Abstract
Background: Human cytomegalovirus (HCMV) is an important pathogen in immunocompromised individuals where it causes end organ diseases leading to increased morbidity and mortality. The aim of this study was to determine the prevalence of HCMV infection and its associated risk factors among HIV patients in Edo State, Nigeria. Methods: A total of 150 HIV patients consisting of 80 males and 70 females were enrolled for this study. About 4 mL of venous blood was aseptically collected from each participant by venipuncture and was centrifuged to obtain serum. The serum was screened by ELISA for HCMV IgG and IgM antibodies. Results: The results obtained were analyzed using SPSS version 20 for data analysis. An overall prevalence of 32.7% for HCMV IgM antibodies was recorded among the HIV patients. Males had a higher prevalence of 55.1% HCMV IgM antibodies than their female counterparts with 44.9% HCMV IgM antibodies. HCMV was more prevalent among the age group <19 years (51.0%), followed by the age group 20–39 years (30.6%), while the age groups >60 years, and 40–59 years recorded a prevalence of 12.2% and 6.1%, respectively. Conclusions: The co-occurrence of HCMV IgG and IgM antibodies, religion and location significantly influenced the acquisition of HCMV infection among HIV patients. Integrating HIV prevention by spreading awareness and early diagnosis of HCMV is key to reducing complications from these viral infections in HIV disease patients.
1. Introduction
Human cytomegalovirus (HCMV) is the largest of the family Herpesviridae. It is prevalent across the globe and affects individuals of various age ranges, genders, and ethnicities [1]. HCMV leads to opportunistic infections (OIs) among individuals who have human immunodeficiency virus (HIV). It is globally endemic and rampant in developing countries [2,3] rather than in developed countries [4]. HCMV has been an important viral pathogen in humans for more than a century with manifestations ranging from asymptomatic in immunocompetent patients to severe end-organ dysfunction in immunocompromised patients with HCMV disease [5]. HCMV causes various life-threatening infections like chorioretinitis, pneumonia, enteritis, encephalitis, colitis and neuropathy [6]. HCMV can be transmitted by direct contact with bodily fluids such as saliva, tears, urine, stool, semen and breast milk [7]. HCMV is latent in most cases, but in immunocompromised individuals, the virus replicates exponentially and spreads throughout the body in the blood, causing cytomegalovirus (CMV) viremia and even organic CMV disease [8]. Detection of HCMV-specific IgM antibody is a diagnostic method for the detection of HCMV DNA by the use of polymerase chain reaction (PCR) test [9]. Below is the structure of HCMV (Figure 1).
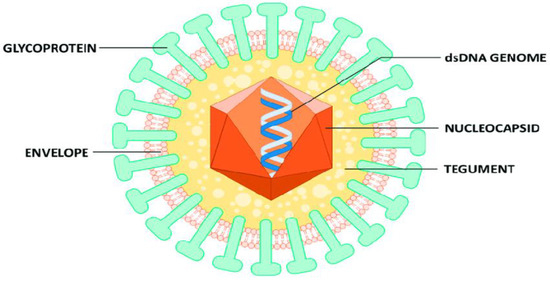
Figure 1.
Structure of HCMV virion [10].
The human immunodeficiency virus (HIV) belongs to the Lentivirus family and is responsible for causing acquired immune deficiency syndrome (AIDS). It leads to the breakdown of the body’s immune system, making it vulnerable to infections [11]. Preventing the spread of infection is crucial, and this involves promoting safe sexual practices, proper needle usage, and open communication with partners. A primary treatment approach for patients during the early phase of the disease involves a combination therapy called antiretroviral therapy [12]. The main test for detecting HIV is an FDA-approved antigen/antibody immunoassay designed to identify HIV-1/2 antibodies and the HIV-1 p24 antigen. An HIV nucleic acid test is also performed to detect the presence of HIV RNA in infected blood [13]. The factors that predispose an individual to cytomegalovirus infection and also lead to the transmission of the infection from person to person can be a result of an immunocompromised system as in HIV patients, engaging in unprotected sexual contact with infected persons, poor hygiene practices, blood transfusions, accidents in health care settings, depreciating socioeconomic standards and also from mother to baby before or during birth [14].
