Abstract
The COVID-19 pandemic has reshaped the global understanding of airborne disease transmission, particularly in healthcare environments. This literature review examines how building ventilation and indoor air quality strategies have evolved in response to SARS-CoV-2, with a specific focus on healthcare settings. A systematic review of 163 post-pandemic studies, alongside a selective review of pre-COVID-19 literature, was conducted to assess how scientific knowledge, practical recommendations, and HVAC-related interventions have changed. The review categorizes studies across detection methods, simulation models, observational analyses, and policy recommendations, drawing attention to novel findings and evidence-supported practices. While the body of research reaffirms the critical role of ventilation, many recommendations remain unevaluated through empirical methods. This study identifies the gaps in evidence and highlights the most impactful advances that can inform future design, maintenance, and operational protocols in healthcare facilities to mitigate airborne infection risks.
1. Introduction
Problem Statement
This study aims to examine how healthcare facility managers, owners, and scientists addressed the pandemic crisis, particularly concerning indoor air quality in the healthcare-built environment. It investigates their research activities, collaborations, and contributions to understanding and mitigating indoor air transmission of infectious diseases within healthcare facilities. We seek to identify the knowledge gained about the built environment and indoor air quality during COVID-19 and to answer the following question: Has our knowledge fundamentally changed? This study investigates whether extreme events, such as a pandemic, foster information acquisition and improve creative problem-solving as evidenced by modifications made, measurements taken, and the scientific outcomes obtained. Are these approaches and knowledge different from what we knew before? Therefore, (1) a systematic literature review will be conducted of the scientific papers submitted in this field after the pandemic, followed by (2) a selective literature review of articles published before the COVID-19 pandemic to compare our knowledge pre- and post-pandemic, and works of significance in producing new knowledge supported by evidence. In this context, research findings for any premise in which healthcare services (inpatient, outpatient, medical offices, examination rooms, etc.) are delivered were collected and analyzed. The research questions in this mixed methods study are
- Has the COVID-19 pandemic improved our knowledge base specific to healthcare settings, and what are the new or novel findings?
- How often do we see recommendations not supported by evidence in the scholarly works published after the COVID-19 pandemic?
- To what extent has the existing knowledge and experience available in the literature shaped the written recommendations and policies during the pandemic?
2. Background
This section is devoted to the previously published literature review papers to pivot the focus of this current work onto the areas that have not been adequately addressed. For ease of reading, such review papers have been categorized into those addressing disease transmission routes, the role of HVAC (Heating, Ventilation, and Air Conditioning), followed by recommended IAQ (Indoor Air Quality) strategies.
2.1. Routes of Disease Transmission
At the beginning of the COVID-19 pandemic, there was considerable discussion regarding the modes of disease transmission. Nearly half of the post-COVID-19 review studies identified in this research were review studies of other experimental and simulation works to confirm airborne transmission of COVID-19. According to Ref. [1], the transmissibility and survivability of the virus might be influenced by the thermal properties of ambient air and relative humidity, as indicated in their comprehensive systematic review of 49 studies. The virus can infect the respiratory system through both direct means, such as droplet and person-to-person transmission, as well as indirect means, including contamination of objects and airborne transmission. In Ref. [2], the authors synthesized 14 indoor studies (11 experimental, 3 simulation); 11 supported airborne transmission. They concluded that airborne transmission of SARS-CoV-2 is likely and recommended improving ventilation, maintaining a minimum relative humidity, and keeping at least two meters between individuals. In another study of eleven reviewed papers, four did not verify SARS-CoV-2 in the air. Still, SARS-CoV-2 in air samples was reported in seven papers, indicating that many other factors, including hospital environmental conditions, sampling methods, and ventilation system efficiency could influence the detection of SARS-CoV-2 in the air [3]. In a similar study, Ref. [4] reported the SARS-CoV-2 virus in several air samples collected from patient rooms, warranting its airborne transmission potential. However, because in most included studies, samples were taken within the patient’s room, it seemed difficult to differentiate between true airborne versus droplet routes. Another research study [5] focused on the spread of infectious droplets and aerosols in various environmental settings and demonstrated how droplets and aerosols from a cough-jet of an infected person behave in different confined settings. Again, airborne transmission played a profound role in confined spaces (airplanes, passenger cars, and healthcare centers) under various ventilation conditions.
2.2. Role of HVAC Systems
A literature review of twenty papers indicated that there is insufficient data to draw a firm conclusion on the correlation between ventilation systems and the transmission of SARS-CoV-2. Yet, effective ventilation was identified as one of the six fundamental methods for controlling COVID-19. Improper ventilation could cause further spread of the disease, and using air disinfection and particle filtration with appropriate ventilation was recommended [6]. In another follow-up study reviewing 52 publications, it was determined that COVID-19 infection may be avoided in part by controlling temperature, relative humidity, and ventilation and air conditioning systems. They provided guidance on ventilation techniques, as well as control and preventative measures and identified effective ventilation as the most significant strategy among the six basic strategies previously identified to control COVID-19 infection [7]. Fourteen SARS-CoV-1, MERS-CoV, and SARS-CoV-2 coronavirus outbreaks linked to air conditioning systems were reported by another review study. In six of the seven SARS-CoV-1 studies, airflow-dynamics models or the spatial and temporal patterns of cases provided indirect evidence of the role of HVAC systems (heating, ventilation, and air conditioning) in the outbreak. One MERS study showed that virus particles had been found in HVAC systems. Computer modeling was used in four of the six SARS-CoV-2 investigations to hypothesize or support the viral agents’ ability to propagate through the HVAC system. The authors concluded that previous outbreaks in Asia have substantial evidence of coronavirus airborne transmission. However, the few COVID-19 experiments available could not substantially support that HVAC systems may transmit the SARS-CoV-2 virus [8]. A review study [9] indicated that a malfunction in the HVAC system could aid in the spread of infectious diseases, but inconclusive evidence was provided to support the role of properly functioning HVAC systems. Submicron virus-laden aerosols could be produced by the resuspension of SARS-CoV-2-infected particles adhering to PPE surfaces; removal could produce the initial velocity; and supermicron virus-laden aerosols could be produced by SARS-CoV-2 that was dropped on the floor and spread by medical personnel as they moved between different locations (e.g., shoe). Another similar study emphasized the importance of the airborne spread of COVID-19, acknowledged HVAC as a significant source of indoor and environmental pollution that may account for the quick viral spread and recommended adjustments to hospitals’ HVAC system, such as bringing 100% fresh air [10].
2.3. IAQ Strategies and Safety Measures
The efforts to review and identify strategies and safety measures to lower the likelihood of infection in the built environment made up another significant number of review studies and included miscellaneous issues [11]. IAQ strategies were discussed in three groups: source control strategies such as masks and social distancing, ventilation improvement strategies such as personal ventilation, displacement ventilation, use of ceiling fans, use of adaptive wall-based supply fans; and filtration and air purification strategies such as HEPA (High Efficiency Particulate Air) filters and UVGI (Ultraviolet Germicidal Irradiation). Various strategy combinations were recommended based on the space type in the literature. For instance, dental clinics should maintain a negative pressure between the exam room and waiting area, with an ideal humidity level between 40 and 60 percent [12]. Lower RH can lead to the evaporation of water inside airborne particles and cause long-term suspension of the particles in the air. In contrast, higher RH would increase the particle sizes and lead to faster settling. In addition, an RH of less than 33% is suitable for the survival of COVID-19, while an RH of ~60% significantly decreases the survival rate of COVID-19 [13].
The use of UVGI and HEPA filters as well as laminar airflow was recommended in operating rooms (ORs) to mitigate the dispersion of virus-laden particles [14]. The operating room should stay vacant for enough time after each procedure to void particles via ventilation and ensure higher than 99.9% aerosol clearing, and it is essential to maintain the anteroom at a significantly lower air pressure than the operating room (at least 2.5 pa with a minimum of 12 ACH). Furthermore, aerosol-generating procedures (AGP) should be done while PPE, such as N95 masks, eye protection, gloves, and gowns are on, and after AGP, additional staff should wait for at least 30 min before entering the OR. Finally, it was mentioned that, if possible, patient recovery should take place in a negative pressure isolation room [15]. Turning the OR into negative pressure to treat COVID patients was also recommended yet there is far from sufficient evidence to advocate large-scale conversion due to cost, time and resource constraints [16].
2.4. Takeaway from the Literature
According to the existing body of literature reviewed above, these attempts largely focus on the modalities of SARS-CoV-2 transmission (i.e., aerosols, airborne) and evaluate the role of HVAC systems in restricting the spread of infection. Once this possibility (role of HVAC systems in viral control or spread) was warranted or at least demonstrated, the next cohort of publications describes the approaches and tactics for mitigating virus expansion in various environments within healthcare facilities, such as operating rooms, dental clinics, etc. In this sense, the COVID-19 pandemic resulted in faster generation of research, particularly on indoor air quality. Dozens of HVAC-related and non-HVAC-related recommendations were made, but of utmost importance is to determine which ones are evidence-based and to what extent new knowledge has been produced during the pandemic. Therefore, this research study aims to conduct a systematic literature review of works published after COVID-19 and, through the selective literature review of works published before the pandemic, identify the new knowledge, skills, and practices supported by scientific evidence.
3. Methodology
3.1. Literature Search Strategy
The data gathering and analysis were done based on finding and categorizing the relevant research papers to deliver a systematic literature review. For collecting papers published after the COVID-19 pandemic, firstly, three categories of keywords were identified, labeled, and assembled, as shown in Table 1.

Table 1.
Used keywords for searching post-COVID-19 papers.
We generated 48 search strings by taking the Cartesian product of the three keyword sets in Table 1 (4 key ventilation terms × 4 space terms × 3 miscellaneous terms = 4 × 4 × 3 = 48). Each string used Boolean AND and quoted phrases, e.g., “ventilation rate” AND “operating room” AND “COVID-19”, “air change rate” AND “isolation room” AND “air quality”, “airflow” AND “hospitals” AND “disease”. Various search engines such as Engineering Village, PubMed, and Google Scholar were used for identifying the relevant papers. The selected time for publishing the articles was 2020 and afterward (2020 to 2024). This thorough search process retrieved 1037 records across Google Scholar, PubMed, Engineering Village, and Clemson Library. Initially, the titles were reviewed; 726 records were excluded as irrelevant, leaving 311 records for abstract screening. Subsequently, the abstracts of these selected papers were carefully studied to determine whether they fell within the scope of the review. This further refined the list to 184 articles. During the initial screening, 20 additional papers were identified either through references in the reviewed papers or by the research team, and these papers were also added to the database for further evaluation. Following a thorough examination of each paper, 41 articles were excluded from the final selection due to their lack of relevance to the scope of this study, as they focused on different space functions unrelated to healthcare environments (the second exclusion criterion).
Also, the simulation papers that lacked clear validation or other forms of assuring modeling quality were excluded from the review process. Ultimately, a total of 163 papers were identified as highly relevant and were retrieved and stored in a reference management software for further analysis and organization. Key information, such as the space function, study objectives, study design, and main findings, was carefully recorded. Furthermore, to provide a broader perspective and draw comparisons, a selective literature review of over 200 pre-COVID publications was conducted based on the identified thrusts, results, and recommendations from the post-COVID publications. The pre-COVID articles were identified and imported into reference management software, allowing for a comprehensive analysis that incorporates both pre- and post-COVID literature (Figure 1).
