Abstract
In recent years, the global resurgence of syphilis has posed significant challenges to public health. This sexually transmitted infection, caused by the bacterium Treponema pallidum, can have severe consequences if left untreated, including neurological and cardiovascular complications. Diagnosing syphilis is complex due to its diverse clinical presentations, necessitating a multifaceted approach, including serological, molecular, and direct techniques such as dark-field microscopy. Penicillin remains the primary and effective treatment, but emerging macrolide-resistant strains have spurred investigations into alternative antibiotics. Syphilis vaccine development faces unique hurdles, yet promising strategies are under investigation. Targeted prevention strategies focus on high-risk populations such as men who have sex with men, pregnant women, and individuals with multiple sexual partners. The integration of syphilis services into primary healthcare enhances accessibility, early detection, and treatment. Innovative point-of-care diagnostics offer rapid, sensitive testing, while ongoing vaccine research holds the potential for long-term prevention. Addressing the global burden of syphilis requires a multifaceted approach, encompassing immunological advancements, innovative diagnostics, targeted prevention, and primary healthcare integration. Collaborative efforts between governments, healthcare systems, researchers, and communities are essential to effectively combat syphilis, striving toward a syphilis-free future that promotes better sexual health and overall well-being.
1. Introduction
Syphilis, caused by the spirochete bacterium Treponema pallidum subsp. pallidum, is a re-emerging global public health concern. The etiologic agent, T. pallidum subsp. pallidum, is one of several subspecies within the T. pallidum complex, which also includes T. pallidum subsp. endemicum, T. pallidum subsp. pertenue, and T. pallidum subsp. carateum. To avoid confusion and accurately identify the causative agent of syphilis, we will consistently refer to it as T. pallidum subsp. pallidum throughout this manuscript, in accordance with the nomenclature guidelines for pathogen names [1].
Despite considerable progress in understanding the disease over the years, challenges in effectively controlling and eradicating syphilis still persist [2]. The emergence of antibiotic-resistant strains, complex immunological interactions, and the lack of an effective vaccine underscore the need for innovative research to address this longstanding public health issue [3,4]. Furthermore, recent publications have shed new light on the epidemiology of syphilis and its interactions with other infectious diseases, particularly in people living with HIV [5].
Immunology plays a pivotal role in syphilis research, as understanding the intricate interplay between the host immune response and T. pallidum infection is essential for identifying novel diagnostic methods, developing effective vaccines, and designing targeted immunotherapies [6]. Unravelling the mechanisms by which the bacterium evades the host’s immune defenses and manipulates the inflammatory response is critical for devising strategies to curtail disease progression [7,8].
In parallel, pharmacological interventions are being explored to complement traditional antibiotic treatments and address challenges posed by antibiotic resistance. Novel drug targets are being identified through an in-depth understanding of the bacterium’s pathogenesis, metabolism, and interactions with the host. This quest for new pharmacological avenues aims to identify agents that can disrupt the bacterium’s survival mechanisms, halt disease progression, and potentially eradicate latent infections [9,10,11].
This article serves as a comprehensive review, providing an in-depth exploration of the latest advancements in syphilis research, focusing on cutting-edge developments in immunology and pharmacology. Within these pages, we delve into the ongoing efforts of scientists who relentlessly strive to address unanswered questions, refine existing approaches, and propel the field forward. Our review places a particular emphasis on the profound implications of a comprehensive understanding of immunological responses and the emergence of novel pharmacological interventions. Ultimately, the goal is to explore potential strategies that can effectively curb the global burden of syphilis and pave the way for its control and eradication.
2. Epidemiology of Syphilis: Current Trends and Future Challenges
Syphilis is a disease with a complex progression involving several distinct phases, each with specific clinical characteristics. These phases play a fundamental role in the epidemiology of syphilis as they affect disease transmission, detection, and management. In the primary stage of syphilis, the initial infection occurs after exposure to T. pallidum. The most characteristic symptom of this stage is the formation of a hard sore at the site of infection. This sore, known as a hard chancre, is a painless ulcer that typically develops on the genital organs, anus, mouth, or another area where the bacterium entered the body. The presence of the hard chancre is highly suggestive of primary syphilis. However, it is important to note that not all patients develop a hard chancre, making early identification of syphilis a challenge in some cases [12,13,14,15].
The secondary stage of syphilis occurs a few weeks after the disappearance of the primary hard chancre. During this stage, the disease becomes systemic, affecting the entire body. Symptoms can include various skin rashes, typically non-itchy, fever, malaise, sore throat, hair loss, muscle aches, and other flu-like symptoms. The diversity of clinical manifestations in secondary syphilis can make its recognition challenging, and it is crucial for healthcare professionals to be aware of these symptoms for accurate diagnosis [12,15,16].
After the secondary stage, syphilis can enter a latent period. During this phase, the patient does not exhibit visible symptoms of the disease, making it asymptomatic. Latent syphilis can be further divided into early latent (less than one year after the initial infection) and late latent (more than one year after the initial infection). While symptoms are absent, the T. pallidum bacterium continues to multiply in the body. The latent stage is relevant to the epidemiology of syphilis as latent patients can be asymptomatic carriers and potential sources of transmission [12,13,14,17].
Tertiary syphilis is the most severe phase of the disease and can develop after a variable period, even decades from the initial infection. During this stage, severe damage occurs to internal organs, including the heart, brain, eyes, bones, and blood vessels [13,14]. Symptoms of tertiary syphilis can vary widely, depending on the affected organs. Additionally, tertiary syphilis can lead to serious complications such as arterial aneurysms, cardiovascular disease, neurological impairment, blindness, and other debilitating health problems [12,18,19].
It is important to acknowledge that syphilis encompasses several phases, some of which can be asymptomatic or present mild symptoms that may go unnoticed. This complicates the collection of accurate data. In 2021, approximately 30% of primary syphilis cases were asymptomatic, and 25% of secondary syphilis cases presented with mild symptoms that often went undiagnosed. The absence of early diagnosis can lead to ongoing transmission, further aggravating the spread of the disease [3,5].
A detailed understanding of these syphilis stages is essential for early diagnosis and effective treatment. Early detection and intervention in the primary and secondary stages are crucial to prevent progression to tertiary syphilis and to interrupt the transmission chain. Therefore, the epidemiology of syphilis should consider not only incidence but also the disease stage to develop effective control strategies [13,20].
2.1. Immunology and Pathogenesis of Syphilis
The interaction between T. pallidum and the host’s immune system plays a pivotal role in the progression of syphilis. Understanding the immunological responses and pathogenic mechanisms involved is crucial for comprehending the disease’s complexity [21]. Syphilis is unique in its ability to evade the immune system. T. pallidum, the causative bacterium, has evolved sophisticated strategies to evade detection. These include altering its surface antigens, limiting its exposure to the host’s immune cells, and suppressing the immune response. This bacterium’s remarkable capacity to avoid detection is further exemplified by its ability to alter surface antigens, limit exposure to the host’s immune cells, and suppress immune responses [22,23].
One of the key mechanisms by which T. pallidum evades the host’s immune system is through antigenic variation of its surface proteins. This variation makes it challenging for the host’s immune system to recognize and target the bacterium effectively. Moreover, it contributes to the chronicity of syphilis, allowing the pathogen to persist in the host for extended periods without clearance [24,25].
The interaction between the bacterium and the host’s immune system significantly impacts the severity of syphilis symptoms. In some cases, an effective immune response can lead to the control of the infection and milder clinical manifestations. Conversely, an inadequate or dysregulated immune response may result in more severe and widespread symptoms [22,26]. The immunological aspects of syphilis also influence the effectiveness of treatment. The bactericidal action of antibiotics, such as penicillin, relies partly on the host’s immune system to help clear the bacteria. Therefore, an intact and appropriately functioning immune response is essential for successful treatment outcomes [27,28].
T. pallidum has evolved to persist within the host, often for years or even decades, by evading immune clearance. This persistence contributes to the disease’s chronic nature and underscores the importance of ongoing research to better understand the mechanisms behind the bacterium’s ability to hide from the immune system [29,30]. Investigating the immunological responses and pathogenesis of syphilis is critical for the development of improved diagnostics and treatments. Understanding how T. pallidum manipulates the immune system provides insights into potential therapeutic targets and strategies to enhance the host’s ability to clear the infection [6,31].
In summary, the interplay between T. pallidum and the host’s immune system is a dynamic and complex aspect of syphilis pathogenesis [32]. The pathogen’s ability to evade detection and influence the immune response has significant implications for the course of the disease, severity of symptoms, and treatment outcomes. Further research into these immunological mechanisms is essential to advance our understanding of syphilis and develop more effective strategies for its prevention and control [3,23].
2.2. Risk Factors
Syphilis is not limited to the key populations mentioned earlier, such as men who have sex with men (MSM), reproductive-age women, and pregnant women. To fully understand the risk factors associated with syphilis, it is crucial to explore various dimensions [33,34].
Epidemiological studies reveal that individuals engaged in high-risk behaviors, including unprotected sex and multiple sexual partners, are at greater risk of contracting syphilis. A national (USA) analysis of sexual health data indicated that approximately 1 in 4 syphilis cases in 2021 was related to unprotected sex with multiple partners. Additionally, about 40% of syphilis cases in young adults in the same year were associated with high-risk sexual behaviors [15,34,35].
Alarmingly, we observed an increase of up to 50% in syphilis rates among individuals with substance use disorders, especially those using methamphetamine and other stimulants, between 2020 and 2021. Substance use can be linked to high-risk sexual behaviors, contributing to the spread of syphilis. For instance, studies conducted in 2021 showed that 60% of methamphetamine users reported engaging in unprotected sexual practices in a recent period [36,37,38].
