Abstract
Pseudogymnoascus destructans is a psychrophilic fungus that causes white-nose syndrome (WNS), an emerging disease in North America. This fungus has caused unprecedented population declines. It has also been described in Europe and Asia, where it has not caused significant mortality. The first evidence of WNS in North America came from a photograph of a hibernating bat taken during the winter of 2005–2006 in a cave near Albany, New York. P. destructans develops when the body temperature decreases during winter hibernation. This fungus thrives in humid and cold conditions characteristic of caves. Infected bats can develop visible white fungal growth on the nose, ears, and wings, and awaken more frequently from torpor. It leads to physiologic changes that result in weight loss, dehydration, electrolyte imbalances, and the death of bats. The fungi can persist in the environments of underground bat hibernation sites and are believed to spread primarily by the natural movements of infected bats. Also, there is a strong possibility that it may also be transmitted by humans inadvertently carrying the fungus from cave to cave on their clothing and gear. WNS has a big impact on bat populations with high levels of mortality, particularly endangered species. Some populations will take many years to recover. The decline of bats also has an impact on the spread of diseases, since many species of bat feed on insect carriers of several pathogens.
1. Introduction
Pseudogymnoascus destructans (formerly known as Geomyces destructans) is a psychrophilic fungus that is the etiologic agent of white-nose syndrome (WNS) in bats [1], a fatal fungal disease that has devastated bat populations in the Northern Hemisphere, particularly in North America [2]. The first report of P. destructans (WNS) was in North America in a photograph of a hibernating bat taken in the winter of 2005–2006 in a hibernaculum near Albany, New York (USA) [3]. In New York, the species affected, little brown bats (Myotis lucifugus), were the first to be infected and experienced high mortality, resulting in population declines of 90–100% in caves [4]. Later, this fungus, with curved conidia, was isolated from the skin of the nose and wing of Myotis lucifugus and Myotis septentrionalis [2,5].
In 2013, after a phylogenic analysis, these fungi were classified in the genus Pseudogymnoascus and the family Pseudeurotiaceae [6]. From there, and over subsequent years, it spread to other regions of North America and has killed millions of bats, threatening some species with extinction [7]. Although millions of bats have died in North America, mass mortality has not been observed among European bats infected by the fungus [8]. This fungus is not transmitted to humans.
2. Distribution and Transmission
P. destructans is present in 19 European countries, including Belgium [9], Croatia [10], Czechia [11], Estonia [12], France [13], Germany, Hungary [14], Latvia [15], Luxembourg [16], the Netherlands, Poland [12], Portugal [17], Russia, Slovenia [15], Slovakia [18], Switzerland [14], Ukraine [12], the United Kingdom [19], and Italy [20]. It has also been detected in China (Beijing, Jilin Liaoning, Shandong) [21]. In North America, the geographic distribution of P. destructans continues to increase, and is currently distributed in 38 states of the USA (Alabama, Arkansas, Connecticut, Delaware, Georgia, Illinois, Indiana, Iowa, Kentucky, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Vermont, Virginia, Washington, West Virginia, and Wisconsin) [4,5,22] and five Canadian provinces (New Brunswick, Nova Scotia, Ontario, Prince Edward Island, and Quebec) [4] (Figure 1).
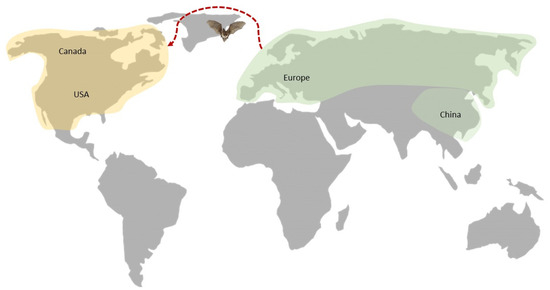
Figure 1.
Distribution of P. destructans (yellow—invasive, green—native).
It is hypothesized that these fungi were introduced to North America from Europe or Asia, where they are native [16,21,23,24]. The route of introduction is still unknown, but the subsequent spread throughout North America probably occurred due to the natural movement of bats [25], contact with contaminated substrates and human clothing/equipment (particularly cave equipment) [26], and host/vector organisms (pathway vector) [27].
