Abstract
Introduction: Rerouting urine flow is often required following radical cystectomy (RC). In this context, the ileal conduit (IC) has become the most common technique for urinary diversion, primarily due to its technical simplicity and suitability for patients with compromised renal function, elderly individuals, and those unable to perform self-catheterization. Objective: This review aims to highlight the complications of IC and categorize them into metabolic and surgical complications, further subdivided by timing (intraoperative, short-term, intermediate-term, and long-term) and anatomical location (uretero-ileal anastomosis, stoma, and ileal segment). Methods: A comprehensive narrative review was conducted to summarize the most common complications of IC, their clinical presentation, and management using Google Scholar, PubMed, and Embase databases to identify studies published from 1950 to 2024. Results: The morbidity associated with IC, especially when compared to continent diversions, remains a subject of debate. Notably, IC-related complications have been described with an incidence rate ranging from 39% to 67%. Conclusions: Providing a comprehensive overview of IC complications and management strategies can enhance clinical practice and improve patient outcomes.
1. Introduction
Urinary diversion involves redirecting the flow of urine, either temporarily or permanently. Typically, a permanent urinary diversion is performed following surgery to treat bladder or pelvic cancer, but it can also be done for other functional or anatomical abnormalities of the urinary tract like neurogenic bladder, radiation cystitis, refractory incontinence, and congenital bladder anomalies [].
There are multiple techniques used for urinary diversion, including continent or non-continent diversions []. Since the introduction of the ileal conduit (IC) in 1950, it has become the most frequently used procedure, accounting for over 60% of such surgeries [,,]. The IC is technically less demanding than continent diversion, as it has a shorter operative time and perioperative risks, and the postoperative care is less complex, which is potentially translated in the potential for fewer complications [,,,]. For these reasons, it is the preferred option for patients with compromised renal function, elderly patients, those with significant comorbidities, and those who are unable to undergo intermittent self-catheterization or who have severe functional impairment [,,]. Nonetheless, these selection criteria have been debated in the literature, with recommendations to tailor the choice to each patient’s needs. Moreover, quality-of-life studies comparing orthotopic neobladder to IC diversion have not shown significant differences []. Thus, emphasis is placed on the importance of discussing patient expectations and providing thorough counseling on the risks and benefits of any type of urinary diversion.
However, although technically simpler to perform, multiple reports differ on whether IC leads to lower morbidity compared to continent diversion. This phenomenon could be attributed to the fact that patients undergoing this procedure usually have more unfavorable oncologic characteristics and a higher prevalence of comorbidities [,,]. Furthermore, a retrospective study demonstrated that up to 66% of patients experienced complications related to the IC [].
Regarding the approaches employed, both robot-assisted radical cystectomy (RARC) and open radical cystectomy (ORC) with extracorporeal urinary diversion have demonstrated comparable oncologic and quality-of-life outcomes. However, robotic techniques have been associated with lower transfusion rates, shorter lengths of stay, reduced risk of Clavien–Dindo grade III–V complications, and lower 90-day rehospitalization rates [,].
Complications are usually categorized into metabolic and surgical types; surgical complications can be further classified based on their timing and anatomical location. In terms of timing, they are categorized as intraoperative and postoperative complications, including short-term (<30 days), intermediate-term (31–90 days), and long-term (>90 days) complications []. Complications have been reported in 63.4%, 27.8%, and 39.7% of patients undergoing IC urinary diversion in the short-term, intermediate-term, and long-term periods, respectively []. Complications according to their anatomical location include issues at the uretero-ileal anastomosis, the ileal segment, and the stoma. Note that most intraoperative complications are not included in these statistics, as they are inherent to the cystectomy procedure itself.
This narrative review describes the most common complications of the IC after RC, including their clinical presentation and management strategies. Through this review, we aim to enhance understanding and guide clinical practice in managing these complications.
2. Methods
A comprehensive narrative review was conducted to summarize the most common complications of IC their clinical presentation, and management.
The literature search was performed using Google Scholar, PubMed, and Embase databases to identify studies published from 1950 to 2024. The search terms included “Ileal conduit,” “IC,” “Complications,” “Urinary diversion,” “Non-continent diversion”, and “Incontinent diversion”. Studies with evidence levels 2 to 4 were included. Extracted data focused on metabolic and surgical complications, surgical techniques and approaches, complication rates, and strategies for prevention and management.
Articles were selected based on relevance, study quality, and methodology. Data were analyzed descriptively, and trends or gaps in current knowledge were identified to highlight areas needing further research.
3. Metabolic Complications
3.1. Hyperchloremic Metabolic Acidosis
Hyperchloremic metabolic acidosis is a complication that occurs in some urinary diversions, particularly in those that utilize distal intestinal segments such as the ileum. Its incidence ranges from 26% to 45% in continent diversions, while for ileal conduits, this incidence decreases to 10–15%. It can occur both in the short and long term, with variations depending on the patient’s renal and hepatic function, as well as the urinary diversion technique employed [].
The pathophysiology underlying this condition involves the absorption of ammonium and chloride from the urine by the intestinal mucosa. The use of a longer bowel segment increases the surface area and the duration of contact between urine and the intestinal mucosa, thereby increasing the risk of metabolic complications. This prolonged exposure facilitates the absorption of urinary solutes through the bowel epithelium, leading to potential electrolyte imbalances and acid-base disturbances. Additionally, the type of intestinal segment utilized and the biochemical composition of the urine significantly influence the development and severity of these complications. Proximal segments, being more permeable, allow for the rapid movement of water and solutes, resulting in the loss of sodium and chloride, which can lead to hypovolemia. In contrast, distal segments such as the ileum and colon are rich in exchange channels, which facilitate the absorption of chloride and ammonium from the urine and the secretion of bicarbonate into the lumen, thereby contributing to the development of hyperchloremic metabolic acidosis [,,,].
The consequences of this chronic condition can lead to metabolic bone disturbances, including osteomalacia, as well as other complications such as muscle weakness and nephrocalcinosis. Preventive strategies emphasize rigorous monitoring of serum pH, electrolytes, bone mineral density, venous blood gases, and parameters of phosphorus and calcium metabolism, with particular attention given to patients with chronic renal disease [].
However, up to 20% of these patients may require treatment with bicarbonate or other alkalinizing agents to correct acidosis and help normalize changes in bone metabolism [,]. Additionally, vitamin D3 has also been shown to improve calcium balance in patients with osteomalacia [].