Socioeconomic inequalities and limited access to healthcare services continued to have a significant impact on syphilis rates in 2021. In economically disadvantaged areas, syphilis rates were up to twice as high as in regions with better healthcare services and education [13,39,40]. A study conducted in a low-income area found that the syphilis rate was 2.5 times higher than in more prosperous areas in the same year [41,42].
Syphilis often coexists with other sexually transmitted infections (STIs), such as HIV, presenting significant challenges in terms of both diagnosis and management. Coinfection with these diseases can exacerbate health risks and transmission, making it a critical concern for public health officials and healthcare providers [43]. It became increasingly evident that individuals living with HIV faced a markedly elevated risk of contracting syphilis, thereby ushering in a formidable challenge at the intersection of these two infections. This worrisome synergy between HIV and syphilis not only imposed a substantial health burden on affected individuals but also placed immense pressure on healthcare systems around the world [44,45].
The findings from these studies demonstrated that, in 2021, the coinfection rate of syphilis among people living with HIV reached alarming levels. In particular, some high-risk populations reported coinfection rates exceeding 20% during that year. This stark increase in coinfection rates raised critical questions about the dynamics of these two sexually transmitted infections, suggesting a complex interplay between them. Understanding the factors driving this epidemiological shift, such as changes in sexual behaviors, disparities in healthcare access, and the potential impact of preventive measures, became paramount in the context of public health
The incidence of syphilis in 2021 was a matter of concern, with an increase of 10% reported globally compared to the previous year. Particularly concerning was the surge in cases among key populations, including MSM and pregnant women, with an increase of 15% during the same period [2,34]. These statistics reflect the urgent need for targeted public health interventions to curb transmission and prevent adverse outcomes, such as congenital syphilis.
Prevalence, representing the total number of individuals with syphilis within a given population at a specific time, continued to be a significant concern in 2021. High prevalence rates suggested a substantial burden of the disease within populations with limited access to healthcare services and sexual health education. Identifying at-risk populations remained essential for effective syphilis control and prevention strategies [33,34].
Accurate epidemiological data on syphilis remained challenging to obtain in 2021 due to several factors [3]. Underreporting of cases, especially in resource-limited settings, led to an underestimation of the true burden of the disease. Furthermore, asymptomatic or mildly symptomatic cases often went undetected, resulting in delayed diagnosis and continued transmission [26,29,46].
Despite these challenges, ongoing efforts by the WHO and other health organizations aimed to strengthen syphilis surveillance systems and improve data collection in 2021. These efforts provided a more comprehensive understanding of the epidemiology and enabled evidence-based interventions [47,48].
The epidemiology of syphilis, characterized by varying incidence and prevalence rates, continued to highlight the need for robust surveillance and targeted public health interventions in 2021 [2,47,49]. Analyzing data from authoritative sources, such as the WHO, provided valuable insights into current trends, at-risk populations, and challenges faced in syphilis control. Implementing evidence-based strategies, promoting sexual health education, and ensuring accessible healthcare services remained key actions for global health authorities to reduce the incidence and prevalence of syphilis and its associated burden on public health [48,50,51].
3. Diagnosis of Syphilis: Advances and Challenges
Syphilis symptoms are not always readily apparent, and the disease often presents diagnostic challenges. Diagnosis relies on a combination of the patient’s clinical and sexual history, physical examinations, laboratory tests, and, in some cases, radiological examinations [13].
3.1. Direct Tests for T. pallidum Detection
Diagnosing primary syphilis can be particularly challenging because not all chancres are externally visible. Some chancres may occur in anatomical sites such as the mouth, anus, or vagina, making them difficult to detect through routine physical examinations. In cases where chancres are visible, the diagnosis of primary syphilis is relatively straightforward [13].
One of the direct tests used for T. pallidum detection is the dark-field microscopy test. This technique allows for the visualization of the spiral morphology of T. pallidum in primary syphilis chancres and boasts a sensitivity ranging from 71% to 100%. However, it is worth noting that only a limited number of bacteria is typically found in smears from the sore due to the rapid and spontaneous healing of primary chancres [52,53,54]. Another direct test is the fluorescent antibody staining technique, which detects pathogenic T. pallidum through an antigen-antibody reaction. This method is suitable for various sample types, including lesions, concentrated fluids, and tissue brushes. The specificity of this test depends on the type of antibody used [55,56,57].
3.2. Serological Tests for Detection of Antibodies against T. pallidum
Serological tests play a pivotal role in diagnosing syphilis by detecting antibodies produced in response to T. pallidum infection. These tests are widely used due to their effectiveness, simplicity, speed, and cost-effectiveness. However, it is important to recognize the variations among different serological tests, including non-treponemal tests (NTT) and treponemal tests (TT) [13,30].
3.2.1. Non-Treponemal Tests (NTT) Limitations, including Special Populations
Non-treponemal tests (NTT), such as the rapid plasma reagin (RPR) test, are invaluable tools for syphilis screening due to their simplicity, speed, and cost-effectiveness. However, it is essential to recognize their limitations, especially in certain special populations, such as individuals living with HIV (PLWH) [58,59].
NTTs quantify immunoglobulin M or G anti-lipid antibodies produced in response to material released by host cells or cardiolipin from T. pallidum [60]. Despite their widespread use, NTTs, including the RPR, may exhibit reduced sensitivity, particularly in cases with low titers [61]. Additionally, false-positive results can occur in specific conditions [62].
In the context of PLWH, several studies have shed light on the potential challenges associated with NTTs in syphilis diagnosis. Notably, PLWH coinfected with syphilis may exhibit specific serological profiles that can complicate the interpretation of NTT results [43,63]. One crucial consideration is the phenomenon known as “serofast status” [3]. In some PLWH coinfected with syphilis, despite appropriate treatment, their NTT results may remain reactive for prolonged periods, even when there is no evidence of active syphilis infection. This phenomenon has raised concerns about the reliability of NTTs in distinguishing between active and treated infections in this population [64].
Another challenge in PLWH is the identification of “serological non-responders”. These individuals may have a delayed or blunted antibody response to T. pallidum infection, leading to false-negative NTT results. This delay in seroconversion can further complicate the timely diagnosis of syphilis in PLWH [3,64].
Understanding the limitations of NTTs in PLWH is critical for appropriate syphilis management. Healthcare providers need to consider the possibility of serofast status or serological non-responders in this population when interpreting NTT results [65].
In such cases, complementary tests, including treponemal tests (TT), molecular techniques, or clinical evaluation, may be necessary to confirm or rule out active syphilis infection [13]. Additionally, close monitoring and a thorough clinical history, including sexual behaviors and previous syphilis treatments, are essential to make informed decisions regarding syphilis diagnosis and treatment in PLWH [3,14,43].
While NTTs are valuable tools for syphilis screening, their limitations, especially in special populations such as PLWH, should be recognized. Serofast status and serological non-responder profiles may complicate the interpretation of NTT results, highlighting the importance of a comprehensive diagnostic approach in syphilis management [54,64,66].
3.2.2. Authorized Tests: aRPR and RPR-S
Among the authorized tests, two noteworthy options are Mediace automated RPR (aRPR) and Sekure rapid plasma reagin (RPR-S), both developed by Sekisui Diagnostics [67]. These tests offer the advantage of automation, potentially streamlining the diagnostic process. However, it is essential to consider their specific characteristics. While Mediace automated RPR (aRPR) provides automation benefits, it is important to note that it has been associated with a limitation—poor sensitivity in cases with low titers [61].
This suggests that aRPR may not be as effective in detecting syphilis in its early stages or in individuals with lower antibody levels. It is particularly important to consider these limitations when dealing with critical key populations, such as sex workers, who may be at increased risk of syphilis but could present with varying antibody levels. Sekure rapid plasma reagin (RPR-S) offers the advantage of rapid results, but its sensitivity and accuracy may require improvement before it can be considered a reliable tool for diagnosing syphilis and evaluating treatment efficacy in clinical practice [62]. Therefore, cautious consideration of its use is warranted.
3.3. Treponemal Tests for Detection of Antibodies
Treponemal tests (TTs) are highly sensitive and are particularly effective in later stages of syphilis. They include the absorbed fluorescent treponemal antibody absorption (FTA-ABS), T. pallidum particle agglutination, T. pallidum particle agglutination assay (TPHA), enzyme immunoassay (EIA), enzyme-linked immunosorbent assay (ELISA), and chemiluminescence immunoassay (CLIA). These tests detect antibodies against T. pallidum proteins [54].
3.4. Molecular Techniques and Mass Spectrometry
Molecular techniques, such as the nucleic acid amplification technique (NAATs), aim to detect T. pallidum infection, with sensitivity and specificity varying based on the stage of syphilis and the specific molecular method employed. Routine molecular methods include conventional PCR, nested PCR (nPCR), real-time PCR (qPCR), reverse transcription PCR (RT-PCR), and loop-mediated isothermal amplification (LAMP) assay [54].
Another method for syphilis detection is mass spectrometry (MS), although it is rarely used in clinical laboratories due to time-consuming data analysis and expensive equipment. This test identifies proteins associated with T. pallidum using a technique in which chemical compounds are ionized into molecules, and the mass-to-charge ratio (m/z) is measured [68]. However, it is important to note that m/z results alone do not have direct diagnostic significance for the disease. They need to be correlated with other data analyses to obtain clinically relevant information.
