Abstract
In the search of new HIV-1 integrase (IN) inhibitors, we synthesized a series of multimeric 5,6-dihydroxyindole-2-carboxylic acid (DHICA) derivatives. Preliminary results indicate that hyperbranched architectures could represent a peculiar molecular requisite for the development of new antiviral lead compounds.
Introduction
In the search for new HIV-1 IN inhibitors, we synthesized and evaluated the biological activity of 5,6-dihydroxyindole-2-carboxylic acid (DHICA, II), an intermediate in the pathway of eumelanin production, and a series of its derivatives (IIIa-i, Figure 1). These compounds were designed as conformationally constrained analogues of the acrylate moiety of caffeic acid phenethyl ester (CAPE, I, Figure 1); several of them showed anti-IN activity in enzyme-based assays at low micromolar concentrations [1a,b].
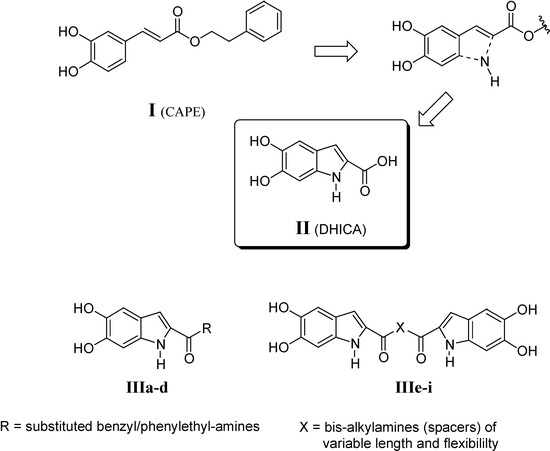
Figure 1.
Design of DHICA from CAPE and DHICA derivatives.
Figure 1.
Design of DHICA from CAPE and DHICA derivatives.
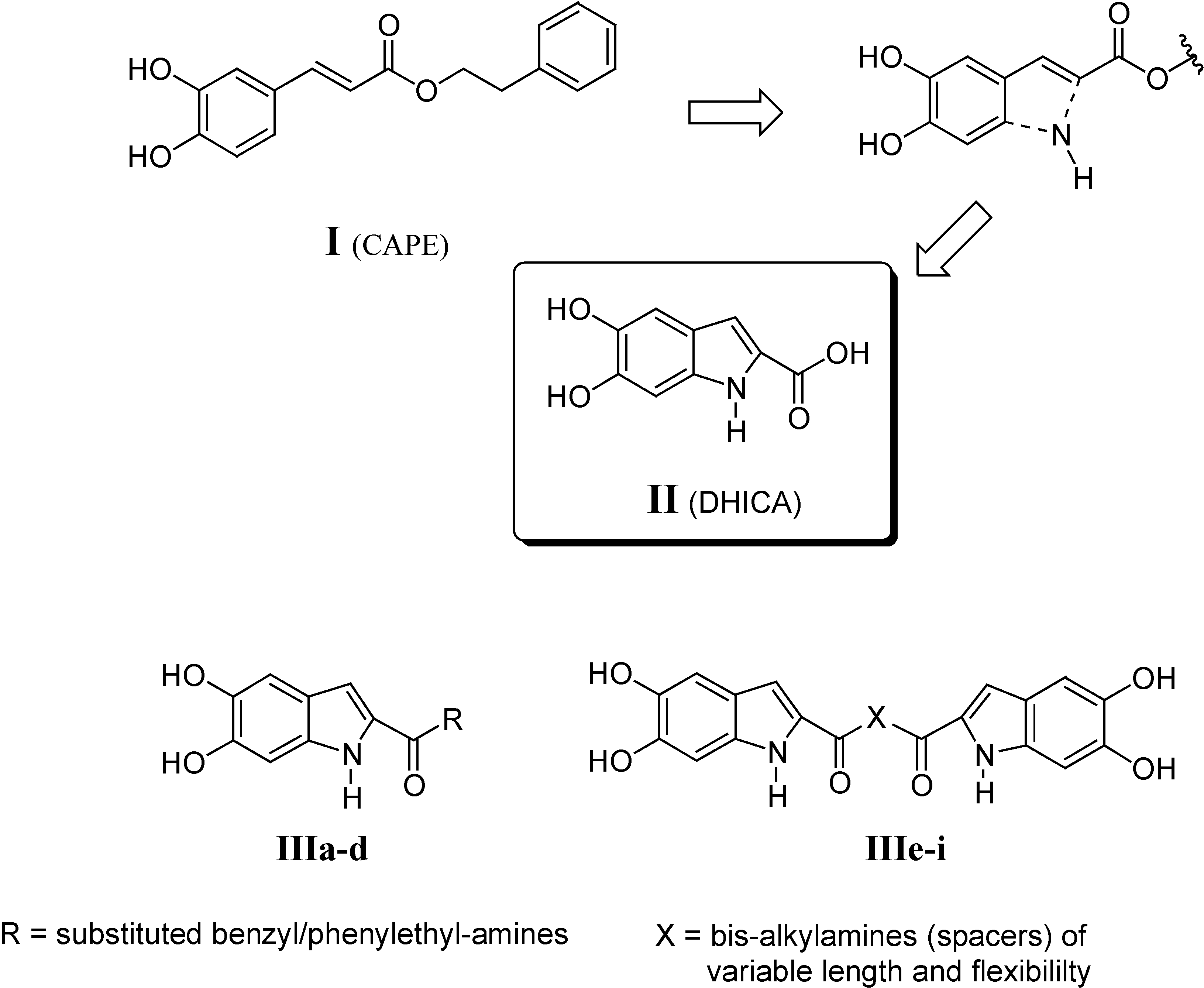
In the course of the preparation of II by a novel synthetic route (see below), we unexpectedly obtained a macromolecular compound as the only product. On the basis of its physico-chemical properties such as solubility and differential scanning calorimetry (DSC) measurements, its structure IVa has been postulated to perhaps correspond to that of a hyperbranched compound. In order to evaluate whether the macromolecular structure of IVa could be endowed with biological activity, we prepared other derivatives in both hyperbranched oligomeric (IVb) and polymeric (IVc) forms (Figure 2). Indeed, since II bears one COOH (A) and two OH (B) groups, which are capable of mutually reacting, it can be regarded as an AB2 monomer, typically giving hyperbranched macromolecules (Figure 3).
