Abstract
Depression is common among patients suffering from cancer, but is often challenging to diagnose due to the overlap of symptoms with cancer and its treatments. Additionally, treating depression in cancer patients is challenging because of the confusion between the adverse effects of antidepressants, cancer treatments, and cancer symptoms. This study aims to evaluate the safety and adverse effects of pharmacological interventions, focusing on antidepressants and psychedelics, in the treatment of depression in cancer patients. The review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, and includes studies published up to July 2024. We searched PubMed, Scielo, and Lilacs databases, and included randomized, double-blinded, controlled clinical trials involving cancer patients with depressive symptoms. A total of 1764 articles were identified, with 21 randomized controlled trials meeting the inclusion criteria. All studies involved cancer patients with depressive symptoms, and only one study included patients with other life-threatening conditions. Serious adverse events related to antidepressant treatment were reported in only two studies, indicating an acceptable safety profile. Most other adverse effects were mild to moderate, and generally well-tolerated. Serious adverse events were infrequent; however, the small sample sizes underscore the necessity of larger, placebo-controlled trials assessing the safety of antidepressants and psychedelics in cancer patients.
1. Introduction
Depression is a commonly diagnosed psychiatric disorder among cancer patients, and both conditions interact in a complex and significant manner. Previous studies indicate a prevalence of depression in oncologic environments that varies from 15% up to 20–25% when considering other depressive diagnoses, such as dysthymia and minor depression [1,2]. However, since multiple variables can affect depression amongst cancer patients, including cancer stage, location, treatment type, and employed diagnosis tools, it can be hard to precisely estimate the prevalence [3].
An overlap between criteria defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the International Classification of Diseases (ICD) complicates the diagnosis of depression in patients facing serious medical conditions, such as cancer. Fatigue, weight loss, and sleep disturbances symptoms are frequent in depression and in cancer itself [4,5]. Apart from physical symptoms, oncologic disease progression is linked to functional and social impacts. It is important to acknowledge that recurrent thoughts of death can be a reasonable reaction considering the limited life expectancy or striking physical suffering [6,7]. Additionally, atypical depressive symptoms, including anxiety, despair, fatigue, post-traumatic stress symptoms, body image distortions, internal restlessness, and social isolation, are more prominent in this population and must be carefully evaluated when identifying depressive symptoms [8,9,10,11].
Providing better interventions is another challenge in diagnosing depression among this population. The last review on this matter found little evidence for antidepressants effects compared to placebo [12]. The decision to prescribe antidepressant for cancer patients must consider each case individually, regarding the drug efficacy and its safety profile. Despite previous studies finding antidepressants as being well-tolerated among this population, the last review found a substantial dropout rate since adverse effects (AEs) caused by treatment are similar to those caused by anticancer therapy, pain syndromes, and to cancer symptoms itself [12,13].
Aside from the lack of evidence for antidepressants’ efficacy, new substances are being investigated to treat depressive symptoms in this population. The first studies investigating psychedelic use in terminal diseases date from the 70s, when lysergic acid diethylamide (LSD) and N,N-dipropyltryotamine (DPT), both serotonergic 5-HT2A receptors agonists, were evaluated in combination with psychotherapy [14]. Meanwhile, war on drugs policies interrupted research being conducted on these substances. More recently, new studies reveal promising results as a treatment option for this population, indicating a decrease in anxiety and depressive symptoms, a reduction in fear of death, and an increased wellbeing, quality of life, and spirituality [15,16]. Psychedelics, also known as hallucinogens, can be divided into four pharmacological classes: (1) classic psychedelics, 5HT2A receptors agonists, such as LSD, DPT, and N,N-Dimethyltryptamine (DMT); (2) empathogens, serotonin and dopamine reuptake inhibitors, such as 3,4-methylenedioxyphenethylamine (MDMA); (3) dissociative anesthetic agents, N-methyl-D-aspartate (NMDA) receptors antagonists, as ketamine; and (4) atypical psychedelics, such as tetrahydrocannabinol, salvinorin A, and ibogaine [17,18,19].
Esketamine is a potent, rapid-acting antidepressant that works by antagonizing NMDA receptors. As the S-enantiomer of ketamine, esketamine disrupts the normal excitatory signaling mediated by glutamate at these receptors, which in turn leads to a cascade of neurobiological effects. While many reviews and meta-analyses have focused on conventional antidepressants or other classes of NMDA receptor modulators, esketamine’s unique profile makes it particularly relevant. Its ability to reduce depressive symptoms within hours or days—as opposed to the several weeks required by standard treatments—offers a critical option for individuals with treatment-resistant depression or acute suicidal ideation. In this context, the rapid efficacy of esketamine offers a promising alternative, expanding the range of treatment options for patients who do not respond adequately to traditional antidepressants.
Currently available reviews and meta-analysis on antidepressants and psychedelics focus on their efficacy, without further elaborating on possible AEs. Therefore, this review aims to systematically investigate adverse effects and safety of currently available pharmacological treatments for depression in oncologic patients to establish suitable safety protocols for their administration.
2. Materials and Methods
Data for this review were collected following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
2.1. Search Strategy
The search was conducted using PubMed, LILACS, and SciELO databases using the following string: (cancer) AND ((antidepressants) OR (psychedelics) OR (psilocybin) OR (LSD) OR (ketamine)) AND ((trial) OR (randomized controlled trials) OR (double-blind procedure)). Studies published until 14 July 2024 were included without language restriction. A flow diagram illustrating the different phases of the systematic search update is presented in Figure 1. The protocol for the systematic review of our study was registered for PROSPERO (CRD42023429203).
