The Social Context of Pregnancy, Respectful Maternity Care, Biomarkers of Weathering, and Postpartum Mental Health Inequities: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Procedure
2.2. Inclusion Criteria
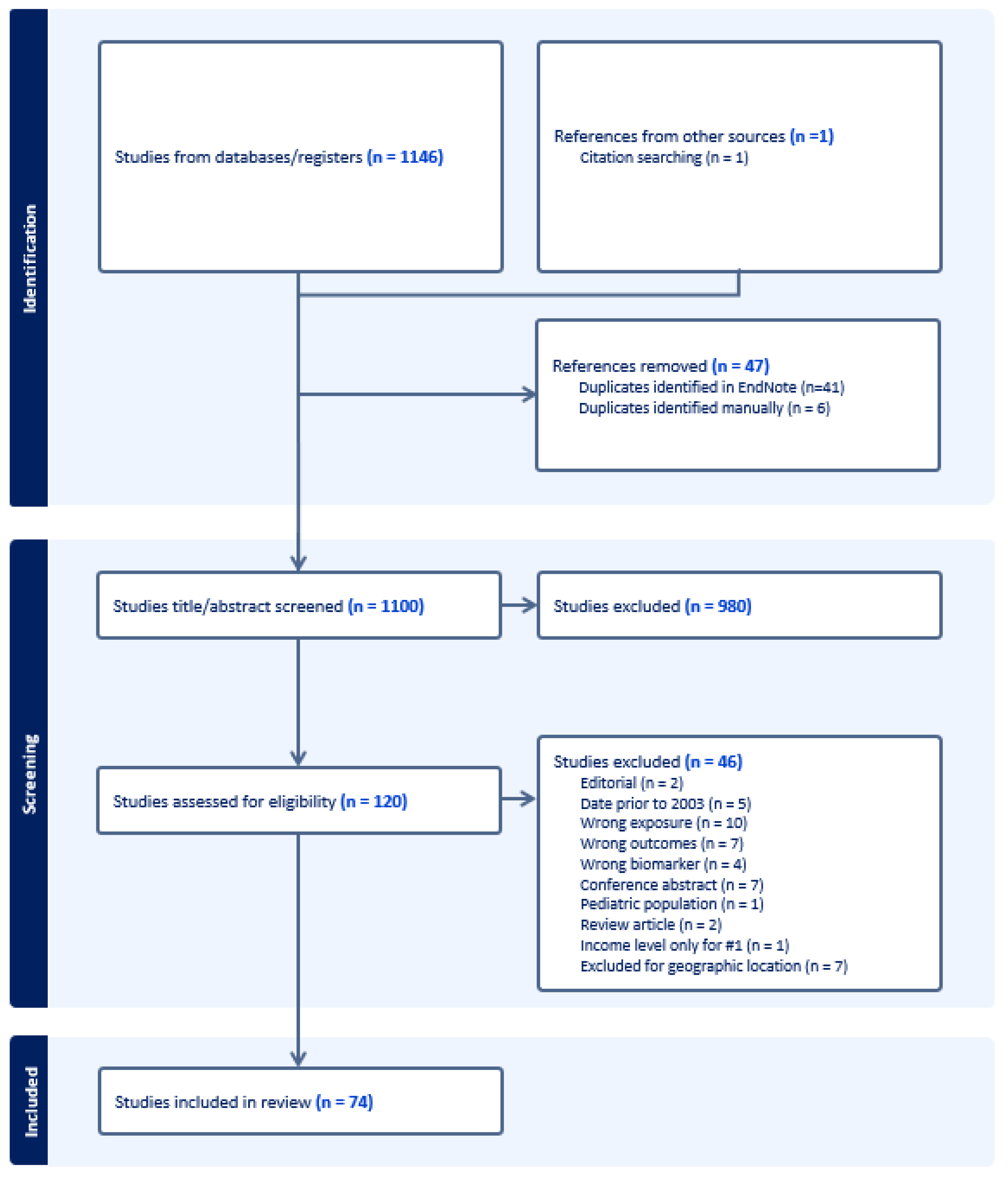
2.3. Study Selection
2.4. Data Extraction
3. Results
3.1. Measurement of Exposures/Experiences during Pregnancy and Birth
3.2. Associations between PMADs and Social Exposures
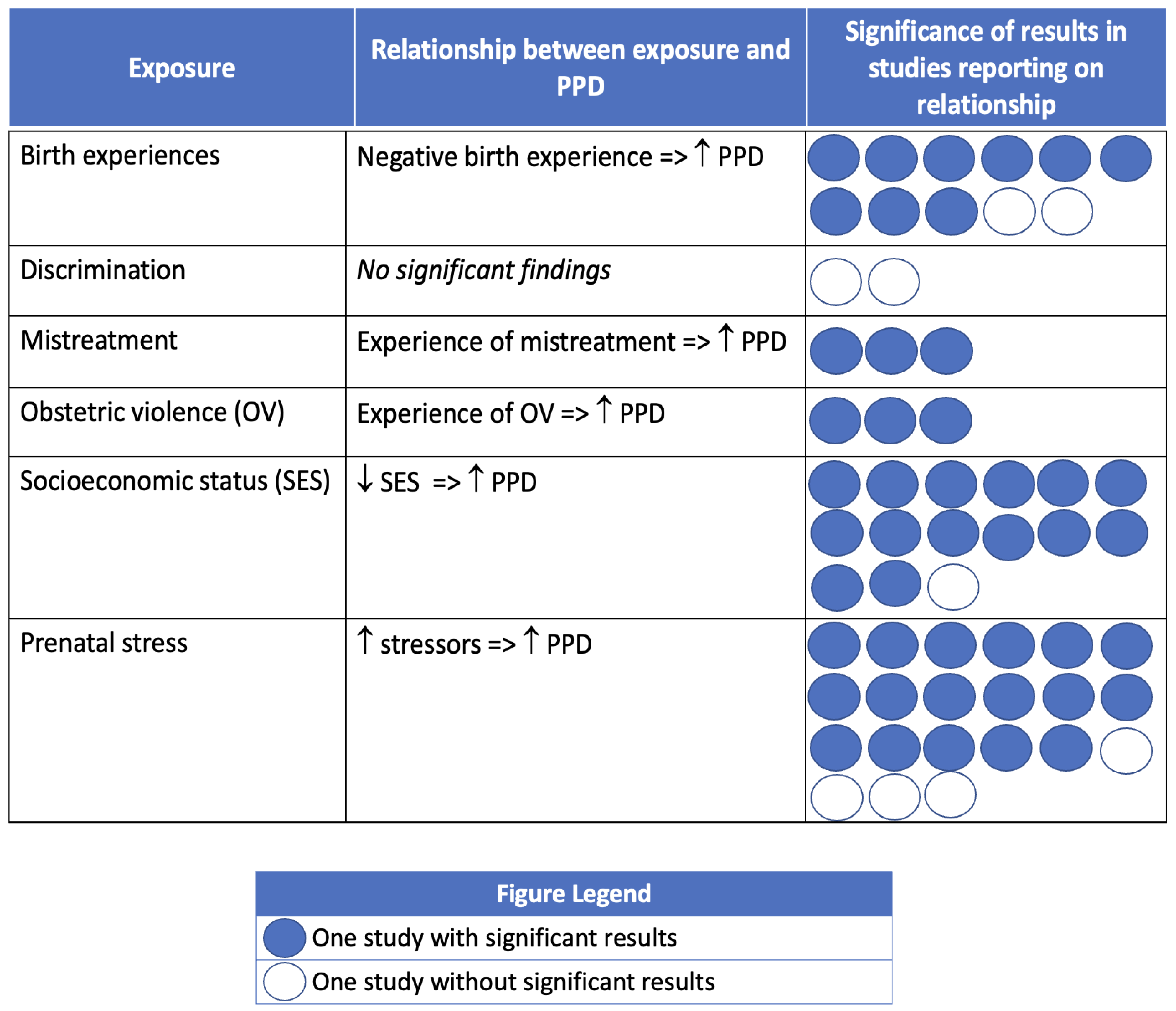
3.3. Postpartum Depression
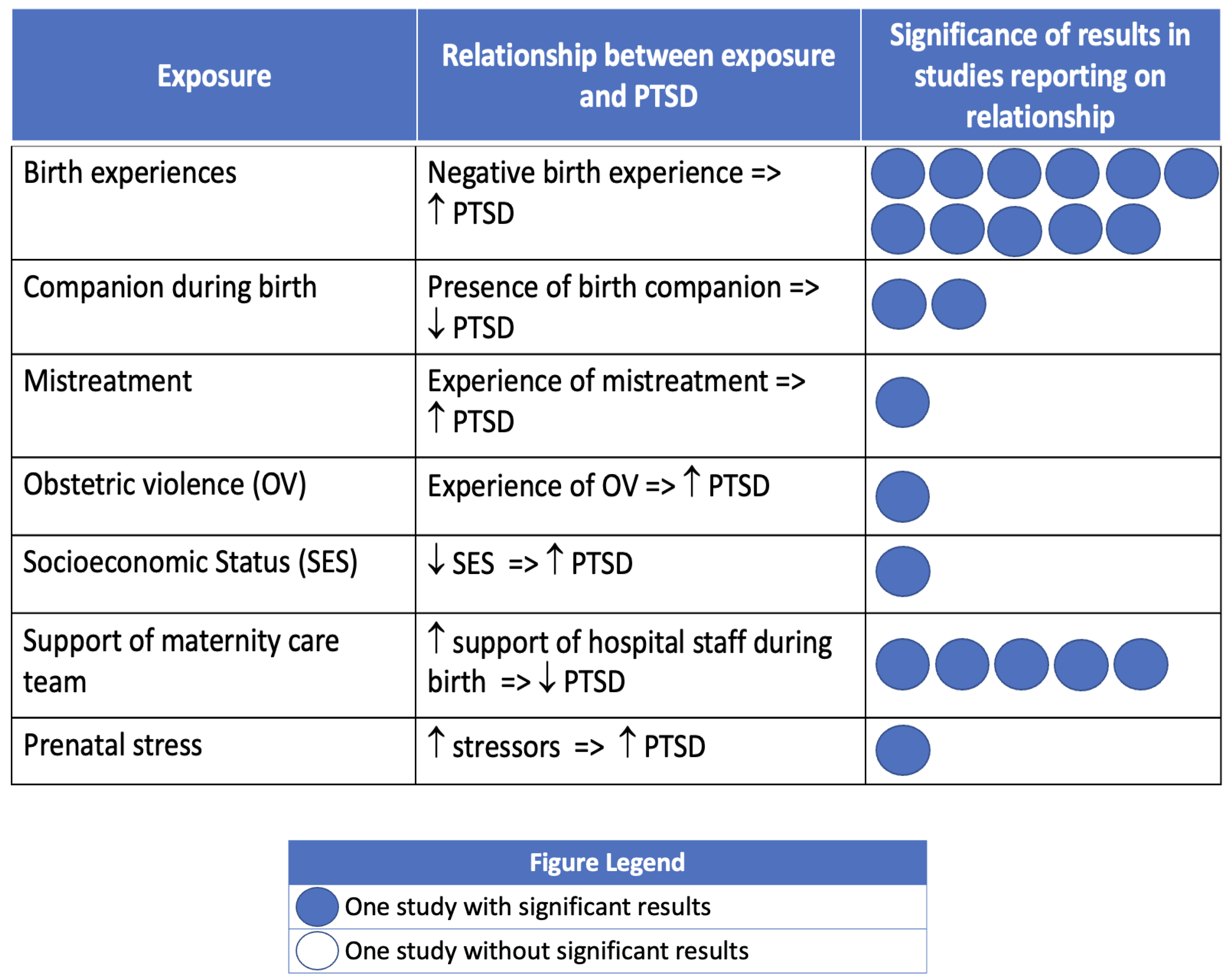
3.4. Birth-Related Post-Traumatic Stress Disorder
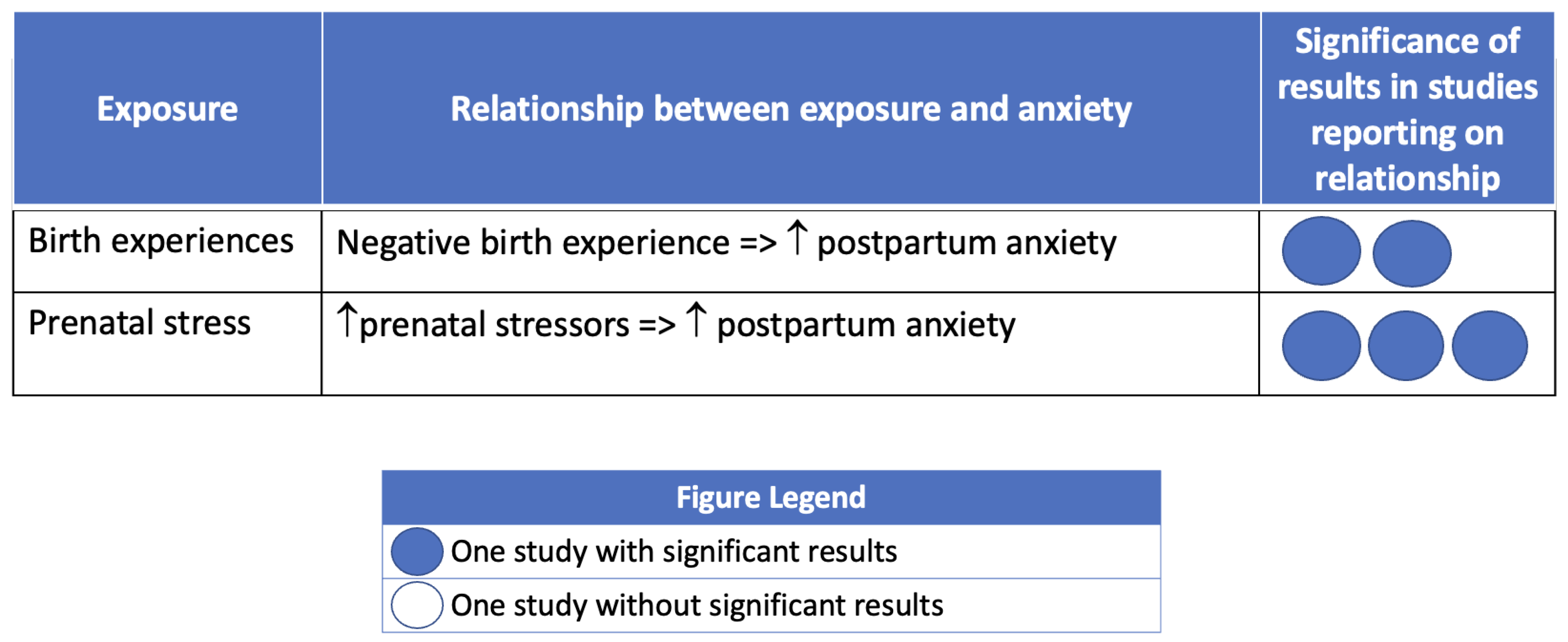
3.5. Postpartum Anxiety
3.6. Studies including Biomarkers of Stress and Weathering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trost, S.; Beauregard, J.; Chandra, G.; Njie, F.; Berry, J.; Harvey, A.; Goodman, D.A. Pregnancy-Related Deaths: Data from Maternal Mortality Review Committees in 36 US States, 2017–2019. Education 2022, 45. [Google Scholar]
- Declercq, E.; Zephyrin, L. Severe Maternal Morbidity in the United States: A Primer. Commonw. Fund 2021, 28. [Google Scholar]
- Luca, D.L.; Margiotta, C.; Staatz, C.; Garlow, E.; Christensen, A.; Zivin, K. Financial Toll of Untreated Perinatal Mood and Anxiety Disorders among 2017 Births in the United States. Am. J. Public Health 2020, 110, 888. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.M.; Chen, J.; Cox, S.; Luo, F.; Robbins, C.L.; Tevendale, H.D.; Li, R.; Ko, J.Y. Healthcare Utilization and Costs Associated with Perinatal Depression among Medicaid Enrollees. Am. J. Prev. Med. 2022, 62, e333–e341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Shuai, H.; Cai, Z.; Fu, X.; Liu, Y.; Xiao, X.; Zhang, W.; Krabbendam, E.; Liu, S.; et al. Mapping Global Prevalence of Depression among Postpartum Women. Transl. Psychiatry 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Slomian, J.; Honvo, G.; Emonts, P.; Reginster, J.-Y.; Bruyère, O. Consequences of Maternal Postpartum Depression: A Systematic review of Maternal and Infant Outcomes. Women’s Health 2019, 15, 1745506519844044. [Google Scholar] [CrossRef] [PubMed]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of Persistent and Severe Postnatal Depression with Child Outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.L.; Jones, E.J.; Bohn, D.; Mccage, S.; Parker, J.G.; Parker, M.; Pierce, S.L.; Campbell, J. Maternal Mortality among American Indian/Alaska Native Women: A Scoping Review. J. Women’s Health 2021, 30, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, D.L.; Sbrilli, M.D.; Laurent, H.K. Impact of Maternal Trauma-Related Psychopathology and Life Stress on HPA Axis Stress Response. Arch. Women’s Ment. Health 2021, 25, 121–128. [Google Scholar] [CrossRef]
