Abstract
Introduction: Calcineurin inhibitors (CNIs), ciclosporin and tacrolimus, are utilized primarily in organ transplantation and the treatment of autoimmune diseases. Since patients depend on these drugs over long periods, they face a potential risk of intoxication. This risk increases substantially when patients are overdosed or inadvertently exposed to cytochrome P450 (CYP) 3A4 inhibitors. Objectives: To analyze the utility of CYP inducers as a plausible treatment modality for acute CNI intoxication using real-world data from the WHO global pharmacovigilance database (VigiBase™) and supporting evidence from published data. Methodology: We explored all individual case safety reports (ICSRs) regarding CNI intoxications registered in VigiBase™. The queries “overdose” or “drug intoxication” were applied against the active ingredients “ciclosporin” and “tacrolimus”. Regarding the utility of CYP inducers, an extensive literature analysis was undertaken. We also report an index clinical case of a 60-year-old liver transplant patient that developed severe tacrolimus intoxication with multiple organ dysfunction at a peak concentration of 33.1 μg/L after a single dose of intravenous fluconazole. Results: Out of 143,710 documented ICSRs reported in VigiBase™ since 1992, 0.26% and 0.02% were registered as CNI overdoses and intoxications, respectively. The main etiological factor for CNI intoxication was the interaction with CYP 3A4 inhibitors (40.0% vs. case reports: 50.0%). The most commonly reported manifestation was acute kidney injury (36.7% vs. case reports: 46.3%). A total of 16.7% of intoxications led to fatal outcomes after drug withdrawal or dose reduction; however, in 43.0% of cases the exact actions undertaken were not reported. In peer-reviewed reports, 34 distinct clinical cases were treated with CYP inducers. Diverse pharmacoenhancement strategies with phenobarbital (5), phenytoin (23) and rifampicin (6) were described with a mean time of achieving the therapeutic target after 2.7 (±0.7), 3.1 (±0.5) and 4.6 (±1.0) days, respectively. In the index case, a therapeutic concentration of 4.9 [4–6 μg/L] was achieved after a 3-day regimen of rifampicin. Conclusion: In addition to general supportive treatment, the administration of phenobarbital, phenytoin, or rifampicin to reverse acute CNI intoxication is a viable treatment modality. The relatively long half-life of phenobarbital coupled with its exclusive renal elimination are potential pitfalls to reckon with. In spite of the favorable pharmacokinetic advantages of rifampicin, phenytoin offers a competitive pharmacodynamic advantage that is indisputable in patients with overt neurotoxicity.
1. Background
Systemically administered calcineurin inhibitors (CNIs), ciclosporin (CsA) and tacrolimus (TAC), are utilized primarily in the field of organ transplantation and for the treatment of autoimmune diseases [1,2,3]. CsA, a cyclic undecapeptide molecule, was first isolated in 1973 from the fungus Tolypocladium inflatum [4,5] whereas TAC, a macrolide molecule (FK 506), was first isolated in 1987 from the bacterium Streptomyces tsukubaensis [6,7]. Both molecules have been shown to demonstrate a somewhat similar mechanism of action by forming complexes with immunophilins (CsA binds to cyclophilin, TAC binds to FK-binding protein 12) [8,9,10]. These complexes together with calcium bind competitively to the phosphatase activity of calcineurin, resulting in a calcium-dependent inhibition of the transduction signal of T cells and the transcription of specific cytokines such as IL-2 [11,12]. Both drugs undergo extensive intestinal and hepatic metabolism through the cytochrome P450 enzyme system (CYP) 3A4 [1,2,3]. Since patients depend on these drugs over