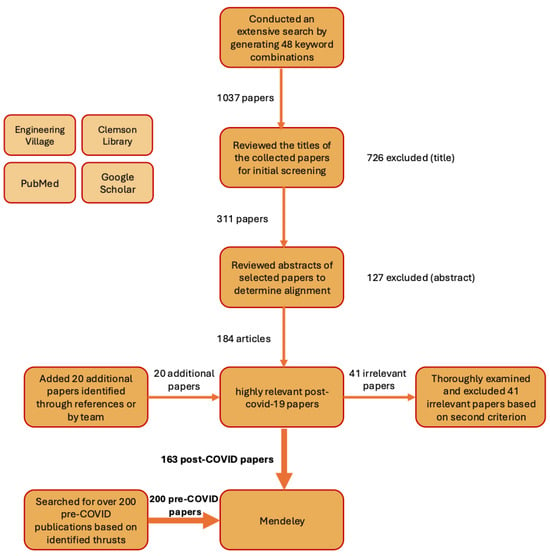
Figure 1.
Procedure for Identifying Post-COVID-19 Papers and Pre-COVID-19 Papers.
3.2. Inclusion and Exclusion Criteria
The following criteria were used for the inclusion of papers published after the COVID-19 pandemic: (1) written in English, (2) centered on healthcare facilities, (3) published after the COVID-19 pandemic (i.e., 2020 onward). The publications found were categorized and given a rating based on data origins and research methodology, as shown in Table 2. Only the PRISMA-compliant papers were tagged as systematic reviews, and other review papers were tagged as selective reviews. Additionally, Figure 2 illustrates the distribution of included papers published after COVID-19 based on research methodology. Note that these ratings are not equivalent to a measure of quality, e.g., Group 3 does not mean greater quality than Group 4 and vice versa.

Table 2.
Metrics for paper rating.
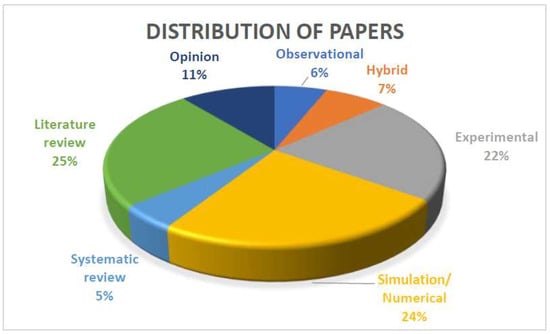
Figure 2.
Distribution of 163 post-COVID-19 reviewed articles according to research method.
4. Results
Upon completing the systematic literature review of post-COVID publications, the research team identified four primary research thrusts, each containing several themes, as shown in Figure 3. The literature grounded theory approach [17] was adopted to map the findings from the literature into relevant thrusts based on their concepts, and further group them into themes that shared related aspects. In this section, findings for each research thrust are separately presented and discussed.
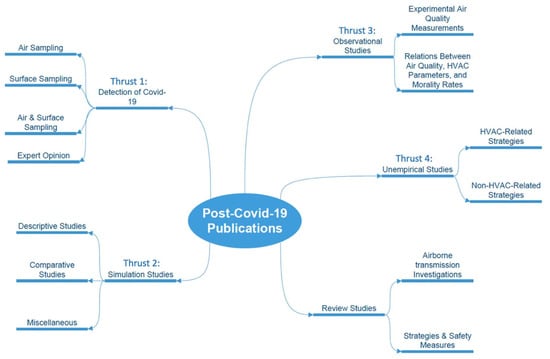
Figure 3.
Post-COVID-19 identified research thrusts and their themes.
4.1. Thrust 1: Detection
After the initial outbreak of COVID-19, there was a debate regarding the various modes of transmission of the virus responsible for causing COVID-19 [18]. Hence, research was focused on recovering the COVID-19 virus strain in the air and on surfaces of various indoor spaces, where the former was indicative of true airborne or droplet transmission.
4.1.1. Air Sampling
In Ref. [19], ten air samples were collected from a hospital with confirmed COVID-19 cases. These samples were taken 2 to 5 m away from patients’ beds. Each air sample was collected into sterile standard midget impingers filled with 20 mL of DMEM containing 100 μg/mL streptomycin, 100 U/mL penicillin, and 1% antifoam reagent, and the collection period lasted for one hour. Viral RNA was extracted from the samples via a reverse-transcription polymerase chain reaction (RT-PCR), and none of the air samples contained the COVID-19 strain. Still, the authors suggested that all healthcare workers at the clinical hospital who come into close contact with patients are required to follow national or international evidence-based precautions. In Ref. [20], the potential aerosol transmission of SARS-CoV-2 was examined in a ward hosting COVID-positive cases. The samples were collected at a height of 1.5 m above the ground floor to represent the breathing zone of individuals and positioned at least 2 m away from the patient beds. The findings indicated two viral RNA-positive air samples (in the intensive care unit (ICU)) out of 14 samples and therefore revealed the possibility of airborne transmission of SARS-CoV-2. In Ref. [21], air sampling was done with all-glass impingers (n = 31) on various parts of a hospital, including the emergency room, ICU, CT-SCAN, and laundry. All of the collected samples in this experiment tested negative, and the SARS-CoV-2 virus was not found in the air. Since the patient rooms maintained a negative pressure against their periphery, the findings were attributed to the pressurization strategy. Another similar experimental study of 33 samples from 8 different hospital locations showed no sign of the virus and therefore their findings led to the conclusion that airborne transmission may not be significant especially if patients were in negative pressure isolation rooms [22].
In Ref. [23], systematic sampling and analysis of airborne SARS-CoV-2 RNA were performed to assess viral spread in different hospital areas, including rooms with COVID patients and corridors adjacent to these rooms. The results revealed that the rooms housing COVID-19 patients had the highest RNA occurrence, with a mean of 2600 c/m3, followed by the adjacent corridor with a mean of 4000 c/m3, which showed statistically significant higher exposure (p < 0.01) due to inadequate supply of fresh air, yet ventilation rates were not explicitly reported in the paper. Table 3 summarizes the air sampling findings.

Table 3.
Summary of findings for air samples.
4.1.2. Surface Sampling
In Ref. [24], swab samples were collected from ceiling ventilation openings and surfaces in central ducts. They also tested central ventilation HEPA filters located several stories above the wards. The results showed that SARS-CoV-2 N and E genes were detected in seven and four out of 19 room vents, respectively. Three samples from the central ventilation HEPA exhaust filters in the ward tested positive. Similarly, corresponding filters from two adjacent COVID-19 wards also showed positive results. Finding SARS-CoV-2 in central ventilation systems up to 50 m away from patient room vent openings suggested that the virus can travel over long distances.
4.1.3. Air & Surface Sampling
In another investigation, 107 surface samples, 46 air samples, two exhaled condensate samples, and two expired air samples (chest air) were collected within and beyond four three-bed isolation rooms. The results showed the toilet was the most contaminated environment among isolation rooms, adjacent corridors, anterooms, and patient rooms [25]. In Ref. [26], 130 surface samples were collected using swabs and 28 air samples using natural sedimentation and air particle sampler techniques to determine the presence of COVID-19 in samples from the area at risk of contamination in Jilin University’s First Hospital. Positive regions were identified on the surfaces of the nursing station inside the isolation room, as well as in the air of the isolation ward. In high-risk areas, such as hospital pre-check triages and the fever clinic, the results showed negative for COVID-19, which was attributed to the open environment and high airflow in these spaces. The study, however, provided no quantitative insights into the airflow characteristics. In an experimental study [27], air samples were collected from a hospital with COVID patients before and after increasing the outside air ratio from 65% to 100%, maintaining the same ACH of 5.9 h−1. Prior to the change in the amount of fresh air, samples obtained up to four meters away from COVID-19 patients in hospital rooms and isolated care units were found to have viable SARS-CoV-2. This is while no positive air samples were found after supplying 100% fresh air to the hospital, and consistently, all air vent swabs were negative. Therefore, supplying fresh air was highlighted as a means to control infection. In another study [28], environmental samples were collected from a ship hospital, a nursing home, three COVID-19 isolation hospital wards, and a long-term care facility where asymptomatic COVID-19 cases were isolated. Swab samples were collected from various surfaces, including food preparation and service areas, hospital isolation wards, an air exhaust duct screen, air-conditioning filter, and a sewage treatment unit, and only one out of twelve air samples tested positive, which was taken from 2.5 m away from a patient with the mask off in an isolation room. To assess the presence of SARS-CoV-2 on air and surfaces within a COVID-19 isolation ward at a hospital in Milan, Italy, Ref. [29] collected five air samples and 37 surface swabs inside five different zones of the ward, including contaminated (i.e., COVID-19 patients’ area and ICU), semi-contaminated (undressing room), and the clean regions (lockers and passage for the medical staff and a dressing room). Results showed that 24.3% of swab samples were positive, but none were collected from a clean area. The most contaminated surfaces were hand sanitizer dispensers, medical equipment, touch screens, shelves for medical equipment, bed rails, and door handles. Table 4 presents a summary of air and surface samples.

Table 4.
Summary of findings for air and surface samples.
In summary, a few notes are outstanding. First, detecting the virus in air samples (~2%) is severely less probable than surface samples (~24%). Admittedly, these values depend on the sample location, vicinity to the virus source, and detection methods. Second, results regarding the airborne transmission of the virus are inconsistent, again mainly due to testing methodology. Nonetheless, the virus was rarely detected at far distances from the source (i.e., infected patient). Third, though it may be immature to generalize the findings, at least one paper shows that providing fresh air improves the quality of air indoors.
4.2. Thrust 2: Simulation Studies
4.2.1. Descriptive Studies
Evaluating the dispersion of particles in various room settings in healthcare facilities was one of the major themes of studies done using computational fluid dynamics (CFD). Studies grouped under this category “described” the dispersion of particles in the environment (i.e., no ventilation parameter manipulation). In Ref. [30], A CFD model was created to assess the ventilation system’s performance in an emergency department inside a university hospital. Results showed that the risk of infection was higher at the patient lying height (1 m), and rooms with higher air velocity, dispersion, and mixing levels (e.g., operating rooms) had a higher probability of contamination. In another study [31], the dispersion of airborne viruses within negative pressure rooms with a pressure differential of 2.5 Pa and an air exchange rate of 12 ACH or higher was explored. The motion of particles towards the circular exhaust opening was observed, indicating that the negative pressure room effectively controlled the spread of virus droplets. Another simulation research study investigated the dispersion of particles in a clinic room using mixing ventilation under two different scenarios: (1) both the patient and doctor were sitting, and (2) the patient had laid on the bed, and the doctor was standing. The risk of infection was lowest when both were sitting and highest when the patient was talking while lying in bed and the doctor was standing [32]. In Ref. [33], a patient’s cough was simulated at 3 patient locations in a cardiac intensive care unit with a positive pressure of 12.5 Pa. The study highlighted that regardless of the patient’s location, air patterns led to dispersion of infectious particles not only on directly exposed surfaces but also on less exposed or concealed areas. In Ref. [34], the entire floor of a private hospital in Paris was modeled, and it was suggested that the ventilation system should be rebalanced to supply more cold air on the sunny side of the building and less cold air on the shaded side to optimize the flow patterns.