3.2. Malabsorption
It is a rare complication and has been reported only in bowel resections greater than 60 cm [,]. However, it is important to consider that patients with significant malabsorption may remain asymptomatic until serious complications arise. Since vitamin B12 and 95% of bile acids are absorbed in the ileum, it is not surprising that vitamin B12 deficiency has been reported in up to 21% of patients in the long-term follow-up. Additionally, cases of hypertriglyceridemia have been observed, although the mechanism and long-term effects remain unknown [].
It is recommended to spare 35 to 50 cm of the distal ileum to prevent vitamin B12 deficiency and bile acid loss []. Furthermore, all patients should be regularly monitored for B12 deficiency and malabsorption. If it is not possible to preserve an appropriate segment of ileum, or if a deficiency is confirmed, lifelong supplementation with monthly intramuscular vitamin B12 injections is required [,,,]
3.3. Kidney Stones
The incidence of kidney stones following an IC is approximately 15%, with the most common stones composed of calcium and oxalate [,]. Contributing factors include hyperchloremic metabolic acidosis, chronic urinary tract infections caused by urea-splitting organisms, dehydration, hypercalciuria, hypersulphaturia, and hypocitruria [,].
Management should focus on addressing the underlying causes. Patients should be advised to maintain a high fluid intake, follow a low-sodium diet, and avoid oxalate-rich foods and beverages. Additional measures, such as oral calcium citrate and a low-fat diet, may be recommended to reduce oxalate absorption. For urinary tract infections, preventive strategies should include encouraging frequent voiding and promptly treating them when diagnosed [].
4. Surgical Complications
4.1. Intraoperative Complications
Several aspects must be considered during the procedure. Resection of the bowel should be performed cautiously to avoid inadvertent bowel injury. Proper preservation of the mesentery is essential to preventing bowel ischemia. When using a linear stapler, it is recommended to reinforce the anastomosis to prevent potential bowel leaks. One should always adhere to the no-touch technique to minimize the risk of ureteral injury during the procedure. Lastly, when fixing the ileal conduit, it is crucial to be aware of a potential conduit torsion and ensure a tension-free uretero-ileal anastomosis [,,,,].
A history of radiation or previous abdominopelvic surgeries can significantly increase the risk of intraoperative complications []. Despite limited evidence supporting these statements, anticipating these risks is crucial for minimizing complications [].
4.2. Ileal Conduit Technique Considerations
During the creation of an IC, a segment of the ileum measuring approximately 15 to 20 cm is harvested (Figure 1). The length of the segment may vary based on the patient’s body habitus to ensure an appropriate conduit length. If a shorter segment is used, tension may occur on the ileoureteral anastomosis, potentially leading to ischemia of the anastomosis or the distal ureter. Conversely, if the ileal segment is too long, complications such as malabsorption, conduit rotation, or kinking may arise, causing intermittent obstruction and urinary stasis, which can lead to recurrent urinary tract infections and the formation of stones [].
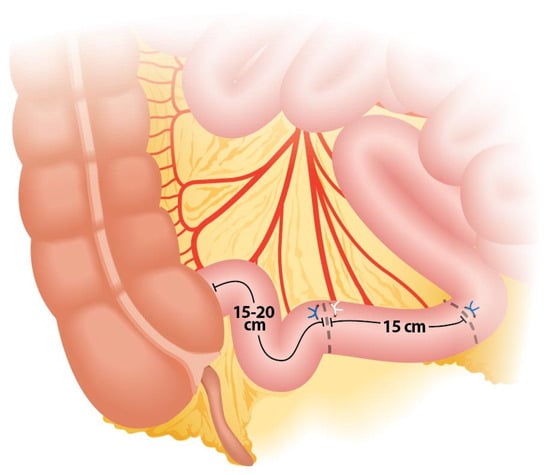
Figure 1.
Ileal segment length and anatomical site for division.
The segment is typically taken approximately 15–20 cm proximal to the ileocecal valve to preserve the terminal ileum, which is crucial for maintaining vitamin B12 absorption and minimizing the risk of malabsorption syndromes and bowel obstruction []. It should be noted that this segment will be between two fixed points, so it cannot be too short (as it would make ileo–ileal anastomosis difficult), nor too long, as this would cause intestinal obstruction due to intestinal kinking.
Crucial steps during the isolation of the bowel segment should be considered to ensure the effectiveness and safety of the procedure. One key step is positioning the stapler in the middle of the mesentery during segment resection, ensuring that the load is fired perpendicular to the bowel to prevent devascularization (Figure 2 and Figure 3). To achieve the correct angulation of the stapler, proper trocar placement is essential. Some authors also recommend discarding 5 cm of ileum proximal to the isolated loop for the same purpose, as this additional space provides mobility and prevents excessive traction (Figure 4) [,,].
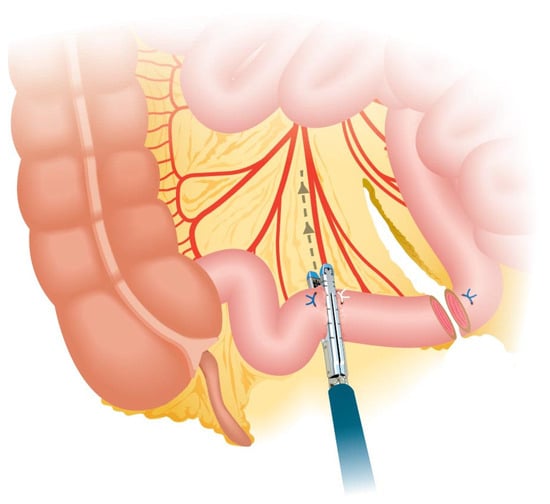
Figure 2.
Perpendicular division of the ileal segment.
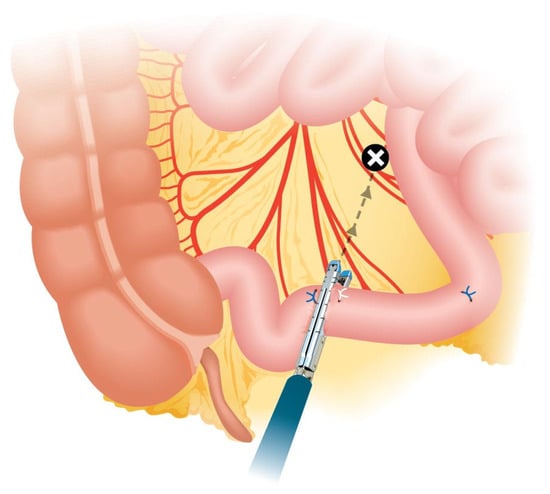
Figure 3.
Non-perpendicular division of the ileal segment. Note the oblique angle of the stapler.
