the residue was isolated by chromatography on a column of neutral Al2O3 column (40 mL) using a 1:100 mixture of 1:100 EtOAc/petroleum ether as eluent to give 4-butoxy-1-methyl-1,2,3,4-tetrahydroquinoline (3a) [4d] as a colorless liquid (158 mg, 72 %); 1H-NMR (CDCl3): δ = 0.90 (t, J = 6.8 Hz, 3H), 1.34-1.44 (m, 2H), 1.52-1.61 (m, 2H), 1.84-1.93 (m, 1H), 2.04-2.12 (m, 1H), 2.89 (s, 3H), 3.04-3.10 (m, 1H), 3.32-3.55 (m, 3H), 4.28 (t, J = 3.8 Hz, 1H), 6.59-6.64 (m, 2H), 7.11-7.16 (m, 2H); 13C-NMR: δ = 13.9, 19.5, 27.3, 32.2, 38.9, 46.3, 67.5, 73.1, 111.3, 115.6, 121.6, 129.2, 130.3, 146.3; ESI-MS: 242 [M+Na]+.
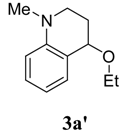
4-Ethoxy-1-methyl-1,2,3,4-tetrahydroquinoline (3a′)[3d]. Colorless liquid (35 %); 1H-NMR: δ = 1.15 (t, 3H), 1.83-1.91 (m, 1H), 2.00-2.06 (m, 1H), 2.85 (s, 3H), 3.01-3.05 (m, 1H), 3.34-3.37 (m, 1H), 3.47-3.53 (m, 2H), 4.25(t, 1H), 6.54-6.56 (m, 2H), 7.04-7.08 (m, 2H); 13C-NMR: δ = 15.7, 27.5, 39.0, 46.4, 63.1, 73.0, 111.4, 115.6, 121.5, 129.4, 130.5, 146.4; ESI-MS: 214 [M+Na]+.
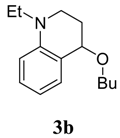
4-Butoxy-1-ethyl-1,2,3,4-tetrahydroquinoline (3b). Colorless liquid (69 %); 1H-NMR: δ = 0.89 (t, J = 7.2 Hz, 3H), 1.27 (t, J = 7.2 Hz, 3H), 1.20-1.30 (m, 2H), 1.52-1.58 (m, 2H), 1.81-1.89 (m, 1H), 2.07-2.15 (m, 1H), 3.08-3.15 (m, 1H), 3.21-3.36 (m, 1H), 3.38-3.54 (m, 4H), 4.28 (t, J = 3.4 Hz, 1H), 6.55-6.65 (m, 2H), 7.10-7.15 (m, 2H); 13C-NMR: δ = 11.0, 13.9, 19.5, 27.2, 32.2, 43.3, 45.3, 67.4, 73.2, 111.0, 114.8, 121.3, 129.2, 130.8, 144.8; ESI-MS: 256 [M+Na]+.
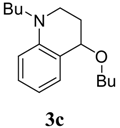
1-Butyl-4-butoxy-1,2,3,4-tetrahydroquinoline (3c). Colorless liquid (78 %); 1H-NMR: δ = 0.89 (t, J = 7.5 Hz, 3H), 0.94 (t, J = 7.2 Hz, 3H), 1.30-1.42 (m, 4H), 1.54-1.61 (m, 4H), 1.80-1.89 (m, 1H), 2.05-2.11 (m, 1H), 3.10-3.35 (m, 3H), 3.41-3.56 (m, 3H), 4.28 (t, J = 3.4 Hz, 1H), 6.54-6.62 (m, 2H), 7.09-7.15 (m, 2H); 13C-NMR: δ = 14.1, 19.6, 20.5, 27.2, 28.7, 32.3, 44.4, 51.2, 67.5, 73.3, 111.0, 114.7, 121.0, 129.3, 130.8, 145.2, 146.0; ESI-MS: 284 [M+Na]+.
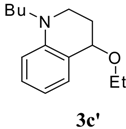
1-Butyl-4-ethoxy-1,2,3,4-tetrahydroquinoline (3c’). Colorless liquid (37 %); 1H-NMR : δ = 0.88 (t, J = 7.5 Hz, 3H), 1.14 (t, J = 7.2 Hz, 3H), 1.22-1.36 (m, 2H), 1.44-1.56 (m, 2H), 1.74-1.84 (m, 1H), 1.98-2.07 (m, 1H), 3.04-3.52 (m, 6H), 4.22-4.24 (m, 1H), 6.48-6.6 3(m, 2H), 7.03-7.16 (m, 2H); 13C-NMR: δ = 14.1, 15.6, 51.1, 44.3, 38.3, 28.6, 20.5, 62.9, 73.1, 111.0, 112.1, 114.6, 129.2, 129.3, 130.8, 145.1; ESI-MS: 256 [M+Na]+.
