Abstract
β-casomorphin 7 (BCM7) is a bioactive peptide that is released during the digestion of the β-casein (in particular, the A1 variant) present in cow’s milk. BCM7 has been linked to several health concerns such as gastrointestinal disorders. Milk processing alters the composition of milk, which in turn may affect its digestion, thus impacting the amount of BCM7 that is released. This study aimed to understand the impact of microfiltration on BCM7 release after the in vitro digestion (mimicking in vivo digestion) of semi-skimmed filtered milk compared to that of pasteurized milk and pasteurized Jersey milk (which does not contain A1 β-casein, the main source of BCM7). LC/MS was used to quantify BCM7. The results indicated that the β-casein variants present in milk, rather than the milk treatments themselves, are the key factors for the release of BCM7. Similar BCM7 levels were found in the filtered and pasteurized milk samples, whereas the Jersey milk released just half the amount.
1. Introduction
β-casein (~30% of total casein) has 12 variants (A1, A2, A3, B, C, D, E, F, G, H1, H2, and I) that differ in their amino acid sequence. Most cattle breeds of European origin have a mix of these variants, while Guernsey and Jersey cows produce milk that has A2 as the major variant [1].
β-casomorphin-7 (BCM7) is an opioid peptide that has effects like those of morphine and has been isolated from an enzymatic digest of β-casein (in particular, A1) and has been linked to several health concerns such as gastrointestinal disorders [2]. Minimal amounts of BCM7 are, however, released from milk that contains A2 β-casein as its main protein [3,4,5]. The difference between A1 and A2 β-casein lies in the specific amino acid at a particular position (the 67th position) in the protein sequence. A1 β-casein contains the amino acid histidine at this position in its protein sequence, whereas A2 β-casein has the amino acid proline [4,5,6]. The pasteurization (85 °C/30 s) and UHT (140 °C/15 s) of milk inhibit the formation of BCM7 during intestinal digestion, which could be due to the protein denaturation altering the protein digestion [3,7,8,9] or due to the formation of radicals during the Maillard reaction that could attack the protein backbone, subsequently modifying some of the peptides that are formed [10]. Traditionally, milk has been subjected to heat treatments that differ in time and temperature, with pasteurization and UHT being the most commonly used [4,7,9,11]. However, more recently, a new filtered milk which undergoes pasteurization and microfiltration, offering a longer shelf life compared to pasteurized milk, has become available in UK supermarkets. In 2020, sales of filtered milk witnessed a significant boost, attributed to its extended shelf life that reduces milk wastage caused by spoilage or expiration when compared to pasteurized milk [12].
Although research has investigated BCM peptides resulting from the digestion of heat-treated milk [7,9], there are gaps in the literature concerning filtered milk and the generation of BCM7 during digestion. Hence, this study aimed to assess the proportions of the main β-casein variant proteins and characterize the release of BCM7 during the in vitro digestion of commercially filtered milk. Pasteurized milk from the same brand of filtered milk and Jersey milk (A2 milk) was used for the comparison of the process effect and A1 variant content, respectively.
2. Materials and Methods
Commercially conventional available semi-skimmed filtered milk samples from major food retailers in the UK were used for this study. Seven brands offered both filtered pasteurized (MF) and non-filtered (pasteurized, P) milk. A Jersey milk sample (A1-free milk) was used as a negative control. Three different batches for each of the milk samples were used to carry out the analysis. All reagents were of analytical grade.
The extraction and the analysis of milk proteins were carried out by the method described by Givens et al. [13] using high-resolution HPLC–MS. Identification was possible as a standard of β-caseins was available and analyzed alongside the milk samples. Identification was carried out by means of the MS spectra and UV chromatograms of the β-casein region, together with extracted ion chromatograms of the A1, A2, and B variants at 1144.95, 1143.05, and 1148.16 m/z, respectively [13,14].
The in vitro gastric and intestinal digestion model used to simulate the fasted state in humans was the one described by Gallier et al. [15]. For the BCM7 analysis, aliquots were collected after the gastric and intestinal stages. Two controls were run with the samples; one was the milk and digestion buffer without enzymes and the other one was the digestion buffer and enzymes without milk.
