Abstract
School-age youth constitute a neglected but highly vulnerable group concerning sexual health risks in low-resource countries. Robust evidence concerning the research landscape of school-based interventions on human papillomavirus in Africa is currently lacking. Therefore, this systematic scoping review (SSR) aims to map evidence about school-based HPV interventions and identify emerging themes, gaps and lessons learned in Africa. This review was guided by Joanna Brigg’s Institute’s guidelines for SSRs and reported based on the Preferred Reporting Items for Systematic Reviews and Metanalysis for Scoping Reviews. Five databases—PubMed, CINAHL, PsycINFO, SCOPUS, and Allied and Complementary Medicine—were searched for relevant literature using appropriate search terms and Boolean operators. The retrieved literature was extracted, deduplicated and screened using the Rayyan software. Only those articles which met the eligibility criteria were included for data charting, collation, and summarization. Ten articles were included in this review. The articles demonstrate that a life-course approach is significant in health intervention. School-based health interventions help reach adolescents in a dynamic life stage, affecting their vulnerability to sexual health risks. The school-based interventions serve as an ideal platform to offer HPV peer education, improving their HPV knowledge and subsequent testing services and enhancing their acceptability for screening and vaccination. Cervical cancer education and screening can be effectively combined in HPV health services for women. While the studies are geographically diverse, such effective interventions, which help reduce bottlenecks in accessing HPV screening and vaccination, are very few in Africa. In conclusion, school-based intervention is a viable strategy that can be adopted for adolescent protection from HPV-induced diseases. However, the current evidence on the impact of these interventions, particularly HPV vaccination, is inadequate.
1. Introduction
The burden of human papillomavirus (HPV) and HPV-related diseases, particularly cervical cancer, are still very high in Africa [1,2]. Unlike HIV/AIDS, the burden is still largely unrecognized in several African countries. HPV is diagnosed in more than 90% of cervical cancers, which are the most common cause of cancer death among women in Africa [2]. Overall, HPV infection and related diseases are more prevalent in developing countries with minimal resources to tackle them. For instance, Africa is characterized by low access to health services and cancer care in particular [3]. Unfortunately, it is projected that cervical cancer incidence and mortality rates will continue to rise due to limited access to prevention services and the concomitant HIV/AIDS epidemic [2]. Without prevention programs through education, vaccination, and early detection, many people might develop related cancers requiring invasive surgical therapies, which might not be available in many African communities [4]. HPV and related diseases could also constitute high costs to the healthcare system.
The defining strategies to reduce the excessive burden of HPV-related diseases include age-appropriate prophylactic HPV vaccination, cervical cancer prevention services for young women, control of HIV/AIDS and health promotion strategies [1]. HPV vaccine uptake is consistently low in many regions due to some misconceptions and low knowledge of its related complications. For instance, Bednarczyk [5] found that many people could not connect the vaccine to cervical cancer prevention, while others doubted the efficacy and safety of the vaccine. While there has been an increase in the introduction of HPV vaccines in African countries, there are challenges with the demand-side and end-user perspectives of HPV vaccination [6]. A study further reported that the knowledge of HPV vaccination and cervical cancer is intertwined with misinformation and fear in sub-Saharan Africa (SSA) [6]. Most African countries face HPV vaccine implementation barriers because vaccine dose completion remains as low as 20% [7]. There is declining public demand for the HPV vaccine in many African countries due to weak social mobilization, low HPV knowledge and vaccine hesitancy [8]. Therefore, understanding HPV vaccination implementation bottlenecks and enablement in real-world settings is critical. While preventive programs, including vaccination, might be effective, there are also challenges of where and how to best deliver such services.
One of the significant concerns about HPV is HIV co-infections among young African women responsible for the early development of invasive cervical cancer [8]. One of the documented strategies is school-based HPV programs targeting adolescents. For instance, South Africa began the school-based HPV vaccination program in 2014. The school-based approach yielded positive results, with 61% of adolescent girls aged 15 receiving the full recommended two-dose schedule. One of the documented strategies is school-based HPV programs targeting adolescents. School-aged adolescents constitute important categories in preventive healthcare. The adolescents operate in a peculiar context at home and school, are exposed to peer pressure and are often curious about their bodies. The world health organization noted that schools are critical for knowledge acquisition and the development of socioemotional, self-regulation and resilience required for a healthy future [9]. However, school-age youth constitute a neglected but highly vulnerable group concerning sexual health risks in low-resource countries. Robust evidence concerning the research landscape of school-based interventions on human papillomavirus in Africa is currently lacking. Therefore, this systematic scoping review (SSR) aims to map evidence about school-based HPV interventions and identify emerging themes, gaps and lessons learned in Africa.
2. Methods
2.1. Study Design
This research adopted an SSR design that seeks to map the existing evidence about school-based HPV interventions in Africa to identify the emerging areas and gaps concerning school-based HPV interventions in Africa.
The procedures and documentation of this review were informed by the Joanna Brigg’s Institute’s guidelines for SSRs and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist [10,11].
2.2. Protocol Registration
This research protocol was not registered with the International Prospective Register of Systematic Reviews (PROSPERO) because PROSPERO does not accept scoping and bibliometric review protocols. Additionally, protocol registration is not a norm in the reporting and methodology of these two review types [12,13,14].
2.3. Research Question
This review seeks to answer this research question: “What are the available evidence and knowledge gaps concerning school-based HPV interventions/programs in Africa?”
2.4. Eligibility Criteria
The following criteria were used to determine the eligibility of a publication for inclusion in this review:
- Refereed original research articles which reported the implementation of a school-based HPV intervention/program.
- Cohort studies, case-control studies, before and after studies, experimental studies, and controlled trials.
- Articles published in the English language.
- Articles with accessible full text.
- Studies conducted in Africa.
Additionally, those publications with the characteristics below were excluded:
- ○
- Non-refereed articles.