4. Pharmacological Interventions
Syphilis treatment has undergone significant transformations over the years. In the past, patients were subjected to prolonged treatment regimens involving arsenic and toxic compounds. However, these treatments often resulted in treatment failure, accompanied by periodic and sporadic outbreaks [20,69].
The pivotal moment in the treatment of syphilis came with the discovery of T. pallidum’s susceptibility to penicillin in 1943. This breakthrough redefined the approach to syphilis treatment, shifting from toxic compounds to antibiotics [69]. The widespread use of penicillin led to a substantial decrease in syphilis cases, marking a significant improvement in syphilis control over the past five decades, especially when compared to the pre-penicillin era [70].
4.1. Penicillin: The Cornerstone of Syphilis Treatment
In the treatment of syphilis, penicillin remains the primary and most effective antimicrobial agent. Penicillin is considered treponemicidal, even at substantially lower concentrations than those required in in vitro tests, which typically demand 0.36 mg/L. This characteristic makes penicillin the prominent therapeutic choice in combating T. pallidum, the causative agent of syphilis. It is important to note that while syphilis can progress to tertiary syphilis if left untreated, it is not an inevitable outcome. In fact, less than 30% of syphilis cases progress to the tertiary stage. The number of bacterial generation times (30–33 h) necessitates a treatment duration of 7–10 days to maintain treponemicidal activity [13,20].
For specific cases, particularly those involving the central nervous system (CNS) and the cerebrospinal fluid (CSF), where the potential persistence of treponemes and the risk of relapses are significant concerns, an extended treatment duration or the administration of long-acting benzathine penicillin G (BPG) at 2.4 million units is recommended. This prolonged presence of treponemicidal penicillin in the bloodstream is crucial for preventing neurosyphilis or relapses in the CNS [13,20].
4.2. Alternative Antibiotics and DoxyPrEP
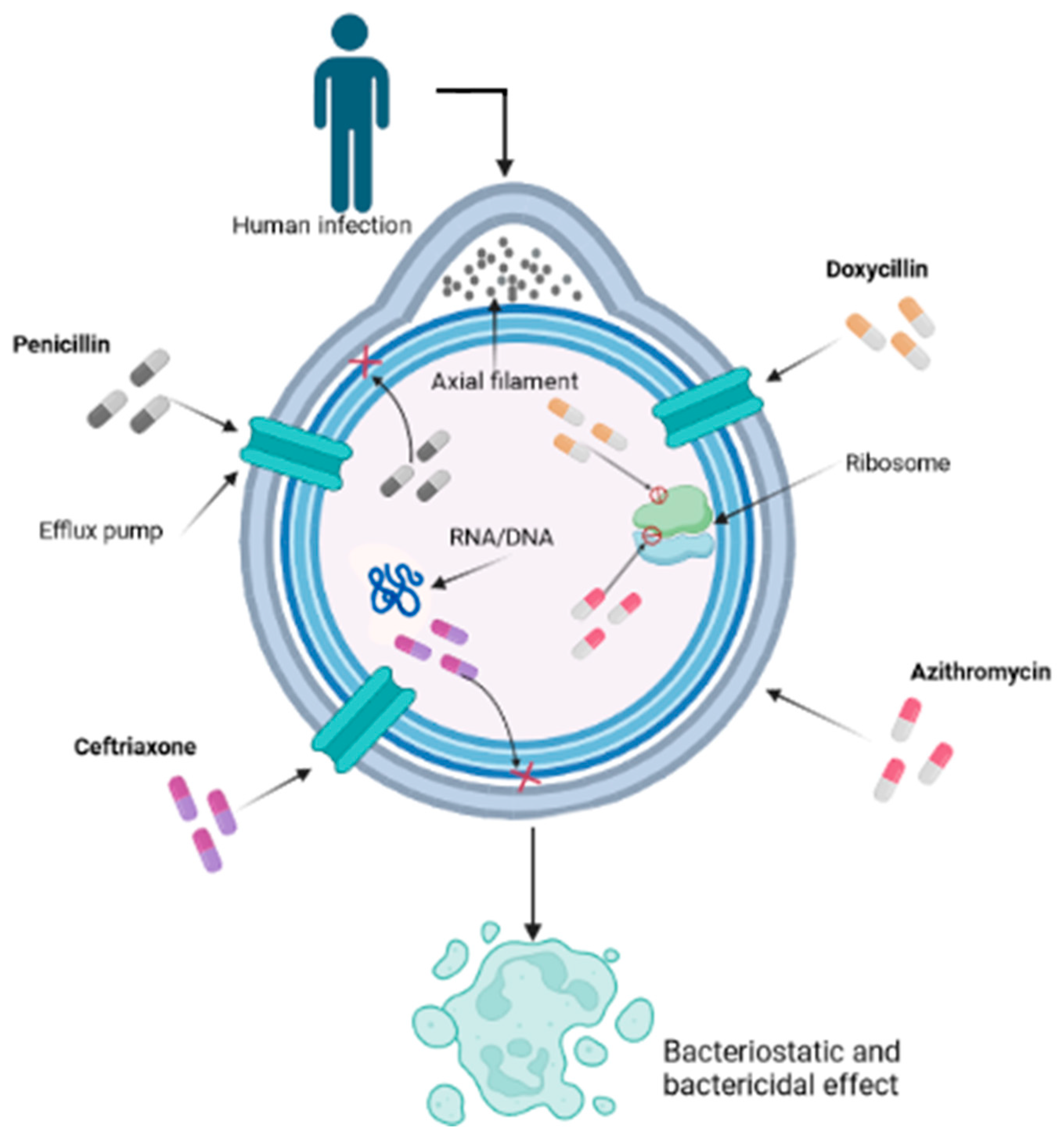
While penicillin remains the gold standard, some patients may have allergies or other contraindications that limit its use. In such cases, alternative antibiotics are considered [71]. Among these alternatives, oral amoxicillin in association with probenecid has shown efficacy in achieving treponemicidal drug levels within the CSF [72]. Additionally, newer anti-treponemal antibiotics such as extended-spectrum cephalosporins (ESC), such as ceftriaxone, administered intramuscularly or intravenously, have been explored (Figure 1) [73,74].
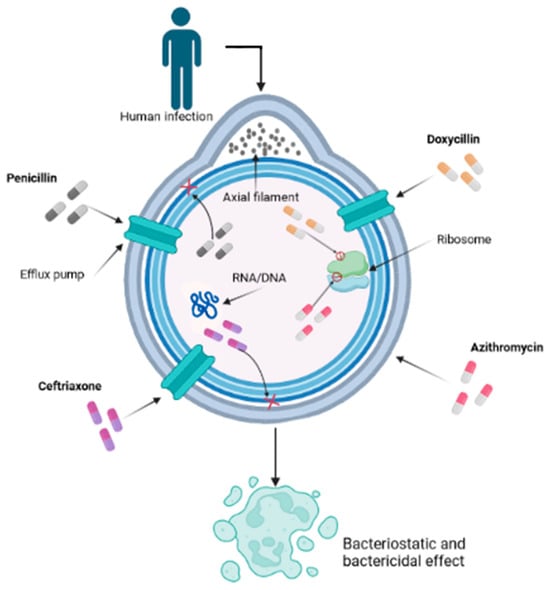
Figure 1.
Mechanism of action of various antibiotics on Treponema pallidum used for syphilis treatment. This cross-section allows us to visualize the inner workings of the bacterial cell, shedding light on its interactions and vital components within the context of the infection process. The intricate mechanism by which various antibiotics, notably penicillin, doxycycline, ceftriaxone, and azithromycin, play pivotal roles in combating the infection caused by T. pallidum, is a nuanced interplay at the forefront of scientific understanding. Penicillin, functioning as a β-lactam compound, disrupts the transpeptidase-mediated synthesis via cross-linking of peptidoglycan, thereby undermining the structural integrity of the cell wall and culminating in bacterial lysis. Doxycycline, classified as a tetracycline derivative, exerts its influence by suppressing protein translation through binding to the 30S subunit of the bacterial ribosome, thereby obstructing the intricate assembly of the translation complex. Ceftriaxone, a member of the cephalosporin class, further augments the multifaceted approach by targeting the bacterial cell wall, thwarting transpeptidase activity, and thereby weakening the structural robustness. In parallel, azithromycin, a representative of the macrolide family, exerts its effect by inhibiting bacterial protein synthesis through impeding the translocation process of the 50S ribosomal subunit, further contributing to the orchestrated disruption of essential bacterial processes.
One notable development in the context of syphilis treatment is the use of doxycycline pre-exposure prophylaxis (DoxyPrEP). DoxyPrEP has gained attention as a potential option, particularly for individuals who may have contraindications to penicillin treatment. Including DoxyPrEP in the discussion, we acknowledge the importance of alternative approaches to syphilis treatment and prevention. However, it is essential to note that the effectiveness of DoxyPrEP may vary based on the specific patient population and their risk factors for syphilis [75,76].
4.3. Azithromycin Resistance in Syphilis
Syphilis treatment strategies have evolved significantly over the years, with penicillin remaining the primary and most effective antimicrobial agent. However, it is crucial to acknowledge the emergence of azithromycin-resistant syphilis as a concern within the pharmacological landscape [20].
Recent reports have indicated cases of syphilis that do not respond effectively to azithromycin, a macrolide antibiotic that has been used as an alternative treatment option, especially in cases of penicillin allergy. Azithromycin resistance poses a challenge to the management of syphilis, as it limits the available treatment options and may result in treatment failures [73,74,75].
The WHO recommends the use of azithromycin for syphilis treatment only in settings where the prevalence of macrolide-resistant T. pallidum is known to be very low [72]. However, the emergence of resistant strains highlights the importance of continuous surveillance and monitoring of syphilis treatment outcomes.