- Elmir, R.; Schmied, V.; Wilkes, L.; Jackson, D. Women’s Perceptions and Experiences of a Traumatic Birth: A Meta-Ethnography. J. Adv. Nurs. 2010, 66, 2142–2153. [Google Scholar] [CrossRef]
- Beck, C.T.; Casavant, S. Synthesis of Mixed Research on Posttraumatic Stress Related to Traumatic Birth. J. Obstet. Gynecol. Neonatal Nurs. 2019, 48, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Soet, J.E.; Brack, G.A.; DiIorio, C. Prevalence and Predictors of Women’s Experience of Psychological Trauma during Childbirth. Birth 2003, 30, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Creedy, D.K.; Shochet, I.M.; Horsfall, J. Childbirth and the Development of Acute Trauma Symptoms: Incidence and Contributing Factors. Birth 2000, 27, 104–111. [Google Scholar] [CrossRef]
- Fenech, G.; Thomson, G. Tormented by Ghosts from Their Past’: A Meta-Synthesis to Explore the Psychosocial Implications of a Traumatic Birth on Maternal Well-Being. Midwifery 2014, 30, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Reno, R.; Burch, J.; Stookey, J.; Jackson, R.; Joudeh, L.; Guendelman, S. Preterm Birth and Social Support Services for Prenatal Depression and Social Determinants. PLoS ONE 2021, 16, e0255810. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Entringer, S.; Buss, C.; Lu, M.C. The Contribution of Maternal Stress to Preterm Birth: Issues and Considerations. In Clinics in Perinatology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 351–384. [Google Scholar] [CrossRef]
- Mukherjee, S.; Coxe, S.; Fennie, K.; Madhivanan, P.; Trepka, M.J. Stressful Life Event Experiences of Pregnant Women in the United States: A Latent Class Analysis. Women’s Health Issues 2017, 27, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sonderlund, A.L.; Schoenthaler, A.; Thilsing, T. The Association between Maternal Experiences of Interpersonal Discrimination and Adverse Birth Outcomes: A Systematic Review of the Evidence. Int. J. Environ. Res. Public Health 2021, 18, 1465. [Google Scholar] [CrossRef] [PubMed]
- Sudhinaraset, M.; Landrian, A.; Golub, G.; Cotter, S.Y.; Afulani, P. Person-Centered Maternity Care and Postnatal Health: Associations with Maternal and Newborn Health Outcomes. AJOG Glob. Rep. 2021, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Moulton, K.; Miller, S.; Persson, P.; Rossin-Slater, M.; Wherry, L.; Aldana, G. Nber Working Paper Series Maternal and Infant Health Inequality: New Evidence from Linked Administrative Data; National Bureau of Economic Research: Cambridge, MA, USA, 2022.