The system used for the identification and quantification of BCM7 released after the in vitro digestion of milk samples was an Agilent 1100 HPLC interfaced with a Bruker micrOTOF-QII QTOF mass spectrometer. Elution solvents A and B were (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile with a gradient elution using LC/MS-grade elution solvents (Fisher Chemical™, Loughborough, UK) on an ACE® C-18 column (300 Å 5 µM 150 mm × 2.1 mm). The quantification of BCM7 in both the samples and in the deuterated BCM7 standards was accomplished by comparing the peak areas in the extracted ion chromatogram at 790.4 m/z. All treatments and measurements were carried out in triplicate. Two-way ANOVA (XLSTAT software version 2022.1.2 (Addinsoft, New York, NY, USA) was used to analyze whether the type of treatment produced significant differences between sample means. Tukey’s test was applied to determine significant differences (p < 0.05) between the means.
3. Results and Discussion
Figure 1 shows the relative content of β-casein variants (as % of total β-casein) present in the conventional milk (MF and P) and Jersey milk samples. An external calibration curve was used for the quantification of the proteins (R2 = 0.98). The spectra were deconvoluted to determine the molecular mass of the proteins in the purified samples, which enabled the identification of the β-casein variants by confirming the genotype with masses of 24,023, 23,968, and 24,092 Da for variants A1, A2, and B, respectively [16]. All conventional milk samples had a higher content of the A2 variant compared to A1. The distribution of the main casein variants A1, A2, and B ranged between 55 and 65, 54 and 68, and 2.5 and 4% for the A1, A2, and B β-casein variants in conventional milk, respectively. As seen in the results shown in Figure 1, the major β-casein variant in conventional milk that was available in the main market in the UK was A2, followed by A1. However, Jersey milk has no A1, and its main β-casein variants were A2 and B (about 75 and 25% of total β-casein, respectively). These results are consistent with the previous data, which demonstrate a characteristic distribution of A2 and B variants in casein separated from Jersey milk. Specifically, the proportions were found to range from 48 to 60 and 28 to 40% of the total β-casein, respectively [17]. In contrast, milk from a different breed exhibited A1, A2, and B variant proportions ranging from 51 to 71, 28 to 40, and 2 to 10% of the total β-casein, respectively [13,17]. Understanding the distribution of β-CN variants in milk samples from different processes is important in order to monitor process-induced changes in Pro67 and His67 balance in milk and dairy products, avoiding a possible excessive increase in His67 in the processed milk due to milk protein denaturation or/and conformation.
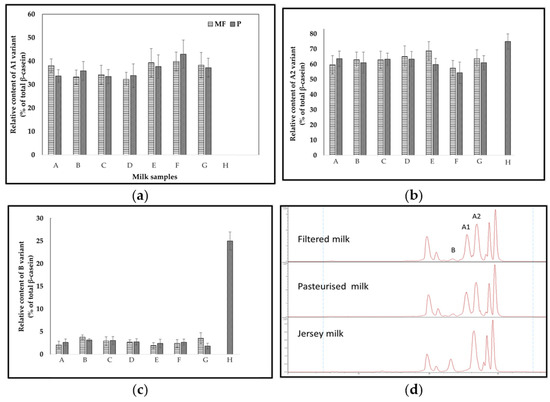
Figure 1.
The relative content of β-casein variants (% of total β-casein) in milk samples (A–H): (a) A1 variant; (b) A2 variant; (c) B variant. MF = filtered. P = pasteurized. Bars are the mean of 3 replicates ± standard deviation. (d) Separation of bovine casein proteins from filtered and pasteurized milk by high resolution HPLC–MS at 214 nm.