long periods without sustainable therapeutic drug monitoring measures, they constantly face a potential risk of supratherapeutic concentrations. This risk increases substantially when patients are inadvertently exposed to CYP 3A4 inhibitors [13,14,15,16,17,18]. In hospitalized patients, cases of iatrogenic overdoses have been reported [19,20,21,22]. Nevertheless, some instances have been described in which an intentional overdose was used as a method of self-harm or suicide [23,24,25]. Persistent diarrhea is a well-known precipitating factor for TAC intoxication [26,27]. Although CNI intoxications are extremely rare, they usually culminate in life-threatening to fatal outcomes [23,24]. Severe CNI intoxication manifests as acute liver and kidney injury, blood glucose and electrolyte derangement, tremors, severe headaches, lethargy, encephalopathy, severe demyelination, leukoencephalopathy with cerebral hemorrhage, paresis, cerebellar ataxia and coma [1,2,3]. Unfortunately, the clinical experience regarding the management of acute CNI intoxication is limited [1,2]. This underscores the importance of understanding the potential consequences and the need for sustainable therapeutic drug monitoring to optimize therapeutic use and avoid toxic supratherapeutic concentrations. TAC is not readily dialyzable due to its high molecular weight, poor aqueous solubility and extensive erythrocyte and plasma protein binding [1,2,3,28]. CsA is also not dialyzable to any appreciable extent due to its unfavorable physicochemical characteristics [1,2,3,29,30]. Due to the absence of validated antidotes, decontamination procedures and effective elimination strategies, the mainstay of management, are restricted to prompt drug withdrawal and general supportive treatment while closely monitoring the risk of graft rejection [1,2,3]. Pharmacoenhancement using potent CYP inducers such as phenobarbital, phenytoin and rifampicin to accelerate the clearance of CNIs offers a potential treatment modality. We hereby report a case in which a 3-day regimen of rifampicin was utilized to counteract fluconazole-induced TAC intoxication. Furthermore, we explored VigiBase™ and thoroughly analyzed the published data for previously reported cases to support the plausibility of this therapeutic approach.
2. Index Clinical Case
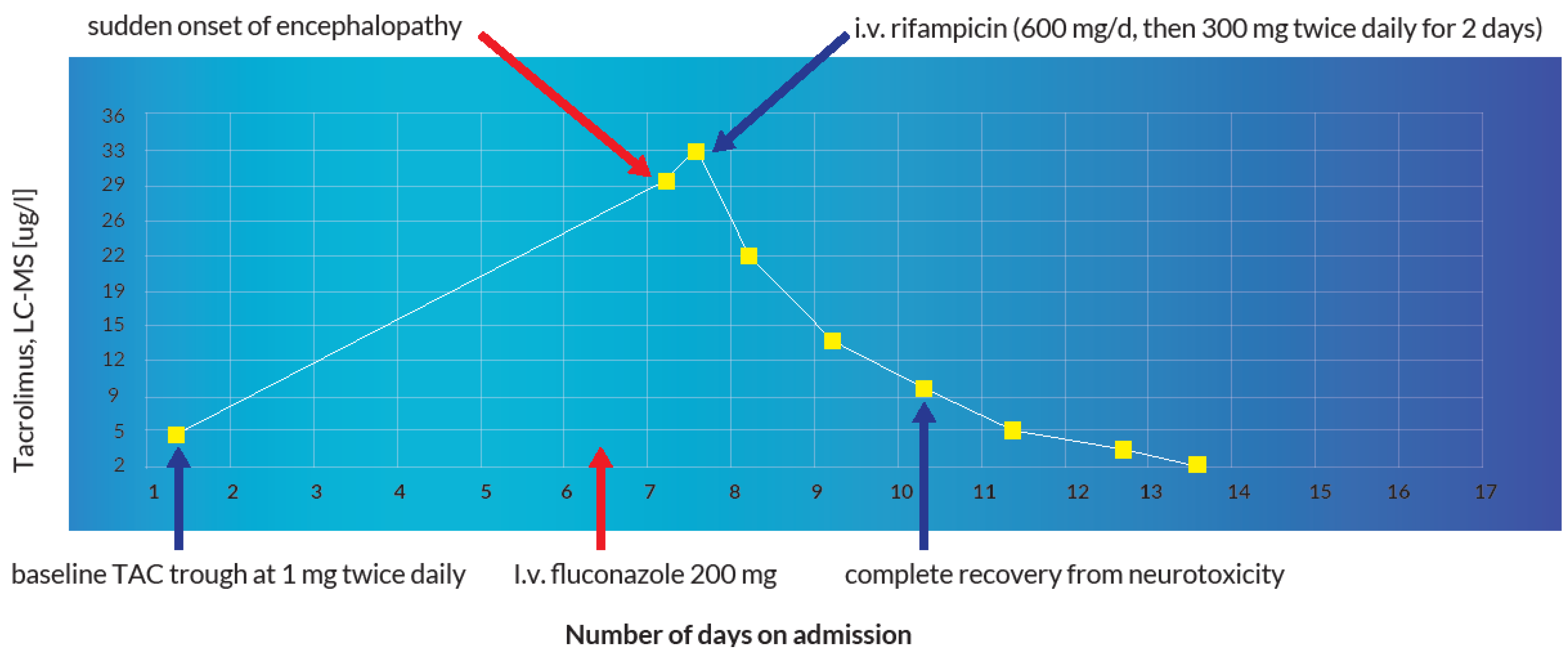
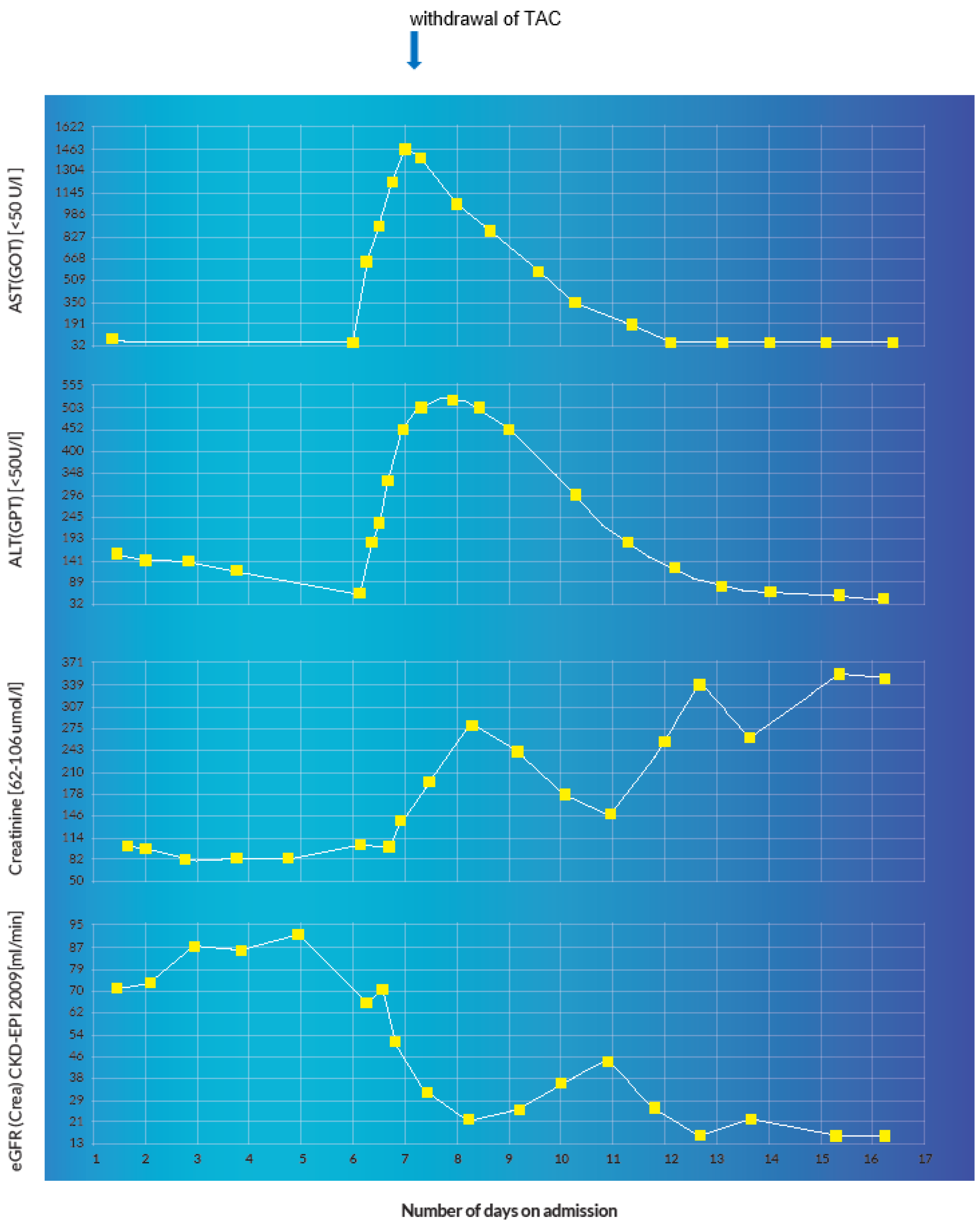
A 60-year-old, 64 kg male was admitted for re-evaluation of an invasive revascularization due to hemorrhagic shock secondary to recurrent upper gastrointestinal bleeding on account of multiple mycotic aneurysms after liver re-transplantation. In the emergency gastroscopy, an exposed duodenal vessel stump was obliterated with an electrocautery and adrenaline injection. A subsequent angiography of the abdomen showed multiple aneurysms of the gastroduodenal and hepatic arteries. Coiling of the aneurysms was performed with subsequent transfusions of two red blood cell concentrates. Apart from apixaban, which remained withheld on the day of admission, all other medications were continued as shown in Table 1. The immunosuppressive therapy with TAC was continued at a dosage of 1 mg twice a day with a baseline trough level of 5 µg/L on admission day 2 (therapeutic range: 4–6 µg/L). As a result of persistent febrile temperatures (up to 39 °C) and increasing inflammatory parameters with a positive history of recurrent cholangitis and previous positive blood cultures for Enterobacter faecium and Pseudomonas aeruginosa, an antibiotic therapy with piperacillin–tazobactam and vancomycin was initiated. On account of persistent hypotension and anemia, the patient was initially transferred to the shock room and then eventually to the intensive care unit. Due