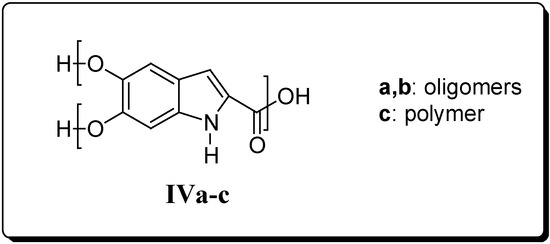
Figure 2.
Title compounds.
Figure 2.
Title compounds.
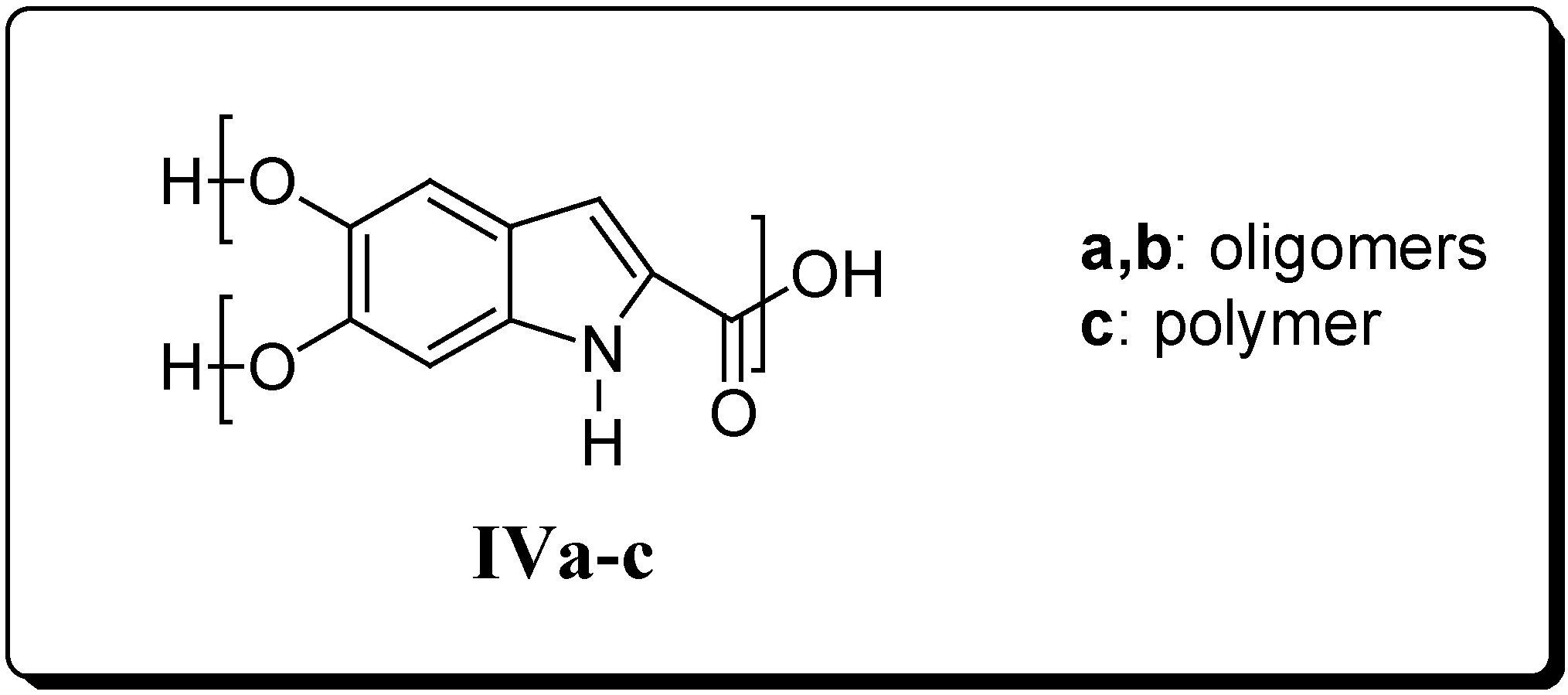
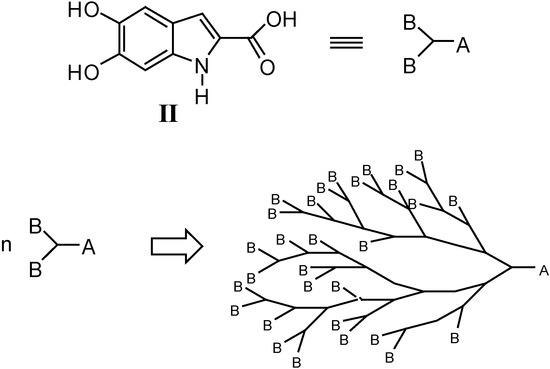
Figure 3.