3. Affected Species
In North America, P. destructans has been described in eleven species of bats: the Indiana bat (Myotis sodalis), the gray bat (Myotis grisescens), the little brown bat (Myotis lucifugus), the northern long-eared bat (Myotis septentrionalis), the big brown bat (Eptesicus fuscus), the tri-colored bat (Perimyotis subflavus), the eastern small-footed bat (Myotis leibii), the Virginia big-eared bat (Corynorhinus townsendii virginianus), the cave bat (Myotis velifer), the Silver-haired bat (Lasionycteris noctivagans), and the South-eastern bat (Myotis austroriparius) [5,14].
In Europe, it has been observed in 13 bat species: Bechstein’s bat (Myotis bechsteinii), the Lesser mouse-eared bat (Myotis blythii oxygnathus), Brandt’s bat (Myotis brandtii), the pond bat (Myotis dasycneme), Daubenton’s bat (Myotis daubentonii), the greater mouse-eared bat (Myotis myotis), the whiskered bat (Myotis mystacinus), Geoffroy’s bat (Myotis emarginatus), the Northern bat (Eptesicus nilssonii), the lesser horseshoe bat (Rhinolophus hipposideros), Barbastell (Barbastella barbastellus), the brown long-eared bat (Plecotus auritus), and Natterer’s bat (Myotis nattereri) [15,18].
In Asia, the species where the fungi have been observed include the greater horseshoe bat (Rhinolophus ferrumequinum), the least horseshoe bat (Rhinolophus pusillus), the large-footed mouse-eared bat (Myotis adversus), the eastern long-fingered bat (Myotis macrodactylus), Rickett’s big-footed bat (Myotis pilosus), the large myotis (Myotis chinensis), the Ussuri tube-nosed bat (Murina usseriensis), the greater tube-nosed bat (Murina leucogaster), and the eastern water bat (Myotis petax) [21].
Rickett’s big-footed bat (Myotis pilosus), the Indiana bat (Myotis sodalis), the grey bat (Myotis grisescens), and the little brown bat (Myotis lucifugus), according to the IUCN Red List of Threatened Species, are considered threatened [28].
4. Pathogen Characteristics and Clinical Presentation
P. destructans is a psychrophilic fungus that grows at temperatures from around 4 °C to 20 °C (the same temperatures that can be found in winter bat hibernacula) [29]. It belongs to the genus Pseudogymnoascus and the family Pseudeurotiaceae [5]. P. destructans can grow and sporulate (reproduce asexually via conidiation) on chitinaceous, keratinaceous, cellulosic, and lipid/protein-rich substrates (e.g., dead fish, mushrooms, fruit, and insects) [30]. It can grow over a wide pH range (pH 5–11), in high levels of sulfur compounds (cysteine, sulfite, sulfide), and in elevated levels of calcium in the environment [30].
The transmission starts during autumn and has a high peak during the months of winter. During the summer, the fungi vanish from the surviving animals and the prevalence during the summer months is almost null. However, the fungus may still be present, as it has the ability to persist in cave sediment, even in the absence of bats [31], and in the skin of bats that utilize contaminated underground sites for daily torpor [26].
4.1. Laboratory Diagnosis
In the laboratory, the fungus has a slow growth on cornmeal agar or Sabouraud dextrose agar incubated at 5 to 15 °C for 16 days [14]. Its colonies have a 1.0 mm diameter and are white marginally, with grey to green powdery centers. The reverse side is uncolored on cornmeal agar or brown in Sabouraud dextrose agar [14,32]. Microscopically, it has moderately thick-walled, curved conidia and erect, hyaline, smooth, narrow, and thin-walled conidiophores [14,33]. Some virulence factors have also been identified in P. destructans that may contribute to skin invasion. These include the over-production of riboflavin (vitamin B2) [34], the secretion of siderophores [35], and the excretion of a subtilisin-like serine protease that reduces collagen [36]. It also produces enzymes such as naphthol-AS-B1-phosphohydrolase, β-glucosidase, leucine and valine arylamidase, esterase/esterase lipase/lipase, N-acetyl-β-glucosaminidase, acid and alkaline phosphatases, proteinases, and ureases [37].
4.2. Clinical Signs in Bats
This fungus almost exclusively affects hibernating bats [1,14]. Gross clinical signals include fungal growth with a 1 to 3 mm diameter, and multifocal to coalescing white foci with a pinpoint black center on the ears, nose, and wing membranes of hibernating bats (Figure 2) [5]. In the wings, it is also possible to observe areas of depigmentation, splitting, and dryness of the patagia [38]. Infected animals also present behavior alterations such as premature emergence from hibernation during the winter period [39]. Other systemic alterations that can be present are increased evaporative water loss through the damaged skin, hypovolemia, hyperkalemia, acidosis, and hypotonic dehydration [24,33].