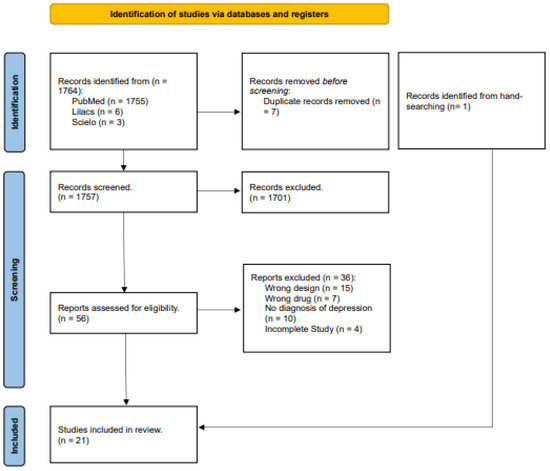
Figure 1.
PRISMA flowchart for the selection of the studies.
Additionally, the review “Antidepressants for the Treatment of Depression in People with Cancer” from the Cochrane Database of Systematic Review by Vita et al. serves as a pivotal reference in this area, with its methodology and findings significantly informing our study’s approach and discussion [12]. Moreover, the article “Safety Issues of Psilocybin and LSD as Potential Rapid Acting Antidepressants and Potential Challenges” by Rossi et al. was also used as a conceptual basis, particularly regarding the safety considerations and challenges associated with rapid-acting antidepressants [20].
2.2. Selection Criteria and Studies Selection
In this review, we systematically analyzed the available randomized double-blinded reports on AEs and safety in pharmacological treatments of depression on adult (≥18 years) cancer patients. All selected studies should contain a control group that could be a different pharmacological treatment or a placebo.
By pharmacological treatment, we meant antidepressants included on Anatomical Therapeutic Chemical Code/Daily Defined Dosage (ATC/DDD), recognized by World Health Organization (WHO) until 2023, but also other substances being tested for depression treatment on cancer patients (ex.: LSD and Psilocybin) [15,16,20,21,22,23,24].
Participants inclusion in studies should be based on a formal major depressive disorder (MDD) diagnosis or symptoms evaluation by a valid instrument. Studies including diagnosis other than MDD or MDD associated with generalized anxiety disorder, persistent depressive disorder, or adjustment disorders were excluded. AEs and safety analysis reported on follow-up studies based on the original ones were also included.
2.3. Data Extraction
The following criteria were defined in order to identify the AEs in the selected articles: (i) AEs reported as such by the authors themselves, which included systematically evaluated AEs (using standardized scales and/or physical and biological measurements), but also those evaluated in a nonsystematic manner (whatever subjective or physical effect described by the authors or reported by the participants as being adverse or negative); (ii) all of the cardiovascular effects reported were included, apart from being considered as AEs by the authors or not; (iii) subjective results measured by effects scales that could be clearly defined as AEs were also included, such as anxiety, derealization, and depersonalization, among other symptoms; (iv) intoxication and death reports that possibly occurred during the experiments were also included.
Extracted information regarding AEs were classified in categories as psychological, neurological, cardiovascular, gastrointestinal, and others, following a previous study [25]. Additionally, relevant information reported on original studies and follow-ups were included, such as the study design, sample, evaluated substances and doses, depressive symptoms scales employed, methods for AEs assessment, and authors’ comments regarding safety and tolerability.
2.4. Quality Assessment
Quality assessment was evaluated by two independent reviewers using the revised Cochrane risk of bias tool for randomized trials (RoB 2 tool) [26]. In case of disagreement in any of the evaluated points, a third reviewer was consulted for the final decision. The evaluation of the studies can be found in Table S1 of the Supplementary Material.
3. Results
A total of 1764 articles were identified from the databases search, from those 21 were selected based on selection criteria. All included studies are randomized, double-blinded, and placebo controlled, and dated from 1985 to 2024. They were developed in 11 different countries (Romania, Belgium, USA, Austria, Canada, Germany, Italy, the Netherlands, Turkey, Switzerland, and China), but most of the research centers were concentrated in the United States, followed by China. Only three studies were multicentered [27,28,29]. Seven of them had a cross-over design, and the remaining had parallel groups [15,16,21,22,23,24,30]. The total sample consisted of 1858 volunteers (210 men and 1648 women).
All studies were performed on cancer-diagnosed patients with associated depressive symptoms, and only one study included patients with other life-threatening clinical conditions [22]. The main type of cancer was breast cancer, followed by cervical cancer. The pharmacological treatments consisted of the following: ketamine, employed by four studies with a dose range from 0.25 to 1.0 mg/kg [31,32,33,34]; psilocybin, employed by four studies with a dose range from 14 to 30 mg (considering a body weight of 70 kg) [15,16,21,30]; fluoxetine, evaluated by four studies with a dose range from 20 to 60 mg a day [27,35,36,37]; LSD, tested by three studies using 20 or 200 µg doses [22,23,38]; mianserin was tested by two studies in a dose range from 10 to 60 mg a day [39,40]; desipramine, tested by a single study employing a dose range from 125 to 200 mg a day [29]; trazadone, tested on a dose of 150 mg a day [41]; paroxetine, tested on a dose range from 20 to 40 mg a day [28,29] amitriptyline, with doses varying from 75 to 150 mg a day [28]; mirtazapine, with doses varying from 5 to 30 mg a day and imipramine tested with doses from 5 up to 100 mg a day [42].
The follow-up periods in each study varied between 3 days and 12 months, and are detailed in Table 1.

Table 1.
Follow-up time for each study.
