Abstract
Studying the current range of species presence is crucial for ecologists and related scientists to understand potential habitats and the influence of environmental factors on species distribution. In this study, we used species distribution modeling (SDM) to look into where the yellow-bellied gecko, also known as the northern house gecko (Hemidactylus flaviviridis Rüppell, 1835), lives in Iran. We achieved this by combining four machine learning algorithms: Random Forest (RF), the Support Vector Machine (SVM), Maximum Entropy (Maxent), and the Generalized Linear Model (GLM). We utilized 19 historical bioclimatic variables, the Digital Elevation Model (DEM), slope, aspect, and the Normalized Difference Vegetation Index (NDVI). After calculating their correlations, we selected variables for modeling with a variance inflation factor (VIF) of less than 10. The findings indicate that the variables “Precipitation of the Coldest Quarter” (BIO19) and “Mean Temperature of Wettest Quarter” (BIO8) have the most significant influence on the species’ distribution. The gecko primarily inhabits low elevations and slopes, particularly those below 400 m above sea level with slopes less than 8 degrees, primarily in southern Iran. Additionally, we found that the NDVI had a minimal impact on the distribution of the species. Therefore, we identify the provinces of Khuzestan, Bushehr, Hormozgan, and Fars, along with parts of the coastal strip of Sistan and Baluchistan, as suitable areas for the current presence of this species.
1. Introduction
Identifying optimal habitats for species is crucial in the face of modern human advancements, including urban expansion [1,2], industrial and agricultural development [3,4,5], climate change [6,7], tourism [8], and migration [9]. Species distribution modeling (SDM) tools are essential for understanding the relationships between environmental and climatic factors and the geographical range of species [10,11,12,13]. These tools have advanced significantly and, with the aid of machine learning algorithms, are capable of quantifying these relationships and predicting species’ presence ranges [14]. Additionally, SDM can identify potential habitats for new species and aid in discovering previously unrecorded species [15].
From a zoogeographical perspective, Iran is located in the Palearctic region, featuring a diverse combination of faunal elements [16]. It is considered the most complex region of southwest Asia, serving as a convergence zone for species from north Africa, south Asia, central Asia, and Europe. Based on the distribution patterns of lizard species, Iran is divided into 13 physiographic regions: the Central Plateau, Sistan Basin, Urmia Basin, Moghan Steppe, Caspian Sea Coast, Khuzestan Plain and Persian Gulf Coast, Baluchistan and Makran Coast, Turkmen Steppe, Zagros Mountain Range, Alborz Mountain Range, the western foothills of the Zagros, Kopet Dag, and the islands of the Persian Gulf [17].
The species Hemidactylus flaviviridis, commonly known as the yellow-bellied gecko and northern house gecko [18], is an introduced species in Iran [19], primarily found in the plains of Khuzestan and along the Persian Gulf coast [20]. Morphologically, this species lacks lateral tail dentition and has external chin scales, no large dorsal tubercles, and 12 lamellae on the underside of the fourth toe [21]. Most recorded occurrences of this species are near human settlements, indicating a close association with human environments. Behaviorally, it is primarily nocturnal and feeds on insects. Native to central India, it is believed that the species spread westward along trade routes through human migration [22]. This study aims to examine the influence of certain environmental parameters on the distribution of H. flaviviridis and identify favorable habitats for its presence in Iran.
2. Materials and Methods
2.1. Occurrence Points
Initially, 117 species presence points were obtained from iNaturalist Research-grade Observations and literary descriptions of Iranian lizards, sourced from the GBIF database [23]. Then, using the CoordinateCleaner package, we filtered and removed records without coordinates or with incomplete coordinate information [24]. Finally, we imported the cleaned points into the ArcGIS Pro environment. Using the Find Identical and Generate Near Table tools, we examined and removed duplicate points and those located less than one kilometer apart. This refinement process resulted in 80 records being used for the species distribution modeling.
