Abstract
Transient Receptor Potential Vanilloid (TRPV) channels represent one of the seven subfamilies of TRP receptors and are widely expressed throughout the human body where they play pivotal roles in various physiological processes. In the gastrointestinal (GI) system, TRPV channels regulate critical functions such as nutrient absorption, motility, and secretions. Beyond maintaining cellular homeostasis, these channels are involved in pain and inflammation, contributing to diverse pathologies. Their central role in the pathophysiology of different digestive system disorders has made TRPV channels a significant focus of research. Moreover, the involvement of TRPV channels in numerous GI cancers has further heightened research interest in the role of these channels. Accordingly, this review elucidates the structural components and intricate signaling pathways of TRPV channels, focusing on the unique characteristics of each family member (TRPV1–6) in GI physiology. Furthermore, we explore the therapeutic potential of targeting these channels to modulate their physiological and pathological roles, highlighting their promise in treating GI disorders. Additionally, we address the challenges associated with their therapeutic application, considering their interactions in different systems, inherent biochemical characteristics, and the alterations required for effective design.
1. Introduction
The gastrointestinal tract (GIT) is indispensable for human well-being as it performs various physiological functions fundamental to overall health. Its efficiency is contingent on a diverse array of components, including gut microbiota, hormone-secreting cells, Gut-Associated Lymphoid Tissue (GALT), and an intricate network of ion channels [1]. Indeed, the GIT constitutes a complex network of diverse ion channels that facilitate the transport of various substances essential for its proper functioning. These channels play a vital role in maintaining the delicate balance of ions, nutrients, and other molecules needed for digestion, nutrient absorption, and overall gut health [2]. Accordingly, the regulation of such channels is finely tuned to respond to an array of stimuli, ranging from changes in the composition of ingested food to temperature, voltage, pressure, osmolarity and tension [3].
As part of the vast network of ion channels present in the human body, one notable family is the Transient Receptor Potential (TRP) channels. Prominently, these non-selective cation channels are involved in mediating various physiological processes. While their significant role in the GIT cannot be overlooked, TRP channels are also found in various other tissues and organs, including the nervous system, skin, bladder, lungs, and cardiovascular system [4,5,6]. TRP channels are involved in sensory perception of temperature, pain, taste, and touch. TRP channels are also pivotal for mediating the responses to chemical, mechanical, and osmotic changes in the environment [7]. Such multifaceted functionality is facilitated by their unique ability to allow transmembrane passage of various ions, including calcium, sodium, potassium, and magnesium [4]. These channels are classified into seven subfamilies based on sequence homology and functional properties, each holding distinct properties and characteristics. These include canonical (TRPC), vanilloid (TRPV), melastatin (TRPM), mucolipins (TRPML), no-mechano-potential (TRPN), ankyrin (TRPA), and polycystin (TRPP) [8].
TRPV channels are named after the first identified member TRPV1, which is sensitive to the vanillylamide capsaicin, the compound responsible for the hot and spicy sensation in chili peppers [9,10]. Such channels bear considerable importance due to widespread expression across the GIT. They play vital roles in the regulation of gut motility, secretion, pain perception, and responses to chemical and thermal stimuli. Among the TRPV subfamily, both non-selective cation channels, TRPV1–4, and selective cation channels, TRPV5–6, are identified to serve distinct yet interrelated functions [11].
Given their intricate involvement in normal body physiology, it comes as no surprise that disruptions in the function or regulation of TRPV channels are involved in numerous gastrointestinal (GI) abnormalities. Their influence on GI disorders has been associated with a broad spectrum of conditions, acting both as protective agents and contributors in disease development, including GI cancers and neuroendocrine tumors (NETs) [12]. Recognizing this, it becomes imperative to emphasize that acquiring a comprehensive understanding of the ramifications of TRPV channels’ malfunctions offers valuable insights into potential therapeutic methods for dealing with related pathologies.
Accordingly, in this comprehensive review, we will delve into the intricate roles and implications of TRPV channels in various GI diseases, aiming to illuminate their significance in pathological contexts and uncover potential avenues for future research and therapeutic interventions.
2. Methods
A comprehensive literature search was conducted on 27 May 2025, and updated on 4 August 2025, to identify studies exploring the role of TRPV channels in gastrointestinal physiology and pathology. Databases searched included PubMed, Scopus, Web of Science, Google Scholar, and Embase. The search strategy used the following string for PubMed and was adapted for other databases:
((TRPV Cation Channels [MeSH Terms]) OR (Transient Receptor Potential Channels [MeSH Terms]) OR (TRPV [Title/Abstract]) OR (Transient Receptor Potential Vanilloid [Title/Abstract])) AND ((Gastrointestinal Tract [MeSH Terms]) OR (Gut [Title/Abstract])).
Additional relevant articles were identified through forward and backward snowballing (citation tracking) of the included studies [13]. Inclusion criteria were peer-reviewed articles published in English from database inception to the search date. Studies were excluded if they were not focused on the GI tract, were not in English, or lacked sufficient scientific detail. Search results were screened by title and abstract for relevance. Included studies were categorized into four TRPV-related thematic areas: architecture, physiology, pathology, and pharmacology. Each thematic area was assigned to a pair of authors who collected and synthesized the evidence. Their synthesized summaries were then critically revised and approved by the senior author to ensure accuracy and coherence.
3. The General Structure of TRPV Channels
TRPV channels are characterized by their tetrameric arrangement, much like voltage-gated ion channels, and have transmembrane and cytoplasmic domains. Each TRPV channel has four monomers that assemble together in a ring-like structure (Figure 1). Each monomer consists of six transmembrane segments labeled S1 to S6 linked to each other through extracellular and intracellular loops [14]. The first four segments, S1 to S4, operate in a manner similar to voltage-sensing domains (VSDs) found in other ion channels, and are therefore collectively referred to as voltage-sensing-like domain (VSLD). This domain is connected to an inner pore region consisting of S5 and S6 bridged by the S4S5-linker. The precise location of this linker is of great importance in regulating channel gating, determining when the channel opens and closes [15]. Together with the S4S5-linker, S5 and S6 are positioned adjacent to other domains, encircling the inner pore to form the lower gate of the channel [11]. On the other hand, the formation of the upper gate is ascribed to the pore helix (PH), formed by a short loop and a helix, which plays an important role in determining the ion selectivity properties of TRPV channels, allowing certain cations in but not others [16].
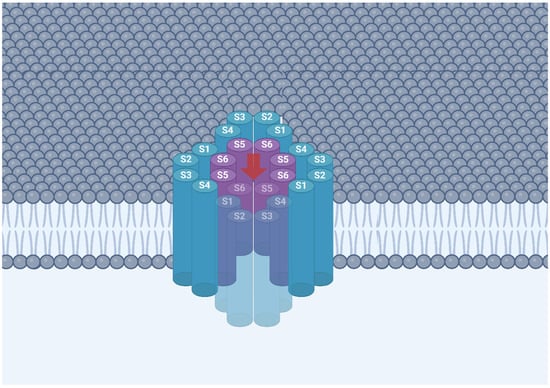
Figure 1.
Schematic representation of a TRPV channel showing its tetrameric arrangement, with each subunit comprising six transmembrane segments (S1–S6). The S1–S4 segments form the voltage sensor–like domain, while S5 and S6 from each subunit contribute to the central ion-conducting pore. Cations enter the channel through the outer pore (red arrow) passing through the central pore and the inner pore sequentially.
Focusing on the cytoplasmic domain of TRPV channels, a distinctive structural arrangement is observed, characterized by a larger N-terminus and a smaller C-terminus (Figure 2). The N-terminus harbors two β-sheets found between a pre-S1 helix and six ankyrin repeats [4]. These repeats collectively form the ankyrin repeat domain (ARD) which stands out due to its interaction with the β-sheets of neighboring monomers, resulting in the formation of a skirt-like structure [17]. This structural motif serves as a platform for intricate engagements with regulatory proteins, thereby exerting a significant influence in modulating the channel’s activity [18]. The C-terminus, on the contrary, contains the TRP domain and is associated with one β-sheet.
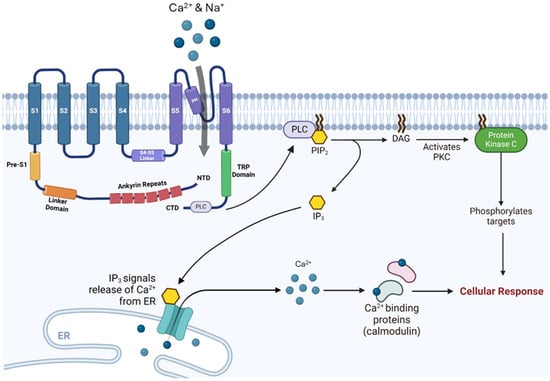
Figure 2.
TRPV channels’ intracellular signaling cascade: The conformational changes in the TRP domain and PLC following TRPV channel’s activation derive PIP2-mediated cellular responses. Abbreviations: TRP, transient receptor potential; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; PKC, protein kinase C; NTD, N-terminal domain; CTD, C-terminal domain; ER, endoplasmic reticulum; IP3, inositol 1,4,5-trisphosphate.
TRPV channels are non-selective cation channels that permit the influx of calcium and other cations upon activation. Their activity can be modulated by G protein-coupled receptors (GPCR) signaling pathways through second messengers such as diacylglycerol (DAG), phospholipase C (PLC), or changes in intracellular calcium levels [19]. Activation of TRPV channels, whether by ligand binding or other stimuli, induces conformational changes that open the channel pore, allowing the entry of ions including sodium and calcium, depending on the channel’s permeability. TRPV stimulation also triggers intracellular signaling cascades, prominently involving PLC. This enzyme catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and DAG. IP3 promotes the release of calcium from intracellular stores in the smooth endoplasmic reticulum, producing downstream cellular effects, while DAG activates protein kinase C (PKC), a serine/threonine kinase, that phosphorylates target proteins and modulates their activity [20].
4. TRPV Channels in GI Physiology
The following section explores each of the TRPV channels individually, outlining their physiological roles, known activators, and involvement in gastrointestinal function. Table 1 summarizes key characteristics of these channels, including where they are expressed, activation mechanisms, and associated GIT-related effects.
4.1. TRPV1
The TRPV1 channel is a polymodal sensor activated by a wide range of stimuli, including temperatures above 42 °C, low pH, hyperosmolarity, capsaicin, 2-arachidonoylglycerol (2-AG) and endovanilloids such as anandamide [21,22]. Additionally, it is activated by endogenous lipids like oleoylethanolamide (OLDA), which binds to the receptor’s vanilloid site to promote calcium influx and nociceptive signaling [23]. It is primarily present in the sensory neurons of the peripheral nervous system, dorsal root ganglia (DRG), and trigeminal ganglia, where it plays a pivotal role in sensing temperature and pain, mediating responses to noxious heat and chemical irritants [9]. Within the GIT, the TRPV1 channel is located on the enteric neurons, contributing to visceral pain perception and the regulation of GI motility and secretion. This is attributed to the eventual release of neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) upon the activation of TRPV1 channel [24]. Additionally, TRPV1 is implicated in modulating vagally mediated contractions of the striated part of the esophagus, as well as regulating gastric acid secretion, gastric emptying, and visceral pain perception in response to inflammatory mediators [25]. Emerging evidence suggests that TRPV1 also plays a role in gastric mucosal defense mechanisms by promoting the release of mucus and bicarbonate, thus protecting the epithelial lining from acidic and mechanical damage [26]. Furthermore, TRPV1 activation has been linked to the modulation of immune responses within the stomach and small intestines of the GIT, influencing inflammatory pathways that can affect conditions such as gastritis and inflammatory bowel disease [27,28].
4.2. TRPV2
The TRPV2 channel is activated by high temperatures, usually above 50–52 °C, mechanical stimuli, and endogenous lipids such as 2-AG and lysophosphatidylcholine (LPC) [29,30,31]. It is also a target for pharmacological agents, most notably Cannabidiol (CBD), a natural compound from Cannabis sativa that acts as a potent and direct agonist of the channel [32]. This channel demonstrates widespread expression across various tissues, thereby contributing to diverse cellular processes, including immune responses, inflammation, and cellular homeostasis [33]. Indeed, its expression in the enteric immune cells including macrophages, dendritic cells, and T cells signifies its role in regulating inflammatory responses and enhancing mucosal immunity within the GIT [34]. Furthermore, in rodents, the TRPV2 channel has also been identified in enteric neurons, where its stimulation influences downstream neurotransmitter release, thereby affecting GI movement and secretion. Specifically, TRPV2 was shown to mediate nitric oxide (NO) release. NO is a key inhibitory neurotransmitter which regulates intestinal motility by inducing smooth muscle relaxation. This was demonstrated through studies showing that TRPV2 activation, by mechanical or chemical stimuli such as probenecid and lysophospholipids, inhibited spontaneous intestinal contractions through enteric neurons [35,36].