4.2.2. Comparative Studies
This group of studies performed a CFD analysis to evaluate the change in one or several ventilation parameters. In Ref. [35], the transport mechanism and deposition patterns of 0.167 μm droplet nuclei (MERS-CoV) inside a six-bed inpatient ward were investigated through CFD. By investigating different ACHs in the range of 3 h−1–13 h−1 and exhausting 0%, 10%, and 50% of the supply air by local exhaust grills. ACH and exhaust airflow rate significantly affect the transport and distribution of particles and the general airflow pattern. Additionally, using a high ACH (i.e., 13) could put occupants in the corridor and other connected amenities at high risk of exposure to pathogens. Ten different air distribution scenarios were raised for minimizing cross-infection in a 3-bed ward in a hospital in China. The scenarios varied in the number of inlets, outlets, and their position. Results showed that a bottom-in-top-out air distribution is the best to minimize cross-infection, and such an appropriate arrangement could decrease particle suspension time by nearly 60% and particle concentrations by 95% [36]. In Ref. [37], several inlet and outlet configurations in a negative pressure isolation chamber were investigated, and the most suitable position was identified: inlet on the patient’s leg side and outlet on the head side. However, if any healthcare worker (including physicians) stands between the bed and the outlet, that person may be exposed to the contaminated air. In another study [38], particle dispersion with a size of 2 to 2000 μm was simulated inside a patient room to assess the filtration effectiveness of a portable ultraviolet (UV) air cleaner on airborne particles. With the two ventilation openings at the ceiling, three main ventilation conditions were employed by switching the location of the inlet and outlet or turning both off, and three different flow rates of 0, 210 m3/h, and 1050 m3/h for the ultraviolet air cleaner were tested. It was shown that transmission of cough droplets less than 20 μm in diameter was sensitive to the ventilation conditions and could be filtered by the UV air cleaner more effectively. In contrast, larger droplets were dominated by the gravitational effect and would be insensitive to ventilation and air cleaner. The study showed that while higher flow rates can improve the filtration efficiency, they can also cause more dispersed virus distribution. A study [39] evaluated the contamination risk of connected rooms via air handling units under two scenarios: (1) exposure from a source within one of the rooms of a building and (2) exposure from a source external to the building that enters through the air intake of an air handling unit. They found that using MERV-13 filters was the safest approach for minimizing contamination in adjacent rooms with a probability of infection of only one in ~7300. It was further shown that increasing the outdoor air from 9% to 33% decreased contamination levels in the connected room by 19%, while this reduction could rise to 93% when the filter media was changed from MERV 8 to MERV 13. A separate study [40] reported minor improvements upon changing the outlet location. In a similar study using CFD, Ref. [41] investigated the effectiveness of a local exhaust ventilation system (LEV) positioned above the coughing patient’s mouth in reducing the spread of infected droplets in hospital waiting areas and patient rooms. They found that the LEV system was able to completely reduce the spread of infected droplets in the first 0.5 s following the cough event. In a separate study, Ref. [42] tested twelve different ventilation configurations, which involved various combinations of air exhaust and supply ducts, including seven displacement ventilation scenarios and five mixing ventilation cases. The ventilation evaluation consisted of three stages. In the first stage, the patient stayed in the test room for five minutes and generated aerosols. In the second stage, the patient exited the unit with a door movement, and ventilation continued without the patient. Finally, in stage 3, ventilation continued without the patient, and the aerosol-generating procedure occurred only in stage 1. The study observed that creating a coherent (preferably upward) airflow structure surrounding the respiratory flow, containing the aerosol cluster, resulted in the removal of 99% of aerosols in the chamber within 4.5 min, which is 4–8 times faster than previous results from single chamber designs.
In summary, the simulation papers aimed at either describing flow patterns or comparing different patterns and configurations. As we will discuss later, the modeling tools and techniques were not significantly different than those developed prior to the pandemic. Hence, the results lack a sense of specificity regarding the COVID-19 transmission.
4.3. Thrust 3: Observational Studies
This thrust includes research on experimental air quality measurements as well as observational studies to draw relations between air quality and HVAC parameters. By measuring ventilation rates in 105 service-categorized outpatient clinic rooms, Ref. [43] investigated whether outpatient clinics in business occupancy settings performed procedures in rooms with ventilation rates above, at, or below thresholds defined by various standards (e.g., ANSI/ASHRAE/ASHE Standard 170 (Ventilation of Health Care Facilities [44]). The study revealed that only 10% of clinic rooms had good air mixing, while 28% of clinic rooms had fair air mixing, and 62% of clinic rooms had poor air mixing. In Ref. [45], an experimental study investigated aerosol exposure inside an airborne infection isolation room (AIIR) with 12 ACH and an operating room with 27 ACH; saline was aerosolized using a nebulizer, and particle concentrations were measured inside the room and immediately outside with a closed door. The results showed that the average time that healthcare workers are exposed to aerosol particles during aerosol-generating medical procedures in AIIR was eight times longer that in ORs (70 s vs. 560 s), and no leakage was observed in both cases with the doors closed. Although the primary goal of recommending that aerosol-generating procedures be carried out in an AIIR as opposed to an OR is to prevent the spread of viral aerosols outside the room, there may be a higher risk of anesthetist exposure to viral aerosols when done in the latter location. In Ref. [46] the temporal fluctuations of particulate matter (PM) and carbon dioxide (CO2) in orthopedic wards and emergency rooms of various hospitals in Lahore, Pakistan (2021) were studied. The study indicated significantly lower PM and CO2 levels in centrally air-conditioned hospitals compared to non-central air-conditioned ones. This observation was attributed to high-efficiency filtration in the central HVAC system. Apart from ventilation type, increased visitor and doctors’ activities and cleaning sessions significantly contributed to indoor air quality. The mean PM2.5 and PM10 were highest during visiting hours, and indoor PM 2.5 concentrations in non-central HVAC systems were associated with outdoor concentrations. Finally, it was recommended that the real-time monitoring of particulates and CO2 can help inform and evaluate the intervention strategies to maintain air hygiene in healthcare environments.
4.4. Thrust 4: Unempirical Studies
In this section, the research team reviewed publications that did not report original data; instead, they reported on data established by third parties or reflected upon by experts in the field. Three categories of research work highlight HVAC-related recommendations, non-HVAC-related recommendations, and design strategies to minimize the risk of infection in healthcare facilities. Engineering solutions that target airborne transmission are recommended as part of a comprehensive plan to reduce the risk of infection inside [47]. Proper building engineering measures, such as minimizing air recirculation, avoiding overcrowding, and ensuring adequate and effective ventilation, filtering, and air disinfection are generally recommended [48].
4.4.1. HVAC-Related Strategies
In Ref. [49] detailed recommendations for primary anesthesiologists on infection prevention in the OR during the COVID-19 pandemic were provided. The article suggests, as an anomaly, the possibility of shutting down the air-conditioning and humidification system during surgery in a positive-pressure operating room for confirmed/suspected COVID-19 patients. It is important to note that this recommendation is not based on a specific study cited in the article but rather presented as a suggestion. If a negative-pressure operating room is available, room pressure should be tested before surgery and maintained below −5 Pa in the main operating room during the surgery. Automated room disinfection, such as using ultraviolet light for one hour, was also a practical measure [50]. In Ref. [51], using recovered heat from a longitudinal air-to-air heat exchanger was proposed to inactivate airborne SARS-CoV-2. In Ref. [52], displacement ventilation, whether through mechanical or natural means, was suggested as a viable alternative to negative-pressure isolation rooms. With air intakes at a low level and outlets at a high level, displacement ventilation creates positive pressure at the ceiling, pushing hot and contaminated air upward, while drawing fresh and conditioned air into the occupied zone. According to [53], filtered air can be measured in equivalent air changes per hour (ACHe) and added to the ACH from the outdoor air. Increasing air exchange rates have trade-offs, such as the additional costs of moving more air as well as excess heating or cooling loads. Using energy-efficient and “smart” systems that distribute air only when the space is occupied leads to energy savings. In Ref. [54], exhaust air volume in the isolation room was approximately 150 m3 per hour per person, much lower than the World Health Organization’s recommended 288 m3 per hour per person. The authors did not mention or study potential impacts of their observation on patient outcomes, yet recommended that using air purifiers as an alternative to increasing ventilation and diluting contaminated air to reduce the possible presence of virus-laden aerosols.
4.4.2. Non-HVAC-Related Strategies
The smallest particle of concern is a single virion (unattached to any fluid droplet from the cough or sneeze of an infected patient), having a diameter of approximately 0.12 microns. While a HEPA filter could theoretically capture them, ULPA filters are superior because they could capture 99.99% of particles measuring 0.12 microns and larger. If SARS-CoV-2 viruses could be brought into contact with an air filter, they could theoretically all be filtered and contained [55]. Furthermore, instead of depending on sporadic and labor-intensive human sterilization of surfaces, a continuous aerosolization of disinfectors was recommended [56]. In contrast, Ref. [57] sought legislation that promotes the integrity of the indoor air environment and put forth some basic strategies, including when to clean to avoid the overuse of chemicals and air fresheners that may simply be contributing additional hazardous substances. Another similar study [58] emphasized the need to put more effort into the importance of the transmission of diseases via the airborne path. Generally, airborne infections are much harder to trace, yet the risk of respiratory illnesses being transmitted through the air must be controlled. In Ref. [59], strategies/guidelines to reduce contamination in the Intensive Care Unit (ICU) and the hospital environments were gathered. The authors stated that there is a lack of sufficient data to specify and quantify the minimum ventilation requirements, especially in hospitals and isolation rooms. In Ref. [60], guidelines were proposed to set a benchmark for current and future viral pandemics. The authors advised using evaporative humidifiers to maintain indoor relative humidity (RH) within the range of 40–60%, portable air cleaners, and an adequate mechanical ventilation system to reduce respiratory infection risk. In order to control SARS-CoV-2 at healthcare facilities, a review study examined strategies and approaches to disinfect surfaces, and recommended advanced strategies, including no-touch techniques, such as engineered antimicrobial surfaces and automated room disinfection systems using hydrogen peroxide vapor or ultraviolet light [61]. The significance of addressing real-time monitoring systems for public health and well-being was stressed by Ref. [62] and later on by Ref. [63]. They addressed how the effectiveness of the building ventilation systems depends on air quality monitoring systems, which include internet of things and wireless sensor networks.
4.4.3. Design-Related Strategies
In Ref. [64] ten considerations were put forth, including 5 for the design phase: (a) strategic site location (an appropriate strategic location may present the potential for disease spread in areas near the healthcare facility); (b) typology configuration (in the event of an infectious emergency, the independence of buildings or the presence of autonomous internal units allows for the separation of various functional areas from the rest of the hospital system, without disrupting regular activities); (c) flexibility (functional areas that can be easily reconverted); (d) functional program; and (e) User-centeredness (it is essential to pay attention to the physical, psychological, and social needs of all users); As well as 5 more strategies for the operation phase: (f) Healthcare network on the territory (establishing community health centers throughout the territory is essential to ensure the provision of primary care services); (g) Patient safety (by utilizing simple visual devices, wayfinding strategies, and design-nudges, it is possible to mitigate the transmission of infections by clearly defining risk areas); (h) HVAC and indoor air quality (HVAC systems should be flexible and capable of being modified in terms of the air used, ranging from recirculation to all-air systems, and pressure, from positive to negative); (i) Innovative finishing materials and furniture (employ high-performance, long-lasting, and easily cleanable materials that cater to the medical requirements); (j) Healthcare digital innovation (continuous monitoring of health status and vital parameters using IT systems) to meet for having resilient hospitals. A study [65] proposed design techniques based on social distancing policy, adaptability, flexibility, and engineering control of novel preventative measures to address present and future pandemics. Separation and distribution of facilities were introduced to limit building users. Furthermore, a hybrid typological method defined by a major embodiment connected to the support ward was indicated to assist in isolating the functional regions that supply internal units from the rest of the hospital system without disrupting routine operations. Separating buildings or separate internal units provides the design of the hospital building mainly with horizontal formations that enable the distribution and separation of areas. Separation, distribution, horizontal formations, separate functions, and circulation were emphasized to allow for more natural lighting, ventilation, and interaction with nature. Outpatient clinic entry sites were indicated to be enlarged to become screening stations to distinguish infected patients from other patients. To help achieve greater flexibility, a modular design was proposed. Solid, non-porous surfaces and smooth curving surfaces were recommended to decrease microorganisms. Reducing manual communication by using automated doors and specific non-touch tools that can be activated by sound, such as automatic doors, bells, and switches, was also recommended.