Schematic architecture of the hyperbranched macromolecules originating from AB2 monomers.
Figure 3.
Schematic architecture of the hyperbranched macromolecules originating from AB2 monomers.
In the last decades, AB2 monomers have been widely used as starting materials for the synthesis of dendritic structures [2,3]. This macromolecular family comprises dendrimers and hyperbranched polymers. Both classes are characterized by having a tree-like architecture in which all linkages converge towards a central core. However, whereas dendrimers are obtained by stepwise growth in long sequences, hyperbranched polymers are obtained in one step. As far as dendrimers are concerned, their applications in treatment of several human diseases are under critical investigation and looks very promising; these macromolecules serve as targeted drug, carriers and delivery agents as well as imaging agents in human systems [4,5,6]. In particular, polymers having a dendrimer-based structure have emerged from several research programs focused on the development of inhibitors of HIV and other enveloped viruses [7,8,9,10,11,12,13,14,15,16,17,18,19].
From a merely structural point of view, purification and deprotection steps for dendrimers are aimed at synthesizing monodisperse, defect-free macromolecules. On the contrary, hyperbranched polymers are characterized by a certain number of defects, which generally do not significantly affect their peculiar features. Although the synthetic chemistry efforts in modern drug research have been focused on the drive to discover orally bioavailable small-molecule drugs, the study