- Pearl, M.; Ahern, J.; Hubbard, A.; Laraia, B.; Shrimali, B.P.; Poon, V.; Kharrazi, M. Life-Course Neighbourhood Opportunity and Racial-Ethnic Disparities in Risk of Preterm Birth. Paediatr. Perinat. Epidemiol. 2018, 32, 412–419. [Google Scholar] [CrossRef]
- Black, K.A.; MacDonald, I.; Chambers, T.; Ospina, M.B. Postpartum Mental Health Disorders in Indigenous Women: A Systematic Review and Meta-Analysis. J. Obstet. Gynaecol. Can. 2019, 41, 1470–1478. [Google Scholar] [CrossRef]
- Estriplet, T.; Morgan, I.; Davis, K.; Crear Perry, J.; Matthews, K. Black Perinatal Mental Health: Prioritizing Maternal Mental Health to Optimize Infant Health and Wellness. Front. Psychiatry 2022, 13, 807235. [Google Scholar] [CrossRef]
- Gadson, A.; Akpovi, E.; Mehta, P.K. Exploring the Social Determinants of Racial/Ethnic Disparities in Prenatal Care Utilization and Maternal Outcome. Semin. Perinatol. 2017, 41, 308–317. [Google Scholar] [CrossRef]
- Vedam, S.; Stoll, K.; Khemet Taiwo, T.; Rubashkin, N.; Cheyney, M.; Strauss, N.; Mclemore, M.; Cadena, M.; Nethery, E.; Rushton, E.; et al. The Giving Voice to Mothers Study: Inequity and Mistreatment during Pregnancy and Childbirth in the United States. Reprod. Health 2019, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Basile Ibrahim, B.; Knobf, M.T.; Shorten, A.; Vedam, S.; Cheyney, M.; Illuzzi, J.; Kennedy, H.P. “I Had to Fight for My VBAC”: A Mixed Methods Exploration of Women’s Experiences of Pregnancy and Vaginal Birth after Cesarean in the United States. Birth 2020, 48, 164–177. [Google Scholar] [CrossRef]
- Basile Ibrahim, B.B.; Kozhimannil, K.B. Racial Disparities in Respectful Maternity Care during Pregnancy and Birth after Cesarean in Rural United States. J. Obstet. Gynecol. Neonatal. Nurs. 2023, 52, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Basile Ibrahim, B.; Kennedy, H.P.; Combellick, J. Experiences of Quality Perinatal Care during the US COVID-19 Pandemic. J. Midwifery Women’s Health 2021, 66, 579–588. [Google Scholar] [CrossRef]
- Basile Ibrahim, B.; Vedam, S.; Illuzzi, J.; Cheyney, M.; Kennedy, H.P. Inequities in Quality Perinatal Care in the United States during Pregnancy and Birth after Cesarean. PLoS ONE 2022, 17, e0274790. [Google Scholar] [CrossRef]
- Logan, R.G.; McLemore, M.R.; Julian, Z.; Stoll, K.; Malhotra, N.; Vedam, S. Coercion and Non-Consent during Birth and Newborn Care in the United States. Birth 2022, 49, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Truong, S.; DeAndrade, S.; Jacober, J.; Medina, M.; Diouf, K.; Meadows, A.; Nour, N.; Schantz-Dunn, J. Respectful Maternity Care in the United States—Characterizing Inequities Experienced by Birthing People. Matern. Child Health J. 2024, 2024, 1–15. [Google Scholar] [CrossRef]
- Mohamoud, Y.A.; Cassidy, E.; Fuchs, E.; Womack, L.S.; Romero, L.; Kipling, L.; Oza-Frank, R.; Baca, K.; Galang, R.R.; Stewart, A.; et al. Vital Signs: Maternity Care Experiences—United States, April 2023. MMWR Morb. Mortal. Wkly Rep. 2023, 72, 961–967. [Google Scholar] [CrossRef]
- Attanasio, L.B.; Ranchoff, B.L.; Paterno, M.T.; Kjerulff, K.H. Person-Centered Maternity Care and Health Outcomes at 1 and 6 Months Postpartum. J. Women’s Health 2022, 31, 1411–1421. [Google Scholar] [CrossRef]
- Geronimus, A.T. The Weathering Hypothesis and the Health of African-American Women and Infants: Evidence and Speculations. Ethn. Dis. 1992, 2, 207–221. [Google Scholar]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and Age Patterns of Allostatic Load Scores among Blacks and Whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Pearson, J.A.; Linnenbringer, E.; Schulz, A.J.; Reyes, A.G.; Epel, E.S.; Lin, J.; Blackburn, E.H. Race-Ethnicity, Poverty, Urban Stressors, and Telomere Length in a Detroit Community-Based Sample. J. Health Soc. Behav. 2015, 56, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.F.; Edwards, E.M.; Horbar, J.D.; Howell, E.A.; Mccormick, M.C.; Pursley, D.M. The Color of Health: How Racism, Segregation, and Inequality Affect the Health and Well-Being of Preterm Infants and Their Families. Pediatr. Res. 2019, 87, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Prather, C.; Fuller, T.R.; Jeffries, W.L.; Marshall, K.J.; Howell, A.V.; Belyue-Umole, A.; King, W. Racism, African American Women, and Their Sexual and Reproductive Health: A Review of Historical and Contemporary Evidence and Implications for Health Equity. In Health Equity; Mary Ann Liebert Inc.: New Rochelle, NY, USA, 2018; pp. 249–259. [Google Scholar] [CrossRef]
- Howell, E.A. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin. Obstet. Gynecol. 2018, 61, 387–399. [Google Scholar] [CrossRef]
- Wildsmith, E. Testing the Weathering Hypothesis among Mexican-Origin Women. Ethn. Dis. 2002, 12, 470–479. [Google Scholar]
- Palacios, J.F.; Portillo, C.J. Understanding Native Women’s Health: Historical Legacies. J. Transcult. Nurs. 2009, 20, 15–27. [Google Scholar] [CrossRef]
- Almeida, J.; Bécares, L.; Erbetta, K.; Bettegowda, V.R.; Ahluwalia, I.B. Racial/Ethnic Inequities in Low Birth Weight and Preterm Birth: The Role of Multiple Forms of Stress. Matern. Child Health J. 2018, 22, 1154–1163. [Google Scholar] [CrossRef]
- Dennis, J.A. Birth Weight and Maternal Age among American Indian/Alaska Native Mothers: A Test of the Weathering Hypothesis. SSM Popul. Health 2019, 7, 100304. [Google Scholar] [CrossRef]
- Evans, L.; Engelman, M.; Mikulas, A.; Malecki, K. How Are Social Determinants of Health Integrated into Epigenetic Research? A Systematic Review. In Social Science and Medicine; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; p. 113738. [Google Scholar] [CrossRef]
- Riggan, K.A.; Gilbert, A.; Allyse, M.A. Acknowledging and Addressing Allostatic Load in Pregnancy Care. In Journal of Racial and Ethnic Health Disparities; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–11. [Google Scholar] [CrossRef]
- Forde, A.T.; Crookes, D.M.; Suglia, S.F.; Demmer, R.T. The Weathering Hypothesis as an Explanation for Racial Disparities in Health: A Systematic Review. Ann. Epidemiol. 2019, 33, 1–18.e3. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Ghastine, L.; Lodge, E.K.; Dhingra, R.; Ward-Caviness, C.K. Understanding Health Inequalities Through the Lens of Social Epigenetics. Annu. Rev. Public Health 2022, 43, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.A.; Jorm, A.F.; Parslow, R.A.; Christensen, H. Is Telomere Length a Biomarker of Aging? A Review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 202–213. [Google Scholar] [CrossRef]
- Pearce, E.E.; Alsaggaf, R.; Katta, S.; Dagnall, C.; Aubert, G.; Hicks, B.D.; Spellman, S.R.; Savage, S.A.; Horvath, S.; Gadalla, S.M. Telomere Length and Epigenetic Clocks as Markers of Cellular Aging: A Comparative Study. GeroScience 2022, 44, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- McEwen, C.A. Connecting the Biology of Stress, Allostatic Load and Epigenetics to Social Structures and Processes. Neurobiol. Stress 2022, 17, 100426. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Seeman, T. Protective and Damaging Effects of Mediators of Stress. Elaborating and Testing the Concepts of Allostasis and Allostatic Load. In Annals of the New York Academy of Sciences; New York Academy of Sciences: New York, NY, USA, 1999; Volume 896, pp. 30–47. [Google Scholar] [CrossRef]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic Load Biomarkers of Chronic Stress and Impact on Health and Cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated Methodological Guidance for the Conduct of Scoping Reviews. JBI Evid. Implement. 2021, 19, 3–10. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- GDP per Capita (Current US$)—OECD Members|Data. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=OE&most_recent_value_desc=true (accessed on 2 February 2024).
- Williams, C.R.; Jerez, C.; Klein, K.; Correa, M.; Belizán, J.M.; Cormick, G. Obstetric Violence: A Latin American Legal Response to Mistreatment during Childbirth. BJOG 2018, 125, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Sadler, M.; Santos, M.J.; Ruiz-Berdún, D.; Rojas, G.L.; Skoko, E.; Gillen, P.; Clausen, J.A. Moving beyond Disrespect and Abuse: Addressing the Structural Dimensions of Obstetric Violence. Reprod. Health Matters 2016, 24, 47–55. [Google Scholar] [CrossRef]
- Garcia, L.M. A Concept Analysis of Obstetric Violence in the United States of America. Nurs. Forum. 2020, 55, 654–663. [Google Scholar] [CrossRef]
- Mallicoat, B.; Uphoff, E.P.; Pickett, K.E. Estimating Social Gradients in Health for UK Mothers and Infants of Pakistani Origin: Do Latent Class Measures of Socioeconomic Position Help? J. Immigr. Minor Health 2020, 22, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, K.L.; O’Donovan, A.; Patrick, J.C.; Creedy, D.; Devilly, G.J. A Prospective Longitudinal Study of the Prevalence of Post-Traumatic Stress Disorder Resulting from Childbirth Events. Psychol. Med. 2010, 40, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Alhasanat, D.; Fry-Mccomish, J.; Yarandi, H.N. Risk For Postpartum Depression among Immigrant Arabic Women in the United States: A Feasibility Study. J. Midwifery Women’s Health 2017, 62, 470–476. [Google Scholar] [CrossRef]
- Catala, P.; Suso-Ribera, C.; Marin, D.; Peñacoba, C. Predicting Postpartum Post-Traumatic Stress and Depressive Symptoms in Low-Risk Women from Distal and Proximal Factors: A Biopsychosocial Prospective Study Using Structural Equation Modeling. Arch. Gynecol. Obstet. 2021, 303, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Cena, L.; Mirabella, F.; Palumbo, G.; Gigantesco, A.; Trainini, A.; Stefana, A. Prevalence of Maternal Antenatal and Postnatal Depression and Their Association with Sociodemographic and Socioeconomic Factors: A Multicentre Study in Italy. J. Affect. Disord. 2021, 279, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Clout, D.; Brown, R. Sociodemographic, Pregnancy, Obstetric, and Postnatal Predictors of Postpartum Stress, Anxiety and Depression in New Mothers. J. Affect. Disord. 2015, 188, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.S.; Gonzales, N.A.; Luecken, L.J.; Crnic, K.A. Multiple Domains of Stress Predict Postpartum Depressive Symptoms in Low-Income Mexican American Women: The Moderating Effect of Social Support. Arch. Women’s Ment. Health 2016, 19, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Cruise, S.M.; Layte, R.; Stevenson, M.; O’Reilly, D. Prevalence and Factors Associated with Depression and Depression-Related Healthcare Access in Mothers of 9-Month-Old Infants in the Republic of Ireland. Epidemiol. Psychiatr. Sci. 2018, 27, 468–478. [Google Scholar] [CrossRef]
