Cerebrovascular Disease as a Manifestation of Tick-Borne Infections: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
| (Organism OR Common Disease Title) AND (Cerebrovascular Disease) such as (“Borrelia burgdorferi” OR “Lyme disease”) AND (“stroke” OR “cerebrovascular accident” OR “transient ischemic attack” OR “TIA” OR “cerebral infarction” OR “brain ischemia” OR “cerebrovascular event” OR “vascular event” OR “cerebral thrombosis” OR “cerebral hemorrhage” OR “cerebral vasculitis” OR “central nervous system vasculitis” OR “neuroborreliosis” OR “neurological Lyme disease” OR “neurovascular”) |
3. The Medical Ecology and Epidemiology of Ticks
4. Pathogenesis of Tick-Derived Vasculopathy
5. Treatment of Cerebrovascular Disease in Tick-Borne Disease
6. Borrelia burgdorferi and Cerebrovascular Disease
7. Borrelia miyamotoi and Cerebrovascular Disease
8. Rickettsia Species and Cerebrovascular Disease
9. Ehrlichia chaffeensis and Cerebrovascular Disease
10. Anaplasma phagocytophilum and Cerebrovascular Disease
11. Francisella tularensis and Cerebrovascular Disease
12. Powassan Virus and Cerebrovascular Disease
13. Crimean–Congo Hemorrhagic Fever Virus and Cerebrovascular Disease
14. Babesia Species as Parasitic Tick-Borne Pathogens Implicated in Cerebrovascular Disease
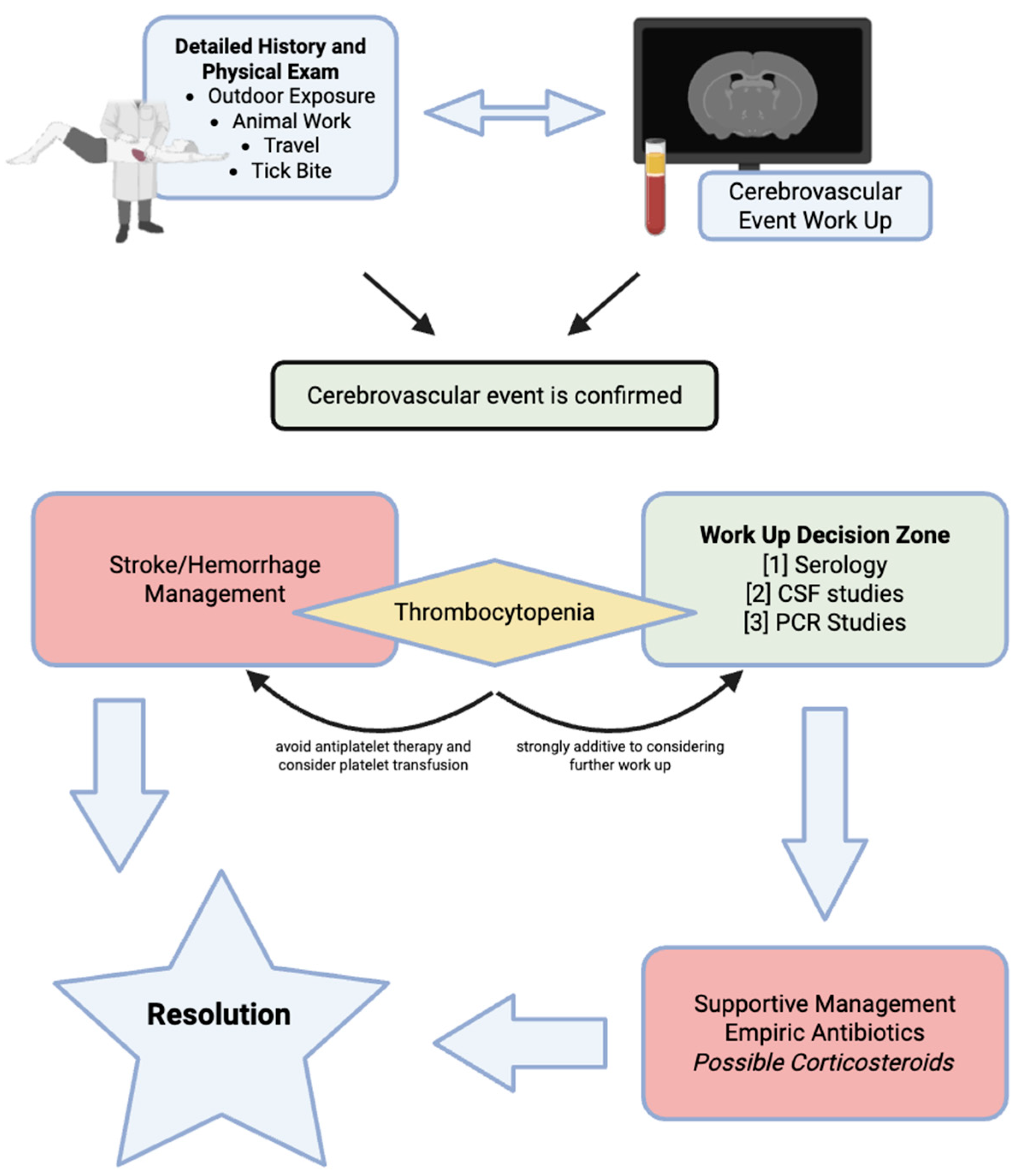
15. Diagnostic and Therapeutic Decision Making
16. Limitations
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBD | Tick-Borne Disease |
| CSF | Cerebrospinal Fluid |
| RMSF | Rocky Mountain Spotted Fever |
| HME | Human Monocytic Ehrlichiosis |
| HGA | Human Granulocytic Anaplasmosis |
| POWV | Powassan Virus |
| LVS | Live Vaccine Strain |
| CCHFV | Crimean–Congo Hemorrhagic Fever Virus |
| BMD | Borrelia miyamotoi Disease |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| MRI | Magnetic Resonance Imaging |
| MRA | Magnetic Resonance Angiography |
| CT | Computed Tomography |
| DIC | Disseminated Intravascular Coagulation |
| SAH | Subarachnoid Hemorrhage |
| TIA | Transient Ischemic Attack |
References
- Merino, O.; Alberdi, P.; Pérez de la Lastra, J.M.; de la Fuente, J. Tick vaccines and the control of tick-borne pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; McCarthy, C.A.; Elias, S.P. Increasing actual and perceived burden of tick-borne disease in Maine. J. Maine Med. Cent. 2019, 1, 13. [Google Scholar] [CrossRef]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, R.; Daszkiewicz, P. About the Greek origin of acarology: A short note on Argas and the Acari. Biol. Lett. 2016, 53, 3–7. [Google Scholar] [CrossRef][Green Version]
- Dhooria, M.S. Historical Account of Acarology. In Fundamentals of Applied Acarology; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Kean, W.F.; Tocchio, S.; Kean, M.; Rainsford, K.D. The musculoskeletal abnormalities of the Similaun Iceman (“ÖTZI”): Clues to chronic pain and possible treatments. Inflammopharmacology 2013, 21, 11–20. [Google Scholar] [CrossRef]
- Huchet, J.B.; Callou, C.; Lichtenberg, R.; Dunand, F. The dog mummy, the ticks and the louse fly: Archaeological report of severe ectoparasitosis in Ancient Egypt. Int. J. Paleopathol. 2013, 3, 165–175. [Google Scholar] [CrossRef]
- Arthur, D. Ticks in Egypt in 1500 B.C.? Nature 1965, 206, 1060–1061. [Google Scholar] [CrossRef]
- Anderson, J.F. The natural history of ticks. Med. Clin. N. Am. 2002, 86, 205–218. [Google Scholar] [CrossRef]
- Walker, D.; Ismail, N. Emerging and re-emerging rickettsioses: Endothelial cell infection and early disease events. Nat. Rev. Microbiol. 2008, 6, 375–386. [Google Scholar] [CrossRef]
- Margini, C.; Maldonado, R.; Keller, P.; Banz, Y.; Escher, R.; Waldegg, G. Fever of Unknown Origin, a Vascular Event, and Immunosuppression in Tick-Endemic Areas: Think About Neoehrlichiosis. Cureus 2023, 15, e40617. [Google Scholar] [CrossRef]
- Vannier, E.G.; Diuk-Wasser, M.A.; Ben Mamoun, C.; Krause, P.J. Babesiosis. Infect. Dis. Clin. N. Am. 2015, 29, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Underwood, J.; Harvey, C.; Lohstroh, E.; Pierce, B.; Chambers, C.; Guzman Valencia, S.; Oliva Chávez, A.S. Anaplasma phagocytophilum Transmission Activates Immune Pathways While Repressing Wound Healing in the Skin. Life 2022, 12, 1965. [Google Scholar] [CrossRef] [PubMed]