- ○
- Non-articles such as dissertations, books, book chapters, bibliometric reviews, scoping reviews, systematic reviews and meta-analyses, editorials, letters, commentaries, opinions, etc.
- ○
- Articles published in Spanish, French, Arabic, Italian, or any other non-English language.
- ○
- Articles reporting school-based cross-sectional studies on HPV.
- ○
- Articles without accessible full text.
- ○
- Articles reporting clinic- or community-based HPV intervention/programs in Africa.
- ○
- Studies conducted among population groups in American, Asian, European, and Australian countries.
2.5. Search Strategy
Between 18 and 20 July 2022, two researchers searched the PubMed, SCOPUS, Allied and Complementary Medicine [AMED] (EBSCO interface), CINAHL (EBSCO interface), and PsycINFO (EBSCO interface) for literature on school-based HPV interventions in Africa, without year limiters, using appropriate search terms and Boolean operators (Table A1). The choice of the search terms used were informed by PICOS (Population, Intervention, Comparison, Outcome and Study Type).
2.6. Deduplication
The literature obtained from the database search were retrieved and imported into the Rayyan software for deduplication [15]. All duplicates were removed.
2.7. Selection of Studies
After deduplication, the remaining literature was then screened to identify those studies eligible for inclusion in the review. The screening process was two-staged, conducted by two independent researchers, and based on the study selection criteria (K.K.K. and E.A.E.). The first stage involved the screening of all literature titles and abstracts—and, at this stage, obviously ineligible publications were excluded (see Figure 1). The potentially eligible publications were retained and subjected to the second-stage screening; at this stage, the full texts of the articles were screened for eligibility. Only those articles meeting the eligibility criteria were selected for this review.
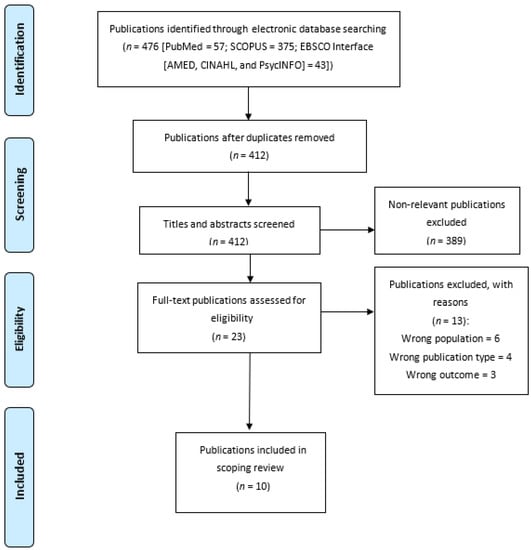
Figure 1.
Flow chart diagram.
2.8. Quality Appraisal
Unlike systematic reviews, the appraisal of the quality of the included articles is not needed in bibliometric and scoping reviews.
2.9. Data Extraction, Collation and Summarization
Two researchers were involved in the data extraction process. The data obtained were citation data (authors’ names and year of publication), study design, country of study, study population, sample size, study objectives, intervention types, findings, and conclusions. The extracted data were thereafter collated and summarized into themes.
3. Results
A synopsis of the ten publications selected for the scoping review is displayed in Table 1. Out of these publications, four were conducted in South Africa, two in Nigeria, and one each in Kenya, Ethiopia and Tanzania. The last one was the only study that focused on two settings, Rwanda and Bhutan. The findings are grouped into six major themes: awareness and knowledge of HPV and CC, determinants of awareness and knowledge, the impact of school-based interventions on health behavior, determinants of post-intervention health behavior, the efficacy of HPV vaccines, and social burdens associated with CC screening and HPV vaccine uptake.

Table 1.
Synopsis of the Reviewed Publications.
3.1. Awareness and Knowledge of Human Papillomavirus and Cervical Cancer
3.1.1. Extent of Knowledge Pre-Intervention
Before the intervention, results obtained from the scoping review are indicative of low awareness of Human Papillomavirus (HPV) and its vaccination [18]. Likewise, understanding of HPV, cancers caused by HPV, its mode of transmission, the HPV vaccine [18,20], that boys could use the HPV vaccine, the sexual infectivity nature of HPV and diseases it causes was inadequate among Africans [20]. Similarly, awareness of cervical cancer (CC) [20] and its prevalence, awareness of the risk factors as well as the broad categories of cervical cancer and HPV was also low in Africa [19,20].
At baseline, sufficient knowledge about CC could not be established, as evidenced by the low overall knowledge score obtained by the majority of the participants [18,19]. In specific terms, knowledge of the various dimensions of cervical cancer such as its symptoms [16,20]. screening [20] and prevention including vaccination was poor prior to educational interventions [16]. Furthermore, an understanding of the causative organism, risk factors [18,19], at-risk populations, and treatment of CC was lacking [19]. Conversely, good knowledge of CC prevention was shown in one of the studies reviewed where adequate knowledge of CC prevention was reported for a group of participants (seminar cohort) but found to be insufficient in another group (school cohort) in the same study. This low knowledge trend was observed in different subsets of the African population such as among mothers [16] and high school students [18].
3.1.2. Change in Knowledge Post-Intervention
Some level of knowledge upswing was reported following educational interventions on HPV and CC in different African settings [16,18,19,20]. Where assessed, a positive change in the global knowledge score was observed after intervention [18,19]. Awareness of CC [19,20] and its prevalence [19], improved greatly post-intervention. Knowledge of CC causative organism, risk factors, population subgroup more predisposed to CC [19], risk factors [20], its major symptoms, screening (Pap smear test) [16,20] and vaccination [16] improved greatly following the intervention.
Likewise, understanding of the STI nature of HPV and awareness about the HPV vaccine became better, whereas knowledge of diseases associated with HPV remained poor [20]. In South Africa, a noticeable positive change in knowledge was recorded among mothers/guardians of school girls [16] and high school learners (73.38%) [18]. While in Nigeria, knowledge of early symptoms of CC, Pap smear, and HPV vaccine remained low among students following an educational intervention. Although a slight but low increase in knowledge of CC risk factors and understanding of CC symptoms was observed [23].