of macromolecular entities could reveal novel and significant scenarios. For example, it is well-known that many of the biological targets are in fact macromolecules which rely heavily on polyvalent/multivalent interactions in their binding and signalling cascades. In this context, with the hope of identifying an original class of antiviral agents, all title compounds will be tested against HIV-1 viral strain.
Results and Discussion
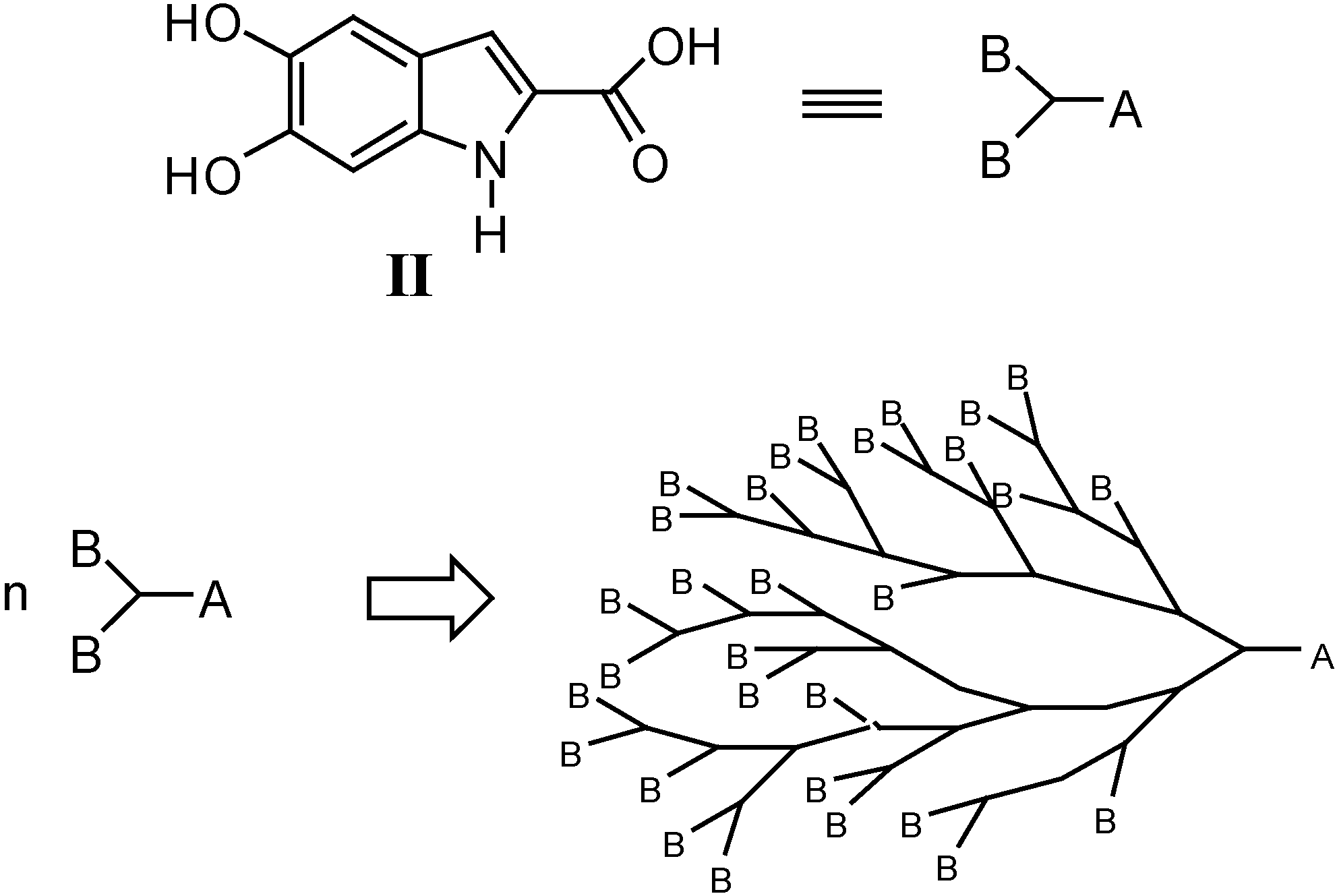
Our synthetic approach to compounds II and IVa-c is depicted in Scheme 1, Scheme 2, Scheme 3 and Scheme 4. Starting from the aldehydes 1a and 1b and methyl azidoacetate (2), azidocinnamates 3a and 3b were prepared in high yields via the Hemetsberger reaction [1a]. Intermediates 3a and 3b were converted in refluxing xylene into the esters 4a and 4b, from which the acids 5a and 5b were obtained in 98% yields on alkaline hydrolysis. Deprotection of the catechol moiety with BBr3 in dichloromethane at –40 °C gave the expected DHICA II (Scheme 1). Compound IVa was obtained by demethylation of intermediate 4b to give methyl 5,6-dihydroxyindole-2-carboxylate (6), which was treated with 12% KOH at reflux for 1 h (Scheme 2).
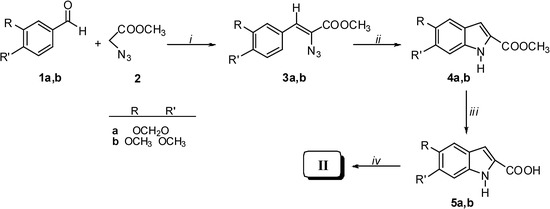
Scheme 1.
Scheme 1.
Reagents and conditions: i) CH3ONa, CH3OH, -15 °C for 4 h; ii) xylene, reflux for 15 min; iii) 12% KOH, reflux for 1 h; vi) BBr3, CH2Cl2, H2O, -40 °C for 4 h.
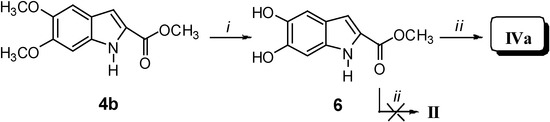
Scheme 2.
Scheme 2.
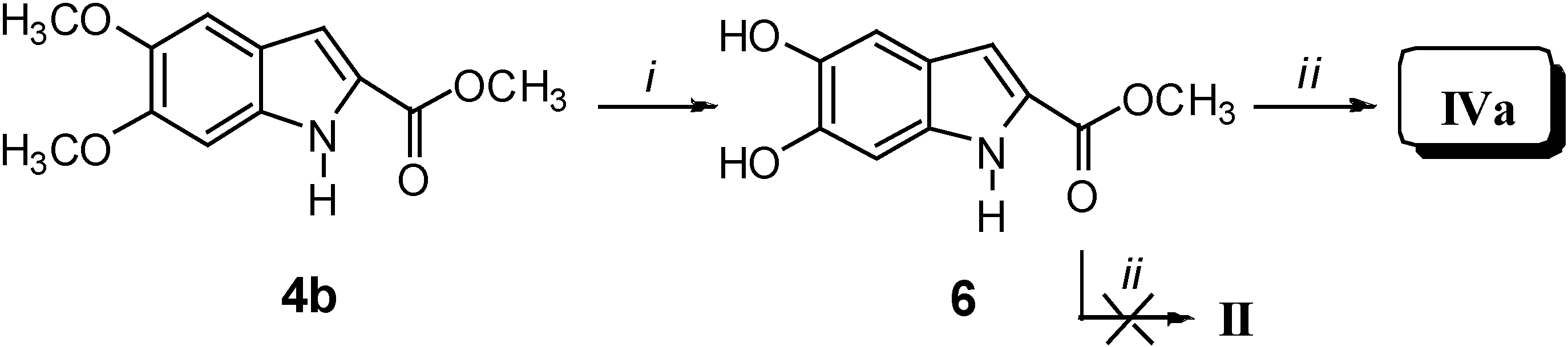
Reagents and conditions: i) BBr3, CH2Cl2, CH3OH, -40 °C, 4 h; ii) 12% KOH, reflux, 1 h.