4.3. TRPV3
TRPV3 channel is activated by innocuous temperatures exceeding 30–33 °C, natural compounds like camphor, 2-aminoethoxydiphenyl borate (2-APB), and endogenous lipids such as 12-hydroxyeicosatetraenoic acid (12-HETE) [37]. It is also robustly activated by a variety of natural monoterpenes found in plants, including menthol, carvacrol, thymol, and carveol [38]. This channel is primarily expressed in epidermal keratinocytes where it plays a vital role in sensing warmth and maintaining skin barrier function [39,40,41]. Although TRPV3 channel has been extensively studied in the context of skin physiology, emerging research suggests its expression in the brain, particularly in regions associated with appetite regulation [42]. Additionally, TRPV3 is expressed in the GIT, particularly in the cecum and colon, where it plays a crucial role in ammonium (NH4+) transport through its divalent-sensitive cation conductance [43]. Thus, TRPV3 contributes to nitrogen metabolism and gut homeostasis. It is localized predominantly to the apical membranes of epithelial cells, influencing epithelial permeability and maintaining barrier integrity by facilitating ion exchange under varying physiological conditions [44]. TRPV3 responds to changes in luminal ionic environments, modulating transepithelial ionic balance and supporting processes such as motility and secretion. Its frequent colocalization with TRPV4 suggests cooperative functions in osmoregulation and mechanosensation, while its presence in regions of high microbial activity highlights its potential role in host-microbe interactions and nutrient absorption [44,45].
4.4. TRPV4
The TRPV4 channel is widely expressed in multiple tissues and cell types, including endothelial cells, sensory neurons, urothelial cells, and chondrocytes [5,46,47,48,49]. Its activation is elicited in response to diverse stimuli, including mechanical stress, osmotic changes, moderate temperatures ranging from 25 to 35 °C, and arachidonic acid metabolites. TRPV4’s activation enhances calcium signaling, influencing ion transport, epithelial barrier function, and sensory transduction pathways across the GI tract [50,51,52,53]. In the GIT milieu, it is distinguished by its involvement in regulating GI motility and fluid secretion across various exocrine glands, including salivary glands, where its activation is essential for regulating salivary flow. This regulation is mediated by the channel’s sensitivity to osmotic changes and mechanical stress, enabling it to facilitate water and ion movement across epithelial cells [54]. The TRPV4 channel’s responsiveness to mechanical stimuli entitles it for the active participation in GI mechanosensation, influencing processes such as gastric emptying and intestinal transit [55]. In the esophagus, the TRPV4 channel regulates calcium influx and ATP release in response to mechanical and chemical stimuli [56]. Similarly, in the stomach, TRPV4 is expressed at both mRNA and protein levels in mouse and rat gastric epithelial cells; upon activation by mechanical stretch or the selective agonist GSK1016790A—a TRPV-agonizing pharmacological tool developed by GlaxoSmithKline (GSK)—it induces calcium entry, generates TRPV4-like currents in the epithelial cells, and promotes ATP release [57]. These activities are crucial for maintaining epithelial integrity, sensory transduction, and protecting against mechanical damage from food passage. TRPV4 also contributes to esophageal mechanosensation, helping regulate peristalsis and coordinating the esophageal response to distension [55,58]. The channel’s activation in the stomach modulates calcium influx and ATP release, processes essential for maintaining gastric epithelial health and protecting against mechanical or chemical injury [57]. Additionally, TRPV4 plays a role in regulating gastric motility and pancreatic fluid secretion, impacting digestion and the maintenance of an optimal environment for enzymatic activity. Dysregulation of TRPV4 activity in the stomach has been associated with gastric dysfunction and potential involvement in inflammatory conditions [59].
4.5. TRPV5 and TRPV6
TRPV5 and TRPV6 channels are predominantly expressed in the GI epithelial cells and are crucial for maintaining calcium homeostasis. These channels are highly selective for calcium ions, distinguishing them from the previously mentioned TRPV channels [60]. Importantly, unlike other TRPV members, TRPV5 and TRPV6 have not been shown to be temperature-sensitive [61]. Their expression and activity are significantly upregulated by the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25[OH]2D3), which is a major hormonal regulator of systemic calcium balance [62,63]. The activity of TRPV5 is tightly regulated by various factors, including the anti-aging hormone Klotho and sialidase, which modulate its expression and function at the cell surface [64]. Furthermore, the membrane phospholipid PIP2 is a critical activator; for TRPV5, its direct binding is essential for channel opening, while for TRPV6, it enhances function by relieving auto-inhibition [65,66]. They are activated by extracellular calcium, enabling the influx of calcium ions across the apical membrane of epithelial cells of the small intestine, particularly in the duodenum and jejunum, where active calcium absorption occurs [60]. This mechanism is instrumental in maintaining systemic calcium balance and supporting various physiological functions, including neuromuscular excitability and bone health. Moreover, the TRPV6 channel participates in the absorption of magnesium within the intestine and serves crucial functions in cellular signaling pathways, which in turn influence pivotal processes including cell proliferation, differentiation, and apoptosis, making the TRPV6 channel increasingly conspicuous as a potential target for cancer therapy [67,68].

Table 1.
A summary of the physiological roles of the different TRPV channels in the GIT.
Table 1.
A summary of the physiological roles of the different TRPV channels in the GIT.
| Channel | Key Activators | Localization and Expression | Physiological Functions in the GI Tract |
|---|---|---|---|
| TRPV1 | Activated by temperatures above 42 °C, hyperosmolarity, low pH, capsaicin, and endovanilloids such as anandamide [21,22]. Moreover, it is activated by the endogenous lipid mediator oleoylethanolamide (OLDA) [38]. | Primarily localized in sensory neurons of the peripheral nervous system, dorsal root ganglia, and trigeminal ganglia [9], as well as in enteric neurons within the gastrointestinal tract (GIT) [24]. | TRPV1 senses temperature and pain, mediating responses to noxious heat and chemical irritants [9]. It contributes to visceral pain perception [25] and regulates GI motility and secretion [25]. It modulates vagally mediated contractions of the striated part of the esophagus, regulates gastric acid secretion and gastric emptying [25], and is implicated in gastric mucosal defense by promoting mucus and bicarbonate release [27,28]. Additionally, it modulates immune responses in the stomach and small intestines [27,28]. |
| TRPV2 | Activated by high temperatures (above 50–52 °C), mechanical stimuli, and endogenous lipids such as 2-arachidonoylglycerol (2-AG) and lysophosphatidylcholine (LPC) [29,30,31]. | Exhibits widespread expression across various tissues [33], including enteric immune cells such as macrophages, dendritic cells, and T cells [34], and has been identified in enteric neurons in rodents [35,36]. | TRPV2 contributes to diverse cellular processes, including immune responses and inflammation [33], and regulates inflammatory responses while enhancing mucosal immunity within the GIT [34]. Its stimulation in enteric neurons influences downstream neurotransmitter release [35,36] and mediates nitric oxide (NO) release, a key inhibitory neurotransmitter that regulates intestinal motility by inducing smooth muscle relaxation [35,36]. |
| TRPV3 | Activated by temperatures exceeding 30–33 °C, natural compounds like camphor and 2-aminoethoxydiphenyl borate (2-APB), and endogenous lipids such as 12-hydroxyeicosatetraenoic acid (12-HETE) [37]. Additionally, Cannabidiol (CBD) potently agonizes TRPV3 [32]. | Primarily expressed in epidermal keratinocytes [39,40,41], with suggested expression in the brain [42]. In the GIT, it is expressed particularly in the cecum and colon [43], and is localized predominantly to the apical membranes of epithelial cells [44]. | TRPV3 plays a vital role in sensing warmth and maintaining skin barrier function [39,40,41]. In the gut, it plays a crucial role in ammonium (NH4+) transport through its divalent-sensitive cation conductance [43], contributing to nitrogen metabolism and gut homeostasis [43]. It influences epithelial permeability and maintains barrier integrity [44], modulates transepithelial ionic balance [44,45], and may have a potential role in host-microbe interactions and nutrient absorption [44,45]. |
| TRPV4 | Activated by mechanical stress, osmotic changes, moderate temperatures (25–35 °C), arachidonic acid metabolites, and the selective pharmacological agonist GSK1016790A [50,51,52,53,57]. | Widely expressed in multiple tissues and cell types [5,46,47,48,49], including endothelial cells, sensory neurons, urothelial cells, and chondrocytes [5,46,47,48,49]. It is present in the GIT milieu [54], and expressed in salivary glands, esophagus, and stomach [54,56,57] | TRPV4 is involved in regulating GI motility [54] and fluid secretion, which is essential for salivary flow [54]. It actively participates in GI mechanosensation, influencing gastric emptying and intestinal transit [55]. It regulates calcium influx and ATP release in the esophagus and stomach [56,57], is crucial for maintaining epithelial integrity and sensory transduction pathways [57], contributes to esophageal mechanosensation and regulation of peristalsis [55,58], and plays a role in regulating gastric motility and pancreatic fluid secretion [57,59]. |
| TRPV5 & TRPV6 | While TRPV5 is positively modulated by Klotho and sialidase [64], both channels are activated by extracellular calcium as well as vitamin D, 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) [60,62,63] and the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), which is essential for TRPV5 opening and enhances TRPV6 function by relieving auto-inhibition [65,66]. | Predominantly expressed in GI epithelial cells [60], specifically on the apical membrane of epithelial cells in the small intestine, particularly in the duodenum and jejunum [60]. | These channels are crucial for maintaining calcium homeostasis [60], being highly selective for calcium ions and enabling calcium influx [60]. They are instrumental in maintaining systemic calcium balance [67,68]. TRPV6 also participates in the absorption of magnesium within the intestine [67,68] and influences cell proliferation, differentiation, and apoptosis via cellular signaling pathways [67,68]. |
5. Potential Role of TRPV Channels in the Pathophysiology of Different GIT Disorders
TRPV channels have been implicated in a multitude of GI pathologies, spanning from pain and dysmotility to secretion abnormalities, inflammation, and ulcer formation. Their diverse roles in modulating sensory perception, cellular signaling, secretion, motility, and immune responses within the GIT underscore their potential role as therapeutic targets for developing novel interventions aimed at improving overall GI health. Targeting TRPV channels has been shown to alleviate GI symptoms, improve motility and secretion, reduce inflammation, and modulate ulcer formation and healing.
5.1. Mediation of Gut Pain
Gut pain is initiated primarily when noxious chemical, mechanical, or inflammatory stimuli activate sensory afferents innervating the intestinal wall. Excitable cells, such as DRG and intrinsic enteric neurons, transduce these stimuli into electrical signals, while non-excitable cells, including epithelial cells, enteroendocrine cells, and immune cells, contribute by releasing mediators such as ATP, histamine, serotonin [69,70,71]. TRPV channels, widespread in the GIT, influence various physiological and sensory functions as previously elucidated [72]. In particular, TRPV1 and TRPV4 are key players in GI nociception [73] (Figure 3). Henceforth, the following sections aim to leverage our understanding of their roles that could potentially guide therapeutic strategies for gut-related pain relief.
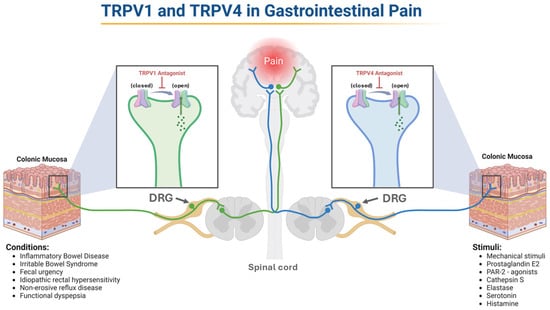
Figure 3.
TRPV1 and TRPV4 in Gut Pain. The increased activation of TRPV1 and TRPV4 expressed by enteric sensory neurons leads to hyperalgesia. Inhibiting these channels offers significant therapeutic potential for managing GI pain. The figure illustrates conditions associated with TRPV1 activation and known stimuli for TRPV4, reflecting differences in how each channel has been characterized in the literature. Abbreviations: TRPV, transient receptor potential vanilloid; PAR-2, proteinase-activated receptor 2; DRG, dorsal root ganglion.
5.1.1. TRPV1 Channel and Its Contribution to Gut Pain
The TRPV1 channel serves as a pivotal player in pain perception and the nociceptive response linked to gut disorders. The core of the relationship between gut inflammation and hyperalgesia lies in the regulation of the TRPV1 channel. In this paradigm, this channel becomes sensitized and upregulated [72]. This was evident in tissue samples from individuals with inflammatory bowel disease (IBD) and rectal hypersensitivity, showing increased TRPV1 expression, correlating with elevated hypersensitivity levels [74]. Moreover, the increase in TRPV1 channel expression is not only limited to conditions marked by overt inflammation; functional gut diseases also demonstrate this surge [73]. For instance, individuals with irritable bowel syndrome (IBS) exhibit elevated TRPV1 levels in the sigmoid colon, correlating with the severity of pain [75]. A parallel narrative emerges in cases of non-active IBD, where continual abdominal pain is associated with an abundance of TRPV1-expressing mucosal nerves [76]. This is similarly seen in cases of fecal urgency, idiopathic rectal hypersensitivity [77], and non-erosive esophageal reflux disease [78]. Furthermore, individuals grappling with functional dyspepsia [79] and diarrhea-dominant IBS [80] exhibit increased pain sensitivity when TRPV1 is activated with capsaicin. These findings suggest that the inhibition and antagonism of the TRPV1 channel offer substantial potential as a therapeutic strategy for mitigating or completely alleviating pain in both inflammatory and functional gut disorders.