5. Discussion
A comprehensive analysis was conducted on the recommendations derived from the post-COVID-19 papers in contrast with the prior knowledge. Similar recommendations within the post-COVID-19 papers were merged to ensure clarity and avoid redundancy. In total, 31 distinct recommendations were identified in Table 5, encompassing aspects aiming to minimize disease transmission in indoor environments. Notably, the recommendations in Table 5 are presented in descending order, ranked by their frequency of occurrence in the post-COVID-19 works, except for those recommendations that have the same frequency (i.e., R5 and R6). This approach emphasizes key areas and recurring themes within the literature.

Table 5.
Comparison of recommendations mentioned in post-COVID-19 (thrusts 1 through 3) and pre-COVID-19 papers.
Table 6 primarily comprises post-COVID-19 studies that do not report original data; rather, they draw upon data established by third parties or reflect the insights of experts in the field (studies grouped under thrust 4). These studies provide valuable perspectives and ideas, which the research team identified as noteworthy and deserving of separate representation. However, it is important to recognize that these ideas, while potentially innovative, require further experimentation and investigation to validate their efficacy. As a result, they have been presented in a distinct table to highlight their speculative nature and the need for additional empirical exploration.

Table 6.
Comparison of recommendations mentioned in post-COVID-19 (thrust 4) and pre-COVID-19 papers.
In response to COVID-19, researchers applied existing techniques—like air and surface sampling—with renewed urgency to detect viral presence in healthcare environments. While these methodologies were well-established, their use during the pandemic helped identify high-risk zones such as toilets and nursing stations, reinforcing the need for robust hygiene and ventilation protocols. However, no major advancements in sampling technology were observed in post-COVID literature, likely due to the longer development timelines such innovations require. Simulation studies, particularly those using computational fluid dynamics (CFD), did not introduce fundamentally new techniques but offered fresh insights by incorporating human behavior and complex geometries. These included modeling how patient positions and healthcare worker movements affect airflow and transmission risk. Although the conclusions largely echoed pre-existing knowledge, the context of COVID-19 pushed researchers to examine dynamic scenarios and transient airflow patterns more rigorously. Experimental air quality and observational studies provided valuable, though less abundant, contributions. Investigations into ventilation compliance in outpatient clinics, aerosol exposure in different clinical rooms, and the influence of temperature, humidity, and particulate matter on infection rates helped clarify environmental impacts on disease transmission. Limited study volume in this category was likely due to logistical challenges, restricted healthcare access, and the urgent demand for rapid insights during the pandemic’s early stages.
Unempirical and review-based publications reinforced foundational infection control practices (ventilation, filtration, disinfection, and minimizing recirculation) while tailoring them to COVID-specific challenges. These studies proposed strategies for makeshift hospitals, HVAC optimization, portable filtration, and flexible facility design. While grounded in existing knowledge, they emphasized adaptability and innovation under crisis conditions. Many recommendations now require empirical validation.
In sum, COVID-era research expanded the application of known techniques, adapted modeling to reflect real-world behavior, and reinforced the value of fundamental infection control strategies. While revolutionary technical breakthroughs were rare, the pandemic catalyzed practical advancements, contextual insights, and renewed attention to the design and operation of safer healthcare environments.
6. Conclusions
This study examined three key research questions regarding the role of the healthcare-built environment and indoor air quality during the COVID-19 pandemic. In this section, specific conclusions for each research question and paths forward are presented.
6.1. COVID-19’s Impact on Indoor Air Quality Knowledge in Healthcare
The systematic literature review and analysis revealed that research conducted during the COVID-19 pandemic led to several advancements in knowledge related to healthcare environments and infection control. Notably, studies utilizing air and surface sampling techniques provided new insights into the potential for airborne and fomite transmission of SARS-CoV-2 in healthcare settings. These methodologies, although existing prior to the pandemic, proved instrumental in detecting viral contamination, identifying high-risk areas, and highlighting hygiene practices to mitigate transmission. These studies demonstrated that SARS-CoV-2 could be recovered from the air and surfaces, even at considerable distances from patient rooms in some instances. By mapping high-risk zones for environmental contamination, such as toilets, patient care areas, and nursing stations, these studies highlighted the importance of routine disinfection and hand hygiene practices among healthcare workers. Moreover, simulation studies and computational fluid dynamics models made significant contributions by incorporating human factors, such as patient positioning and healthcare worker interactions, suggesting minimum distances between patients and healthcare providers to reduce the transmission of infectious agents. While CFD methodology was not new, these recent simulation studies have brought forward new ideas and results in understanding the dynamics of airflow and the dispersion of airborne particles. By simulating various scenarios involving patient and staff movements, researchers gained deeper insights into how individual behaviors and room configurations influence air flow patterns and dispersion of infectious particles.
Experimental approaches in the field of air quality measurements and observational studies also expanded knowledge by investigating the relationship between environmental parameters and infection transmission rates. Assessments of ventilation system performance provided data on potential deficiencies in meeting air exchange requirements during aerosol-generating procedures in outpatient clinical settings. These findings underscore the importance of proper ventilation rates and efficient ventilation systems in healthcare environments. Collectively, the studies conducted during the pandemic generated valuable evidence-based insights and solutions tailored to the specific challenges posed by COVID-19 in healthcare settings. They addressed key knowledge gaps and provided targeted investigations into the transmission dynamics of the virus and the effectiveness of infection control measures. Still, it is evident that pre-existing principles and foundational knowledge played a pivotal role in facilitating this rapid ascent of understanding.
6.2. Unsubstantiated Recommendations in Post-COVID Scholarly Works
The analysis of scholarly works published after the COVID-19 pandemic revealed several instances where authors proposed recommendations or strategies lacking substantive empirical evidence or rigorous validation. This tendency was particularly noticeable among non-empirical studies, which often presented speculative ideas or novel approaches without comprehensive supporting data. For instance, some authors recommended unconventional techniques, such as utilizing recovered heat from HVAC systems to sanitize exhaust air or converting this heat into electrical energy to eliminate viral particles. While these proposals may be theoretically feasible, they lacked concrete data on efficacy and safety, making them speculative. Similarly, other studies suggested innovative solutions like specialized UV treatment or no-touch technologies without thorough investigation. Furthermore, the comparative analysis found examples of ventilation-related recommendations that aligned more with pre-existing guidelines rather than being based on new evidence. This indicates that certain proposed measures were reiterated rather than being novel solutions. However, the urgency of the pandemic likely catalyzed the promotion of these strategies before a comprehensive substantiation of under-validated proposals. Fast solutions appeared to sometimes outweigh the rigors of careful evidence gathering.
Furthermore, it is not uncommon to encounter recommendations that lack comprehensive empirical evidence due to the challenging nature of conducting fully randomized controlled experiments for certain interventions. For instance, investigations into recommendations such as hospitals providing personal protective equipment (PPE) to healthcare workers would ideally require a completely randomized design test to provide robust evidence. However, conducting such experiments may not be ethically feasible as it could potentially endanger the lives of participants, particularly in high-risk environments like healthcare settings. Similarly, recommendations related to increasing surface cleaning, disinfection, and promoting hand hygiene are widely accepted as critical infection control measures during the pandemic. While these measures align with well-established infection control principles, they may not always be supported by extensive empirical evidence, especially in the context of a rapidly evolving and novel pathogen like SARS-CoV-2.
6.3. How Existing Knowledge and Experience Impact Recommendations
Throughout the COVID-19 pandemic, scientists and practitioners have heavily relied on existing knowledge, experience, and organizational practices to address the challenges posed by the virus. The urgency of the pandemic demanded rapid responses, and as a result, leveraging established principles and practices became a crucial aspect of formulating effective strategies.
Firstly, fundamental infection control recommendations formed the cornerstone of the scientific approach. Measures such as adequate ventilation, physical distancing, and frequent disinfection were consistently emphasized, aligning with established principles in the field of public health. These well-known strategies were prioritized as they have proven efficacy in mitigating the spread of infectious diseases. Secondly, scientific investigations leveraged familiar methodologies rather than entirely novel approaches. For instance, simulation-based studies extensively used computational fluid dynamics (CFD) to analyze airflow patterns and assess ventilation systems. CFD simulations were not new to the field, as they were previously employed to understand airflow dynamics, but during the pandemic, they were adapted to explore the transmission of SARS-CoV-2. Similarly, experimental approaches relied on established techniques like air and surface sampling, which have been used for environmental monitoring in various contexts. Thirdly, practical constraints and resource limitations played a role in guiding the scientific response. The urgency of the pandemic necessitated quick decision-making and mobilization of resources, leaving limited room for radical departures from existing practices. For example, hospitals faced challenges in swiftly converting to negative pressure isolation rooms due to infrastructure limitations, leading to the adoption of alternative mitigation strategies. Progress often occurred through incremental advancements and improvements to existing protocols and workflows, rather than revolutionary transformations. This tendency reflects the difficulty of rapidly shifting from prior practices and thinking during times of crisis.
6.4. Path Forward
While the research conducted during the COVID-19 pandemic significantly improved our understanding of infection control measures and transmission dynamics, there are several paths forward to continue enhancing our knowledge base in healthcare environments, with the promise of building a more resilient healthcare infrastructure (Table 7). Each conclusion theme arose from a specific research question, and Table 7 lists potential paths forward pertaining to each theme.

Table 7.
Future Research and Path Forward.
Author Contributions
Conceptualization, E.M. and M.S.N.T.; methodology, E.M.; software, E.M.; validation, E.M. and M.S.N.T.; formal analysis, M.S.N.T.; resources, E.M.; writing—original draft preparation, M.S.N.T. and E.M.; writing—review and editing, E.M. and M.S.N.T.; visualization, M.S.N.T. and E.M.; supervision, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the conclusions are from previously published sources cited in the manuscript; no new data were generated.