3.2. Determinants of Awareness and Knowledge of Cervical Cancer and Human Papillomavirus: Pre- and Post-Intervention
At baseline, the determinants of awareness and extent of knowledge about cervical cancer were mostly demographic factors. For instance, cervical cancer awareness was linked with gender and age. Whereas an understanding of CC symptoms was also linked to demographic factors (these include gender (female), level of study (3rd year and above) and family income (5000>) [20], an understanding of the CC risk factors was predicted by gender (female) and family income (5000>) only. Maternal education (low level) was linked to poor knowledge of CC risk factors. Paternal educational level was directly associated with good knowledge of CC screening [20].
As for HPV knowledge at baseline, this was positively associated with age and CGPA. The lower the age and CGPA, the poorer the knowledge. Appropriate understanding of HPV vaccination was associated with higher family income and vice versa [20]. For knowledge change post-intervention, differences in age, gender, level of study and place of residence were important determinants. Female and older students had better knowledge of HPV than their male and younger colleagues, respectively [18].
The post-intervention change in HPV understanding was significantly influenced by the year of study, level of education, and CGPA. Those studying at the postgraduate level, second year and above, and students with higher CGPA had a better understanding of HPV [20].
The improvement in cervical cancer global knowledge score was a function of a higher level of study (>3rd year), higher CGPA and urban residence [20]. While another study found a positive relationship between adequate general knowledge of CC and poor rural residence [18]. Good understanding of the symptoms of CC after the educational intervention was determined by older age, year of study (3rd year), and maternal job status (employed) [20]. Understanding of CC screening was predicated on being an urban resident, being a postgraduate student and year of study. The level of study (4th year >) also determined the understanding of CC vaccination [20]. Being ‘engaged’ either as attendees or handout readers during interventions was a determinant of knowledge improvement on CC screening, predisposing factors and HPV vaccine [23].
3.3. Impact of School-Based Interventions on Health Behavior
Post-intervention health behavior reported by the selected publications in our scoping review indicated a high rate of vaccine acceptance and uptake as well as cervical self-screening acceptance [16]. Vaccine acceptance by parents/guardians and their children/wards was indicated through consent and assent by the parents and children, respectfully. Parents’ participation in a health education event had a positive influence on consent to vaccinate their children/wards [16]. Most studies reported a high rate of vaccine acceptability with a consent rate ranging between 59% [25] and 59.7% [16] as reported in South Africa. In Kenya, a rate of 88.1% was recorded [17].
Although not all consent did translate into the uptake of the vaccine’s first dose as a high baseline, vaccine acceptance failed to match vaccine uptake after intervention [16,17] in some settings. Vermandere et al. (2014) observed an uptake of 31.1% in Kenya, where negative vaccine behavior occurred among 17.7% of the participants and 51.2% experienced difficulties which prevented them from getting their daughters vaccinated.
Conversely, the total rate of uptake of the first vaccine dose among those who consented was quite high in some countries. In South Africa, this ranges from 99.2% [25] to 99.3% [16], while 89% and 95% of school girls indicated being vaccinated in Rwanda and Bhutan, respectively. A high proportion (**) of them had more than one dose of the vaccine [21].
Complete/sufficient vaccination (a minimum of two doses within six months) was reported for the majority (91.6%) of those who initiated it in South Africa, this represents 53.7% of the targeted population [25]. Similarly, Dreyer et al. (2022) observed a completion rate of 90.5% in the same country. Some local differences in the rate of vaccine completion were observed, 93.3% in the Western Cape and 82.6% in Gauteng. [25]. While Dreyer et al. indicated that the number of vaccine doses influenced its completion as three doses attracted a slightly lower rate (91.6%) than two doses (95.9%) [16]. Where indicated, an impressive proportion of the vaccinated population (87.8%) received all three doses of the HPV vaccine [25].
Some negative screening behavior was also found among some mothers whose rate of self-screening uptake was comparatively lower than the initial acceptance [16]. Contrariwise, in a similar population, a good attitude towards CC screening as well as a positive attitude towards and trust in vaccines was previously observed by researchers [22].
3.4. Determinants of Post-Intervention Health Behavior: Screening and Vaccination
Some of the post-intervention health behavior expected in the studies include an increase in the rate of CC screening, vaccine acceptability and uptake. Certain underlying factors were highly influential in these health behaviors across settings. These determinants were documented by only a few of the studies sampled.
In South Africa, Dreyer et al. reported that invitation to partake in a screening exercise, especially at a health training, access to screening facilities and scoring high in knowledge tests were significantly associated with positive screening behavior. Additionally, receiving a self-screening kit at home or at a health education program was statistically associated with screening uptake than visiting a health facility [16]. Parental informed consent determined vaccine uptake, while the number of required vaccine doses was also positively associated with vaccine completion [16].
As for vaccine behavior, individual characteristics did not reflect vaccine acceptance in Kenya [17]. However, baseline concerns about the HPV vaccine, such as its efficacy, side effects, and possible effects on fertility, negatively affected vaccine acceptance among Kenyans. Likewise, apprehensions about wrong vaccine administration, foreseeing partner’s disapproval, as well as religion (Muslims accepted less) determined vaccine acceptance. Furthermore, concerns about child age, inadequate information about CC, and the belief that vaccine promotes unprotected sex negatively affected vaccine acceptability in the studied group [17].
The authors further observed that older age was positively associated with vaccine acceptance [17]. Moreover, having an urban background, baseline awareness of CC, and vaccine acceptance were all positively linked to vaccine uptake [17]. Prior knowledge or awareness of CC and partner disapproval were the major predictors of vaccine uptake [17]. In contrast, hindrances to vaccine uptake include lack of information/invitation about the vaccine (where and when to vaccinate), vaccine disapproval by significant others (e.g., partner and the daughter herself), non-availability, fear of side effects, time constraint, and failure to recall the vaccination [17].