Many different scales were employed for depressive symptoms evaluation, such as the Hamilton Depression Rating Scale (HDRS), the Hospital Anxiety and Depression Scale (HADS), the Montgomery–Åsberg Depression Rating Scale (MADRS), the Brief Zung Self Rating Depression Scale (BZSDS), the Zung Self Rating Depression Scale (ZSRDS), the Beck Depression Inventory (BDI) and the Patient Health Questionnaire-9 (PHQ-9). All tested antidepressants had positive results, shown by a decrease in depressive symptoms scale scores and good safety and tolerability profiles, besides improving life quality and reducing the stress related to the cancer treatment. In studies employing ketamine, besides the improvement of depressive symptoms, patients also had a reduction in post-operatory pain [31,32,33,34]. Classic psychedelics are presented as a new alternative, with promising results for the improvement of depression and anxiety symptoms in this population.
AEs were documented based on researchers’ evaluations, patients report, laboratory exams, and vital signs monitoring, such as heart rate and blood pressure. Subjective scales, such as the 5-Dimensional Altered States of Consciousness Rating Scale (5D-ASC), the Brief Psychiatric Rating Scale (BPRS), and the Monitor Rating Questionnaire (MRQ), were also utilized regarding the specificity of AEs in each study to offer a broader approach for the comprehension of depressive symptoms and the identification of potential AEs.
Regarding the types of cancer, ten studies had a sample with mixed cancer types, and breast cancer was predominant among all participants (as presented in Table 2). Participants enrolled in studies investigating traditional antidepressants use were in active oncologic treatment phase, including chemotherapy, hormone therapy, and/or radiotherapy [27,28,29,35,36,37,39,40,41].

Table 2.
Number of patients (n) by cancer type in each publication.
Costa and collaborators treated patients with cytotoxic drugs and/or radiotherapy simultaneously with mianserin without any detrimental interactions’ reports [39]. Another study investigating mianserin had four patients simultaneously going through chemotherapy without any detrimental interactions or alterations on white cells counting, which concluded the trial without complications [40]. Studies concerning fluoxetine, trazadone, and mirtazapine had participants submitted to oncologic treatment with chemotherapy and/or hormone therapy without any AEs reported [27,35,36,37,41,42]. In Pezzella and collaborators study, participants were submitted to different regimens of chemotherapy, and trials analyzing classic psychedelics (psilocybin and LSD) and ketamine did not include participants going through treatment with chemotherapy, hormone therapy, and/or radiotherapy [28].
3.1. Adverse Effects
3.1.1. Classical Antidepressants
Details regarding each study and its reported AEs are presented in Table 3. A more detailed table with details can be found in the Supplementary Material in Table S2.

Table 3.
AEs reported by publication.
The most frequent AEs, considering all evaluated substances, were gastrointestinal symptoms. Following the timeline of included reports, the first trial did not find a significant difference on the number of patients presenting AEs in the mianserin (n = 17) compared to the placebo (n = 1) group. Miaserin most frequently reported AE was sleepiness, which was generally mild and was reported for six patients during the first week. Sleepiness incidence sharply decreased and was not addressed at day 28 [39]. Razavi and collaborators study reported digestive and neuropsychiatric AEs more frequently in fluoxetine (24% and 49%, respectively) compared to the placebo group (13% and 35%, respectively); however, this difference was not statistically significant [27]. In Van Heeringen and collaborators’ study, the trial mianserin and placebo groups did not have statistically significant differences regarding AEs [40].
Differently from previous studies, Holland et al. used a standardized method to record the AEs, namely the Food and Drugs Administration’s coding symbol and the Thesaurus for Adverse Event Terminology (COSTART) dictionary. The single AE presenting a statistically significant difference between treatments was dry mouth, recorded in 14 patients (66.6%) in the fluoxetine group and 4 patients (23.5%) in the desipramine group [35]. Another study from Razavi and collaborators compared trazodone to clorazepate, and did not find any significant differences between groups regarding safety and AEs frequency. However, a patient in trazodone group had to be withdrawn due to an event of severe vertigo and pulsation in the head. In addition, 13 patients in the trazodone group and 3 patients in the clorazepate group had a dose adjustment due to AEs (sleepiness, aggressiveness, and disinhibition) [41].
Next, in Pezzella et al.’s study, 47 (53.4%) patients in the paroxetine group reported at least one AE compared to 53 (59.6%) patients in the amitriptyline group. The most frequently reported AEs for paroxetine were nausea (13.6%) and leucopenia (10.2%), both are commonly observed in patients undergoing chemotherapy. As for amitriptyline group, the most frequent AEs were dry mouth (14.6%) and constipation (11.2%). Anticholinergic effects (dry mouth, constipation, urinary retention, and impaired urination) were more frequent in the amitriptyline group (19.1%, n = 17) than in the paroxetine group (11.4%, n = 10). The AEs most frequently reported as related to the depression treatment were sleepiness (8 patients in the amitriptyline group and 3 in the paroxetine group) and dry mouth (13 patients in the amitriptyline group and 4 patients in paroxetine group), although their incidence in the amitriptyline group was at least twice that reported in the paroxetine group [28].
Four patients had to withdraw from Fisch et al.’s fluoxetine study (two because of daily headaches and two because of nausea or vomiting). In addition, fifteen patients had sudden hospitalizations during the trial, nine in the fluoxetine group and six in placebo group (there was no statistically significant difference between hospitalizations for each group) [36]. In Musselman et al. trial patients treated with desipramine had higher incidence of dry mouth when compared to the placebo, but this difference was not statistically significant (p = 0.09). The most frequent AEs reported for desipramine were dry mouth (73%, n = 8), constipation (36%, n = 4), headache (36%, n = 4), and pain (36%, n = 4). As for paroxetine, dry mouth (46%, n = 6), nausea (38%, n = 8), and pain (38%, n = 5) were more frequently reported [29].