5.1.2. TRPV4 Channel and Its Contribution to Gut Pain
The TRPV4 channel plays a crucial role in mediating mechanical hyperalgesia in various GI disorders. It has been demonstrated that activators of the TRPV4 channel intensify the response of sensory neurons within the colon of mice as well as increase mechanosensitivity in mesenteric and serosal sensory nerves [81]. Conversely, in TRPV4-deficient mice, there is a significant reduction in mechanosensitivity in these sensory nerves, along with diminished behavioral responses to nociceptive distention of the colon [82]. Henceforth, the increased mechanical responses to colorectal distension suggest that the activation of the TRPV4 channel contributes to a heightened responsiveness of the sensory nerves [83]. This link to hyperalgesia triggered by mechanical forces emphasizes the essential role of the TRPV4 channel as a primary detector of mechanical stimuli in the context of nociception.
Moreover, the TRPV4 channel plays a role in transmitting pain induced by inflammation, acting as secondary transducers for heightened sensitivity to mechanical stimuli. Sensitization of TRPV4-expressing sensory neurons is triggered by inflammatory mediators such as prostaglandin E2 (PGE2), which activates Proteinase-Activated Receptor 2 (PAR-2). In the context of inflammation, particularly in allergy-induced inflammation, PAR-2 serves as a crucial integrator of signaling cascades, contributing to an increased perception of pain in the presence of hypotonic, mildly hypertonic, or mechanical stimuli [84]. Notably, the TRPV4 channel coexists with PAR-2 in rat DRG neurons. The administration of PAR-2 agonists elicits mechanically induced hyperalgesia in mice through a TRPV4-dependent mechanism [85]. Additionally, PAR-2 agonism enhances TRPV4 channel-mediated sensory nerve responses in the colon [81], where even sub-nociceptive doses of PAR-2 agonists sensitize colonic sensory nerve fibers to TRPV4 agonists [83]. Further studies demonstrate that activating PAR-2 through cathepsin S [86] or elastase [87] leads to TRPV4 activation, sensitizing nociceptors and enhancing sensitivity to mechanical stimuli during inflammation. Moreover, substances like serotonin and histamine can influence TRPV4 expression in murine colonic DRG neurons, intensifying mechanically induced hyperalgesia due to TRPV4 agonism [88]. Furthermore, TRPV4 regulates pain signaling indirectly through ATP release. TRPV4 activation triggers calcium influx, which promotes ATP release from gastric epithelial cells via vesicular nucleotide transporter (VNUT). This ATP then acts on purinergic receptors on sensory neurons, amplifying nociceptive signaling and contributing to visceral hypersensitivity and inflammatory pain. Thus, the inhibition of TRPV4 or VNUT significantly reduces ATP release and mucosal inflammation [56]. Considering all this information, it is of no doubt that TRPV4 plays a significant role in the pathophysiology of inflammation, hyperalgesia, and pain. Therefore, it emerges as a promising and innovative therapeutic target for individuals experiencing gut pain.
5.2. Facilitation of Gut Motility and Regulation of Secretions
TRPV channels, notably TRPV1, TRPV2 and TRPV4, regulate gut motility (Figure 4) and secretions [73]. Therefore, understanding their roles offers therapeutic potential for these channels to be targeted in the context of managing gut conditions related to motility and fluid secretion.
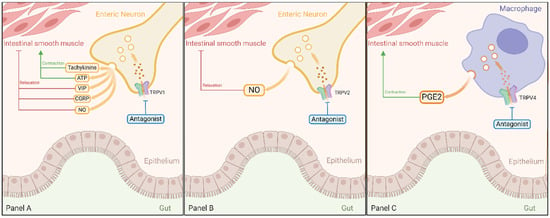
Figure 4.
Roles of TRPV1, TRPV2, and TRPV4 Channels in Gut Motility. TRPV1 channel influences gut motor activity by releasing signaling molecules like tachykinins, ATP, VIP, CGRP, and NO upon activation (Panel A). TRPV2 channel promotes intestinal relaxation and enhances peristaltic movements through NO synthesis (Panel B). TRPV4 channel promotes colonic contractions via PGE2 release from tunica muscularis macrophages (Panel C). Antagonizing these TRPV channels presents therapeutic opportunities for managing GI motility disorders. Abbreviations: TRPV, transient receptor potential vanilloid; ATP, adenosine triphosphate; VIP, vasoactive intestinal peptide, CGRP, calcitonin gene-related peptide; NO, nitric oxide; PGE2, prostaglandin E2.
5.2.1. Role of TRPV1 Channel in the Motor Activity and Secretory Processes of the Gut
The TRPV1 channel plays a crucial role in regulating gut motor activity (Figure 4 Panel A). When activated by stimuli such as capsaicin, this channel initiates the release of signaling molecules, influencing various gut components like esophageal striated muscles, smooth muscles, nervous system, and epithelial cells. This modulation, in turn, affects secretion and motility processes [89]. In terms of motility, TRPV1 activation results in the release of motor stimulants like tachykinins and ATP, or inhibitors such as vasoactive intestinal peptide (VIP), CGRP, and NO [25,90,91]. Moreover, in the mouse esophagus, capsaicin has been demonstrated to produce a significant inhibitory effect on vagal contractions in esophageal preparations with an intact mucosa, while no such effect is observed in preparations lacking mucosa. The TRPV1 channels have been identified as the key mediators of this inhibitory action of capsaicin on vagally induced striated muscle contractions, primarily through mucosal primary afferents. These afferents, located within the mucosa and containing TRPV1-positive fibers, subsequently activate an inhibitory local reflex arc [90]. Indeed, it was demonstrated that the relaxing effect of capsaicin on the intestinal circular muscles of the distal colon, achieved by stimulating NO release, is markedly diminished in mice lacking the TRPV1 channel in comparison to their wildtype controls [92]. Under pathological conditions, the TRPV1 channel demonstrates critical importance in the modulation of GI motility, with its activation on afferent nerve fibers resulting in the initiation of sympathetic reflex pathways. This mechanistic response has been documented in clinical scenarios such as peritoneal irritation and laparotomy-induced impairment of GI transit [93]. This notion is further supported by observations indicating that TRPV1 blockers can alleviate experimental colitis-associated gastroparesis [94]. Additionally, the TRPV1 channel plays a regulatory role in secretory processes throughout the alimentary canal, influencing both neurons and epithelial cells. This includes their involvement in saliva secretion, esophageal platelet-activating factor production, release of somatostatin and gastrin, and stimulation of prosecretory effects in the colon [95,96,97,98]. Collectively, these examples highlight TRPV1 channel essential role in digestive system motility and secretory processes.
5.2.2. Role of TRPV2 Channel in Motor Activity of the Gut
The TRPV2 channel plays a vital role in gut motility, influencing both intestinal relaxation and peristaltic movement to enhance gut transit speed. These motile changes are attributed to TRPV2-mediated release of NO from the inhibitory motor neurons, potentially involving intrinsic sensory neurons as well (Figure 4 Panel B). Supporting this, TRPV2 activation in isolated intestinal tissue inhibited spontaneous circular muscle contractions in a manner dependent on NO and neuronal activity, and these responses were blocked by the TRPV2 antagonist tranilast [36]. This underscores the crucial involvement of TRPV2 channel activation in inducing intestinal relaxation, peristaltic movements, and enhanced transit speed via NO synthesis. Taken together, this growing body of evidence firmly establishes the essential role of TRPV2 in modulating gastrointestinal motility through NO-dependent regulatory pathways, positioning TRPV2 channels as promising therapeutic targets whose activation may offer novel solutions for managing GI motility disorders.
5.2.3. Role of TRPV4 Channel in Motor Activity and Secretory Processes of the Gut
The TRPV4 channel plays a pivotal role in the secretory and mechanical functions of the gut, with their influence evident in various physiological processes (Figure 4 Panel C). In the hepatobiliary secretory system, both rat intrahepatic bile duct units and murine cholangiocytes exhibit heightened intracellular calcium levels under hypotonic conditions, a response that is magnified by TRPV4 agonists and attenuated with reduced TRPV4 expression [99]. Furthermore, the activation of TRPV4 in cholangiocytes induces bicarbonate secretion essential for bile synthesis. This is underscored by TRPV4 agonists enhancing bile flow and luminal secretion in rats, an effect counteracted by TRPV4 blockade [99]. Additionally, observations in a rat model of polycystic kidney disease reveal a correlation between cholangiocyte hyperproliferation and excessive, mislocalized TRPV4 expression. TRPV4 agonists effectively mitigate this proliferation and cyst development [100].
Moreover, the importance of the TRPV4 channel in gut motility is closely tied to its interaction with intestinal muscularis macrophages (MMs). These TRPV4-expressing MMs establish connections with smooth muscle cells (SMCs) independent of neuronal input. Essentially, MMs release PGE2, initiating contractions in the colon that occur outside the influence of the enteric nervous system. Accordingly, under conditions of excessive gut motility, the removal or inhibition of TRPV4 from intestinal MMs results in diminished intestinal motility [101]. These findings open potential avenues for therapeutic interventions targeting TRPV4, particularly in the context of gut disorders associated with hypermotility.
5.3. Role of TRPV Channels in Gut Inflammation
TRPV channels, especially TRPV1, TRPV2, TRPV4, and TRPV6, play crucial roles in modulating gut inflammation, suggesting innovative therapeutic possibilities for various gut inflammatory disorders. Consequently, this section explores these inflammatory mechanisms governed by TRPV channels.
5.3.1. Role of TRPV1 Channel in Gut Inflammation
The activation of the TRPV1 channel has been closely implicated in heightened inflammation observed in conditions such as pancreatitis, colitis, and ileitis [73]. The impact extends to esophageal health, where capsaicin and acid activation of TRPV1 have been linked to the development of esophagitis [96]. Notably, ethanol-induced damage to the stomach mucosa in murine models is attributed to TRPV1-mediated release of neuronal substance P [102]. In this context, substance P can modulate various physiological processes (Figure 5), including inflammation and pain perception, suggesting that its release in the stomach mucosa may be part of the response to ethanol exposure, potentially exacerbating the damage to the stomach lining. This is supported by an experiment conducted on mice with colitis, demonstrating that TRPV1 antagonism or genetic knockout contributes to the mitigation of inflammation, offering a promising avenue for therapeutic intervention [73]. The influence of TRPV1 is not confined to the gut alone, as it is implicated in pancreatic inflammation associated with types I and II diabetes, and obesity. Furthermore, TRPV1 exerts its impact on CD4+ T cells, influencing their activation and pro-inflammatory characteristics. Modulation of TRPV1 leads to reduced IL-2 production, coupled with elevated levels of IL-17 and IFN-γ production, all of which are pro-inflammatory mediators [103]. Consequently, the inhibition of TRPV1 emerges as a prospective and strategic therapeutic approach for mitigating inflammation within the GIT, particularly those of an autoimmune nature.
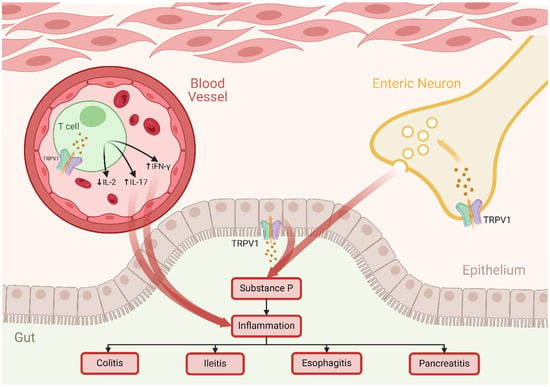
Figure 5.
The Role of TRPV1 Channel in Gut Inflammation. Activation of TRPV1 channels on enteric neurons and colonic epithelial cells triggers the release of the pro-inflammatory substance P. Additionally, TRPV1 activation in T cells leads to increased release of cytokines IL-17 and IFN-γ and a decrease in IL-2. These inflammatory responses are implicated in GI conditions such as esophagitis, ileitis, colitis, and pancreatitis. Abbreviations: TRPV, transient receptor potential vanilloid; IL-2, interleukin 2; IL-17, interleukin 17; IFN-γ, interferon-gamma.