It is noteworthy that, due to transesterification reactions, compound II was not obtained, thus confirming that oligomerization was favoured. The synthesis of IVb was carried out by reacting equimolar amounts of 7 and II in anhydrous DMF for 60 h, in the presence of a catalytic amount of triethanolamine (Scheme 3).
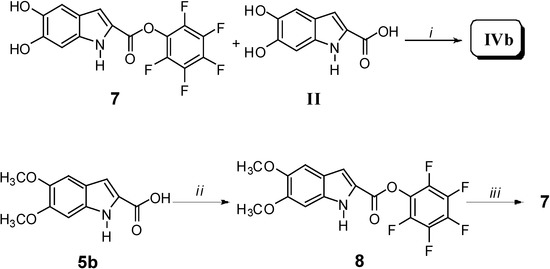
Scheme 3.
Scheme 3.
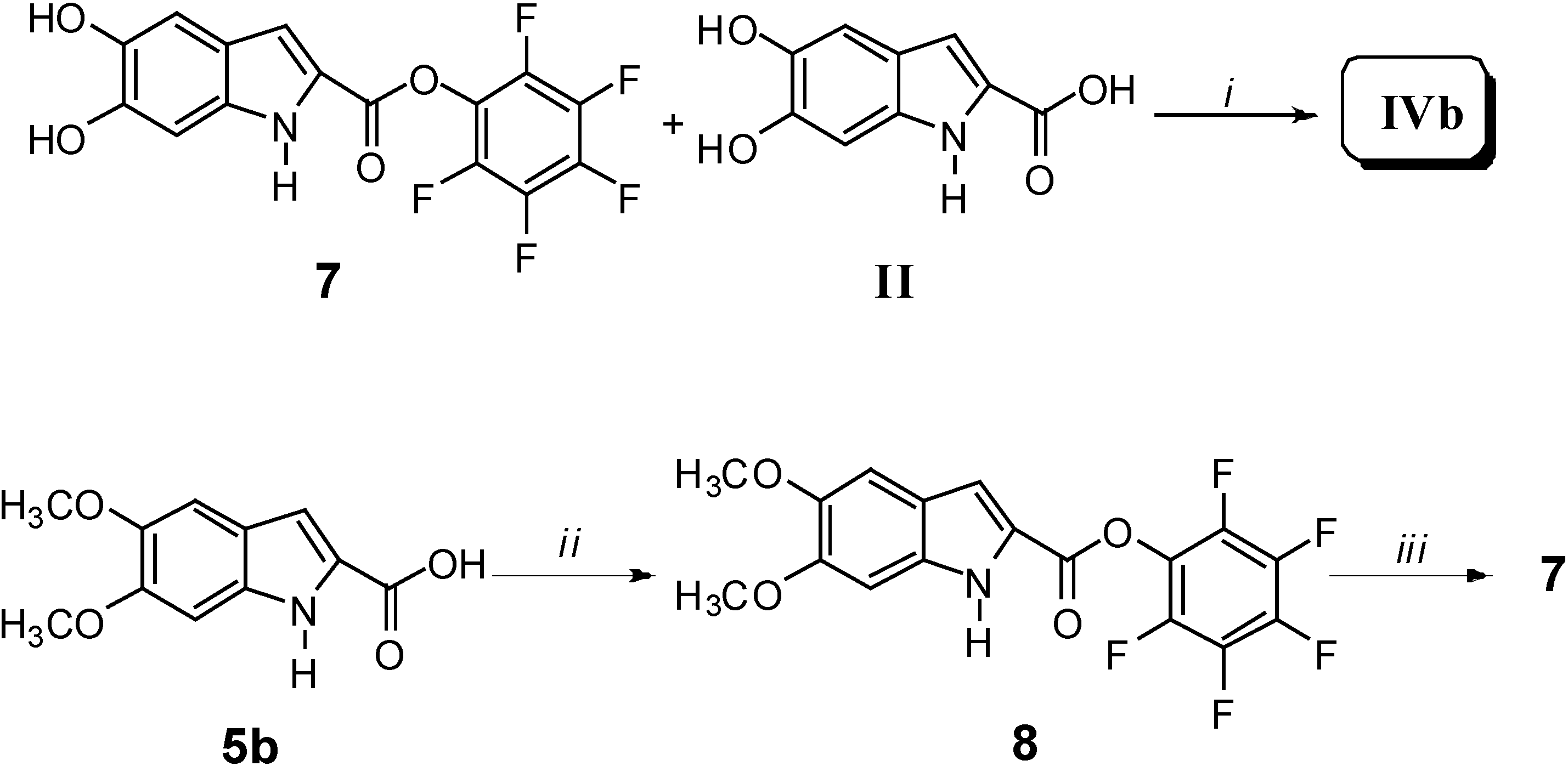
Reagents and conditions: i) Triethanolamine, anhydrous DMF, 80 °C, 60 h; ii) Pentafluoro-phenol, dioxane, r.t. for 4 h; iii) BBr3, CH2Cl2, H2O, -40 °C for 4 h.