Navari et al. compared fluoxetine to placebo and reported two increases in hepatic function exams [37]. Finally, the trial of Cankurtaran et al. evaluated mirtazapine (n = 20) compared to imipramine (n = 13) and placebo (n = 20) groups. There was no difference between groups regarding pain, nausea/vomit, and appetite. Initial, middle, and late insomnia scores only improved in the mirtazapine group [42].
3.1.2. New Treatments
Beyond traditional approaches using classic antidepressants, new substances are being investigated for their possible antidepressive properties. Unlike the previously mentioned medicines, these new treatments do not demand daily doses, but rather comprise experimental sessions where patients’ intake of the psychoactive substances occur under observation. An advantage of these interventions is the fast improvement of depressive symptoms.
Grob and collaborators trial comprised two sessions spaced by many weeks. In one of them, participants received a psilocybin dose (0.2 mg/kg), and in the other, they received niacin (used as active placebo; 250 mg). Reported AEs were mostly psychological; during sessions, psilocybin induced moderate ego dissolution and auditive alterations (more details on Table 2), but subjective effects during sessions were well tolerated [21]. In another study, patients again participated on two sessions, a psilocybin one (0.3 mg/kg) and a niacin one (250 mg), separated by a 7-week interval. In both cases, patients went through a therapy session during substance effects. No serious AEs, neither clinic nor psychiatric, were reported, and pharmacological intervention was not necessary when handling psychological effects [16]. In the same year, another trial compared high (22 or 30 mg/70 kg) to low doses (1 or 3 mg/70 kg) of psilocybin. Again, no serious AEs were recorded, and psychological subjective effects were transient and improved at the end of sessions [15].
In Gasser et al.’s trial, two doses were evaluated (200 µg vs. 20 µg, the latter serving as an active placebo) in a two-arm protocol, each receiving a single intervention. At the end of the two-month follow-up, the placebo group was offered to receive the experimental dose. The group receiving the experimental dose reported a wider range of AEs when compared to the placebo group, such as affective lability, anxiety, emotional distress, depersonalization, derealization, euphoric mood, feeling abnormal, feeling cold, gait disturbance, hallucinations, hyperhidrosis, illusions, mydriasis, and abnormal thinking. Additionally, AEs were more frequent and intense in sessions evaluating the 200 µg dose. Still, patients receiving the experimental dose reported anxiety less frequently during the session than those receiving the placebo [22,23].
Another substance that has been tested in the last few years is ketamine. Fan and collaborators’ study did not report any AEs when comparing R-ketamine to mildazolam [31]. Wang and collaborators compared various doses of both ketamine enantiomers, R and S-ketamine, without any serious AEs reported. The most frequent AE reported was nausea, followed by dizziness and vomit, respectively [32]. A study comparing ketamine and placebo (saline) in patients with depressive symptoms submitted to intracerebral tumor resection did not find any statistically significant difference regarding anxiety and delirium. Additionally, three days following the surgery, no statistically relevant difference was observed in patients presenting mania, psychotic, or dissociative symptoms [33]. In the same year, Liu et al. compared R to S-ketamine, and concluded that the latter has less complications and better tolerability regarding effects like nausea, vomit, and dizziness [34].
Concerning the follow-up studies, patients from Ross et al.’s study did not report any lasting AEs related to the sessions of psilocybin-assisted therapy [16,30]. Qualitative analysis based on Gasser et al.’s study did not mention any negative reports from patients regarding the LSD sessions, and nor were any AEs reported during follow-ups [22,23].
3.2. Serious Adverse Effects
One serious AE was reported in a patient receiving an LSD intervention (200 µg); during sessions, the volunteer experienced an acute anxiety episode and delusions [24]. The patient was then successfully treated with lorazepam and olanzapine (doses not informed; olanzapine single dose was administered since lorazepam alone was not effective in blocking symptoms). The following session patient’s dose was reduced to 100 µg, and no AEs were reported.
Further mentioned serious AEs were considered unrelated to depression pharmacological treatment: one patient was hospitalized due to obsessive–compulsive disorder (the patient’s previous comorbidity) around 6 months after LSD treatment (no temporal relationship); two had unexpected pregnancies followed by spontaneous abortions, one between treatment sessions with LSD and the other approximately 12 weeks after last LSD treatment; a radius fracture 16 weeks after the last LSD treatment (occurred during a private party); surgical correction of nasal septum deviation 11 weeks after last LSD administration (previously planned surgery); suspected transient ischemic attack 2 weeks after the last LSD session (patient suffered from Marfan syndrome and had previously experienced similar attacks); hospitalization due to disorientation 6 weeks after placebo treatment (episode occurred before LSD treatment and is attributed to chemotherapy); and one patient deceased due to cancer progression 10 weeks after the last placebo intervention and before LSD treatment onset [24].
In Costa et al. trial, one patient allocated to the mianserin group deceased, but this event was considered unrelated to the intervention [39]. Pezzella and collaborators also reported serious AEs unrelated to depression pharmacological treatment. One patient in the paroxetine group developed severe leukopenia and a mild reaction at the breast implant site, which was deemed to be unrelated to the medication administered by the study. This patient underwent chemotherapy during the trial, and received a combination of fluorouracil, mesna, cyclophosphamide, mitozantrone hydrochloride, ondansetron hydrochloride, dexamethasone, metaclopromide, and haloperidol. Also, the patients’ treatment included lenograstim, piritramide, and tramadol hydrochloride. In the amitriptyline group, three patients presented non-fatal serious AEs; that is, moderate persistent lesion, intense pain, severe leukopenia, and moderate respiratory infection, but none were considered related to the study’s medication [28].