5.3.2. Role of TRPV2 Channel in Gut Inflammation
In the gut, the expression of the TRPV2 channel extends to the enteric neurons, SMCs, and immune cells, suggesting a possible role in modulating inflammation [104]. The role of TRPV2 in sensory neurons remains somewhat ambiguous; however, it was recently proven that there is an increase in TRPV2 expression in the dorsal root ganglia, needed for the transmission of sensory information. This implies a potential involvement in sensitization during inflammation, particularly in transmitting pain in response to noxious temperatures [105]. Moreover, in a study involving mice with colitis, it was found that mice lacking TRPV2 displayed a milder form of colitis compared to their wild-type counterparts. This observation could be attributed to a potential decrease in macrophage recruitment, pointing to the involvement of TRPV2 in the onset of colitis. Despite these findings, the precise mechanisms driving this effect remain to be fully understood [106].
5.3.3. Role of TRPV4 Channel in Gut Inflammation
Recent investigations spotlight the pro-inflammatory role of TRPV4 within the colonic mucosa. Specifically, the activation of TRPV4 by the agonist 4-α-phorbol 12, 13-di-decanoate (4αPDD) instigates an elevation in intracellular calcium levels, prompting the release of chemokines from intestinal epithelial cells. This agonist further increases paracellular permeability, leading to colitis when applied to murine colons [107]. Moreover, augmented TRPV4 expression manifests in mice with dextran sulfate sodium (DSS)-induced colitis and in the blood vessels of actively inflamed colonic tissues, as compared to their healthy controls [82]. There is also considerable promise in the observation that patients with IBD exhibited infiltration of TRPV4-positive and CD45-positive white blood cells in their colonic mucosa [107]. This indicates a potential involvement of the TRPV4 channel in recruiting or activating immune cells, thereby contributing to the inflammatory processes observed in IBD. In both mouse and human colon, TRPV4 localizes to epithelial cells and certain unidentified cells within the submucosal and muscular layers. TRPV4 agonists can increase intracellular calcium concentrations and promote chemokine release in human colon cancer cell lines and induce colitis in mice [56]. Having said this, numerous research initiatives focusing on the TRPV4 channel blockers have been initiated to explore the therapeutic potential of targeting TRPV4 in the context of gut inflammation [108].
5.3.4. Role of TRPV6 Channel in Gut Inflammation
Although not extensively explored, the TRPV6 channel has emerged as a significant player in alcohol-induced gut inflammation and barrier dysfunction. Experiments involving Caco-2 cells, a line of colorectal adenocarcinoma cells, and mice intestinal organoids demonstrate that inhibiting TRPV6 mitigates the effects of ethanol and acetaldehyde, including calcium influx and barrier dysfunction [109]. Remarkably, mice lacking TRPV6 display resistance to alcohol-induced intestinal issues, underscoring the critical role of TRPV6 in this context. These findings emphasize the potential of targeting TRPV6 as prospective treatments for inflammatory gut disorders triggered by alcohol.
5.4. Role of TRPV Channels in Ulcer Formation and Healing
Ulcer formation and healing are dynamic and complex processes that involve the interplay of multiple cellular signaling pathways. These pathways are crucial for responding to injury, inflammation, and tissue repair. Emerging evidence underscores the significant involvement of TRPV channels in both the formation and healing of ulcers in the GIT. By influencing epithelial cell proliferation, migration, and mucosal repair mechanisms, these channels present promising therapeutic targets for improving recovery from ulcers and reducing their recurrence.
5.4.1. TRPV Channels and Ulcer Formation
Ulcers typically result from an imbalance between protective factors, such as the mucosal barrier and blood flow, and damaging factors, such as gastric acid, mechanical injury, and inflammation. In this context, TRPV channels, particularly TRPV1, TRPV2, and TRPV4, play critical roles in sensing noxious stimuli and triggering inflammatory responses that contribute to ulcer formation.
TRPV1 responds to gastric acid and mechanical damage in sensory neurons and epithelial cells in the GIT. It contributes to the protection of the GIT by enhancing mucosal blood flow and strengthening the epithelial barrier through the release of substance P and CGRP [96]. However, sustained or excessive TRPV1 activation can exacerbate inflammation and sensitivity, contributing to mucosal injury. This occurs through mechanisms including enhanced vasodilation and increased vascular permeability, resulting in the recruitment of immune cells to the affected area [110,111]. Additionally, the neuropeptides released stimulate pro-inflammatory cytokines, such as IL-1β and TNF-α, further amplifying inflammation and worsening mucosal damage [112]. This dysregulation is particularly evident in conditions of chronic stress such as Helicobacter pylori infection, acid reflux, and peptic ulcers, where heightened inflammatory responses exacerbate mucosal damage.
TRPV2 plays a multifaceted role in the GIT, extending beyond inflammatory pathways to include responses to mechanical stretch and thermal stimuli. This makes it a key player in ulcer formation, particularly under conditions of acid-induced injury or physical stress. TRPV2 channels are expressed in various GIT cell types, including epithelial cells, where their activation promotes the release of pro-inflammatory mediators and enhances cellular responses to mechanical stress [113]. By facilitating calcium influx, TRPV2 activates downstream signaling cascades involved in inflammation, contributing to tissue damage and disruption of mucosal integrity [114]. Although TRPV2 has not been as extensively studied as TRPV1, its capacity to sense mechanical stress and regulate inflammatory processes underscores its potential role in the early phases of ulcer pathogenesis.
TRPV4 has been implicated in the response to both mechanical stress and inflammatory mediators, playing a critical role in regulating inflammation in ulcerative conditions. Its activation stimulates PGE2 release from macrophages, thereby increasing mucosal permeability and contributing to tissue damage [101]. In esophageal epithelial cells, TRPV4 further exacerbates inflammation by enhancing calcium influx, which triggers the release of IL-1β and TNF-α, leading to greater tissue injury [115]. This mechanism is particularly relevant in conditions like gastroesophageal reflux disease (GERD) and peptic ulcers. Conversely, the inhibition of TRPV4 has been shown to attenuate inflammation, protect against mucosal injury and promote healing, highlighting its potential as a therapeutic target for ulcer-related disorders [55].
5.4.2. Role of TRPV Channels in Ulcer Healing
TRPV channels play multifaceted roles in wound healing within the GIT by modulating inflammation, cellular migration, and tissue regeneration. Among these, TRPV4 plays a dual and complex role in epithelial repair processes. Its activation in epithelial cells enhances intracellular calcium signaling, driving cytoskeletal reorganization and promoting cell migration across wounded areas [116]. TRPV4 activation in esophageal keratinocytes stimulates both proliferation and migration by upregulating key proteins involved in cell adhesion and movement, such as E-cadherin and integrins [117]. Additionally, calcium influx through TRPV4 channels supports the activation of crucial signaling pathways, including extracellular signal–regulated kinases 1 and 2 (ERK1/2) and phosphoinositide 3-kinase/protein kinase B (Akt) (PI3K/Akt), which are essential for cell survival and proliferation [116]. Paradoxically, TRPV4 can also act as a negative regulator of wound healing. Murine models have demonstrated that its deletion in esophageal keratinocytes significantly accelerates tissue repair by enhancing proliferation and speeding migration to the wound site [59]. This inhibitory role aligns with findings suggesting that TRPV4 modulates responses to mechanical stress and regulates tissue regeneration, potentially limiting effective epithelial repair [118].
Conversely, TRPV3 promotes epithelial repair by facilitating calcium influx and activating signaling pathways involved in cytokine release, cell proliferation, and migration. Expressed in both skin and GI tissues, TRPV3 activation enhances keratinocyte migration and accelerates wound closure, suggesting a similar role in GIT ulcer healing by promoting mucosal regeneration [119,120]. Similarly, TRPV2 supports wound healing by promoting keratinocyte migration, as demonstrated in skin models, indicating analogous function in GI ulcer repair [121]. However, its role in inflammatory processes suggests that excessive activation may prolong inflammation and impede healing, emphasizing the need for precise modulation.
Additionally, TRPV1 contributes to ulcer healing by promoting angiogenesis. Its activation on endothelial cells triggers the release of vasodilatory factors like CGRP and NO, enhancing blood flow to damaged tissues [122,123]. This vascularization is essential for delivering nutrients and immune cells necessary for effective repair, underscoring the therapeutic potential of targeting TRPV channels to optimize both inflammation and tissue regeneration.
5.4.3. Implications for Therapeutic Targeting of TRPV Channels in Ulcerative Diseases
The involvement of TRPV channels in both inflammation and ulcer healing positions them as promising targets for therapeutic intervention in ulcerative diseases. These channels contribute to the initiation of inflammatory processes that can worsen ulcer formation, while also playing key roles in epithelial regeneration and tissue repair. Targeting these pathways offers the potential to both prevent ulcer development and promote faster healing, making them valuable in managing conditions such as peptic ulcers, inflammatory bowel disease, and GERD.
TRPV1 antagonists have shown promise in reducing ulcer formation in animal models, with compounds that block TRPV1 reducing acid-induced injury and inflammatory responses [124]. Similarly, TRPV4 inhibitors also show promise for promoting ulcer healing, as studies on TRPV4 knockout mice demonstrated accelerated wound repair. Blocking TRPV4 activity may facilitate faster recovery of epithelial lesions, particularly in chronic ulcers where persistent inflammation frequently hinders the healing process [59]. Additionally, TRPV3 modulators could be useful in promoting ulcer healing by enhancing cell migration and proliferation, essential steps in mucosal repair. However, the challenge lies in balancing the activation of TRPV3, as its excessive activation could lead to uncontrolled proliferation or sustained inflammation. As such, the therapeutic application of TRPV3-targeting drugs would require a nuanced approach to prevent adverse effects while promoting healing. Lastly, the therapeutic targeting of TRPV2 remains an area for further exploration, especially in the context of mechanotransduction and wound healing. As TRPV2 is involved in both inflammation and cell migration, its modulation could offer benefits in managing both the inflammatory and repair phases of ulcerative diseases.
6. Role of TRPV Channels in GIT Tumors
TRPV channels play a pronounced role in digestive system tumors, of which oral, esophageal, gastric, intestinal and colorectal tumors are studied [125]. These channels are implicated in the uncontrolled proliferation, increased invasion, migration, and immortality of such tumors [126]. The contribution of TRPV channels to cancer progression is primarily mediated through the dysregulation of intracellular Ca2+ levels, affecting cellular processes, including cell cycle progression and apoptosis. Alterations in TRPV channel activity in these malignancies are typically attributed to changes in TRPV mRNA and protein expression levels, whether increased or decreased, rather than mutations in TRP genes [127].
6.1. Oral Cavity Cancer
TRPV1 expression is notably heightened in both pre-malignant leukoplakia and tongue squamous cell carcinoma (SCC) [128]. Interestingly, while capsaicin demonstrates cytotoxic effects on oral SCC cells, this mechanism operates independently of TRPV1’s typical Ca2+ conductivity pathway, as evidenced by the absence of intracellular Ca2+ elevation upon stimulation [129]. Instead, capsaicin-induced cytotoxicity has been associated with the generation of reactive oxygen species (ROS), mitochondrial dysfunction, and the activation of apoptotic signaling pathways, leading to cell death [130]. A study conducted in 2016 reported that patients with a history of smoking and alcohol consumption exhibited increased expression levels of TRPV1–4 mRNA. Moreover, elevated protein levels of TRPV1–4 have been observed in various regions of the oral cavity affected by SCC, including the tongue, gingiva, oral floor, and buccal mucosa, compared to normal tissue [131]. Among these, TRPV1 and TRPV3 are expressed at higher levels than TRPV2 and TRPV4 are. Activation of TRPV1 by its agonists, such as capsaicin, can induce cancer cell apoptosis via the generation of ROS. Conversely, TRPV1 activation may also stimulate cancer cell migration and invasiveness in certain contexts [131]. For instance, in hepatoblastoma, TRPV1 activation induced by hepatocyte growth factor (HGF) leads to an influx of Ca2+ into hepatoblastoma cells following capsaicin treatment, which enhances cell migration [132]. Similarly, TRPV4 in SCC has been implicated in promoting cancer cell proliferation and migration through activation of the calcium/calmodulin-dependent protein kinase II (CaMKII)-Akt signaling pathway [133].
6.2. Esophageal Cancer
Dysregulation of TRPV1, TRPV2, and TRPV4 has been observed in esophageal squamous cell carcinoma (ESCC), with these channels being overexpressed at both mRNA and protein levels compared to normal esophageal tissues. Hyperactive TRPV1 and TRPV4 have been shown to promote ESCC cell proliferation and migration [134]. Specifically, TRPV1 activation by capsaicin or heat exposure significantly increased both proliferation and migration, while these effects were effectively suppressed by the TRPV1 antagonist AMG9810, highlighting its therapeutic potential. Similar inhibitory effects have also been observed with other TRPV1 antagonists such as BCTC, which reduces capsaicin-induced responses and suppresses TRPV1-mediated cell signaling [135], and SB705498, a compound that potently blocks TRPV1 activation by capsaicin, heat, and protons, showing promise in both preclinical and clinical settings [136,137]. Moreover, TRPV4 activation enhanced cell migration but had no significant effect on proliferation; this pro-migratory effect was suppressed by the inhibitor ruthenium red [134]. In contrast, TRPV2 overexpression correlates with poor prognosis and increased tumor aggression [138,139]. Inhibiting TRPV2 in ESCC cells reduces proliferation, migration, and invasion, emphasizing its pivotal role in cancer progression [140,141].