The intermediate 7 was obtained by treating 5b with pentafluorophenol to afford the pentaflurophenyl ester 8. The latter was then deprotected to give 7 in good yield. Since hydroxy and pentafluorophenolic ester were the only reactive groups under such reaction conditions, a typical AB2 + B2 polymerization occurred, thus giving rise to dendritic oligomeric structures of relatively low molecular weight. This procedure was carried out with the aim of obtaining, by a different synthetic procedure, an oligomer comparable to IVa. As a matter of fact, when compared to II, both IVa and IVb showed a larger solubility in many polar solvents (water included). Besides, a further synthesis was designed and carried out: polymer IVc was prepared by reacting monomer II following a well-known polymerization route commonly used in the lab-scale direct preparation of high molecular weight polyesters from carboxylic acids (Scheme 4) [2].
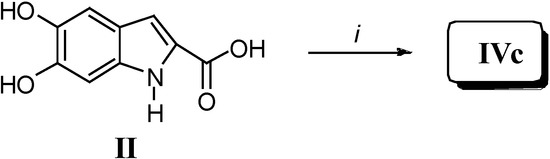
Scheme 4.
Scheme 4.
Reagents and conditions: i) Anhydrous N-methyl pyrrolidone/pyridine (5:1, v/v); triphenyl-phosphine, hexachloroethane, 25 °C, 4 h.
Compound IVc was characterized by a solubility even larger than that of II, IVa, and IVb, thus suggesting the presence of more numerous OH groups and confirming a (highly-)branched structure which behaves as a unimolecular micelle. In order to obtain information about the polymeric structure of the title compounds, a full characterization of IVa-c is under way and will be reported elsewhere. However, some preliminary results can be here briefly outlined. DSC experiments show broad melting peaks for all samples, thus indicating the typical polydispersion state of a one-step-obtained synthetic oligo- or polymeric compound.
According to the expected increase of polydispersity as the molecular weight increases, typical for step-growth polymerization, melting peaks become larger in the following order: IVa < IVb << IVc; this behavior being due to the increasing number of different molecular species that characterize any of the above compounds. Furthermore, also melting temperatures give some indications; indeed, their values increase in the following order: IVa < IVb < IVc (i.e. 75 < 80 < 85 °C, respectively) that is what expected on the basis of the molecular weight of the three compounds, from the lowest to the highest.
Biological Activity
Preliminary biological data on cytotoxicity and antiviral activity of the title compounds are reported in Table 1. The compound concentrations required to reduce the viability of mock-infected cells by 50% (CC50) and to achieve 50% protection of MT-4 cells from the HIV-1 induced cytopathogenicity (EC50), were determined by the MTT method [20]. In cell-based assays, IVa-c showed cytotoxicity and antiviral activity which were inversely related to their molecular weight. In particular, with comparable EC50 values, the oligomers IVa and IVb (EC50s = 0.5 and 1.5 µg/mL for IVa and IVb, respectively) seem significantly capable to prevent the HIV-1 multiplication in acutely infected MT-4 cells with respect to the polymer IVc (EC50 = >30 µg/mL). Meanwhile, cytotoxicity values support this observation (CC50s = 5, 15, and >100 µg/mL for IVa, IVb and IVc, respectively). Interestingly, with respect to the monomer II and its derivatives IIIa-i, which were toxic and did not yield therapeutic efficacy [1a], the multimeric compounds have been proved to be effective in the HIV-1 multiplication, thus showing cytoprotection activity in cell-based assays. Although IVa and IVb proved significantly less potent than the reference compound Efavirenz (EC50 = 0.5 and 1.5 µg/mL v. 0.0031 µg/mL), a non-nucleoside reverse transcriptase inhibitor (NNRTI) commonly used in therapeutic protocols [21], they showed a similar biological profile when compared to Merck L-731,988 [22] (an important HIV-1 integrase inhibitor lead compound).

Table 1.
Cytotoxicities and Antiviral Activities of ΙVa-c.
| Cpd | aCC50 | bEC50 | cTI |
|---|---|---|---|
| II | 0.9 | >0.9 | - |
| IVa | 5 | 0.5 | 10 |
| IVb | 15 | 1.5 | 10 |
| IVc | >100 | >100 | - |
| dL-731,988 | 15.6 | 0.43 | 36 |
| eEFV | 11 | 0.0031 | >1000 |
aCC50: Cytotoxic concentration 50%; bEC50: Effective Concentration 50%; cTI: Therapeutic index = CC50/EC50; dL-731,988: Merck’s lead compound; eEFV: Efavirenz. Due to their oligo/polymeric nature, data referring to biological activities of multimeric derivatives (and those of the reference compounds for comparison) are expressed in µg/mL.
Conclusions
These preliminary results for IVa,b are consistent with the conclusion that a hyperbranched oligomeric backbone with relatively low molecular weight and relatively high degree of branching could represent an interesting structural archetype for HIV-1 inhibition as well as for other potential antiviral activities. Moreover, the possibility that hyperbranched derivatives might target the adsorption/fusion steps of HIV multiplication cycle in cells could be considered. Detailed cytotoxicity and antiviral activity as well as inhibition activities toward cellular targets of these compounds are under investigation and will be discussed in due course.