No serious AEs were reported in psilocybin and ketamine trials.
3.3. Efficacy
Although this review does not focus on the efficacy of classical and psychedelic antidepressants in improving depressive symptoms, Table 4 summarizes the evaluated treatments along with a brief description of their efficacy and safety.

Table 4.
Key differences between classic antidepressants and psychedelics.
3.4. Quality Assessment
All included articles were randomized, double-blinded, and had a control group (as required by the selection criteria). The quality assessment tool employed evaluates studies using five domains: bias arising from randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of reported results. Biases mostly arose from lack of data regarding randomization process, deviations from intended intervention, loss of participants during follow-up, and the lack of standardization for AEs evaluation. Nonetheless, the design of included studies entailed good bias control.
4. Discussion
The present review evaluated adverse effects documented in double-blinded, randomized, controlled trials investigating pharmacological treatment for depression in cancer patients. Only one serious AE related to the pharmacological intervention was reported, and consisted of acute transient anxiety and delusion during LSD administration to a patient enrolled in Holze et al.’s study [24]. The symptoms ceased after pharmacological intervention. LSD, a classic psychedelic, can promote mental and thought alterations through 5-HT2A receptor agonism, and it can induce anxiety, especially in high doses. The dose employed by this study was considered high, increasing the risk for this particular AE [38,43,44].
In a previous systematic review evaluating AEs induced by classic psychedelics, like LSD and psilocybin, administration in double-blinded, randomized, controlled studies did not find any documented serious AEs. The most reported AEs were headaches/migraine, nausea/vomit, cardiovascular alterations, and psychological alterations, similarly to what was described for the oncological sample [25].
Through interactions with the serotonergic system, these substances can lead to psychological effects, causing changes in thoughts, perceptions, and emotions [17,45]. However, the subjective effects were limited to the experimental sessions, without any reported symptoms persisting after the intervention. The activation of 5-HT2A receptors also explains the headaches and migraines AEs, which could result from serotonin toxicity [46]; additionally, interactions with gastrointestinal receptors can account for the nausea and vomit [47,48].
Another evaluated substance, also considered psychedelic due to its perception-altering properties, is ketamine, which acts as an NMDA receptors antagonist. In the last few years, ketamine has been approved as a new depression treatment, and two meta-analysis concerning treatment-resistant depression support its antidepressant properties [49,50,51]. The approved medicine, esketamine, employs intranasal administration. Esketamine has proven effective for treating treatment-resistant depression (TRD), although it must be used with caution. The robust evidence supporting its efficacy, safety, and tolerability makes it a valuable, innovative treatment option in clinical practice [52]. Esketamine nasal spray was identified as the preferred option for patients who are unresponsive to two antidepressants, with strong agreement on its use in outpatient settings [53].
However, the four studies included in this review employed intravenous administration and considered both ketamine enantiomers (S- and R-ketamine) [32]. The reported AEs were nausea/vomit, dizziness, and transient dissociative, psychotic, and maniac symptoms. There were no reports of lasting AEs. Although studies on intranasal esketamine were not included in this review, it nonetheless demonstrates promising potential as a treatment option.
Regarding the remaining antidepressants, the main AEs for selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine and paroxetine, were gastrointestinal, that is, nausea, vomit, abdominal pain, and diarrhea. These effects arise from SSRIs interaction with serotonin receptors in the gastrointestinal tract [54]. Additionally, SSRIs can induce psychiatric effects, like agitation, anxiety, nervousness and neurological effects, such as tremors, weight loss or gain, and sexual dysfunction.
Tricyclic antidepressants—such as imipramine, desipramine and amitriptyline—are known for their efficacy in depression treatment when compared to other classes that block monoamines reuptake, mainly norepinephrine and serotonin, and in a smaller range, dopamine [55]. Their post-synaptic activity varies according to the neurotransmission system involved, and generally entails AEs. Tricyclics block muscarinic (cholinergic), type-1 histaminergic, α-2 and β-adrenergic, and serotonergic receptors. More rarely, they can also block dopamine receptors as well. These actions are not necessarily related to the antidepressive effects, but with AEs like dry mouth, constipation, urinary retention (anticholinergic effects), sedation, somnolence, dizziness (histaminergic effects), reflexive tachycardia, vertigo, and tremors (α-1 and α-1 adrenergic effects) [56]. Generally, the AEs profile found in this review and those pointed out by systematic reviews evaluating acceptance and tolerability in patients without cancer are not different [12]. SSRIs have better acceptance compared to tricyclics, since they induce less AEs due to receptor selectivity [57].
Another antidepressant evaluated was mianserin, a tetracyclic medicines that does not induce anticholinergic and cardiovascular effects. Costa et al. and Van Heeringen et al.’s studies found that sedative effects were the most frequently reported, especially during treatment onset, improving with time [39,40]. Another tetracyclic antidepressant, mirtazapine, was investigated due to histaminergic effects caused by the actions of H1 receptors, which can increase appetite and induce somnolence [42]. In this case, AEs were used to benefit oncologic patients with anorexia and insomnia [58,59].
Lastly, trazadone, a selective serotonin reuptake inhibitor with antagonistic action over 5-HT2A-C post-synaptic receptors, was evaluated for its sedative effects. In Razavi et al.’s trial, a patient was withdrawn due to pulsation in the head and severe vertigo after five days of trazadone administration. Other patients demanded a dose adjustment due to somnolence, disinhibition, and aggressiveness [27].