2.2. Data Collection
To assess the impact of various environmental parameters on the distribution of H. flaviviridis in Iran, three topographical variables—elevation, slope, and aspect—were included, along with the vegetation cover index obtained via Google Earth Engine. Additionally, 19 bioclimatic variables were sourced from the WorldClim database [25], all at a resolution of 1 square kilometer (Table 1). Then, the variables were clipped and masked to the boundaries of the study area, Iran, between 25° and 40° N latitude and 44° and 64° E longitude. Next, we calculated the variance inflation factor (VIF) between variables based on Pearson correlation metrics [26] and removed those with VIF < 10 to address multicollinearity issues. Eleven out of the twenty-three input variables exhibited collinearity problems: BIO1, BIO3, BIO5, BIO6, BIO7, BIO10, BIO11, BIO12, BIO13, BIO16, and BIO17. These variables were excluded from further analysis. After removing the collinear variables, the remaining environmental variables showed linear correlation coefficients ranging from a minimum of 0.0002 (between BIO22 and BIO8) to a maximum of −0.787 (between BIO20 and BIO8).

Table 1.
List of variables and their description. Variables marked with an asterisk (*) were used in the modeling process.
2.3. Species Distribution Model
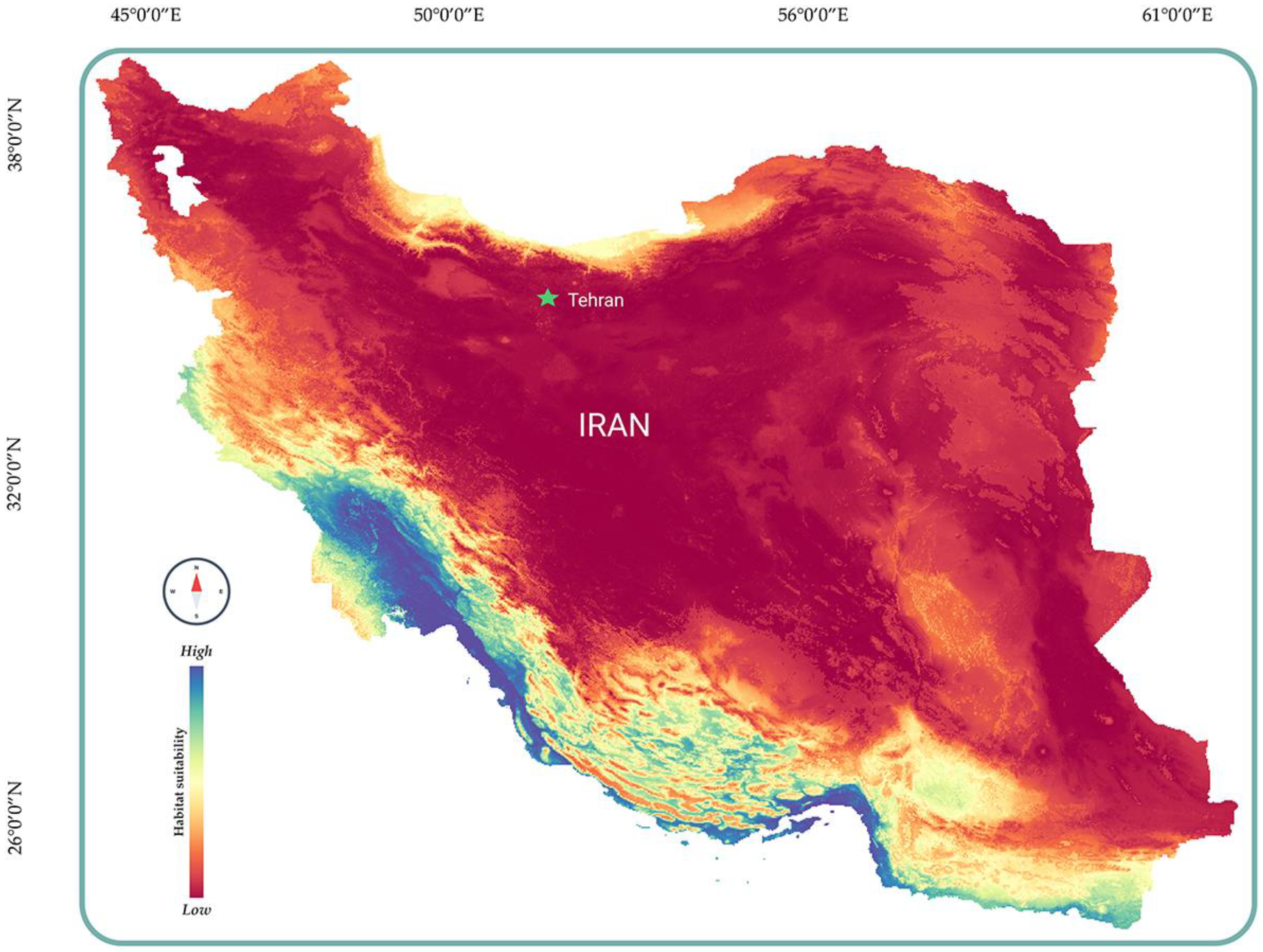