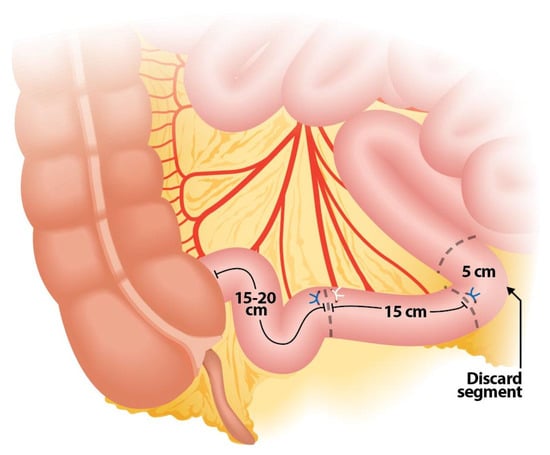
Figure 4.
Ileal segment configuration showing a 5 cm portion to discard.
Another technical aspect is identifying and preserving a reliable vascular supply, which is essential for ensuring the viability of the IC due to its terminal blood supply. During the procedure, the ileal segment is dissected along with its vascular pedicles, avoiding large feeding vessels to prevent vascular damage to both the segment and the ileo-ileal anastomosis [,]. To maintain structural integrity and prevent an internal transmesenteric ileal hernia, the mesenteric window created during the ileal anastomosis is closed (Figure 5) [,,]. The viability of the IC can then be assessed using indocyanine green (ICG). If perfusion during or after IC creation is found to be inadequate, a reevaluation of the vascular supply is essential. In such cases, consultation with general surgery may be required to assess the viability of the bowel segment and perform interventions such as further resection or revision of the conduit to ensure adequate blood flow and prevent ischemia or necrosis.
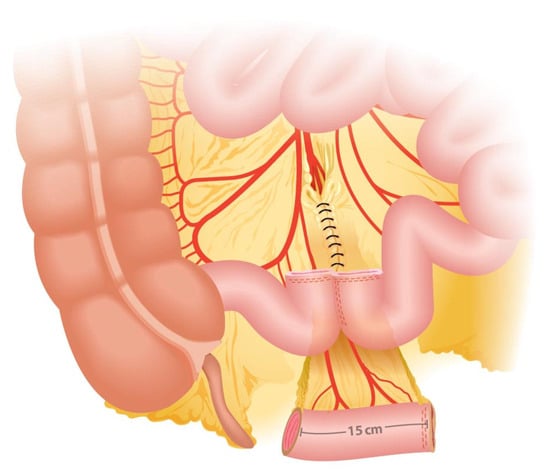
Figure 5.
Closure of the mesenteric window, ileo-ileal anastomosis achieving fecal continuity, and orientation of the ileal segment.
4.3. Bowel-Related Complications
Among the most common early complications, paralytic ileus is reported in up to 22% of cases. Factors contributing to its occurrence include preoperative bowel preparation, pain control medications, and surgical technique. Treatment typically involves nasogastric decompression, intravenous fluids, and bowel rest, with surgical intervention required in cases such as hernias [].
In contrast, leakage of the ileo-enteric anastomosis is one of the most serious complications due to its high mortality rates. This complication is primarily linked to the development of sepsis or septic shock, both of which can rapidly lead to multiorgan failure if not promptly identified and treated [].
Although the specific incidence of leakage in the context of IC creation has not been reported, studies of other gastrointestinal surgeries have documented leakage rates of 1% to 3% for ileocolonic anastomoses [,]. This complication is often associated with errors in surgical technique during the creation of the entero-enteric anastomosis. While no significant differences have been observed between staplers and standard sutures, or between side-to-side and end-to-end anastomosis, ensuring a watertight and tension-free closure remains critical to minimizing the risk of leakage and its associated complications [,].
Other complications include accidental injury to the bowel during ileal resection. The most common injuries occurred due to thermal injuries, accounting for approximately 50% of the reported cases, and occur when electrocautery unintentionally raises the temperature of surrounding tissues, even when the instrument is not directly in contact or during the lysis of adhesions. Access-related injuries, primarily from Trocar or Veress needle placement, account for 32% of intestinal injuries [].
Rectal injury during ORC occurs in approximately 4–9.6% of cases. Patients with prior pelvic radiation, pelvic surgery, or inflammatory diseases of the rectosigmoid colon are at higher risk. Small rectal injuries are typically managed by closing the defect with two layers of sutures. For larger defects, or in cases where contamination or impaired healing is anticipated, sigmoid loop colostomy is recommended [,]. Serosal tears may be less severe but still need repair to avoid fistula formation or leaks [].
4.4. Ureteric Complications
Ureteral injuries during mobilization for ileal conduit creation are particularly concerning in cases of tumor involvement or fibrosis from prior radiation therapy. Although inadvertent ureteral transections are rare, as noted by Hautmann et al., they can occur during the ureteroileal anastomosis []. Additionally, ureteral devascularization, which is more common on the left side due to the more extensive dissection, mobilization, and tunneling under the sigmoid mesentery, may lead to necrosis of the ureter [,].
Many of these complications, such as leaks, necrosis, and stenosis, tend to present postoperatively and are often associated with inadequate suture strength, excessive tension, or compromised blood supply to the ureter. Applying a “no-touch” principle during surgery, along with using ICG fluorescence imaging before performing the anastomosis, can help verify adequate perfusion and minimize the risk of ureteral necrosis and subsequent stenosis [].
4.5. Vascular Complications
As previously mentioned, the complications discussed are more closely associated with RC itself, rather than being specifically related to the IC. Intraoperative bleeding is a significant concern when dissecting pelvic tissues and mobilizing the ileum. The pelvic area contains large blood vessels, such as the iliac arteries and veins, which can be accidentally injured during the procedure, resulting in serious blood loss. Proper identification and careful dissection are essential to avoid these injuries []. Similarly, as noted earlier, when creating an IC, there is a rare but serious risk of ischemia in the ileal segment following RC. This occurs when blood flow to the isolated bowel segment is insufficient, potentially resulting in tissue necrosis and conduit failure. Several studies highlight the importance of meticulous surgical technique to preserve the blood supply, thereby reducing the risk of ischemia and its associated complications [].
4.6. Anesthesia-Related Complications
This includes hemodynamic instability, which can occur due to blood loss or manipulation of major anatomical structures. Proper control of blood pressure and administration of intravenous fluids are essential to prevent hypovolemic shock during surgery [].