Future research and pharmacological interventions should focus on addressing azithromycin-resistant syphilis, exploring alternative treatment options, and developing strategies to combat this emerging challenge. This includes studying the mechanisms of resistance, optimizing treatment regimens, and evaluating the efficacy of new antibiotics or combination therapies to ensure effective management of syphilis in the face of evolving resistance patterns [10,77].
By addressing azithromycin resistance within the context of pharmacological interventions, healthcare providers and researchers can work together to develop more effective treatment strategies and mitigate the impact of drug-resistant syphilis strains.
4.4. Challenges and Future Directions in Syphilis Treatment
The challenges associated with developing new antibiotics and vaccines for syphilis treatment are multifaceted. T. pallidum’s unique characteristics, such as its inability to be cultured in a lab setting, the scarcity of experimental models, and high research costs, pose significant obstacles [34]. Recent studies have explored the susceptibility of T. pallidum to alternative antibiotics such as linezolid, moxifloxacin, and clofazimine, offering promise for potential candidates in human clinical trials [78,79].
The ongoing development of new antibiotics, along with the pursuit of effective vaccines, offers hope for expanding therapeutic options and improving the outlook for syphilis treatment. Achieving this goal requires a comprehensive understanding of the immune system’s role, a thorough assessment of the severity of syphilis-related diseases, the selection of antimicrobials with the correct spectrum of action, determination of optimal dosages, treatment durations, and assessing overall efficacy [22,27,73].
5. Future Approaches in Syphilis Prevention and Control
Syphilis remains a global public health concern, necessitating continuous efforts to develop innovative strategies for prevention and control [13]. As the incidence of syphilis continues to rise in certain populations, there is an urgent need to explore new approaches to combat this sexually transmitted infection (STI). In this section, we discuss potential future approaches in syphilis prevention and control, focusing on various aspects of research and intervention.
5.1. Development of Effective Vaccines
Researchers are continually exploring innovative vaccine formulations to enhance the immunogenicity and efficacy of syphilis vaccines. This includes investigating different adjuvants, delivery systems, and antigen combinations to elicit a robust and long-lasting immune response. Promising results have been obtained in preclinical studies with new adjuvants and delivery platforms, offering opportunities for more effective vaccine candidates in the future [76,80], such as the proteins endoflagella, FlaB3, Gpd, Tp92 (BamA), Tp0136, Tp0751, TprI, TprK, and TprF1 [81,82]. The development of a syphilis vaccine poses unique challenges due to the complex nature of the T. pallidum bacterium, which is the causative agent of syphilis [76,83]. Understanding the mechanisms of immune evasion employed by the bacterium is essential in guiding vaccine design. Researchers must address the antigenic variation of T. pallidum to ensure broad protection against different strains of the pathogen [84].
The focus of developing a syphilis vaccine has evolved over the past decade or so. Initially, efforts were aimed at stimulating a protective humoral response, involving opsonic antibodies, to target and eliminate T. pallidum through phagocytosis. For instance, in a study from 2006, Cullen and Cameron explored various T. pallidum vaccine approaches, including whole-cell, subunit, and non-protein carbohydrate antigens, which showed promise in facilitating ‘opsonophagocytosis’ [85]. In a significant publication in 2011, Carlson et al. presented a perspective on the clinical course of syphilis, suggesting that it hinges on the delicate balance between delayed-type hypersensitivity (DTH) and humoral immunity against T. pallidum. They proposed that a robust DTH response initially clears the organism from the body, while a strong humoral response later on may lead to ‘prolonged infection and progression to tertiary disease’ [22]. They concluded that an effective syphilis vaccine should be oriented towards enhancing the DTH response, which could potentially be bolstered by incorporating BCG into the vaccine formulation. Finally, in 2014, Cameron and Lukehart underscored the significance of a comprehensive approach to vaccine development, one that encompasses both the DTH response and opsonic antibodies/phagocytosis [76]. Consequently, the current landscape does not exhibit a consensus on the creation of an effective syphilis vaccine [86].
As the target population for a syphilis vaccine may differ from that of other STI vaccines, considerations must be given to factors such as age, gender, and risk behaviors. Determining the optimal age for vaccination, identifying high-risk groups, and tailoring the vaccine to address specific transmission patterns are critical aspects of designing an effective vaccination strategy [83,87].
While there is currently no available vaccine for syphilis, it is important to emphasize that, when a successful vaccine candidate is identified, overcoming logistical and financial barriers in vaccine distribution and uptake will be crucial to ensure equitable protection against syphilis. The development of an effective syphilis vaccine is a complex yet vital endeavor to combat the growing burden of this sexually transmitted infection [83]. Advances in vaccine formulation, understanding the challenges in vaccine design, considerations for target populations, ethical and safety aspects, collaboration, cross-protection potential, integration with existing control strategies, and vaccine implementation are all critical factors that deserve attention in syphilis vaccine research. A successful vaccine holds the promise of transforming syphilis prevention and control efforts, ultimately contributing to improved global sexual health outcomes [76,83,84].
5.2. Innovative Point-of-Care Diagnostics
Early and accurate diagnosis of syphilis is crucial for timely treatment and prevention of further transmission. Future approaches in syphilis diagnostics involve the development of innovative point-of-care tests that are rapid, sensitive, and easy to use. A notable example of this advancement is the development of a highly sensitive molecular assay capable of detecting T. pallidum DNA in clinical samples [88]. This innovative diagnostic tool not only offers greater accuracy but also enables the detection of the bacterium in the early stages of infection, facilitating timely intervention. Additionally, an innovative multiplex immunoassay was introduced that was capable of simultaneously detecting multiple syphilis-specific antibodies in the patient’s serum, enhancing diagnostic accuracy and providing information about the stage of infection [89]. It also features a portable and rapid diagnostic device designed for use in resource-limited settings [90]. This device utilizes cutting-edge microfluidic technology to detect syphilis markers in blood samples within minutes, enabling immediate clinical decisions and reducing the time-to-treatment initiation. These advanced diagnostic tools have significant potential for on-the-spot testing in various healthcare settings, ultimately enabling early detection and immediate treatment initiation to contain the spread of syphilis [88,89,90].
Researchers are exploring various technologies to develop cutting-edge point-of-care tests for syphilis, including lateral flow assays, rapid immunochromatographic tests, loop-mediated isothermal amplification (LAMP), and nucleic acid amplification techniques. These technologies offer faster and more reliable results, making them suitable for use in diverse healthcare settings, including remote and resource-limited areas [91,92,93].
Moreover, the integration of multiple tests into a single platform, enabling simultaneous detection of different sexually transmitted infections, including syphilis, is being pursued. Multiplexed assays have the potential to enhance the efficiency of screening programs, reducing the burden on healthcare facilities and allowing for comprehensive testing in one simple procedure [94,95].
Additionally, future approaches in syphilis diagnostics are emphasizing user-friendliness and non-invasive sampling techniques. Saliva-based or urine-based point-of-care tests are being explored as alternatives to blood-based tests, making sample collection less invasive and more acceptable to individuals, particularly in high-risk populations. Integration of diagnostics with digital health solutions presents an exciting avenue for improving syphilis management [88,96]. Point-of-care tests equipped with connectivity features could allow for real-time data transmission to central databases, facilitating disease surveillance, monitoring treatment outcomes, and informing public health interventions [97].
5.3. Targeted Prevention Strategies
Targeted prevention strategies are vital components of syphilis control efforts, aiming to address the specific needs of high-risk populations and areas with elevated transmission rates. By focusing on key populations and tailoring interventions to their unique challenges, these strategies seek to optimize resource allocation and maximize the impact of preventive measures [98,99].
One of the primary targets of these prevention strategies is MSM and transgender individuals. These groups often face higher rates of syphilis transmission due to various factors, including social stigma, discrimination, and limited access to healthcare services. Tailored interventions for MSM and transgender individuals involve community engagement and peer-led initiatives to create safe spaces for discussions about sexual health and promote regular testing and treatment-seeking behavior [100].
In addition to MSM and transgender individuals, other high-risk populations, such as commercial sex workers, people who use drugs, and individuals with multiple sexual partners, also require focused prevention efforts. Partner notification and contact tracing are essential in these cases to identify undiagnosed cases and interrupt the transmission chain. Utilizing technology and community health workers can enhance the effectiveness of partner notification efforts and facilitate timely testing and treatment for those at risk [34,100].
Antenatal screening and prevention play a crucial role in preventing congenital syphilis, which occurs when syphilis is transmitted from an infected mother to her unborn child. Early detection and treatment during pregnancy are critical to protecting the health of both the mother and the baby. Integrating syphilis testing and treatment into existing antenatal care services ensures that pregnant women receive the necessary care and preventive measures [101].
Furthermore, community-based organizations and healthcare providers play a significant role in delivering targeted prevention interventions. These organizations are often well-positioned to reach at-risk populations, provide health education, and facilitate access to testing and treatment services [50,102,103]. Culturally sensitive and non-stigmatizing approaches are essential in building trust and promoting engagement with these communities [104,105].
Comprehensive health education and awareness campaigns are essential components of targeted prevention strategies [50]. By raising awareness about syphilis transmission, prevention methods, and the importance of regular testing, these campaigns empower the public to take charge of their sexual health [51]. Promoting open discussions about sexual health and reducing the stigma surrounding syphilis and other sexually transmitted infections can foster a more supportive environment for prevention behaviors [51,102].