6.3. Gastric Cancer
TRPV1 expression is markedly downregulated in primary gastric cancer (GC) tissues compared to adjacent normal tissues. This downregulation is correlated with larger tumor size, higher histological grade, advanced clinical stage, and poor survival in GC patients. TRPV1 inhibits GC development through the or calcium/calmodulin-dependent protein kinase kinase β/AMP-activated protein kinase (CaMKKβ/AMPK) pathway, highlighting its upregulation as a promising target for GC prevention and treatment [142]. Furthermore, elevated TRPV2 expression in GC patients has been identified as a prognostic biomarker and possible therapeutic target [143]. Additionally, research has shown that the calcium-sensing receptor (CaSR) and the TRPV4 channel are co-expressed in GC cells; CaSR activation induces TRPV4-mediated Ca2+ entry, which promotes GC cell proliferation, migration, and invasion [144].
6.4. Intestinal Adenoma
While TRPV channels are involved in many malignant tumors, their role in benign tumors remains obscure. A study published in 2014 highlighted that TRPV1 deficiency promotes the formation of intestinal adenomas by impairing the regulatory interaction between TRPV1 and protein tyrosine phosphatase 1B. Under normal conditions, TRPV1 activation enhances this enzyme’s ability to suppress epidermal growth factor receptor (EGFR) signaling through the activation of Ca2+/calpain, thereby controlling epithelial cell proliferation in the intestinal lining. However, in the absence of TRPV1, this regulatory pathway is disrupted, leading to unchecked EGFR activation, excessive cell proliferation, and the development of adenomas [145]. Of the oncogenic pathways associated with TRPV1-EGFR signaling, signal transducer and activator of transcription 3 (STAT3), ERK1/2, and PI3K stand out. Without appropriate EGFR phosphorylation, oncogenesis is a likely destiny to many epithelial cells [145,146]. Beyond its role in adenoma formation, aberrant EGFR signaling is a well-established driver of tumor progression, contributing to increased proliferation, survival, and resistance to apoptosis in various cancers [147]. Similarly, VEGF promotes angiogenesis, which is critical for tumor growth and metastasis by ensuring adequate blood supply to the expanding tumor mass [148,149]. TRPV1’s role goes beyond intracellular signaling; these channels promote immune regulation by promoting the release of VIP and pituitary adenylate cyclase-activating peptide (PACAP), two important modularity cytokines. TRPV1 knockout mice showed enhanced release of IL-6 and IL-11, on top of the increased activity of the protumorigenic STAT3 and NF-κB signaling pathways [146]. Through our literature review, we witnessed that other TRPV channels have not been exclusively explored in the pathophysiology of intestinal adenoma, highlighting a significant gap in this area.
6.5. Colorectal Cancer
TRPV1 acts as a tumor suppressor in colorectal cancer (CRC), showing lower expression levels in CRC tissues compared to non-cancerous colorectal tissues. Activation of TRPV1 by capsaicin inhibits CRC growth and induces apoptosis via the p53 pathway [150]. Conversely, TRPV4 is upregulated in colon cancer, correlating with poorer prognosis. Its inhibition induces apoptosis and autophagy in colon cancer cells through phosphatase and tensin homolog deleted on chromosome 10 (PTEN) pathway activation. In addition, TRPV6 is overexpressed in CRC, correlating with early-stage cancer and increased invasiveness. Inhibition of TRPV6 expression suppresses proliferation and induces apoptosis in CRC cells [150].
6.6. Neuroendocrine Tumors
NETs represent a diverse group of neoplasms with clinical behavior ranging from benign and low-grade malignant to high-grade malignant forms [151]. Within this spectrum, TRP channels have been shown to play functionally relevant roles, including in insulinomas [152]. The role of TRPV channels is most clearly defined in pancreatic neuroendocrine tumors (PanNETs), where both TRPV1 and TRPV6 have distinct and significant functions. In human PanNET cell lines like BON-1 and QGP-1, TRPV1 activation by agonists such as capsaicin induces a robust calcium influx that directly stimulates the exocytosis of secretory granules, thereby increasing the release of hormones and neuroendocrine markers like chromogranin A. This effect is specifically blocked by TRPV1 antagonists like capsazepine [153]. Furthermore, TRPV1 transcripts and protein are detected in BON and CNDT2.5 carcinoid/NET lines, suggesting the existence of additional Ca2+-coupled growth pathways [125]. Pharmacologically, high-dose capsaicin exerts cytotoxic effects on PanNET cells by inducing mitochondrial dysfunction, loss of membrane potential, and caspase-3 activation leading to apoptosis, although these effects are only partially dependent on TRPV1 itself [154]. In contrast to this secretory role, TRPV6 drives tumor proliferation by maintaining elevated pro-growth calcium signals. Functional studies show that knocking down TRPV6 in BON-1 cells inhibits proliferation and reduces activity of the calcium-dependent transcription factor nuclear factor of activated T-cells (NFAT), confirming its role as a key oncogenic channel in PanNETs [125].
Similarly, the functions of TRPV channels in gastrointestinal carcinoid tumors (e.g., midgut NETs) represent a significant knowledge gap, with no direct studies available. By analogy to the serotonin-secreting BON-1 cell line, it is hypothesized that TRPV1 activation could potentially provoke serotonin release from enterochromaffin-derived carcinoid cells, potentially exacerbating carcinoid syndrome symptoms like flushing and diarrhea [153,155]. Notably, another channel, TRPA1, is highly expressed in enterochromaffin-like QGP-1 cells, where agonists such as allyl-isothiocyanate and cinnamaldehyde induce Ca2+ influx and robust serotonin release; this effect is blocked both by TRPA1 antagonists and siRNA, blocking channel function and knocking down the channel’s expression, respectively [156]. This mechanism is physiologically relevant as native enterochromaffin cells in human and rodent guts also use TRPA1 to trigger 5-HT secretion, reinforcing its role as a chemosensory channel [157]. A TRPV1 or TRPA1 antagonist could therefore theoretically help stabilize hormone release. Furthermore, given that TRPV6 is often upregulated in secretory cancers, a pro-proliferative role analogous to its function in PanNETs is possible, but this has not been investigated.
7. Potential Challenges with Targeting TRPVs for Therapy
The approach of addressing TRPV channels for therapeutic purposes seems to represent a noteworthy advancement in managing disorders within the GIT. However, several obstacles exist that impede the swift realization of its clinical implementation.
By now, it must be appreciated that TRPV channels are active across multiple body systems. This unquestionably needs to be considered when contemplating the feasibility of targeting these channels for therapeutic purposes. An illustrative instance involves the presence of the TRPV4 channel not only within the GIT, but also within blood vessels and the heart, where it contributes to the regulation of vascular tone and blood pressure [158,159]. Consequently, if a therapeutic strategy is designed to antagonize the TRPV4 channel in the GIT, there is a potential for this antagonism to also impact the TRPV4 channel in cardiovascular tissues. As a result, unintended outcomes could arise, including alterations in blood vessel tone, blood pressure regulation, and other functional dysregulations. This could ultimately lead to the development of conditions like hypertension and arrhythmias [158]. This situation highlights the imperative for meticulous delivery methods as well as precision in the development of therapies targeting TRPV channels in an organ-specific approach.
Not only are TRPV channels involved in interconnected signaling pathways, but they also exhibit a polymodal activation characteristic. For instance, TRPV1 exhibits sensitivity to a range of stimuli including capsaicin, extracellular pH, and heat. This responsiveness, in turn, is intricately influenced by the presence and intensity of other stimuli, necessitating meticulous control and consideration of the existing microenvironment [160]. This nuanced interplay underscores the heightened risk and intricate nature of antagonizing TRPV channels; blocking one stimulus can lead to altered channel’s responses to other stimuli, thereby yielding unintended adverse outcomes—a challenge for pharmaceutical companies to tackle. Moreover, given the complexity of the TRPV channels’ involvement, any modification to their engagement with the numerous signaling pathways they participate in carries the potential for disruption. To illustrate, the TRPV4 channel plays a pivotal role in gut function by perceiving mechanical forces and osmotic pressure changes. When the gut experiences mechanical stretching due to factors like food movement or intestinal wall expansion, the TRPV4 channel is activated. This activation prompts the entry of calcium ions into cells, initiating intracellular signaling pathways that influence smooth muscle contractions, mucosal secretion, and overall gut motility [73]. Conversely, the activation of the TRPV4 channel is also linked to the regulation of epithelial barrier integrity, which is critical for maintaining the selective permeability of the intestinal lining [161]. Navigating these complexities presents a challenge when it comes to modulating or targeting TRPV channels. Any such interventions must account for the multifaceted roles within the gut and the potential ramifications for various signaling pathways.
The need for extensive research and efforts to develop agents targeting TRPV channels influenced by gene polymorphism might hinder the creation of successful targeted treatments. For instance, the human TRPV1 gene has been documented to possess six non-synonymous polymorphisms, leading to variations in responses to agonists like capsaicin and anandamide [162]. Some of these variations might heighten sensitivity to particular drugs, while others could lead to diminished responsiveness. Additionally, specific gene polymorphisms could elevate the risk of adverse effects in some patients but not in others. This underscores the necessity of personalized medicine strategies, where tailoring treatments based on an individual’s genetic composition concerning the targeted TRPV variants can enhance therapeutic outcomes. However, this endeavor is not without its challenges. Personalized medicine introduces its own complexities and financial demands, which restrict accessibility for individuals and healthcare systems alike. These factors, in addition to the long development timelines, not only hinder the practical implementation of personalized treatments, but also complicate the creation of the necessary drugs.
Research has revealed that when TRPV channels are blocked or manipulated, the body responds by attempting to regain its normal function. This was evident in a clinical trial involving AMG 517, a specific TRPV1 antagonist administrated to mitigate pain. Notably, since this antagonist does not hinder heat-induced receptor activation, the suppression of TRPV1 led to hyperthermia in humans, a pattern similarly observed in rats, dogs, and monkeys [163]. This notion finds support in a separate study using SB-705498, a compound that antagonizes all modalities of TRPV1 activation, where no hyperthermia was observed [164]. This compelling evidence could potentially indicate how compensatory mechanisms played a role in causing hyperthermia in the first study, in contrast to the second one. Consequently, another concern arises, pertaining to the gradual decline in cells’ sensitivity to TRPV-targeted drugs over time. This development of tolerance and desensitization could lead to a diminished therapeutic response, potentially necessitating elevated drug doses for comparable effects or causing a decline in treatment effectiveness. Consequently, this poses a challenge for pharmaceutical companies to create enduring and safe drugs. Furthermore, compensatory responses can differ among individuals, reigniting the concerns associated with personalized medicine discussed earlier.
Finally, emerging evidence in the field of TRPV channel research brings to light the intricacies of these channels’ functions, posing both opportunities and challenges for therapeutic interventions. As our understanding of TRPV channels’ roles continues to evolve, the potential for unexpected interactions and off-target effects becomes a significant challenge. These channels are found in various tissues throughout the body, and as new roles are uncovered, the risk of unintended consequences when targeting them for therapy increases. Emerging research might reveal that a TRPV channel assumes a role not solely within the intended therapeutic pathway but also in other unforeseen systems, leading to unpredicted outcomes. This underscores the importance of comprehensive exploration, cautious therapeutic design, and constant adaptation as we navigate the evolving landscape of TRPV channel functions in the pursuit of effective and safe treatments.
8. Conclusions
In conclusion, TRPV channels, being extensively distributed throughout the GIT, assume crucial roles in its function and are thus subject to tight regulation. TRPV channels are integral in governing both normal physiological functions and pathological conditions. In normal body physiology, they modulate gut motility, secretions, and nutrient absorption. Conversely, in pathological conditions, they contribute to gut pain sensation and inflammation. TRPV channels are also involved in key cellular processes, such as proliferation and migration, which are critical in both ulcer formation and mucosal healing. Hence, their involvement underscores the therapeutic potential of targeting TRPV channels for treating GI disorders. Moreover, TRPV channels have been associated with various GI tumors, presenting potential breakthrough targets for innovative cancer therapies. Nonetheless, several challenges hinder the clinical application of TRPV-targeted therapies. These include potential unintended systemic effects due to their widespread distribution, altered responses to different stimuli, loss of essential physiological roles, compensatory mechanisms following channel antagonism, and the necessity of a personalized approach to therapy. Despite significant progress, important gaps remain. Future studies should aim to clarify the cell-type-specific and context-dependent roles of TRPV channels, better delineate the systemic consequences of TRPV modulation, and develop more selective modulators and targeted delivery systems. It will also be important to account for patient-specific factors that may influence therapeutic responses. Addressing these gaps will be critical to fully harnessing the therapeutic potential of TRPV channels and translating experimental insights into effective, safe, and personalized interventions for gastrointestinal disease.