3.5. Efficacy of HPV Vaccine and Other Issues
Only one of the ten papers reviewed measured the efficacy of the HPV vaccine campaign in Africa [21]. The authors tested the presence of vaccine-targeted HPV gene in the urine samples provided by the recipients of the HPV vaccine and reported 58% and 60% overall efficacy in Rwanda and Bhutan, respectively. It was indicated that the HPV vaccine reduced the prevalence of both vaccine-targeted HPV types and the untargeted variants in the population. Thus, offering an additional layer of protection. The total effectiveness against other HPV types of infection or cross-protection was 63% in Bhutan and 58% in Rwanda, while the overall efficacy was put at 80% and above. The study affirms the efficacy of HPV vaccine campaigns when the coverage is widespread [21].
That HPV vaccine uptake and cervical screening could pose a burden to healthcare providers [24] and certain groups of service users [17,24] were established in some of the studies. For the service providers, the period of the HPV vaccine campaign was characterized by extended working hours, work overload and job stress. Health workers providing services at the facilities during the vaccine campaign also reported that the quality of care was impacted due to long waiting hours [24]. At the same time, the cost of transportation to the health facility was a major burden to service users [17]. The health workers delivering HPV vaccine at schools reported long travel time as a problem [24]. For the two HPV vaccine doses administered, the number of clinical consultations, routine activities and work burden due to low staff capacity at the sampled facilities were comparable to the off-campaign period [24].
4. Discussion
This scoping review examined publications on school-based HPV interventions as documented across African settings. This review established a dearth of scientific research on the subject in Africa. The ten publications included in the current review focused on seven countries, and a majority (40%) of the research was conducted in South Africa, followed by 20% from Nigeria and 10% each from the other five countries. They all covered a range of topics, including acceptability, knowledge and awareness, CC screening and vaccine uptake, impacts and burdens of HPV vaccination.
Many documented interventions were characterized by the initiation of educational interventions and cervical cancer screening during HPV vaccine campaigns in schools. The educational interventions include formal health talks/education, the use of written and audiovisual materials to deliver health talks, as well as formal lecture presentations using PowerPoint. The constant variable in these studies was that they were school-based and the theme was designed around HPV screening and vaccination. In some studies, experts organized a training seminar on the subject and complemented the physical training with the distribution of educational materials, while peer training was likewise adopted to scale down the knowledge shared in one of the studies [16,18,19]. Other studies also found that peer training has always been an important strategy for HPV education [19,26,27].
Interventions on CC screening adopted strategies such as inviting female parents/guardians to participate in a health talk where they were educated on CC and HPV. This was combined with a CC screening exercise, as participants were offered self-sampling kits or directed to a health facility for the screening [16]. The diverse intervention strategies implemented in the reviewed studies yielded varying outcomes. However, in the main, the pre- and post-intervention assessments prove the efficacy of HPV school-based interventions in Africa (see also, [28,29,30]). Low awareness and knowledge levels at the reference point improved significantly once educational interventions were introduced. Mostly, personal factors, such as age, gender, income, and education, were associated with knowledge improvement. However, none of the studies was countrywide in scope. Therefore, inter-country and sub-regional comparison of the findings was not considered in our review.
The probable influence of differential timing of post-intervention assessment on the outcome is vague as no study methodically examined this. One study conducted the post-test on the same day and one week for peer educators and the general student population, respectively [19]. While the post-intervention scores for the whole sample improved significantly, the peer educators who took the test on the same day as the seminar fared better in some aspects of the test. Another Nigerian study passively mentioned that deferring the post-intervention test to six months may have negatively affected knowledge change [23]. These are suggestive of some methodological implications concerning knowledge transfer.
The high rate of HPV vaccine and CC screening uptake in the reviewed studies is reassuring. On the other hand, the World Health Organization (WHO) data on the global coverage of the HPV vaccine suggests a downward trend. A meager 15% of eligible girls worldwide have received the first dose so far [31]. In Africa, a populous country such as Nigeria, despite its population size, has yet to include it in the government-approved routine immunization plan [32]. The same applies to CC screening coverage. This explains the reason for the rising incidence of CC-related morbidity and mortality on the continent [33,34,35].
The only study that examined the impact of the HPV vaccine affirmed its efficacy when the coverage is widespread, further reiterating the importance of the government’s commitment to the reduction of the vaccine-preventable diseases caused by HPV, especially in countries where no-cost HPV vaccination has not been instituted. The success of routine childhood immunization is worthy of note here (WHO, 2021). Furthermore, there was no study on how the experience of vaccine side effects [36,37,38] could determine the HPV vaccine completion rate. Such significant evidence would have been worthwhile in understanding and forestalling the vaccine attrition rate reported in some studies.
5. Conclusions
All the studies reviewed were school-based, with interventions concerning HPV screening and vaccination. Most of the interventions were characterized by the initiation of educational interventions and cervical cancer screening during HPV vaccine campaigns in schools. Overall, there were significant positive results concerning HPV knowledge. Awareness about CC and its prevalence improved greatly post-intervention. Knowledge of CC causative organisms, risk factors, population subgroup more predisposed to CC, its major symptoms, screening (Pap smear test) and vaccination improved significantly following the school-based interventions. There was also a high rate of vaccine acceptance and uptake as well as cervical self-screening acceptance. In short, school-based intervention is a viable strategy that can be adopted for adolescent protection from HPV-induced diseases.
Author Contributions
Study conceptualization—J.A. and K.K.K.; study protocol design—K.K.K. and J.A.; data collection—K.K.K. and E.A.E.; data analysis—J.A. and K.A.; manuscript drafting—J.A., K.K.K. and K.A.; review of drafts—J.A., K.K.K., and K.A. Acceptance of the final draft—J.A., K.K.K., K.A. and E.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was self-funded.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Search strategy.
Table A1.