- Miura KMatsuo, J.; Rahman, M.A.; Kumagai, Y.; Li, X.; Rikihisa, Y. Ehrlichia chaffeensis Induces Monocyte Inflammatory Responses through MyD88, ERK, and NF-κB but Not through TRIF, Interleukin-1 Receptor 1 (IL-1R1)/IL-18R1, or Toll-Like Receptors. Infect. Immun. 2011, 79, 4947–4956. [Google Scholar] [CrossRef]
- Tominello, T.R.; Oliveira, E.R.A.; Hussain, S.S.; Elfert, A.; Wells, J.; Golden, B.; Ismail, N. Emerging Roles of Autophagy and Inflammasome in Ehrlichiosis. Front. Immunol. 2019, 10, 1011. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Anderson, C.C.; Brissette, C.A. Borrelia miyamotoi: A Comprehensive Review. Pathogens 2023, 12, 267. [Google Scholar] [CrossRef]
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A prototypical emerging pathogen. Clin. Microbiol. Rev. 2003, 16, 37–64. [Google Scholar] [CrossRef]
- Dumic, I.; Jevtic, D.; Veselinovic, M.; Nordstrom, C.W.; Jovanovic, M.; Mogulla, V.; Veselinovic, E.M.; Hudson, A.; Simeunovic, G.; Petcu, E.; et al. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Microorganisms 2022, 10, 1433. [Google Scholar] [CrossRef]
- Degabriel, M.; Valeva, S.; Boisset, S.; Henry, T. Pathogenicity and virulence of Francisella tularensis. Virulence 2023, 14, 2274638. [Google Scholar] [CrossRef]
- Khan, M.; Beckham, J.D.; Piquet, A.L.; Tyler, K.L.; Pastula, D.M. An Overview of Powassan Virus Disease. Neurohospitalist 2019, 9, 181–182. [Google Scholar] [CrossRef]
- Hawman, D.W.; Feldmann, H. Crimean–Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Ord, R.L.; Lobo, C.A. Human Babesiosis: Pathogens, Prevalence, Diagnosis and Treatment. Curr. Clin. Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Girschick, H.J. Tick-host interactions and their immunological implications in tick-borne diseases. Curr. Sci. 2003, 85, 1284–1298. [Google Scholar]
- Brites-Neto, J.; Duarte, K.M.; Martins, T.F. Tick-borne infections in human and animal population worldwide. Vet. World 2015, 8, 301–315. [Google Scholar] [CrossRef]
- McCoy, K.D.; Léger, E.; Dietrich, M. Host specialization in ticks and transmission of tick-borne diseases: A review. Front. Cell. Infect. Microbiol. 2013, 3, 57. [Google Scholar] [CrossRef]
- Carroll, J.F.; Schmidtmann, E.T. Dispersal of blacklegged tick (Acari:Ixodidae) nymphs and adults at the woods-pasture interface. J. Med. Entomol. 1996, 33, 554–558. [Google Scholar] [CrossRef]
- Estrada-Peña, A. The relationships between habitat topology, critical scales of connectivity and tick abundance Ixodes ricinus in a heterogeneous landscape in northern Spain. Ecography 2003, 26, 661–671. [Google Scholar] [CrossRef]
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]
- Nasirian, H. Detailed new insights about tick infestations in domestic ruminant groups: A global systematic review and meta-analysis. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2022, 46, 526–601. [Google Scholar] [CrossRef]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- İnci, A.; Yıldırım, A.; Düzlü, Ö. The Current Status of Ticks in Turkey: A 100-Year Period Review from 1916 to 2016. Turkiye Parazitol. Derg. 2016, 40, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends Parasitol. 2014, 30, 205–214. [Google Scholar] [CrossRef]
- Kleissl, L.; Weninger, S.; Winkler, F.; Ruivo, M.; Wijnveld, M.; Strobl, J. Ticks’ tricks: Immunomodulatory effects of ixodid tick saliva at the cutaneous tick-host interface. Front. Immunol. 2025, 16, 1520665. [Google Scholar] [CrossRef]
- Weninger, S.; Müller, S.; Markowicz, M.; Schötta, A.-M.; Unterluggauer, L.; Kleissl, L.; Redl, A.; Stockinger, H.; Strobl, J.; Stary, G. Borrelia burgdorferi spirochetes cause vascular damage and neurogenic inflammation in Erythema migrans. J. Investig. Dermatol. 2023, 143, S350. [Google Scholar] [CrossRef]
- Ebady, R.; Niddam, A.F.; Boczula, A.E.; Kim, Y.R.; Gupta, N.; Tang, T.T.; Odisho, T.; Zhi, H.; Simmons, C.A.; Skare, J.T.; et al. Biomechanics of Borrelia burgdorferi Vascular Interactions. Cell Rep. 2016, 16, 2593–2604. [Google Scholar] [CrossRef]
- Fischer, J.R.; LeBlanc, K.T.; Leong, J.M. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 2006, 74, 435–441. [Google Scholar] [CrossRef]
- Steere, A.C.; Coburn, J.; Glickstein, L. The emergence of Lyme disease. J. Clin. Investig. 2004, 113, 1093–1101. [Google Scholar] [CrossRef]