Figure 2.
Lesions in the skin of a bat due to P. destructans (License: common use).
Post-mortem, the animals are emaciated and the fungi growing on the skin can be present or not. Histological examinations of the skin can reveal cupping erosions and ulcers that are filled with densely packed, PAS- and GMS-positive fungal hyphae [7]. Animals that die during hibernation do not present inflammatory responses surrounding the invading hyphae, but in animals that emerged from hibernation, it is possible that they may exhibit a neutrophilic reaction as well edema and necrosis [7]. Although mortality is very high, some animals can survive and recover from the damage [7,14].
4.3. Diagnosis and Treatment
Methods to identify the fungus include fungal culture, histopathological examination, and PCR [4]. There is no practical treatment for colonies affected at the moment.
5. Social, Environmental, and Economic Impacts
5.1. Social Impacts
Some insectivorous bat species (e.g., M. septentrionalis) consume large quantities of mosquitoes each night [40]. Many of these mosquitos and other insects are carriers of many diseases that can affect humans. With the decline of bats due to P. destructans, there is a reduction in the elimination of these vectors and a higher risk for the transmission of vector-borne diseases [40].
5.2. Environmental Impact
P. destructans affects all life stages of hibernating bats, and the mortality in hibernacula can be very high, resulting in significant and rapid decreases in bat abundance in the regions affected by this fungi [23]. In North America, millions of bats have already died due to this fungi, leading to the decline of populations, with some common species (e.g., Muotis lucifugus) becoming almost inexistent in some regions [23,41]. Populations that are affected by P. destructans have a slow recovery due to their already low annual fecundity (one juvenile a year) and other causes of mortality such as wind-turbine collisions [42]. Some may never recover to the number before infection. The alteration in bat populations can lead to alterations at the trophic level; damaged ecosystem services (e.g., pollination); negative impacts on agriculture, forestry, human, and animal health; and reductions in native biodiversity and threatened species [23].
5.3. Economic Impacts
Insectivorous bats offer pest control services by consuming insects that damage crops and forests or vectors that carry diseases [43]. Bat species are also important pollinators and dispersers of seeds in tropical and subtropical regions [43]. These services save farmers millions of dollars every year. The mortality of bats due to these fungi is estimated to reduce these valuable and irreplaceable ecosystem services [44].
6. Conclusions
P. destructans has an enormous impact on the bat population, especially in North America, with levels of mortality so high that previously common bat species have become almost extinct.
There is limited understanding of both fungal pathogens and wildlife hosts, since fungal species, in general, are very poorly investigated compared to other taxa of pathogens. Existing antifungal medications are limited, and those in use often confer considerable side effects to the animals, and anti-fungal vaccines are still not available. There is also inadequate knowledge about the immunology, physiology, and ecology of P. destructans on the multiple species of bats infected. This has led to many challenges in the control and prevention of this disease [45].
Efforts to attend to this devastating disease have already been made, but in the future, more research and development of new drugs will be necessary to combat this disease.
Author Contributions
Conceptualization, A.G. and I.P.; methodology, A.G. and I.P.; software, A.G. and I.P.; validation, A.G. and I.P.; formal analysis, A.G. and I.P.; investigation, A.G. and I.P.; resources, A.G. and I.P.; data curation, A.G. and I.P.; writing—original draft preparation, A.G. and I.P.; writing—review and editing, A.G. and I.P.; visualization, A.G. and I.P.; supervision, A.G. and I.P.; project administration, A.G. and I.P.; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
The participation of Pires, I., was supported by the projects UIDB/CVT/00772/2020 and LA/P/0059/2020, funded by the Portuguese Foundation for Science and Technology (FCT). (Project UIDB/CVT/0772/2020.) The participation of Garcês, A., was supported by National Funds from the FCT-Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lorch, J.M.; Meteyer, C.U.; Behr, M.J.; Boyles, J.G.; Cryan, P.M.; Hicks, A.C.; Ballmann, A.E.; Coleman, J.T.H.; Redell, D.N.; Reeder, D.M.; et al. Experimental Infection of Bats with Geomyces Destructans Causes White-Nose Syndrome. Nature 2011, 480, 376–378. [Google Scholar] [CrossRef]
- Gargas, A.; Trest, M.; Christensen, M.; Volk, T.; Blehert, D. Geomyces Destructans Sp. Nov. Associated with Bat White-Nose Syndrome. Mycotaxon 2009, 108, 147–154. [Google Scholar] [CrossRef]
- Lindner, D.L.; Gargas, A.; Lorch, J.M.; Banik, M.T.; Glaeser, J.; Kunz, T.H.; Blehert, D.S. DNA-Based Detection of the Fungal Pathogen Geomyces Destructans in Soils from Bat Hibernacula. Mycologia 2011, 103, 241–246. [Google Scholar] [CrossRef]
- White-Nose Syndrome. Available online: https://www.whitenosesyndrome.org/where-is-wns (accessed on 4 July 2023).