After gathering the necessary data, we began constructing the species distribution model. The modeling was carried out in the R programming environment [27] using the sdm package [28]. In the sdmData step, pseudo-absence or background data (bg = 100) were generated in RStudio. To build the model, we employed four algorithms: Random Forest (RF), Maximum Entropy (Maxent), the Support Vector Machine (SVM), and the Generalized Linear Model (GLM), with five bootstrap replications for each [29,30,31,32]. In the final step, we generated the projected species distribution using the Ensemble function, incorporating results from all four algorithms. The final distribution was refined by applying a threshold value based on the maximum sensitivity and specificity (max(se + sp) (Figure 1) [33,34].
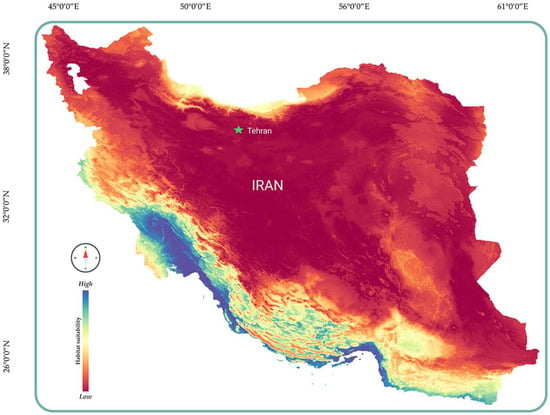
Figure 1.
Predicted distribution of H. flaviviridis in Iran.
3. Results
3.1. Model Performance
To evaluate the model, two parameters were considered: TSS and AUC. The closer the AUC value is to 1, the better the model’s performance, whereas values closer to 0.5 indicate a random prediction [35]. The TSS ranges from minus one to one, with values closer to one signifying excellent model performance [36]. In this study, the AUC and TSS values are presented in Table 2, showing that the SVM algorithm had the best performance, while the GLM performed the worst.

Table 2.
Model performance based on Area Under the Curve and True Skill Statistics.