Infection with human cytomegalovirus is widespread, with its occurrence being less common in developed nations and more common in economically disadvantaged countries. In the year 2022, an average of 630,000 individuals succumbed to causes linked to HIV/AIDS on a global scale. The region most heavily impacted is Sub-Saharan Africa, where 4.2 to 5.5 million individuals are living with HIV in western and central Sub-Saharan Africa, and 17.4 to 24.5 million individuals are living with the virus in eastern and southern Sub-Saharan Africa according to the 2022 global HIV and AIDS statistics [15]. Also, previous studies have reported HCMV infection rates of 92% among prospective blood donors [16] and 96% among non-HIV patients in separate studies previously carried out in Nigeria [17,18]. Moreover, the reports from Nigeria show that the HCMV seroprevalence varies greatly with a variety of epidemiological factors such as age, geographical distribution, socioeconomic status and marital status [18]. Research has shown that HCMV co-infections increase the danger of human immunodeficiency virus transmissions [19]. The seminal shedding of HCMV has been found to be partially liable for a large amount of new HIV infections, even more so than common STIs, such as gonorrhea, syphilis, chlamydia, or herpes simplex virus type 2 (HSV-2) [19]. Understanding how HCMV works with HIV transmission can facilitate the development of improved HIV prevention strategies in the future.
HIV prevalence in Nigeria is notably lower compared to certain other African nations like South Africa and Zambia. In Nigeria alone, approximately 49,000 individuals lost their lives to AIDS in the year 2020 [20]. AIDS, a condition stemming from HIV infection, remains a significant global public health concern. Statistical projections for the year 2022 indicated that there were around 39.0 (33.1 million–45.7 million) individuals living with HIV worldwide, with 1.3 million newly identified HIV-positive cases and 0.63 million fatalities attributed to HIV/AIDS-related illnesses [15]. In industrialized countries such as USA, Australia and Europe, HCMV sero-prevalence among the adult population is between 35% and 80% [21,22], in contrast to highly endemic areas of countries in Africa and Asia, where sero-prevalence approaches 100% [4,21].
Previous studies have reported HCMV infection rates of 93.2% and 19.5% in Ghana and Nigeria, respectively [4,17]. Also, a prevalence rate of 92% and 96% were obtained in separate studies previously carried out in Nigeria [16,17]. HCMV prevalence rates in some parts of Nigeria were from 92% in Jos to 96% in Lagos, both in 2009 [16,17]. Research conducted in the past has indicated that individuals who are simultaneously infected with both HIV and HCMV tend to experience a swift advancement towards AIDS and, ultimately, demise. This outcome remains consistent even among those who are undergoing highly active antiretroviral therapy. The impact of the infection on organ systems in individuals who are HIV-positive with diminished CD4+ cell counts resembles the effects observed in recipients of organ transplants. Laboratory diagnosis of HCMV is rarely performed as a routine test by individuals especially in underdeveloped or developing countries [4,22]. This may result in under diagnosis of the disease and the negligence of treatment, which invariably contributes to the transmission, morbidity and subsequent mortality in HIV patients. The objective of this research was to ascertain the occurrence rate of cytomegalovirus infections within HIV-positive individuals in Edo state, Nigeria. The study was poised to answer questions on the prevalence of HCMV infection among people living with HIV in Edo State, Nigeria, the possible risk factors of HCMV infection and the effective strategies to improve access and uptake of HCMV testing among individuals in the State.
2. Materials and Methods
2.1. The Study Area and Population
This is a single-center study conducted in Edo State, a multicultural metropolitan state located in the southern part of Nigeria. The study covered a period of 5 months and consisted of one hundred and fifty (150) HIV positive patients attending the HIV clinics at the central hospital, Benin City, Edo State, Nigeria. The state is estimated to have an average population of 3,233,366 [23]. The people living in this area are mainly involved in trading and farming while the majority of them work in the public sector. Known HIV patients attending the HIV clinics at the central hospital, Edo State were recruited into this study. A total of one hundred and fifty (80 males and 70 females) HIV positive patients attending the HIV clinics at the central hospital, Edo State constituted the study population while 50 non-HIV positive apparently healthy individuals served as controls.
2.2. Inclusion Criteria
The study included only known HIV positive patients attending the HIV clinics at the central hospital that consented to participate in this study.