- Blehert, D.S.; Hicks, A.C.; Behr, M.; Meteyer, C.U.; Berlowski-Zier, B.M.; Buckles, E.L.; Coleman, J.T.H.; Darling, S.R.; Gargas, A.; Niver, R.; et al. Bat White-Nose Syndrome: An Emerging Fungal Pathogen? Science 2009, 323, 227. [Google Scholar] [CrossRef] [PubMed]
- Minnis, A.M.; Lindner, D.L. Phylogenetic Evaluation of Geomyces and Allies Reveals No Close Relatives of Pseudogymnoascus Destructans, Comb. Nov., in Bat Hibernacula of Eastern North America. Fungal Biol. 2013, 117, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Meteyer, C.U.; Verant, M.L. 72-White-Nose Syndrome: Cutaneous Invasive Ascomycosis in Hibernating Bats. In Fowler’s Zoo and Wild Animal Medicine Current Therapy, Volume 9; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2019; pp. 508–513. ISBN 978-0-323-55228-8. [Google Scholar]
- Johnson, J.R. Virulence Factors in Escherichia Coli Urinary Tract Infection. Clin. Microbiol. Rev. 1991, 4, 49. [Google Scholar] [CrossRef]
- Puechmaille, S.J.; Wibbelt, G.; Korn, V.; Fuller, H.; Forget, F.; Mühldorfer, K.; Kurth, A.; Bogdanowicz, W.; Borel, C.; Bosch, T.; et al. Pan-European Distribution of White-Nose Syndrome Fungus (Geomyces Destructans) Not Associated with Mass Mortality. PLoS ONE 2011, 6, e19167. [Google Scholar] [CrossRef]
- Igor, P.; Đaković, M. Identification of Four Plecotus Species (Chiroptera, Vespertilionidae) in Croatia Based on Cranial Characters. Mammalia 2015, 80, 385–394. [Google Scholar] [CrossRef]
- Pikula, J.; Bandouchova, H.; Novotny, L.; Meteyer, C.U.; Zukal, J.; Irwin, N.R.; Zima, J.; Martínková, N. Histopathology Confirms White-Nose Syndrome in Bats in Europe. J. Wildl. Dis. 2012, 48, 207–211. [Google Scholar] [CrossRef]
- Puechmaille, S.J.; Frick, W.F.; Kunz, T.H.; Racey, P.A.; Voigt, C.C.; Wibbelt, G.; Teeling, E.C. White-Nose Syndrome: Is This Emerging Disease a Threat to European Bats? Trends Ecol. Evol. 2011, 26, 570–576. [Google Scholar] [CrossRef]
- Puechmaille, S.J.; Verdeyroux, P.; Fuller, H.; Gouilh, M.A.; Bekaert, M.; Teeling, E.C. White-Nose Syndrome Fungus (Geomyces destructans) in Bat, France. Emerg. Infect. Dis. 2010, 16, 290–293. [Google Scholar] [CrossRef]
- Wibbelt, G.; Kurth, A.; Hellmann, D.; Weishaar, M.; Barlow, A.; Veith, M.; Prüger, J.; Görföl, T.; Grosche, L.; Bontadina, F.; et al. White-Nose Syndrome Fungus (Geomyces destructans) in Bats, Europe. Emerg. Infect. Dis. 2010, 16, 1237–1243. [Google Scholar] [CrossRef]
- Zukal, J.; Bandouchova, H.; Brichta, J.; Cmokova, A.; Jaron, K.S.; Kolarik, M.; Kovacova, V.; Kubátová, A.; Nováková, A.; Orlov, O.; et al. White-Nose Syndrome without Borders: Pseudogymnoascus Destructans Infection Tolerated in Europe and Palearctic Asia but Not in North America. Sci. Rep. 2016, 6, 19829. [Google Scholar] [CrossRef]
- Leopardi, S.; Blake, D.; Puechmaille, S.J. White-Nose Syndrome Fungus Introduced from Europe to North America. Curr. Biol. 2015, 25, R217–R219. [Google Scholar] [CrossRef]