- Ma, Y.; Sturrock, A.; Weis, J.J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 1991, 59, 671–678. [Google Scholar] [CrossRef]
- Burns, M.J.; Sellati, T.J.; Teng, E.I.; Furie, M.B. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted [corrected] IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect. Immun. 1997, 65, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Shelite, T.; Mei, F.C.; Ha, T.; Hu, Y.; Xu, G.; Chang, Q.; Wakamiya, M.; Ksiazek, T.G.; Boor, P.J.; et al. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc. Natl. Acad. Sci. USA 2013, 110, 19615–19620. [Google Scholar] [CrossRef]
- Chan, Y.G.-Y.; Riley, S.P.; Martinez, J.J. Adherence to and invasion of host cells by spotted fever group Rickettsia species. Front. Microbiol. 2010, 1, 139. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, G.; Walker, D.H. Changes in the adherens junctions of human endothelial cells infected with spotted fever group rickettsiae. Virchows Arch. Int. J. Pathol. 2005, 446, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Clifton, D.R.; Rydkina, E.; Huyck, H.; Pryhuber, G.; Freeman, R.S.; Silverman, D.J.; Sahni, S.K. Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: Regulation by nuclear transcription factor NF-kappaB. Int. J. Med. Microbiol. 2005, 295, 267–278. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, C.; Su, Z.; Chang, Q.; Qiu, Y.; Bei, J.; Gaitas, A.; Xiao, J.; Drelich, A.; Khanipov, K.; et al. Endothelial Exosome Plays a Functional Role during Rickettsial Infection. mBio 2021, 12, e00769-21. [Google Scholar] [CrossRef]
- Forestal, C.A.; Benach, J.L.; Carbonara, C.; Italo, J.K.; Lisinski, T.J.; Furie, M.B. Francisella tularensis Selectively Induces Proinflammatory Changes in Endothelial Cells. J. Immunol. 2003, 171, 2563–2570. [Google Scholar] [CrossRef]
- Hestvik, G.; Uhlhorn, H.; Södersten, F.; Åkerström, S.; Karlsson, E.; Westergren, E.; Gavier-Widén, D. Tularaemia in European Brown Hares (Lepus europaeus) and Mountain Hares (Lepus timidus) Characterized by Histopathology and Immunohistochemistry: Organ Lesions and Suggestions of Routes of Infection and Shedding. J. Comp. Pathol. 2017, 157, 103–114. [Google Scholar] [CrossRef]
- Conde, J.N.; Sanchez-Vicente, S.; Saladino, N.; Gorbunova, E.E.; Schutt, W.R.; Mladinich, M.C.; Himmler, G.E.; Benach, J.; Kim, H.K.; Mackow, E.R. Powassan Viruses Spread Cell to Cell during Direct Isolation from Ixodes Ticks and Persistently Infect Human Brain Endothelial Cells and Pericytes. J. Virol. 2022, 96, e0168221. [Google Scholar] [CrossRef]
- Mladinich, M.C.; Himmler, G.E.; Conde, J.N.; Gorbunova, E.E.; Schutt, W.R.; Sarkar, S.; Tsirka, S.-A.E.; Kim, H.K.; Mackow, E.R. Age-dependent Powassan virus lethality is linked to glial cell activation and divergent neuroinflammatory cytokine responses in a murine model. J. Virol. 2024, 98, e0056024. [Google Scholar] [CrossRef] [PubMed]
- Connolly-Andersen, A.M.; Moll, G.; Andersson, C.; Akerström, S.; Karlberg, H.; Douagi, I.; Mirazimi, A. Crimean-Congo hemorrhagic fever virus activates endothelial cells. J. Virol. 2011, 85, 7766–7774. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Didier, P.J.; England, J.D.; Santana-Gould, L.; Doyle-Meyers, L.A.; Martin, D.S.; Jacobs, M.B.; Philipp, M.T. Inflammation in the pathogenesis of lyme neuroborreliosis. Am. J. Pathol. 2015, 185, 1344–1360. [Google Scholar] [CrossRef] [PubMed]
- Herbst, J.; Crissinger, T.; Baldwin, K. Diffuse ischemic strokes and sickle cell crisis induced by disseminated anaplasmosis: A case report. Case Rep. Neurol. 2020, 11, 271–276. [Google Scholar] [CrossRef]
- Back, T.; Grünig, S.; Winter, Y.; Bodechtel, U.; Guthke, K.; Khati, D.; von Kummer, R. Neuroborreliosis-associated cerebral vasculitis: Long-term outcome and health-related quality of life. J. Neurol. 2013, 260, 1569–1575. [Google Scholar] [CrossRef]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and ‘Candidatus Neoehrlichia’ bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, C.M.; Kim, D.M.; Yun, N.R. Manifestation of anaplasmosis as cerebral infarction: A case report. BMC Infect. Dis. 2018, 18, 409. [Google Scholar] [CrossRef]
- Diaz, J.H. 298-Ticks, Including Tick Paralysis. Mand. Douglas Bennett’s Princ. Pract. Infect. Dis. 2015, 2, 3266–3279.e1. [Google Scholar]