to multiple mycotic aneurysms, the anti-infective therapy with piperacillin–tazobactam was changed to meropenem and further augmented with co-trimoxazole, valganciclovir, caspofungin and a single dose of 200 mg of intravenous fluconazole on admission day 7. Additionally, the oral administration of TAC and pantoprazole was changed to intravenous at a dosage of 0.072 mg/d and 40 mg/d, respectively, as depicted in Table 1 and Table 2. After the withdrawal of all sedative medications in the intensive care unit on admission day 8, the patient developed encephalopathy, acutely deranged renal and liver function that manifested in a persistent comatose state. A possible status epilepticus and other neurologic, infectious and metabolic etiologic factors were ruled out after the detection of a TAC blood trough level of 31.0 µg/L (sampling was undertaken at 07:50 a.m., 10 min before the next administration of TAC). A peak TAC concentration of 33.1 µg/L was detected on the same day at 04:16 p.m. This prompted the immediate withdrawal of TAC. There was no evidence of an iatrogenic overdose or intentional poisoning. The cause of the sudden supratherapeutic TAC concentrations was attributed to an inhibition of hepatic cytochrome enzyme due to the concomitant administration of fluconazole. Under pharmacoenhancement with rifampicin (600 mg on day one, followed by 300 mg twice a day for 2 days), the TAC blood trough level reduced to 4.9 µg/L. Instantaneously, the patient recovered from the coma, his cognition was adequate without any focal neurological deficits and he was successfully extubated that same day. At a subtherapeutic trough level of 2.1 µg/L on admission day 14, TAC was restarted with a dose of 1 mg twice a day. However, the trough level remained subtherapeutic at 1.8 µg/L. He was eventually discharged home with a doubled TAC dose of 2 mg twice a day on admission day 25. The dose was reduced to 2 mg/d in the first follow-up visit. During subsequent follow-up visits the TAC blood trough levels remained in the desired therapeutic range. The evolution of the TAC blood trough levels and the corresponding laboratory values during the first two weeks of hospitalization is summarized in Table 3.

Table 1.
List of medications on admission.

Table 2.
List of medications administered during the initial phase of hospitalization.

Table 3.
Evolution of the TAC and vancomycin blood trough levels and laboratory parameters.
3. Results
3.1. Demographics of CNI Overdose and Intoxication in VigiBase™
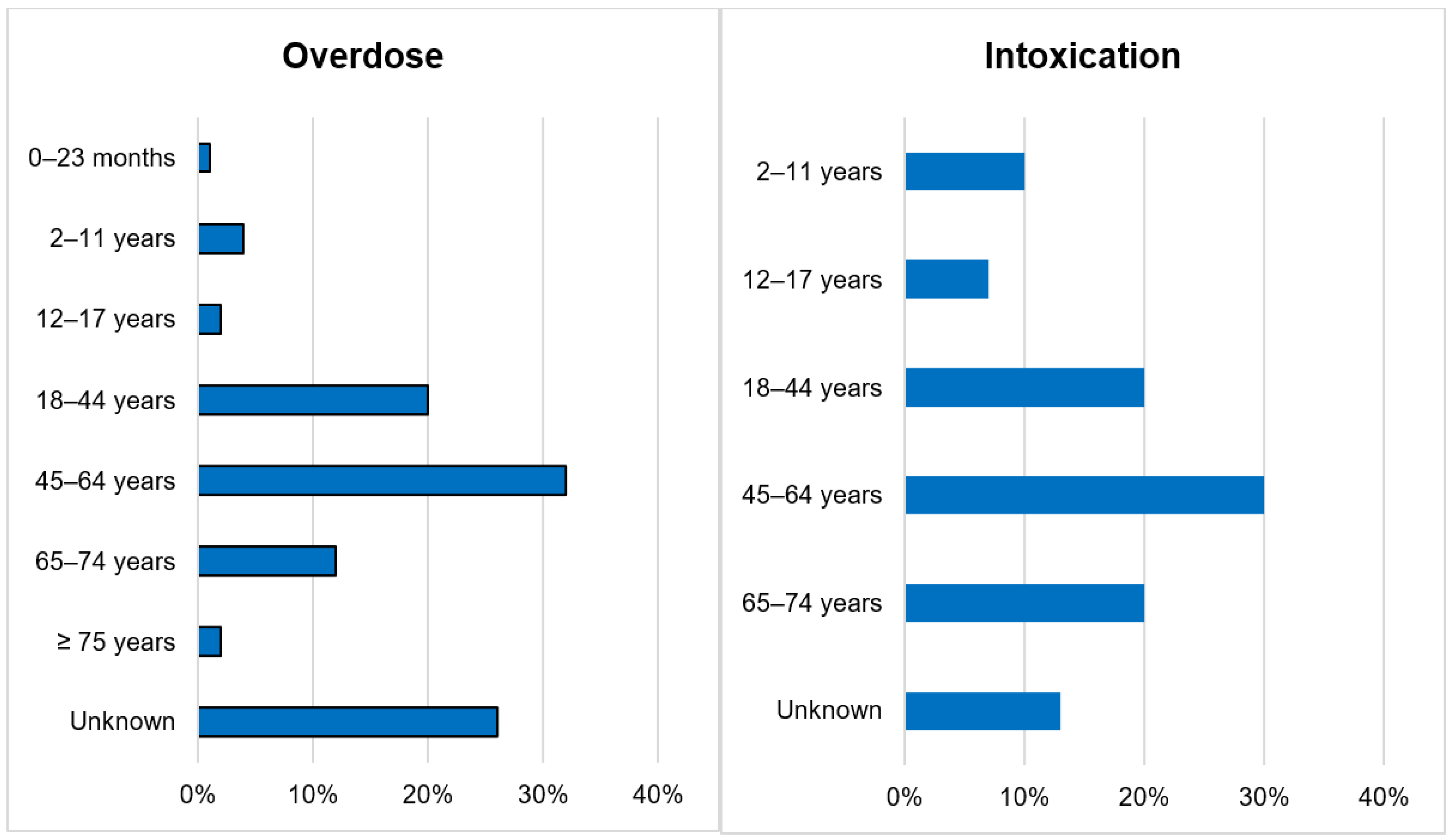
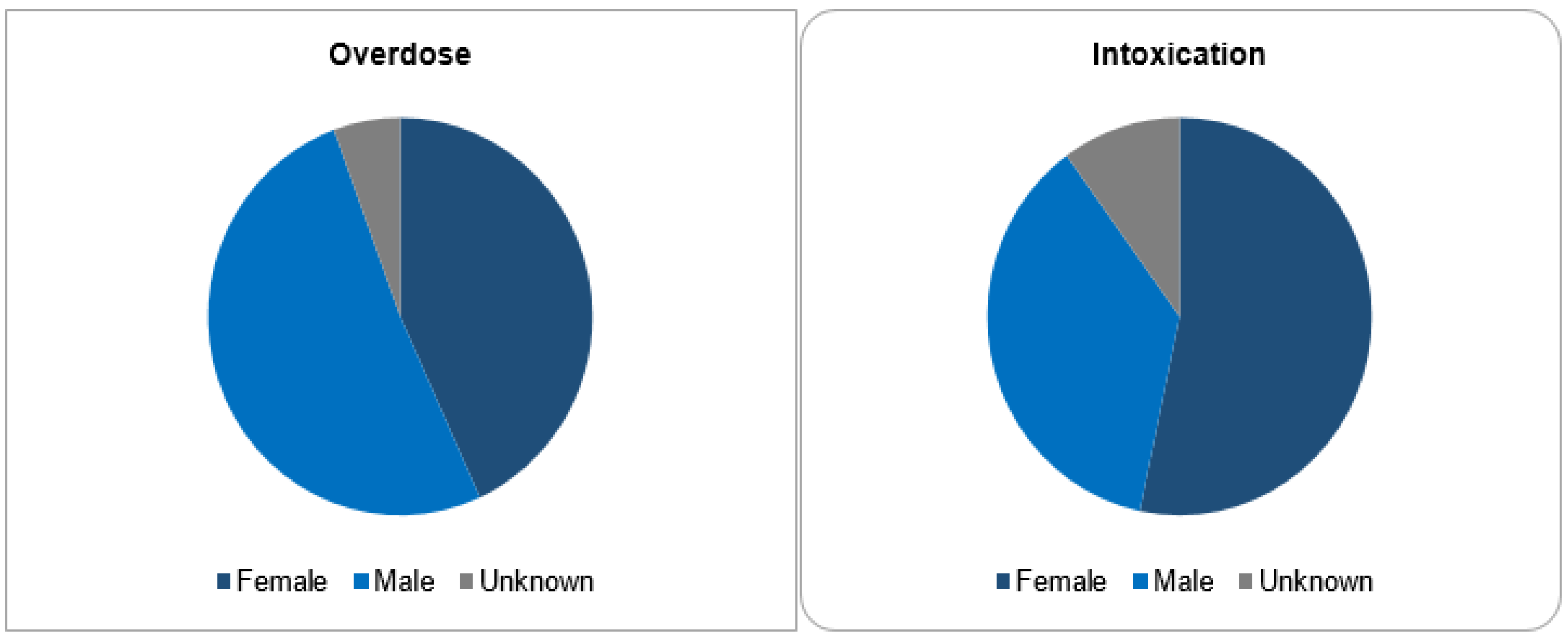
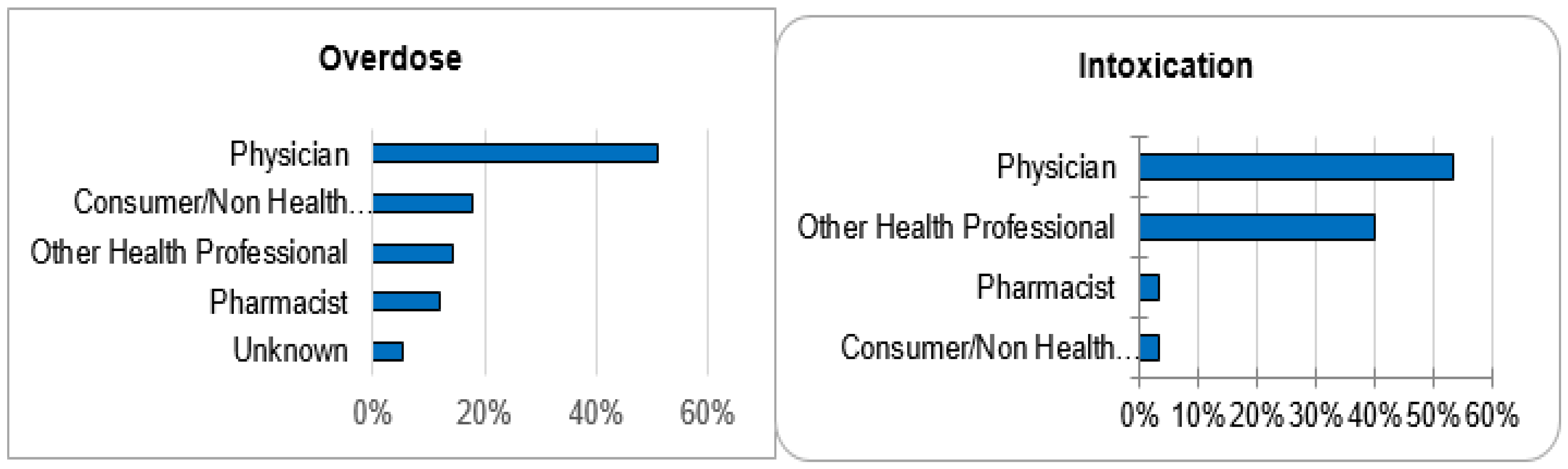
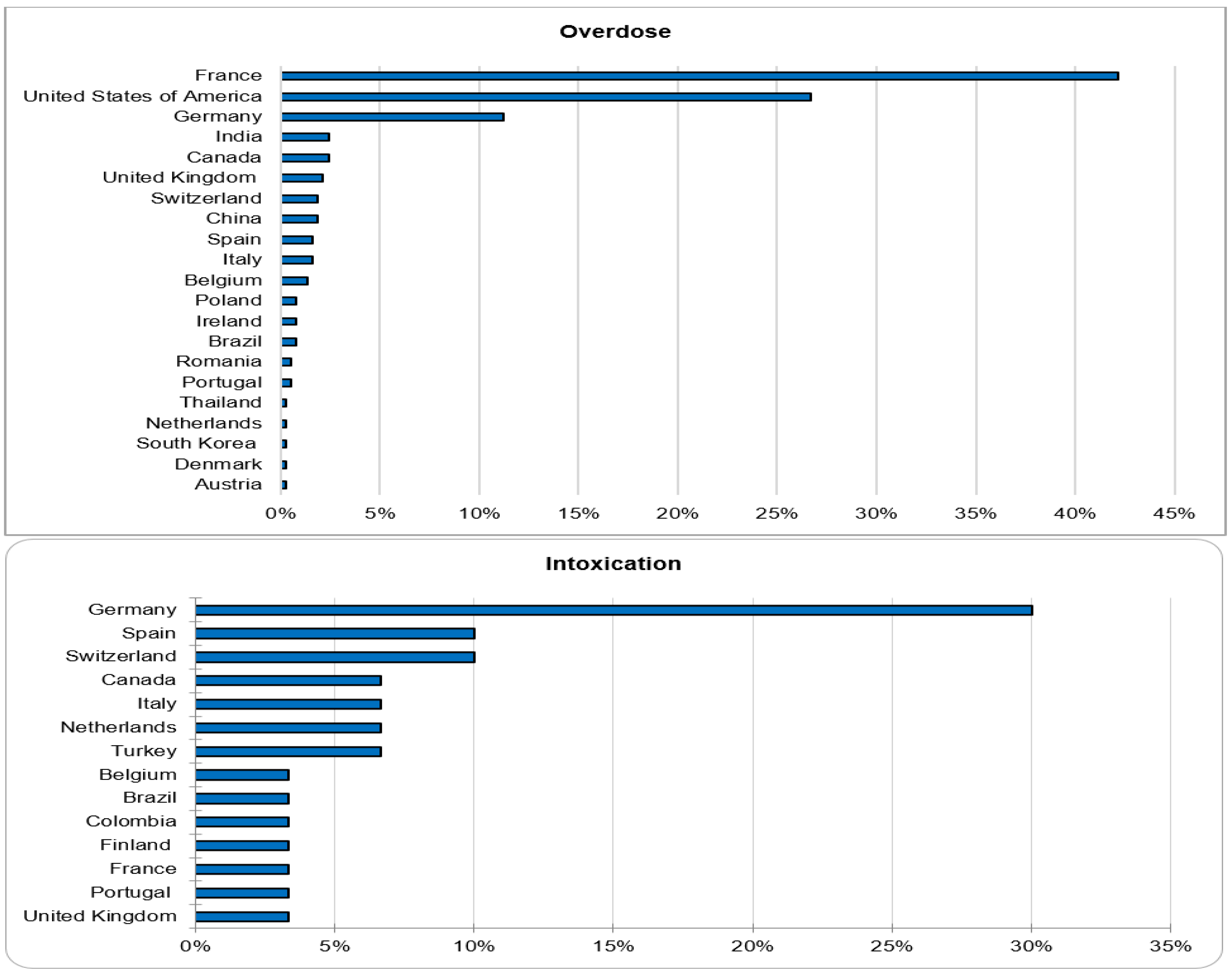
Out of 143,710 documented ICSRs on ciclosporin and tacrolimus since January 1992, 375 (0.26%) and 30 (0.02%) were registered as CNI overdoses and intoxications, respectively. In both instances, the majority of patients (32% vs. 30%) were within the age group of 45–64 years. Whilst the majority of overdose cases were males (51.2%), the majority of intoxication cases were females (53.3%). More than 50% of all cases were reported by physicians. Cases of overdose were mostly reported from France (42.1%), the United States of America (26.7%) and Germany (11.2%). The countries of primary source from which most cases of intoxication were reported were Germany (30%), Spain (10%) and Switzerland (10%). The demographical data are represented in Figure 1, Figure 2, Figure 3 and Figure 4.
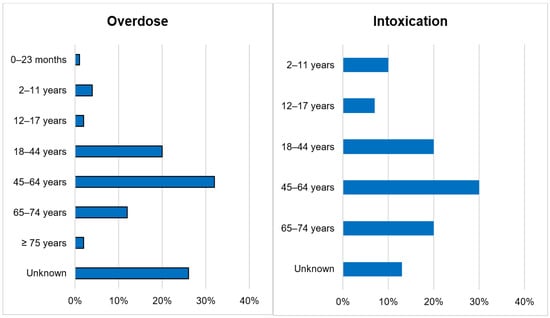
Figure 1.
Age categories.
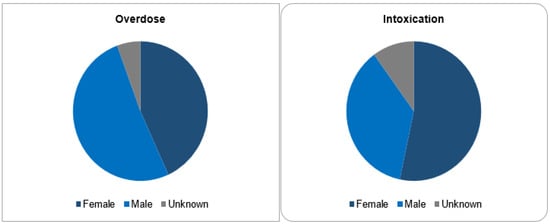
Figure 2.
Sex categories.
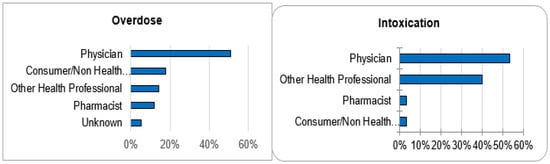
Figure 3.
Reporter qualifications.
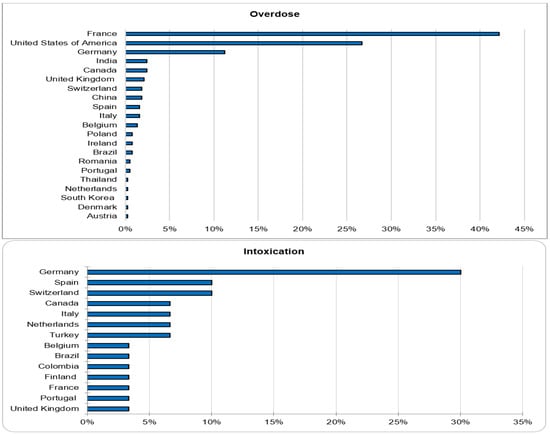
Figure 4.
Countries of primary source.