5. Post-Operative Complications
5.1. Ureteroileal Anastomosis Complications
Anastomotic Leak
Urine leakage is an early postoperative complication that typically occurs between the 7th and 10th postoperative day [], with a reported incidence of 1–2% [,]. It can arise from any suture line within the IC but is more commonly seen at the ureteroileal anastomosis []. Its etiology is related to tension at the anastomosis and ischemia of the distal ureter.
Although some authors suggest that the use of ureteral stents placed during surgery may reduce the rate of urine leakage following ureteroileal anastomosis [,], prospective studies and meta-analyses comparing leakage rates in patients with and without stents have found no statistically significant differences between the groups [,].
When present, urine leakage is associated with increased drainage output (often with elevated creatinine levels in the fluid coming from the drain, if analyzed), decreased urinary output, paralytic ileus, and/or urinary sepsis [].
The diagnosis can initially be made using the drain fluid creatinine-to-serum creatinine ratio (DCSCR), which has a sensitivity of 68.8% and a specificity of 80.9% when the ratio is >1.18. If there is a high suspicion of this complication, DCSCR should be monitored regularly. Imaging studies, such as CT intravenous pyelogram or CT loopogram, may be used; however, the choice depends on the surgeon’s preference or criteria [,].
For management, algorithms recommend dividing the complication into those occurring within 6 days postoperatively and those after 6 days. When the leak occurs within the first 6 days, an early revision can be considered. When it occurs after 6 days, conservative management is initiated, which includes options like percutaneous nephrostomy, a fenestrated conduit catheter, a drain tube, or keeping ureteric stents in situ. If the leak is not controlled, surgical revision or a bilateral nephrostomy with ureteric occlusion may be considered. However, surgical reintervention with reconstruction is rare nowadays [].
It should be considered that urinary leakage can lead to fibrosis, which, over time, increases the risk of developing a uretero-ileal stricture [].
5.2. Ureteroileal Stricture
Uretero-ileal anastomosis stricture is a late surgical complication that usually occurs 3 months after surgery. Between 7% and 25% of patients experience this complication during follow-up [,,,].
Predisposing factors include excessive tension at the anastomosis site, short ureteral spatulation, over-dissection, urinary leakage, prior radiotherapy or chemotherapy, higher BMI, ASA score > 2, lymph node involvement, male sex, and a history of previous abdominal surgery [,].
It is more frequent in the left ureter []. This is attributed to the transposition of the left ureter, which leads to greater ureteral mobilization and angulation, with an increased risk of ischemic stricture. Left stenosis accounts for approximately 45–63%, compared to 29–40% of right stenosis and 8–13% of bilateral stenosis [,].
Strictures usually manifest with flank pain, impaired renal function, and/or urinary infection. Diagnosis is often made via CT scan, demonstrating hydronephrosis and delayed contrast passage to the IC. Initial treatment typically involves urinary diversion through percutaneous nephrostomies [].
There are various management options for this condition with varying success rates, such as endoscopic dilation and anastomosis reconstruction. Endoscopic dilation is a less invasive technique but has a slightly lower success rate (30–70%). It is suitable for complex patients with short stenoses (<1 cm) [,,].
Ureteroileal anastomosis reconstruction is more complex and invasive but has higher success rates, reaching 80–100% [,]. Reconstruction is especially indicated in patients with bilateral and/or long strictures (>1 cm) [], it can be performed either open or robotic with comparable success rates [].
Regarding the techniques employed, there are multiple types of uretero-ileal anastomosis, with the most commonly used being those described by Bricker and Wallace [,]. Various studies have attempted to determine which technique has a lower rate of stricture. Some studies indicate a higher rate of stricture associated with Bricker-type anastomosis []. In contrast, others find no significant differences and note that bilateral stricture is more frequently observed in patients undergoing Wallace-type anastomosis [].
Additionally, it has been reported that using a mucosa-to-mucosa running suture for the conduit anastomosis, supplemented with interrupted serosa-to-serosa sutures, is recommended, as it has been shown to reduce the incidence of stricture from 12% to 3% [].
In terms of surgical approach, several studies have attempted to compare the rates of strictures between open and robot-assisted surgery. Some retrospective series report higher rates of strictures in robot-assisted surgery [], while others find no significant differences between the two approaches []. This discrepancy may be explained by the initial practice of mobilizing the ureter for extracorporeal anastomosis. With minimal ureteral mobilization, a shorter ileal segment, proper ureteral spatulation, and the use of ICG to verify adequate perfusion, the incidence of strictures is expected to be lower in robot-assisted surgery [].
5.3. Stoma Related Complications
5.3.1. Parastomal Hernia
Information on the incidence of parastomal hernias (PH) is limited in the medical literature, as is information regarding patients undergoing surgical hernia repair following RC and IC placement. It is common to encounter multiple complications simultaneously, which complicates the assessment of more precise outcomes.
Most data are retrospective. In the largest available review, a total of 3170 patients treated with RC and IC were analyzed, and the clinical diagnosis of PH ranged from 4% to 28% [,]. The study by Dewulf et al. demonstrated that the prophylactic use of a lightweight mesh in the subcutaneous position reduced the incidence of PHs within the first 24 months of surgery from 23% to 11%, with a mean follow-up of 3 years [,].
Other researchers have expanded the range to 5% to 65% depending on the duration of follow-up and whether the diagnosis is made clinically or radiographically [].
Risk factors for this complication are numerous, with the most commonly described being the presence of a large stoma with an associated fascial defect (>24 mm). Patients with this condition have up to a 6.8-fold increased risk compared to those with smaller stomas [,].
An increase in BMI has been associated with a higher risk of PH. Specifically, patients with a BMI ≥ 22.9 kg/m2 have a 2.17-fold increased risk of PH compared to those with a lower BMI []. To decrease the risk of stoma-related complications, modifiable risk factors such as obesity, chronic obstructive pulmonary disease (COPD), and malnutrition should be addressed. Additionally, certain surgical techniques have been proposed to reduce the risk of stoma retraction. However, according to the literature, these techniques have not consistently demonstrated a clear improvement or reduction in risk, and further research is needed to establish their effectiveness [].
Other significantly reported risk factors included female gender, low preoperative albumin levels, prolonged operative time, postoperative eGFR less than 60 mL/min, previous exploratory laparotomy, and history of tobacco use [,,,].
The clinical presentation of PH includes symptoms such as pain, bowel incarceration, complications with appliances, discomfort, or stoma leakage [,,].
Surgical treatment for PHs ranges from local repairs with sutures, stoma relocation, to mesh-based repairs. The surgical approach can be open, laparoscopic, or robot-assisted. It should be noted that recurrence rates after PH repair can reach up to 69% [,,,].