- De Schepper, S.; Vercauteren, T.; Tersago, J.; Jacquemyn, Y.; Raes, F.; Franck, E. Post-Traumatic Stress Disorder after Childbirth and the Influence of Maternity Team Care during Labour and Birth: A Cohort Study. Midwifery 2016, 32, 87–92. [Google Scholar] [CrossRef]
- Desmarais, S.L.; Pritchard, A.; Lowder, E.M.; Janssen, P.A. Intimate Partner Abuse before and during Pregnancy as Risk Factors for Postpartum Mental Health Problems. BMC Pregnancy Childbirth 2014, 14, 132. [Google Scholar] [CrossRef]
- Edge, D. Ethnicity, Psychosocial Risk, and Perinatal Depression-a Comparative Study among Inner-City Women in the United Kingdom. J. Psychosom. Res. 2007, 63, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.; Galletly, C.; Semmler-Booth, T.; Dekker, G. Does Antenatal Screening for Psychosocial Risk Factors Predict Postnatal Depression? A Follow-up Study of 154 Women in Adelaide, South Australia. Aust. N. Z. J. Psychiatry 2008, 42, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ertan, D.; Hingray, C.; Burlacu, E.; Sterlé, A.; El-Hage, W. Post-Traumatic Stress Disorder Following Childbirth. BMC Psychiatry 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.L.; Dietz, P.M.; O’Hara, M.W.; Burley, K.; Ko, J.Y. Postpartum Anxiety and Comorbid Depression in a Population-Based Sample of Women. J. Women’s Health 2014, 23, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Ayers, S. Support during Birth Interacts with Prior Trauma and Birth Intervention to Predict Postnatal Post-Traumatic Stress Symptoms. Psychol. Health 2011, 26, 1553–1570. [Google Scholar] [CrossRef] [PubMed]
- Garthus-Niegel, S.; Von Soest, T.; Vollrath, M.E.; Eberhard-Gran, M. The Impact of Subjective Birth Experiences on Post-Traumatic Stress Symptoms: A Longitudinal Study. Arch. Women’s Ment. Health 2013, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Handelzalts, J.E.; Levy, S.; Ayers, S.; Krissi, H.; Peled, Y. Two Are Better than One? The Impact of Lay Birth Companions on Childbirth Experiences and PTSD. Arch. Women’s Ment. Health 2022, 25, 797–805. [Google Scholar] [CrossRef]
- Handelzalts, J.E.; Levy, S.; Krissi, H.; Peled, Y. Epidural Analgesia Associations with Depression, PTSD, and Bonding at 2 Months Postpartum. J. Psychosom. Obstet. Gynecol. 2022, 43, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.E.; Ayers, S.; Quigley, M.A.; Stein, A.; Alderdice, F. Prevalence and Factors Associated with Postpartum Posttraumatic Stress in a Population-Based Maternity Survey in England. J. Affect. Disord. 2021, 279, 749–756. [Google Scholar] [CrossRef]
- Hein, A.; Rauh, C.; Engel, A.; Häberle, L.; Dammer, U.; Voigt, F.; Fasching, P.A.; Faschingbauer, F.; Burger, P.; Beckmann, M.W.; et al. Socioeconomic Status and Depression during and after Pregnancy in the Franconian Maternal Health Evaluation Studies (FRAMES). Arch. Gynecol. Obstet. 2014, 289, 755–763. [Google Scholar] [CrossRef]
- Hernández-Martínez, A.; Rodríguez-Almagro, J.; Molina-Alarcón, M.; Infante-Torres, N.; Rubio-Álvarez, A.; Martínez-Galiano, J.M. Perinatal Factors Related to Post-Traumatic Stress Disorder Symptoms 1–5 Years Following Birth. Women Birth 2020, 33, e129–e135. [Google Scholar] [CrossRef]
- Holt, L.; Sellwood, W.; Slade, P. Birth Experiences, Trauma Responses and Self-Concept in Postpartum Psychotic-like Experiences. Schizophr. Res. 2018, 197, 531–538. [Google Scholar] [CrossRef]
- Janssen, P.A.; Heaman, M.I.; Urquia, M.L.; O’Campo, P.J.; Thiessen, K.R. Risk Factors for Postpartum Depression among Abused and Nonabused Women. Am. J. Obstet. Gynecol. 2012, 207, 489.e1–489.e8. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Tang, M.; Folger, A.; Ammerman, R.T.; Hossain, M.M.; Short, J.; Van Ginkel, J.B. Neighborhood Effects on PND Symptom Severity for Women Enrolled in a Home Visiting Program. Community Ment. Health J. 2018, 54, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.; Russo, J.; Gavin, A. Predictors of Postpartum Depression. J. Women’s Health 2014, 23, 753–759. [Google Scholar] [CrossRef]
- Kim, H.G.; Geppert, J.; Quan, T.; Bracha, Y.; Lupo, V.; Cutts, D.B. Screening for Postpartum Depression among Low-Income Mothers Using an Interactive Voice Response System. Matern. Child Health J. 2012, 16, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kjerulff, K.H.; Attanasio, L.B.; Sznajder, K.K.; Brubaker, L.H. A Prospective Cohort Study of Post-Traumatic Stress Disorder and Maternal-Infant Bonding after First Childbirth HHS Public Access. J. Psychosom. Res. 2021, 144, 110424. [Google Scholar] [CrossRef] [PubMed]
- Kothari, C.L.; Liepman, M.R.; Shama Tareen, R.; Florian, P.; Charoth, R.M.; Haas, S.S.; McKean, J.W.; Moe, A.; Wiley, J.; Curtis, A. Intimate Partner Violence Associated with Postpartum Depression, Regardless of Socioeconomic Status. Matern. Child Health J. 2016, 20, 1237–1246. [Google Scholar] [CrossRef]
- Kress, V.; Von Soest, T.; Kopp, M.; Wimberger, P.; Garthus-Niegel, S. Differential Predictors of Birth-Related Posttraumatic Stress Disorder Symptoms in Mothers and Fathers-A Longitudinal Cohort Study. J. Affect. Disord. 2021, 292, 121–130. [Google Scholar] [CrossRef]
- LaCoursiere, D.Y.; Hirst, K.P.; Barrett-Connor, E. Depression and Pregnancy Stressors Affect the Association between Abuse and Postpartum Depression. Matern. Child Health J. 2012, 16, 929–935. [Google Scholar] [CrossRef]
- Leavy, E.; Cortet, M.; Huissoud, C.; Desplanches, T.; Sormani, J.; Viaux-Savelon, S.; Dupont, C.; Pichon, S.; Gaucher, L. Disrespect during Childbirth and Postpartum Mental Health: A French Cohort Study. BMC Pregnancy Childbirth 2023, 23, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Phan, J.; Yasui, M.; Doan, S. Prenatal Life Events, Maternal Employment, and Postpartum Depression across a Diverse Population in New York City. Community Ment. Health J. 2018, 54, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Luecken, L.J.; Crnic, K.A.; Gonzales, N.A.; Winstone, L.K.; Somers, J.A. Mother-Infant Dyadic Dysregulation and Postpartum Depressive Symptoms in Low-Income Mexican-Origin Women. Biol. Psychol. 2019, 147, 107614. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.E.L.; Urbina, E.; D’Anna-Hernandez, K.L. Sociocultural Stressors Across the Perinatal Period and Risk for Postpartum Depressive Symptoms in Women of Mexican Descent. Cultur. Divers. Ethn. Minor Psychol. 2020, 26, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vázquez, S.; Hernández-Martínez, A.; Rodríguez-Almagro, J.; Delgado-Rodríguez, M.; Martínez-Galiano, J.M. Relationship between Perceived Obstetric Violence and the Risk of Postpartum Depression: An Observational Study. Midwifery 2022, 108, 103297. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Coxe, S.; Fennie, K.; Madhivanan, P.; Trepka, M.J. Antenatal Stressful Life Events and Postpartum Depressive Symptoms in the United States: The Role of Women’s Socioeconomic Status Indices at the State Level. J. Women’s Health 2017, 26, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Fennie, K.; Coxe, S.; Madhivanan, P.; Trepka, M.J. Racial and Ethnic Differences in the Relationship between Antenatal Stressful Life Events and Postpartum Depression among Women in the United States: Does Provider Communication on Perinatal Depression Minimize the Risk? Ethn. Health 2018, 23, 542–565. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; El-Khoury Lesueur, F.; Sutter-Dallay, A.L.; Franck, J.; Thierry, X.; Melchior, M.; van der Waerden, J. The Role of Prenatal Social Support in Social Inequalities with Regard to Maternal Postpartum Depression According to Migrant Status. J. Affect. Disord. 2020, 272, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Nakić Radoš, S.; Martinić, L.; Matijaš, M.; Brekalo, M.; Martin, C.R. The Relationship between Birth Satisfaction, Posttraumatic Stress Disorder and Postnatal Depression Symptoms in Croatian Women. Stress Health 2022, 38, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.P.; Phipps, M.G. Postpartum Depression in Adolescent and Adult Mothers: Comparing Prenatal Risk Factors and Predictive Models. Matern. Child Health J. 2013, 17, 1071–1079. [Google Scholar] [CrossRef]
- O’Donovan, A.; Alcorn, K.L.; Patrick, J.C.; Creedy, D.K.; Dawe, S.; Devilly, G.J. Predicting Posttraumatic Stress Disorder after Childbirth. Midwifery 2014, 30, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Ogbo, F.A.; Kingsley Ezeh, O.; Dhami, M.V.; Naz, S.; Khanlari, S.; Mckenzie, A.; Agho, K.; Page, A.; Ussher, J.; Perz, J.; et al. Perinatal Distress and Depression in Culturally and Linguistically Diverse (CALD) Australian Women: The Role of Psychosocial and Obstetric Factors. Int. J. Environ. Res. Public Health 2019, 16, 2945. [Google Scholar] [CrossRef] [PubMed]
- Paiz, J.C.; de Jezus Castro, S.M.; Giugliani, E.R.J.; dos Santos Ahne, S.M.; Aqua, C.B.D.; Giugliani, C. Association between Mistreatment of Women during Childbirth and Symptoms Suggestive of Postpartum Depression. BMC Pregnancy Childbirth 2022, 22, 664. [Google Scholar] [CrossRef] [PubMed]
- Tavares Pinheiro, R.; Monteiro Da Cunha Coelho, F.; Azevedo Da Silva, R.; Amaral Tavares Pinheiro, K.; Pierre Oses, J.; De Ávila Quevedo, L.; Dias De Mattos Souza, L.; Jansen, K.; Maria Zimmermann Peruzatto, J.; Gus Manfro, G.; et al. Association of a Serotonin Transporter Gene Polymorphism (5-HTTLPR) and Stressful Life Events with Postpartum Depressive Symptoms: A Population-Based Study. J. Psychosom. Obstet. Gynecol. 2013, 34, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Price, S.K.; Proctor, E.K. A Rural Perspective on Perinatal Depression: Prevalence, Correlates, and Implications for Help-Seeking among Low-Income Women. J. Rural. Health 2009, 25, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Qobadi, M.; Collier, C.; Zhang, L. The Effect of Stressful Life Events on Postpartum Depression: Findings from the 2009–2011 Mississippi Pregnancy Risk Assessment Monitoring System. Matern. Child Health J. 2016, 20, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Razurel, C.; Kaiser, B.; Antonietti, J.P.; Epiney, M.; Sellenet, C. Relationship between Perceived Perinatal Stress and Depressive Symptoms, Anxiety, and Parental Self-Efficacy in Primiparous Mothers and the Role of Social Support. Women Health 2017, 57, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Henry, A.; Harvey, S.B.; Homer, C.S.E.; Davis, G.K. Depression, Anxiety and Posttraumatic Stress Disorder Six Months Following Preeclampsia and Normotensive Pregnancy: A P4 Study. BMC Pregnancy Childbirth 2022, 22, 108. [Google Scholar] [CrossRef] [PubMed]