- Paiva-Cardoso, M.D.N.; Morinha, F.; Barros, P.; Vale-Gonçalves, H.; Coelho, A.C.; Fernandes, L.; Travassos, P.; Faria, A.S.; Bastos, E.; Santos, M.; et al. First Isolation of Pseudogymnoascus Destructans in Bats from Portugal. Eur. J. Wildl. Res. 2014, 60, 645–649. [Google Scholar] [CrossRef]
- Martínková, N.; Bačkor, P.; Bartonička, T.; Blažková, P.; Červený, J.; Falteisek, L.; Gaisler, J.; Hanzal, V.; Horáček, D.; Hubálek, Z.; et al. Increasing Incidence of Geomyces Destructans Fungus in Bats from the Czech Republic and Slovakia. PLoS ONE 2010, 5, e13853. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.M.; Worledge, L.; Miller, H.; Drees, K.P.; Wright, P.; Foster, J.T.; Sobek, C.; Borman, A.M.; Fraser, M. First Confirmation of Pseudogymnoascus Destructans in British Bats and Hibernacula. Vet. Rec. 2015, 177, 73. [Google Scholar] [CrossRef]
- Garzoli, L.; Bozzetta, E.; Varello, K.; Cappelleri, A.; Patriarca, E.; Debernardi, P.; Riccucci, M.; Boggero, A.; Girometta, C.; Picco, A.M. White-Nose Syndrome Confirmed in Italy: A Preliminary Assessment of Its Occurrence in Bat Species. J. Fungi 2021, 7, 192. [Google Scholar] [CrossRef]
- Hoyt, J.R.; Sun, K.; Parise, K.L.; Lu, G.; Langwig, K.E.; Jiang, T.; Yang, S.; Frick, W.F.; Kilpatrick, A.M.; Foster, J.T.; et al. Widespread Bat White-Nose Syndrome Fungus, Northeastern China. Emerg. Infect. Dis. 2016, 22, 140–142. [Google Scholar] [CrossRef]
- Lorch, J.M.; Knowles, S.; Lankton, J.S.; Michell, K.; Edwards, J.L.; Kapfer, J.M.; Staffen, R.A.; Wild, E.R.; Schmidt, K.Z.; Ballmann, A.E.; et al. Snake Fungal Disease: An Emerging Threat to Wild Snakes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150457. [Google Scholar] [CrossRef] [PubMed]
- Frick, W.F.; Pollock, J.F.; Hicks, A.C.; Langwig, K.E.; Reynolds, D.S.; Turner, G.G.; Butchkoski, C.M.; Kunz, T.H. An Emerging Disease Causes Regional Population Collapse of a Common North American Bat Species. Science 2010, 329, 679–682. [Google Scholar] [CrossRef]
- Warnecke, L.; Turner, J.M.; Bollinger, T.K.; Lorch, J.M.; Misra, V.; Cryan, P.M.; Wibbelt, G.; Blehert, D.S.; Willis, C.K.R. Inoculation of Bats with European Geomyces Destructans Supports the Novel Pathogen Hypothesis for the Origin of White-Nose Syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 6999–7003. [Google Scholar] [CrossRef]
- Maher, S.P.; Kramer, A.M.; Pulliam, J.T.; Zokan, M.A.; Bowden, S.E.; Barton, H.D.; Magori, K.; Drake, J.M. Spread of White-Nose Syndrome on a Network Regulated by Geography and Climate. Nat. Commun. 2012, 3, 1306. [Google Scholar] [CrossRef]
- Ballmann, A.E.; Torkelson, M.R.; Bohuski, E.A.; Russell, R.E.; Blehert, D.S. Dispersal hazards of pseudogymnoascus destructans by bats and human activity at hibernacula in summer. J. Wildl. Dis. 2017, 53, 725–735. [Google Scholar] [CrossRef]
- Carpenter, G.M.; Willcox, E.V.; Bernard, R.F.H.; Stiver, W. Detection of Pseudogymnoascus Destructans on Free-Flying Male Bats Captured During Summer in the Southeastern USA. J. Wildl. Dis. 2016, 52, 922–926. [Google Scholar] [CrossRef] [PubMed]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 14 November 2022).