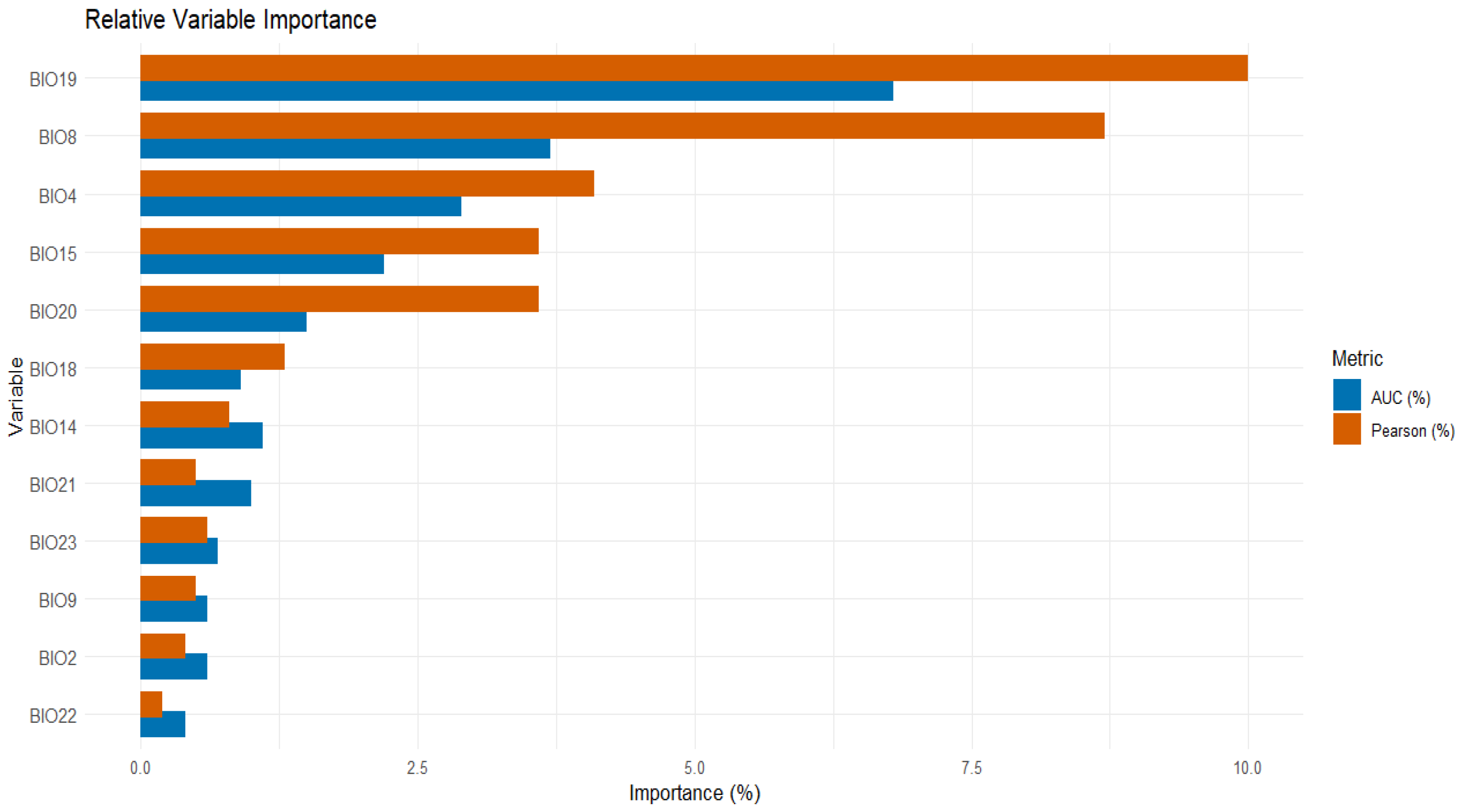
3.2. Relative Variable Contribution
In terms of variable contributions, the analysis was conducted using Pearson correlation and AUC methods. BIO19 contributed the most, with values of 10% and 6.8%, followed by BIO8 with 8.7% and 3.7%. BIO22 had the lowest contribution, with values of 0.2% and 0.4% (Figure 2).
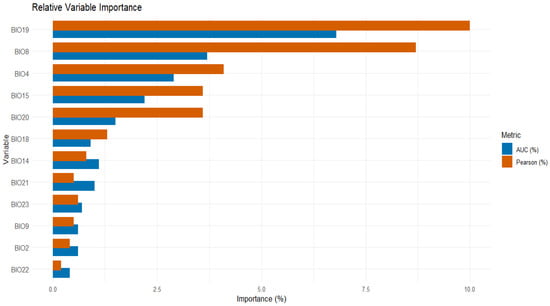
Figure 2.
Relative variable importance in the modeling process based on Pearson correlation and AUC metrics.