Use of Artificial Intelligence
AI or AI-assisted tools were not used in drafting any aspect of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACH | Air changes per hour |
| ACHe | Equivalent air changes per hour |
| AGP | Aerosol-generating procedure |
| AIIR | Airborne infection isolation room |
| CFD | Computational fluid dynamics |
| CO2 | Carbon dioxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ER | Emergency room |
| HEPA | High efficiency particulate air |
| HVAC | Heating, ventilation, and air conditioning |
| IAQ | Indoor air quality |
| ICU | Intensive care unit |
| IoT | Internet of things |
| LEV | Local exhaust ventilation |
| MERV | Minimum efficiency reporting value |
| ML | Machine learning |
| N95 | N95 filtering facepiece respirator |
| OPC | Overall particle counter |
| OR | Operating room |
| PM10 | Particulate matter ≤ 10 μm |
| PM2.5 | Particulate matter ≤ 2.5 μm |
| PPE | Personal protective equipment |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RH | Relative humidity |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TMT | Total maximum time |
| ULPA | Ultra-low penetration air |
| UVGI | Ultraviolet germicidal irradiation |
References
- Rahimi, N.R.; Fouladi-Fard, R.; Aali, R.; Shahryari, A.; Rezaali, M.; Ghafouri, Y.; Ghalhari, M.R.; Asadi-Ghalhari, M.; Farzinnia, B.; Gea, O.C.; et al. Bidirectional association between COVID-19 and the environment: A systematic review. Environ. Res. 2021, 194, 110692. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Jaafarzadeh, N.; Martínez, S.S.; Mirzaee, S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2021, 193, 110612. [Google Scholar] [CrossRef]
- Aghalari, Z.; Dahms, H.-U.; Sosa-Hernandez, J.E.; Oyervides-Muñoz, M.A.; Parra-Saldívar, R. Evaluation of SARS-CoV-2 transmission through indoor air in hospitals and prevention methods: A systematic review. Environ. Res. 2021, 195, 110841. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Leili, M.; Azarian, G.; Poormohammadi, A. Sampling and detection of corona viruses in air: A mini review. Sci. Total Environ. 2020, 740, 140207. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Fadaei, A. Ventilation Systems and COVID-19 Spread: Evidence from a Systematic Review Study. Eur. J. Sustain. Dev. Res. 2021, 5, em0157. [Google Scholar] [CrossRef]
- Fadaei, A. Influence of Ventilation in Healthcare Facilities Prevention of Infection COVID-19: Systematic Review Study. Eur. J. Sustain. Dev. Res. 2021, 5, em0170. [Google Scholar] [CrossRef]
- Chirico, F.; Sacco, A.; Bragazzi, N.L.; Magnavita, N. Can Air-Conditioning Systems Contribute to the Spread of SARS/MERS/COVID-19 Infection? Insights from a Rapid Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 6052. [Google Scholar] [CrossRef]
- Hirota, K. Air contamination with SARS-CoV-2 in the operating room. J. Anesth. 2021, 35, 333–336. [Google Scholar] [CrossRef]
- Correia, G.; Rodrigues, L.; da Silva, M.G.; Gonçalves, T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses 2020, 141, 109781. [Google Scholar] [CrossRef]
- Nair, A.N.; Anand, P.; George, A.; Mondal, N. A review of strategies and their effectiveness in reducing indoor airborne transmission and improving indoor air quality. Environ. Res. 2022, 213, 113579. [Google Scholar] [CrossRef]
- Güven, Y.; Sepet, E.; Sezgin, B.I.; Çelik, E.G. The Importance and Improvement of Indoor Air Quality in Dental Clinics within the context of COVID-19. EC Dent. Sci. 2021, 20, 32–50. [Google Scholar]
- Ahlawat, A.; Wiedensohler, A.; Mishra, S.K. An overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol Air Qual. Res. 2020, 20, 1856–1861. [Google Scholar] [CrossRef]
- Maulani, C.; Masulili, S.L.C.; Auerkari, E.I. COVID-19 transmission and dental treatment: A narrative review. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, ML, USA, 2021. [Google Scholar] [CrossRef]
- Engelman, D.T.; Lother, S.; George, I.; Funk, D.J.; Ailawadi, G.; Atluri, P.; Grant, M.C.; Haft, J.W.; Hassan, A.; Legare, J.-F.; et al. Adult Cardiac Surgery and the COVID-19 Pandemic: Aggressive Infection Mitigation Strategies Are Necessary in the Operating Room and Surgical Recovery. Ann. Thorac. Surg. 2020, 110, 707–711. [Google Scholar] [CrossRef]
- Theodorou, C.; Simpson, G.; Walsh, C. Theatre ventilation. R. Coll. Surg. Engl. 2021, 103, 151–154. [Google Scholar] [CrossRef]
- Dunne, C. The place of the literature review in grounded theory research. Int. J. Soc. Res. Methodol. 2011, 14, 111–124. [Google Scholar] [CrossRef]
- Ram, K.; Thakur, R.C.; Singh, D.K.; Kawamura, K.; Shimouchi, A.; Sekine, Y.; Nishimura, H.; Singh, S.K.; Pavuluri, C.M.; Singh, R.; et al. Why airborne transmission hasn’t been conclusive in case of COVID-19? An atmospheric science perspective. Sci. Total Environ. 2021, 773, 145525. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020, 725, 138401. [Google Scholar] [CrossRef]
- Kenarkoohi, A.; Noorimotlagh, Z.; Falahi, S.; Amarloei, A.; Mirzaee, S.A.; Pakzad, I.; Bastani, E. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci. Total Environ. 2020, 748, 141324. [Google Scholar] [CrossRef]
- Masoumbeigi, H.; Ghanizadeh, G.; Arfaei, R.Y.; Heydari, S.; Goodarzi, H.; Sari, R.D.; Tat, M. Investigation of hospital indoor air quality for the presence of SARS-CoV-2. J. Environ. Health Sci. Eng. 2020, 18, 1259–1263. [Google Scholar] [CrossRef]
- Vosoughi, M.; Karami, C.; Dargahi, A.; Jeddi, F.; Jalali, K.M.; Hadisi, A.; Haghighi, S.B.; Dogahe, H.P.; Noorimotlagh, Z.; Mirzaee, S.A. Investigation of SARS-CoV-2 in hospital indoor air of COVID-19 patients’ ward with impinger method. Environ. Sci. Pollut. Res. 2021, 28, 50480–50488. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Vílchez, H.; Fraile-Ribot, P.A.; Marco, E.; Campins, A.; Orfila, J.; van Drooge, B.L.; Fanjul, F. Spread of SARS-CoV-2 in hospital areas. Environ. Res. 2021, 204, 112074. [Google Scholar] [CrossRef]
- Nissen, K.; Krambrich, J.; Akaberi, D.; Hoffman, T.; Ling, J.; Lundkvist, Å.; Svensson, L.; Salaneck, E. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci. Rep. 2020, 10, 19589. [Google Scholar] [CrossRef]
- Ding, Z.; Qian, H.; Xu, B.; Huang, Y.; Miao, T.; Yen, H.-L.; Xiao, S.; Cui, L.; Wu, X.; Shao, W.; et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2020, 753, 141710. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Chen, Y.; He, J.; Chen, L.; Liu, Y.; Hu, X.; Li, A.; Liu, S.; Zhang, P.; et al. Clinical Data on Hospital Environmental Hygiene Monitoring and Medical Staff Protection during the Coronavirus Disease 2019 Outbreak. medRxiv 2020. [Google Scholar] [CrossRef]
- Alkalamouni, H.; Hitti, E.; Zaraket, H. Adopting fresh air ventilation may reduce the risk of airborne transmission of SARS-CoV-2 in COVID-19 unit. J. Infect. 2021, 83, e4–e5. [Google Scholar] [CrossRef]
- Mouchtouri, V.A.; Koureas, M.; Kyritsi, M.; Vontas, A.; Kourentis, L.; Sapounas, S.; Rigakos, G.; Petinaki, E.; Tsiodras, S.; Hadjichristodoulou, C. Environmental contamination of SARS-CoV-2 on surfaces, air-conditioner and ventilation systems. Int. J. Hyg. Environ. Health 2020, 230, 113599. [Google Scholar] [CrossRef]
- Razzini, K.; Castrica, M.; Menchetti, L.; Maggi, L.; Negroni, L.; Orfeo, N.V.; Pizzoccheri, A.; Stocco, M.; Muttini, S.; Balzaretti, C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 2020, 742, 140540. [Google Scholar] [CrossRef]
- Alrebi, O.F.; Obeidat, B.; Abdallah, I.A.; Darwish, E.F.; Amhamed, A. Airflow dynamics in an emergency department: A CFD simulation study to analyse COVID-19 dispersion. Alex. Eng. J. 2021, 61, 3435–3445. [Google Scholar] [CrossRef]
- Anuraghava, C.; Abhiram, K.; Reddy, V.N.S.; Rajan, H. CFD modelling of airborne virus diffusion characteristics in a negative pressure room with mixed mode ventilation. Int. J. Simul. Multidiscip. Des. Optim. 2021, 12, 1. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, S. Experimental and numerical study on the transport of droplet aerosols generated by occupants in a fever clinic. Build. Environ. 2021, 187, 107402. [Google Scholar] [CrossRef]
- Anghel, L.; Popovici, C.-G.; Stătescu, C.; Sascău, R.; Verdeș, M.; Ciocan, V.; Șerban, I.-L.; Mărănducă, M.A.; Hudișteanu, S.-V.; Țurcanu, F.-E. Impact of HVAC-systems on the dispersion of infectious aerosols in a cardiac intensive care unit. Int. J. Environ. Res. Public Health 2020, 17, 6582. [Google Scholar] [CrossRef]
- Beaussier, M.; Vanoli, E.; Zadegan, F.; Peray, H.; Bezian, E.; Jilesen, J.; Gandveau, G.; Gayraud, J.-M. Aerodynamic analysis of hospital ventilation according to seasonal variations. A simulation approach to prevent airborne viral transmission pathway during COVID-19 pandemic. Environ. Int. 2022, 158, 106872. [Google Scholar] [CrossRef]
- Satheesan, M.K.; Mui, K.W.; Wong, L.T. A numerical study of ventilation strategies for infection risk mitigation in general inpatient wards. Build. Simul. 2020, 13, 887–896. [Google Scholar] [CrossRef]
- Wang, J.-X.; Cao, X.; Chen, Y.-P. An air distribution optimization of hospital wards for minimizing cross-infection. J. Clean. Prod. 2021, 279, 123431. [Google Scholar] [CrossRef]
- Ghorui, S. CFD Study of Ventilation in a Room Maintained Under Negative-Pressure to Prevent Airborne Contamination; FOSSEE IIT Bombay: Mumbai, Maharashtra, India, 2020. [Google Scholar]
- Feng, Y.; Zhao, J.; Spinolo, M.; Lane, K.; Leung, D.; Marshall, D.; Mlinaric, P. Assessing the filtration effectiveness of a portable ultraviolet air cleaner on airborne SARS-CoV-2 laden droplets in a patient room: A numerical study. Aerosol Air Qual. Res. 2021, 21, 200608. [Google Scholar] [CrossRef]
- Pease, L.F.; Wang, N.; Salsbury, T.I.; Underhill, R.M.; Flaherty, J.E.; Vlachokostas, A.; Kulkarni, G.; James, D.P. Investigation of potential aerosol transmission and infectivity of SARS-CoV-2 through central ventilation systems. Build. Environ. 2021, 197, 107633. [Google Scholar] [CrossRef]
- Wang, F.; Chaerasari, C.; Rakshit, D.; Permana, I.; Kusnandar. Performance Improvement of a Negative-Pressurized Isolation Room for Infection Control. Healthcare 2021, 9, 1081. [Google Scholar] [CrossRef]
- Borro, L.; Mazzei, L.; Raponi, M.; Piscitelli, P.; Miani, A.; Secinaro, A. The role of air conditioning in the diffusion of SARS-CoV-2 in indoor environments: A first computational fluid dynamic model, based on investigations performed at the Vatican State Children’s hospital. Environ. Res. 2021, 193, 110343. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.S.; Park, H. Optimized mechanism for fast removal of infectious pathogen-laden aerosols in the negative-pressure unit. J. Hazard. Mater. 2022, 435, 128978. [Google Scholar] [CrossRef]
- King, K.G.; Delclos, G.L.; Brown, E.L.; Emery, S.T.; Yamal, J.M.; Emery, R.J. An assessment of outpatient clinic room ventilation systems and possible relationship to disease transmission. Am. J. Infect. Control 2021, 49, 808–812. [Google Scholar] [CrossRef]
- ANSI/ASHRAE/ASHE Standard 170-2017; Ventilation of Health Care Facilities. ASHRAE: Atlanta, GA, USA, 2017.