5.4. Integration of Syphilis Services into Primary Healthcare
The integration of syphilis services into primary healthcare is a pivotal step in enhancing accessibility and delivery of syphilis prevention, diagnosis, and treatment. This integration offers comprehensive and timely care, leading to improved health outcomes and a reduction in syphilis transmission [106].
A significant advantage of this integration is the expanded reach to diverse populations. Primary healthcare facilities serve as the initial point of contact for individuals seeking medical care, regardless of their socioeconomic status or healthcare needs. By incorporating syphilis services into these facilities, individuals are more likely to access testing and treatment services, especially in underserved and remote regions where specialized clinics may be limited [107,108]
Moreover, integration enables a comprehensive approach to sexual health. Primary healthcare providers are well-positioned to address various health needs of their patients, including sexual health. By including syphilis services in routine health check-ups and screenings, primary healthcare providers can offer comprehensive care and encourage regular testing for sexually transmitted infections, including syphilis [108,109,110].
The integration of syphilis services also facilitates early detection and prompt treatment. Primary healthcare providers can identify syphilis cases during routine medical visits, enabling timely intervention and reducing the risk of complications. Additionally, integration promotes regular syphilis testing among high-risk populations, such as pregnant women, men who have sex with men, and individuals with multiple sexual partners, thereby enhancing the likelihood of early diagnosis and treatment [51,99,110,111,112,113].
Incorporating syphilis services into primary healthcare enhances continuity of care. Patients who test positive for syphilis can receive follow-up care, monitoring, and treatment from the same healthcare provider, ensuring they receive appropriate and consistent support throughout their healthcare journey [31,106].
Furthermore, integration contributes to destigmatization and normalizes discussions regarding sexual health. By providing syphilis services within primary healthcare settings, the stigma often associated with seeking specialized care for sexually transmitted infections is diminished. This normalization of sexual health conversations can encourage individuals to be more open about their sexual behaviors, enabling healthcare providers to offer tailored prevention strategies and support [34,114,115,116,117,118,119].
Training and capacity-building for primary healthcare providers are essential components of successful integration. Equipping healthcare professionals with the knowledge and skills to provide comprehensive syphilis services ensures accurate diagnoses, appropriate treatment, and effective prevention counseling. Additionally, incorporating syphilis services into medical education curricula can further promote awareness and expertise among future healthcare providers.
6. Future Directions
Advancing syphilis research and implementing innovative approaches are paramount in the global efforts to prevent and control syphilis. The comprehensive understanding of the immunological aspects of syphilis is opening new avenues for the development of effective vaccines, which could potentially revolutionize syphilis prevention strategies. These vaccines have the potential to provide long-lasting protection and significantly reduce the burden of syphilis worldwide.
Moreover, the development of innovative point-of-care diagnostics is transforming syphilis testing and diagnosis. Rapid and sensitive diagnostic tools that are easily accessible and deployable in resource-limited settings can lead to early detection and immediate treatment initiation, reducing transmission rates and preventing complications.
Targeted prevention strategies, tailored to specific at-risk populations, are crucial in curbing the spread of syphilis. By addressing individual risk factors and promoting behavioral changes, targeted interventions can effectively reduce syphilis incidence in vulnerable communities.
The integration of syphilis services into primary healthcare systems plays a vital role in expanding access to syphilis prevention and treatment services. By incorporating syphilis care into routine healthcare visits, we can destigmatize discussions around sexual health and reach diverse populations, ensuring that everyone has access to quality and timely care.
Looking ahead, a multi-faceted approach that combines immunological advancements, innovative diagnostics, targeted prevention strategies, and integration into primary healthcare settings can strengthen syphilis control efforts and contribute to achieving global health goals. Continued investment in research, capacity-building, and awareness campaigns will be key to sustaining progress in syphilis prevention and control.
In summary, addressing the challenges posed by syphilis requires a collaborative effort from governments, healthcare systems, researchers, and communities. By harnessing the potential of immunology, diagnostics, prevention strategies, and integration into primary healthcare, we can move towards a syphilis-free future, promoting better sexual health and well-being for all.
Author Contributions
Conceptualization, O.N.S. and J.O.F.; writing—original draft preparation, O.N.S.; writing—review and editing, J.O.F., O.N.S., A.L.E.M., C.R.M.I., E.A.C., R.M.Q., O.J.I., C.V.M., R.F.C., C.C.T. and M.J.S.B.; Figures and captions: C.V.M. and R.M.Q. visualization, O.J.I.; supervision, O.N.S. and J.O.F.; project administration, O.N.S. and J.O.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no relevant financial relationship or other conflict of interest to disclose pertaining to the subject matter.
References
- Marks, M.; Solomon, A.W.; Mabey, D.C. Endemic Treponemal Diseases. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 601–607. [Google Scholar] [CrossRef]
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018, 5, 24–38. [Google Scholar] [CrossRef]
- Tiecco, G.; Degli Antoni, M.; Storti, S.; Marchese, V.; Focà, E.; Torti, C.; Castelli, F.; Quiros-Roldan, E. A 2021 Update on Syphilis: Taking Stock from Pathogenesis to Vaccines. Pathogens 2021, 10, 1364. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Jin, Y.; Wu, B.; Wu, Y. Advancements in the Development of Nucleic Acid Vaccines for Syphilis Prevention and Control. Hum. Vaccin. Immunother. 2023, 19, 2234790. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Chen, X.-S.; Garcia, P.J. Syphilis. Lancet 2023, 402, 336–346. [Google Scholar] [CrossRef]
- Osias, E.; Hung, P.; Giacani, L.; Stafylis, C.; Konda, K.A.; Vargas, S.K.; Reyes-Díaz, E.M.; Comulada, W.S.; Haake, D.A.; Haynes, A.M.; et al. Investigation of Syphilis Immunology and Treponema Pallidum Subsp. Pallidum Biology to Improve Clinical Management and Design a Broadly Protective Vaccine: Study Protocol. BMC Infect. Dis. 2020, 20, 444. [Google Scholar] [CrossRef]
- Mues, N.; Chu, H.W. Out-Smarting the Host: Bacteria Maneuvering the Immune Response to Favor Their Survival. Front. Immunol. 2020, 11, 819. [Google Scholar] [CrossRef]
- Scott, B.N.V.; Sarkar, T.; Kratofil, R.M.; Kubes, P.; Thanabalasuriar, A. Unraveling the Host’s Immune Response to Infection: Seeing Is Believing. J. Leukoc. Biol. 2019, 106, 323–335. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Cohen, S.E.; Klausner, J.D.; Engelman, J.; Philip, S. Syphilis in the Modern Era. Infect. Dis. Clin. N. Am. 2013, 27, 705–722. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Primers 2017, 3, 17073. [Google Scholar] [CrossRef]
- Avelleira, J.C.R.; Bottino, G. Sífilis: Diagnóstico, Tratamento e Controle. An. Bras. Dermatol. 2006, 81, 111–126. [Google Scholar] [CrossRef]
- Singh, A.E.; Romanowski, B. Syphilis: Review with Emphasis on Clinical, Epidemiologic, and Some Biologic Features. Clin. Microbiol. Rev. 1999, 12, 187–209. [Google Scholar] [CrossRef]
- Tsimis, M.E.; Sheffield, J.S. Update on Syphilis and Pregnancy. Birth Defects Res. 2017, 109, 347–352. [Google Scholar] [CrossRef]