5.3.2. Stomal Prolapse
A stomal prolapse is considered when there is a significant increase in the size of the stoma after maturation. Studies define a prolapse as a protrusion greater than 30 mm, typically occurring after the first year of the postoperative period []. Its incidence varies between 1.5% and 8% in patients with IC [].
Risk factors vary and can be related to patient-specific characteristics, such as weakened abdominal muscles, obesity, ongoing constipation, or chronic coughing [].
A patient with a prolapse may experience voiding dysfunction, pain, bulging, poor appliance fitting, obstruction, strangulation, and urinary infections. The management approach will be determined by the severity of the symptoms, ranging from conservative strategies to the resection of the prolapsed segment or relocation [].
A patient with a prolapse who remains asymptomatic is an ideal candidate for conservative management []. Non-surgical treatment includes the use of a prolapse belt to reduce the prolapse, though this carries the risk of stoma necrosis. If the stoma prolapse is associated with a PH, it should be repaired with a tension-free mesh [,,,,,].
5.3.3. Stoma Retraction
Stoma retraction is defined as a stoma surface that is more than 0.5 cm below the level of the skin; however, there is limited information in the literature regarding this complication in relation to IC []. The reported incidence of stoma retraction ranges from 9% to 15% for IC and from 1% to 11% for all ostomies [,].
The causes of stoma retraction are related to both the surgeon’s technique and patient-specific factors. These include inadequate intestinal mobilization, a high BMI, and persistent tension on the stoma due to a short mesentery or a thick abdominal wall, all of which can make conduit exteriorization more difficult. Additionally, postoperative weight gain can alter the distance between the fascia and skin, further contributing to retraction [,]. Similarly, this complication has also been associated with Crohn’s disease [].
Clinically retraction can lead to leakage and skin irritation, including dermatitis, which in turn creates difficulty in adhering devices to the skin. The use of a convex pouch can help optimize sealing and prevent leakage by flattening the peristomal skin, allowing the stoma to protrude outward above the skin and enabling effluent to discharge into the pouch [,]. However, it is important to consider that the convex shape of the device applies additional pressure on the abdomen, so the stoma should be monitored closely for the potential development of ulcers [].
Treatment is typically conservative and involves the use of a convex pouch system and patient education []. However, surgical intervention may be necessary, which can be particularly challenging for the surgeon, especially if there is a history of previous surgeries [].
5.3.4. Stoma Stenosis
Stoma stenosis is defined as a narrowing of the lumen, which may occur at the level of the skin or fascia. This condition leads to poor drainage [,].
The incidence of stoma stenosis occurs in 20% to 25% of cases; most occurrences are late, typically around 10 years after the intervention [].
It is associated with potential ischemia leading to retraction and scarring, particularly in the context of a circumferential mucocutaneous separation. Other contributing factors include chronic local inflammation and hyperkeratosis [,,,]. Stenosis at the skin level can be treated by dilating the stoma opening, generally by inserting a lubricated finger up to the fascial layer for several minutes [,]. This technique is taught to patients, who should perform it frequently and regularly. Digital dilation carries the risk of creating false passages in the mucosa. If stenosis is very severe, surgical management, revision, and/or relocation may be necessary [].
6. Conclusions
While cystectomy with ileal conduit formation is a well-established and safe procedure, there are still potential complications that can arise. Awareness of these complications and their management can optimize patient outcomes. We encourage high-level evidence research in this area, as the evidence is primarily retrospective and exhibits considerable variability in reporting.
Author Contributions
Conceptualization, L.K.F., S.J., A.G.-B. and L.G.M.; methodology, L.K.F.; software, L.K.F.; validation, L.G.M., R.S. and R.S.S.; formal analysis, L.K.F.; investigation, L.K.F.; resources, R.S.; data curation, L.K.F.; writing—original draft preparation, L.K.F., F.E., S.J., C.A.G. and A.G.-B.; writing—review and editing, L.K.F. and F.E.; visualization, L.K.F. and L.G.M.; supervision, L.G.M. and R.S.; project administration, L.G.M.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| IC | Ileal Conduit |
| RARC | Robot-Assisted Radical Cystectomy |
| ORC | Open Radical Cystectomy |
| RC | Radical Cystectomy |
| ICG | Indocyanine Green |
| DCSCR | Drain Fluid Creatinine-to-Serum Creatinine Ratio |
| BMI | Body Mass Index |
| PH | Parastomal Hernia |
| COPD | Chronic Obstructive Pulmonary Disease |
References
- Tanna, R.J.; Powell, J.; Mambu, L.A. Ileal Conduit. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Liu, Y.L.; Luo, H.-L.; Chiang, P.-H.; Chang, Y.-C.; Chiang, P.-H. Long-term urinary tract effect of ileal conduit after radical cystectomy compared with bladder preservation: A nationwide, population-based cohort study with propensity score-matching analysis. BMJ Open 2018, 8, e023136. [Google Scholar] [CrossRef]
- Medina, L.G.; Baccaglini, W.; Hernández, A.; Rajarubendra, N.; Winter, M.; Ashrafi, A.N.; Tafuri, A.; Cacciamani, G.E.; Sotelo, R. Robotic intracorporeal ileal conduit: Technical aspects. Arch. Esp. Urol. 2019, 72, 299–308. [Google Scholar]