Author Contributions
Conceptualization, O.T., A.A. (Ahmed Arabi), H.E.R., L.A., F.A.Z., M.H., A.A. (Ayeda Abuqaba) and A.B.; methodology, O.T., A.A. (Ahmed Arabi), H.E.R., L.A. and A.B.; validation, O.T., A.A. (Ahmed Arabi), H.E.R., L.A. and A.B.; writing—original draft preparation, O.T., A.A. (Ahmed Arabi), H.E.R., L.A., F.A.Z., M.H. and A.A. (Ayeda Abuqaba); writing—review and editing, O.T., A.A. (Ahmed Arabi), H.E.R., L.A. and A.B.; visualization, O.T. and H.E.R.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Ammar Boudaka was supported by a Qatar University Student Grant, “QUST-1-CMED-2025-262”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-AG | 2-Arachidonoylglycerol |
| 2-APB | 2-Aminoethoxydiphenyl Borate |
| 12-HETE | 12-Hydroxyeicosatetraenoic Acid |
| Akt | Protein Kinase B |
| AMPK | AMP-activated protein kinase |
| ARD | Ankyrin Repeat Domain |
| ATP | Adenosine Triphosphate |
| Ca2+ | Calcium Ion |
| CaMKII | Calcium/Calmodulin-Dependent Protein Kinase II |
| CaSR | Calcium-Sensing Receptor |
| CBD | Cannabidiol |
| CGRP | Calcitonin Gene-Related Peptide |
| CRC | Colorectal Cancer |
| DAG | Diacylglycerol |
| DRG | Dorsal Root Ganglia |
| DSS | Dextran Sulfate Sodium |
| EGFR | Epidermal Growth Factor Receptor |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| ESCC | Esophageal Squamous Cell Carcinoma |
| GALT | Gut-Associated Lymphoid Tissue |
| GC | Gastric Cancer |
| GERD | Gastroesophageal Reflux Disease |
| GIT | Gastrointestinal Tract |
| GPCR | G Protein-Coupled Receptors |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable Bowel Syndrome |
| IFN-γ | Interferon Gamma |
| IL-11 | Interleukin-11 |
| IL-17 | Interleukin-17 |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IP3 | Inositol 1,4,5-Trisphosphate |
| LPC | Lysophosphatidylcholine |
| MMs | Muscularis Macrophages |
| NFAT | Nuclear Factor of Activated T-cells |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NH4+ | Ammonium ion |
| NO | Nitric Oxide |
| OLDA | Oleoylethanolamide |
| PACAP | Pituitary Adenylate Cyclase-Activating Peptide |
| PanNETs | Pancreatic Neuroendocrine Tumors |
| PAR-2 | Proteinase-Activated Receptor 2 |
| PH | Pore Helix |
| PI3K | Phosphoinositide 3-Kinase |
| PIP2 | Phosphatidylinositol 4,5-Bisphosphate |
| PKC | Protein Kinase C |
| PLC | Phospholipase C |
| PTEN | Phosphatase and Tensin Homolog |
| PGE2 | Prostaglandin E2 |
| ROS | Reactive Oxygen Species |
| SCC | Squamous Cell Carcinoma |
| SMCs | Smooth Muscle Cells |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TRP | Transient Receptor Potential |
| TRPA | Transient Receptor Potential Ankyrin |
| TRPC | Transient Receptor Potential Canonical |
| TRPM | Transient Receptor Potential Melastatin |
| TRPML | Transient Receptor Potential Mucolipin |
| TRPN | Transient Receptor Potential No-Mechano-Potential |
| TRPP | Transient Receptor Potential Polycystin |
| TRPV | Transient Receptor Potential Vanilloid |
| VIP | Vasoactive Intestinal Peptide |
| VNUT | Vesicular Nucleotide Transporter |
| VSLD | Voltage-Sensing-Like Domain |
References
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Boudaka, A.; Al-Suleimani, M.; Al-Lawati, I.; Baomar, H.; Al-Siyabi, S.; Zadjali, F. Downregulation of endothelial transient receptor potential vanilloid type 4 channel underlines impaired endothelial nitric oxide-mediated relaxation in the mesenteric arteries of hypertensive rats. Physiol. Res. 2019, 68, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Boudaka, A.; Al-Yazeedi, M.; Al-Lawati, I. Role of Transient Receptor Potential Vanilloid 4 Channel in Skin Physiology and Pathology. Sultan Qaboos Univ. Med. J. 2020, 20, e138–e146. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Seebohm, G.; Schreiber, J.A. Beyond Hot and Spicy: TRPV Channels and their Pharmacological Modulation. Cell Physiol. Biochem. 2021, 55, 108–130. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Fluck, E.C., 3rd; Ahmed, T.; Moiseenkova-Bell, V.Y. Structural insights into the gating mechanisms of TRPV channels. Cell Calcium 2020, 87, 102168. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of Inflammatory Bowel Diseases: A Population Study in a Healthcare District of North-West Italy. J. Clin. Med. 2023, 12, 641. [Google Scholar] [CrossRef]
- Gusenbauer, M. Beyond Google Scholar, Scopus, and Web of Science: An evaluation of the backward and forward citation coverage of 59 databases’ citation indices. Res. Synth. Methods 2024, 15, 802–817. [Google Scholar] [CrossRef]
- Hellmich, U.A.; Gaudet, R. Structural biology of TRP channels. Handb. Exp. Pharmacol. 2014, 223, 963–990. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Dosey, T.L.; Wang, Z.; Fan, G.; Zhang, Z.; Serysheva, I.I.; Chiu, W.; Wensel, T.G. Structures of TRPV2 in distinct conformations provide insight into role of the pore turret. Nat. Struct. Mol. Biol. 2019, 26, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.B.; Wang, R.R.; Choo, S.S.; Gaudet, R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J. Biol. Chem. 2010, 285, 731–740. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Islas, L.D. Molecular Physiology of TRPV Channels: Controversies and Future Challenges. Annu. Rev. Physiol. 2023, 85, 293–316. [Google Scholar] [CrossRef]
- Veldhuis, N.A.; Poole, D.P.; Grace, M.; McIntyre, P.; Bunnett, N.W. The G protein-coupled receptor-transient receptor potential channel axis: Molecular insights for targeting disorders of sensation and inflammation. Pharmacol. Rev. 2015, 67, 36–73. [Google Scholar] [CrossRef]
- Adapala, R.K.; Katari, V.; Teegala, L.R.; Thodeti, S.; Paruchuri, S.; Thodeti, C.K. TRPV4 mechanotransduction in fibrosis. Cells 2021, 10, 3053. [Google Scholar] [CrossRef]
- Holzer, P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br. J. Pharmacol. 2008, 155, 1145–1162. [Google Scholar] [CrossRef]
- Ciura, S.; Liedtke, W.; Bourque, C.W. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: A mechanical process involving TRPV1 but not TRPV4. J. Neurosci. 2011, 31, 14669–14676. [Google Scholar] [CrossRef] [PubMed]
- Laleh, P.; Yaser, K.; Alireza, O. Oleoylethanolamide: A novel pharmaceutical agent in the management of obesity-an updated review. J. Cell Physiol. 2019, 234, 7893–7902. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Zimmermann, K.; Filipović, M.R.; Izydorczyk, I.; Eberhardt, M.; Kichko, T.I.; Mueller-Tribbensee, S.M.; et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Boudaka, A.; Wörl, J.; Shiina, T.; Neuhuber, W.L.; Kobayashi, H.; Shimizu, Y.; Takewaki, T. Involvement of TRPV1-dependent and -independent components in the regulation of vagally induced contractions in the mouse esophagus. Eur. J. Pharmacol. 2007, 556, 157–165. [Google Scholar] [CrossRef]
- Holzer, P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G699–G705. [Google Scholar] [CrossRef]
- Holzer, P. TRPV1: A new target for treatment of visceral pain in IBS? Gut 2008, 57, 882–884. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Q.; Chen, C.; Yang, X.; Xie, R.; Xu, J. The Role of Transient Receptor Potential Vanilloid 1 in Common Diseases of the Digestive Tract and the Cardiovascular and Respiratory System. Front. Physiol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Park, U.; Vastani, N.; Guan, Y.; Raja, S.N.; Koltzenburg, M.; Caterina, M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 2011, 31, 11425–11436. [Google Scholar] [CrossRef]
- Shibasaki, K.; Ishizaki, Y.; Mandadi, S. Astrocytes express functional TRPV2 ion channels. Biochem. Biophys. Res. Commun. 2013, 441, 327–332. [Google Scholar] [CrossRef]
- Ciardo, M.G.; Ferrer-Montiel, A. Lipids as central modulators of sensory TRP channels. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1615–1628. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Samanta, A.; Liu, Y.; Hughes, T.E.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife 2019, 8, e48792. [Google Scholar] [CrossRef]
- Parenti, A.; De Logu, F.; Geppetti, P.; Benemei, S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 2016, 173, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V.; Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Amantini, C. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front. Immunol. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Kashiba, H.; Uchida, Y.; Takeda, D.; Nishigori, A.; Ueda, Y.; Kuribayashi, K.; Ohshima, M. TRPV2-immunoreactive intrinsic neurons in the rat intestine. Neurosci. Lett. 2004, 366, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Boudaka, A.; Shibasaki, K.; Yamanaka, A.; Sugiyama, T.; Tominaga, M. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J. Neurosci. 2010, 30, 16536–16544. [Google Scholar] [CrossRef]
- Doerner, J.F.; Hatt, H.; Ramsey, I.S. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J. Gen. Physiol. 2011, 137, 271–288. [Google Scholar] [CrossRef]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef]
- Nilius, B.; Bíró, T.; Owsianik, G. TRPV3: Time to decipher a poorly understood family member! J. Physiol. 2014, 592, 295–304. [Google Scholar] [CrossRef]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef]
- Colton, C.K.; Zhu, M.X. 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb. Exp. Pharmacol. 2007, 179, 173–187. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, Y.; Sodhi, R.K.; Khare, P.; Bishnoi, M.; Kondepudi, K.K.; Medhi, B.; Kuhad, A. Role of TRPV1/TRPV3 channels in olanzapine-induced metabolic alteration: Possible involvement in hypothalamic energy-sensing, appetite regulation, inflammation and mesolimbic pathway. Toxicol. Appl. Pharmacol. 2020, 402, 115124. [Google Scholar] [CrossRef] [PubMed]
- Schrapers, K.T.; Sponder, G.; Liebe, F.; Liebe, H.; Stumpff, F. The bovine TRPV3 as a pathway for the uptake of Na(+), Ca(2+), and NH(4)(+). PLoS ONE 2018, 13, e0193519. [Google Scholar] [CrossRef] [PubMed]
- Liebe, F.; Liebe, H.; Kaessmeyer, S.; Sponder, G.; Stumpff, F. The TRPV3 channel of the bovine rumen: Localization and functional characterization of a protein relevant for ruminal ammonia transport. Pflug. Arch. 2020, 472, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Manneck, D.; Braun, H.S.; Schrapers, K.T.; Stumpff, F. TRPV3 and TRPV4 as candidate proteins for intestinal ammonium absorption. Acta Physiol. 2021, 233, e13694. [Google Scholar] [CrossRef]
- Mochizuki, T.; Sokabe, T.; Araki, I.; Fujishita, K.; Shibasaki, K.; Uchida, K.; Naruse, K.; Koizumi, S.; Takeda, M.; Tominaga, M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J. Biol. Chem. 2009, 284, 21257–21264. [Google Scholar] [CrossRef]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef]
- Darby, W.G.; Grace, M.S.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. Int. J. Biochem. Cell Biol. 2016, 78, 217–228. [Google Scholar] [CrossRef]
- Maqboul, A.; Elsadek, B. Expression profiles of TRPV1, TRPV4, TLR4 and ERK1/2 in the dorsal root ganglionic neurons of a cancer-induced neuropathy rat model. PeerJ 2018, 6, e4622. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef] [PubMed]