Search strategy.
| Scopus-Based Search | |||
|---|---|---|---|
| Search Focus | No. | Search String | Hits |
| Publications on schools | #1 | (TITLE-ABS-KEY (school) OR TITLE-ABS-KEY (students) OR TITLE-ABS-KEY (adolescents) OR TITLE-ABS-KEY (youth)) | 4,508,053 |
| Publications on HPV | #2 | (TITLE-ABS-KEY (hpv) OR TITLE-ABS-KEY ( human AND papillomavir *) OR TITLE-ABS-KEY (human AND papilloma AND vir *)) | 79,064 |
| Publications on interventions | #3 | (TITLE-ABS-KEY (program *) OR TITLE-ABS-KEY (intervention) OR TITLE-ABS-KEY (implementation) OR TITLE-ABS-KEY (“case-control”) OR TITLE-ABS-KEY (“cohort study”) OR TITLE-ABS-KEY (“before and after study”) OR TITLE-ABS-KEY (“controlled trial”)) | 7,862,182 |
| Publications on African countries, territories, and dependencies | #4 | ((TITLE-ABS-KEY (angola) OR TITLE-ABS-KEY (benin) OR TITLE-ABS-KEY (botswana) OR TITLE-ABS-KEY (burkina AND faso) OR TITLE-ABS-KEY (burundi) OR TITLE-ABS-KEY (cameroon) OR TITLE-ABS-KEY (cabo AND verde) OR TITLE-ABS-KEY (cape AND verde) OR TITLE-ABS-KEY (central AND african AND republic) OR TITLE-ABS-KEY (chad) OR TITLE-ABS-KEY (comoros) OR TITLE-ABS-KEY (congo) OR TITLE-ABS-KEY (ivory AND coast) OR TITLE-ABS-KEY (democratic AND republic AND of AND congo) OR TITLE-ABS-KEY (djibouti) OR TITLE-ABS-KEY (equatorial AND guinea) OR TITLE-ABS-KEY (eritrea) OR TITLE-ABS-KEY (ethiopia) OR TITLE-ABS-KEY (gabon) OR TITLE-ABS-KEY (gambia) OR TITLE-ABS-KEY (ghana) OR TITLE-ABS-KEY (guinea) OR TITLE-ABS-KEY (guinea-bissau) OR TITLE-ABS-KEY (kenya) OR TITLE-ABS-KEY (lesotho) OR TITLE-ABS-KEY (liberia) OR TITLE-ABS-KEY (madagascar) OR TITLE-ABS-KEY (malawi) OR TITLE-ABS-KEY (mali) OR TITLE-ABS-KEY (mauritania) OR TITLE-ABS-KEY (mauritius) OR TITLE-ABS-KEY (mayotte) OR TITLE-ABS-KEY (mozambique) OR TITLE-ABS-KEY (namibia) OR TITLE-ABS-KEY (niger) OR TITLE-ABS-KEY (nigeria) OR TITLE-ABS-KEY (reunion) OR TITLE-ABS-KEY (rwanda) OR TITLE-ABS-KEY (saint AND helena) OR TITLE-ABS-KEY (sao AND tome AND principe) OR TITLE-ABS-KEY (senegal) OR TITLE-ABS-KEY (seychelles) OR TITLE-ABS-KEY (sierra AND leone) OR TITLE-ABS-KEY (somalia) OR TITLE-ABS-KEY (south AND africa) OR TITLE-ABS-KEY (south AND sudan))) OR ((TITLE-ABS-KEY (eswatini) OR TITLE-ABS-KEY (togo) OR TITLE-ABS-KEY (uganda) OR TITLE-ABS-KEY (zambia) OR TITLE-ABS-KEY (zimbabwe) O TITLE-ABS-KEY (egypt) OR TITLE-ABS-KEY (libya) OR TITLE-ABS-KEY (algeria) OR TITLE-ABS-KEY (tunisia) OR TITLE-ABS-KEY (morocco) OR TITLE-ABS-KEY (western AND sahara) OR TITLE-ABS-KEY (sudan) OR TITLE-ABS-KEY (tunisia))) | 1,148,745 |
| Publications on school-based HPV interventions in Africa | #5 (#1 AND #2 AND #3 AND #4) | ((TITLE-ABS-KEY (hpv) OR TITLE-ABS-KEY (human AND papillomavir *) OR TITLE-ABS-KEY (human AND papilloma AND vir *))) AND ((TITLE-ABS-KEY (program *) OR TITLE-ABS-KEY (intervention) OR TITLE-ABS-KEY (implementation) OR TITLE-ABS-KEY (“case-control”) OR TITLE-ABS-KEY (“cohort study”) OR TITLE-ABS-KEY (“before and after study”) OR TITLE-ABS-KEY ("controlled trial”))) AND ((TITLE-ABS-KEY (school) OR TITLE-ABS-KEY (students) OR TITLE-ABS-KEY (adolescents) OR TITLE-ABS-KEY (youth))) AND (((TITLE-ABS-KEY (angola) OR TITLE-ABS-KEY (benin) OR TITLE-ABS-KEY (botswana) OR TITLE-ABS-KEY (burkina AND faso) OR TITLE-ABS-KEY (burundi) OR TITLE-ABS-KEY (cameroon) OR TITLE-ABS-KEY (cabo AND verde) OR TITLE-ABS-KEY (cape AND verde) OR TITLE-ABS-KEY (central AND african AND republic) OR TITLE-ABS-KEY (chad) OR TITLE-ABS-KEY (comoros) OR TITLE-ABS-KEY (congo) OR TITLE-ABS-KEY (ivory AND coast) OR TITLE-ABS-KEY (democratic AND republic AND of AND congo) OR TITLE-ABS-KEY (djibouti) OR TITLE-ABS-KEY (equatorial AND guinea) OR TITLE-ABS-KEY (eritrea) OR TITLE-ABS-KEY (ethiopia) OR TITLE-ABS-KEY (gabon) OR TITLE-ABS-KEY (gambia) OR TITLE-ABS-KEY (ghana) OR TITLE-ABS-KEY (guinea) OR TITLE-ABS-KEY (guinea-bissau) OR TITLE-ABS-KEY (kenya) OR TITLE-ABS-KEY (lesotho) OR TITLE-ABS-KEY (liberia) OR TITLE-ABS-KEY (madagascar) OR TITLE-ABS-KEY (malawi) OR TITLE-ABS-KEY (mali) OR TITLE-ABS-KEY (mauritania) OR TITLE-ABS-KEY (mauritius) OR TITLE-ABS-KEY (mayotte) OR TITLE-ABS-KEY (mozambique) OR TITLE-ABS-KEY (namibia) OR TITLE-ABS-KEY (niger) OR TITLE-ABS-KEY (nigeria) OR TITLE-ABS-KEY (reunion) OR TITLE-ABS-KEY (rwanda) OR TITLE-ABS-KEY (saint AND helena) OR TITLE-ABS-KEY (sao AND tome AND principe) OR TITLE-ABS-KEY (senegal) OR TITLE-ABS-KEY (seychelles) OR TITLE-ABS-KEY (sierra AND leone) OR TITLE-ABS-KEY (somalia) OR TITLE-ABS-KEY (south AND africa) OR TITLE-ABS-KEY (south AND sudan))) OR ((TITLE-ABS-KEY (eswatini) OR TITLE-ABS-KEY (togo) OR TITLE-ABS-KEY (uganda) OR TITLE-ABS-KEY (zambia) OR TITLE-ABS-KEY (zimbabwe) OR TITLE-ABS-KEY (egypt) OR TITLE-ABS-KEY (libya) OR TITLE-ABS-KEY (algeria) OR TITLE-ABS-KEY (tunisia) OR TITLE-ABS-KEY (morocco) OR TITLE-ABS-KEY (western AND sahara) OR TITLE-ABS-KEY (sudan) OR TITLE-ABS-KEY (tunisia) | 375 |