2.3. Sample Size
The sample size of the study was determined using the HCMV IgM antibodies’ prevalence from previous studies on HCMV infection among HIV positive patients in Benin City, Nigeria, which was 7.0% [24]. The sample size for this study was then obtained using the formula described by [25].
where;
N = (Z)2 P (1 − P)/(D)2
- N = the necessary sample size
- Z = confidence level set at 95% (standardized value of 1.96)
- P = projected HCMV prevalence among HIV patients in Benin City (7.0%)
- D = acceptable margin of error of 5% (standardized value = 0.05)
- N = 1.96 × 0.07(1 − 0.07)/(0.05)2
- = 3.8416 × 0.07 × 0.93/0.0025
- = 100 samples
A total of 150 HIV patients (samples) were recruited and participated in the research.
2.4. Specimen Collection
Approximately 4 mL of venous blood were collected from each participant using a sterile technique through venipuncture. The blood was then placed into clean, unaltered containers and subjected to centrifugation at a speed of 3000× g revolutions per minute for a duration of 10 min in order to obtain the serum. The sera obtained were stored at −20 °C before sample analysis.
2.5. Laboratory Analysis
Prior to the analysis, the ice-covered sera were defrosted at room temperature or about 45 min and all reagents were brought to room temperature. The thawed sera were then homogenously mixed and screened for HCMV antibodies using ELISA test kits from Elabscience, Huston, TX, USA in accordance with the manufacturer’s directives.
2.6. Quality Control
The usage was in accordance with the manufacturer’s directives. The kits were stored at the manufacturer’s recommended temperature (2–27 °C). The quality control tag was checked to ensure it was not tampered with before purchase. Data precision was ensured through the process of entering all acquired data twice.
2.7. Statistical Analysis
The data collected from the questionnaire and the findings from the laboratory analysis were assessed using SPSS version 20 software. To establish the connection between demographic information and prevalence rates, the Pearson Chi-square test was employed with a 95% confidence interval and a significance level set at 0.05.
3. Results
An overall prevalence of 32.7% for HCMV IgM antibodies was recorded among HIV patients in Edo State, Nigeria (Table 1).

Table 1.
The prevalence of HCMV co-infection among HIV patients in Edo State, Nigeria.
Table 2 shows some demographic characteristics of the patients. Gender of participants in this study did not influence the prevalence of HCMV infection among HIV patients (p = 0.8618). The prevalence of HCMV infection was higher in males (55.1%) than their female counterparts (44.9%). The patients’ age also did not significantly influence the prevalence of HCMV infection among the study participants (p = 0.083). The prevalence of HCMV infection was higher among the <19 years age group (51.0%), followed by the 20–39 years age group (30.6%), then by the >60 years age group (12.2%) and the 40–59 years age group recording the least prevalence (6.1%). The place of residence of the study participants significantly affected the prevalence of HCMV infection among HIV infected patients (p = 0.002). The prevalence of HCMV infection was however higher in those residing in the urban areas (61.2%) than those in the rural areas (38.8%). Religion significantly impacted on the prevalence of HCMV infection among HIV patients (p = 0.0024). However, the prevalence of HCMV infection was higher in Christians (77.9%) than their Muslim counterparts (22.1%).

Table 2.
Participant’s socio-demographic characteristics.
Table 3 shows the co-detection of HCMV IgM and IgG antibodies among the HIV patients. The co-detection of HCMV IgG and IgM antibodies in this study statistically significantly influenced the prevalence of HCMV infection among HIV patients (p ≤ 0.001) and (p = 0.019) IgG and IgM, respectively. The prevalence of HCMV was higher in IgG (40.7%), compared to its prevalence in IgM (32.7%), whereas the co-existence of both IgG and IgM among the positive patients was 14.0% (Table 3).

Table 3.
Co-detection of HCMV IgM and IgG antibodies among the HIV patients.