- Salm Ward, T.; Kanu, F.A.; Robb, S.W. Prevalence of Stressful Life Events during Pregnancy and Its Association with Postpartum Depressive Symptoms. Arch. Women’s Ment. Health 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Silveira, M.F.; Mesenburg, M.A.; Bertoldi, A.D.; De Mola, C.L.; Bassani, D.G.; Domingues, M.R.; Stein, A.; Coll, C.V.N. The Association between Disrespect and Abuse of Women during Childbirth and Postpartum Depression: Findings from the 2015 Pelotas Birth Cohort Study. J. Affect. Disord. 2019, 256, 441–447. [Google Scholar] [CrossRef]
- Sommerlad, S.; Schermelleh-Engel, K.; La Rosa, V.L.; Louwen, F.; Oddo-Sommerfeld, S. Trait Anxiety and Unplanned Delivery Mode Enhance the Risk for Childbirth-Related Posttraumatic Stress Disorder Symptoms in Women with and without Risk of Preterm Birth: A Multi Sample Path Analysis. PLoS ONE 2021, 16, e0256681. [Google Scholar] [CrossRef] [PubMed]
- de Souza, K.J.; Rattner, D.; Gubert, M.B. Institutional Violence and Quality of Service in Obstetrics Are Associated with Postpartum Depression. Rev. Saude Publica 2017, 51, 69. [Google Scholar] [CrossRef]
- Steetskamp, J.; Treiber, L.; Roedel, A.; Thimmel, V.; Hasenburg, A.; Skala, C. Post-Traumatic Stress Disorder Following Childbirth: Prevalence and Associated Factors—A Prospective Cohort Study. Arch. Gynecol. Obstet. 2022, 306, 1531–1537. [Google Scholar] [CrossRef]
- Stone, S.L.; Diop, H.; Declercq, E.; Cabral, H.J.; Fox, M.P.; Wise, L.A. Stressful Events during Pregnancy and Postpartum Depressive Symptoms. J. Women’s Health 2015, 24, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.; Yakupova, V. Past Traumatic Life Events, Postpartum PTSD, and the Role of Labor Support. Int. J. Environ. Res. Public Health 2023, 20, 6048. [Google Scholar] [CrossRef] [PubMed]
- Tebeka, S.; Le Strat, Y.; Mandelbrot, L.; Benachi, A.; Dommergues, M.; Kayem, G.; Lepercq, J.; Luton, D.; Ville, Y.; Ramoz, N.; et al. Early- and Late-Onset Postpartum Depression Exhibit Distinct Associated Factors: The IGEDEPP Prospective Cohort Study. BJOG 2021, 128, 1683–1693. [Google Scholar] [CrossRef]
- Waller, R.; Kornfield, S.L.; White, L.K.; Chaiyachati, B.H.; Barzilay, R.; Njoroge, W.; Parish-Morris, J.; Duncan, A.; Himes, M.M.; Rodriguez, Y.; et al. Clinician-Reported Childbirth Outcomes, Patient-Reported Childbirth Trauma, and Risk for Postpartum Depression. Arch. Women’s Ment. Health 2022, 25, 985–993. [Google Scholar] [CrossRef]
- Wikman, A.; Axfors, C.; Iliadis, S.I.; Cox, J.; Fransson, E.; Skalkidou, A. Characteristics of Women with Different Perinatal Depression Trajectories. J. Neurosci. Res. 2020, 98, 1268–1282. [Google Scholar] [CrossRef]
- Yakupova, V.; Suarez, A. Postpartum PTSD and Birth Experience in Russian-Speaking Women. Midwifery 2022, 112, 103385. [Google Scholar] [CrossRef]
- Adynski, H.; Zimmer, C.; Thorp, J.; Santos, H.P. Predictors of Psychological Distress in Low-Income Mothers over the First Postpartum Year. Res. Nurs. Health 2019, 42, 205–216. [Google Scholar] [CrossRef]
- Bianciardi, E.; Barone, Y.; Lo Serro, V.; de Stefano, A.; Giacchetti, N.; Aceti, F.; Niolu, C. Inflammatory Markers of Perinatal Depression in Women with and without History of Trauma. Riv. Psichiatr. 2021, 56, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bränn, E.; Papadopoulos, F.; Fransson, E.; White, R.; Edvinsson, Å.; Hellgren, C.; Kamali-Moghaddam, M.; Boström, A.; Schiöth, H.B.; Sundström-Poromaa, I.; et al. Inflammatory Markers in Late Pregnancy in Association with Postpartum Depression-A Nested Case-Control Study. Psychoneuroendocrinology 2017, 79, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Buglione-Corbett, R.; Deligiannidis, K.; Leung, K.; Zhang, N.; Lee, M.; Rosal, M.; Moore Simas, T. Expression of Inflammatory Markers in Women with Perinatal Depressive Symptoms. Arch. Women’s Ment. Health 2018, 21, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Caparros-Gonzalez, R.A.; Romero-Gonzalez, B.; Strivens-Vilchez, H.; Gonzalez-Perez, R.; Martinez-Augustin, O.; Peralta-Ramirez, M.I. Hair Cortisol Levels, Psychological Stress and Psychopathological Symptoms as Predictors of Postpartum Depression. PLoS ONE 2017, 12, e0182817. [Google Scholar] [CrossRef] [PubMed]
- Corwin, E.J.; Pajer, K.; Paul, S.; Lowe, N.; Weber, M.; McCarthy, D.O. Bidirectional Psychoneuroimmune Interactions in the Early Postpartum Period Influence Risk of Postpartum Depression. Brain Behav. Immun. 2015, 49, 86. [Google Scholar] [CrossRef] [PubMed]
- Incollingo Rodriguez, A.C.; Polcari, J.J.; Nephew, B.C.; Harris, R.; Zhang, C.; Murgatroyd, C.; Santos, H.P. Acculturative Stress, Telomere Length, and Postpartum Depression in Latinx Mothers. J. Psychiatr. Res. 2022, 147, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Katrinli, S.; Smith, A.K.; Drury, S.S.; Covault, J.; Ford, J.D.; Singh, V.; Reese, B.; Johnson, A.; Scranton, V.; Fall, P.; et al. Cumulative Stress, PTSD, and Emotion Dysregulation during Pregnancy and Epigenetic Age Acceleration in Hispanic Mothers and Their Newborn Infants. Epigenetics 2023, 18, 2231722. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, E.E.; Lapato, D.M.; Jackson-Cook, C.; Strauss, J.F.; Roberson-Nay, R.; York, T.P. Maternal Biological Age Assessed in Early Pregnancy Is Associated with Gestational Age at Birth. Sci. Rep. 2021, 11, 15440. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakatochi, M.; Kunimoto, S.; Okada, T.; Aleksic, B.; Toyama, M.; Shiino, T.; Morikawa, M.; Yamauchi, A.; Yoshimi, A.; et al. Methylation Analysis for Postpartum Depression: A Case Control Study. BMC Psychiatry 2019, 19, 190. [Google Scholar] [CrossRef]
- Ono, C.T.; Yu, Z.; Obara, T.; Ishikuro, M.; Murakami, K.; Kikuya, M.; Kikuchi, S.; Kobayashi, N.; Kudo, H.; Ogishima, S.; et al. Association between Low Levels of Anti-Inflammatory Cytokines during Pregnancy and Postpartum Depression. Psychiatry Clin. Neurosci. 2023, 77, 434–441. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Mahieu, B.; Nowak, G.; Maes, M. Lower Serum Zinc and Higher CRP Strongly Predict Prenatal Depression and Physio-Somatic Symptoms, Which All Together Predict Postnatal Depressive Symptoms. Mol. Neurobiol. 2017, 54, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Simpson, W.; Steiner, M.; Coote, M.; Frey, B.N. Relationship between Inflammatory Biomarkers and Depressive Symptoms during Late Pregnancy and the Early Postpartum Period: A Longitudinal Study. Rev. Bras. Psiquiatr. 2016, 38, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Stickel, S.; Eickhoff, S.B.; Habel, U.; Stickeler, E.; Goecke, T.W.; Lang, J.; Chechko, N. Endocrine Stress Response in Pregnancy and 12 Weeks Postpartum—Exploring Risk Factors for Postpartum Depression. Psychoneuroendocrinology 2021, 125, 105122. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression. Development of the 10-Item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Ayers, S.; Wright, D.B.; Thornton, A. Development of a Measure of Postpartum PTSD: The City Birth Trauma Scale. Front. Psychiatry 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A Measure of Subjective Stress. Psychosom. Med. 1979, 41, 209–218. [Google Scholar] [CrossRef]
- Weiss, D.S.; Marmar, C.R. The Impact of Event Scale—Revised. In Assessing Psychological Trauma and PTSD; Wilson, J.P., Keane, K., Eds.; The Guilford Press: New York, NY, USA, 1997; pp. 399–411. [Google Scholar]
- Spielberger, C.D.; Sydeman, S.J. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In The Use of Psychological Testing for Treatment Planning and Outcome Assessment; Maruish, M.E., Ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1994; pp. 292–321. [Google Scholar]
- Bohren, M.A.; Tunçalp, Ö.; Miller, S. Transforming Intrapartum Care: Respectful Maternity Care. In Best Practice and Research: Clinical Obstetrics and Gynaecology; Bailliere Tindall Ltd.: Paris, France, 2020; pp. 113–126. [Google Scholar] [CrossRef]
- Tunçalp, O.; Were, W.M.; Maclennan, C.; Oladapo, O.T.; Gülmezoglu, A.M.; Bahl, R.; Daelmans, B.; Mathai, M.; Say, L.; Kristensen, F.; et al. Quality of Care for Pregnant Women and Newborns—The WHO Vision. BJOG 2015, 122, 1045–1049. [Google Scholar] [CrossRef]
- Miller, S.; Lalonde, A. The Global Epidemic of Abuse and Disrespect during Childbirth: History, Evidence, Interventions, and FIGO’s Mother-Baby Friendly Birthing Facilities Initiative. Int. J. Gynecol. Obstet. 2015, 131, S49–S52. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations: Intrapartum Care for a Positive Childbirth Experience; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Basile Ibrahim, B.; Cheyney, M.; Vedam, S.; Kennedy, H.P. “I Was Able to Take It Back”: Seeking VBAC after Experiencing Dehumanizing Maternity Care in a Primary Cesarean. SSM Qual. Res. Health 2023, 4, 100339. [Google Scholar] [CrossRef]
- Altman, M.R.; McLemore, M.R.; Oseguera, T.; Lyndon, A.; Franck, L.S. Listening to Women: Recommendations from Women of Color to Improve Experiences in Pregnancy and Birth Care. J. Midwifery Women’s Health 2020, 65, 466–473. [Google Scholar] [CrossRef]
- McLemore, M.R.; Altman, M.R.; Cooper, N.; Williams, S.; Rand, L.; Franck, L. Health Care Experiences of Pregnant, Birthing and Postnatal Women of Color at Risk for Preterm Birth. Soc. Sci. Med. 2018, 201, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.R.; Oseguera, T.; McLemore, M.R.; Kantrowitz-Gordon, I.; Franck, L.S.; Lyndon, A. Information and Power: Women of Color’s Experiences Interacting with Health Care Providers in Pregnancy and Birth. Soc. Sci. Med. 2019, 238, 112491. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernandez, C.S.; de la Calle, M.; Arribas, S.M.; Garrosa, E.; Ramiro-Cortijo, D. Factors Associated with Obstetric Violence Implicated in the Development of Postpartum Depression and Post-Traumatic Stress Disorder: A Systematic Review. Nurs. Rep. 2023, 13, 1553–1576. [Google Scholar] [CrossRef] [PubMed]