| PubMed-Based Search | |||
| Search Focus | No. | Search String | Hits |
| Publications on schools | #1 | (((school[MeSH Terms]) OR (students[MeSH Terms])) OR (adolescents[MeSH Terms])) OR (youth[MeSH Terms]) | 2,378,151 |
| Publications on HPV | #2 | ((HPV[MeSH Terms]) OR (human papillomavirus[MeSH Terms])) OR (human papilloma virus[MeSH Terms]) | 36,659 |
| Publications on interventions | #3 | ((((((program * [MeSH Terms]) OR (intervention[MeSH Terms])) OR (implementation[MeSH Terms])) OR (case-control[MeSH Terms])) OR (cohort study[MeSH Terms])) OR (before and after study[MeSH Terms])) OR (controlled trial[MeSH Terms]) | 3,496,068 |
| Publications on African countries, territories, and dependencies | #4 | (((((((((((((((((((((((((((((((((((((((((((((((((((((((((((Algeria[MeSH Terms]) OR (Angola[MeSH Terms])) OR (Benin[MeSH Terms])) OR (Botswana[MeSH Terms])) OR (burkina faso[MeSH Terms])) OR (burundi[MeSH Terms])) OR (cabo verde[MeSH Terms])) OR (cape verde[MeSH Terms])) OR (cameroon[MeSH Terms])) OR (central african republic[MeSH Terms])) OR (chad[MeSH Terms])) OR (comoros[MeSH Terms])) OR (congo[MeSH Terms])) OR (ivory coast[MeSH Terms])) OR (cote d ivoire[MeSH Terms])) OR (djibouti[MeSH Terms])) OR (democratic republic of congo[MeSH Terms])) OR (egypt[MeSH Terms])) OR (equatorial guinea[MeSH Terms])) OR (eritrea[MeSH Terms])) OR (eswatini[MeSH Terms])) OR (ethiopia[MeSH Terms])) OR (gabon[MeSH Terms])) OR (gambia[MeSH Terms])) OR (ghana[MeSH Terms])) OR (guinea[MeSH Terms])) OR (guinea bissau[MeSH Terms])) OR (kenya[MeSH Terms])) OR (lesotho[MeSH Terms])) OR (liberia[MeSH Terms])) OR (libya[MeSH Terms])) OR (madagascar[MeSH Terms])) OR (malawi[MeSH Terms])) OR (mali[MeSH Terms])) OR (mauritania[MeSH Terms])) OR (mauritius[MeSH Terms])) OR (morocco[MeSH Terms])) OR (mozambique[MeSH Terms])) OR (namibia[MeSH Terms])) OR (niger[MeSH Terms])) OR (nigeria[MeSH Terms])) OR (rwanda[MeSH Terms])) OR (sao tome and principe[MeSH Terms])) OR (senegal[MeSH Terms])) OR (seychelles[MeSH Terms])) OR (sierra leone[MeSH Terms])) OR (somalia[MeSH Terms])) OR (south africa[MeSH Terms])) OR (south sudan[MeSH Terms])) OR (sudan[MeSH Terms])) OR (tanzania[MeSH Terms])) OR (togo[MeSH Terms])) OR (tunisia[MeSH Terms])) OR (uganda[MeSH Terms])) OR (zambia[MeSH Terms])) OR (zimbabwe[MeSH Terms])) OR (reunion[MeSH Terms])) OR (saint helena[MeSH Terms])) OR (western sahara[MeSH Terms])) OR (mayotte[MeSH Terms]) | 262,847 |
| Publications on school-based HPV interventions in Africa | #5 (#1 AND #2 AND #3 AND #4) | (((#1) AND (#2)) AND (#3)) AND (#4) | 57 |
| Allied and Complementary Medicine (AMED), CINAHL, And PsycInfo using the EBSCO Interface | |||
| Publications on schools | S1 | AB School OR AB students OR AB adolescents OR AB youth | 1,290,085 |
| Publications on HPV | S2 | AB human papillomavirus OR AB human papilloma virus OR AB hpv | 14,277 |
| Publications on interventions | S3 | AB program* OR AB intervention OR AB implementation OR AB case-control OR AB cohort study OR AB (before and after study) OR AB controlled trial | 1,883,416 |
| Publications on African countries, territories, and dependencies | S4 | AB algeria OR AB angola OR AB benin OR AB botswana OR AB burkina faso OR AB burundi OR AB cape verde OR AB cabo verde OR AB cameroon OR AB central african republic OR AB chad OR AB comoros OR AB congo OR AB cote d’ivoire OR AB ivory coast OR AB djibouti OR AB democratic republic of congo OR AB egypt OR AB equatorial guinea OR AB eritrea OR AB eswatini OR AB ethiopia OR AB gabon OR AB gambia OR AB ghana OR AB guinea OR AB guinea bissau OR AB kenya OR AB lesotho OR AB liberia OR AB libya OR AB madagascar OR AB malawi OR AB mali OR AB mauritania OR AB mauritius OR AB morocco OR AB mozambique OR AB namibia OR AB niger OR AB nigeria OR AB rwanda OR AB (sao tome and principe) OR AB senegal OR AB seychelles OR AB sierra leone OR AB somalia OR AB south Africa OR AB south sudan OR AB sudan OR AB tanzania OR AB togo OR AB tunisia OR AB uganda OR AB zambia OR AB zimbabwe OR AB reunion OR AB saint helena OR AB western sahara OR AB mayotte | 106,753 |