- Kobayashi, K.; Goel, A.; Coelho, M.P.; Medina Perez, M.; Klumpp, M.; Tewari, S.O.; Appleton-Figueira, T.; Pinter, D.J.; Shapiro, O.; Jawed, M. Complications of ileal conduits after radical cystectomy: Interventional radiologic management. RadioGraphics 2021, 41, 249–267. [Google Scholar] [CrossRef]
- Madersbacher, S.; Schmidt, J.; Eberle, J.M.; Thoeny, H.C.; Burkhard, F.; Hochreiter, W.; Studer, U.E. Long-term outcome of ileal conduit diversion. J. Urol. 2003, 169, 985–990. [Google Scholar] [CrossRef]
- Philip, J.; Manikandan, R.; Venugopal, S.; Desouza, J.; Javlé, P.M. Orthotopic Neobladder versus Ileal Conduit Urinary Diversion after Cystectomy—A Quality-of-Life Based Comparison. Annals 2009, 91, 565–569. [Google Scholar] [CrossRef]
- Lee, R.K.; Abol-Enein, H.; Artibani, W.; Bochner, B.; Dalbagni, G.; Daneshmand, S.; Fradet, Y.; Hautmann, R.E.; Lee, C.T.; Lerner, S.P.; et al. Urinary diversion after radical cystectomy for bladder cancer: Options, patient selection, and outcomes. BJU Int. 2014, 113, 11–23. [Google Scholar] [CrossRef]
- Demaegd, L.; Albersen, M.; Muilwijk, T.; Milenkovic, U.; Moris, L.; Everaerts, W.; Van Poppel, H.; Van Der Aa, F.; Joniau, S.; Akand, M. Comparison of postoperative complications of ileal conduits versus orthotopic neobladders. Transl. Androl. Urol. 2020, 9, 2541–2554. [Google Scholar] [CrossRef]
- Han, J.H.; Ku, J.H. Robot-assisted radical cystectomy: Where we are in 2023. Investig. Clin. Urol. 2023, 64, 107. [Google Scholar] [CrossRef]
- Beirnaert, J.; Benarroche, D.; Pinar, U.; Roupret, M.; Phé, V.; Vaessen, C.; Parra, J.; Chartier-Kastler, E.; Seisen, T. Robotic versus open cystectomy with ileal conduit for the management of neurogenic bladder: A comparative study. World J. Urol. 2022, 40, 2963–2970. [Google Scholar] [CrossRef]
- Cano Megías, M.; Muñoz Delgado, E.G. Bone and metabolic complications of urinary diversions. Endocrinol. Nutr. 2015, 62, 100–105. [Google Scholar] [CrossRef]
- Giannini, S.; Nobile, M.; Sartori, L.; Aragona, F.; Ruffato, A.; Dalle Carbonare, L.; Ciuffreda, M.; Liberto, L.; Artibani, W.; D’Angelo, A.; et al. Bone density and skeletal metabolism in patients with orthotopic ileal neobladder. J. Am. Soc. Nephrol. 1997, 8, 1553–1559. [Google Scholar] [CrossRef]
- Cruz, D.N.; Huot, S.J. Metabolic complications of urinary diversions: An overview. Am. J. Med. 1997, 102, 477–484. [Google Scholar] [CrossRef]
- Mills, R.D.; Studer, U.E. Metabolic Consequences of Continent Urinary Diversion. J. Urol. 1999, 161, 1057–1066. [Google Scholar] [CrossRef]
- Colombo, R.; Naspro, R. Ileal Conduit as the Standard for Urinary Diversion After Radical Cystectomy for Bladder Cancer. Eur. Urol. Suppl. 2010, 9, 736–744. [Google Scholar] [CrossRef]
- Regmi, S.K.; Bearrick, E.N.; Hannah, P.T.F.; Sathianathen, N.; Kalapara, A.; Konety, B.R. Drain fluid creatinine-to-serum creatinine ratio as an initial test to detect urine leakage following cystectomy: A retrospective study. Indian J. Urol. 2021, 37, 153–158. [Google Scholar] [CrossRef]
- Vasdev, N.; Moon, A.; Thorpe, A. Metabolic complications of urinary intestinal diversion. Indian J. Urol. 2013, 29, 310. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Tyson, M.D.; Chang, S.S. Conduit Urinary Diversion. Urol. Clin. N. Am. 2018, 45, 25–36. [Google Scholar] [CrossRef]
- Anderson, C.B.; McKiernan, J.M. Surgical Complications of Urinary Diversion. Urol. Clin. N. Am. 2018, 45, 79–90. [Google Scholar] [CrossRef]
- Bishoff, J.T.; Allaf, M.E.; Kirkels, W.; Moore, R.G.; Kavoussi, L.R.; Schroder, F. Laparoscopic bowel injury: Incidence and clinical presentation. J. Urol. 1999, 161, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Flechner, S.M.; Spaulding, J.T. Management of rectal injury during cystectomy. Urology 1982, 19, 143–147. [Google Scholar] [CrossRef]
- Kozminski, M.; Konnak, J.W.; Grossman, H.B. Management of rectal injuries during radical cystectomy. J. Urol. 1989, 142, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Frazier, H.A.; Robertson, J.E.; Paulson, D.F. Complications of radical cystectomy and urinary diversion: A retrospective review of 675 cases in 2 decades. J. Urol. 1992, 148, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.M.; de Abreu, A.L.C.; Goh, A.C.; Fairey, A.; Berger, A.; Leslie, S.; Xie, H.W.; Gill, K.S.; Miranda, G.; Aron, M.; et al. Robotic intracorporeal urinary diversion: Technical details to improve time efficiency. J. Endourol. 2014, 28, 1320–1327. [Google Scholar] [CrossRef]
- Gabriel, P.-É.; Siebert, M.; Le Fouler, A.; Van Glabeke, E.; Trésallet, C. Management of gastro-intestinal emergencies in patients with ileal conduit ureteral diversion. J. Visc. Surg. 2022, 159, 399–408. [Google Scholar] [CrossRef]
- Ellis, C.T.; Maykel, J.A. Defining Anastomotic Leak and the Clinical Relevance of Leaks. Clin. Colon. Rectal. Surg. 2021, 34, 359–365. [Google Scholar] [CrossRef]
- Kavaric, P.; Eldin, S.; Nenad, R.; Dragan, P.; Vukovic, M. Modified Wallace anastomotic technique reduces ureteroenteric stricture rates after ileal conduit urinary diversion. Int. Braz. J. Urol. 2020, 46, 446–455. [Google Scholar] [CrossRef]
- Ghoneim, M.A.; el-Mekresh, M.M.; el-Baz, M.A.; el-Attar, I.A.; Ashamallah, A. Radical cystectomy for carcinoma of the bladder: Critical evaluation of the results in 1026 cases. J. Urol. 1997, 158, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Nassar, O.A.H.; Alsafa, M.E.S. Experience with ureteroenteric strictures after radical cystectomy and diversion: Open surgical revision. Urology 2011, 78, 459–465. [Google Scholar] [CrossRef]
- Hautmann, R.E.; de Petriconi, R.; Gottfried, H.W.; Kleinschmidt, K.; Mattes, R.; Paiss, T. The ileal neobladder: Complications and functional results in 363 patients after 11 years of followup. J. Urol. 1999, 161, 422–427; discussion 427–428. [Google Scholar] [CrossRef]