- Zhang, Y.; Lafontant, G.; Bonner, F.J., Jr. Lyme neuroborreliosis mimics stroke: A case report. Arch. Phys. Med. Rehabil. 2000, 81, 519–521. [Google Scholar] [CrossRef]
- Kurian, M.; Pereira, V.M.; Vargas, M.I.; Fluss, J. Stroke-like Phenomena Revealing Multifocal Cerebral Vasculitis in Pediatric Lyme Neuroborreliosis. J. Child. Neurol. 2015, 30, 1226–1229. [Google Scholar] [CrossRef]
- Garkowski, A.; Zajkowska, J.; Zajkowska, A.; Kułakowska, A.; Zajkowska, O.; Kubas, B.; Jurgilewicz, D.; Hładuński, M.; Łebkowska, U. Cerebrovascular Manifestations of Lyme Neuroborreliosis-A Systematic Review of Published Cases. Front. Neurol. 2017, 8, 146. [Google Scholar] [CrossRef]
- Kumar, P.; Pramod, K. Neurorickettsioses: A rare presentation with stroke in a young adult. J. Clin. Diagn. Res. 2014, 8, MD03–MD04. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Akkurt, B.H.; Kraehling, H.; Nacul, N.G.; Elsharkawy, M.; Schmidt-Pogoda, A.; Minnerup, J.; Stracke, C.P.; Schwindt, W. Vasculitis and Ischemic Stroke in Lyme Neuroborreliosis-Interventional Management Approach and Literature Review. Brain Sci. 2023, 13, 1388. [Google Scholar] [CrossRef] [PubMed]
- Topakian, R.; Stieglbauer, K.; Aichner, F.T. Unexplained cerebral vasculitis and stroke: Keep Lyme neuroborreliosis in mind. Lancet Neurol. 2007, 6, 756–757. [Google Scholar] [CrossRef]
- Palleis, C.; Forbrig, R.; Lehner, L.; Quach, S.; Albert, N.L.; Brendel, M.; Schöberl, F.; Straube, A. Lyme neuroborreliosis: An unusual case with extensive (peri)vasculitis of the middle cerebral artery. Eur. J. Neurol. 2023, 30, 785–787. [Google Scholar] [CrossRef]
- Garc Ía-Baena, C.; Cárdenas, M.F.; Ramón, J.F. Cerebral haemorrhage as a clinical manifestation of human ehrlichiosis. BMJ Case Rep. 2017, 2017, bcr2016219054. [Google Scholar] [CrossRef]
- Bushnell, C.; Kernan, W.N.; Sharrief, A.Z.; Chaturvedi, S.; Cole, J.W.; Cornwell, W.K., III; Cosby-Gaither, C.; Doyle, S.; Goldstein, L.B.; Lennon, O.; et al. 2024 Guideline for the Primary Prevention of Stroke: A Guideline From the American Heart Association/American Stroke Association. Stroke 2024, 55, e344–e424. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., III; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Moreno Legast, G.; Schnider, A.; Nicastro, N. Ischemic Stroke: Do Not Forget Lyme Neuroborreliosis. Case Rep. Neurol. Med. 2018, 2018, 1720725. [Google Scholar] [CrossRef]
- Li, S.; Vytopil, M.; Hreib, K.; Craven, D.E. Lyme disease presenting as multiple ischaemic strokes. Pract. Neurol. 2015, 15, 284–288. [Google Scholar] [CrossRef]
- Almoussa, M.; Goertzen, A.; Fauser, B.; Zimmermann, C.W. Stroke as an unusual first presentation of Lyme disease. Case Rep. Neurol. Med. 2015, 2015, 389081. [Google Scholar] [CrossRef]
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and Phenotypic Analysis of Borrelia Miyamotoi Sp. Nov., Isolated from the Ixodid Tick Ixodes Persulcatus, the Vector for Lyme Disease in Japan. Int. J. Syst. Bacteriol. 1995, 45, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans Infected with Relapsing Fever Spirochete Borrelia Miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- Hovius, J.W.; de Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, A.J.; Asgeirsson, H.; Hammas, B.; Karlsson, E.; Parke, Å.; Hoornstra, D.; Wilhelmsson, P.; Hovius, J.W. Two Cases of Borrelia miyamotoi Meningitis, Sweden, 2018. Emerg. Infect. Dis. 2019, 25, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.S.; Ard, K.L.; Schaefer, P.W.; Branda, J.A. Case 32-2020: A 63-year-old man with confusion, fatigue, and garbled speech. N. Engl. J. Med. 2020, 383, 1578–1586. [Google Scholar] [CrossRef]
- Gandhi, S.; Narasimhan, S.; Workineh, A.; Mamula, M.; Yoon, J.; Krause, P.J.; Farhadian, S.F. Borrelia Miyamotoi Meningoencephalitis in an Immunocompetent Patient. Open Forum Infect. Dis. 2022, 9, ofac295. [Google Scholar] [CrossRef]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R. Meningoencephalitis from Borrelia Miyamotoi in an Immunocompromised Patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef]
- Boden, K.; Lobenstein, S.; Hermann, B.; Margos, G.; Fingerle, V. Borrelia Miyamotoi-Associated Neuroborreliosis in Immunocompromised Person. Emerg. Infect. Dis. 2016, 22, 1617–1620. [Google Scholar] [CrossRef]