- Berna-Erro, A.; Izquierdo-Serra, M.; Sepúlveda, R.V.; Rubio-Moscardo, F.; Doñate-Macián, P.; Serra, S.A.; Carrillo-Garcia, J.; Perálvarez-Marín, A.; González-Nilo, F.; Fernández-Fernández, J.M.; et al. Structural determinants of 5′,6′-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci. Rep. 2017, 7, 10522. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Anishkin, A.; Zinkevich, N.S.; Nishijima, Y.; Korishettar, A.; Wang, Z.; Fang, J.; Wilcox, D.A.; Zhang, D.X. Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation. J. Biol. Chem. 2018, 293, 5307–5322. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, D.; Zhu, X.; Wu, Z.; An, Q.; Zhao, J.; Gao, X.; Zhang, L. Roles of TRP and PIEZO receptors in autoimmune diseases. Expert. Rev. Mol. Med. 2024, 26, e10. [Google Scholar] [CrossRef]
- Boudaka, A.; Tominaga, M. Physiological and Pathological Significance of Esophageal TRP Channels: Special Focus on TRPV4 in Esophageal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 4550. [Google Scholar] [CrossRef]
- Mihara, H.; Boudaka, A.; Tominaga, M.; Sugiyama, T. Transient Receptor Potential Vanilloid 4 Regulation of Adenosine Triphosphate Release by the Adenosine Triphosphate Transporter Vesicular Nucleotide Transporter, a Novel Therapeutic Target for Gastrointestinal Baroreception and Chronic Inflammation. Digestion 2020, 101, 6–11. [Google Scholar] [CrossRef]
- Mihara, H.; Suzuki, N.; Boudaka, A.A.; Muhammad, J.S.; Tominaga, M.; Tabuchi, Y.; Sugiyama, T. Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia. World J. Gastroenterol. 2016, 22, 5512–5519. [Google Scholar] [CrossRef]
- Mihara, H.; Boudaka, A.; Sugiyama, T.; Moriyama, Y.; Tominaga, M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J. Physiol. 2011, 589 Pt 14, 3471–3482. [Google Scholar] [CrossRef]
- Boudaka, A.; Saito, C.T.; Tominaga, M. Deletion of TRPV4 enhances in vitro wound healing of murine esophageal keratinocytes. Sci. Rep. 2020, 10, 11349. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef]
- Peng, J.B.; Suzuki, Y.; Gyimesi, G.; Hediger, M.A. TRPV5 and TRPV6 Calcium-Selective Channels. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press/Taylor & Francis, LLC.: Boca Raton, FL, USA, 2018; pp. 241–274. [Google Scholar]
- Na, T.; Peng, J.B. TRPV5: A Ca(2+) channel for the fine-tuning of Ca(2+) reabsorption. Handb. Exp. Pharmacol. 2014, 222, 321–357. [Google Scholar] [CrossRef] [PubMed]
- Fecher-Trost, C.; Weissgerber, P.; Wissenbach, U. TRPV6 channels. Handb. Exp. Pharmacol. 2014, 222, 359–384. [Google Scholar] [CrossRef]
- Leunissen, E.H.; Nair, A.V.; Büll, C.; Lefeber, D.J.; van Delft, F.L.; Bindels, R.J.; Hoenderop, J.G. The epithelial calcium channel TRPV5 is regulated differentially by klotho and sialidase. J. Biol. Chem. 2013, 288, 29238–29246. [Google Scholar] [CrossRef]
- Hughes, T.E.; Del Rosario, J.S.; Kapoor, A.; Yazici, A.T.; Yudin, Y.; Fluck, E.C., 3rd; Filizola, M.; Rohacs, T.; Moiseenkova-Bell, V.Y. Structure-based characterization of novel TRPV5 inhibitors. Elife 2019, 8, e49572. [Google Scholar] [CrossRef]
- Cai, R.; Wang, L.; Liu, X.; Michalak, M.; Tang, J.; Peng, J.-B.; Chen, X.-Z. Auto-inhibitory intramolecular S5/S6 interaction in the TRPV6 channel regulates breast cancer cell migration and invasion. Commun. Biol. 2021, 4, 990. [Google Scholar] [CrossRef]
- Stewart, J.M. TRPV6 as A Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef]
- Khattar, V.; Wang, L.; Peng, J.B. Calcium selective channel TRPV6: Structure, function, and implications in health and disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef]
- Lai, N.Y.; Mills, K.; Chiu, I.M. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J. Intern. Med. 2017, 282, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, V.N.; Kostic, A.D.; Chiu, I.M. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol. Pain. 2021, 9, 100056. [Google Scholar] [CrossRef]
- Alaimo, A.; Rubert, J. The Pivotal Role of TRP Channels in Homeostasis and Diseases throughout the Gastrointestinal Tract. Int. J. Mol. Sci. 2019, 20, 5277. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. TRP channels in the digestive system. Curr. Pharm. Biotechnol. 2011, 12, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Hicks, G.A. TRP channels as therapeutic targets: Hot property, or time to cool down? Neurogastroenterol. Motil. 2006, 18, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.; Anand, P.; Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008, 57, 923–929. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.; Anand, P.; Ghosh, S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef]
- Chan, C.L.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Bhat, Y.M.; Bielefeldt, K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 263–270. [Google Scholar] [CrossRef]
- Hammer, J.; Führer, M.; Pipal, L.; Matiasek, J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol. Motil. 2008, 20, 125–133. [Google Scholar] [CrossRef]
- Gonlachanvit, S.; Mahayosnond, A.; Kullavanijaya, P. Effects of chili on postprandial gastrointestinal symptoms in diarrhoea predominant irritable bowel syndrome: Evidence for capsaicin-sensitive visceral nociception hypersensitivity. Neurogastroenterol. Motil. 2009, 21, 23–32. [Google Scholar] [CrossRef]
- Sipe, W.E.; Brierley, S.M.; Martin, C.M.; Phillis, B.D.; Cruz, F.B.; Grady, E.F.; Liedtke, W.; Cohen, D.M.; Vanner, S.; Blackshaw, L.A.; et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1288–G1298. [Google Scholar] [CrossRef]
- Brierley, S.M.; Page, A.J.; Hughes, P.A.; Adam, B.; Liebregts, T.; Cooper, N.J.; Holtmann, G.; Liedtke, W.; Blackshaw, L.A. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 2008, 134, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Altier, C.; Chapman, K.; Liedtke, W.; Zamponi, G.; Vergnolle, N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 2008, 135, 937–946, 946.e1–946e2. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Joseph, E.K.; Reichling, D.; Levine, J.D. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J. Neurosci. 2006, 26, 3864–3874. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578 Pt 3, 715–733. [Google Scholar] [CrossRef]
- Zhao, P.; Lieu, T.; Barlow, N.; Metcalf, M.; Veldhuis, N.A.; Jensen, D.D.; Kocan, M.; Sostegni, S.; Haerteis, S.; Baraznenok, V.; et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 2014, 289, 27215–27234. [Google Scholar] [CrossRef]
- Zhao, P.; Lieu, T.; Barlow, N.; Sostegni, S.; Haerteis, S.; Korbmacher, C.; Liedtke, W.; Jimenez-Vargas, N.N.; Vanner, S.J.; Bunnett, N.W. Neutrophil Elastase Activates Protease-activated Receptor-2 (PAR2) and Transient Receptor Potential Vanilloid 4 (TRPV4) to Cause Inflammation and Pain. J. Biol. Chem. 2015, 290, 13875–13887. [Google Scholar] [CrossRef]
- Cenac, N.; Altier, C.; Motta, J.P.; d’Aldebert, E.; Galeano, S.; Zamponi, G.W.; Vergnolle, N. Potentiation of TRPV4 signalling by histamine and serotonin: An important mechanism for visceral hypersensitivity. Gut 2010, 59, 481–488. [Google Scholar] [CrossRef]
- Weber, E.; Neunlist, M.; Schemann, M.; Frieling, T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J. Physiol. 2001, 536 Pt 3, 741–751. [Google Scholar] [CrossRef]
- Boudaka, A.; Wörl, J.; Shiina, T.; Saito, S.; Atoji, Y.; Kobayashi, H.; Shimizu, Y.; Takewaki, T. Key role of mucosal primary afferents in mediating the inhibitory influence of capsaicin on vagally mediated contractions in the mouse esophagus. J. Vet. Med. Sci. 2007, 69, 365–372. [Google Scholar] [CrossRef]
- Boudaka, A.; Wörl, J.; Shiina, T.; Shimizu, Y.; Takewaki, T.; Neuhuber, W.L. Galanin modulates vagally induced contractions in the mouse oesophagus. Neurogastroenterol. Motil. 2009, 21, 180–188. [Google Scholar] [CrossRef]
- Benko, R.; Lazar, Z.; Undi, S.; Illenyi, L.; Antal, A.; Horvath, O.P.; Rumbus, Z.; Wolf, M.; Maggi, C.A.; Bartho, L. Inhibition of nitric oxide synthesis blocks the inhibitory response to capsaicin in intestinal circular muscle preparations from different species. Life Sci. 2005, 76, 2773–2782. [Google Scholar] [CrossRef]
- Holzer, P.; Lippe, I.T.; Holzer-Petsche, U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology 1986, 91, 360–363. [Google Scholar] [CrossRef]
- De Schepper, H.U.; De Man, J.G.; Ruyssers, N.E.; Deiteren, A.; Van Nassauw, L.; Timmermans, J.P.; Martinet, W.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G245–G253. [Google Scholar] [CrossRef]
- Ding, Q.W.; Zhang, Y.; Wang, Y.; Wang, Y.N.; Zhang, L.; Ding, C.; Wu, L.L.; Yu, G.Y. Functional vanilloid receptor-1 in human submandibular glands. J. Dent. Res. 2010, 89, 711–716. [Google Scholar] [CrossRef]
- Cheng, L.; de la Monte, S.; Ma, J.; Hong, J.; Tong, M.; Cao, W.; Behar, J.; Biancani, P.; Harnett, K.M. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G135–G143. [Google Scholar] [CrossRef] [PubMed]
- Ericson, A.; Nur, E.M.; Petersson, F.; Kechagias, S. The effects of capsaicin on gastrin secretion in isolated human antral glands: Before and after ingestion of red chilli. Dig. Dis. Sci. 2009, 54, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Schicho, R.; Krueger, D.; Zeller, F.; Von Weyhern, C.W.; Frieling, T.; Kimura, H.; Ishii, I.; De Giorgio, R.; Campi, B.; Schemann, M. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 2006, 131, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; Masyuk, A.I.; Splinter, P.L.; Banales, J.M.; Huang, B.Q.; Tietz, P.S.; Masyuk, T.V.; Larusso, N.F. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc. Natl. Acad. Sci. USA 2007, 104, 19138–19143. [Google Scholar] [CrossRef]
- Gradilone, S.A.; Masyuk, T.V.; Huang, B.Q.; Banales, J.M.; Lehmann, G.L.; Radtke, B.N.; Stroope, A.; Masyuk, A.I.; Splinter, P.L.; LaRusso, N.F. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology 2010, 139, 304–314.e302. [Google Scholar] [CrossRef]
- Luo, J.; Qian, A.; Oetjen, L.K.; Yu, W.; Yang, P.; Feng, J.; Xie, Z.; Liu, S.; Yin, S.; Dryn, D.; et al. TRPV4 Channel Signaling in Macrophages Promotes Gastrointestinal Motility via Direct Effects on Smooth Muscle Cells. Immunity 2018, 49, 107–119.e104. [Google Scholar] [CrossRef]
- Gazzieri, D.; Trevisani, M.; Springer, J.; Harrison, S.; Cottrell, G.S.; Andre, E.; Nicoletti, P.; Massi, D.; Zecchi, S.; Nosi, D.; et al. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic. Biol. Med. 2007, 43, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, J.; Shen, L.; Wang, B. TRP channels in inflammatory bowel disease: Potential therapeutic targets. Biochem. Pharmacol. 2022, 203, 115195. [Google Scholar] [CrossRef]
- Shibasaki, K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. 2016, 66, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.D.; Alessandri-Haber, N. TRP channels: Targets for the relief of pain. Biochim. Biophys. Acta 2007, 1772, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Issa, C.M.; Hambly, B.D.; Wang, Y.; Maleki, S.; Wang, W.; Fei, J.; Bao, S. TRPV2 in the development of experimental colitis. Scand. J. Immunol. 2014, 80, 307–312. [Google Scholar] [CrossRef]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.P.; Vergnolle, N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 2011, 140, 275–285. [Google Scholar] [CrossRef]
- Vincent, F.; Acevedo, A.; Nguyen, M.T.; Dourado, M.; DeFalco, J.; Gustafson, A.; Spiro, P.; Emerling, D.E.; Kelly, M.G.; Duncton, M.A. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009, 389, 490–494. [Google Scholar] [CrossRef]
- Meena, A.S.; Shukla, P.K.; Bell, B.; Giorgianni, F.; Caires, R.; Fernández-Peña, C.; Beranova, S.; Aihara, E.; Montrose, M.H.; Chaib, M.; et al. TRPV6 channel mediates alcohol-induced gut barrier dysfunction and systemic response. Cell Rep. 2022, 39, 110937. [Google Scholar] [CrossRef]