3.3. Ensemble Prediction
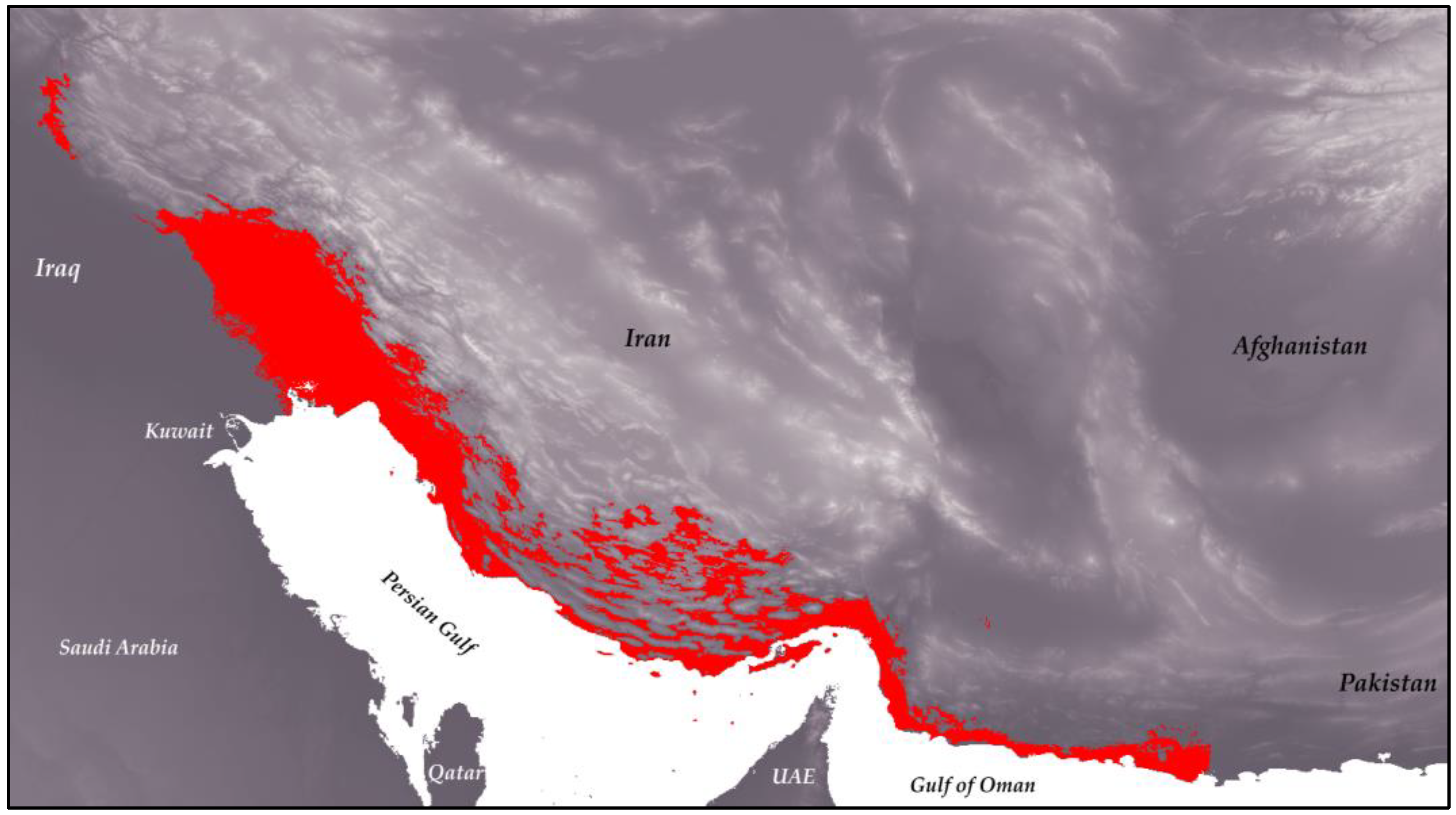
Finally, the output map from the modeling process was converted into binary form using ArcGIS Pro (Figure 3), with the threshold determined based on max(se + sp). The elevation range of the species indicates that it can inhabit areas between −29 m and 3450 m above sea level, with the highest favorability at elevations below 413 m, averaging 266 m. In terms of vegetation, the species is found in low-density areas, suggesting a preference for habitats near human settlements.