- Chaturvedi, M.; Sharma, C.; Chaturvedi, M. Effects of Pesticides on Human Beings and Farm Animals: A Case Study. Res. J. Chem. Environ. Sci. 2013, 1, 6. [Google Scholar]
- Raudabaugh, D.B.; Miller, A.N. Nutritional Capability of and Substrate Suitability for Pseudogymnoascus Destructans, the Causal Agent of Bat White-Nose Syndrome. PLoS ONE 2013, 8, e78300. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Ryan, P.G. Micro-Plastic Ingestion by Waterbirds from Contaminated Wetlands in South Africa. Mar. Pollut. Bull. 2018, 126, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Shuey, M.M.; Drees, K.P.; Lindner, D.L.; Keim, P.; Foster, J.T. Highly Sensitive Quantitative PCR for the Detection and Differentiation of Pseudogymnoascus Destructans and Other Pseudogymnoascus Species. Appl. Environ. Microbiol. 2014, 80, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Verant, M.L.; Boyles, J.G.; Waldrep, W., Jr.; Wibbelt, G.; Blehert, D.S. Temperature-Dependent Growth of Geomyces Destructans, the Fungus That Causes Bat White-Nose Syndrome. PLoS ONE 2012, 7, e46280. [Google Scholar] [CrossRef]
- Flieger, M.; Bandouchova, H.; Cerny, J.; Chudíčková, M.; Kolarik, M.; Kovacova, V.; Martínková, N.; Novák, P.; Šebesta, O.; Stodůlková, E.; et al. Vitamin B2 as a Virulence Factor in Pseudogymnoascus Destructans Skin Infection. Sci. Rep. 2016, 6, 33200. [Google Scholar] [CrossRef]
- Mascuch, S.J.; Moree, W.J.; Hsu, C.-C.; Turner, G.G.; Cheng, T.L.; Blehert, D.S.; Kilpatrick, A.M.; Frick, W.F.; Meehan, M.J.; Dorrestein, P.C.; et al. Direct Detection of Fungal Siderophores on Bats with White-Nose Syndrome via Fluorescence Microscopy-Guided Ambient Ionization Mass Spectrometry. PLoS ONE 2015, 10, e0119668. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Risch, T.S.; Savary, B.J. Isolation and Identification of an Extracellular Subtilisin-Like Serine Protease Secreted by the Bat Pathogen Pseudogymnoascus Destructans. PLoS ONE 2015, 10, e0120508. [Google Scholar] [CrossRef]
- Smyth, C.; Schlesinger, S.; Overton, B.; Butchkoski, C. The Alternative Host Hypothesis and Potential Virulence Genes in Geomyces Destructans. Bat Res. News 2013, 54, 17–24. [Google Scholar]
- Turner, G.G.; Meteyer, C.U.; Barton, H.; Gumbs, J.F.; Reeder, D.M.; Overton, B.; Bandouchova, H.; Bartonička, T.; Martínková, N.; Pikula, J.; et al. Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome. J. Wildl. Dis. 2014, 50, 566–573. [Google Scholar] [CrossRef]
- Reeder, D.M.; Frank, C.L.; Turner, G.G.; Meteyer, C.U.; Kurta, A.; Britzke, E.R.; Vodzak, M.E.; Darling, S.R.; Stihler, C.W.; Hicks, A.C.; et al. Frequent Arousal from Hibernation Linked to Severity of Infection and Mortality in Bats with White-Nose Syndrome. PLoS ONE 2012, 7, e38920. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Wund, M.A. Experimental Assessment of the Impacts of Northern Long-Eared Bats on Ovipositing Culex (Diptera: Culicidae) Mosquitoes. J. Med. Entomol. 2009, 46, 1037–1044. [Google Scholar] [CrossRef]
- Reynolds, H.T.; Ingersoll, T.; Barton, H.A. Modeling the environmental growth of pseudogymnoascus destructans and its impact on the white-nose syndrome epidemic. J. Wildl. Dis. 2015, 51, 318–331. [Google Scholar] [CrossRef]
- Russell, R.E.; Thogmartin, W.E.; Erickson, R.A.; Szymanski, J.; Tinsley, K. Estimating the Short-Term Recovery Potential of Little Brown Bats in the Eastern United States in the Face of White-Nose Syndrome. Ecol. Model. 2015, 314, 111–117. [Google Scholar] [CrossRef]
- Boyles, J.; Cryan, P.; Mccracken, G.; Kunz, T. Economic Importance of Bats in Agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Fenton, B. Bat Ecology; The University of Chicago Press: Chicago, IL, USA, 2006; ISBN 0-226-46207-2. [Google Scholar]
- Blehert, D.; Lankau, E. Pseudogymnoascus Destructans (White-Nose Syndrome Fungus). CABI Compend. 2017, 1, 119002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