- Stoltey, J.E.; Cohen, S.E. Syphilis Transmission: A Review of the Current Evidence. Sex. Health 2015, 12, 103. [Google Scholar] [CrossRef]
- Bilman, V.; Bertoglio, L.; Melissano, G.; Chiesa, R. Contained Rupture of an Aortic Arch Aneurysm in a Patient with Syphilitic Aortitis. A Case Report. J. Vasc. Bras. 2021, 20, e20210160. [Google Scholar] [CrossRef]
- Cocora, M.; Nechifor, D.; Lazar, M.-A.; Mornos, A. Impending Aortic Rupture in a Patient with Syphilitic Aortitis. Vasc. Health Risk Manag. 2021, 17, 255–258. [Google Scholar] [CrossRef]
- Stamm, L.V. Syphilis: Re-Emergence of an Old Foe. Microb. Cell 2016, 3, 363–370. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; He, B.; Cao, T.; Zhou, X.; Ning, L.; Chen, E.; Li, Y.; Xie, X.; Peng, B.; et al. Investigation of the Immune Escape Mechanism of Treponema Pallidum. Infection 2023, 51, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Dabiri, G.; Cribier, B.; Sell, S. The Immunopathobiology of Syphilis: The Manifestations and Course of Syphilis Are Determined by the Level of Delayed-Type Hypersensitivity. Am. J. Dermatopathol. 2011, 33, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; La Vake, C.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PLoS Negl. Trop. Dis. 2012, 6, e1717. [Google Scholar] [CrossRef] [PubMed]
- Giacani, L.; Molini, B.J.; Kim, E.Y.; Godornes, B.C.; Leader, B.T.; Tantalo, L.C.; Centurion-Lara, A.; Lukehart, S.A. Antigenic Variation in Treponema Pallidum: TprK Sequence Diversity Accumulates in Response to Immune Pressure during Experimental Syphilis. J. Immunol. 2010, 184, 3822–3829. [Google Scholar] [CrossRef]
- Liu, D.; Tong, M.-L.; Lin, Y.; Liu, L.-L.; Lin, L.-R.; Yang, T.-C. Insights into the Genetic Variation Profile of TprK in Treponema Pallidum during the Development of Natural Human Syphilis Infection. PLoS Negl. Trop. Dis. 2019, 13, e0007621. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Tang, K.; Liu, R.; Li, J. An Updated Review of Recent Advances in Neurosyphilis. Front. Med. 2022, 9, 800383. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.E.; Okeke, N.L.; Hicks, C.B. Treatment of Syphilis A Systematic Review. JAMA 2014, 312, 1905. [Google Scholar] [CrossRef]
- Liu, H.; Han, Y.; Chen, X.; Bai, L.; Guo, S.; Li, L.; Wu, P.; Yin, Y. Comparison of Efficacy of Treatments for Early Syphilis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials and Observational Studies. PLoS ONE 2017, 12, e0180001. [Google Scholar] [CrossRef]
- LaFond, R.E.; Lukehart, S.A. Biological Basis for Syphilis. Clin. Microbiol. Rev. 2006, 19, 29–49. [Google Scholar] [CrossRef]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema Pallidum, the Syphilis Spirochete: Making a Living as a Stealth Pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef]
- Morshed, M.G.; Singh, A.E. Recent Trends in the Serologic Diagnosis of Syphilis. Clin. Vaccine Immunol. 2015, 22, 137–147. [Google Scholar] [CrossRef]
- Church, B.; Wall, E.; Webb, J.R.; Cameron, C.E. Interaction of Treponema Pallidum, the Syphilis Spirochete, with Human Platelets. PLoS ONE 2019, 14, e0210902. [Google Scholar] [CrossRef]
- Pereira Nogueira, W.; Figueiredo Nogueira, M.; de Almeida Nogueira, J.; Freire, M.E.M.; Gir, E.; de Oliveira e Silva, A.C. Syphilis in Riverine Communities: Prevalence and Associated Factors. Rev. Esc. Enferm. USP 2022, 56, e20210258. [Google Scholar] [CrossRef]
- Schmidt, R.; Carson, P.J.; Jansen, R.J. Resurgence of Syphilis in the United States: An Assessment of Contributing Factors. Infect. Dis. Res. Treat. 2019, 12, 117863371988328. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 187. [Google Scholar] [CrossRef] [PubMed]
- Copen, C.E.; Brookmeyer, K.A.; Haderxhanaj, L.T.; Hogben, M.; Torrone, E.A. Sexual Risk Behaviors Among Persons Diagnosed With Primary and Secondary Syphilis Who Reported High-Risk Substance Use: Data from the National Notifiable Diseases Surveillance System, 2018. Sex. Transm. Dis. 2022, 49, 99–104. [Google Scholar] [CrossRef]
- Ye, X.; Li, F.-R.; Pan, Q.; Li, Z.; Yu, G.-Q.; Liu, H.; Liu, J.; Huai, P.-C.; Zhang, F.-R. Prevalence and Associated Factors of Sexually Transmitted Infections among Methamphetamine Users in Eastern China: A Cross-Sectional Study. BMC Infect. Dis. 2022, 22, 7. [Google Scholar] [CrossRef]
- Jennings, J.M.; Wagner, J.; Tilchin, C.; Schumacher, C.M.; Thornton, N.; Hamill, M.M.; Rompalo, A.; Ruhs, S.; Rives, S.; Ghanem, K.G.; et al. Methamphetamine Use, Syphilis, and Specific Online Sex Partner Meeting Venues Are Associated with HIV Status among Urban Black Gay and Bisexual Men Who Have Sex Men. Sex. Transm. Dis. 2021, 48, S32–S39. [Google Scholar] [CrossRef] [PubMed]
- da Costa Dantas, J.; Marinho, C.D.S.R.; Pinheiro, Y.T.; Fernandes Ferreira, M.Â.; Rosendo da Silva, R.A. Spatial Distribution of Gestational Syphilis in Brazil: Socioeconomic and Health Services Inequalities. Am. J. Trop. Med. Hyg. 2023, 109, 42–49. [Google Scholar] [CrossRef]
- Ramos, R.D.S.P.D.S.; Carneiro, G.R.; Oliveira, A.L.S.D.; Cunha, T.N.D.; Ramos, V.P. Incidence of Congenital Syphilis According to Inequalities and Living Conditions in the City of Recife, Pernambuco, Brazil. Rev. Bras. Saúde Matern. Infant. 2021, 21, 785–794. [Google Scholar] [CrossRef]
- Smock, L.; Caten, E.; Hsu, K.; DeMaria, A. Economic Disparities and Syphilis Incidence in Massachusetts, 2001–2013. Public Health Rep. 2017, 132, 309–315. [Google Scholar] [CrossRef]
- Marques dos Santos, M.; Lopes, A.K.B.; Roncalli, A.G.; Lima, K.C.D. Trends of Syphilis in Brazil: A Growth Portrait of the Treponemic Epidemic. PLoS ONE 2020, 15, e0231029. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Dashwood, T.; Walmsley, S. The Intersection of HIV and Syphilis: Update on the Key Considerations in Testing and Management. Curr. HIV/AIDS Rep. 2021, 18, 280–288. [Google Scholar] [CrossRef]
- Ribeiro, A.; Trevizol, A.; Oluwoye, O.; McPherson, S.; McDonell, M.G.; Briese, V.; Miguel, A.C.; Fratzinger, R.C.; Laranjeira, R.R.; Alonso, A.L.; et al. HIV and Syphilis Infections and Associated Factors among Patients in Treatment at a Specialist Alcohol, Tobacco, and Drugs Center in São Paulo’s “Cracolândia”. Trends Psychiatry Psychother. 2020, 42, 1–6. [Google Scholar] [CrossRef]
- Simões, L.A.; Mendes, J.C.; Silveira, M.R.; Costa, A.M.G.D.; Lula, M.D.; Ceccato, M.D.G.B. Fatores Associados à Coinfecção HIV/Sífilis No Início Da Terapia Antirretroviral. Rev. Saude Publica 2022, 56, 59. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Izzo, I.; Carriero, C.; Antoni, M.D.; Storti, S.; Tiecco, G.; Gardini, G.; Focà, E.; Castelli, F. Decrease in New Diagnosis of HIV/AIDS in the Two Years Period 2019-2020: Impact of COVID-19 Pandemic. J. Public health Res. 2022, 11, jphr.2021.2256. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Pellowski, J.; Turner, C. Prevalence of Sexually Transmitted Co-Infections in People Living with HIV/AIDS: Systematic Review with Implications for Using HIV Treatments for Prevention. Sex. Transm. Infect. 2011, 87, 183–190. [Google Scholar] [CrossRef]
- Schillinger, D. Social Determinants, Health Literacy, and Disparities: Intersections and Controversies. HLRP Health Lit. Res. Pract. 2021, 5, e234–e243. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Granger, J.; Espadafor López, B.; Cobo, F.; Blasco Morente, G.; Sampedro Martinez, A.; Tercedor Sánchez, J.; Aliaga-Martinez, L.; Padilla-Malo de Molina, A.; Navarro-Marí, J.M. Update on the Diagnosis of Sexually Transmitted Infections. Actas Dermo-Sifiliográficas (Engl. Ed.) 2020, 111, 711–724. [Google Scholar] [CrossRef]
- Caitano, A.R.; Gusmão, C.M.G.; Dias-Trindade, S.; Barbalho, I.M.P.; Morais, P.S.G.; Caldeira-Silva, G.J.P.; Romão, M.H.; Valentim, J.L.R.S.; Dias, A.P.; Alcoforado, J.L.M.; et al. Massive Health Education through Technological Mediation: Analyses and Impacts on the Syphilis Epidemic in Brazil. Front. Public Health 2022, 10, 944213. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, V.C.D.; Lira, P.I.C.D.; Frias, P.G.D.; Romaguera, L.M.D.; Caires, S.D.F.F.; Ximenes, R.A.D.A. Risk Factors for Syphilis in Women: Case-Control Study. Rev. Saude Publica 2017, 51, 78. [Google Scholar] [CrossRef]