1-Allyl-4-butoxy-1,2,3,4-tetrahydroquinoline (3d). Colorless liquid (45 %); 1H-NMR : δ = 0.94 (t, J = 7.2 Hz, 3H), 1.36-1.48 (m, 2H), 1.56-1.66 (m, 2H), 1.86-1.98 (m, 1H), 2.12-2.18 (m, 1H), 2.95 (s, 1H), 3.12-3.20 (m, 1H), 3.42-3.57 (m, 3H), 3.92 (s, 1H), 4.34 (s, 1H), 5.18-5.24 (m, 2H), 5.82-5.88 (m, 1H), 6.61-6.66 (m, 2H), 7.11-7.19 (m, 2H); 13C-NMR: δ = 14.1, 19.6, 27.3, 32.3, 44.2, 53.7, 67.5, 73.3, 111.5, 115.2, 115.9, 121.3, 129.3, 130.6, 133.5, 145.2; ESI-MS: 268 [M+Na]+.
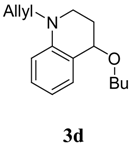
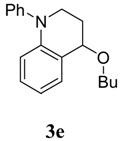
4-Butoxy-1-phenyl-1,2,3,4-tetrahydroquinoline (3e). Colorless liquid (37 %); 1H-NMR : δ = 0.99 (t, J = 7.2 Hz, 3H), 1.36-1.52 (m, 2H), 1.56-1.74 (m, 2H), 2.02-2.14 (m, 1H), 2.21-2.28 (m, 1H), 3.58-3.72 (m, 3H), 3.79-3.90 (m, 1H), 4.46 (s, 1H), 6.76-6.82 (m, 2H), 7.02-7.12 (m, 1H), 7.18-7.24 (m, 1H), 7.30-7.35 (m, 3H), 7.40-7.46 (m, 2H); 13C-NMR: δ = 14.0, 19.6, 27.6, 32.3, 46.5, 67.8, 73.0, 115.1, 117.5, 122.9, 124.6, 125.9, 128.5, 129.6, 130.6, 144.4, 147.9.
4-Butoxy-6-chloro-1-methyl-1,2,3,4-tetrahydroquinoline (3f). Colorless liquid (74 %); 1H-NMR: δ = 0.93 (t, J = 7.2 Hz, 3H), 1.37-1.45 (m, 2H), 1.57-1.63 (m, 2H), 1.86-1.94 (m, 1H), 2.08-2.13 (m, 1H), 2.90 (s, 3H), 3.09-3.15 (m, 1H), 3.36-3.42 (m, 1H), 3.48-3.58 (m, 2H), 4.26 (t, J = 3.6 Hz, 1H), 6.52-6.54 (m, 1H), 7.08-7.13 (m, 2H); 13C-NMR: δ = 14.0, 19.6, 27.2, 32.2, 39.0, 46.4, 67.9, 72.9, 112.5, 120.3, 123.2, 128.9, 129.7, 144.9; ESI-MS: 276 [M+Na]+.
4-Butoxy-1,6-dimethyl-1,2,3,4-tetrahydroquinoline (3g) [4d]. Colorless liquid (35 %), 1H-NMR: δ = 0.93 (t, J = 7.4 Hz, 3H), 1.34-1.45 (m, 2H), 1.64-1.87 (m, 2H), 1.87-1.96 (m, 1H), 2.08-2.16 (m, 1H), 2.25 (s, 3H), 2.89 (s, 3H), 3.05-3.11 (m, 1H), 3.29-3.38 (m, 1H), 3.49-3.59 (m, 2H), 4.29 (t, J = 3.6 Hz, 1H), 6.56-6.59 (m, 1H), 6.98 (s, 2H); 13C‑NMR: δ = 14.1, 19.6, 20.4, 27.6, 29.8, 32.3, 39.4, 46.7, 67.8, 73.3, 111.9, 122.0, 125.0, 129.8, 130.9, 144.5; ESI-MS: 256 [M+Na]+.