- Tsui, B.C.; Pan, S. Are aerosol-generating procedures safer in an airborne infection isolation room or operating room? Br. J. Anaesth. 2020, 125, e485–e487. [Google Scholar] [CrossRef]
- Nimra, A.; Ali, Z.; Nasir, Z.A.; Tyrrel, S.; Sidra, S. Characterization of Indoor Air Quality in Relation to Ventilation Practices in Hospitals of Lahore, Pakistan. Sains Malays. 2021, 50, 1609–1620. [Google Scholar] [CrossRef]
- Tang, J.W.; Marr, L.C.; Li, Y.; Dancer, S.J. COVID-19 has redefined airborne transmission. BMJ 2021, 373, n913. [Google Scholar] [CrossRef]
- Morawska, L.; Tang, J.W.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F.; et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020, 142, 105832. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, Y.; Liu, P.; Wang, S.; Xu, X.; Wang, X.; Huang, Y. Infection Prevention Strategy in Operating Room during Coronavirus Disease 2019 (COVID-19) Outbreak. Chin. Med. Sci. J. 2020, 35, 114–120. [Google Scholar] [CrossRef]
- Al-Benna, S. Negative pressure rooms and COVID-19. J. Perioper. Pract. 2021, 31, 18–23. [Google Scholar] [CrossRef]
- Rezaei, N.; Jafari, M.; Nazari, A.; Salehi, S.; Talati, F.; Torab, R.; Nejad-Rahim, R. A novel methodology and new concept of SARS-CoV-2 elimination in heating and ventilating air conditioning systems using waste heat recovery. AIP Adv. 2020, 10, 085308. [Google Scholar] [CrossRef]
- Bhagat, R.K.; Linden, P.F. Displacement ventilation: A viable ventilation strategy for makeshift hospitals and public buildings to contain COVID-19 and other airborne diseases: Ventilation strategy for COVID-19. R. Soc. Open Sci. 2020, 7, 200680. [Google Scholar] [CrossRef]
- Allen, J.G.; Ibrahim, A.M. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA 2021, 325, 2112–2113. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, B. Makeshift hospitals for COVID-19 patients: Where health-care workers and patients need sufficient ventilation for more protection. J. Hosp. Infect. 2020, 105, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.M.; Goring, R. Practical Steps to Improve Air Flow in Long-Term Care Resident Rooms to Reduce COVID-19 Infection Risk. J. Am. Med. Dir. Assoc. 2020, 21, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Elias, B.; Bar-Yam, Y. Could Air Filtration Reduce COVID-19 Severity and Spread? New England Complex Systems Institute: Cambridge, MA, USA, 2020; Volume 9, pp. 1–4. [Google Scholar]
- Nwanaji-Enwerem, J.C.; Allen, J.G.; Beamer, P.I. Another invisible enemy indoors: COVID-19, human health, the home, and United States indoor air policy. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 773–775. [Google Scholar] [CrossRef]
- Morawska, L.; Allen, J.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.J.; Floto, A.; Franchimon, F.; et al. A paradigm shift to combat indoor respiratory infection. Science 2021, 372, 689–691. [Google Scholar] [CrossRef]
- Santos, A.F.; Gaspar, P.D.; Hamandosh, A.; de Aguiar, E.B.; Filho, A.C.G.; de Souza, H.J.L. Best Practices on HVAC Design to Minimize the Risk of COVID-19 Infection within Indoor Environments. Braz. Arch. Biol. Technol. 2020, 63, e20200335. [Google Scholar] [CrossRef]
- Ahlawat, A.; Mishra, S.K.; Birks, J.W.; Costabile, F.; Wiedensohler, A. Preventing airborne transmission of SARS-CoV-2 in hospitals and nursing homes. Int. J. Environ. Res. Public Health 2020, 17, 8553. [Google Scholar] [CrossRef]
- Choi, H.; Chatterjee, P.; Lichtfouse, E.; Martel, J.A.; Hwang, M.; Jinadatha, C.; Sharma, V.K. Classical and alternative disinfection strategies to control the COVID-19 virus in healthcare facilities: A review. Environ. Chem. Lett. 2021, 19, 1945–1951. [Google Scholar] [CrossRef]
- Ho, Y.-H.; Li, P.-E.; Chen, L.-J.; Liu, Y.-L. Indoor air quality monitoring system for proactive control of respiratory infectious diseases: Poster abstract. In Proceedings of the SenSys 2020—2020 18th ACM Conference on Embedded Networked Sensor Systems, Virtual, 16 November 2020; Association for Computing Machinery, Inc.: New York, NY, USA, 2020; pp. 693–694. [Google Scholar] [CrossRef]
- Saini, J.; Dutta, M.; Marques, G. Indoor Air Quality Monitoring Systems and COVID-19. In Emerging Technologies During the Era of COVID-19 Pandemic; Studies in Systems, Decision and Control; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2021; Volume 348, pp. 133–147. [Google Scholar] [CrossRef]
- Capolongo, S.; Gola, M.; Brambilla, A.; Morganti, A.; Mosca, E.I.; Barach, P. COVID-19 and healthcare facilities: A decalogue of design strategies for resilient hospitals. Acta Bio-Medica Atenei Parm. 2020, 91, 50–60. [Google Scholar] [CrossRef]
- Makram, A.; El-Ashmawy, R.A. Future Hospital Building Design Strategies Post COVID-19 Pandemic. Int. J. Sustain. Dev. Plan. 2022, 17, 1169–1179. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, O.; Li, A.; Hou, L.; Olofsson, T.; Zhang, L.; Lei, W. Adaptive wall-based attachment ventilation: A comparative study on its effectiveness in airborne infection isolation rooms with negative pressure. Engineering 2022, 8, 130–137. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Li, Y.; Buus, M.; Winther, F.V. Risk of cross-infection in a hospital ward with downward ventilation. Build. Environ. 2010, 45, 2008–2014. [Google Scholar] [CrossRef]
- Qian, H.; Li, Y.; Nielsen, P.V.; Hyldgaard, C.E. Dispersion of exhalation pollutants in a two-bed hospital ward with a downward ventilation system. Build. Environ. 2008, 43, 344–354. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Li, Y.; Buus, M.; Winther, F.V.; Qian, H. Cross infection in a hospital ward and deposition of particles exhaled from a source manikin. In Proceedings of the Fifth International Workshop on Energy and Environment of Residential Buildings and the Third International Conference on Built Environment and Public Health, Guilin, China, 29–31 May 2009. [Google Scholar]
- Qian, H.; Zheng, X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J. Thorac. Dis. 2018, 10, S2295–S2304. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.; Yang, R. Virus diffusion in isolation rooms. J. Hosp. Infect. 2006, 62, 338–345. [Google Scholar] [CrossRef]
- Reddy, S.C.; Valderrama, A.L.; Kuhar, D.T. Improving the Use of Personal Protective Equipment: Applying Lessons Learned. Clin. Infect. Dis. 2019, 69, S165–S170. [Google Scholar] [CrossRef]
- John, A.; Tomas, M.E.; Cadnum, J.L.; Mana, T.S.; Jencson, A.; Shaikh, A.; Zabarsky, T.F.; Wilson, B.M.; Donskey, C.J. Are health care personnel trained in correct use of personal protective equipment? Am. J. Infect. Control 2016, 44, 840–842. [Google Scholar] [CrossRef]
- Reid, S.M.; Farion, K.J.; Suh, K.N.; Audcent, T.; Barrowman, N.J.; Plint, A.C. Use of personal protective equipment in Canadian pediatric emergency departments. Can. J. Emerg. Med. 2011, 13, 71–78. [Google Scholar] [CrossRef][Green Version]
- Sargent, E.V.; Gallo, F. Use of Personal Protective Equipment for Respiratory Protection. ILAR J. 2003, 44, 52–56. [Google Scholar] [CrossRef]
- Fischer, W.A.; Weber, D.J.; Wohl, D.A. Personal Protective Equipment: Protecting Health Care Providers in an Ebola Outbreak. Clin. Ther. 2015, 37, 2402–2410. [Google Scholar] [CrossRef]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- Houghton, C.; Meskell, P.; Delaney, H.; Smalle, M.; Glenton, C.; Booth, A.; Chan, X.H.S.; Devane, D.; Biesty, L.M. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: A rapid qualitative evidence synthesis. Cochrane Database Syst. Rev. 2020, 2020, CD013582. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.A.; Fukuda, K.; Uyeki, T.M.; Cox, N.J.; Bridges, C.B. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2005, 54, 1–41. [Google Scholar] [PubMed]
- Arnold, F.W.; LaJoie, A.S.; Brock, G.N.; Peyrani, P.; Rello, J.; Menéndez, R.; Lopardo, G.; Torres, A.; Rossi, P.; Ramirez, J.A.; et al. Improving Outcomes in Elderly Patients With Community-Acquired Pneumonia by Adhering to National Guidelines Community-Acquired Pneumonia Organization International Cohort Study Results. Arch. Intern. Med. 2009, 169, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.A.; Maxwell, D.J.; Pulver, L.K.; Horn, F.; Robertson, M.B.; Kaye, K.I.; Peterson, G.M.; Dollman, W.B.; Wai, A.; Tett, S.E. A quality improvement initiative to improve adherence to national guidelines for empiric management of community-acquired pneumonia in emergency departments. Int. J. Qual. Health Care 2011, 23, 142–150. [Google Scholar] [CrossRef]
- Durando, P.; Bassetti, M.; Orengo, G.; Crimi, P.; Battistini, A.; Bellina, D.; Talamini, A.; Tiberio, G.; Alicino, C.; Iudici, R.; et al. Adherence to international and national recommendations for the prevention of surgical site infections in Italy: Results from an observational prospective study in elective surgery. Am. J. Infect. Control 2012, 40, 969–972. [Google Scholar] [CrossRef]
- Landers, T.; Abusalem, S.; Coty, M.-B.; Bingham, J. Patient-centered hand hygiene: The next step in infection prevention. Am. J. Infect. Control 2012, 40, S11–S17. [Google Scholar] [CrossRef]
- Nancy, B. The Evolution Handwashing to Hand Hygiene Guidance. Crit. Care Nurs. Q. 2004, 27, 295–307. [Google Scholar]
- Bloomfield, S.F.; Aiello, A.E.; Cookson, B.; O’BOyle, C.; Larson, E.L. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am. J. Infect. Control 2007, 35, S27–S64. [Google Scholar] [CrossRef]
- Jumaa, P. Hand hygiene: Simple and complex. Int. J. Infect. Dis. 2005, 9, 3–14. [Google Scholar] [CrossRef]
- Allegranzi, B.; Pittet, D. Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect. 2009, 73, 305–315. [Google Scholar] [CrossRef]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef]
- Dancer, S. The role of environmental cleaning in the control of hospital-acquired infection. J. Hosp. Infect. 2009, 73, 378–385. [Google Scholar] [CrossRef]
- Escombe, A.R.; Oeser, C.C.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Pan, W.; Martínez, C.; Chacaltana, J.; Rodríguez, R.; Moore, D.A.J.; et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007, 4, e68. [Google Scholar] [CrossRef]
- Mehta, Y.; Gupta, A.; Todi, S.; Myatra, S.; Samaddar, D.P.; Patil, V.; Bhattacharya, P.K.; Ramasubban, S. Guidelines for prevention of hospital acquired infections. Indian J. Crit. Care Med. 2014, 18, 149–163. [Google Scholar] [CrossRef]
- Guo, Y.; Qian, H.; Sun, Z.; Cao, J.; Liu, F.; Luo, X.; Ling, R.; Weschler, L.B.; Mo, J.; Zhang, Y. Assessing and controlling infection risk with Wells-Riley model and spatial flow impact factor (SFIF). Sustain. Cities Soc. 2021, 67, 102719. [Google Scholar] [CrossRef]