- Sekeyová, Z.; Danchenko, M.; Filipčík, P.; Fournier, P.E. Rickettsial infections of the central nervous system. PLoS Negl. Trop. Dis. 2019, 13, e0007469. [Google Scholar] [CrossRef] [PubMed]
- Horney, L.F.; Walker, D.H. Meningoencephalitis as a major manifestation of Rocky Mountain spotted fever. South Med. J. 1988, 81, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Raoult, D. Rickettsia rickettsii and other spotted fever group rickettsiae (Rocky Mountain spotted fever and other spotted fevers). In Principles and Practice of Infectious Diseases, 5th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone: New York, NY, USA, 2000; Volume 2, pp. 2035–2045. [Google Scholar]
- Baganz, M.D.; Dross, P.E.; Reinhardt, J.A. Rocky Mountain spotted fever encephalitis: MR findings. AJNR Am. J. Neuroradiol. 1995, 16, 919–922. [Google Scholar] [PubMed]
- Massey, E.W.; Thames, T.; Coffey, C.E.; Gallis, H.A. Neurologic complications of Rocky Mountain spotted fever. South Med. J. 1985, 78, 1288–1290, 1303. [Google Scholar] [CrossRef]
- Steinfeld, H.J.; Silverstein, J.; Weisburger, W.; Rattner, F. Deafness associated with Rocky Mountain spotted fever. Md. Med. J. 1988, 37, 287–288. [Google Scholar]
- Kirk, J.L.; Fine, D.P.; Sexton, D.J.; Muchmore, H.G. Rocky Mountain spotted fever. A clinical review based on 48 confirmed cases, 1943–1986. Medicine 1990, 69, 35–45. [Google Scholar] [CrossRef]
- Archibald, L.K.; Sexton, D.J. Long-term sequelae of Rocky Mountain spotted fever. Clin. Infect. Dis. 1995, 20, 1122–1125. [Google Scholar] [CrossRef]
- Sexton, D.J.; Kirkland, K.B. Rickettsial infections and the central nervous system. Clin. Infect. Dis. 1998, 26, 247–248. [Google Scholar] [CrossRef]
- Srinivasa Murthy, C.L.; Namitha, P.; Raghavendra, K.; Kumar, N.; Pejaver, R. An unusual case of typhus group rickettsial infection presenting as cerebrovascular stroke. Pediatr. Infect. Dis. 2015, 7, 74–77. [Google Scholar] [CrossRef]
- Boulahri, T.; Taous, A.; Berri, M.A.; Traibi, I.; Elbenaye, J.; Rouimi, A. Rickettsiosis associated with cerebral infarction: A new case study. Pan Afr. Med. J. 2017, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, Z.N.; Saker, Z.; Rahhal, H.; Nasserddine, A.; Younes, M. Rickettsial infection causing non-aneurysmal subarachnoid hemorrhage with transient corpus callosum lesion. J. Med. Surg. Public Health 2024, 2, 100093. [Google Scholar] [CrossRef]
- Laura, D.; Quynh, V.; Muhammad, A. Microvessel Cerebral Vasculitis and Peripheral Neuropathy as Neurologic Manifestations in Rocky Mountain Spotted Fever (P5.9-008). Neurology 2019, 92, P5.9-008. [Google Scholar] [CrossRef]
- Feng, H.; Popov, V.L.; Yuoh, G.; Walker, D.H. Role of T lymphocyte subsets in immunity to spotted fever group rickettsiae. J. Immunol. 1997, 158, 5314–5320. [Google Scholar] [CrossRef] [PubMed]
- Elghetany, M.T.; Walker, D.H. Hemostatic changes in Rocky Mountain spotted fever and Mediterranean spotted fever. Am. J. Clin. Pathol. 1999, 112, 159–168. [Google Scholar] [CrossRef]
- Rydkina, E.; Turpin, L.C.; Sahni, S.K. Rickettsia rickettsii Infection of Human Macrovascular and Microvascular Endothelial Cells Reveals Activation of Both Common and Cell Type-Specific Host Response Mechanisms. Infect. Immun. 2010, 78, 2599–2606. [Google Scholar] [CrossRef]
- Hamilton, K.S.; Standaert, S.M.; Kinney, M.C. Characteristic peripheral blood findings in human ehrlichiosis. Mod. Pathol. 2004, 17, 512–517. [Google Scholar] [CrossRef]
- Epidemiology and Statistics: Centers for Disease Control and Prevention. 2022. Available online: https://www.cdc.gov/ehrlichiosis/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/ehrlichiosis/stats/index.html (accessed on 1 May 2025).
- Abernathy, H.; Alejo, A.; Arahirwa, V.; Mansour, O.; Brown-Marusiak, A.; Giandomenico, D.; Boyce, R.M. Leopards do not change their spots: Tick borne disease symptomology case report. BMC Infect. Dis. 2022, 22, 699. [Google Scholar] [CrossRef]
- Hongo, I.; Bloch, K.C. Ehrlichia infection of the central nervous system. Curr. Treat. Opt. Neurol. 2006, 8, 179–184. [Google Scholar] [CrossRef]
- Iyamu, O.; Ciccone, E.J.; Schulz, A.; Sung, J.; Abernathy, H.; Alejo, A.; Tyrlik, K.; Arahirwa, V.; Mansour, O.; Giandomenico, D. Neurological manifestations of ehrlichiosis among a cohort of patients: Prevalence and clinical symptoms. BMC Infect. Dis. 2024, 24, 701. [Google Scholar] [CrossRef]