- Solaimalai, D.; Gupta, A.; George, L.; Manesh, A.; Karthik, R.; Sathishkumar, D.; Peter, C.V.D.; Varghese, G.M.; Pulimood, S.A.; Kannangai, R.; et al. Upward Trends of Syphilis in the Non-Pregnant Adults: A Six-Year Report on Clinical and Epidemiological Profile of Syphilis from a Tertiary Care Center, India. Front. Public Health 2022, 10, 908591. [Google Scholar] [CrossRef]
- Varshney, K.; Ikanovic, A.; Ghosh, P.; Shet, P.; Di Sipio, M.; Khatri, C.; Mahmood, M.Q. A Global Scoping Review of the Factors Associated with HIV and Syphilis Co-Infection: Findings from 40 Countries. Venereology 2022, 1, 98–113. [Google Scholar] [CrossRef]
- Peterman, T.A.; Furness, B.W. Public Health Interventions to Control Syphilis. Sex. Health 2015, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, Y.; Xiao, Y. Laboratory Diagnostic Tools for Syphilis: Current Status and Future Prospects. Front. Cell. Infect. Microbiol. 2021, 10, 574806. [Google Scholar] [CrossRef] [PubMed]
- Wolgemuth, C.W. Flagellar Motility of the Pathogenic Spirochetes. Semin. Cell Dev. Biol. 2015, 46, 104–112. [Google Scholar] [CrossRef]
- Forrestel, A.K.; Kovarik, C.L.; Katz, K.A. Sexually Acquired Syphilis. J. Am. Acad. Dermatol. 2020, 82, 17–28. [Google Scholar] [CrossRef]
- Hook, E.W.; Roddy, R.E.; Lukehart, S.A.; Hom, J.; Holmes, K.K.; Tam, M.R. Detection of Treponema Pallidum in Lesion Exudate with a Pathogen-Specific Monoclonal Antibody. J. Clin. Microbiol. 1985, 22, 241–244. [Google Scholar] [CrossRef]
- Ito, F.; Hunter, E.F.; George, R.W.; Pope, V.; Larsen, S.A. Specific Immunofluorescent Staining of Pathogenic Treponemes with a Monoclonal Antibody. J. Clin. Microbiol. 1992, 30, 831–838. [Google Scholar] [CrossRef]
- Morshed, M.G. Current Trend on Syphilis Diagnosis: Issues and Challenges. In Infectious Diseases and Nanomedicine II; Advances in Experimental Medicine and Biology; Springer: New Delhi, India, 2014; pp. 51–64. [Google Scholar]
- Mallma, P.; Garcia, P.; Carcamo, C.; Torres-Rueda, S.; Peeling, R.; Mabey, D.; Terris-Prestholt, F. Rapid Syphilis Testing Is Cost-Effective Even in Low-Prevalence Settings: The CISNE-PERU Experience. PLoS ONE 2016, 11, e0149568. [Google Scholar] [CrossRef]
- García Luna, J.A.; Romero-Rosas, N.; Silva Pena, S.A.; Oviedo Sarmiento, O.J.; Galindo Orrego, X.; Lenis Quintero, W.; Perea, L.C.; Buitrago, E.M.; Osorio, L.; Salazar, J.C.; et al. Smith Diagnostic performance of two rapid tests for syphilis screening in people living with HIV in Cali, Colombia. PLoS ONE 2023, 18, e0282492. [Google Scholar] [CrossRef]
- Gao, K.; Shen, X.; Lin, Y.; Zhu, X.-Z.; Lin, L.-R.; Tong, M.-L.; Xiao, Y.; Zhang, H.-L.; Liang, X.-M.; Niu, J.-J.; et al. Origin of Nontreponemal Antibodies During Treponema Pallidum Infection: Evidence From a Rabbit Model. J. Infect. Dis. 2018, 218, 835–843. [Google Scholar] [CrossRef]
- Leroy, A.-G.; Robert, M.; Carpentier, M.; Bastidon, C.; Gautreau, B.; Lefebvre, M.; Bonnet, B.; Bernier, C.; Corvec, S.; Guillouzouic, A. Assessment of a Fully Automated RPR Assay (Mediace RPR) for Serological Diagnosis and Follow-up of Syphilis: A Retrospective Study. Diagn. Microbiol. Infect. Dis. 2022, 104, 115767. [Google Scholar] [CrossRef]
- Osbak, K.; Abdellati, S.; Tsoumanis, A.; Van Esbroeck, M.; Kestens, L.; Crucitti, T.; Kenyon, C. Evaluation of an Automated Quantitative Latex Immunoturbidimetric Non-Treponemal Assay for Diagnosis and Follow-up of Syphilis: A Prospective Cohort Study. J. Med. Microbiol. 2017, 66, 1130–1139. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W.; Sun, C.; Yue, X.; Zheng, M.; Fu, G.; Gong, X. Prevalence of Syphilis among People Living with HIV and Its Implication for Enhanced Coinfection Monitoring and Management in China: A Meta-Analysis. Front. Public Health 2022, 10, 1002342. [Google Scholar] [CrossRef]
- Marchese, V.; Tiecco, G.; Storti, S.; Degli Antoni, M.; Calza, S.; Gulletta, M.; Viola, F.; Focà, E.; Matteelli, A.; Castelli, F.; et al. Syphilis Infections, Reinfections and Serological Response in a Large Italian Sexually Transmitted Disease Centre: A Monocentric Retrospective Study. J. Clin. Med. 2022, 11, 7499. [Google Scholar] [CrossRef]
- Sena, A.C.; Wolff, M.; Martin, D.H.; Behets, F.; Van Damme, K.; Leone, P.; Langley, C.; McNeil, L.; Hook, E.W. Predictors of Serological Cure and Serofast State After Treatment in HIV-Negative Persons With Early Syphilis. Clin. Infect. Dis. 2011, 53, 1092–1099. [Google Scholar] [CrossRef]
- Ramos, A.N., Jr. Persistence of Syphilis as a Challenge for the Brazilian Public Health: The Solution Is to Strengthen SUS in Defense of Democracy and Life. Cad. Saúde Pública 2022, 38, PT069022. [Google Scholar] [CrossRef]
- Shukla, M.R.; Pereira, L.; Gaynor, A.M.; Sun, Y.; Edwards, D.; Simmons, T.; Andrews, C.W.; Park, I.U.; Hong, J.; Cao, W.; et al. Evaluation of Three Automated Nontreponemal Rapid Plasma Reagin (RPR) Tests for the Laboratory Diagnosis of Syphilis. J. Clin. Microbiol. 2023, 61, e00168-23. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Douglas, J.M. Penicillin Treatment of Syphilis. JAMA 2009, 301, 769. [Google Scholar] [CrossRef] [PubMed]
- Janier, M.; Unemo, M.; Dupin, N.; Tiplica, G.S.; Potočnik, M.; Patel, R. 2020 European Guideline on the Management of Syphilis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, B.; Moyers, A.; Hinkle, T.; Kessler, R.; Russell, N.G. 2021 CDC Update: Treatment and Complications of Sexually Transmitted Infections (STIs). Venereology 2022, 1, 23–46. [Google Scholar] [CrossRef]
- Katanami, Y.; Hashimoto, T.; Takaya, S.; Yamamoto, K.; Kutsuna, S.; Takeshita, N.; Hayakawa, K.; Kanagawa, S.; Ohmagari, N. Amoxicillin and Ceftriaxone as Treatment Alternatives to Penicillin for Maternal Syphilis. Emerg. Infect. Dis. 2017, 23, 827–829. [Google Scholar] [CrossRef]
- Bolan, R.K.; Beymer, M.R.; Weiss, R.E.; Flynn, R.P.; Leibowitz, A.A.; Klausner, J.D. Doxycycline Prophylaxis to Reduce Incident Syphilis among HIV-Infected Men Who Have Sex With Men Who Continue to Engage in High-Risk Sex. Sex. Transm. Dis. 2015, 42, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fan, Y.; Chen, J.; Yang, J.; Gao, L.; Wu, X.; Xu, X.; Zhang, Y.; Yue, P.; Cao, W.; et al. Efficacy and Safety of Treatments for Different Stages of Syphilis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials and Observational Studies. Microbiol. Spectr. 2022, 10, e02977-22. [Google Scholar] [CrossRef]
- Hamill, M.M.; Onzia, A.; Wang, T.-H.; Kiragga, A.N.; Hsieh, Y.-H.; Parkes-Ratanshi, R.; Gough, E.; Kyambadde, P.; Melendez, J.H.; Manabe, Y.C. High Burden of Untreated Syphilis, Drug Resistant Neisseria Gonorrhoeae, and Other Sexually Transmitted Infections in Men with Urethral Discharge Syndrome in Kampala, Uganda. BMC Infect. Dis. 2022, 22, 440. [Google Scholar] [CrossRef]
- Grant, J.S.; Stafylis, C.; Celum, C.; Grennan, T.; Haire, B.; Kaldor, J.; Luetkemeyer, A.F.; Saunders, J.M.; Molina, J.-M.; Klausner, J.D. Doxycycline Prophylaxis for Bacterial Sexually Transmitted Infections. Clin. Infect. Dis. 2020, 70, 1247–1253. [Google Scholar] [CrossRef]
- Stamm, L.V. Hope for New Antibiotics for Syphilis Treatment. EBioMedicine 2021, 66, 103320. [Google Scholar] [CrossRef]
- Haynes, A.M.; Giacani, L.; Mayans, M.V.; Ubals, M.; Nieto, C.; Pérez-Mañá, C.; Quintó, L.; Romeis, E.; Mitjà, O. Efficacy of Linezolid on Treponema Pallidum, the Syphilis Agent: A Preclinical Study. EBioMedicine 2021, 65, 103281. [Google Scholar] [CrossRef]
- Katz, K.A.; Klausner, J.D. Azithromycin Resistance in Treponema Pallidum. Curr. Opin. Infect. Dis. 2008, 21, 83–91. [Google Scholar] [CrossRef]
- Chen, X.-S.; Yin, Y.-P.; Wei, W.-H.; Wang, H.-C.; Peng, R.-R.; Zheng, H.-P.; Zhang, J.-P.; Zhu, B.-Y.; Liu, Q.-Z.; Huang, S.-J. High Prevalence of Azithromycin Resistance to Treponema Pallidum in Geographically Different Areas in China. Clin. Microbiol. Infect. 2013, 19, 975–979. [Google Scholar] [CrossRef]
- Orbe-Orihuela, Y.C.; Sánchez-Alemán, M.Á.; Hernández-Pliego, A.; Medina-García, C.V.; Vergara-Ortega, D.N. Syphilis as Re-Emerging Disease, Antibiotic Resistance, and Vulnerable Population: Global Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1546. [Google Scholar] [CrossRef]