- Yu, I.T.; Li, Y.; Wong, T.W.; Tam, W.; Chan, A.T.; Lee, J.H.; Leung, D.Y.; Ho, T. Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. N. Engl. J. Med. 2004, 350, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Ha, L.D.; Bloom, S.A.; Hien, N.Q.; Maloney, S.A.; Mai, L.Q.; Leitmeyer, K.C.; Anh, B.H.; Reynolds, M.G.; Montgomery, J.M.; Comer, J.A.; et al. Lack of SARS Transmission among Public Hospital Workers, Vietnam. Emerg. Infect. Dis. 2004, 10, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Gao, N. The airborne transmission of infection between flats in high-rise residential buildings: A review. Build. Environ. 2015, 94, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Brankston, G.; Gitterman, L.; Hirji, Z.; Lemieux, C.; Gardam, M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007, 7, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Seto, W. Airborne transmission and precautions: Facts and myths. J. Hosp. Infect. 2015, 89, 225–228. [Google Scholar] [CrossRef]
- Zhou, L.; Yao, M.; Zhang, X.; Hu, B.; Li, X.; Chen, H.; Zhang, L.; Liu, Y.; Du, M.; Sun, B.; et al. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol. Sci. 2020, 152, 105693. [Google Scholar] [CrossRef]
- Daisey, J.M.; Angell, W.J.; Apte, M.G. Indoor air quality, ventilation and health symptoms in schools: An analysis of existing information. Indoor Air 2003, 13, 53–64. [Google Scholar] [CrossRef]
- Goyal, R.; Khare, M. Indoor Air Quality: Current Status, Missing Links and Future Road Map for India. J. Civ. Environ. Eng. 2012, 2, 2–4. [Google Scholar] [CrossRef]
- Kennedy, M.; Lee, S.J.; Epstein, M. Modeling aerosol transmission of SARS-CoV-2 in multi-room facility. J. Loss Prev. Process Ind. 2021, 69, 104336. [Google Scholar] [CrossRef]
- Miller-Leiden, S.; Lohascio, C.; Nazaroff, W.W.; Macher, J. Effectiveness of In-Room Air Filtration and Dilution Ventilation for Tuberculosis Infection Control. J. Air Waste Manag. Assoc. 1996, 46, 869–882. [Google Scholar] [CrossRef]
- Adal, K.A.; Anglim, A.M.; Palumbo, C.L.; Titus, M.G.; Coyner, B.J.; Farr, B.M. The Use of High-Efficiency Particulate Air-Filter Respirators to Protect Hospital Workers from Tuberculosis--A Cost-Effectiveness Analysis. N. Engl. J. Med. 1994, 331, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Salam, Z.-H.A.; Karlin, R.B.; Ling, M.L.; Yang, K.S. The impact of portable high-efficiency particulate air filters on the incidence of invasive aspergillosis in a large acute tertiary-care hospital. Am. J. Infect. Control 2010, 38, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, A.A.; Rogak, S.N.; Bartlett, K.H.; Green, S.I. Preventing Airborne Disease Transmission: Review of Methods for Ventilation Design in Health Care Facilities. Adv. Prev. Med. 2011, 2011, 124064. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Stephens, B. HVAC filtration for controlling infectious airborne disease transmission in indoor environments: Predicting risk reductions and operational costs. Build. Environ. 2013, 70, 150–160. [Google Scholar] [CrossRef]
- Jefferson, T.; Foxlee, R.; Del Mar, C.; Dooley, L.; Ferroni, E.; Hewak, B.; Prabhala, A.; Nair, S.; Rivetti, A. Physical interventions to interrupt or reduce the spread of respiratory viruses: Systematic review. BMJ 2008, 336, 77–80. [Google Scholar] [CrossRef]
- Lee, K.; Shukla, V.; Clark, M.; Mierzwinski-Urban, M.; Pessoa-Silva, C.; Conly, J. Physical Interventions to Interrupt or Reduce the Spread of Respiratory Viruses—Resource Use Implications: A Systematic Review. CADTH Technol. Overv. 2011, 2, e2302. [Google Scholar]
- Larson, E.; Kretzer, E. Compliance with handwashing and barrier precautions. J. Hosp. Infect. 1995, 30, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Garbey, M.; Joerger, G.; Furr, S. A systems approach to assess transport and diffusion of hazardous airborne particles in a large surgical suite: Potential impacts on viral airborne transmission. Int. J. Environ. Res. Public Health 2020, 17, 5404. [Google Scholar] [CrossRef]
- Tang, J.W.; Nicolle, A.; Pantelic, J.; Klettner, C.A.; Su, R.; Kalliomaki, P.; Saarinen, P.; Koskela, H.; Reijula, K.; Mustakallio, P.; et al. Different Types of Door-Opening Motions as Contributing Factors to Containment Failures in Hospital Isolation Rooms. PLoS ONE 2013, 8, e66663. [Google Scholar] [CrossRef]
- Kalliomäki, P.; Saarinen, P.; Tang, J.W.; Koskela, H. Airflow patterns through single hinged and sliding doors in hospital isolation rooms—Effect of ventilation, flow differential and passage. Build. Environ. 2016, 107, 154–168. [Google Scholar] [CrossRef]
- Sadrizadeh, S.; Pantelic, J.; Sherman, M.; Clark, J.; Abouali, O. Airborne particle dispersion to an operating room environment during sliding and hinged door opening. J. Infect. Public Health 2018, 11, 631–635. [Google Scholar] [CrossRef]
- Cheong, K.; Chong, K. Development and application of an indoor air quality audit to an air-conditioned building in Singapore. Build. Environ. 2001, 36, 181–188. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, X.; Villalba-Díez, J.; Ordieres-Meré, J. Indoor air-quality data-monitoring system: Long-term monitoring benefits. Sensors 2019, 19, 4157. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Chu, C.-H.; Shin, S.-M. ISSAQ: An integrated sensing systems for real-time indoor air quality monitoring. IEEE Sens. J. 2014, 14, 4230–4244. [Google Scholar] [CrossRef]
- Yang, C.-T.; Liao, C.-J.; Liu, J.-C.; Den, W.; Chou, Y.-C.; Tsai, J.-J. Construction and application of an intelligent air quality monitoring system for healthcare environment. J. Med. Syst. 2014, 38, 15. [Google Scholar] [CrossRef]
- Asthana, P.; Mishra, S. IoT Enabled Real Time Bolt based Indoor Air Quality Monitoring System. In Proceedings of the 2018 International Conference on Computational and Characterization Techniques in Engineering & Sciences (CCTES) Integral University, Lucknow, India, 14–15 September 2018. [Google Scholar]
- Memarzadeh, F.; Olmsted, R.N.; Bartley, J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: Effective adjunct, but not stand-alone technology. Am. J. Infect. Control 2010, 38, S13–S24. [Google Scholar] [CrossRef]
- Ethington, T.; Newsome, S.; Waugh, J.; Lee, L.D. Cleaning the air with ultraviolet germicidal irradiation lessened contact infections in a long-term acute care hospital. Am. J. Infect. Control 2018, 46, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.G. The History of Ultraviolet Germicidal Irradiation for Air Disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Green, C.F.; Scarpino, P.V. The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria. Environ. Eng. Policy 2001, 3, 101–107. [Google Scholar] [CrossRef]
- Kujundzic, E.; Matalkah, F.; Howard, C.J.; Hernandez, M.; Miller, S.L. UV air cleaners and upper-room air ultraviolet germicidal irradiation for controlling airborne bacteria and fungal spores. J. Occup. Environ. Hyg. 2006, 3, 536–546. [Google Scholar] [CrossRef]
- Hsiao, T.-C.; Chuang, H.-C.; Griffith, S.M.; Chen, S.-J.; Young, L.-H. COVID-19: An aerosol’s point of view from expiration to transmission to viral-mechanism. Aerosol Air Qual. Res. 2020, 20, 905–910. [Google Scholar] [CrossRef]
- Li, Y.; Leung, G.M.; Tang, J.W.; Yang, X.; Chao, C.Y.H.; Lin, J.Z.; Lu, J.W.; Nielsen, P.V.; Niu, J.; Qian, H.; et al. Role of ventilation in airborne transmission of infectious agents in the built environment? A multidisciplinary systematic review. Indoor Air 2007, 17, 2–18. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Eames, I.; Chan, P.; Ridgway, G. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006, 64, 100–114. [Google Scholar] [CrossRef]
- Luongo, J.C.; Fennelly, K.P.; Keen, J.A.; Zhai, Z.J.; Jones, B.W.; Miller, S.L. Role of mechanical ventilation in the airborne transmission of infectious agents in buildings. Indoor Air 2016, 26, 666–678. [Google Scholar] [CrossRef]
- Knibbs, L.D.; Morawska, L.; Bell, S.C.; Grzybowski, P. Room ventilation and the risk of airborne infection transmission in 3 health care settings within a large teaching hospital. Am. J. Infect. Control 2011, 39, 866–872. [Google Scholar] [CrossRef]
- Vonci, N.; De Marco, M.F.; Grasso, A.; Spataro, G.; Cevenini, G.; Messina, G. Association between air changes and airborne microbial contamination in operating rooms. J. Infect. Public Health 2019, 12, 827–830. [Google Scholar] [CrossRef]
- Nielsen, P.V. Control of airborne infectious diseases in ventilated spaces. J. R. Soc. Interface 2009, 6, S747–S755. [Google Scholar] [CrossRef]
- Sundell, J.; Levin, H.; Nazaroff, W.W.; Cain, W.S.; Fisk, W.J.; Grimsrud, D.T.; Gyntelberg, F.; Li, Y.; Persily, A.K.; Pickering, A.C.; et al. Ventilation rates and health: Multidisciplinary review of the scientific literature. Indoor Air 2011, 21, 191–204. [Google Scholar] [CrossRef]
- Cooke, M.W.; Higgins, J.; Kidd, P. Use of emergency observation and assessment wards: A systematic literature review. Emerg. Med. J. 2003, 20, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Chartier, Y.; Pessoa-Silva, C.L.; Jensen, P.; Li, Y.; Seto, W.-H. Natural Ventilation for Infection Control in Healthcare Settings; WHO Publication: Geneva, Switzerland, 2009. [Google Scholar]
- Qian, H.; Li, Y.; Seto, W.; Ching, P.; Ching, W.; Sun, H. Natural ventilation for reducing airborne infection in hospitals. Build. Environ. 2010, 45, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hobday, R.; Dancer, S. Roles of sunlight and natural ventilation for controlling infection: Historical and current perspectives. J. Hosp. Infect. 2013, 84, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Escombe, A.R.; Ticona, E.; Chávez-Pérez, V.; Espinoza, M.; Moore, D.A.J. Improving natural ventilation in hospital waiting and consulting rooms to reduce nosocomial tuberculosis transmission risk in a low resource setting. BMC Infect. Dis. 2019, 19, 88. [Google Scholar] [CrossRef]
- Chaivisit, P.; Fontana, A.; Galindo, S.; Strub, C.; Choosong, T.; Kantachote, D.; Suksaroj, T.T. Airborne bacteria and fungi distribution characteristics in natural ventilation system of a university hospital in Thailand. EnvironmentAsia 2018, 11, 53–66. [Google Scholar] [CrossRef]
- Haas, C.N.; Rycroft, T.; Bibby, K.; Casson, L. Risks from Ebolavirus Discharge from Hospitals to Sewer Workers. Water Environ. Res. 2017, 89, 357–368. [Google Scholar] [CrossRef]
- Teixeira, J.V.; Miranda, S.; Monteiro, R.A.R.; Lopes, F.V.S.; Madureira, J.; Silva, G.V.; Pestana, N.; Pinto, E.; Vilar, V.J.P.; Boaventura, R.A.R. Assessment of indoor airborne contamination in a wastewater treatment plant. Environ. Monit. Assess. 2013, 185, 59–72. [Google Scholar] [CrossRef]
- Goldmann, D.A. Transmission of viral respiratory infections in the home. Pediatr. Infect. Dis. J. 2000, 19, S97–S102. [Google Scholar] [CrossRef] [PubMed]
- Beggs, C.B. The Airborne Transmission of Infection in Hospital Buildings: Fact or Fiction? Indoor Built Environ. 2003, 12, 9–18. [Google Scholar] [CrossRef]