4. Discussion
The findings derived from this investigation revealed a seroprevalence of 32.7% of HCMV IgM antibodies among HIV patients in Edo State. This prevalence rate is considerable, suggesting a heightened susceptibility to factors prompting the reactivation of HCMV infection. Additionally, the elevated rates of social engagement, involvement in commercial sex activities, engagement with multiple sexual partners, declining socioeconomic conditions, and inadequate hygiene practices are aspects that could contribute to the elevated prevalence levels. Our result is similar to the findings obtained in Iran with a prevalence of 33% [26] among pregnant women; 29.3% among women of reproductive age attending selected hospitals in Zaria, Kaduna State, Nigeria [27]. This seroprevalence is higher when compared to the 3.1% obtained among HIV infected adult patients on HAART, in 2012 [24]; 19.5% obtained in a study among healthy blood donors at Lagos University Teaching Hospital in 2009 [17]; 9.52% recorded among AIDS patients in India [28] and 2.5% recorded among pregnant women in western Sudan [29].
Analysis of the results based on gender shows that there was no significant association between HCMV IgM antibodies and gender, though the male participants had a higher prevalence of 55.1% compared to the females at 44.9%. This study recorded a significantly higher percentage of males having HCMV IgM antibodies than their female counterparts. The noticeable increase in the rate of active infection among males compared to females in this research implies that males might encounter more factors that contribute to the reactivation or reinfection of HCMV among the participants in the study. Immunosuppression and patients who have immune compromising diseases such as HIV, by engaging in multiple sexual relationships makes them prone to HCMV re-activation and disease. A similar trend was reported by Fotowade and colleagues in 2015 [30].
The prevalence based on age revealed no significant difference in HCMV IgM antibodies and age. This finding agrees with a previous report of Adeola and co-workers among patients in Ilorin, Nigeria [30]. This study recorded a prevalence of HCMV IgM antibodies of 51.0% among patients in the age range <19 years; 30.6% among the 20–39 years age group; 12.2% among the >60 years age group; whereas 6.1% among the 40–59 years age group recorded the lowest prevalence [30] This finding of 51% prevalence of HCMV IgM antibodies, among the <19 years age group indicates that this is the age range where HCMV infection commonly develops and can be attributed to a low level of education in this age group. Furthermore, these patients fall within the adolescent age group, which could make them more prone to engaging in risky behaviors that facilitate transmission. Additionally, the substantial occurrence of HCMV IgM antibodies at a rate of 30.6% among individuals aged 20 to 39, signifies the prevalence of active infection within sexually active young and mature adults who are more likely to exhibit promiscuous behavior and therefore have an increased likelihood of infection. Moreover, the prevalence of HCMV IgM antibodies observed among patients aged between 40 and 59, as well as those above 60, illustrates the cumulative nature of the infection due to the latent presence of the virus in the body; once infected, an individual remains so for life. Hence, this might be attributed to reactivation, as the virus is capable of resurfacing when the immune system is compromised. This trend aligns with the conclusions drawn in a previous study [31].
When examining the outcomes according to geographical region, it becomes evident that a noteworthy correlation exists between HCMV IgM antibodies and location. Nevertheless, the prevalence was more pronounced in urban locales, at 61.2%, as opposed to rural regions, where it stood at 38.8%. The high levels of HCMV infection in urban areas rather than rural areas may be due to a massive increase in urban population causing basic infrastructure to be insufficient, coupled with social and economic inequalities. Higher rates of social interaction, commercial sex activity and multiple sexual partners, depreciating socioeconomic standards, poor hygienic practices, low standards of education and racial differences between the populations are valid factors that might be responsible for this variation in prevalence rates obtained by certain studies [32,33]. Analysis of the results based on religion shows that there was a significant association between HCMV IgM antibodies with religion. The prevalence was however higher in Christian 77.9% than their Muslim counterparts 22.1%. More so, religion is not a known predisposing factor to the acquisition of HCMV infection among HIV patients. Therefore, this higher prevalence observed among the Christian population compared to their Muslim counterparts may be due to population differences. It was observed that there were many more study participants who identified as Christians than those who identified as Muslims.