- Grant, A.C.; Hunter, S.; Partin, W.C. A case of acute monocytic ehrlichiosis with prominent neurologic signs. Neurology 1997, 48, 1619–1623. [Google Scholar] [CrossRef]
- Dumler, J.S.; Walker, D.H. Tick-borne ehrlichioses. Lancet Infect. Dis. 2001, 1, 21–28. [Google Scholar] [CrossRef]
- Guzman, N.; Yarrarapu, S.N.S.; Beidas, S.O. Anaplasma Phagocytophilum. In StatPearls [Internet]; Updated 8 August 2023; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513341/ (accessed on 1 May 2025).
- Guo, W.P.; Huang, B.; Zhao, Q.; Xu, G.; Liu, B.; Wang, Y.H.; Zhou, E.M. Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an, China. PLoS Negl. Trop. Dis. 2018, 12, e0006916. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Westblade, L.F.; Kessler, D.A.; Sfeir, M.; Slavinski, S.; Backenson, B.; Gebhardt, L.; Kane, K.; Laurence, J.; Scherr, D.; et al. Death from Transfusion-Transmitted Anaplasmosis, New York, USA, 2017. Emerg. Infect. Dis. 2018, 24, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.J.; Weil, A.A.; Branda, J.A. Case 16-2018: A 45-Year-Old Man with Fever, Thrombocytopenia, and Elevated Aminotransferase Levels. N. Engl. J. Med. 2018, 378, 2023–2029. [Google Scholar] [CrossRef]
- Guru, S.; Mahar, M.; Guru, N.; Parent, L. A Neurological Manifestation of Anaplasmosis: A Case Report. Cureus 2025, 17, e77877. [Google Scholar] [CrossRef]
- Schmaier, A.H.; Srikanth, S.; Elghetany, M.T.; Normolle, D.; Gokhale, S.; Feng, H.M.; Walker, D.H. Hemostatic/fibrinolytic protein changes in C3H/HeN mice infected with rickettsia conorii--a model for Rocky Mountain spotted fever. Thromb. Haemost. 2001, 86, 871–879. [Google Scholar]
- Zellner, B.; Huntley, J.F. Ticks and Tularemia: Do We Know What We Don’t Know? Front. Cell Infect. Microbiol. 2019, 9, 146. [Google Scholar] [CrossRef]
- Çoban, E.; Serindağ, H.C.; Kara, E.S.; Selcuk, H.H.; Eren, F.; Albay, V.B.; Soysal, A. Central Nervous System Vasculitis Due to an Endemic Zoonosis in Turkey; Tularemia. Noro Psikiyatr. Ars. 2019, 58, 73–76. [Google Scholar] [CrossRef]
- Kalyan, M.; Tousif, A.H.; Sonali, S.; Vichitra, C.; Sunanda, T.; Praveenraj, S.S.; Ray, B.; Gorantla, V.R.; Rungratanawanich, W.; Mahalakshmi, A.M. Role of endogenous lipopolysaccharides in neurological disorders. Cells 2022, 11, 4038. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Brackney, D.E.; Dupuis, A.P., 2nd; Robich, R.M.; Fauver, J.R.; Brito, A.F.; Williams, S.C.; Anderson, J.F.; Lubelczyk, C.B.; Lange, R.E.; et al. Phylogeographic reconstruction of the emergence and spread of Powassan virus in the northeastern United States. Proc. Natl. Acad. Sci. USA 2023, 120, e2218012120. [Google Scholar] [CrossRef]
- Bazer, D.A.; Orwitz, M.; Koroneos, N.; Syritsyna, O.; Wirkowski, E. Powassan Encephalitis: A Case Report from New York, USA. Case Rep. Neurol. Med. 2022, 2022, 8630349. [Google Scholar] [CrossRef]
- Santos, R.I.; Hermance, M.E.; Gelman, B.B.; Thangamani, S. Spinal Cord Ventral Horns and Lymphoid Organ Involvement in Powassan Virus Infection in a Mouse Model. Viruses 2016, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Feldmann, H. Recent advances in understanding Crimean-Congo hemorrhagic fever virus. F1000Research 2018, 7, F1000 Faculty Rev-1715. [Google Scholar] [CrossRef] [PubMed]
- Kleib, A.S.; Salihy, S.M.; Ghaber, S.M.; Sidiel, B.W.; Sidiya, K.C.; Bettar, E.S. Crimean-Congo Hemorrhagic Fever with Acute Subdural Hematoma, Mauritania, 2012. Emerg. Infect. Dis. 2016, 22, 1305–1306. [Google Scholar] [CrossRef]
- Ulusoy, E.K. Association of ischemic brainstem stroke following tick bite and Crimean-Congo hemorrhagic fever: A rare presentation. J. Clin. Res. Bioeth. 2018, 9, 1000319. [Google Scholar] [CrossRef]
- Metanat, M.; Sharifi-Mood, B.; Alavi-Naini, R.; Kermansaravi, F.; Hamzehnezhad, M. Crimean-Congo Hemorrhagic Fever and Hypertension: A Case Report. Arch. Clin. Infect. Dis. 2014, 9, e20696. [Google Scholar]
- Meisel, C.; Prass, K.; Braun, J.; Victorov, I.; Wolf, T.; Megow, D.; Halle, E.; Volk, H.D.; Dirnagl, U.; Meisel, A. Preventive antibacterial treatment improves the general medical and neurological outcome in a Mouse model of stroke. Stroke 2004, 35, 2–6. [Google Scholar] [CrossRef]