- Seto, W.; Conly, J.; Silva, C.P.; Malik, M.; Eremin, S. Infection prevention and control measures for acute respiratory infections in healthcare settings: An update. East. Mediterr. Health J. 2013, 19, S39–S47. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control 2016, 44, S102–S108. [Google Scholar] [CrossRef]
- Fernstrom, A.; Goldblatt, M. Aerobiology and Its Role in the Transmission of Infectious Diseases. J. Pathog. 2013, 2013, 493960. [Google Scholar] [CrossRef]
- Lai, A.C.K.; Poon, C.K.M.; Cheung, A.C.T. Effectiveness of facemasks to reduce exposure hazards for airborne infections among general populations. J. R. Soc. Interface 2012, 9, 938–948. [Google Scholar] [CrossRef]
- Johnson, D.F.; Druce, J.D.; Birch, C.; Grayson, M.L. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin. Infect. Dis. 2009, 49, 275–277. [Google Scholar] [CrossRef]
- Bischoff, W.E.; Reid, T.; Russell, G.B.; Peters, T.R. Transocular entry of seasonal influenza-attenuated virus aerosols and the efficacy of N95 respirators, surgical masks, and eye protection in humans. J. Infect. Dis. 2011, 204, 193–199. [Google Scholar] [CrossRef]
- Bin-Reza, F.; Chavarrias, V.L.; Nicoll, A.; Chamberland, M.E. The use of masks and respirators to prevent transmission of influenza: A systematic review of the scientific evidence. Influ. Other Respir. Viruses 2012, 6, 257–267. [Google Scholar] [CrossRef]
- Smith, J.D.; MacDougall, C.C.; Johnstone, J.; Copes, R.A.; Schwartz, B.; Garber, G.E. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: A systematic review and meta-analysis. Can. Med. Assoc. J. 2016, 188, 567–574. [Google Scholar] [CrossRef]
- Offeddu, V.; Yung, C.F.; Low, M.S.F.; Tam, C.C. Effectiveness of Masks and Respirators Against Respiratory Infections in Healthcare Workers: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017, 65, 1934–1942. [Google Scholar] [CrossRef]
- Pryor, F.; Messmer, P.R. The Effect of Traffic Patterns in the OR on Surgical Site Infections. AORN J. 1998, 68, 649–660. [Google Scholar] [CrossRef]
- Andersson, A.E.; Bergh, I.; Karlsson, J.; Eriksson, B.I.; Nilsson, K. Traffic flow in the operating room: An explorative and descriptive study on air quality during orthopedic trauma implant surgery. Am. J. Infect. Control 2012, 40, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.B.; Raphael, I.J.; Maltenfort, M.G.; Honsawek, S.; Dolan, K.; Younkins, E.A. The effect of laminar air flow and door openings on operating room contamination. J. Arthroplast. 2013, 28, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, K.; Lee, B.; Ferrand, Y.; Fredendall, L.; San, D.; Salgado, C.; Shvorin, D.; Khoshkenar, A.; Reeves, S.; the Realizing Improved Patient Care through Human-Centered Design in the Operating Room (RIPCHDOR) Study Group. The Influence of Traffic, Area Location, and Other Factors on Operating Room Microbial Load. Infect. Control Hosp. Epidemiol. 2018, 39, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Kaye, K.S.; Classen, D.; Arias, K.M.; Podgorny, K.; Burstin, H.; Calfee, D.P.; Coffin, S.E.; Dubberke, E.R.; Fraser, V.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals. Infect. Control. Hosp. Epidemiol. 2008, 29, S51–S61. [Google Scholar] [CrossRef]
- Sun, C.; Zhai, Z. The efficacy of social distance and ventilation effectiveness in preventing COVID-19 transmission. Sustain. Cities Soc. 2020, 62, 102390. [Google Scholar] [CrossRef]
- Hall, C.B.; Douglas, R.G., Jr. Modes of transmission of respiratory syncytial virus. J. Pediatr. 1981, 99, 100–103. [Google Scholar] [CrossRef]
- Kelso, J.K.; Milne, G.J.; Kelly, H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 2009, 9, 117. [Google Scholar] [CrossRef]
- Halder, N.; Kelso, J.K.; Milne, G.J. Analysis of the effectiveness of interventions used during the 2009 A/H1N1 influenza pandemic. BMC Public Health 2010, 10, 168. [Google Scholar] [CrossRef]
- Ahmed, F.; Zviedrite, N.; Uzicanin, A. Effectiveness of workplace social distancing measures in reducing influenza transmission: A systematic review. BMC Public Health 2018, 18, 518. [Google Scholar] [CrossRef]
- Naskar, I.; Pal, A.K. Automatic Indoor Air Quality Control of COVID-19 Patient Facilities Using Type-2 Fuzzy Controller. J. Phys. Conf. Ser. 2021, 1937, 012035. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Kansal, H. Fuzzy logic controller based operating room air condition control system. Int. J. Innov. Res. Electr. Electron. Instrum. Control Eng. 2014, 2, 510–514. [Google Scholar]
- Pradityo, F.; Surantha, N. Indoor Air Quality Monitoring and Controlling System based on IoT and Fuzzy Logic. In Proceedings of the 2019 7th International Conference on Information and Communication Technology (ICoICT), Kuala Lumpur, Malaysia, 24–26 July 2019; IEEE: New York, NY, USA, 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Li, W.; Koo, C.; Cha, S.H.; Hong, T.; Oh, J. A novel real-time method for HVAC system operation to improve indoor environmental quality in meeting rooms. Build. Environ. 2018, 144, 365–385. [Google Scholar] [CrossRef]
- Ahamed, N.U.; Bin Taha, Z.; Khairuddin, I.B.M.; Rabbi, M.F.; Rahaman, S.A.M.M.; Sundaraj, K. Fuzzy logic controller design for intelligent air-conditioning system. In Proceedings of the 2016 2nd International Conference on Control Science and Systems Engineering (ICCSSE), Singapore, 27–29 July 2016; IEEE: New York, NY, USA, 2016; pp. 232–236. [Google Scholar] [CrossRef]
- Yu, T.-C.; Lin, C.-C. An Intelligent Wireless Sensing and Control System to Improve Indoor Air Quality: Monitoring, Prediction, and Preaction. Int. J. Distrib. Sens. Netw. 2015, 11, 140978. [Google Scholar] [CrossRef]
- Chow, T.; Yang, X. Ventilation performance in operating theatres against airborne infection: Review of research activities and practical guidance. J. Hosp. Infect. 2004, 56, 85–92. [Google Scholar] [CrossRef]
- Romano, F.; Marocco, L.; Gustén, J.; Joppolo, C.M. Numerical and experimental analysis of airborne particles control in an operating theater. Build. Environ. 2015, 89, 369–379. [Google Scholar] [CrossRef]
- Haque, S.E.; Rahman, M. Association between temperature, humidity, and COVID-19 outbreaks in Bangladesh. Environ. Sci. Policy 2020, 114, 253–255. [Google Scholar] [CrossRef]
- Chan, K.H.; Peiris, J.S.M.; Lam, S.Y.; Poon, L.L.M.; Yuen, K.-Y.; Seto, W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef]
- Songer, J.R. Influence of Relative Humidity on the Survival of Some Airborne Viruses. Appl. Microbiol. 1967, 15, 35–42. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Bouvier, N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012, 2, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Crijns, F.; Keinänen-Toivola, M.; Dunne, C. Antimicrobial coating innovations to prevent healthcare-associated infection. J. Hosp. Infect. 2017, 95, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.; Small, B.A.; Thom, K.A.; CDC Prevention Epicenters Program. Innovative Methods of Hospital Disinfection in Prevention of Healthcare-Associated Infections. Curr. Treat. Options Infect. Dis. 2018, 10, 65–77. [Google Scholar] [CrossRef]
- Fleming, M.; Patrick, A.; Gryskevicz, M.; Masroor, N.; Hassmer, L.; Shimp, K.; Cooper, K.; Doll, M.; Stevens, M.; Bearman, G. Deployment of a touchless ultraviolet light robot for terminal room disinfection: The importance of audit and feedback. Am. J. Infect. Control 2018, 46, 241–243. [Google Scholar] [CrossRef]
- Doll, M.; Morgan, D.J.; Anderson, D.; Bearman, G. Touchless Technologies for Decontamination in the Hospital: A Review of Hydrogen Peroxide and UV Devices. Curr. Infect. Dis. Rep. 2015, 17, 44. [Google Scholar] [CrossRef]
- Weber, D.J.; Kanamori, H.; Rutala, W.A. ‘No touch’ technologies for environmental decontamination. Opin. Infect. Dis. 2016, 29, 424–431. [Google Scholar] [CrossRef]
- Arundel, A.V.; Sterling, E.M.; Biggin, J.H.; Sterling, T.D. Indirect health effects of relative humidity in indoor environments. Environ. Health Perspect. 1986, 65, 351–361. [Google Scholar] [CrossRef]
- Nerandzic, M.M.; Thota, P.; Sankar, T.; Jencson, A.; Cadnum, J.L.; Ray, A.J.; Salata, R.A.; Watkins, R.R.; Donskey, C.J. Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infect. Control Hosp. Epidemiol. 2015, 36, 192–197. [Google Scholar] [CrossRef]
- Nerandzic, M.M.; Cadnum, J.L.; Pultz, M.J.; Donskey, C.J. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect. Dis. 2010, 10, 197. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Moore, B.A. Terminal Decontamination of Patient Rooms Using an Automated Mobile UV Light Unit. Infect. Control Hosp. Epidemiol. 2011, 32, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Mahida, N.; Vaughan, N.; Boswell, T. First UK evaluation of an automated ultraviolet-C room decontamination device (Tru-D™). J. Hosp. Infect. 2013, 84, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Gergen, M.F.; Smathers, E.; Sexton, D.J.; Chen, L.F.; Weber, D.J.; Rutala, W.A. Decontamination of Targeted Pathogens from Patient Rooms Using an Automated Ultraviolet-C-Emitting Device. Infect. Control Hosp. Epidemiol. 2013, 34, 466–471. [Google Scholar] [CrossRef]
- Eames, I.; Shoaib, D.; Klettner, C.A.; Taban, V. Movement of airborne contaminants in a hospital isolation room. J. R. Soc. Interface 2009, 6, S757–S766. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Shih, Y.-C.; Hu, S.-C. Numerical study on the dispersion of airborne contaminants from an isolation room in the case of door opening. Appl. Therm. Eng. 2009, 29, 1544–1551. [Google Scholar] [CrossRef]
- Rosero-Montalvo, P.D.; Caraguay-Procel, J.A.; Jaramillo, E.D.; Michilena-Calderon, J.M.; Umaquinga-Criollo, A.C.; Mediavilla-Valverde, M.; Ruiz, M.A.; Beltran, L.A.; Peluffo, D.H. Air Quality Monitoring Intelligent System Using Machine Learning Techniques. In Proceedings of the 3rd International Conference on Information, Systems and Computer Science (INCISCOS 2018), Quito, Ecuador, 14–16 November 2018; IEEE: New York, NY, USA, 2018; pp. 75–80. [Google Scholar] [CrossRef]
- Taştan, M.; Gökozan, H. Real-Time Monitoring of Indoor Air Quality with Internet of Things-Based E-Nose. Appl. Sci. 2019, 9, 3435. [Google Scholar] [CrossRef]
- Salamone, F.; Belussi, L.; Currò, C.; Danza, L.; Ghellere, M.; Guazzi, G.; Lenzi, B.; Megale, V.; Meroni, I. Integrated Method for Personal Thermal Comfort Assessment and Optimization through Users’ Feedback, IoT and Machine Learning: A Case Study. Sensors 2018, 18, 1602. [Google Scholar] [CrossRef]
- Iqbal, U.; Dar, M.A.; Bukhari, S.N. Intelligent Hospitals based on IOT. In Proceedings of the 2018 Fourth International Conference on Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), Chennai, India, 27–28 February 2018; IEEE: New York, NY, USA, 2018; pp. 1–3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).