- Cameron, C.E.; Lukehart, S.A. Current Status of Syphilis Vaccine Development: Need, Challenges, Prospects. Vaccine 2014, 32, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N. Immunity in Experimental Syphilis. J. Immunol. 1973, 110, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.S.; Dhama, K.; Thanigaivel, S.; Chakraborty, S.; Anbarasu, K.; Dey, N.; Karunakaran, R. Strategies for Successful Designing of Immunocontraceptive Vaccines and Recent Updates in Vaccine Development against Sexually Transmitted Infections—A Review. Saudi J. Biol. Sci. 2022, 29, 2033–2046. [Google Scholar] [CrossRef]
- Ávila-Nieto, C.; Pedreño-López, N.; Mitjà, O.; Clotet, B.; Blanco, J.; Carrillo, J. Syphilis Vaccine: Challenges, Controversies and Opportunities. Front. Immunol. 2023, 14, 1126170. [Google Scholar] [CrossRef]
- Xu, M.; Xie, Y.; Zheng, K.; Luo, H.; Tan, M.; Zhao, F.; Zeng, T.; Wu, Y. Two Potential Syphilis Vaccine Candidates Inhibit Dissemination of Treponema Pallidum. Front. Immunol. 2021, 12, 759474. [Google Scholar] [CrossRef]
- Cameron, C.E. Syphilis Vaccine Development: Requirements, Challenges, and Opportunities. Sex. Transm. Dis. 2018, 45, S17–S19. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Konda, K.A.; Klausner, J.D. Notes on Syphilis Vaccine Development. Front. Immunol. 2022, 13, 952284. [Google Scholar] [CrossRef]
- Cullen, P.A.; Cameron, C.E. Progress towards an Effective Syphilis Vaccine: The Past, Present and Future. Expert Rev. Vaccines 2006, 5, 67–80. [Google Scholar] [CrossRef]
- Ambrose, C.T. Vaccines and the Looming Threat of Syphilis. Glob. Vaccines Immunol. 2015, 1, 8–13. [Google Scholar] [CrossRef][Green Version]
- Giacani, L. Strategies for Syphilis Vaccine Development. J. Bras. Doenças Sex. Transm. 2022, 34, e22341249. [Google Scholar] [CrossRef]
- Pham, M.D.; Ong, J.J.; Anderson, D.A.; Drummer, H.E.; Stoové, M. Point-of-Care Diagnostics for Diagnosis of Active Syphilis Infection: Needs, Challenges and the Way Forward. Int. J. Environ. Res. Public Health 2022, 19, 8172. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.C.; Sollis, K.A.; Kelly, H.A.; Benzaken, A.S.; Bitarakwate, E.; Changalucha, J.; Chen, X.-S.; Yin, Y.-P.; Garcia, P.J.; Strasser, S.; et al. Point-of-Care Tests to Strengthen Health Systems and Save Newborn Lives: The Case of Syphilis. PLoS Med. 2012, 9, e1001233. [Google Scholar] [CrossRef]
- Causer, L.M.; Kaldor, J.M.; Conway, D.P.; Leslie, D.E.; Denham, I.; Karapanagiotidis, T.; Ryan, C.; Wand, H.; Anderson, D.A.; Robertson, P.W.; et al. An Evaluation of a Novel Dual Treponemal/Nontreponemal Point-of-Care Test for Syphilis as a Tool to Distinguish Active From Past Treated Infection. Clin. Infect. Dis. 2015, 61, 184–191. [Google Scholar] [CrossRef]
- Caya, C.; Maheu-Giroux, M.; Xia, Y.; Serhir, B.; Morin, V.; Libman, M.; Corsini, R.; Goldfarb, D.M.; Wong, T.; Singh, A.E.; et al. Stopping Syphilis Transmission in Arctic Communities through Rapid Diagnostic Testing: The STAR Study Protocol. PLoS ONE 2022, 17, e0273713. [Google Scholar] [CrossRef]
- Toskin, I.; Blondeel, K.; Peeling, R.W.; Deal, C.; Kiarie, J. Advancing Point of Care Diagnostics for the Control and Prevention of STIs: The Way Forward. Sex. Transm. Infect. 2017, 93, S81–S88. [Google Scholar] [CrossRef]
- Basing, L.A.W.; Simpson, S.V.; Adu-Sarkodie, Y.; Linnes, J.C. A Loop-Mediated Isothermal Amplification Assay for the Detection of Treponema Pallidum Subsp. Pertenue. Am. J. Trop. Med. Hyg. 2020, 103, 253–259. [Google Scholar] [CrossRef]
- Tharakan, S.; Faqah, O.; Asghar, W.; Ilyas, A. Microfluidic Devices for HIV Diagnosis and Monitoring at Point-of-Care (POC) Settings. Biosensors 2022, 12, 949. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Kaur, B.; Chorti, P. From Point-of-Care Testing to EHealth Diagnostic Devices (EDiagnostics). ACS Cent. Sci. 2018, 4, 1600–1616. [Google Scholar] [CrossRef]
- Naeem, F.; Karellis, A.; Nair, S.; Routy, J.-P.; Yansouni, C.P.; Kim, J.; Pai, N. Multiplexed Technologies for Sexually Transmitted Infections: Global Evidence on Patient-Centered and Clinical Health Outcomes. BMJ Glob. Health 2021, 6, e005670. [Google Scholar] [CrossRef]
- Karellis, A.; Naeem, F.; Nair, S.; Mallya, S.D.; Routy, J.-P.; Gahagan, J.; Yansouni, C.P.; Kim, J.; Pai, N.P. Multiplexed Rapid Technologies for Sexually Transmitted Infections: A Systematic Review. Lancet Microbe 2022, 3, e303–e315. [Google Scholar] [CrossRef]
- Osbak, K.K.; Van Raemdonck, G.A.; Dom, M.; Cameron, C.E.; Meehan, C.J.; Deforce, D.; Ostade, X.V.; Kenyon, C.R.; Dhaenens, M. Candidate Treponema Pallidum Biomarkers Uncovered in Urine from Individuals with Syphilis Using Mass Spectrometry. Future Microbiol. 2018, 13, 1497–1510. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.A.; Bolan, G.A. Syphilis Elimination: Lessons Learned Again. Sex. Transm. Dis. 2018, 45, S80–S85. [Google Scholar] [CrossRef] [PubMed]
- Peterman, T.A.; Cha, S. Context-Appropriate Interventions to Prevent Syphilis: A Narrative Review. Sex. Transm. Dis. 2018, 45, S65–S71. [Google Scholar] [CrossRef] [PubMed]
- Copen, C.E.; Rushmore, J.; De Voux, A.; Kirkcaldy, R.D.; Fakile, Y.F.; Tilchin, C.; Duchen, J.; Jennings, J.M.; Spahnie, M.; Norris Turner, A.; et al. Factors Associated With Syphilis Transmission and Acquisition Among Men Who Have Sex With Men: Protocol for a Multisite Egocentric Network Study. JMIR Res. Protoc. 2022, 11, e40095. [Google Scholar] [CrossRef] [PubMed]
- Welch, J. Antenatal Screening for Syphilis. BMJ 1998, 317, 1605–1606. [Google Scholar] [CrossRef][Green Version]
- Paiva, J.C.D.L.; Dias-Trindade, S.; Gonzalez, M.O.A.; Barros, D.M.D.S.; Cardoso, P.H.; Bezerra, P.H.C.; Lima, T.G.F.D.M.S.; Lacerda, J.D.S.; Muneiro, L.C.; Cunha-Oliveira, A.; et al. Analysis of the Impact of Communication Campaigns under the Project “Syphilis No”: A National Tool for Inducing and Promoting Health. Int. J. Environ. Res. Public Health 2022, 19, 15884. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Dang, A.K.; Vu, G.T.; Nguyen, C.T.; Le, T.H.T.; Truong, N.T.; Hoang, C.L.; Tran, T.T.; Tran, T.H.; Pham, H.Q.; et al. Lack of Knowledge about Sexually Transmitted Diseases (STDs): Implications for STDs Prevention and Care among Dermatology Patients in an Urban City in Vietnam. Int. J. Environ. Res. Public Health 2019, 16, 1080. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.A.; Delgado, L.F.; Haderxhanaj, L.T.; Hogben, M. Improving Sexual Health in U.S. Rural Communities: Reducing the Impact of Stigma. AIDS Behav. 2022, 26, 90–99. [Google Scholar] [CrossRef]
- Grieb, S.M.; Jackman, K.-M.; Jennings, J.M. Recommendations From Black Sexual Minority Men: Building Trust to Improve Engagement and Impact of HIV/STI Research. Health Promot. Pract. 2021, 22, 395–403. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Saes, M.; Duro, S.M.S.; de Souza Gonçalves, C.; Tomasi, E.; Facchini, L.A. Assessment of the Appropriate Management of Syphilis Patients in Primary Health Care in Different Regions of Brazil from 2012 to 2018. Cad. Saúde Pública 2022, 38, EN231921. [Google Scholar] [CrossRef]
- Santos, M.M.D.; Rosendo, T.M.S.D.S.; Lopes, A.K.B.; Roncalli, A.G.; Lima, K.C.D. Weaknesses in Primary Health Care Favor the Growth of Acquired Syphilis. PLoS Negl. Trop. Dis. 2021, 15, e0009085. [Google Scholar] [CrossRef]
- van Weel, C.; Kidd, M.R. Why Strengthening Primary Health Care Is Essential to Achieving Universal Health Coverage. Can. Med. Assoc. J. 2018, 190, E463–E466. [Google Scholar] [CrossRef]
- McCormack, H.; Guy, R.; Bourne, C.; Newman, C.E. Integrating Testing for Sexually Transmissible Infections into Routine Primary Care for Aboriginal Young People: A Strengths-based Qualitative Analysis. Aust. N. Z. J. Public Health 2022, 46, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.L.d.L.; Guimarães, D.C.d.S.; Sarkis, D.J.; Gabriel, T.T.; Delgado, C.S.; Campos, A.A.L.; Nogueira, M.C.; Ribeiro, L.C. Factors Associated with Women Diagnosed with Syphilis Who Received Prenatal Care in a Primary Healthcare Unit. Einstein (São Paulo) 2023, 31, eAO0046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).