3.2. Seriousness Criteria of CNI Overdose and Intoxication
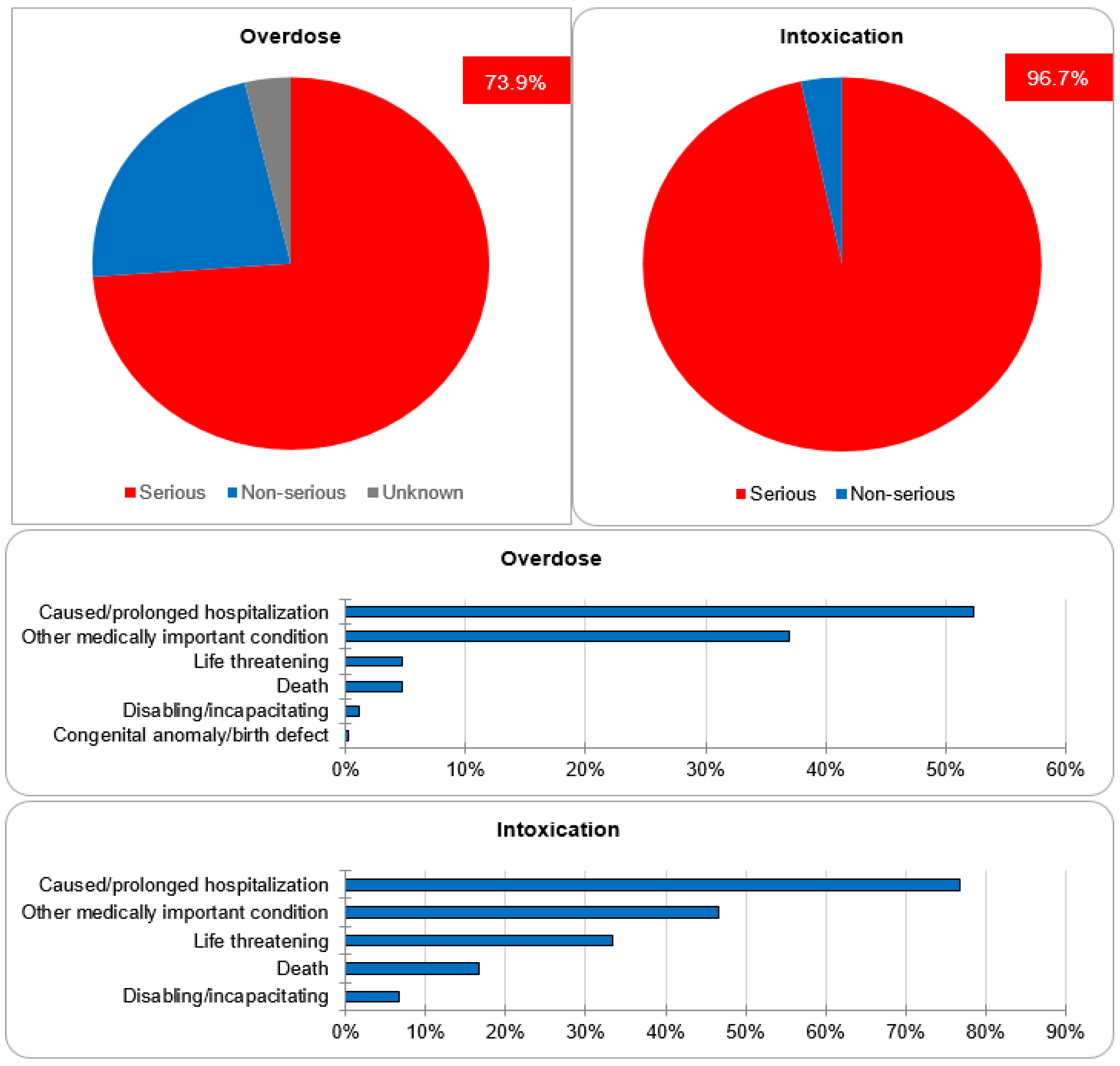
Regarding the assessment of seriousness, 73.9% and 22.4% of cases of overdose were categorized as serious and non-serious, respectively. The seriousness category of the remaining 3.7% was unknown. The majority of the cases were classified as serious because they either caused or prolonged hospitalization (52.3%), caused other medically important conditions (36.9%), or resulted in life-threatening complications (4.7%). One case resulted in a congenital anomaly/birth defect. For the cases of intoxication, 96.7% were categorized as serious, whereas 3.3% were non-serious. Out of the serious cases, 42.6% caused or prolonged hospitalization, 25.9% caused other medically important conditions and 18.52% resulted in life-threatening complications. The data regarding seriousness are depicted in Figure 5.
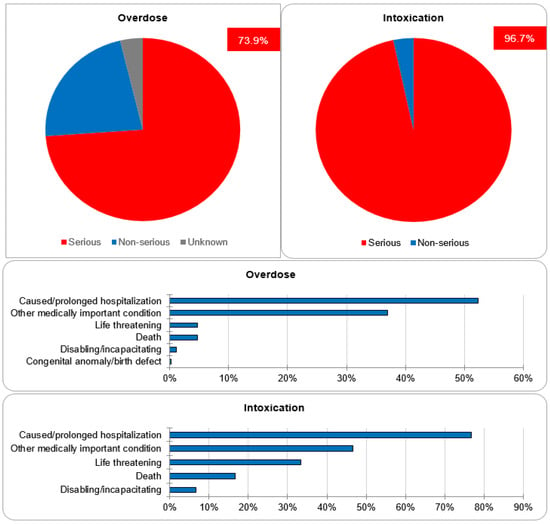
Figure 5.
Seriousness criteria.
3.3. Causality Assessments, Actions Taken and Outcomes of CNI Intoxication
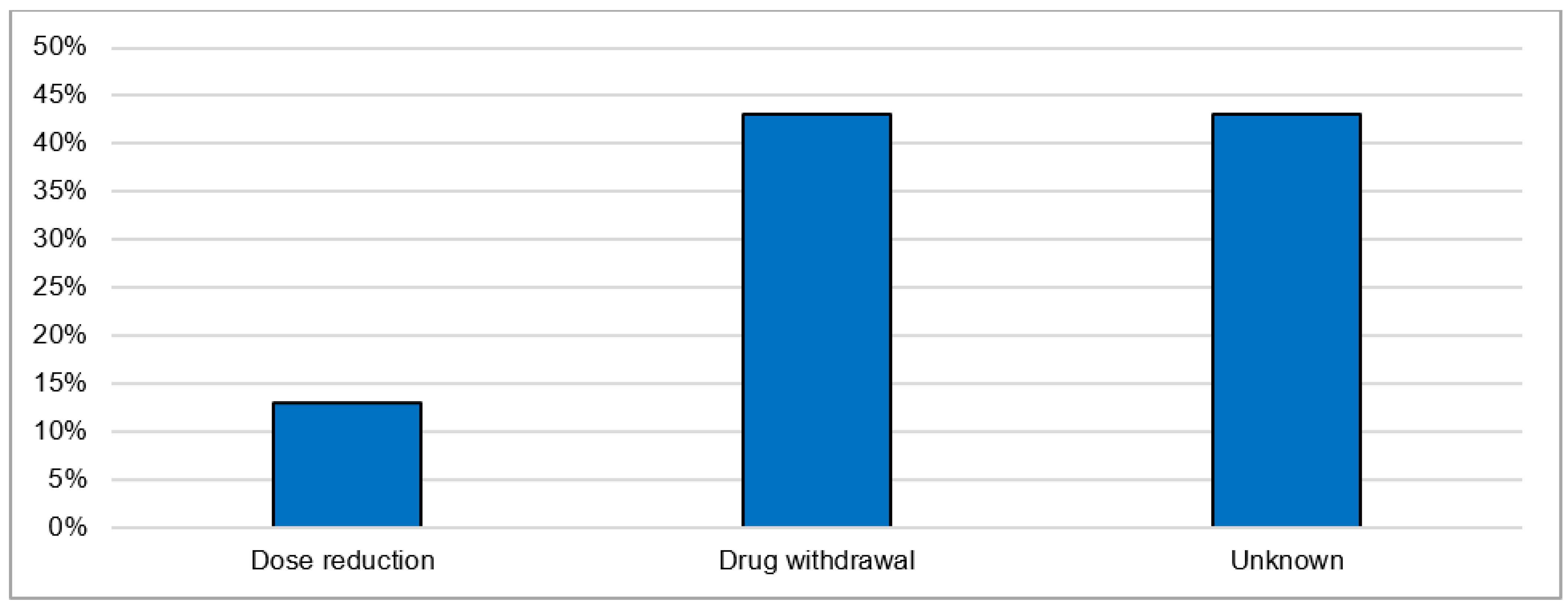
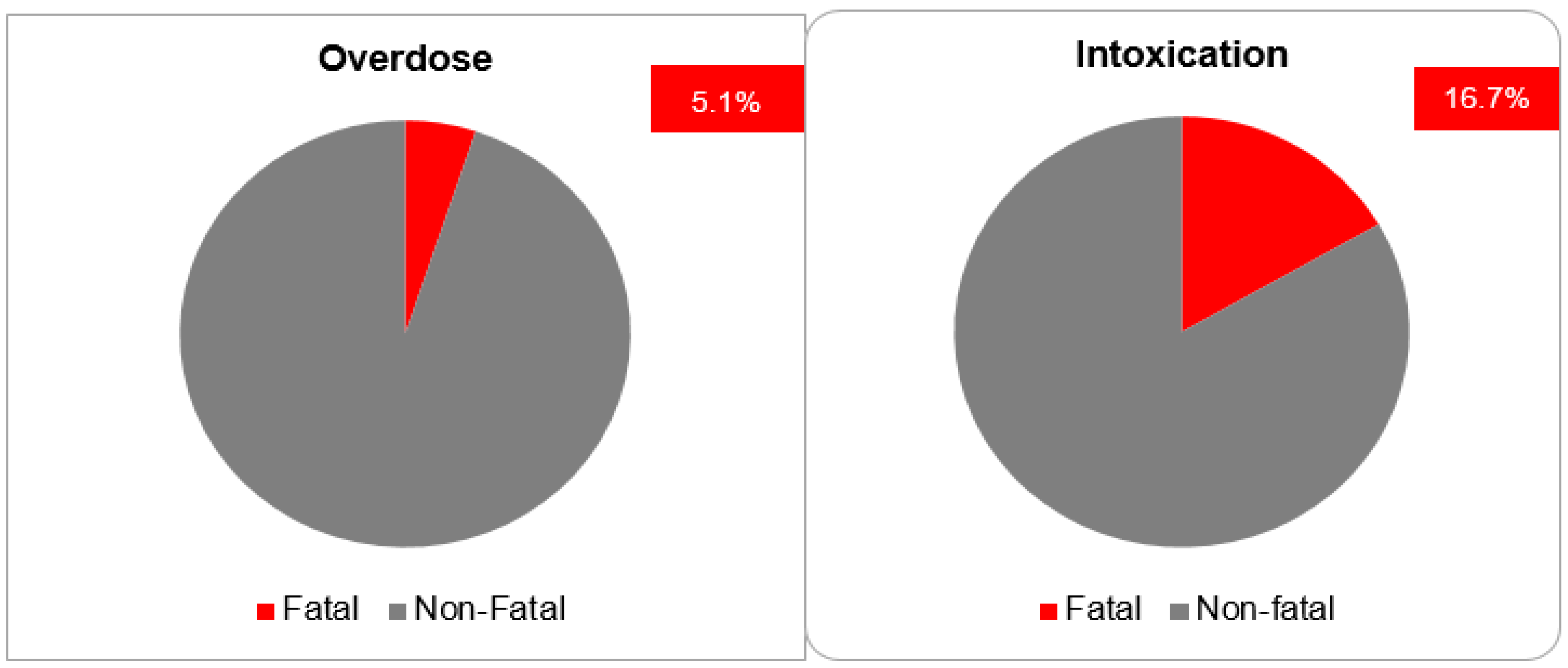
The most postulated causality of CNI intoxication was the interaction with CYP 3A4 inhibitors (40.0%). The exact cause in the majority of cases (50.0%) is unknown. The most frequently co-reported active ingredients that are known CYP 3A4 inhibitors are clarithromycin, voriconazole, itraconazole, amlodipine and dasabuvir. The most commonly reported manifestations were acute kidney failure (36.7%) and gastrointestinal disturbance: diarrhea (16.7%) and vomiting (10.0%). As general supportive measures, the actions taken after intoxication were immediate drug withdrawal (43.3%) and dose reduction (13.3%). For the remaining cases, the exact treatments undertaken were not reported (Figure 6). The mortality rate after CNI intoxication was relatively high at 16.7% compared to 5.1% after overdose (Figure 7).
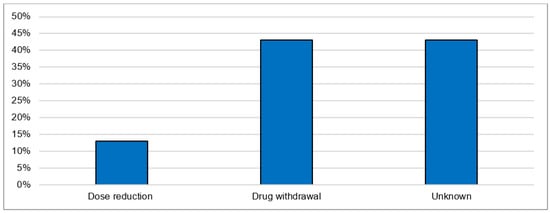
Figure 6.
Actions undertaken after acute intoxication.
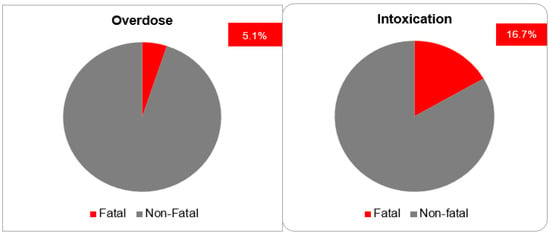
Figure 7.