- Liese, K.L.; Davis-Floyd, R.; Stewart, K.; Cheyney, M. Obstetric Iatrogenesis in the United States: The Spectrum of Unintentional Harm, Disrespect, Violence, and Abuse. Anthropol. Med. 2021, 28, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Illich, I. Medical Nemesis: The Expropriation of Health; Calder & Boyars: London, UK, 1975. [Google Scholar]
- Attanasio, L.; Kozhimannil, K.B. Health Care Engagement and Follow-up after Perceived Discrimination in Maternity Care. Med. Care 2017, 55, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, L.B.; Ranchoff, B.L.; Geissler, K.H. Perceived Discrimination during the Childbirth Hospitalization and Postpartum Visit Attendance and Content: Evidence from the Listening to Mothers in California Survey. PLoS ONE 2021, 16, e0253055. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.; Putt, M.; Halbert, C.H.; Grande, D.; Schwartz, J.S.; Liao, K.; Marcus, N.; Demeter, M.B.; Shea, J.A. Prior Experiences of Racial Discrimination and Racial Differences in Health Care System Distrust. Med. Care 2013, 51, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Prairie, E.; Côté, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The Determinant Role of IL-6 in the Establishment of Inflammation Leading to Spontaneous Preterm Birth. Cytokine Growth Factor Rev. 2021, 59, 1359–6101. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.L.; Christian, L.M.; Mackos, A.R.; Nolan, T.S.; Gondwe, K.W.; Anderson, C.M.; Hall, M.W.; Williams, K.P.; Slavich, G.M. Lifetime Stressor Exposure, Systemic Inflammation during Pregnancy, and Preterm Birth among Black American Women. Brain Behav. Immun. 2022, 101, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Giurgescu, C.; Engeland, C.G.; Zenk, S.N.; Kavanaugh, K. Stress, Inflammation and Preterm Birth in African American Women. Newborn Infant Nurs. Rev. 2013, 13, 171–177. [Google Scholar] [CrossRef]
- Nowak, A.L.; Anderson, C.M.; MacKos, A.R.; Neiman, E.; Gillespie, S.L. Stress during Pregnancy and Epigenetic Modifications to Offspring DNA: A Systematic Review of Associations and Implications for Preterm Birth. J. Perinat. Neonatal. Nurs. 2020, 34, 134. [Google Scholar] [CrossRef] [PubMed]
- Hux, V.J.; Catov, J.M.; Roberts, J.M. Allostatic Load in Women with a History of Low Birth Weight Infants: The National Health and Nutrition Examination Survey. J. Women’s Health 2014, 23, 1039. [Google Scholar] [CrossRef] [PubMed]
- Phillippe, M. Telomeres, Oxidative Stress, and Timing for Spontaneous Term and Preterm Labor. Am. J. Obstet. Gynecol. 2022, 227, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From Basics to Birth and Beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Basile Ibrahim, B. Operationalising the Quality Maternal and Newborn Care Framework to Improve Maternity Care Quality and Health Outcomes for Marginalised Women and Childbearing People. Int. J. Birth Parent. Educ. 2023, 10, 8. [Google Scholar]
- Chinkam, S.; Ibrahim, B.B.; Diaz, B.; Steer-Massaro, C.; Kennedy, H.P.; Shorten, A. Learning from Women: Improving Experiences of Respectful Maternity Care during Unplanned Caesarean Birth for Women with Diverse Ethnicity and Racial Backgrounds. Women Birth 2023, 36, e125–e133. [Google Scholar] [CrossRef] [PubMed]
- Combellick, J.L.; Basile Ibrahim, B.; Julien, T.; Scharer, K.; Jackson, K.; Powell Kennedy, H. Birth during the COVID-19 Pandemic: What Childbearing People in the United States Needed to Achieve a Positive Birth Experience. Birth 2022, 49, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Royse, J.; Lange-Maia, B.; Murray, L.; Shah, R.C.; DeMaio, F. Structural Racism, Socio-Economic Marginalization, and Infant Mortality. Public Health 2021, 190, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural Racism and Health Inequities in the USA: Evidence and Interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- Wallace, M.; Crear-Perry, J.; Richardson, L.; Tarver, M.; Theall, K. Separate and Unequal: Structural Racism and Infant Mortality in the US. Health Place 2017, 45, 140–144. [Google Scholar] [CrossRef]
- Gee, G.C.; Hicken, M.T. Structural Racism: The Rules and Relations of Inequity. Ethn. Dis. 2021, 31, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.A.; Arkin, E.; Proctor, D.; Kauh, T.; Holm, N. Systemic and Structural Racism: Definitions, Examples, Health Damages, and Approaches to Dismantling. Health Aff. 2022, 41, 171–178. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) [Citation] | N | Loc | Des | Exp | PMAD | Results |
|---|---|---|---|---|---|---|
| Alcorn (2010) [60] | 776 | Australia | PL | TB | PTSD ANX PPD | A total of 45.5% of the sample (n = 394) reported a traumatic birth event at 4–6 weeks post-partum. All participants who met full PTSD criteria at 4–6 weeks postpartum had experienced at least one birthing event during which they believed either their life/well-being or the life/well-being of their baby was in danger. |
| Alhasanat (2017) [61] | 50 | USA | R | ST | PPD | Among Arab-American WIC recipients, life stress significantly correlated to EPDS score (p = 0.02). |
| Catala (2021) [62] | 116 | Spain | PL | BES | PPD PTSD | In bivariate analysis, significant association between PTSD and satisfaction with the professionals at labor (p = 0.036). 14.6% (n = 17) of sample had EPDS scores indicating probable depression and 7.7% (n = 9) had probable PTSD. |
| Cena (2021) [63] | 1251 | Italy | PL | SES | PPD | PPD is associated with participants reporting “several to many” economic problems (p < 0.01). |
| Clout (2015) [64] | 105 | Australia | PL | ST | PPD | Stressful life events were associated with EPDS score (p < 0.05). |
| Coburn (2016) [65] | 269 | USA | PL | ST | PPD | Higher levels of daily hassles (p = 0.013), partner stress (p < 0.001), and family stress (p = 0.013) were associated with more postpartum depressive symptoms. |
| Cruise (2018) [66] | 10,827 | Ireland | PL | SES | PPD | Tenants were almost twice as likely to be depressed as owner occupiers (OR 1.80; 95% CI 1.59–2.03). Participants in the lowest income quintile were over three times more likely to be depressed as their more affluent peers (OR 3.53; 95% CI 2.81–4.42). |
| De Schepper (2016) [67] | 229 | Belgium | PL | TB SES | PTSD | Participants who experienced birth as a traumatic event had a 46% higher likelihood of developing PTSD. Odds of PTSD were 6X more likely in women with an annual family income of less than EUR 2500. |
| DesMarais (2014) [68] | 100 | Canada | R | ST IPV | PPD PTSD ANX | Corrected univariate models for IPV before and during pregnancy were significant for stress (p < 0.05), anxiety (p < 0.01), and posttraumatic stress disorder (p < 0.001). The corrected univariate model for IPV during, but not before, pregnancy was additionally significant for depression (p < 0.05). |
| Edge (2007) [69] | 200 | UK | PL | SES | PPD | Black Caribbean women were twice as likely as White British women to live in the most deprived areas of the city (p < 0.001), to be living on benefits (p < 0.05), and to be single parents (p < 0.05). Black Caribbean women (27%, n = 19) were not more likely than White women (21%, n = 27) (p = 0.307) to screen positive for PPD. |
| Edwards (2008) [70] | 154 | Australia | PL | ST | PPD | Among socially disadvantaged women in Australia, the only antenatal risk factor found to predict PPD was childhood emotional abuse. |
| Ertan (2021) [71] | 916 | France | CS | TB | PTSD PPD | A traumatic experience of birth was associated with higher postpartum EPDS scores. |
| Farr (2014) [72] | 4451 | USA | CS | ST SES | ANX PPD | Women with the highest prevalence of postpartum anxiety symptoms experienced 6–13 (16.1%) or 3–5 (15.9%) stressors during pregnancy. Women with the highest prevalence of comorbid postpartum anxiety and depressive symptoms experienced 6–13 stressors during pregnancy (17.3%). Women with the highest prevalence of only postpartum depressive symptoms were those experiencing 6–13 stressors during pregnancy (5.8%) and women with annual household incomes between USD 10,000 and USD 14,999 (4.2%). |
| Ford (2011) [73] | 138 | UK | PL | BES | PTSD | At three months postpartum, maternity care staff support was a significant predictor of PTSD symptoms (p < 0.05). |
| Garthus-Niegal (2013) [74] | 1499 | Norway | PL | BES | PTSD | Birth experiences were significantly associated with post-traumatic stress symptoms. |
| Handelzalts (2022) [75] | 246 | Israel | PL | BC | PTSD | Women who were accompanied by their partners and an additional companion were lower in birth-related PTSD symptoms than women accompanied by only their partner (p < 0.05). |
| Handelzalts (2022) [76] | 254 | Israel | PL | BES | PTSD PPD | Perception of birth experience (p < 0.05) significantly predicted birth-related PTSD (BiTS) in linear regression models. No significant findings for birth experience and PPD. |
| Harrison (2021) [77] | 16,000 | UK | CS | BES | PTSD | Factors significantly associated with birth related PTSD: higher level of deprivation, not having a health care professional to talk to about sensitive issues during pregnancy, having an instrumental or caesarean birth, experiencing childbirth as worse than expected. |
| Hein (2014) [78] | 1100 | Germany | PL | SES | PPD | Having a low income, renting home vs. owning, and less than high school education were all significantly associated with higher EPDS scores, but few participants screened positive for PPD. |
| Hernández-Martínez (2020) [79] | 1531 | Spain | CS | RMC | PTSD | The presence of traumatic stress symptoms was identified in 7.2% of the study population. Variables found to be protective factors against PTSD symptoms included having the birth plan respected (aOR 0.44; 95% CI 0.24–0.80). |
| Holt (2018) [80] | 1393 | UK | R | BES | PTSD PPD | Fearful birth experiences and traumatic birth appraisals were both significantly correlated with PTSD symptoms and PPD. |