One of the main limitations of the evaluated studies is the lack of standardization in the assessment of adverse effects (AEs). Most AEs were documented based on patient reports, with only a few studies utilizing standardized instruments. A significant issue is the heterogeneity of the studies themselves, which impacts the reliability of findings. In addition to the diversity of cancer types, stages, and treatments, the severity of the treatment regimen also influences the occurrence and intensity of AEs. Some studies suggest that pancreatic, lung, head, neck, and ovarian cancers are more strongly associated with depressive symptoms [60]. Moreover, cancer treatments—including multiple cytotoxic drugs, radiotherapy, opioids, and hormonal therapies—act as confounding factors, further complicating the evaluation of AEs. Regarding new pharmacological interventions, such as psilocybin and LSD, while they show promising antidepressant effects, larger studies are required to better establish their safety and tolerability. Altogether, these factors limit the generalizability of the safety profile of these treatments.
5. Conclusions
Overall, pharmacological treatment for depression in cancer patients appears to be relatively safe. Classic antidepressants predominantly cause gastrointestinal adverse events—such as nausea, vomiting, abdominal pain, and dry mouth—which, while common, are generally not serious. In contrast, psychedelic treatments have been observed to induce subjective and psychological symptoms during experimental sessions; these symptoms typically resolve by the end of the session, although a notable exception was found with LSD treatment, where a serious adverse event required pharmacological intervention and a subsequent dose reduction.
Given these findings, future trials should be designed to directly compare the efficacy and safety profiles of esketamine, psychedelics, and traditional antidepressants. Such head-to-head comparisons will be crucial in identifying optimal treatment modalities for depression in cancer patients. Furthermore, there remains a significant gap in the current literature regarding standardized evaluation of adverse events and the impact of sample heterogeneity, particularly concerning the various types of cancer. This highlights the need for multicentric, randomized, and placebo-controlled studies that employ uniform methods for adverse event assessment. Addressing these gaps will not only enhance our understanding of treatment effects, but also pave the way for more precise and effective interventions in this vulnerable population.
Future research should focus on direct comparative studies among esketamine, psychedelics, and traditional antidepressants; larger, multicentric trials that can adequately address sample heterogeneity and the development and application of standardized methodologies for adverse event evaluation. These research directions are essential for advancing our knowledge and improving treatment strategies for depression among cancer patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/psychoactives4010006/s1, Table S1 Quality assessment; Table S2 Design, sample, substances and dose, depression assessment, AEs assessment, AEs reported by publication.
Author Contributions
Original draft preparation and systematic search: R.M.M. and L.T.L.G.; review and editing: R.G.d.S. and J.C.B.; supervision: J.E.C.H. and R.G.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
R.M.M. and L.T.L.G. received funding from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). J.E.C.H. is a recipient of a CNPq 1A productivity fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of Depression, Anxiety, and Adjustment Disorder in Oncological, Haematological, and Palliative-Care Settings: A Meta-Analysis of 94 Interview-Based Studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Riedl, D.; Schüßler, G. Factors Associated with and Risk Factors for Depression in Cancer Patients—A Systematic Literature Review. Transl. Oncol. 2022, 16, 101328. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Nanni, M.G.; Riba, M.B.; Sabato, S.; Grassi, L. The Burden of Psychosocial Morbidity Related to Cancer: Patient and Family Issues. Int. Rev. Psychiatry 2017, 29, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.M.A.; Bobonis Babilonia, M. Distinguishing Depressive Symptoms From Similar Cancer-Related Somatic Symptoms: Implications for Assessment and Management of Major Depression after Breast Cancer. S. Med. J. 2017, 110, 667–672. [Google Scholar] [CrossRef]
- Panjwani, A.A.; Li, M. Recent Trends in the Management of Depression in Persons with Cancer. Curr. Opin. Psychiatry 2021, 34, 448–459. [Google Scholar] [CrossRef]
- Breitbart, W. Depression, Hopelessness, and Desire for Hastened Death in Terminally Ill Patients With Cancer. JAMA 2000, 284, 2907. [Google Scholar] [CrossRef]
- Vehling, S.; Kissane, D.W.; Lo, C.; Glaesmer, H.; Hartung, T.J.; Rodin, G.; Mehnert, A. The Association of Demoralization with Mental Disorders and Suicidal Ideation in Patients with Cancer. Cancer 2017, 123, 3394–3401. [Google Scholar] [CrossRef]
- Brenne, E.; Loge, J.H.; Kaasa, S.; Heitzer, E.; Knudsen, A.K.; Wasteson, E. Depressed Patients with Incurable Cancer: Which Depressive Symptoms Do They Experience? Palliat. Support. Care 2013, 11, 491–501. [Google Scholar] [CrossRef]
- Diaz-Frutos, D.; Baca-Garcia, E.; García-Foncillas, J.; López-Castroman, J. Predictors of Psychological Distress in Advanced Cancer Patients under Palliative Treatments. Eur. J. Cancer Care 2016, 25, 608–615. [Google Scholar] [CrossRef]
- Yi, J.C.; Syrjala, K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. N. Am. 2017, 101, 1099–1113. [Google Scholar] [CrossRef]
- Ebede, C.C.; Jang, Y.; Escalante, C.P. Cancer-Related Fatigue in Cancer Survivorship. Med. Clin. N. Am. 2017, 101, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Vita, G.; Compri, B.; Matcham, F.; Barbui, C.; Ostuzzi, G. Antidepressants for the Treatment of Depression in People with Cancer. Cochrane Database Syst. Rev. 2023, 2023, CD011006. [Google Scholar]
- Rayner, L.; Price, A.; Evans, A.; Valsraj, K.; Higginson, I.J.; Hotopf, M. Antidepressants for Depression in Physically Ill People. Cochrane Database Syst. Rev. 2010, 2010, CD007503. [Google Scholar] [CrossRef] [PubMed]
- Petranker, R.; Anderson, T.; Farb, N. Psychedelic Research and the Need for Transparency: Polishing Alice’s Looking Glass. Front. Psychol. 2020, 11, 1681. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Kersgaard, B.; Addy, P.H. Clinical Applications of Hallucinogens: A Review. Exp. Clin. Psychopharmacol. 2016, 24, 229–268. [Google Scholar] [CrossRef]
- Carlini, E.A.; Maia, L.O. Plant and Fungal Hallucinogens as Toxic and Therapeutic Agents. In Plant Toxins; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–44. [Google Scholar]
- Norwegian Institute of Public Health WHO Collaborating Centre for Drug Statistics Methodoly. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 14 July 2024).