N-Methylaniline (4a) [4a]. Colorless liquid (46%), 1H-NMR: δ = 2.85 (s, 3H), 3.67 (br, 1H), 6.61-6.65 (m, 2H), 6.70-6.76 (m, 1H), 7.18-7.24 (m, 2H); 13C-NMR: δ = 26.6, 30.8, 112.5, 117.3, 129.3, 148.4; ESI-MS: 108 [M+H]+.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (20372041) (20572058) and Beijing Department of Education (XK100030514).
References and Notes
- Perry, N. B.; Blunt, J. W.; McCombs, J. D.; Munro, M. H. G. Discorhabdin C, A Highly Cytotoxic Pigment From A Sponge of the Genus Latrunculia. J. Org. Chem. 1986, 51, 5476–5478. [Google Scholar] Williamson, N. M.; March, P. R.; Ward, A. D. An Improved Synthesis of 2,2-Disubstituted-1,2-dihydroquinolines and Their Conversion to 3-Chloro-2,2-disubstituted-tetra-hydroquinolines. Tetrahedron Lett. 1995, 36, 7721–7724. [Google Scholar] Romesh, M.; Moham, P. S.; Shanmugam, P. A Convenient Synthesis of Flindersine, Atanine and Their Analogues. Tetrahedron 1984, 40, 4041–4049. [Google Scholar] Mertes, M. P.; Lin, A. J. Cofactor inhibitors of Thymidylate Synthetase. Piperidine and Tetrahydroquinoline Analogs of Tetrahydrofolic Acid. J. Med. Chem. 1970, 13, 276–279. [Google Scholar] Padwa, A.; Brodney, M. A.; Liu, B.; Satake, K.; Wu, T. A Cycloaddition Approach Toward the Synthesis of Substituted Indolines and Tetrahydroquinolines. J. Org. Chem. 1999, 64, 3595–3607. [Google Scholar] De Kempe, N.; Keppens, M. Novel Routes to Indoles, Indolines, Quinolines And Tetrahydroquinolines From N-(Cyclohexylidene)amines. Tetrahedron 1996, 52, 3705–3718. [Google Scholar] Hiessbock, R.; Wolf, C.; Richter, E.; Hitzler, M.; Chiba, P.; Kratzel, M.; Ecker, G. Synthesis and in Vitro Multidrug Resistance Modulating Activity of a Series of Dihydrobenzopyrans and Tetrahydroquinolines. J. Med. Chem. 1999, 42, 1921–1926. [Google Scholar]
- For leading references see: Nishio, T.; Tabata, M.; Koyama, H.; Sakamoto, M. Photochemistry of N-(2-Acylphenyl)-2-methylprop-2-enamides: Competition Between Photocyclization and Long-Range Hydrogen Abstraction. Helv. Chim. Acta. 2005, 88, 78–86. [Google Scholar] Katritzky, A. R.; Rachwal, S.; Rachwal, B. Recent Progress in the Synthesis of 1,2,3,4,-Tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070. [Google Scholar] Isabelle, G.-D.; Gastaud, P.; RajanBabu, T. V. Asymmetric Synthesis of Functionalized 1,2,3,4-Tetrahydroquinolines. Org, Lett. 2001, 3, 2053–2056. [Google Scholar]
- Lautens, M.; Tayama, E.; Herse, C. Palladium-Catalyzed Intramolecular Coupling Between Aryl Iodides and Allyl Moieties via Thermal and Microwave-Assisted Conditions. J. Am. Chem. Soc. 2005, 127, 72–73. [Google Scholar] Yi, C. S.; Yun, S. Y. Ruthenium-catalyzed Intermolecular Coupling Reactions of Arylamines with Ethylene and 1,3-Dienes: Mechanistic Insight on Hydroamination vs ortho-C-H Bond Activation. Org, Lett. 2005, 7, 2181–2183. [Google Scholar] Zhang, J.; Li, C.-J. InCl3-Catalyzed Domino Reaction of Aromatic Amines with Cyclic Enol Ethers in Water: A Highly Efficient Synthesis of New 1,2,3,4-Tetrahydroquinoline Derivatives. J. Org. Chem. 2002, 67, 3969–3971. [Google Scholar] Chen, R.; Qian, C. One-pot Synthesis of Tetrahydroquinolines Catalyzed by Dy(OTf)3 in Aqueous Solution. Synthetic Commun. 2002, 32, 2543–2548. [Google Scholar] Luo, Y.; Li, Z.; Li, C.