Fatal outcomes.
3.4. Analysis of Case Reports
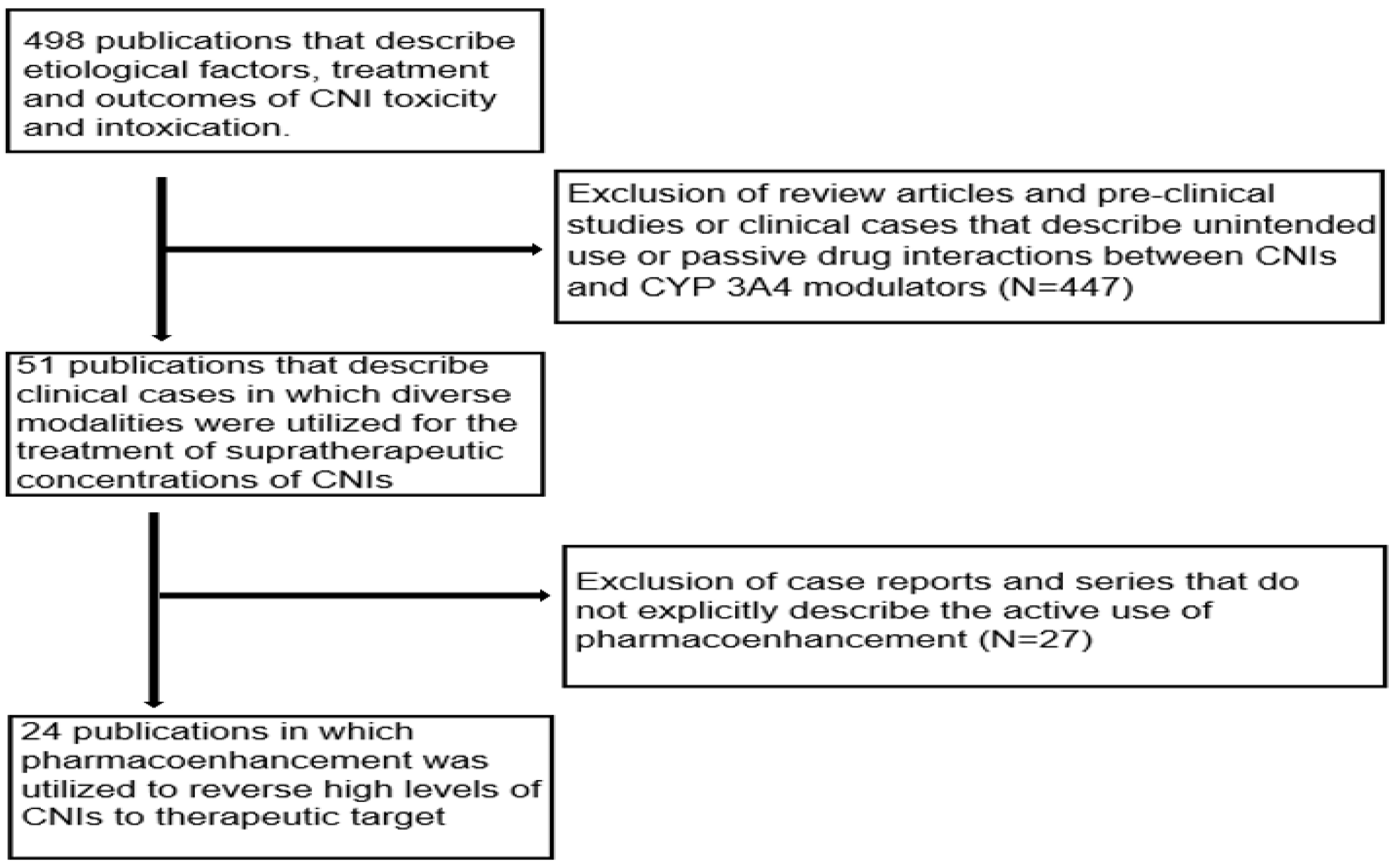
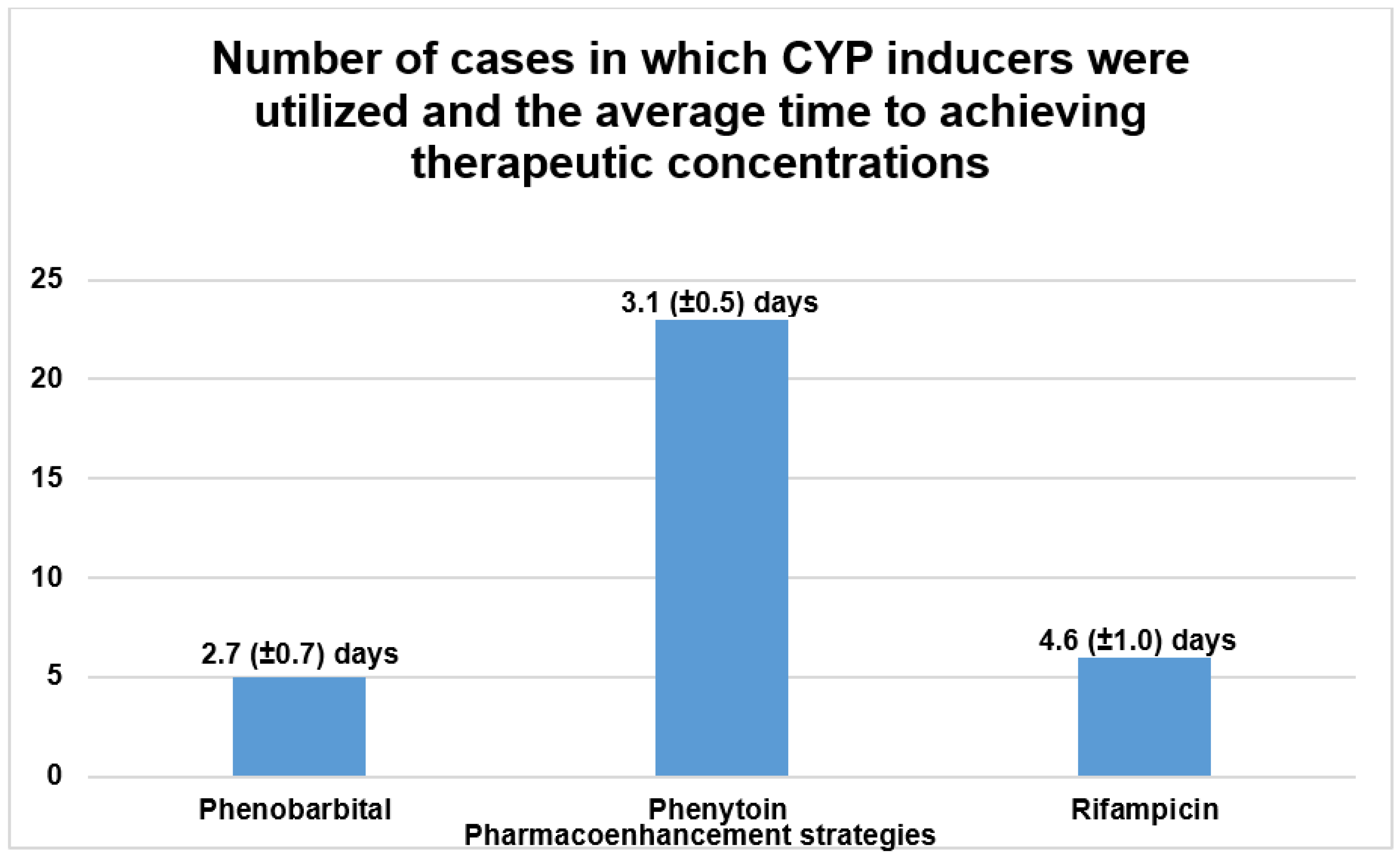
The initial literature search yielded a total of 498 publications that described the causes, treatment and outcomes of CNI toxicity. All review articles and publications concerning pre-clinical studies or clinical cases that described the unintended use or passive drug interactions between CNIs and CYP 3A4 modulators were excluded. The publications that did not explicitly describe the use of pharmacoenhancement were also excluded. After extensive screening, a total of 24 publications in which CYP inducers were used to counteract supratherapeutic concentrations of CNIs were identified as illustrated in Figure 8 below [13,14,15,16,17,18,19,20,21,22,23,26,31,32,33,34,35,36,37,38,39,40,41]. These publications described 34 distinct clinical cases in which pharmacoenhancement was used to treat supratherapeutic concentrations of TAC (91.2% of cases) and CsA (8.8% of cases). The utility of phenobarbital (n = 5), phenytoin (n = 23) and rifampicin (n = 6) was described with a mean time to achieving therapeutic target after 2.7 (±0.7), 3.1 (±0.5) and 4.6 (±1.0) days, respectively, as illustrated in Figure 9. The commonest indications for CNI therapy were renal transplant (n = 15) and liver transplant (n = 9). In one case, an overdose of TAC was mistakenly administered to a non-transplant patient. Two stem cell transplant patients were under treatment with TAC. The adjudged etiologic factors for the supratherapeutic concentrations were the interaction with CYP 3A4 inhibitors (50.0%), iatrogenic overdose (23.5%) and a genetic defect in CYP 3A4 enzyme activity (5.8%). Prolonged diarrhea was the precipitating factor in one case. In 17.6% of cases, the exact cause was unknown. The culprit CYP 3A4-inhibiting drugs were nirmatrelvir, cobicistat, darunavir, dasabuvir, ritonavir, clarithromycin, atazanavir, voriconazole and fluconazole. The commonest manifestations of intoxication were nephrotoxicity (46.3%), neurotoxicity (22.0%) and gastrointestinal disturbance (14.6%). A summary of the clinical cases can be found in the Supplementary Material, Table S1.
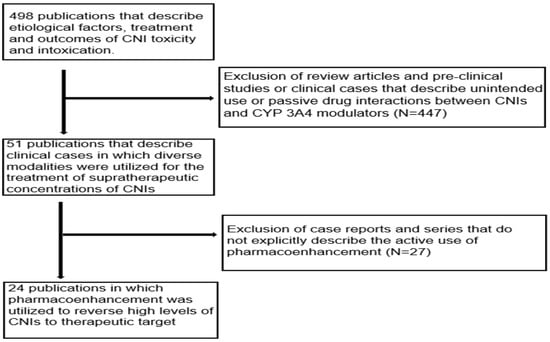
Figure 8.
Flow chart of publications that were extensively screened to obtain the clinical cases.
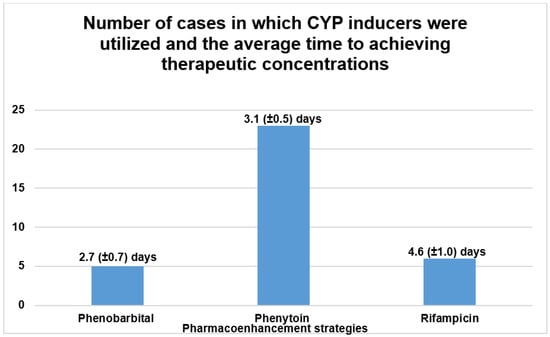
Figure 9.
Summary of peer-reviewed case reports on the treatment of CNI intoxication using CYP inducers.