The presence of specific HCMV IgG and IgM antibodies concurrently, demonstrated that there was no substantial link between the prevalence of HCMV co-antibodies among the HIV patients under investigation. The prevalence of HCMV IgM antibodies was 32.7%, while that of IgG antibodies was 40.7%. This suggests a heightened rate of anti-CMV IgM antibodies due to reactivation of the infection; since the virus remains latent, individuals already carry the virus when exposed, particularly those with compromised immune systems. Additionally, when examining the co-infection of specific HCMV antibodies (IgM and IgG), there was a significant correlation observed with the prevalence of HCMV co-antibodies in the studied HIV patients. The prevalence of both HCMV IgM and IgG antibodies was 14.0%. This underscores the increased risk of HIV transmission associated with HCMV co-infection. HCMV has been identified as a partial contributor to a substantial number of new HIV infections, surpassing even common sexually transmitted infections like gonorrhea, syphilis, chlamydia, or herpes simplex virus type 2. The co-infection of HIV and HCMV often accelerates the progression to AIDS. The compromised immune systems in HIV patients make them susceptible to virus re-infection, leading to long-term medical complications and potential fatality. These findings are in accordance with the research by Gianella and colleagues [19].
5. Conclusions
Our study recorded a prevalence of 32.7% HCMV IgM antibodies among HIV patients indicating that the seroprevalence of HCMV infection is widespread among males and females in Edo State, Southern Nigeria. The high prevalence was attributable to co-infection, location and religion. It is advisable to adopt strategies aimed at managing the transmission of HCMV infection through addressing the risk factors identified in this study. HCMV holds significance as a prominent pathogen within African populations, serving as a source of acute illness, especially in individuals with weakened immune systems and those living with HIV. Enhancing the implementation of diagnostic tests and clinical trials for treating acute HCMV-related conditions is crucial, particularly in patient groups such as HIV-infected or exposed children with HCMV, as well as in HIV-infected adults exhibiting signs of active HCMV infection or disease. Establishing routine laboratory diagnoses plays a pivotal role in proactively preventing the onset of this disease.
Author Contributions
Conceptualization, N.F.O., I.M.M.-O., O.J.N. and C.I.N.; methodology, N.F.O. and C.I.N.; software, N.F.O., I.M.M.-O. and C.I.N.; validation, N.F.O., I.M.M.-O., O.J.N. and C.I.N.; formal analysis, N.F.O., I.M.M.-O., O.J.N. and C.I.N.; investigation, N.F.O., I.M.M.-O. and C.I.N.; resources, N.F.O., I.M.M.-O., O.J.N. and C.I.N.; data curation, N.F.O.; writing—original draft preparation, N.F.O., I.M.M.-O. and C.I.N.; writing—review and editing, N.F.O., I.M.M.-O., O.J.N. and C.I.N.; visualization, N.F.O., I.M.M.-O. and C.I.N.; supervision, I.M.M.-O. and O.J.N.; project administration, I.M.M.-O., O.J.N. and C.I.N.; funding acquisition, N.F.O. and C.I.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical authorization was secured from the Edo State Ministry of Health Research and Ethics Committee, identified by the Ethical Clearance Protocol Number HM.1208/199. Participants in the study were assigned code numbers, and personal names were not documented. The data gathered remained entirely detached from the individuals, and their identities will never be disclosed in any publication arising from this study. Participants were guaranteed that choosing not to partake in the study or withdrawing from it would have no adverse consequences on their privileges or benefits.
Informed Consent Statement
Permission was acquired from the individuals partaking in the research, and the study’s aims were effectively conveyed to them. Engagement was entirely optional, and no manipulation or inappropriate persuasion was applied to the participants. The research upheld confidentiality and privacy, with all participants being guaranteed that their personal details and provided information would remain undisclosed and safeguarded.
Data Availability Statement
The data employed in this investigation are not accessible to the general public due to a confidentiality arrangement with the study participants. Nonetheless, the data can be obtained by reaching out to the designated autho upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Policy Recommendations
Further investigations involving a sizable participant pool on a national scale are warranted to gain a comprehensive understanding of HCMV–HIV co-infection. This holistic view can then serve as a foundation for the development of national strategies for disease prevention and treatment. Moreover, the use of anti-CMV therapy in conjunction with ART for individuals with HCMV viremia is advisable, as this approach could alleviate the burden of HCMV-related complications and subsequently extend the life expectancy of individuals living with HIV. Efforts should also be made to educate HIV-infected patients about the transmission mechanisms of HCMV, similar to the practices in place for other sexually transmitted diseases. Additionally, promoting preventive measures such as opting for HCMV-free blood transfusions for those who test negative for HCMV, when feasible, can be beneficial. Alongside safe sexual practices and the avoidance of potentially contaminated needles, the emphasis on effective antiretroviral treatment remains a prime method for preventing HCMV disease in HIV-infected patients. Identifying individuals at risk and closely monitoring cases of HCMV end-organ disease are other pertinent strategies for prevention. We also recommend CMV viral load testing to better monitor the infection and treatment of HIV-positive patients.