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot Study of Psilocybin Treatment for Anxiety in Patients With Advanced-Stage Cancer. Arch. Gen. Psychiatry 2011, 68, 71. [Google Scholar] [CrossRef]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated With Life-Threatening Diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef]
- Gasser, P.; Kirchner, K.; Passie, T. LSD-Assisted Psychotherapy for Anxiety Associated with a Life-Threatening Disease: A Qualitative Study of Acute and Sustained Subjective Effects. J. Psychopharmacol. 2015, 29, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Holze, F.; Gasser, P.; Müller, F.; Dolder, P.C.; Liechti, M.E. Lysergic Acid Diethylamide–Assisted Therapy in Patients With Anxiety With and Without a Life-Threatening Illness: A Randomized, Double-Blind, Placebo-Controlled Phase II Study. Biol. Psychiatry 2023, 93, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.N.; Hallak, J.E.C.; Bouso Saiz, J.C.; Dos Santos, R.G. Safety Issues of Psilocybin and LSD as Potential Rapid Acting Antidepressants and Potential Challenges. Expert Opin. Drug Saf. 2022, 21, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Razavi, D.; Allilaire, J.-F.; Smith, M.; Salimpour, A.; Verra, M.; Desclaux, B.; Saltel, P.; Piollet, I.; Gauvain-Piquard, A.; Trichard, C.; et al. The Effect of Fluoxetine on Anxiety and Depression Symptoms in Cancer Patients. Acta Psychiatr. Scand. 1996, 94, 205–210. [Google Scholar] [CrossRef]
- Pezzella, G.; Moslinger-Gehmayr, R.; Contu, A. Treatment of Depression in Patients with Breast Cancer: A Comparison between Paroxetine and Amitriptyline. Breast Cancer Res. Treat. 2001, 70, 1–10. [Google Scholar] [CrossRef]
- Musselman, D.L.; Somerset, W.I.; Guo, Y.; Manatunga, A.K.; Porter, M.; Penna, S.; Lewison, B.; Goodkin, R.; Lawson, K.; Lawson, D.; et al. A Double-Blind, Multicenter, Parallel-Group Study of Paroxetine, Desipramine, or Placebo in Breast Cancer Patients (Stages I, II, III, and IV) With Major Depression. J. Clin. Psychiatry 2006, 67, 288–296. [Google Scholar] [CrossRef]
- Agin-Liebes, G.I.; Malone, T.; Yalch, M.M.; Mennenga, S.E.; Ponté, K.L.; Guss, J.; Bossis, A.P.; Grigsby, J.; Fischer, S.; Ross, S. Long-Term Follow-up of Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Patients with Life-Threatening Cancer. J. Psychopharmacol. 2020, 34, 155–166. [Google Scholar] [CrossRef]
- Fan, W.; Yang, H.; Sun, Y.; Zhang, J.; Li, G.; Zheng, Y.; Liu, Y. Ketamine Rapidly Relieves Acute Suicidal Ideation in Cancer Patients: A Randomized Controlled Clinical Trial. Oncotarget 2017, 8, 2356–2360. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xu, X.; Peng, S.; Xu, F.; Liu, P. Use of Various Doses of S-Ketamine in Treatment of Depression and Pain in Cervical Carcinoma Patients with Mild/Moderate Depression After Laparoscopic Total Hysterectomy. Med. Sci. Monit. 2020, 26, e922028. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Zhang, G.; Wang, A.; Lin, S.; Chan, M.T.V.; Peng, Y.; Wang, G.; Han, R. Ketamine Alleviates Depressive Symptoms in Patients Undergoing Intracranial Tumor Resection: A Randomized Controlled Trial. Anesth. Analg. 2021, 133, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, P.; Li, Q.; Yan, H.; Shi, X.; Liu, C.; Zhang, Y.; Peng, S. Effect of Pretreatment of S-Ketamine On Postoperative Depression for Breast Cancer Patients. J. Investig. Surg. 2021, 34, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.C.; Romano, S.J.; Heiligenstein, J.H.; Tepner, R.G.; Wilson, M.G. A Controlled Trial of Fluoxetine and Desipramine in Depressed Women with Advanced Cancer. Psycho-Oncology 1998, 7, 291–300. [Google Scholar] [CrossRef]
- Fisch, M.J.; Loehrer, P.J.; Kristeller, J.; Passik, S.; Jung, S.-H.; Shen, J.; Arquette, M.A.; Brames, M.J.; Einhorn, L.H. Fluoxetine Versus Placebo in Advanced Cancer Outpatients: A Double-Blinded Trial of the Hoosier Oncology Group. JCO 2003, 21, 1937–1943. [Google Scholar] [CrossRef]
- Navari, R.M.; Brenner, M.C.; Wilson, M.N. Treatment of Depressive Symptoms in Patients with Early Stage Breast Cancer Undergoing Adjuvant Therapy. Breast Cancer Res. Treat. 2008, 112, 197–201. [Google Scholar] [CrossRef]
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S.; et al. Acute Dose-Dependent Effects of Lysergic Acid Diethylamide in a Double-Blind Placebo-Controlled Study in Healthy Subjects. Neuropsychopharmacology 2021, 46, 537–544. [Google Scholar] [CrossRef]