4. Discussion
4.1. Clinical Pharmacological Evaluation of the Index Case
The immediate-release formulation of Prograf® (TAC, Astellas Pharma AG, Wallisellen, Switzerland) is incompletely absorbed in the upper gastrointestinal tract with a bioavailability of approximately 22% (±6) in adult liver transplant patients [3]. With a mean standard dose of 0.30 mg/kg/day at steady-state, the average Cmax and Tmax amount to 74.1 µg/L and 3.0 h, respectively [1]. TAC has an extremely high protein binding capacity of about 99% to plasma proteins (albumin and α-1 acid glycoprotein) and erythrocytes [1,2,3]. Intravenously administered TAC exhibits a two-compartment pharmacokinetic model [1,2,42]. Pharmacokinetic studies with healthy volunteers demonstrate a blood/plasma ratio of about 20/1 with a mean volume of distribution of about 11.5 L/kg (±4.3) [43]. In adult liver transplant patients, this amounts to about 30.1 L/kg (±14.7) [44]. TAC is extensively metabolized by intestinal and hepatic CYP 3A4 as well as CYP3A5 [1,2,3]. Out of the 8 known metabolites of TAC, only 31-O-demethyl-tacrolimus demonstrates similar activity to TAC in in vitro studies [3,45]. The metabolites are excreted primarily in the bile [1,2,3]. Furthermore, it is a substrate as well as a weak inhibitor of the drug transporter P-glycoprotein (P-gp) [3]. The average half-life is reported to be approximately 43 h [1] but can sometimes be longer due to inter-individual variations [46,47]. In adult liver transplant patients, the mean elimination half-life after intravenous infusion is about 12.1 h (±4.7) [44], this is why a 12-hourly dosage interval is routinely implemented. Less than 1% undergoes renal elimination in the unchanged form at a renal clearance of less than 1 mL/min [1,2,3]. This explains why TAC is not significantly eliminated through renal replacement therapy [48]. The concomitant administration of drugs or herbal products that are modulators of CYP3A4 can drastically affect the metabolism and clearance of TAC, which can consequently disrupt therapeutic concentrations [49,50,51,52]. Diflucan® (fluconazole, Pfizer AG, Zurich, Switzerland) is a moderate inhibitor of CYP3A4 and a potent inhibitor of CYP2C9 and CYP2C19 [1,2,3,53]. It is also a weak inhibitor of CYP1A2 [3]. The concomitant administration of fluconazole and TAC led to a clinically significant increase in the mean TAC blood trough concentrations in previous pharmacokinetic studies [54,55]. In the index patient, the concomitant administration of intravenous fluconazole 200 mg (at 06:49 a.m.) and intravenous TAC 0.072 mg (at 07:40 a.m.) on admission day 7 resulted in a sudden elevation (6-fold) of the baseline TAC blood trough concentration accompanied by an acute derangement of the hepatic and renal parameters (see illustration in Figure 10 and Figure 11). A possible iatrogenic overdose was ruled out because the administered intravenous dose of 0.072 mg/d was just about 18% of the expected plasma exposure from the baseline oral dose of 2 mg/d (considering an oral bioavailability of 20%, 2 mg oral formulation is equivalent to 0.4 mg intravenous) [56]. In the measured trough concentration at 07:50 a.m. on admission day 8, no TAC was administered approximately 24 h before the blood sample was withdrawn, which is why a pre-analytical error in the framework of a non-trough level determination was ruled out. Moreover, the quantification was performed using the liquid chromatography technique coupled with mass spectrometry (LC-MS), which is why interfering factors due to co-medication were unlikely [57,58]. In the Swiss drug information of Prograf®, coma and encephalopathy are documented as adverse drug reactions (ADRs) with an incidence of about 0.1–1% whereas acute hepatocellular injury and renal failure are documented with an incidence of about 1–10%. The international databanks, UpToDate™ and Micromedex™, describe these ADRs as life-threatening toxidromes of TAC. VigiBase™ registered 160 cases of coma (PT), 420 cases of encephalopathy (PT), 21 cases of hepatocellular injury (PT) and 1169 cases of renal failure (PT) out of a total of 79,223 ICSRs on tacrolimus since 1992. The repeat TAC concentration of 33.1 µg/L at 4:16 p.m. confirmed the diagnosis of a tacrolimus intoxication and pharmacoenhancement with rifampicin was started at 8:00 p.m. that same day. Under fluconazole-induced CYP inhibition, the effective half-life of TAC reduced from 30.4 h to 27.7 h after 4 days of initiating rifampicin. This was less likely to occur after drug withdrawal alone, in the presence of CYP inhibition. The initial CYP-inducing effect of rifampicin, even within the first few days of treatment, can result in a clinically significant increase in the total clearance of tacrolimus [59]. The administration of rifampicin may result in a reduction of the efficacy of caspofungin through its modulatory effect on transport proteins.
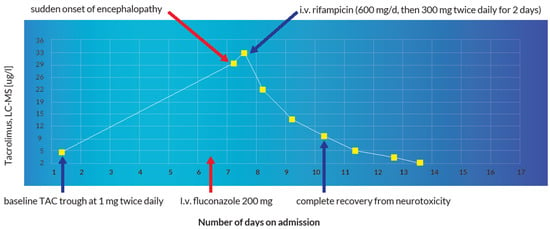
Figure 10.
Illustration of the reversal of fluconazole-induced TAC intoxication using rifampicin.
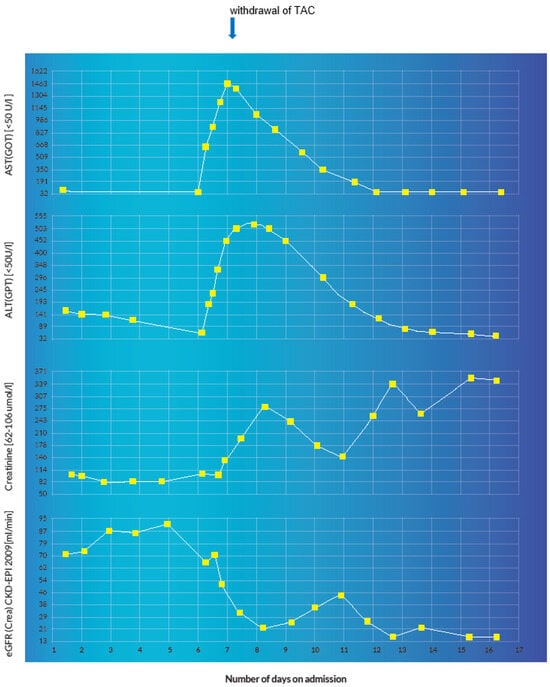
Figure 11.
Graphical representation of acute hepatocellular and renal injury manifested by a sudden derangement of the transaminases and renal function parameters with a positive correlation to the administration of fluconazole and the detection of the supratherapeutic TAC concentration.
It is important to note that although the cumulative risk of liver injury by the concomitant medications, especially the anti-infective agents (see Table 4 below), may have partly contributed to the pre-existing reduction in the overall hepatic metabolic capacity, the supratherapeutic TAC level is more suggestive of a concentration-dependent hepatic injury and most likely explains the constellation of adverse reactions in this case. Below is a table showing the incidences of hepatic injury from the administered antimicrobial agents.

Table 4.
Incidence of hepatic injury from administered antimicrobial agents according to LiverTox.
4.2. Retrospective Analysis of CNI Intoxications from VigiBase™ Versus Previously Published Peer-Reviewed Clinical Cases
To the best of our knowledge, this is the first study exploring the demographics and outcomes of CNI intoxication using VigiBase™. The results from VigiBase™ affirm those obtained from previous epidemiological studies regarding the toxicity of CNIs [23,60,61]. Due to underreporting or unreported cases, the actual proportion of CNI intoxications in ICSRs at the global level remains unknown. Based on the fact that the majority of the ICSRs were reported by physicians, pharmacists and other health care professionals, the reports are expected to be generally reliable. Although the exact causality in the majority of cases could not be established, the most adjudged causality was the interaction with CYP 3A4 inhibitors (40.0%). A similar but more objective trend was observed in the analysis of the previously published clinical cases, whereby the main cause of supratherapeutic concentrations was the interaction with CYP 3A4 inhibitors (50.0%) [31,32,33,34,35,36], followed by overdose (23.5%) [20,21,37]. Moreover, the results from both analyses showed that the majority of patients (VigiBase™: 30.0% vs. case reports: 50.1%) were within the age group of 45–64 years. Organ transplant patients in this age group have shown in previous studies to face the highest risk of drug-drug interactions due to polypharmacy [62,63,64]. The most commonly reported manifestation of intoxication was nephrotoxicity (VigiBase™: 36.7% vs. case reports: 46.3%). This is in line with the prevalence figures of nephrotoxic adverse reactions of CNIs from the international databases: Micromedex™ and UpToDate™, as well as in the respective summary of product characteristics.
Even though the details of treatment measures were not explicitly reported in VigiBase, in the majority of cases, either immediate drug withdrawal or dose reduction was undertaken. Perhaps other treatment modalities were not reported because they are either off-label, non-validated or not standardized. This explains why in about 43.0% of reported cases, the exact actions taken were not declared. The high mortality rate of 16.7% underscores the importance of exploring alternative treatment modalities apart from general supportive measures to optimize treatment outcomes. Although the administration of phenobarbital, phenytoin and rifampicin in organ transplant patients may come along with the increased risk of potential drug interactions with concomitant medications and adverse outcomes such as graft rejection [51,65], the overall benefit of averting the high risk of mortality from severe CNI toxicity using these inducers is indispensable. Moreover, in some selected patient groups with pre-existing or new indications, such as bacterial infections in the case of rifampicin and neurological conditions in the case of phenytoin or phenobarbital, the benefits of pharmacoenhancement further outweigh the risks. The relatively long half-life of phenobarbital coupled with its exclusive renal elimination are pitfalls to reckon with regarding the potential risk of adverse drug reactions. In spite of the favorable pharmacokinetic advantages of rifampicin, such as relatively short half-life, extensive induction potential (including P-gp for orally administered CNIs) and predominant extra-renal elimination, phenytoin offers a competitive anticonvulsive pharmacodynamic advantage that is indisputable in patients with CNI-induced neurotoxicity. This explains why in the majority of cases phenytoin was the CYP inducer of choice. Based on the results obtained from the analysis of the clinical cases, the mean time required to achieve therapeutic concentrations after initiation of CYP-induction appears to be inversely proportional to the half-life of the inducing agent. However, due to the heterogeneity of the cases and small sample size, further studies are required to validate these findings.
5. Methodology
5.1. Study Design
We explored all ICSRs regarding CNI toxicities that were registered in VigiBase™ since January 1992. VigiBase™ is a unique international database of the WHO that contains reported potential adverse reactions to medicinal products [66]. It is the largest global database, with over 30 million anonymized reports of suspected adverse reactions, submitted since 1968 by over 150 member countries (as of October 2023) of the WHO Program for International Drug Monitoring (WHO PIDM). It was developed by the Uppsala Monitoring Centre (UMC) in Sweden and is continuously maintained and updated with an incoming structured set of patient safety records [67]. Since the information is retrieved from a variety of registry databases from different countries of primary source without access to detailed clinical history, and the probability that the suspected adverse reactions are drug-related is not the same in all cases, the individual causal relationships between suspected adverse reactions and drugs cannot be definitively ascertained [66]. Moreover, it is important to note that the information is inhomogeneous and does not represent the opinion of the UMC or WHO. Since all the reports in VigiBase™ are anonymized, ethical approval was not required for this study. However, written informed consent was obtained from the index case.