References
- Sijmons, S.; Thys, K.; Mbong Ngwese, M.; Van Damme, E.; Dvorak, J.; Van Loock, M.; Li, G.; Tachezy, R.; Busson, L.; Aerssens, J. High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination. J. Virol. 2015, 89, 7673–7695. [Google Scholar] [CrossRef]
- Grønborg, H.L.; Jespersen, S.; Hønge, B.L.; Jensen-Fangel, S.; Wejse, C. Review of cytomegalovirus coinfection in HIV-infected individuals in Africa. Rev. Med. Virol. 2017, 27, e1907. [Google Scholar] [CrossRef]
- Udeze, A.; Odebisi-Omokanye, M.; Ajileye, T. Cytomegalovirus infection among Human Immunodeficiency Virus (HIV) infected individuals on highly active anti-retroviral therapy in North-Central Nigeria. Afr. Health Sci. 2018, 18, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.A.; Armah, H.B.; Narter-Olaga, E.G. Seroprevalence of Cytomegalovirus among some voluntary blood donors at the 37 Military Hospital, Accra, Ghana. Ghana. Med. J. 2006, 40, 99–104. [Google Scholar] [CrossRef][Green Version]
- Ngai, J.J.; Chong, K.L.; Mohammed, S.O. Cytomegalovirus Retinitis in Primary Immune Deficiency Disease. Case Rep. Opthalmol. Med. 2018, 2018, 8125806. [Google Scholar] [CrossRef] [PubMed]
- Coats, D.K.; Demmler, G.J.; Paysse, E.A.; Du, L.T.; Libby, C. Ophthalmologic findings in children with congenital cytomegalovirus infection. J. AAPOS 2000, 4, 110–116. [Google Scholar] [CrossRef]
- Sia, I.G.; Patel, R. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin. Microbiol. Rev. 2000, 13, 83–121. [Google Scholar] [CrossRef]
- Tagarro, A.; Del Valle, R.; Dominguez-Rodríguez, S.; Baquero-Artigao, F.; Noguera-Julian, A.; Vives-Oñós, I.; Santos, M.; Hawkins, M.M.; Pérez-Seoane, B.; Medina, G.; et al. For Spanish Registry of Infants with Congenital Cytomegalovirus Infection (REDICCMV) Study Group. Growth Patterns in Children with Congenital Cytomegalovirus Infection. Ped. Infect. Dis. J. 2019, 38, 1230–1235. [Google Scholar] [CrossRef]
- Yinon, Y.; Farine, D.; Yudin, M.H. Screening, diagnosis, and management of cytomegalovirus infection in pregnancy. Obstet. Gynecol. Surv. 2010, 65, 736–743. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Anglemyer, A.; Horvath, T.; Rutherford, G. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA 2013, 310, 1619–1620. [Google Scholar] [CrossRef]
- Lu, D.Y.; Wu, H.Y.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.R. HAART in HIV/AIDS treatments: Future trends. Infect. Disord. Drug Targets 2018, 18, 15–22. [Google Scholar] [CrossRef]
- Parekh, B.S.; Ou, C.Y.; Fonjungo, P.N.; Kalou, M.B.; Rottinghaus, E.; Puren, A.; Alexander, H.; Hurlston Cox, M.; Nkengasong, J.N. Diagnosis of human immunodeficiency virus infection. Clin. Microbiol. Rev. 2019, 32, e00064-18. [Google Scholar] [CrossRef] [PubMed]
- Friedland, G.H.; Klein, R.S. Transmission of the human immunodeficiency virus. N. Engl. J. Med. 1987, 317, 1125–1135. [Google Scholar] [CrossRef]
- UNAIDS. Global HIV/AIDS Statistics-Fact Sheet. 2023. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 20 August 2023).