-J. A Silver-Catalyzed Domino Route toward 1,2-Dihydroquinoline Derivatives from Simple Anilines and Alkynes. Org, Lett. 2005, 7, 2675–2678. [Google Scholar] Sundararajan, G.; Prabagaran, N.; Varghese, B. First Asymmetric Synthesis of Quinoline Derivatives by Inverse Electron Demand (IED) Diels-Alder Reaction Using Chiral Ti(IV) Complex. Org, Lett. 2001, 3, 1973–1976. [Google Scholar] Talukdar, S.; Chen, C.-T.; Fang, J.-M. A Stereoselective Route to Polysubstituted Tetrahydro-quinolines by Benzotriazole-Promoted Condensation of Aliphatic Aldehydes and Aromatic Amines. J. Org. Chem. 2000, 65, 3148–3153. [Google Scholar]
- Shono, T.; Matsumura, Y.; Inoue, K.; Ohmizu, H.; Kashimura, S. Electroorganic Chemistry. 62. Reaction of Iminium Ion with Nucleophile: A Versatile Synthesis of Tetrahydroquinolines and Julolidines. J. Am. Chem. Soc. 1982, 104, 5753–5757. [Google Scholar] Murahashi, S.-I.; Naota, T.; Nakato, T. Convenient Method for the Construction of Quinoline Skeletons from N-Methylarylamines via N-(t-Butyldioxymethyl)arylamines. Synlett. 1992, 835–836. [Google Scholar] Murahashi, S.-I.; Naota, T.; Yonemura, K. Ruthenium-catalyzed Cytochrome P-450 Type Oxidation of Tertiary Amines with Alkylhydroperoxides. J. Am. Chem. Soc. 1988, 110, 8256–8258. [Google Scholar] Murata, S.; Miura, M.; Nomura, M. Oxidation of 3- or 4-Substituted N,N-Dimethylanilines with Molecular Oxygen in the Presence of Either Ferric Chloride or [Fe(salen)]OAc. J. Org. Chem. 1989, 54, 4700–4702. [Google Scholar] Murata, S.; Miura, M.; Nomura, M. Iron-catalyzed Oxidation of N,N-Dimethylaniline with Molecular Oxygen. J. Chem. Soc. Chem. Commun. 1989, 116–118. [Google Scholar]
- Krause, N. Modern Organocopper Chemistry; Wiley-VCH Verlag GmbH: Weinheim, 2002. [Google Scholar] Hathaway, B. J. Comprehensive Coordination Chemistry; Wilkinson, G., Ed.; Pergamon: Oxford, 1987; Vol. 5, pp. 533–774. [Google Scholar]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] Kwong, F. Y.; Klapars, A.; Buchwald, S. L. Copper-Catalyzed Coupling of Alkylamines and Aryl Iodides: An Efficient System Even in an Air Atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] Rispens, T.; Engberts, Jan B. F. N. Efficient Catalysis of a Diels-Alder Reaction by Metallo-Vesicles in Aqueous Solution. Org. Lett. 2001, 3, 941–943. [Google Scholar] Smrčina, M.; Vyakočil, Š.; Máca, B.; Polášek, M.; Claxton, T. A.; Abbott, A. P.; Kočovský, P. Selective Cross-coupling of 2-Naphthol and 2-Naphthylamine Derivatives. A Facile Synthesis of 2,2',3-Trisubstituted and 2,2',3,3'-Tetrasubstituted 1,1'-Binaphthyls. J. Org. Chem. 1994, 59, 2156–2163. [Google Scholar] Smrčina, M.; Lorenc, M.; Hanuš, V.; Sedmera, P.; Kočovsky, P. Synthesis of Enantiomerically Pure 2,2'-Dihydroxy-1,1'-binaphthyl, 2,2'-diamino-1,1'-binaphthyl and 2-amino-2'-hydroxy-1,1'-binaphthyl. Comparison of Processes Operating as Diastereoselective Crystallization and as Second Order Asymmetric Transformation. J. Org. Chem. 1992, 57, 1917–1920. [Google Scholar]
- Billman, J. H.; Radike, A.; Mundy, B. W. Alkylation of Amines. I. J. Am. Chem. Soc. 1942, 64, 2977–2978. [Google Scholar] Thomas, D. G.; Billman, J. H.; Davis, C. E. Alkylation of Amines. II. N,N-Dialkylation of Nuclear Substituted Anilines. J. Am. Chem. Soc. 1946, 68, 895–896. [Google Scholar]
- Sample Availability: Contact the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