| Publications on school-based HPV interventions in Africa | S5 | S1 AND S2 AND S3 AND S4 | 51 |
References
- De Vuyst, H.; Alemany, L.; Lacey, C.; Chibwesha, C.J.; Sahasrabuddhe, V.; Banura, C.; Denny, L.; Parham, G.P. The burden of human papillomavirus infections and related diseases in sub-saharan Africa. Vaccine 2013, 31 (Suppl. 5), F32–F46. [Google Scholar] [CrossRef]
- Kombe, A.J.K.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Boyle, P.; Ngoma, T.; Sullivan, R.; Brawley, O. Cancer in Africa: The way forward. Ecancermedicalscience 2019, 13, 953. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-related oropharyngeal cancer: A review on burden of the disease and opportunities for prevention and early detection. Hum. Vaccin Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef]
- Bednarczyk, R.A. Addressing HPV vaccine myths: Practical information for healthcare providers. Hum. Vaccin Immunother. 2019, 15, 1628–1638. [Google Scholar] [CrossRef]
- Deignan, C.; Swartz, A.; Cooper, S.; Colvin, C.J. Stakeholders’ Understandings of Human Papillomavirus (HPV) Vaccination in Sub-Saharan Africa: A Rapid Qualitative Systematic Review. Vaccines 2021, 9, 496. [Google Scholar] [CrossRef]
- Lubeya, M.K.; Mwanahamuntu, M.; Chibwesha, C.; Mukosha, M.; Wamunyima, M.M.; Kawonga, M. Implementation strategies to increase human papillomavirus vaccination uptake for adolescent girls in sub-Saharan Africa: A scoping review protocol. PLoS ONE 2022, 17, e0267617. [Google Scholar] [CrossRef]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human Papillomavirus Vaccination in South Africa: Programmatic Challenges and Opportunities for Integration with Other Adolescent Health Services? Front. Public Health 2022, 10, 799984. [Google Scholar] [CrossRef] [PubMed]
- WHO [World Health Organization]. WHO Guideline on School Health Services. 2021. Available online: https://www.who.int/publications/i/item/9789240029392 (accessed on 13 September 2022).
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. -Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Nastri, L.; Moretti, A.; Migliaccio, S.; Paoletta, M.; Annunziata, M.; Liguori, S.; Toro, G.; Bianco, M.; Cecoro, G.; Guida, L. Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review. Nutrients 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Drikite, L.; Bedford, J.P.; O’Bryan, L.; Petrinic, T.; Rajappan, K.; Doidge, J.; Harrison, D.A.; Rowan, K.M.; Mouncey, P.R.; Young, D.; et al. Treatment strategies for new onset atrial fibrillation in patients treated on an intensive care unit: A systematic scoping review. Crit. Care (Lond. Engl.) 2021, 25, 257. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Botha, M.H.; Snyman, L.C.; Visser, C.; Burden, R.; Laubscher, N.; Grond, B.; Richter, K.; Becker, P.J.; Harvey, J.; et al. Combining cervical cancer screening for mothers with schoolgirl vaccination during human papillomavirus (HPV) vaccine implementation in South Africa: Results from the VACCS1 and VACCS2 trials. Int. J. Gynecol. Cancer. 2022, 32, 592–598. [Google Scholar] [CrossRef]
- Vermandere, H.; Naanyu, V.; Mabeya, H.; Vanden Broeck, D.; Michielsen, K.; Degomme, O. Determinants of acceptance and subsequent uptake of the HPV vaccine in a cohort in Eldoret, Kenya. PLoS ONE 2014, 9, e109353. [Google Scholar] [CrossRef] [PubMed]
- Mbulawa, Z.Z.A.; Somdyala, N.I.; Mabunda, S.A.; Williamson, A.L. Effect of Human Papillomavirus (HPV) Education Intervention on HPV Knowledge and Awareness Among High School Learners in Eastern Cape Province of South Africa. J. Cancer Educ. 2021. [Google Scholar] [CrossRef]
- Sadoh, A.E.; Okonkwobo, C.; Nwaneri, D.U.; Ogboghodo, B.C.; Eregiea, C.; Oviawe, O.; Famuyiwa, O. Effect of Peer Education on Knowledge of Human Papilloma Virus and Cervical Cancer among Female Adolescent Students in Benin City, Nigeria. Ann. Glob. Health 2018, 84, 121–128. [Google Scholar] [CrossRef]
- Indracanti, M.; Berhane, N.; Minyamer, T. Factors associated with pre-and post-educational intervention knowledge levels of hpv and cervical cancer among the male and female university students, northwest ethiopia. Cancer Manag. Res. 2021, 13, 7149–7163. [Google Scholar] [CrossRef]