5.2. Extraction of Data from VigiBase™
The queries “overdose” or “drug intoxication” (as reaction terms) were applied against the active ingredients “ciclosporin” and “tacrolimus”. In the MedDRA (version 26.0, English language), the queries “overdose” and “drug intoxication” are categorized as preferred term (PT) and lowest level term (LLT), respectively. Using the qualitative global view settings with drugs as the therapeutic scope, an automatic vigiMatch duplicate scope algorithm was used to de-duplicate all hits [68].
5.3. Extraction of Case Reports
Regarding the utility of CYP inducers as rescue therapy, an extensive literature search in PubMed, Google Scholar, Scopus and Science Direct was undertaken using Boolean operators with the aid of the following key words: “calcineurin inhibitors”, “tacrolimus”, “ciclosporin”, “cytochrome 3A4 inducers”, “rifampicin”, “phenobarbital”, “carbamazepine”, “phenytoin”, “management of acute toxicity, overdose or intoxication” and “pharmacoenhancement”. All peer-reviewed publications in the English language that describe the use of pharmacoenhancement with CYP inducers to counteract supratherapeutic concentrations of CNIs were analyzed systematically.
5.4. Statistical Analyses
The case details showing all ICSRs, drug-reaction pairs, the corresponding treatments and outcomes were exported from the VigiLyze software version 26.0 and analyzed descriptively using Microsoft Excel© version 6.2.14 for Windows and R version 4.3.2. For the mean time to achieving therapeutic target under the CYP inducers a confidence level of 95% was applied.
6. Conclusions
Although acute CNI intoxications are rare, they can lead to life-threatening complications such as multiple organ dysfunction that can potentially result in fatal outcomes. The commonest cause is the interaction with CYP 3A4 inhibitors rather than overdose. In the index case, the concomitant administration of a single intravenous dose of fluconazole resulted in a 6-fold increase in the baseline TAC blood trough level due to the inhibition of the hepatic CYP3A4 metabolism. TAC intoxication manifested as acute neurotoxicity, nephrotoxicity and hepatotoxicity as depicted in Figure 10 and Figure 11. The benefit of pharmacoenhancement with rifampicin outweighed the risk of further liver injury in this patient with an imminent case of severe acute TAC intoxication. Although rifampicin is contraindicated in patients with severe pre-existing or acute liver injury, it was administered as an off-label therapy after rigorous risk-benefit analysis. In addition to prompt drug withdrawal and general supportive measures, pharmacoenhancement with a 3-day regimen of intravenous rifampicin accelerated the metabolism and clearance of TAC, which resulted in an instantaneous and remarkable recovery of the patient. In VigiBase™, apart from drug withdrawal or dose reduction, there are no ICSRs whereby CYP inducers were utilized as treatments. In about 43% of reported cases, the exact treatments or actions taken were not reported. In published articles, diverse pharmacoenhancement strategies with phenobarbital, phenytoin and rifampicin were described in 34 distinct clinical cases. Due to the lack of clinical cases whereby the effect of TAC withdrawal alone compared to that of withdrawal and pharmacoenhancement, multicenter clinical investigations are required to validate the safety and effectiveness of the various pharmacoenhancement strategies. For individual cases, a critical patient-oriented risk-benefit analysis regarding the choice of CYP inducer as well as the dosage and route of administration is highly recommended. For patient safety purposes, when any of the pharmacoenhancement strategies are utilized, the respective CNI concentrations should be closely monitored and the enzyme-inducing therapy should be kept as short as possible (3–4 days). Furthermore, the increased dosage of concomitant medications that are substrates of the induced enzymes should be proactively implemented. The relatively high occurrence of polypharmacy in organ transplant patients under treatment with CNIs requires that health care providers consider drug-drug interactions as potential causes of severe acute CNI intoxication. In this category of patients, it is highly recommended to critically review and consider the dose adjustment of relevant concomitant medications to prevent the need of using other drugs to reverse toxicity.
Real-world data from VigiBase™ is continuously updated with incoming structured set of patient safety records. The data consist of spontaneous reports from diverse countries of primary source with different reporting policies and various reporting errors. Due to the heterogeneity of reports and potential confounding factors such as patient demographic characteristics, comorbidities and concomitant medications, the results obtained from VigiBase™ should be interpreted with utmost caution. Since the analysis of the case reports did not include cases of CNI intoxication whereby the management was solely restricted to drug withdrawal or dose reduction, it was not possible to evaluate the actual benefit of pharmacoenhancement. Moreover, in some cases, data on patient-specific CYP 3A4-genotyping and pharmacokinetic analyses were unavailable. As expected in rare medical events, the relatively small sample size of clinical cases evaluated in this article (n = 34) is not representative. It must be assumed that the number of unreported or unpublished cases is much higher.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharma3010002/s1, Table S1: Summary of peer-reviewed case reports.
Author Contributions
Conceptualization, S.D., K.E., C.G. and G.A.K.-U.; Data curation, S.D.; Formal analysis, S.D.; Investigation, S.D.; Methodology, S.D.; Project administration, S.D.; Resources, K.E., C.G., P.P., A.S. and G.A.K.-U.; Software, S.D. and G.A.K.-U.; Supervision, G.A.K.-U.; Validation, G.A.K.-U.; Visualization, S.D., K.E., C.G. and A.S.; Writing—original draft, S.D.; Writing—review and editing, K.E., C.G., A.S. and G.A.K.-U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Since all the reports in VigiBase™ are anonymized, ethical approval was not required for this study.
Informed Consent Statement
A written informed consent statement from the index case is available.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest. The published results and conclusions do not represent the general opinion of the UMC or WHO.
References
- Swiss Drug Information. Available online: www.swissmedicinfo.ch (accessed on 4 October 2023).
- Micromedex™. Available online: www.micromedexsolutions.com (accessed on 4 October 2023).
- UpToDate™. Available online: www.uptodate.com (accessed on 4 October 2023).
- Tribe, H.T. The discovery and development of cyclosporin. Mycologist 1998, 12, 20–22. [Google Scholar] [CrossRef]
- Borel, J.F.; Kis, Z.L. The discovery and development of cyclosporine (Sandimmune). Transpl. Proc. 1991, 23, 1867–1874. [Google Scholar]
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. 1987, 40, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Farmer, J.D., Jr.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Handschumacher, R.E.; Harding, M.W.; Rice, J.; Drugge, R.J.; Speicher, D.W. Cyclophilin: A specific cytosolic binding protein for cyclosporin A. Science 1984, 226, 544–547. [Google Scholar] [CrossRef]
- Braun, W.; Kallen, J.; Mikol, V.; Walkinshaw, M.D.; Wüthrich, K. Three-dimensional structure and actions of immunosuppressants and their immunophilins. FASEB J. 1995, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Klee, C.B.; Bierer, B.E.; Burakoff, S.J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc. Natl. Acad. Sci. USA 1992, 89, 3686–3690. [Google Scholar] [CrossRef]
- Kung, L.; Halloran, P.F. Immunophilins may limit calcineurin inhibition by cyclosporine and tacrolimus at high drug concentrations. Transplantation 2000, 70, 327–335. [Google Scholar] [CrossRef]
- Snee, I.; Drobina, J.; Mazer-Amirshahi, M. Tacrolimus toxicity due to enzyme inhibition from ritonavir. Am. J. Emerg. Med. 2023, 69, e5–e218. [Google Scholar] [CrossRef]
- Rose, D.T.; Gandhi, S.M.; Bedard, R.A.; Mondy, K.E.; Chu, A.L.; Gamble, K.C.; Gee, A.T.; Kundra, M.A.; Williams, A.L.; Lee, B.K. Supratherapeutic Tacrolimus Concentrations With Nirmatrelvir/Ritonavir in Solid Organ Transplant Recipients Requiring Hospitalization: A Case Series Using Rifampin for Reversal. Open Forum. Infect Dis. 2022, 9, ofac238. [Google Scholar] [CrossRef]
- Hoppe, J.M.; Holderied, A.; Schönermarck, U.; Vielhauer, V.; Anders, H.J.; Fischereder, M. Drug-induced CYP induction as therapy for tacrolimus intoxication. Clin. Nephrol. Case Stud. 2022, 10, 42–46. [Google Scholar] [CrossRef]
- Cadley, S.M.; Sethi, A.; Knorr, J.P. CYP induction to reverse tacrolimus toxicity resulting from concomitant Paxlovid use. Transpl. Infect Dis. 2022, 24, e13982. [Google Scholar] [CrossRef]
- Sharma, A.; Wahby, K.A.; Inany, M.; Lee, S.J. Use of phenytoin for treatment of tacrolimus toxicity with superimposed sepsis. BMJ Case Rep. 2020, 13, e234839. [Google Scholar] [CrossRef]
- Patel, S.J.; Kuten, S.A.; Musick, W.L.; Gaber, A.O.; Monsour, H.P.; Knight, R.J. Combination Drug Products for HIV-A Word of Caution for the Transplant Clinician. Am. J. Transplant. 2016, 16, 2479–2482. [Google Scholar] [CrossRef]
- Nghiem, D.D. Tacrolimus Induced Organ Failure: Reversal by Activation of the Cytochrome P450-3a System. Uro 2021, 1, 222–227. [Google Scholar] [CrossRef]
- O’Connor, A.D.; Rusyniak, D.E.; Mowry, J. Acute tacrolimus toxicity in a non-transplant patient. Clin. Toxicol. 2008, 46, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Karasu, Z.; Gurakar, A.; Carlson, J.; Pennington, S.; Kerwin, B.; Wright, H.; Nour, B.; Sebastian, A. Acute tacrolimus overdose and treatment with phenytoin in liver transplant recipients. J. Okla State Med. Assoc. 2001, 94, 121–123. [Google Scholar] [PubMed]
- Nghiem, D.D. Role of pharmacologic enhancement of p-450 in cyclosporine overdose. Transplantation 2002, 74, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Ceschi, A.; Rauber-Lüthy, C.; Kupferschmidt, H.; Banner, N.R.; Ansari, M.; Krähenbühl, S.; Taegtmeyer, A.B. Acute calcineurin inhibitor overdose: Analysis of cases reported to a national poison center between 1995 and 2011. Am. J. Transpl. 2013, 13, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Arellano, F.; Monka, C.; Krupp, P.F. Acute cyclosporin overdose. A review of present clinical experience. Drug. Saf. 1991, 6, 266–276, Erratum in Drug. Saf. 1991, 6, 338. [Google Scholar] [CrossRef] [PubMed]