- McDougal, W.S.; Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C.A. Campbell-Walsh Urology; Elsevier: Philadelphia, PA, USA, 2016; pp. 2434–2435. [Google Scholar]
- Farnham, S.B.; Cookson, M.S. Surgical complications of urinary diversion. World J. Urol. 2004, 22, 157–167. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Ning, K.; Wu, Z.-S.; Li, Z.-Y.; Deng, M.-H.; Xiong, L.-B.; Yu, C.-P.; Zhang, Z.-L.; Liu, Z.-W.; Lu, H.-M.; et al. Ureteral stents cannot decrease the incidence of ureteroileal anastomotic stricture and leakage: A systematic review and meta-analysis. Int. J. Surg. 2021, 93, 106058. [Google Scholar] [CrossRef]
- Regan, J.B.; Barrett, D.M. Stented versus nonstented ureteroileal anastomoses: Is there a difference with regard to leak and stricture? J. Urol. 1985, 134, 1101–1103. [Google Scholar] [CrossRef]
- Mullins, J.K.; Guzzo, T.J.; Ball, M.W.; Pierorazio, P.M.; Eifler, J.; Jarrett, T.W.; Schoenberg, M.P.; Bivalacqua, T.J. Ureteral stents placed at the time of urinary diversion decreases postoperative morbidity. Urol. Int. 2012, 88, 66–70. [Google Scholar] [CrossRef]
- Mattei, A.; Birkhaeuser, F.D.; Baermann, C.; Warncke, S.H.; Studer, U.E. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? results of a prospective randomized trial. J. Urol. 2008, 179, 582–586. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Consensus Conference on Bladder Cancer; Hautmann, R.E.; Abol-Enein, H.; Hafez, K.; Haro, I.; Mansson, W.; Mills, R.D.; Montie, J.D.; Sagalowsky, A.I.; Stein, J.P.; et al. Urinary diversion. Urology 2007, 69 (Suppl. S1), 17–49. [Google Scholar] [CrossRef]
- Brown, K.G.M.; Koh, C.E.; Vasilaras, A.; Eisinger, D.; Solomon, M.J. Clinical algorithms for the diagnosis and management of urological leaks following pelvic exenteration. Eur. J. Surg. Oncol. 2014, 40, 775–781. [Google Scholar] [CrossRef]
- Al-Nader, M.; Krafft, U.; Hess, J.; Kesch, C.; AbdelRazek, M.; Abolyosr, A.; Alsagheer, G.A.; Mohamed, O.; Fathi, A.; Tschirdewahn, S.; et al. Bricker versus Wallace ureteroileal anastomosis: A multi-institutional propensity score-matched analysis. Int. J. Urol. 2024, 31, 813–818. [Google Scholar] [CrossRef]
- Christoph, F.; Herrmann, F.; Werthemann, P.; Janik, T.; Schostak, M.; Klopf, C.; Weikert, S. Ureteroenteric strictures: A single center experience comparing bricker versus wallace ureteroileal anastomosis in patients after urinary diversion for bladder cancer. BMC Urol. 2019, 19, 100. [Google Scholar] [CrossRef]
- Adnan, S.; Abu Bakar, M.; Khalil, M.A.I.; Fiaz, S.; Ahmad Cheema, Z.; Ali, A.; Mir, K. Outcomes of Uretero-Ileal Anastomosis in Bladder Cancer Cystectomies: Bricker vs. Wallace 1. Cureus 2022, 14, e22782. [Google Scholar] [CrossRef]
- Hosseini, A.; Dey, L.; Laurin, O.; Adding, C.; Hoijer, J.; Ebbing, J.; Collins, J.W. Ureteric stricture rates and management after robot-assisted radical cystectomy: A single-centre observational study. Scand. J. Urol. 2018, 52, 244–248. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, B.; Hu, Y.; Li, H.; Qin, X.; Hu, X.; Wang, S.; Wang, H.; Zhang, P.; Wo, Q.; et al. The cause analysis of benign uretero-ileal anastomotic stricture after radical cystectomy and urinary diversion. Front. Oncol. 2022, 12, 1070141. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Chen, Q.; Xia, D.; Zhang, H.; Chen, M. Endoscopic Procedures in the Treatment of Ureteroenteric Anastomotic Strictures: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 626939. [Google Scholar] [CrossRef]
- Narita, S.; Saito, M.; Numakura, K.; Habuchi, T. Incidence, Etiology, Prevention and Management of Ureteroenteric Strictures after Robot-Assisted Radical Cystectomy: A Review of Published Evidence and Personal Experience. Curr. Oncol. 2021, 28, 4109–4117. [Google Scholar] [CrossRef]
- Gin, G.E.; Ruel, N.H.; Parihar, J.S.; Warner, J.N.; Yuh, B.E.; Yamzon, J.; Wilson, T.G.; Lau, C.S.; Chan, K.G. Ureteroenteric anastomotic revision as initial management of stricture after urinary diversion. Int. J. Urol. 2017, 24, 390–395. [Google Scholar] [CrossRef]
- Albisinni, S.; Aoun, F.; Mjaess, G.; Abou Zahr, R.; Diamand, R.; Porpiglia, F.; Esperto, F.; Autorino, R.; Fiori, C.; Tubaro, A.; et al. Contemporary management of benign uretero-enteric strictures after cystectomy: A systematic review. Minerva Urol. Nephrol. 2021, 73, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Bricker, E.M. Bladder substitution after pelvic evisceration. Surg. Clin. N. Am. 1950, 30, 1511–1521. [Google Scholar] [CrossRef]
- Wallace, D.M. Uretero-ileostomy. Br. J. Urol. 1970, 42, 529–534. [Google Scholar] [CrossRef]
- Anderson, C.B.; Morgan, T.M.; Kappa, S.; Moore, D.; Clark, P.E.; Davis, R.; Penson, D.F.; Barocas, D.A.; Smith, J.A.; Cookson, M.S.; et al. Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter? J. Urol. 2013, 189, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Kobayashi, Y.; Maruyama, Y.; Kawada, T.; Sadahira, T.; Oiwa, Y.; Katayama, S.; Nishimura, S.; Takamoto, A.; Sako, T.; et al. Comparison of intracorporeal versus extracorporeal urinary diversion after robot-assisted radical cystectomy at a medium-sized facility. Int. J. Clin. Oncol. 2021, 26, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Hemal, A.K.; Abol-Enein, H.; Tewari, A.; Shrivastava, A.; Shoma, A.M.; Ghoneim, M.A.; Menon, M. Robotic radical cystectomy and urinary diversion in the management of bladder cancer. Urol. Clin. N. Am. 2004, 31, 719–729. [Google Scholar] [CrossRef]
- Themes UFO. Ileal Conduit Urinary Diversion [Internet]. Abdominal Key, 2020. Available online: https://abdominalkey.com/ileal-conduit-urinary-diversion/ (accessed on 13 November 2024).