- Costa, D.; Mogos, I.; Toma, T. Efficacy and Safety of Mianserin in the Treatment of Depression of Women with Cancer. Acta Psychiatr. Scand. 1985, 72, 85–92. [Google Scholar] [CrossRef]
- Van Heeringen, K.; Zivkov, M. Pharmacological Treatment of Depression in Cancer Patients: A Placebo-Controlled Study of Mianserin. Br. J. Psychiatry 1996, 169, 440–443. [Google Scholar] [CrossRef]
- Razavi, D.; Kormoss, N.; Collard, A.; Farvacques, C.; Delvaux, N. Comparative Study of the Efficacy and Safety of Trazodone versus Clorazepate in the Treatment of Adjustment Disorders in Cancer Patients: A Pilot Study. J. Int. Med. Res. 1999, 27, 264–272. [Google Scholar] [CrossRef]
- Cankurtaran, E.S.; Ozalp, E.; Soygur, H.; Akbiyik, D.I.; Turhan, L.; Alkis, N. Mirtazapine Improves Sleep and Lowers Anxiety and Depression in Cancer Patients: Superiority over Imipramine. Support. Care Cancer 2008, 16, 1291–1298. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.I.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin Induces Schizophrenia-like Psychosis in Humans via a Serotonin-2 Agonist Action. NeuroReport 1998, 9, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L. Recent Advances in the Neuropsychopharmacology of Serotonergic Hallucinogens. Behav. Brain Res. 2015, 277, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A. Headache May Be Caused by Activation of 5-HT2A Receptors in Serotonin Toxicity. BMJ 2014, 348, g2159. [Google Scholar] [CrossRef]
- Minami, M.; Endo, T.; Hirafuji, M. Role of serotonin in emesis. Folia Pharmacol. Jpn. 1996, 108, 233–242. [Google Scholar] [CrossRef]
- Basak, S.; Kumar, A.; Ramsey, S.; Gibbs, E.; Kapoor, A.; Filizola, M.; Chakrapani, S. High-Resolution Structures of Multiple 5-HT3AR-Setron Complexes Reveal a Novel Mechanism of Competitive Inhibition. eLife 2020, 9, e57870. [Google Scholar] [CrossRef]
- Young, A.H. Ketamine: A New Chapter for Clinical Psychopharmacology? J. Psychopharmacol. 2023, 37, 755–756. [Google Scholar] [CrossRef]
- Scott, F.; Hampsey, E.; Gnanapragasam, S.; Carter, B.; Marwood, L.; Taylor, R.W.; Emre, C.; Korotkova, L.; Martín-Dombrowski, J.; Cleare, A.J.; et al. Systematic Review and Meta-Analysis of Augmentation and Combination Treatments for Early-Stage Treatment-Resistant Depression. J. Psychopharmacol. 2023, 37, 268–278. [Google Scholar] [CrossRef]
- Strawbridge, R.; Hodsoll, J.; Powell, T.R.; Hotopf, M.; Hatch, S.L.; Breen, G.; Cleare, A.J. Inflammatory Profiles of Severe Treatment-Resistant Depression. J. Affect. Disord. 2019, 246, 42–51. [Google Scholar] [CrossRef]
- Di Vincenzo, M.; Martiadis, V.; Della Rocca, B.; Arsenio, E.; D’Arpa, A.; Volpicelli, A.; Luciano, M.; Sampogna, G.; Fiorillo, A. Facts and myths about use of esketamine for treatment-resistant depression: A narrative clinical review. Front. Psychiatry 2024, 15, 1394787. [Google Scholar] [CrossRef]
- Maina, G.; Adami, M.; Ascione, G.; Bondi, E.; De Berardis, D.; Delmonte, D.; Maffezzoli, S.; Martinotti, G.; Nivoli, A.; Ottavianelli, E.; et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: A Delphi panel. Ann. Gen. Psychiatry 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Goodnick, P.J. Selective Serotonin Reuptake Inhibitors in the Treatment of Affective Disorders—III. Tolerability, Safety and Pharmacoeconomics. J. Psychopharmacol. 1998, 12 (Suppl. S4), S55–S87. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.M. Meta-Analytical Studies on New Antidepressants. Br. Med. Bull. 2001, 57, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.I.; Sadock, B.J. Comprehensive Textbook of Psychiatry, 6th ed.; Williams and Wilkins: Baltimore, MD, USA, 1995. [Google Scholar]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Gorman, J.M. Mirtazapine: Clinical Overview. J. Clin. Psychiatry 1999, 60 (Suppl. S17), 9–13; discussion 46–48. [Google Scholar]
- Kast, R. Mirtazapine May Be Useful in Treating Nausea and Insomnia of Cancer Chemotherapy. Support. Care Cancer 2001, 9, 469–470. [Google Scholar] [CrossRef]
- Walker, J.; Holm Hansen, C.; Martin, P.; Sawhney, A.; Thekkumpurath, P.; Beale, C.; Symeonides, S.; Wall, L.; Murray, G.; Sharpe, M. Prevalence of Depression in Adults with Cancer: A Systematic Review. Ann. Oncol. 2013, 24, 895–900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).