- Ozkurt, Z.; Ozden, K.; Kiki, I.; Usanmaz, M. Prognostic significance of antithrombin activity in patients with crimean-congo hemorrhagic Fever. Eurasian J. Med. 2011, 43, 83–86. [Google Scholar] [CrossRef]
- Locke, S.; O’Bryan, J.; Zubair, A.S.; Rethana, M.; Moffarah, A.S.; Krause, P.J.; Farhadian, S.F. Neurologic Complications of Babesiosis, United States, 2011–2021. Emerg. Infect. Dis. 2023, 29, 1127–1135. [Google Scholar] [CrossRef]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Evolution, Biologic Functions, and Recommended Dietary Allowances for Vitamin D. In Nutrition and Health; Holick, M.F., Ed.; Humana Press: Totowa, NJ, USA, 1999; Available online: https://link.springer.com/chapter/10.1007/978-1-4757-2861-3_1#citeas (accessed on 1 May 2025).
- Kidwell, C.S.; Chalela, J.A.; Saver, J.L.; Starkman, S.; Hill, M.D.; Demchuk, A.M.; Butman, J.A.; Patronas, N.; Alger, J.R.; Latour, L.L.; et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004, 292, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Dos, A.H.; Stenor, C. Exploring corticosteroid therapy in tick-borne encephalitis: A case report and rationale for future research. Diagn. Microbiol. Infect. Dis. 2025, 22, 116964. [Google Scholar] [CrossRef]
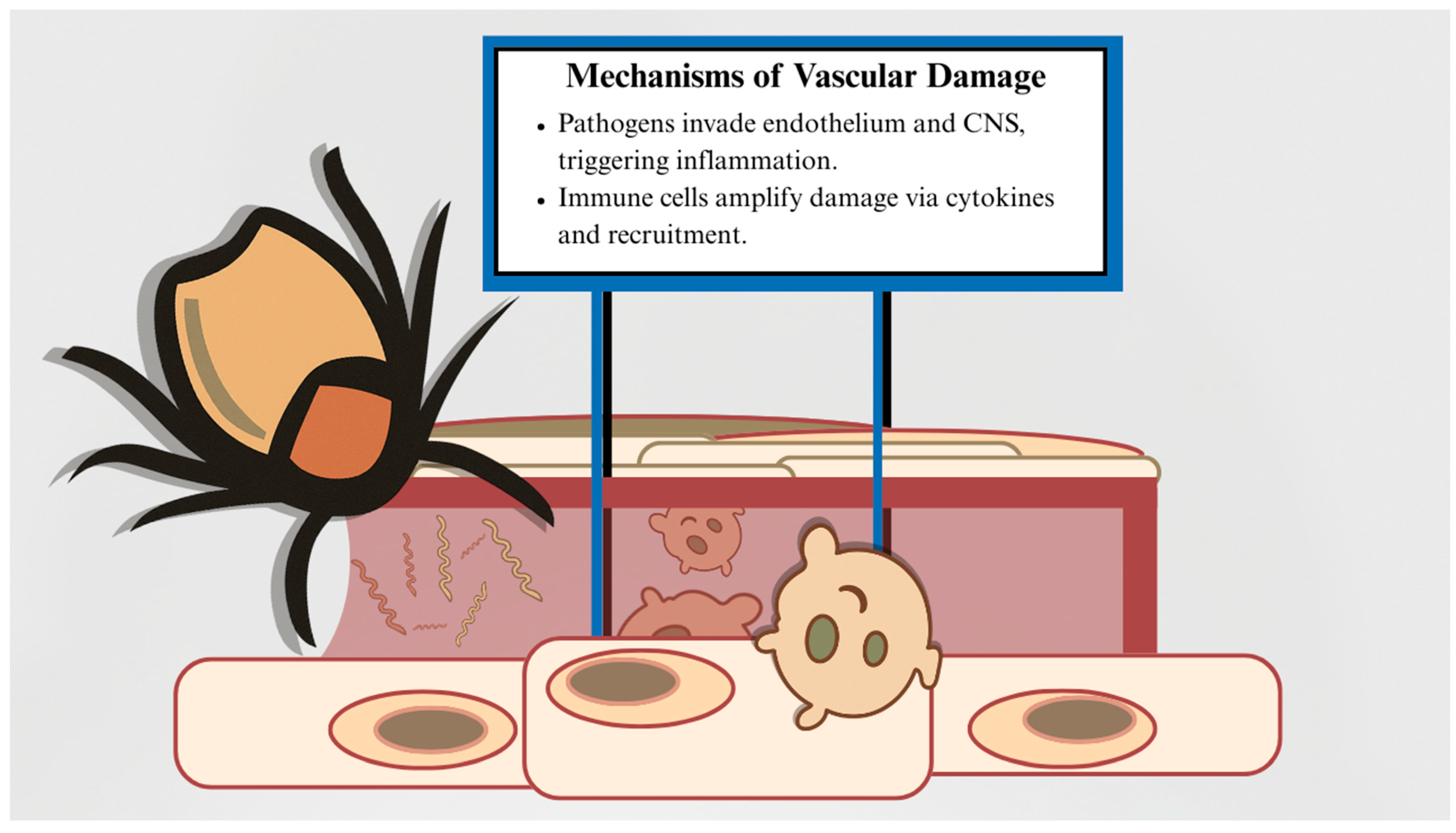
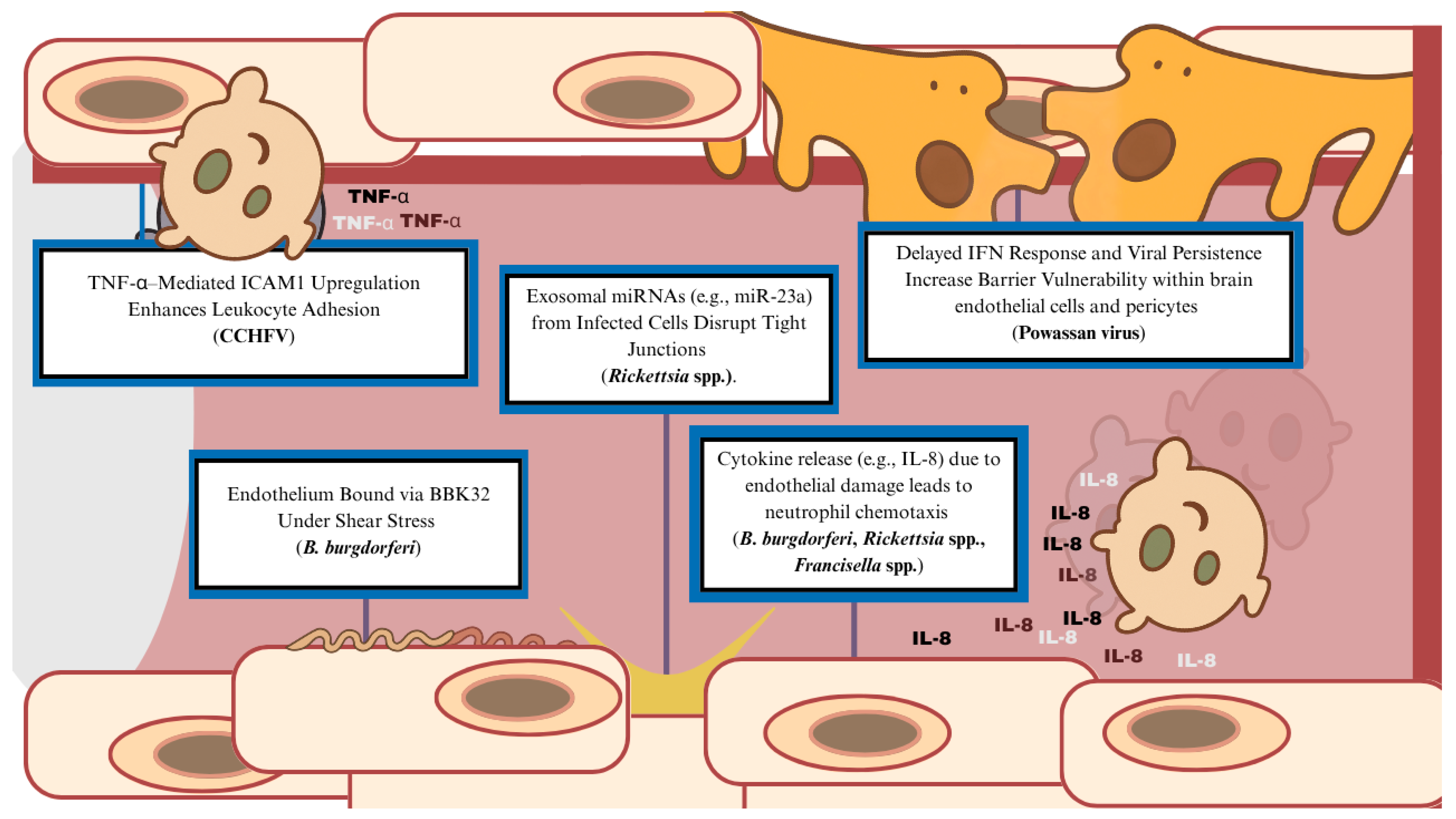
| Organism | Classification | Core Associated Condition | Hallmarks of Infection |
|---|---|---|---|
| Borrelia burgdorferi | Spirochete Bacteria | Lyme Disease [17] |
|
| Borrelia miyamotoi | Spirochete Bacteria | Relapsing Fever [18] |
|
| Rickettsia species | Obligate Intracellular Bacteria | Spotted Fever Rickettsioses (e.g., RMSF) [19] |
|
| Ehrlichia chaffeensis | Obligate Intracellular Bacteria | Human Monocytic Ehrlichiosis (HME) [20] |
|
| Anaplasma phagocytophilum | Obligate Intracellular Bacteria | Human Granulocytic Anaplasmosis (HGA) [21] |
|
| Francisella tularensis | Gram-negative Coccobacillus | Tularemia [22] |
|
| Powassan Virus | Flavivirus (RNA Virus) | Powassan Encephalitis [23] |
|
| Crimean–Congo Hemorrhagic Fever Virus | Nairovirus (RNA Virus, Bunyaviridae family) | Crimean-Congo Hemorrhagic Fever [24] |
|
| Babesia Species | Protozoa (Apicomplexan Parasite) | Babesiosis [25] |
|
| Organism | Representative Mechanisms |
|---|---|
| Borrelia burgdorferi |
|
| Rickettsia spp. |
|
| Francisella tularensis |
|
| Powassan virus |
|
| Crimean–Congo hemorrhagic fever virus |
|
| Tick saliva components |
|
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| Kurian et al., 2014 [62] | 12-year-old boy Previously healthy Tick bite history | Multifocal cerebral vasculitis with stroke-like symptoms (no infarct). | Multifocal arterial stenoses (MCA, ACA, basilar), vessel wall enhancement (MRI) | IV ceftriaxone (4 wks), steroids, aspirin → full recovery. |
| Almoussa et al., 2015 [74] | 43-year-old man No known risk factors Tick bite 4 months prior | Right thalamic infarct Cognitive deficits, hemiparesis | Right thalamic infarct Hyperintense signals in periventricular, periaqueductal areas Bilateral vascular abnormalities (MRI) | IV ceftriaxone (3 wks) → motor recovery, persistent amnesia. |
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| Kumar et al., 2014 [64] | 25-year-old male from India with no comorbidities | Left MCA territory infarct | Left frontotemporal infarct (CT) | Doxycycline → motor recovery |
| Srinivasa Murthy et al., 2015 [92] | 18-month-old male child with no prior conditions | Right MCA territory multifocal infarcts, left hemiplegia and UMN facial palsy | Infarct of the right corona radiata, right basal ganglia, right frontal gyri, insular cortex, and right anterior temporal lobe (MRI) | Doxycycline → rapid recovery |
| Boulahri et al., 2017 [93] | 50-year-old female from Morocco without any cerebrovascular risk factors | Deep left MCA infarct, aphasia, hemiplegia | Left sylvian infarct (MRI), normal CTA | Doxycycline + ciprofloxacin (10 days) → marked recovery |
| El Moussaoui et al., 2024 [94] | 23-year-old male from Lebanon with no prior conditions | Non-aneurysmal SAH with splenial lesion | Transient splenial lesion, no aneurysm (CTA) | Doxycycline (2 wks) → full recovery |