- Narang, S.K.; Alam, N.N.; Campain, N.J.; Pathak, S.; McGrath, J.S.; Daniels, I.R.; Smart, N.J. Parastomal hernia following cystectomy and ileal conduit urinary diversion: A systematic review. Hernia 2017, 21, 163–175. [Google Scholar] [CrossRef]
- Donahue, T.F.; Bochner, B.H.; Sfakianos, J.P.; Kent, M.; Bernstein, M.; Hilton, W.M.; Cha, E.K.; Yee, A.M.; Dalbagni, G.; Vargas, H.A. Risk Factors for the Development of Parastomal Hernia after Radical Cystectomy. J. Urol. 2014, 191, 1708–1713. [Google Scholar] [CrossRef]
- Dewulf, M.; Hildebrand, N.D.; Bouwense, S.A.W.; Bouvy, N.D.; Muysoms, F. Parastomal hernias after cystectomy and ileal conduit urinary diversion: Surgical treatment and the use of prophylactic mesh: A systematic review. BMC Surg. 2022, 22, 118. [Google Scholar] [CrossRef]
- Feng, D.; Wang, Z.; Yang, Y.; Li, D.; Wei, W.; Li, L. Incidence and risk factors of parastomal hernia after radical cystectomy and ileal conduit diversion: A systematic review and meta-analysis. Transl. Cancer Res. TCR 2021, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Ahmed, Y.E.; May, P.; Ali, T.; Ahmad, B.; Raheem, S.; Stone, K.; Hasasnah, A.; Rana, O.; Cole, A.; et al. Natural History and Predictors of Parastomal Hernia after Robot-Assisted Radical Cystectomy and Ileal Conduit Urinary Diversion. J. Urol. 2018, 199, 766–773. [Google Scholar] [CrossRef]
- Liu, N.W.; Hackney, J.T.; Gellhaus, P.T.; Monn, M.F.; Masterson, T.A.; Bihrle, R.; Gardner, T.A.; House, M.G.; Koch, M.O. Incidence and risk factors of parastomal hernia in patients undergoing radical cystectomy and ileal conduit diversion. J. Urol. 2014, 191, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Djaladat, H.; Ghoreifi, A.; Tejura, T.; Miranda, G.; Cai, J.; Sheybaee Moghaddam, F.; Aldana, I.; Sotelo, R.; Gill, I.; Bhanvadia, S.; et al. Prophylactic Use of Biologic Mesh in Ileal Conduit (PUBMIC): A Randomized Clinical Trial. J. Urol. 2024, 211, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kouba, E.; Sands, M.; Lentz, A.; Wallen, E.; Pruthi, R.S. Incidence and Risk Factors of Stomal Complications in Patients Undergoing Cystectomy With Ileal Conduit Urinary Diversion for Bladder Cancer. J. Urol. 2007, 178, 950–954. [Google Scholar] [CrossRef]
- Antoniou, S.A.; Agresta, F.; Garcia Alamino, J.M.; Berger, D.; Berrevoet, F.; Brandsma, H.-T.; Bury, K.; Conze, J.; Cuccurullo, D.; Dietz, U.A.; et al. European Hernia Society guidelines on prevention and treatment of parastomal hernias. Hernia 2018, 22, 183–198. [Google Scholar] [CrossRef]
- DeAsis, F.J. Current state of laparoscopic parastomal hernia repair: A meta-analysis. WJG 2015, 21, 8670. [Google Scholar] [CrossRef]
- Hansson, B.M.E.; Slater, N.J.; van der Velden, A.S.; Groenewoud, H.M.M.; Buyne, O.R.; de Hingh, I.H.J.T.; Bleichrodt, R.P. Surgical techniques for parastomal hernia repair: A systematic review of the literature. Ann. Surg. 2012, 255, 685–695. [Google Scholar] [CrossRef]
- Odensten, C.; Strigård, K.; Dahlberg, M.; Gunnarsson, U.; Näsvall, P. Parastomal Hernia Repair; Seldom Performed and Seldom Reported: Results From a Nationwide Survey. Scand. J. Surg. 2020, 109, 96–101. [Google Scholar] [CrossRef]
- Szymanski, K.M.; St-Cyr, D.; Alam, T.; Kassouf, W. External stoma and peristomal complications following radical cystectomy and ileal conduit diversion: A systematic review. Ostomy Wound Manag. 2010, 56, 28–35. [Google Scholar]
- O’Flynn, S.K. Care of the stoma: Complications and treatments. Br. J. Community Nurs. 2018, 23, 382–387. [Google Scholar] [CrossRef]
- Wani, M.; Bhat, T.; Sheriff, M. Conduit over conduit reconstruction of retracted and fibrosed ileal conduit in severe abdominal adhesions. Curr. Urol. 2022, 16, 50–52. [Google Scholar] [CrossRef]
- Colwell, J. The state of the standard diversion. J. WOCN 2001, 28, 6–17. [Google Scholar] [CrossRef]
- Cheung, M.T. Complications of an Abdominal Stoma: An Analysis of 322 Stomas. Aust. N. Z. J. Surg. 1995, 65, 808–811. [Google Scholar] [CrossRef]
- Nordström, G.M.; Borglund, E.; Nyman, C.R. Local Status of the Urinary Stoma-the Relation to Peristomal Skin Complications. Scand. J. Urol. Nephrol. 1990, 24, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.N.; Allen, S.E.; Hussain, M.; Greenwell, T.J.; Shah, P.J.R. Stomal Complications of Ileal Conduits Are Significantly Higher When Formed in Women with Intractable Urinary Incontinence. J. Urol. 2004, 172 Pt 1, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.J.; Bevan, L.; Macdonald, L.; Watkins, A.J.; Morgan, A.R.; Beynon, J.; Carr, N.D. A prospective audit of stomas--analysis of risk factors and complications and their management. Color. Dis. 2003, 5, 49–52. [Google Scholar] [CrossRef]
- Tankel, J.; Edden, Y. Stoma Retraction and Stenosis. In Clinical Decision Making in Colorectal Surgery; Steele, S.R., Maykel, J.A., Wexner, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 623–628. Available online: http://link.springer.com/10.1007/978-3-319-65942-8_79 (accessed on 13 November 2024).
- Daneshmand, S.; Bartsch, G. Improving selection of appropriate urinary diversion following radical cystectomy for bladder cancer. Expert Rev. Anticancer Ther. 2011, 11, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Colwell, J.C.; Fichera, A. Care of the Obese Patient With an Ostomy. J. Wound Ostomy Cont. Nurs. 2005, 32, 378–383. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).