Experimental
General
The synthesis of monomer II and intermediates 3a,b, 4a,b, 5a,b, 7 and 8 used for the preparation of the title compounds was previously reported [1a]. These compounds have been prepared by using the same experimental procedure. Anhydrous solvents and all reagents were purchased from Aldrich, Merck or Carlo Erba. All reactions involving air- or moisture-sensitive compounds were performed under nitrogen atmosphere using oven-dried glassware and syringes to transfer solutions. Melting points (m.p.) were determined using an Electrothermal melting point or a Köfler apparatus and are uncorrected. Infrared (IR) spectra were recorded as thin films or nujol mulls on NaCl plates with a Perkin-Elmer 781 IR spectrophotometer and are expressed in ν (cm-1). Nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were determined in 1:3 CDCl3/DMSO-d6 or DMSO-d6 and were recorded on a 200 MHz Varian XL-200 instrument. Chemical shifts (δ scale) are reported in parts per million (ppm) downfield from tetramethylsilane (TMS) used as an internal standard. Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet; brs, broad singlet; dd, double doublet. The assignment of changeable protons (OH and NH) was confirmed by the addition of D2O. Analytical thin-layer chromatography (TLC) was done on Merck silica gel F-254 plates. For flash chromatography Merck Silica gel 60 was used with a particle size 0.040-0.063 mm (230-400 mesh ASTM). Elemental analyses were performed on a Perkin-Elmer 2400 spectrometer, and were within ±0.4% of the theoretical values. Differential Scanning Calorimetry (DSC) measurements were performed using a DSC Q100 V9.0 (TA Instrument, New Castle, USA) as detailed below.
Preparation of methyl 5,6-dihydroxy-1H-indole-2-carboxylate (6). To a solution of 5,6-dimethoxy-1H-indole-2-carboxylic acid methyl ester 4b (1.0 mmol) in dichloromethane (130 mL), a 1M BBr3 sol. in dichloromethane (4 mmol) was added at -40 °C and under nitrogen atmosphere. The mixture was stirred at the same temperature for 4 h, then, the reaction was quenched in methanol and the solvents were removed under reduced pressure. The residue was washed three times with methanol. After purification by silica gel flash column chromatography, the product was recrystallized from isopropyl alcohol. Yield = 80 %; m.p. = 248 - 249 °C (lit. [23] 255 – 260 °C); IR (nujol) ν cm-1 = 3480 (OH); 3320 (NH); 1690 (C=O); 1H-NMR (CDCl3/DMSO-d6) δ 10.98 (s, 1H, NH), 8.76 (s, 1H, OH), 8.35 (s, 1H, OH), 6.93 (s, 1H, Ar-H), 6.88 (s, 2H, Ar-H), 3.83 (s, 3H, CH3); 1H-NMR (DMSO-d6) δ 11.28 (s, 1H, NH), 9.15 (s, 1H, OH), 8.65 (s, 1H, OH), 6.89 (s, 2H, Ar-H), 6.79 (s, 1H, Ar-H), 3.81 (s, 3H, CH3); 13C-NMR (DMSO-d6): δ 161.7, 146.5, 142.2, 132.9, 124.5, 119.8, 107.5, 104.9, 96.9, 51.3; Anal. Calcd. for C10H9NO4: C, 57.97; H, 4.38; N, 6.76. Found: C, 58.11; H, 4.22; N, 6.89.
Preparation of IVa. In a 25 mL round bottom vessel, 6 (0.200 g, 0.960 mmol) was dissolved in 12% KOH (9 mL, 19.20 mmol) and the mixture was allowed to stir for 30 min at reflux. The dark solution formed was then poured onto ice and acidified with 6N HCl. Thereafter, the mixture was poured into diethyl ether and the precipitated oligomer IVa was filtered to provide a dark powder that was washed with water and diethyl ether and dried in a vacuum oven for 24 h at 40 °C. DSC: Tm = ~75 °C.
Preparation of IVb. Compound II (0.054 g, 0.27 mmol) and 7 (0.096 g, 0.27 mmol) in anhydrous DMF (7.5 mL) containing anhydrous triethylamine (0.1 mL) were placed in a 10 mL round bottom vessel at room temperature. The vessel was sealed and heated in an oil bath set at 80 °C. The mixture was allowed to react under stirring for 60 h. The mixture was then poured into diethyl ether and the precipitated oligomer IVb was filtered to give a dark powder that was washed with diethyl ether and dried in a vacuum oven for 24 h at 40 °C. DSC: Tm = ~80 °C.
Preparation of IVc. In a 5 mL round bottom vessel compound II (0.050 g, 0.26 mmol) was dissolved in an N-methylpyrrolidone/pyridine mixture (1.5 mL, 5:1, v/v) and triphenylphosphine (0.080 g, 0.49 mmol) and hexachloroethane (0.093 g, 0.57 mmol) were added. The vessel was sealed and the mixture was allowed to stir for 4 h at room temperature, then diethyl ether (15 mL) was added and the precipitated polymer IVc was recovered and washed several times by centrifugation in the presence of methanol to give a brown powder. DSC: Tm = ~85 °C.