| Janssen (2012) [81] | 6421 | Canada | CS | IPV SES | PPD | Among abused women: age extremes were associated with PPD. Among non-abused women: unemployment (aOR 1.41; 95% CI 1.06–1.84), foreign birth (aOR 2.04; 95% CI 1.35–3.09], and low income (aOR 1.68; 95% CI 1.25–2.25) were associated with PPD. PPD was significantly associated with abuse occurring only prior to pregnancy (aOR 3.28; 95% CI 1.86–5.81), starting postpartum (aOR 4.76; 95% CI 1.41–16.02), and resuming postpartum (aOR 3.81; 95% CI 1.22–11.88). |
| Jones (2018) [82] | 295 | USA | R | SES | PPD | Residential stability was found to be statistically significantly associated with PPD symptom severity based on the 3-month EPDS total score (p = 0.01). Social disadvantage was not found to be significant (p = 0.12). |
| Katon (2014) [83] | 1423 | USA | PL | ST | PPD | In a cohort of high-risk prenatal clinic patients, the total stress score was significantly associated with PPD [OR 1.14; 95% CI 1.09–1.19]. |
| Kim (2012) [84] | 838 | USA | CS | SES | PPD | Postpartum depression symptoms were present in 17% (n = 55), and were associated with being single (aOR 2.41; 95% CI 1.29–4.50), first time mother status (aOR 2.43; 95% CI 1.34–4.40), and living in temporary housing (aOR 2.35; 95% CI 1.30–4.26). |
| Kjerulff (2021) [85] | 3006 | USA | PL | BES ST | PTSD | Scores on the FBS Birth Experience Scale strongly associated with childbirth-related PTSD (CR-PTSD). Women who reported CR-PTSD symptoms were less positive about their childbirth experience than women who did not experience CR-PTSD. Shared decision-making (SDM) was also strongly associated with CR-PTSD. Women who reported experiencing CR-PTSD reported lower levels of SDM than women who did not experience CR-PTSD. Women with low social support and high stress during the third trimester before childbirth were more likely to report CR-PTSD symptoms. |
| Kothari (2016) [86] | 301 | USA | CS | SES IPV | PPD | Poor participants had significantly higher postpartum depression scores than nonpoor participants (mean EPDS 6.0 and 4.7, respectively, p = 0.017). IPV and poverty were positively associated with each other (p < 0.001) and with EPDS score (IPV: p < 0.001; poverty: p = 0.017). In multiple linear regression, IPV remained significantly associated, but poverty did not (IPV: adjusted p < 0.001; poverty: adjusted p = 0.141). |
| Kress (2021) [87] | 1146 | Germany | C | TB | PTSD PPD | Poorer subjective birth experience predicted PTSD symptoms with small to moderately sized effects. Level of support during birth was moderately associated with PTSD symptoms (low support associated with higher PTSD symptoms). |
| LaCoursiere (2012) [88] | 1054 | USA | R | ST | PPD | Women reporting a physical fight during pregnancy had a fourfold increased odds of screening positive for PPD (OR = 4.09; 95% CI 1.23–13.54). As the frequency of stressors increased, the prevalence of screening positive for PPD also increased. Approximately 10% (30 of 293) of women without any stressors screened positive for PPD, 15.4% (62 of 402) of women with one stressor category, 17.2% (39 of 188) with two stressor categories, 41.3% (31 of 75) with three different categories of stressors, and 61.9% (13 of 21) of women reporting stressors in all four categories (Chi-square test for trend p < 0.001). |
| Leavy (2023) [89] | 123 | France | C | MIST | PTSD PPD | Reported disrespect during childbirth was significantly associated with higher childbirth-related PTSD (CB-PTSD) 2 months after birth (p < 0.001). PPD at 2 months after childbirth was positively associated with reported disrespect in the birth room (p = 0.01). PPD and CB-PTSD were significantly associated 2 months after childbirth (p < 0.01). |
| Liu (2018) [90] | 3010 | USA | R | ST | PPD | Factors associated with PPD included having at least one stressor, with a 1.8 X increase with 1–2 stressors endorsed, 3.4 X increase with 3–5 stressors, and 10.6 X increase with 6+ stressors. |
| Luecken (2019) [91] | 322 | USA | PL | ST | PPD | Depressive symptoms at 24 weeks postpartum were associated with prenatal economic hardship and prenatal stressful life events. |
| Luis Sanchez (2020) [92] | 159 | USA | PL | DISC ST | PPD | Increases in general perceived stress symptoms from pregnancy to postpartum contributed to depressive symptoms postpartum. |
| Mallicoat (2020) [59] | 4245 | UK | C | SES | Maternal Mental Health | In a cohort of women of Pakistani ethnicity, three different SES measures showed statistically significant relationships with mental health (all p < 0.0005). |
| Martinez-Vázquez (2022) [93] | 782 | Spain | CS | OV | PPD | Risk factors for PPD included experiencing verbal obstetric violence (aOR 2.02; 95% CI 1.35–3.02), and psycho-affective obstetric violence (aOR 2.65; 95% CI 1.79–3.93). The perception of support during pregnancy, birth, and postpartum was protective against PPD (aOR 0.15; 95% CI 0.04–0.54) for women who perceived “enough support” and further protective for women who received “much support” (aOR 0.13; 95% CI 0.0–0.45). |
| Mukherjee (2017a) [17] | 115,704 | USA | R | ST | PPD | The highest prevalence of postpartum depression was in the multiple stress class, followed by illness/death, and low-stress classes. |
| Mukherjee (2017b) [94] | 91,253 | USA | R | SES | PPD | Women who experienced all four stressor categories, including partner-related, traumatic, emotional, and financial, had the highest odds of PPD symptoms (aOR 5.43; 95% CI 5.36–5.51). The odds of experiencing PPD symptoms decreased with an increase in the state-level social/economic autonomy index (aOR 0.75; 95% CI 0.64–0.88). There was significant cross-level interaction between number of stressor categories experienced and state-level SES index. |
| Mukherjee (2018) [95] | 87,565 | USA | R | ST SES | PPD | Women in the lower income and education categories generally had a higher prevalence of PPD than those in the highest categories. Financial stress was a significant risk factor for PPD. Those who experienced a stressful life event had a higher unadjusted prevalence of PPD than those that did not experience it. |
| Nakamura (2020) [96] | 14,587 | France | C | SES | PPD | SES was negatively associated with EPDS score, with an increase of one unit of SES associated with a reduction of, respectively, 6%, 10%, and 16% of EPDS score in non-migrant women (RR = 0.94; 95% CI 0.91–0.96), second generation migrant women (RR = 0.90; 95% CI 0.86–0.96) and first-generation migrant women (RR = 0.84; 95% CI 0.76–0.95). |
| Nakic Rados (2021) [97] | 603 | Croatia | CS | BES | PTSD PPD | In an online study of women within 12 months postpartum, low birth satisfaction (including stress during labor and quality of care provided) was associated with higher PTSD symptoms, but not PPD symptoms. |
| Nunes (2013) [98] | 6283 | USA | R | ST | PPD | Among mothers over the age of 25, stressors were associated with increased odds of reporting PPD symptoms. The strongest risk factors among the stressor variables included “argue more than usual”, “physical fight”, “couldn’t pay bills”, “use of drugs by others”, and “partner did not want pregnancy”. Across all age ranges, combined stress score was strongly associated with PPD symptoms. Participants reporting six or more stressors had over 20X odds of reporting PPD symptoms as compared to those reporting no stressors. |
| O’Donovan (2014) [99] | 933 | Australia | PL | TB | PTSD | Of the 45.5% of women who reported that their birth experience was traumatic (n = 394), 7.9% developed PTSD between 4 and 6 weeks postpartum (n = 31). Primagravidas were more likely to report that their birth was traumatic. Event-related psychological variables that predicted birth trauma were perceived lack of control during labor, low self-efficacy, discrepancy in expectations around the birthing event, and feeling unprepared for the birth. |
| Ogbo (2019) [100] | 25,407 | Australia | R | SES | PPD | Higher SES had a protective effect on PPD symptoms. |
| Paiz (2022) [101] | 287 | Brazil | CS | MIST SES | PPD | Women who experienced mistreatment during childbirth had a higher prevalence of symptoms suggestive of PPD (PR 1.55; 95% CI 1.07–2.25). Higher socioeconomic status had an inverse association with PPD (PR 0.53; 95% CI 0.33–0.83). |
| Pinheiro (2012) [102] | 276 | Brazil | CS | ST | PPD | EPDS score > 13 was associated with the presence of SLE (p < 0.01). |
| Price (2009) [103] | 1086 | USA | R | SES | PPD | Significantly increased odds of PPD when eligible for Temporary Aid for Needy Families (TANF; signifier of low SES). |
| Qobadi (2016) [104] | 3695 | USA | R | ST | PPD | Mothers who experienced high relational stress, low financial stress and high trauma-related stresses had the highest likelihood of PPD diagnosis after adjusting for confounders (aOR 8.6; 95% CI 3.5–21.3), followed by those who reported high relational stress with low financial and trauma-related stress (OR 5.9; 95% CI 3.5–10.2) compared to women with low stress in all three categories. |
| Razurel (2017) [105] | 235 | Switzerland | PL | ST | PPD ANX | In a mainly high-income, well-educated sample, health professional support immediately post-birth displayed a significant interaction with mental health outcomes. Perceived stress was significantly associated with EPDS scores. |
| Roberts (2022) [106] | 392 | Ireland | PL | TB | PTSD ANX PPD | For the 6 month postpartum EPDS, prenatal EPDS, any maternal mental health history, anxiety screening at 6 months, and experience of traumatic birth explained 56.2% of variability (p < 0.001). |
| Salm Ward (2017) [107] | 10,231 | USA | R | ST | PPD | Significantly higher odds of reporting PPD symptoms were identified among mothers with less than a high school education (compared to college graduates, OR 1.70; 95% CI 1.13–2.54), living in a rural area (versus urban, OR 1.28; 95% CI 1.03–1.60), using Medicaid for delivery (versus private insurance, OR 1.45; 95% CI 1.07–1.95), with an unintended pregnancy (versus intended, OR 1.78; 95% CI 1.31–2.41). As the number of cumulative SLEs increased, the odds of reporting PPD symptoms also increased, with the greatest odds of reporting PPD symptoms among mothers who have experienced six or more SLEs (OR 5.77; 95% CI 3.89–8.55). After controlling for significant sociodemographic variables, each SLE was still associated with increased odds of PPD symptoms. |