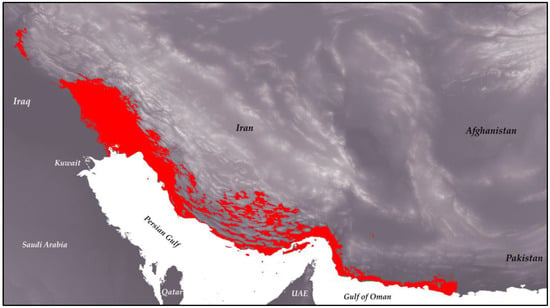
Figure 3.
Binary distribution map of H. flaviviridis overlaid on an elevation map of Iran. The red areas indicate the suitable habitat range for the species.
4. Discussion
Species distribution modeling is a valuable tool for identifying and predicting the potential range of species presence, and its results can inform more effective species management. As mentioned, H. flaviviridis is a non-native species in Iran, believed to have arrived in west Asia and southern Iran from east Asia via trade routes, particularly through sea routes and southern ports. Alien species can disrupt local biodiversity and create competition for resources such as food and habitat [37,38,39], which in turn increases environmental stress [40]. Therefore, it is anticipated that the growing population of this non-native species may pose a threat to the habitats of native geckos in Iran.
Our species distribution modeling results indicated that the most significant climatic factor influencing the distribution of this species is the Precipitation of the Coldest Quarter, while among the topographical variables, elevation above sea level plays the most important role. The final distribution map shows that the provinces of Khuzestan, Fars, Hormozgan, Bushehr, and the coastal region of Sistan and Baluchistan, along with the Iranian islands, exhibit high suitability for the species’ presence. In a study conducted by Hosseinzadeh et al. (2016), they projected the impact of future climate change on this species, showing that by 2040, southern regions of Iran, particularly Hormozgan, Bushehr, and Khuzestan, will continue to have high suitability for this species, with potential expansion to higher altitudes [21].
The findings of our research contribute to the better management and more precise control of H. flaviviridis. It is recommended that proactive measures be taken to control the excessive spread of this species, as it may negatively impact the native gecko populations in the region.
Author Contributions
Conceptualization, S.G.S. and M.H.; methodology, S.G.S.; software, N.G.S. and S.G.S.; validation, S.G.S.; formal analysis, S.G.S. and M.H.; investigation, N.G.S. and S.G.S.; resources, S.G.S.; data curation, S.G.S.; writing—original draft preparation, S.G.S.; writing—review and editing, S.G.S. and M.H.; supervision, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in [Fick, S.E. and R.J. Hijmans, 2017. WorldClim 2: new 1 km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37 (12): 4302–4315, accessed via https://worldclim.org on 27 July 2024] and [Hemidactylus flaviviridis Rüppell, 1835 in GBIF Secretariat (2023). GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei accessed via GBIF.org on 30 July 2024].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kondratyeva, A.; Knapp, S.; Durka, W.; Kühn, I.; Vallet, J.; Machon, N.; Martin, G.; Motard, E.; Grandcolas, P.; Pavoine, S. Urbanization Effects on Biodiversity Revealed by a Two-Scale Analysis of Species Functional Uniqueness vs. Redundancy. Front. Ecol. Evol. 2020, 8, 73. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Nilon, C.H.; Lepczyk, C.A.; Parker, T.S.; Warren, P.S.; Cilliers, S.S.; Goddard, M.A.; Hahs, A.K.; Herzog, C.; Katti, M.; et al. Hierarchical Filters Determine Community Assembly of Urban Species Pools. Ecology 2016, 97, 2952–2963. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, J.A.; Watson, J.E.M.; Lane, J.L.; Sonter, L.J.; Venter, O.; Atkinson, S.C.; Allan, J.R. Renewable Energy Development Threatens Many Globally Important Biodiversity Areas. Glob. Change Biol. 2020, 26, 3040–3051. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Verma, A. Anthropogenic Activities and Biodiversity Threats. Int. J. Biol. Innov. 2022, 4, 94–103. Available online: https://ssrn.com/abstract=4048276 (accessed on 24 September 2024). [CrossRef]
- Perrings, C.; Halkos, G. Agriculture and the Threat to Biodiversity in Sub-Saharan Africa. Environ. Res. Lett. 2015, 10, 095015. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple Dimensions of Climate Change and Their Implications for Biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef]