Differential Scanning Calorimetry (DSC) measurements
The DSC instrument was calibrated with indium (calibration standard, purity 99.999%) for melting point and heat of fusion. A heating rate of 10 °C/min was employed in the range 20–250 °C. Analyses were performed under an Ar purge (50 mL/min). Standard aluminium sample pans were used. About 8-9 mg sample was taken for analysis. An empty pan was used as reference.
Acknowledgements
The authors thank Dr Maria Orecchioni for assistance with NMR spectroscopy, and Dr Gianfranco Angotzi for the preparation of some intermediates. Financial support to M.S. from the “Ministero dell'Istruzione, dell'Università e della Ricerca” (MIUR), Rome, Italy, is gratefully acknowledged.
References and Notes
- Sechi, M.; Angotzi, G.; Dallocchio, R.; Dessì, A.; Carta, F.L.; Sannia, Mariani A.; Fiori, S.; Sanchez, T.; Movssessian, L.; Plasencia, C.; Neamati, N. Design and synthesis of novel dihydroxyindole-2-carboxylic acids as HIV-1 integrase inhibitors. Antivir. Chem. & Chemother. 2004, 15, 67–82. [Google Scholar]b)d’Ischia, N.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and Indole-5,6-diones. In Advances in Heterocyclic Chemistry; Academic Press: New York, 2005; Vol. 89, Chapter 1; pp. 1–65. [Google Scholar]
- Jikei, M.; Kakimoto, M. Hyperbranched polymers: a promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–85. [Google Scholar] [CrossRef]
- Monticelli, O.; Mendichi, R.; Bissano, S.; Mariani, A.; Russo, S. Synthesis, characterization and properties of a hyperbranched aromatic polyamide: poly(ABZAIA). Macromol. Chem. Phys. 2000, 201, 2123–27. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Taylor, A.M.; Goddard, W.A., III. Starburst dendrimers: Control of size, shape, surface, chemistry, and topology. Angew. Chem., Int. Ed. Engl. 1990, 102, 119–157. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–36. [Google Scholar] [CrossRef] [PubMed]
- Stiriba, S.E.; Frey, H.; Haag, R. Dendritic Polymers in Biomedical Applications: From Potential to Clinical Use in Diagnostics and Therapy. Angew. Chem., Int. Ed. 2002, 41, 1329–34. [Google Scholar]
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–8. [Google Scholar]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Cloninger, M.J. Biological applications of dendrimers. Curr. Opin. Chem. Biol. 2002, 6, 742–8. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.R.; Holan, G. US Patent No. 6,190,650, 2001.
- Bourne, N.; Stanberry, L.R.; Kern, E.R.; Holan, G.; Matthews, B.; Bernstein, D.I. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 2000, 44, 2471–4. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Matthews, B.; Cheung, D.; Tam, T.; Gadawski, I.; Leung, D.; Holan, G.; Raff, J.; Sacks, S. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus replication. Antiviral Res. 2002, 55, 319–29. [Google Scholar] [CrossRef] [PubMed]
- Luscher-Mattli, M. Polyanions - a lost chance in the fight against HIV and other virus diseases? Antiviral Chem. Chemother. 2000, 11, 249–59. [Google Scholar]
- Witvrouw, M.; Fikkert, V.; Pluymers, W.; Matthews, B.; Mardel, K.; Schols, D.; Raff, J.; Debyser, Z.; De Clercq, E.; Holan, G.; Pannecouque, C. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 2000, 58, 1100–8. [Google Scholar]
- Turpin, J.A. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Invest. Drugs 2002, 11, 1077–97. [Google Scholar] [CrossRef]
- D'Cruz, O.J.; Uckun, F.M. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 2004, 10, 315–36. [Google Scholar]
- Dezzutti, C.S.; James, V.N.; Ramos, A.; Sullivan, S.T.; Siddig, A.; Bush, T.J.; Grohskopf, L.A.; Paxton, L.; Subbarao, S.; Hart, C.E. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 2004, 48, 3834–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Emau, P.; Cairns, J.S.; Flanary, L.; Morton, W.R.; McCarthy, T.D.; Tsai, C.C. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses 2005, 21, 207–13. [Google Scholar]
- Gong, E.; Matthews, B.; McCarthy, T.; Chu, J.; Holan, G.; Raff, J.; Sacks, S. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antiviral Res. 2005, 68, 139–46. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R.J. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar]
- Barbaro, G.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr. Pharm. Des. 2005, 11, 1850–1843. [Google Scholar] [CrossRef]
- Hazuda, D.J.; Felock, P.; Witmer, M.; Wolfe, A.; Stillmock, K.; Grobler, J.A.; Espeseth, A.; Gabryelski, L.; Schleif, W.; Blau, C.; Miller, M.D. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000, 287, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Wyler, H.; Dreiding, A.S. Darstellung und Abbauprodukte des Betanidins. 3. Über die Konstitution des Randenfarbstoffes Betanin. Helv. Chim. Acta 1959, 42, 1699–702. [Google Scholar]
- Sample Availability: Samples of the compounds I-IV and 1-8 are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