- Alao, O.O.; Joseph, D.E.; Mamman, A.; Banwat, E.B. The seroprevalence of cytomegalovirus antibodies among prospective blood donors in Jos. Niger. J. Med. 2008, 17, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, A.A.; Akanmu, A.S.; Adeyemo, T.A.; Wright, K.O.; Dada, M.O.; Dosunmu, A.O. Seroprevalence of CMV antibodies among HIV patients and blood donors at Lagos State University Teaching Hospital. S. Afr. Med. J. 2009, 99, 528–530. [Google Scholar]
- Ojide, C.K.; Ophori, E.A.; Eghafona, N.O.; Omoti, C. Seroprevalence of Cytomegalovirus (CMV) amongst voluntary blood donors in University of Benin Teaching Hospital (UBTH), Edo State, Nigeria. Br. J. Med. Med. Res. 2012, 2, 15–20. [Google Scholar] [CrossRef]
- Gianella, S.; Scheffler, K.; Mehta, S.R.; Little, S.J.; Freitas, L.; Morris, S.R.; Smith, D.M. Seminal Shedding of CMV and HIV Transmission among Men Who Have Sex with Men. Int. J. Environ. Res. Public Health 2015, 12, 7585–7592. [Google Scholar] [CrossRef] [PubMed]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV & AIDS Statistics—2020 Fact Sheet. UNAIDS.org. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 18 August 2023).
- Al Mana, H.; Yassine, H.M.; Nadin, N.Y.; Al-Mohannadi, A.; Al-Sadeq, D.W.; Alhababi, D.; Nasser, E.A.; Nasrallah, G.K. The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review. Pathogens 2019, 8, 213. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of Cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Federal Government of Nigeria. Federal Republic of Nigeria Official Gazette. 2009. Available online: https://gazettes.africa/gazettes/ng/2009 (accessed on 19 August 2023).
- Ojide, C.; Kalu, E.; Nwadike, V.; Ogbaini-Emovon, E.; Omoti, C. Seroprevalence of cytomegalovirus among HIV-infected adult patients on HAART. Int. J. Trop. Dis. Health 2013, 3, 233–241. [Google Scholar] [CrossRef]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences, 7th ed.; John Wiley and Sons: New York, NY, USA, 1999; pp. 552–560. ISBN 0-471. [Google Scholar]
- Arabzadeh, A.M.; Mosavat, S.A.; Eftekhari, N. Seroepidemiology of Human Cytomegalovirus in Pregnant Women and their Neonates in Kerman City During 2005. J. Kerman Univ. Med. Sci. 2007, 14, 279–288. [Google Scholar]
- Yusuf, I.T.; Olonitola, O.S.; Jatau, E.D. Seroprevalence of Cytomegalovirus among Women of Reproductive Age Attending Selected Hospitals in Zaria Metropolis, Kaduna State, Nigeria. Int. J. Sci. Res. Publ. 2018, 8, 462–471. [Google Scholar] [CrossRef]
- Basawaraju, A.; Mane, P.M.; Vijayadurga, S. The reactivation of the cytomegalovirus (CMV) infection in HIV infected patients. J. Clin. Diagnos Res. 2011, 5, 749–751. [Google Scholar]
- Hamdan, Z.H.; Abdelbagi, I.E.; Nasser, N.M.; Adam, I. Seroprevalence of cytomegalovirus and rubella among pregnant women in western Sudan. Virol. J. 2011, 8, 217–220. [Google Scholar] [CrossRef]
- Fotowade, A.; Okonko, I.O.; Agbede, O.O.; Suileman, S.T. High seropositivity of IgG and IgM antibodies against cytomegalovirus (CMV) among HIV-1 seropositive patients in Ilorin, Nigeria. Afr. Health Sci. 2015, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Neto, E.C.; Rubin, R.; Schulte, J.; Giugliani, R. Newborn screening for congenital infectious diseases. Emerg. Inf. Dis. 2004, 10, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Kimura, H.; Yabuta, Y.; Tanaka, N.; Ito, Y.; Ishikawa, K.; Suzuki, C.; Morishima, T. Prevalence of maternal cytomegalovirus (CMV) antibody and detection of CMV DNA in amniotic fluid. Microbiol. Immunol. 1999, 43, 781–784. [Google Scholar] [CrossRef]
- Adland, E.; Klenerman, P.; Goulder, P.; Matthews, P.C. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front. Microbiol. 2015, 6, 1016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).