- Trew, B.T.; Maclean, I.M.D. Vulnerability of Global Biodiversity Hotspots to Climate Change. Glob. Ecol. Biogeogr. 2021, 30, 768–783. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Chong, C.W.; Radam, A. Tourism and Biodiversity Loss: Implications for Business Sustainability. Procedia Econ. Financ. 2016, 35, 166–172. [Google Scholar] [CrossRef]
- Cripps, G.; Gardner, C.J. Human Migration and Marine Protected Areas: Insights from Vezo Fishers in Madagascar. Geoforum 2016, 74, 49–62. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Austin, M. Species Distribution Models and Ecological Theory: A Critical Assessment and Some Possible New Approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can Species Distribution Models Really Predict the Expansion of Invasive Species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, E.; Ranjbaran, Y.; Sayahnia, R.; Ahmadzadeh, F. Assessing the climate change effects on the distribution pattern of the Azerbaijan Mountain Newt (Neurergus crocatus). Ecol. Complex. 2022, 50, 100997. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Applying various algorithms for species distribution modelling. Integr. Zool. 2012, 8, 124–135. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Noori, S.; Zahiri, R.; Yusefi, G.H.; Rajabizadeh, M.; Hawlitschek, O.; Rakhshani, E.; Husemann, M.; Rajaei, H. Patterns of Zoological Diversity in Iran—A Review. Diversity 2024, 16, 621. [Google Scholar] [CrossRef]
- Anderson, S.C. The Lizards of Iran. Contribution to Herpetology, 15; SSAR: Oxford, OH, USA, 1999; 442p. [Google Scholar]
- Mahendra, B.C. Sexual dimorphism in the Indian House-gecko Hemidactylus flaviviridis Ruppel. Curr. Sci. Bangalore 1935, 4, 178–179. [Google Scholar]
- Anderson, J. A Contribution to the Herpetology of Arabia: With A Preliminary List of the Reptiles and Batrachians of Egypt; R.H. Porter: London, UK, 1896; 122p. [Google Scholar]
- Gholamifard, A.; Rastegar-Pouyani, N. Distribution of Hemidactylus geckos (Reptilia: Gekkonidae) in Fars Province, Southern Iran. Amphib. Reptile Conserv. 2011, 5, 1–6. [Google Scholar]
- Moghbeli Mehni, M. The Species Diversity of Lizards in Anbarabad City, Located in Southeastern Kerman Province. Master’s Thesis, Shahid Bahonar University of Kerman, Kerman, Iran, 2016. [Google Scholar]
- Hosseinzadeh, M.S.; Aliabadian, M.; Rastegar-Pouyani, E. Predicting Geographical Distribution of Invasive Species, Hemidactylus flaviviridis Ruppell, 1840 in Current and Future Time in Iran Using Species Distribution Modeling. J. Anim. Res. 2016, 28, 431–440. [Google Scholar]
- GBIF Secretariat. Hemidactylus flaviviridis Rüppell, 1835. In Global Biodiversity Information Facility Backbone Taxonomy; Checklist dataset. 2023. Available online: https://www.gbif.org/dataset/d7dddbf4-2cf0-4f39-9b2a-bb099caae36c (accessed on 30 July 2024).
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Ritter, C.D.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 10 July 2024).
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2005, 190, 231–259. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- McCullagh, P. Generalized linear models. Eur. J. Oper. Res. 1984, 16, 285–292. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2015, 6, 337–348. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Manchester, S.J.; Bullock, J.M. The impacts of non-native species on UK biodiversity and the effectiveness of control. J. Appl. Ecol. 2000, 37, 845–864. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists' warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Santicchia, F.; Wauters, L.A.; Tranquillo, C.; Villa, F.; Dantzer, B.; Palme, R.; Preatoni, D.; Martinoli, A. Invasive alien species as an environmental stressor and its effects on coping style in a native competitor, the Eurasian red squirrel. Hormones Behav. 2022, 140, 105127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).