- Baussano, I.; Sayinzoga, F.; Tshomo, U.; Tenet, V.; Vorsters, A.; Heideman, D.A.M.; Gheit, T.; Tommasino, M.; Umulisa, M.C.; Franceschi, S.; et al. Impact of Human Papillomavirus Vaccination, Rwanda and Bhutan. Emerg. Infect. Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Dreyer, G.; Van Der Merwe, F.H.; Botha, M.H.; Snyman, L.C.; Constant, D.; Visser, C.; Harvey, J. School-based human papillomavirus vaccination: An opportunity to increase knowledge about cervical cancer and improve uptake of screening. S. Afr. Med. J. 2015, 105, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Ifediora, C.O.; Azuike, E.C. Targeting cervical cancer campaigns on teenage high schoolers in resource-limited economies: Lessons from an intervention study of Nigerian senior secondary school girls. Fam. Pract. 2019, 36, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.E.; Erio, T.; Baisley, K.; Lees, S.; Watson-Jones, D. The impact of a human papillomavirus (HPV) vaccination campaign on routine primary health service provision and health workers in Tanzania: A controlled before and after study. BMC Health Serv. Res. 2018, 18, 173. [Google Scholar] [CrossRef]
- Botha, M.H.; Van der Merwe, F.H.; Snyman, L.C.; Dreyer, G. The Vaccine and Cervical Cancer Screen (VACCS) project: Acceptance of human papillomavirus vaccination in a school-based programme program in two provinces of South Africa. S. Afr. Med. J. 2015, 105, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Suarez Mora, A.; Madrigal, J.M.; Jordan, L.; Patel, A. Effectiveness of an Educational Intervention to Increase Human Papillomavirus Knowledge in High-Risk Minority Women. J. Low Genit. Tract Dis. 2018, 22, 288–294. [Google Scholar] [CrossRef]
- Cory, L.; Cha, B.; Ellenberg, S.; Bogner, H.R.; Hwang, W.T.; Smith, J.S.; Haggerty, A.; Morgan, M.; Burger, R.; Chu, C. Effects of Educational Interventions on Human Papillomavirus Vaccine Acceptability: A Randomized Controlled Trial. Obstet Gynecol. 2019, 134, 376–384. [Google Scholar] [CrossRef]
- Sitaresmi, M.N.; Rozanti, N.M.; Simangunsong, L.B.; Wahab, A. Improvement of Parent’s awareness, knowledge, perception, and acceptability of human papillomavirus vaccination after a structured-educational intervention. BMC Public Health 2020, 20, 1836. [Google Scholar] [CrossRef] [PubMed]
- AlRadini, F.; El-Sheikh, A.; Bin Jamaan, N.; Hushan, H.; Binhuwaimel, W.; Alhedaithy, F.; Alanzi, S. Prevalence of Over-the-Counter Cosmeceutical Usage and the Impact of a Health Education Intervention in Female Saudi University Students. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1867–1877. [Google Scholar] [CrossRef]
- Rani, U.; Darabaner, E.; Seserman, M.; Bednarczyk, R.A.; Shaw, J. Public Education Interventions and Uptake of Human Papillomavirus Vaccine: A Systematic Review. J. Public Health Manag. Pract. 2022, 28, E307–E315. [Google Scholar] [CrossRef]
- WHO [World Health Organization]. Immunization Coverage: Fact Sheet. 2021, p. 1. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 18 September 2022).
- Ezeanochie, M.; Olasimbo, P. Awareness and uptake of human papilloma virus vaccines among female secondary school students in Benin City, Nigeria. Afr. Health Sci. 2020, 20, 45–50. [Google Scholar] [CrossRef]
- Jedy-Agba, E.; Joko, W.Y.; Liu, B.; Buziba, N.G.; Borok, M.; Korir, A.; Masamba, L.; Manraj, S.S.; Finesse, A.; Wabinga, H.; et al. Trends in cervical cancer incidence in sub-Saharan Africa. Br. J. Cancer 2020, 123, 148–154. Available online: https://www.nature.com/articles/s41416-020-0831-9 (accessed on 18 September 2022). [CrossRef] [PubMed]
- Kamaraju, S.; Drope, J.; Sankaranarayanan, R.; Shastri, S. Cancer Prevention in Low-Resource Countries: An Overview of the Opportunity. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, Y.; Abdeljaoued-Tej, I.; Zatchi, A.A.; Abdelhak, S.; Boubaker, S.; Brown, J.S.; Benkahla, A. Cancer in Africa: The Untold Story. Front. Oncol. 2021, 11, 650117. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.F.; Andrade, C.V.; Russomano, F.B.; Rodrigues, L.L.; Oliveira, N.S.; Provance, D.W., Jr. HPV vaccines: A controversial issue? Braz. J. Med. Biol. Res. 2016, 49, e5060. [Google Scholar] [CrossRef]
- Ko, L.K.; Taylor, V.M.; Mohamed, F.B.; Do, H.H.; Gebeyaw, F.A.; Ibrahim, A.; Ali, A.A.; Winer, R.L. “We brought our culture here with us”: A qualitative study of perceptions of HPV vaccine and vaccine uptake among East African immigrant mothers. Papillomavirus Res. 2019, 7, 21–25. [Google Scholar] [CrossRef]
- Nabirye, J.; Okwi, L.A.; Nuwematsiko, R.; Kiwanuka, G.; Muneza, F.; Kamya, C.; Babirye, J.N. Health system factors influencing uptake of Human Papilloma Virus (HPV) vaccine among adolescent girls 9-15 years in Mbale District, Uganda. BMC Public Health 2020, 20, 171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).