| Silveira (2019) [108] | 3065 | Brazil | C | MIST | PPD | Verbal abuse from maternity care personnel increased odds of having moderate PPD (OR 1.58; 95% CI 1.06–2.33) and severe PPD (OR 1.69; 95% CI 1.06–2.70). Physical abuse increased the odds of having marked/severe PPD (OR 2.28; 95% CI 1.26–4.12). Having experienced three or more mistreatment types increased the odds of moderate PPD (OR 2.90; 95% CI 1.30–35.74) and severe PPD (OR 3.86; 95% CI 1.58–9.42). |
| Sommerlad (2021) [109] | 284 | Germany | PL | BES | PTSD | A positive birth experience directly reduced CB-PTSD symptoms (p < 0.01). |
| Souza (2017) [110] | 10,468 | Brazil | CS | OV | PPD | Experiencing obstetric violence was an independent predictor of postpartum Edinburgh Postnatal Depression Scale scores. |
| Steetskamp (2022) [111] | 278 | Germany | PL | BC | PTSD | A total of 6.3% (13/206) of those without a companion during labor had PTSD symptoms vs. 1% (4/383) of those who had a companion (p < 0.001). PTSD was seen more often in patients with a migrant background (p = 0.007). Maternal age (p < 0.001), parity (p < 0.001), migrant background (p < 0.001), assistance during labor (p < 0.001) and the mode of delivery (p = 0.001) influence PTSD symptom severity. |
| Stone (2015) [112] | 5395 | USA | R | ST | PPD | Reporting of one or more stressors was associated with increased prevalence of PPD (PR 1.68; 95% CI 1.42–1.98). The strongest association was observed for partner stress (PR 1.90; 95% CI 1.51–2.38). |
| Suarez (2023) [113] | 2579 | Russia | CS | TB BC | PTSD PPD | A total of 37.5% of participants had clinically significant depressive symptoms (EPDS scores > 10). In total, 20.5% of women fulfilled all the DSM-5 diagnostic criteria for PTSD (according to CBiTS scores). Both PPD (Pearson correlation = 0.34, p < 0.001) and PTSD (Pearson correlation = 0.46, p < 0.001) significantly correlated with the subjective birth trauma. Women who gave birth in the presence of a support person scored lower on both the postpartum PTSD scale and the subjective scale of traumatic birth experience and had a significantly lower risk of having a clinical postpartum-PTSD diagnosis. |
| Tebeka (2021) [114] | 3310 | France | PL | ST | PPD | Early and late onset PPD were significantly associated with stressful life events in pregnancy (early onset OR 2.0; 95% CI 1.5–2.7; late onset OR 2.4, 95% CI 1.8–3.2). |
| Waller (2022) [115] | 1082 | USA | PL | TB | PPD | Patient-reported childbirth trauma is significantly associated with postpartum depression (OR 1.33; 95% CI 1.10–1.60). |
| Wikman (2020) [116] | 2466 | Sweden | R | BES | PPD | Participants with a self-reported negative experience with delivery had 4.3X the likelihood of early postpartum depression (OR 4.3, 95% CI 2.9–6.4). |
| Yakupova (2022) [117] | 611 | Russia | CS | OV BC | PTSD PPD | Postpartum PTSD symptoms were higher among women who experienced obstetric violence (p < 0.001) during childbirth. The more interventions they had (p = 0.012), and the more instances of obstetric violence they experienced (p < 0.001), the higher the PTSD symptoms were. The presence of a partner or a personal midwife/doula at birth was associated with lower rates of cesarean birth, fewer medical interventions, and less obstetric violence (p < 0.017 for all). |
| Author (Year) [Citation] | N | Loc | Des | Exp | BioM | PMAD | Results |
|---|---|---|---|---|---|---|---|
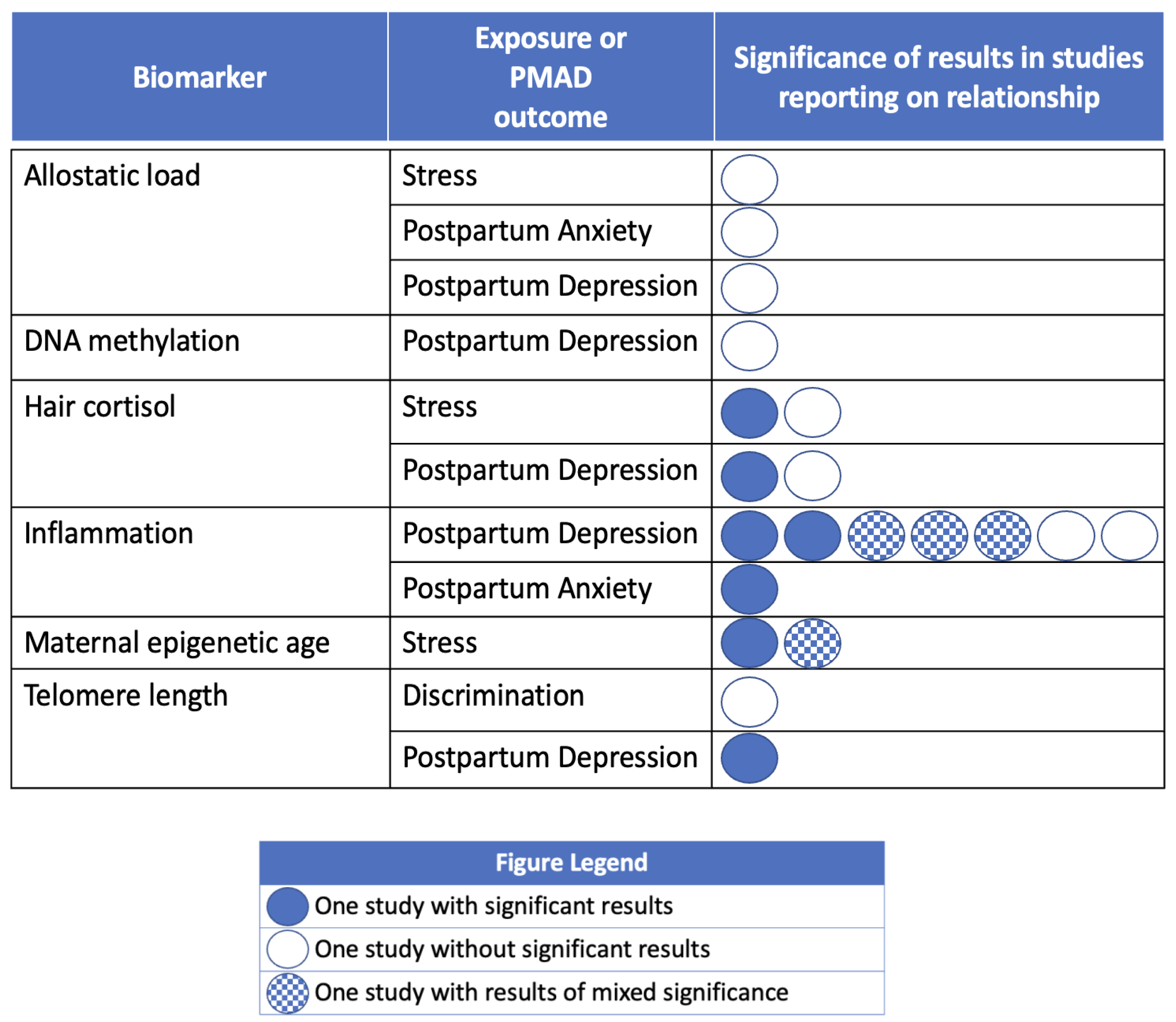
| Adynski (2019) [118] | 845 | USA | PL | ST SES | AL | PPD ANX | Odds of elevated depressive symptoms in women with food insecurity were 2.12 (95% CI 1.33–3.37) compared to those without perceived food insecurity. Allostatic load score (OR 0.98; 95% CI 0.91–1.05) and the remaining demographic and social determinants of health predictor variables were not significantly predictive of elevated depressive symptomology. |
| Bianciardi (2021) [119] | 79 | Italy | PL | - | INF | PPD | The EPDS total score of the two groups with PPD (with and without trauma) was the dependent variable and the biological markers (cortisol, IL-6, TNF-α) were the independent variables. The results were not significant. The TNF-α OR was 1.856 (p = 0.053). |
| Brann (2017) [120] | 291 | Sweden | C | - | INF | PPD | The sole significant value on Bonferroni correction was higher IL-10 in controls than those with PPD (p = 0.029); this did not hold in sensitivity analyses where those with depression in pregnancy were excluded. |
| Buglione-Corbett (2018) [121] | 110 | USA | PL | - | INF | PPD | Elevated serum TNF-α was associated with lower EPDS total score (p = 0.046) after adjusting for demographics and medication use. In contrast, IL-6, CRP, and IL-1β did not demonstrate statistically significant associations with depressive symptoms by the EPDS in either crude or adjusted models. |
| Caparros-Gonzalez (2017) [122] | 44 | Spain | PL | ST | CORT | PPD | Hair cortisol at the first trimester (p < 0.05) and third trimester (p < 0.05) significantly predicted EPDS scores. The group with postpartum depression symptoms had higher hair cortisol levels during the first, second, and third trimesters. |
| Corwin (2015) [123] | 152 | USA | PL | - | INF | PPD | TNFα levels were significantly different between groups (those symptomatic of PPD and those without PPD) with lower TNFα levels (p < 0.05) at all time points in women symptomatic of PPD. There were no differences in any other cytokine or in the ratios of any pro- to anti-inflammatory cytokine among women who did or did not score symptomatic of PPD. |
| Incollingo Rodriguez (2022) [124] | 150 | USA | PL | DISC | TL | PPD | TL was a significant negative predictor of postpartum EPDS scores (p = 0.024). There were no significant differences in TL based on ethnicity (US born or non-US born), spoken language (English or Spanish), income, welfare status, education, or marital status (all p’s > 0.110). The Everyday Discrimination Scale score did not predict TL (p = 0.208). |
| Katrinli (2023) [125] | 89 | USA | CS | ST | EA | - | Exposure to past-year stressful life events was significantly associated with accelerated epigenetic age in mothers. |
| Lancaster (2021) [126] | 229 | USA | PL | ST | EA | - | In the African American/Black subset only, early pregnancy EA [using Horvath’s clock calculator] was inversely related to early perinatal Perceived Stress Scale score. European/White participants did not have significant findings. |
| Nakamura (2019) [127] | 36 | Japan | PL | - | DM | PPD | The difference in methylation frequency between the postpartum non-depressed group and the postpartum depressed group was small, and sites with genome-wide significant differences were not confirmed. |
| Ono (2023) [128] | 490 | Japan | PL | - | INF | PPD | IL-4 and IL-10 higher during pregnancy in controls than +PPD. All others null. |
| Roomruangwong (2017) [129] | 71 | Thailand | PL | - | INF | PPD ANX | ↑ CRP: ↑ STAI score (anx), (p = 0.003) ↑ CRP: ↑ Multivariable outcome (postnatal depressive symptoms on EPDS, BDI, STAI, Hamilton) (p = 0.001). Postnatal EDPS was not predicted by CRP. |
| Simpson (2016) [130] | 33 | Canada | PL | - | INF | PPD | IL-6 (p = 0.025), and IL-10 (p = 0.006) were significant predictors of postpartum EPDS score. |
| Stickel (2021) [131] | 196 | Germany | PL | ST | CORT | PPD | Neither SLE nor CORT was associated with PPD. |
| Exposure | Measurement Tool | Included Studies Using Measurement Tool |
|---|---|---|
| Perinatal stress/stressors | Perceived Stress Scale (PSS) | [118,122,123,126] |
| PRAMS questions about stressors | [17,72,88,90,94,95,98,104,107,112] | |
| Economic Hardship Scale | [91] | |
| Antenatal Perceived Stress Inventory (APSI) | [105] | |
| Postpartum Depression Predictors Inventory (PDPI)-Revised | [61] | |
| Paykel Scale | [114] | |
| Prenatal Psychosocial Profile Stress Scale | [83] | |
| Symptoms Screener for Adults (STRESS-A) Turner Life Events Scale | [125] | |
| Discrimination | Everyday Discrimination Scale (EDS) | [124] |
| Discrimination Stress Scale | [92] | |
| Birth experiences/satisfaction | Childbirth Experience Questionnaire | [75,76] |
| Birth Satisfaction Scale-Revised (BSS-R) | [97] | |
| Wijma Delivery Experience Questionnaire | [80] | |
| Women’s View of Birth Labor Satisfaction Questionnaire (WOMBLSQ) | [62] | |
| First Baby Study Birth Experiences Scale | [85] | |
| Birth Expectation and Experience Scale | [99] | |
| Interactions with the maternity care team | Support and Control in Birth (SCIB) Questionnaire | [73] |
| Mistreatment/disrespect during childbirth | Behavior of the Mother’s Caregivers–Satisfaction Questionnaire (BMC-SQ) | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile-Ibrahim, B.; Combellick, J.; Mead, T.L.; Sorensen, A.; Batten, J.; Schafer, R. The Social Context of Pregnancy, Respectful Maternity Care, Biomarkers of Weathering, and Postpartum Mental Health Inequities: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 480. https://doi.org/10.3390/ijerph21040480
Basile-Ibrahim B, Combellick J, Mead TL, Sorensen A, Batten J, Schafer R. The Social Context of Pregnancy, Respectful Maternity Care, Biomarkers of Weathering, and Postpartum Mental Health Inequities: A Scoping Review. International Journal of Environmental Research and Public Health. 2024; 21(4):480. https://doi.org/10.3390/ijerph21040480
Chicago/Turabian StyleBasile-Ibrahim, Bridget, Joan Combellick, Thomas L. Mead, Alee Sorensen, Janene Batten, and Robyn Schafer. 2024. "The Social Context of Pregnancy, Respectful Maternity Care, Biomarkers of Weathering, and Postpartum Mental Health Inequities: A Scoping Review" International Journal of Environmental Research and Public Health 21, no. 4: 480. https://doi.org/10.3390/ijerph21040480
APA StyleBasile-Ibrahim, B., Combellick, J., Mead, T. L., Sorensen, A., Batten, J., & Schafer, R. (2024). The Social Context of Pregnancy, Respectful Maternity Care, Biomarkers of Weathering, and Postpartum Mental Health Inequities: A Scoping Review. International Journal of Environmental Research and Public Health, 21(4), 480. https://doi.org/10.3390/ijerph21040480