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| García-Baena et al., 2017 [69] | 16-year-old male who was previously healthy | Intracerebral hemorrhage | Right frontoparietal intracerebral hemorrhage with surrounding edema causing mild ventricular compression, without midline shift (CT) | Surgical drainage + oral doxycycline (6 months) → complete recovery |
| Grant et al., 1997 [104] | 54-year-old male from Georgia with no previous cardiovascular risk factors | Subacute ischemic stroke | Nonenhancing right frontal subcortical white matter lesion (MRI) | Intravenous doxycycline → partial recovery |
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| Herbst et al., 2020 [56] | 26-year-old female with HbSC sickle-cell disease | Diffuse bilateral ischemic strokes | Widespread acute + subacute cortical and subcortical ischemic lesions in both hemispheres (MRI) | Doxycycline + clindamycin + atovaquone + eculizumab → poor recovery |
| Kim et al., 2018 [59] | 70-year-old female from Korea with no comorbidities | Lacunar cerebral infarction in the left basal ganglia | Small lacunar infarct in left basal ganglia (MRI); normal MRA | Doxycycline → complete recovery |
| Guru et al., 2025 [110] | 65-year-old female with past medical history of hypertension, type II diabetes, and previous cerebrovascular accident | Acute infarction likely due to embolism | Ill-defined hypodensity in posterior right corona radiata (CT); 3 cm area of restricted diffusion between subcortical white matter and lateral wall of right lateral ventricle ± additional watershed lesions (MRI) | Oral doxycycline (14 days) → complete recovery |
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| Çoban et al., 2019 [113] | 42-year-old female, previously healthy | Acute pontine infarction with central nervous-system vasculitis | Hyperintense non-contrast enhancing lesions in bilateral periventricular, peritrigonal, and pericallosal regions, centrum semiovale, and corona radiata (MRI); narrowing and irregularities of bilateral medial and anterior cerebral arteries (MRA) | Streptomycin and doxycycline (3 weeks + 5 days) → regression of lesions on imaging |
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| Bazer et al., 2022 [116] | 62-year-old male from USA with previous right putamen infarct, hepatitis C, hypertension, and substance abuse | Acute diffuse infarction | Acute left putamen infarct (MRI); normal MRA; small foci of acute infarct involving cerebellum, left basal ganglia, and splenium of corpus callosum (2-week in-patient MRI) | Ceftriaxone + acyclovir → IVIG → no recovery |
| Case | Patient Demographics | Cerebrovascular Event | Imaging Findings | Therapy and Outcome |
|---|---|---|---|---|
| Kleib et al., 2016 [119] | 58-year-old male shepherd with no previous history | Left subdural hematoma | Left subdural hematoma without midline shift (CT); left subdural hematoma with midline shift (repeat 2-week CT) | Corticosteroids + supportive care → complete recovery |
| Ulosoy, 2018 [120] | 60-year-old male livestock worker without previous history | Ischemic stroke | Restricted diffusion in anterior left hemi-pons compatible with acute infarct (MRI) | Ribavirin + platelet transfusion → complete recovery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, D.; Kim, S.; Berry, A.; Belle, M.; Panico, N.; Kaura, S.; Price, A.; Reardon, T.; Ellen, M. Cerebrovascular Disease as a Manifestation of Tick-Borne Infections: A Narrative Review. J. Vasc. Dis. 2025, 4, 33. https://doi.org/10.3390/jvd4030033
Doyle D, Kim S, Berry A, Belle M, Panico N, Kaura S, Price A, Reardon T, Ellen M. Cerebrovascular Disease as a Manifestation of Tick-Borne Infections: A Narrative Review. Journal of Vascular Diseases. 2025; 4(3):33. https://doi.org/10.3390/jvd4030033
Chicago/Turabian StyleDoyle, David, Samuel Kim, Alexis Berry, Morgan Belle, Nicholas Panico, Shawn Kaura, Austin Price, Taylor Reardon, and Margaret Ellen. 2025. "Cerebrovascular Disease as a Manifestation of Tick-Borne Infections: A Narrative Review" Journal of Vascular Diseases 4, no. 3: 33. https://doi.org/10.3390/jvd4030033
APA StyleDoyle, D., Kim, S., Berry, A., Belle, M., Panico, N., Kaura, S., Price, A., Reardon, T., & Ellen, M. (2025). Cerebrovascular Disease as a Manifestation of Tick-Borne Infections: A Narrative Review. Journal of Vascular Diseases, 4(3), 33. https://doi.org/10.3390/jvd4030033