- Sein Anand, J.; Chodorowski, Z.; Kujawska, H. Acute suicidal intoxication with tacrolimus in a kidney transplant patient. Przegl. Lek. 2005, 62, 517–518. [Google Scholar] [PubMed]
- Bax, K.; Tijssen, J.; Rieder, M.J.; Filler, G. Rapid resolution of tacrolimus intoxication-induced AKI with a corticosteroid and phenytoin. Ann. Pharmacother. 2014, 48, 1525–1528. [Google Scholar] [CrossRef]
- Hochleitner, B.W.; Bösmüller, C.; Nehoda, H.; Frühwirt, M.; Simma, B.; Ellemunter, H.; Steurer, W.; Hochleitner, E.O.; Königsrainer, A.; Margreiter, R. Increased tacrolimus levels during diarrhea. Transpl. Int. 2001, 14, 230–233. [Google Scholar] [CrossRef]
- Kishino, S.; Takekuma, Y.; Sugawara, M.; Shimamura, T.; Furukawa, H.; Todo, S.; Miyazaki, K. Influence of continuous venovenous haemodiafiltration on the pharmacokinetics of tacrolimus in liver transplant recipients with small-for-size grafts. Clin. Transpl. 2003, 17, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Ptachcinski, R.J.; Burckart, G.J.; Yang, S.L.; Starzl, T.E.; Van Theil, D.H. The clearance of cyclosporine by hemodialysis. J. Clin. Pharmacol. 1984, 24, 528–531. [Google Scholar] [CrossRef]
- Lemaire, M.; Tillement, J.P. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J. Pharm. Pharmacol. 1982, 34, 715–718. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, X.X.; Li, S.; Zhang, Q.; Guo, L.J.; Chen, W.; Liu, L.H. Supratherapeutic Tacrolimus Concentrations With Nirmatrelvir/Ritonavir in a Lung Transplant Patient: A Case Report Using Rifampin for Reversal. Front. Pharmacol. 2022, 14, 1285078. [Google Scholar] [CrossRef]
- Kwon, E.J.; Yun, G.A.; Park, S.; Kim, S.; Chae, D.W.; Park, H.S.; Lee, T.; Jeong, J.C. Treatment of acute tacrolimus toxicity with phenytoin after Paxlovid (nirmatrelvir/ritonavir) administration in a kidney transplant recipient. Kidney Res. Clin. Pract. 2022, 41, 768–770. [Google Scholar] [CrossRef]
- Shah, A.; Nasrullah, A.; Butt, M.A.; Young, M. Paxlovid with Caution: Novel Case of Paxlovid-Induced Tacrolimus Toxicity in a Cardiac Transplant Patient. Eur. J. Case Rep. Intern. Med. 2022, 9, 003528. [Google Scholar] [CrossRef]
- Meaney, C.J.; O’Connor, M.; McGowan, M.; Hamid, M.; Su, W. Treatment of prolonged tacrolimus toxicity using phenytoin in a haemodialysis patient. J. Clin. Pharm. Ther. 2019, 44, 640–643. [Google Scholar] [CrossRef]
- Jantz, A.S.; Patel, S.J.; Suki, W.N.; Knight, R.J.; Bhimaraj, A.; Gaber, A.O. Treatment of acute tacrolimus toxicity with phenytoin in solid organ transplant recipients. Case Rep. Transplant. 2013, 2013, 375263. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.E.; Rossique-Gonzalez, M.; Gelman, B.; Kato, T. Use of phenobarbital in the management of acute tacrolimus toxicity: A case report. Transplant. Proc. 2000, 32, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Tejeira, R.E.; Chang, I.F.; Bristow, L.J.; Karpen, S.J.; Goss, J.A. Treatment of acute tacrolimus whole-blood elevation with phenobarbital in the pediatric liver transplant recipient. Pediatr. Transplant. 2005, 9, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Naylor, H.; Robichaud, J. Decreased tacrolimus levels after administration of rifampin to a patient with renal transplant. Can. J. Hosp. Pharm. 2013, 66, 388–392. [Google Scholar] [CrossRef]
- Jindal, R.M.; Pescovitz, M.D.; Cummings, O.W.; Book, B.; Lumeng, L.; Milgrom, M.L.; Leapman, S.B.; Filo, R.S. Persistence of cyclosporine after withdrawal of the drug in a patient with chronic liver transplant rejection. Role of the monoethylglycinexylidine test. Transplantation 1996, 61, 1657–1658. [Google Scholar] [CrossRef]
- Lucey, M.R.; Kolars, J.C.; Merion, R.M.; Campbell, D.A.; Aldrich, M.; Watkins, P.B. Cyclosporin toxicity at therapeutic blood levels and cytochrome P-450 IIIA. Lancet 1990, 335, 11–15. [Google Scholar] [CrossRef]
- Yeh, C.N.; Hsieh, C.H.; Chao-Ming, H.; Long-Bin, B.J. Acute overdoses of tacrolimus (FK 506). Dig. Dis. Sci. 1999, 44, 1650. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, H.; Ma, S.; Rui, J.Z.; Miao, L.Y. Population pharmacokinetics and pharmacogenetics of tacrolimus in healthy Chinese volunteers. Pharmacology 2011, 88, 288–294. [Google Scholar] [CrossRef]
- Hebert, M.F.; Park, J.M.; Chen, Y.L.; Akhtar, S.; Larson, A.M. Effects of St. John’s wort (Hypericum perforatum) on tacrolimus pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 2004, 44, 89–94. [Google Scholar] [CrossRef]
- Jusko, W.J.; Piekoszewski, W.; Klintmalm, G.B.; Shaefer, M.S.; Hebert, M.F.; Piergies, A.A.; Lee, C.C.; Schechter, P.; Mekki, Q.A. Pharmacokinetics of tacrolimus in liver transplant patients. Clin. Pharmacol. Ther. 1995, 57, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Cook, M.; Alak, A.M. In-vitro metabolic studies of tacrolimus using precision-cut rat and human liver slices. J. Pharm. Biomed. Anal. 1996, 15, 349–357. [Google Scholar] [CrossRef]
- Antignac, M.; Hulot, J.S.; Boleslawski, E.; Hannoun, L.; Touitou, Y.; Farinotti, R.; Lechat, P.; Urien, S. Population pharmacokinetics of tacrolimus in full liver transplant patients: Modelling of the post-operative clearance. Eur. J. Clin. Pharmacol. 2005, 61, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Oteo, I.; Lukas, J.C.; Leal, N.; Suarez, E.; Valdivieso, A.; Gastaca, M.; Ortiz de Urbina, J.; Calvo, R. Tacrolimus pharmacokinetics in the early post-liver transplantation period and clinical applicability via Bayesian prediction. Eur. J. Clin. Pharmacol. 2013, 69, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.A.; Hewitt, J.M.; Sorenson, A.L.; Barber, D.L.; Bowers, L.; Rynders, G.; Arrazola, L.; Matas, A.J.; Rosenberg, M.E.; Canafax, D.M. Pharmacokinetics of FK506 after intravenous and oral administration in patients awaiting renal transplantation. J. Clin. Pharmacol. 1994, 34, 859–864. [Google Scholar] [CrossRef] [PubMed]
- López-Montes, A.; Gallego, E.; López, E.; Pérez, J.; Lorenzo, I.; Llamas, F.; Serrano, A.; Andrés, E.; Illescas, L.; Gómez, C. Treatment of tuberculosis with rifabutin in a renal transplant recipient. Am. J. Kidney Dis. 2004, 44, e59–e63. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Latorre, A.; Manzanares, C.; Morales, E.; Herrero, J.C.; Dominguez-Gil, B.; Carreño, A.; Cubas, A.; Delgado, M.; Andres, A.; et al. Clinical management of tacrolimus drug interactions in renal transplant patients. Transpl. Proc. 1999, 31, 2252–2253. [Google Scholar] [CrossRef]
- Chenhsu, R.Y.; Loong, C.C.; Chou, M.H.; Lin, M.F.; Yang, W.C. Renal allograft dysfunction associated with rifampin-tacrolimus interaction. Ann. Pharmacother. 2000, 34, 27–31. [Google Scholar] [CrossRef]
- Mori, T.; Aisa, Y.; Kato, J.; Nakamura, Y.; Shimizu, T.; Okamoto, S. Overcoming the effect of rifampin on the tacrolimus metabolism by itraconazole administration in an allogeneic hematopoietic stem cell transplant recipient. Int. J. Hematol. 2010, 91, 553–554. [Google Scholar] [CrossRef]
- Niwa, T.; Shiraga, T.; Takagi, A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol. Pharm. Bull. 2005, 28, 1805–1808. [Google Scholar] [CrossRef]
- Lumlertgul, D.; Noppakun, K.; Rojanasthien, N.; Kanchanarattanakorn, K.; Jittikanont, S.; Manoyot, A.; Bunnachak, D.; Ophascharoensuk, V. Pharmacokinetic study of the combination of tacrolimus and fluconazole in renal transplant patients. J. Med. Assoc Thai. 2006, 89 (Suppl. S2), S73–S78. [Google Scholar]
- Osowski, C.L.; Dix, S.P.; Lin, L.S.; Mullins, R.E.; Geller, R.B.; Wingard, J.R. Evaluation of the drug interaction between intravenous high-dose fluconazole and cyclosporine or tacrolimus in bone marrow transplant patients. Transplantation 1996, 61, 1268–1272. [Google Scholar] [CrossRef]
- Kuan, W.J.; Châteauvert, N.; Leclerc, V.; Drolet, B. Tacrolimus Dose-Conversion Ratios Based on Switching of Formulations for Patients with Solid Organ Transplants. Can. J. Hosp. Pharm. 2021, 74, 317–326. [Google Scholar] [CrossRef]
- Marcelín-Jiménez, G.; García-González, A.; Angeles-Moreno, A.P.; Contreras-Zavala, L.; Rivera, L.; Morales, M. Development of an ultra-performance liquid chromatography technique coupled with mass spectrometry for the measurement of tacrolimus in micro-samples of whole blood, and its application on a pharmacokiinetic trial. Arzneimittelforschung 2007, 57, 659–664. [Google Scholar] [CrossRef]
- USZ Analysenauskunftssystem. Available online: https://vademecum.usz.ch/Analysis/?entryID=4962 (accessed on 9 October 2023).
- Hebert, M.F.; Fisher, R.M.; Marsh, C.L.; Dressler, D.; Bekersky, I. Effects of rifampin on tacrolimus pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 1999, 39, 91–96. [Google Scholar] [CrossRef]
- Riva, N.; Schaiquevich, P.; Cáceres Guido, P.; Halac, E.; Dip, M.; Imventarza, O. Pharmacoepidemiology of tacrolimus in pediatric liver transplantation. Pediatr. Transplant. 2017, 21, e12982. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, I.; Oyama, S.; Inada, A.; Wakabayashi, T.; Iida, T.; Kambara, H.; Uchida, M.; Sano, Y.; Hosohata, K. Current Status of Adverse Event Profile of Cyclosporine in Kidney, Stem Cell, and Heart Transplantations Using the Japanese Pharmacovigilance Database. Cureus 2022, 14, e29383. [Google Scholar] [CrossRef] [PubMed]
- Amkreutz, J.; Koch, A.; Buendgens, L.; Muehlfeld, A.; Trautwein, C.; Eisert, A. Prevalence and nature of potential drug-drug interactions among kidney transplant patients in a German intensive care unit. Int. J. Clin. Pharm. 2017, 39, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Castro, V.; Centurion, I.G.; Espinosa, J.; Keller, G.A.; Gonzalez, C.D.; Riera, M.C.; Saubidet, C.L.; Di Girolamo, G.; Pujol, G.S.; et al. A Systematic Approach to Assess the Burden of Drug Interactions in Adult Kidney Transplant Patients. Curr. Drug. Saf. 2016, 11, 156–163. [Google Scholar] [CrossRef]
- Gago-Sánchez, A.I.; Font, P.; Cárdenas, M.; Aumente, M.D.; Del Prado, J.R.; Calleja, M.Á. Real clinical impact of drug-drug interactions of immunosuppressants in transplant patients. Pharmacol. Res. Perspect. 2021, 9, e00892. [Google Scholar] [CrossRef] [PubMed]
- Formea, C.M.; Evans, C.G.; Karlix, J.L. Altered cytochrome p450 metabolism of calcineurin inhibitors: Case report and review of the literature. Pharmacotherapy 2005, 25, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- VigiBase™. Available online: https://who-umc.org/vigibase/ (accessed on 4 October 2023).
- VigiLyze™. Available online: https://vigilyze.who-umc.org/ (accessed on 4 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).