- Munjuluri, S.; Wilkerson, D.A.; Sooch, G.; Chen, X.; White, F.A.; Obukhov, A.G. Capsaicin and TRPV1 Channels in the Cardiovascular System: The Role of Inflammation. Cells 2021, 11, 18. [Google Scholar] [CrossRef]
- Chen, J.; Sun, W.; Zhu, Y.; Zhao, F.; Deng, S.; Tian, M.; Wang, Y.; Gong, Y. TRPV1: The key bridge in neuroimmune interactions. J. Intensive Med. 2024, 4, 442–452. [Google Scholar] [CrossRef]
- Devesa, I.; Planells-Cases, R.; Fernández-Ballester, G.; González-Ros, J.M.; Ferrer-Montiel, A.; Fernández-Carvajal, A. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J. Inflamm. Res. 2011, 4, 67–81. [Google Scholar] [CrossRef]
- Dunne, O.M.; Martin, S.L.; Sergeant, G.P.; McAuley, D.F.; O’Kane, C.M.; Button, B.; McGarvey, L.P.; Lundy, F.T. TRPV2 modulates mechanically Induced ATP Release from Human bronchial epithelial cells. Respir. Res. 2024, 25, 188. [Google Scholar] [CrossRef] [PubMed]
- Dvornikova, K.A.; Platonova, O.N.; Bystrova, E.Y. The Role of TRP Channels in Sepsis and Colitis. Int. J. Mol. Sci. 2024, 25, 4784. [Google Scholar] [CrossRef] [PubMed]
- Pacífico, D.M.; Costa, D.; Lima Barbosa, M.L.; Rebouças, C.S.M.; Simonato, S.G.; Warren, C.A.; Morais, M.; Leitao, R.F.C.; Brito, G.A.C. TRPV4 modulates inflammatory responses and apoptosis in enteric glial cells triggered by Clostridioides difficile toxins A and B. J. Inflamm. 2025, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Zhang, R.; Xu, Y.; Luo, C.; Wang, R.; Xu, S.; Wei, L. Inhibition of TRPV4 remodels single cell polarity and suppresses the metastasis of hepatocellular carcinoma. Cell Death Dis. 2023, 14, 379. [Google Scholar] [CrossRef]
- Jiang, D.; Guo, R.; Dai, R.; Knoedler, S.; Tao, J.; Machens, H.G.; Rinkevich, Y. The Multifaceted Functions of TRPV4 and Calcium Oscillations in Tissue Repair. Int. J. Mol. Sci. 2024, 25, 1179. [Google Scholar] [CrossRef]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 369. [Google Scholar] [CrossRef]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015, 29, 182–192. [Google Scholar] [CrossRef]
- Ishii, T.; Uchida, K.; Hata, S.; Hatta, M.; Kita, T.; Miyake, Y.; Okamura, K.; Tamaoki, S.; Ishikawa, H.; Yamazaki, J. TRPV2 channel inhibitors attenuate fibroblast differentiation and contraction mediated by keratinocyte-derived TGF-β1 in an in vitro wound healing model of rats. J. Dermatol. Sci. 2018, 90, 332–342. [Google Scholar] [CrossRef]
- Varela-López, E.; Del Valle-Mondragón, L.; Castrejón-Téllez, V.; Pérez-Torres, I.; Arenas, A.P.; Rojas, F.M.; Guarner-Lans, V.; Vargas-González, A.; Pastelín-Hernández, G.; Torres-Narváez, J.C. Role of the Transient Receptor Potential Vanilloid Type 1 (TRPV1) in the Regulation of Nitric Oxide Release in Wistar Rat Aorta. Oxid. Med. Cell Longev. 2021, 2021, 8531975. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, Y.; Li, Z.; Du, Y.; Chen, Q.; Guo, Q.; Ban, Y.; Gong, P. Sensory neuron transient receptor potential vanilloid-1 channel regulates angiogenesis through CGRP in vivo. Front. Bioeng. Biotechnol. 2024, 12, 1338504. [Google Scholar] [CrossRef]
- Peles, S.; Medda, B.K.; Zhang, Z.; Banerjee, B.; Lehmann, A.; Shaker, R.; Sengupta, J.N. Differential effects of transient receptor vanilloid one (TRPV1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience 2009, 161, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Stokłosa, P.; Borgström, A.; Kappel, S.; Peinelt, C. TRP Channels in Digestive Tract Cancers. Int. J. Mol. Sci. 2020, 21, 1877. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Titiz, M.; Souza Monteiro de Araújo, D.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules 2023, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Kärki, T.; Tojkander, S. TRPV Protein Family-From Mechanosensing to Cancer Invasion. Biomolecules 2021, 11, 1019. [Google Scholar] [CrossRef]
- Marincsák, R.; Tóth, B.I.; Czifra, G.; Márton, I.; Rédl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral. Dis. 2009, 15, 328–335. [Google Scholar] [CrossRef]
- Gonzales, C.B.; Kirma, N.B.; De La Chapa, J.J.; Chen, R.; Henry, M.A.; Luo, S.; Hargreaves, K.M. Vanilloids induce oral cancer apoptosis independent of TRPV1. Oral. Oncol. 2014, 50, 437–447. [Google Scholar] [CrossRef]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef]
- Sakakibara, A.; Sakakibara, S.; Kusumoto, J.; Takeda, D.; Hasegawa, T.; Akashi, M.; Minamikawa, T.; Hashikawa, K.; Terashi, H.; Komori, T. Upregulated Expression of Transient Receptor Potential Cation Channel Subfamily V Receptors in Mucosae of Patients with Oral Squamous Cell Carcinoma and Patients with a History of Alcohol Consumption or Smoking. PLoS ONE 2017, 12, e0169723. [Google Scholar] [CrossRef]
- Waning, J.; Vriens, J.; Owsianik, G.; Stüwe, L.; Mally, S.; Fabian, A.; Frippiat, C.; Nilius, B.; Schwab, A. A novel function of capsaicin-sensitive TRPV1 channels: Involvement in cell migration. Cell Calcium 2007, 42, 17–25. [Google Scholar] [CrossRef]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Lab. Investig. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Huang, R.; Wang, F.; Yang, Y.; Ma, W.; Lin, Z.; Cheng, N.; Long, Y.; Deng, S.; Li, Z. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio 2019, 9, 206–225. [Google Scholar] [CrossRef]
- Valenzano, K.J.; Grant, E.R.; Wu, G.; Hachicha, M.; Schmid, L.; Tafesse, L.; Sun, Q.; Rotshteyn, Y.; Francis, J.; Limberis, J.; et al. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 2003, 306, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.J.; Hannan, S.L.; Smart, D.; Jerman, J.C.; Arpino, S.; Smith, G.D.; Brough, S.; Wright, J.; Egerton, J.; Lappin, S.C.; et al. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and heat-mediated activation of the receptor. J. Pharmacol. Exp. Ther. 2007, 321, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Rami, H.K.; Thompson, M.; Stemp, G.; Fell, S.; Jerman, J.C.; Stevens, A.J.; Smart, D.; Sargent, B.; Sanderson, D.; Randall, A.D.; et al. Discovery of SB-705498: A potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med. Chem. Lett. 2006, 16, 3287–3291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, S.S.; Yan, Y.; Zhao, S. Overexpression of transient receptor potential vanilloid 2 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Med. Oncol. 2014, 31, 17. [Google Scholar] [CrossRef]
- Kudou, M.; Shiozaki, A.; Yamazato, Y.; Katsurahara, K.; Kosuga, T.; Shoda, K.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. The expression and role of TRPV2 in esophageal squamous cell carcinoma. Sci. Rep. 2019, 9, 16055. [Google Scholar] [CrossRef]
- Shiozaki, A.; Kudou, M.; Ichikawa, D.; Fujiwara, H.; Shimizu, H.; Ishimoto, T.; Arita, T.; Kosuga, T.; Konishi, H.; Komatsu, S.; et al. Esophageal cancer stem cells are suppressed by tranilast, a TRPV2 channel inhibitor. J. Gastroenterol. 2018, 53, 197–207. [Google Scholar] [CrossRef]
- Huang, R.; Li, S.; Tian, C.; Zhou, P.; Zhao, H.; Xie, W.; Xiao, J.; Wang, L.; Habimana, J.D.; Lin, Z.; et al. Thermal stress involved in TRPV2 promotes tumorigenesis through the pathways of HSP70/27 and PI3K/Akt/mTOR in esophageal squamous cell carcinoma. Br. J. Cancer 2022, 127, 1424–1439. [Google Scholar] [CrossRef]
- Gao, N.; Yang, F.; Chen, S.; Wan, H.; Zhao, X.; Dong, H. The role of TRPV1 ion channels in the suppression of gastric cancer development. J. Exp. Clin. Cancer Res. 2020, 39, 206. [Google Scholar] [CrossRef] [PubMed]
- Zoppoli, P.; Calice, G.; Laurino, S.; Ruggieri, V.; La Rocca, F.; La Torre, G.; Ciuffi, M.; Amendola, E.; De Vita, F.; Petrillo, A.; et al. TRPV2 Calcium Channel Gene Expression and Outcomes in Gastric Cancer Patients: A Clinically Relevant Association. J. Clin. Med. 2019, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Xu, J.; Xiao, Y.; Wu, J.; Wan, H.; Tang, B.; Liu, J.; Fan, Y.; Wang, S.; Wu, Y.; et al. Calcium Promotes Human Gastric Cancer via a Novel Coupling of Calcium-Sensing Receptor and TRPV4 Channel. Cancer Res. 2017, 77, 6499–6512. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.R.; Takahashi, N.; Harris, A.R.; Lee, J.; Bertin, S.; Jeffries, J.; Jung, M.; Duong, J.; Triano, A.I.; Lee, J.; et al. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J. Clin. Investig. 2014, 124, 3793–3806. [Google Scholar] [CrossRef]
- de Jong, P.R.; Bertin, S.; Raz, E. TRPV1: Turning up the heat on intestinal tumorigenesis. Mol. Cell Oncol. 2015, 2, e975619. [Google Scholar] [CrossRef][Green Version]
- Du, Y.; Karatekin, F.; Wang, W.K.; Hong, W.; Boopathy, G.T.K. Cracking the EGFR code: Cancer biology, resistance mechanisms, and future therapeutic frontiers. Pharmacol. Rev. 2025, 77, 100076. [Google Scholar] [CrossRef]
- Dumitru, C.S.; Raica, M. Vascular Endothelial Growth Factor Family and Head and Neck Squamous Cell Carcinoma. Anticancer. Res. 2023, 43, 4315–4326. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, W.; Chen, F.L.; Zhang, Y.J.; Liu, X.C.; Yang, X.Y.; Hu, S.S.; Jiang, Y.; Yuan, J. Expression and significance of pigment epithelium-derived factor and vascular endothelial growth factor in colorectal adenoma and cancer. World J. Gastrointest. Oncol. 2024, 16, 670–686. [Google Scholar] [CrossRef]
- Rizopoulos, T.; Assimakopoulou, M. Transient receptor potential (TRP) channels in human colorectal cancer: Evidence and perspectives. Histol. Histopathol. 2021, 36, 515–526. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology 2004, 80 (Suppl. S1), 12–15. [Google Scholar] [CrossRef]
- Islam, M.S. Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells. Cells 2020, 9, 685. [Google Scholar] [CrossRef]
- Mergler, S.; Skrzypski, M.; Sassek, M.; Pietrzak, P.; Pucci, C.; Wiedenmann, B.; Strowski, M.Z. Thermo-sensitive transient receptor potential vanilloid channel-1 regulates intracellular calcium and triggers chromogranin A secretion in pancreatic neuroendocrine BON-1 tumor cells. Cell Signal 2012, 24, 233–246. [Google Scholar] [CrossRef]
- Skrzypski, M.; Sassek, M.; Abdelmessih, S.; Mergler, S.; Grötzinger, C.; Metzke, D.; Wojciechowicz, T.; Nowak, K.W.; Strowski, M.Z. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell Signal 2014, 26, 41–48. [Google Scholar] [CrossRef]
- Menon, G.; Pandit, S.; Annamaraju, P.; Bhusal, K. Carcinoid Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Doihara, H.; Nozawa, K.; Kojima, R.; Kawabata-Shoda, E.; Yokoyama, T.; Ito, H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol. Cell Biochem. 2009, 331, 239–245. [Google Scholar] [CrossRef]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Chaigne, S.; Barbeau, S.; Ducret, T.; Guinamard, R.; Benoist, D. Pathophysiological Roles of the TRPV4 Channel in the Heart. Cells 2023, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Li, J.; Zhang, H.R.; Bai, S.W.; Yang, H.Y.; Shen, B.; Du, J.; Xia, X.M. The effect of transient receptor potential vanilloid 4 on the intestinal epithelial barrier and human colonic cells was affected by tyrosine-phosphorylated claudin-7. Biomed. Pharmacother. 2020, 122, 109697. [Google Scholar] [CrossRef]
- Khairatkar-Joshi, N.; Szallasi, A. TRPV1 antagonists: The challenges for therapeutic targeting. Trends Mol. Med. 2009, 15, 14–22. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Chizh, B.A.; O’Donnell, M.B.; Napolitano, A.; Wang, J.; Brooke, A.C.; Aylott, M.C.; Bullman, J.N.; Gray, E.J.; Lai, R.Y.; Williams, P.M.; et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 2007, 132, 132–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).