Milk samples were also subjected to in vitro digestion, and BCM7 was quantified by LC-MS (Figure 2). In the present work, no BCM7 was detected in either conventional or Jersey milk before or after digestion with Pepsin alone (in vitro gastric digestion), irrespective of the milk type or processing method employed. This differs from earlier research that indicated the formation of BCM7 in the gastric digests of purified β-casein variants and milk containing A1 and A2 variants [11,18]. However, our findings align with recent studies that used a similar experimental setup and more physiologically relevant digestion conditions [7,9]. These discrepancies in results are likely attributed to variations in digestive conditions and analytical methods used to identify BCM7. However, BCM7 was detected in all milk samples after the intestinal stage. The BCM7 released after the intestinal stage from filtered and pasteurized milk samples ranged between 4.55 to 7.05 and 4.99 to 6.89 mg g−1 β-casein, respectively. No significant differences were found in the BCM7 content between the filtered and pasteurized milk samples from the same brand, where the p-values were more than 0.05. The amount of BCM7 released from conventional milk samples was approximately double that released by Jersey milk (2.5–4.5 mg g−1 β-casein). While most studies correlate the BCM7 released after intestinal digestion with the presence of A1 [7,9], the absence of A1 in Jersey milk was not sufficient to eliminate the presence of BCM7. These results showed that the A1 variant is not solely responsible for the release of BCM7 after milk digestion. More investigation is needed to study the effect of other β-casein variants and milk matrix on the release of BCM7.
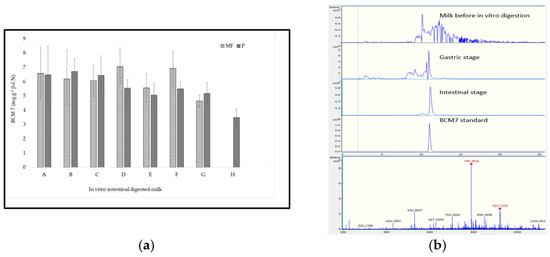
Figure 2.
(a) Concentration of BCM7 detected after in vitro gastrointestinal digestion (mg BCM7 mg−1 β-casein). MF = filtered. P = pasteurized. Bars are the mean of 3 replicates ± standard deviation. Star denotes a significant difference (p < 0.05). (b) Extracted chromatographic peak of BCM7 (at mass 790.4 Da) in milk samples before and after gastrointestinal digestion.
4. Conclusions
BCM7 is released during the intestinal digestion stage. Through the simulated in vitro digestion of milk and utilization of a BCM7 standard for peptide quantification, it was clear that BCM7 formation can arise from both conventional and Jersey milk. Among the filtered and pasteurized milk samples, there was no significant difference in the content of BCM7. This suggests that microfiltration has no significant effect on the proportions of β-casein variants. However, Jersey milk exhibited a significantly lower BCM7 content. This study suggests that the relatively lower concentration of BCM7 in Jersey milk compared to conventional samples may be attributed more to differences in β-casein composition than to the effect of microfiltration. More investigation is needed to understand the effect of β-casein composition on the BCM7 released after milk digestion.
Author Contributions
Conceptualization, M.-J.O.-C. and M.C.; methodology, N.M. and R.B.; software, Burker Data Analysis software 4.3 and XLSTAT version 2022.1.2 (Addinsoft, New York, NY, USA); formal analysis, R.B.; investigation, R.B.; resources, M.-J.O.-C., M.C. and R.B.; data curation, R.B.; writing—original draft preparation, R.B.; writing—review and editing, M.-J.O.-C., M.C. and N.M.; supervision, M.-J.O.-C. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by the Ministry of Higher Education and Scientific Research, Libya, under JACS Code 100527 at the University of Reading. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Mintel database (dairy and non-dairy drinks, milk, and cream, UK, June, 2021) is not publicly available; however, select data for research purposes can be made available upon request to the corresponding author. Milk and Non-Dairy Milk-US-2021: Consumer market research report | Mintel.com.
Conflicts of Interest
M.C. has received funding from Arla Foods and Apetito. The remaining authors declare that research was conducted in the absence of any commercial or financial relationships that could be construe as a potential conflict of interest.
References
- ul Haq, M.R.; Kapila, R.; Shandilya, U.K.; Kapila, S. Impact of milk derived β-casomorphins on physiological functions and trends in research: A review. Int. J. Food Prop. 2014, 17, 1726–1741. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Review of the potential health impact of β-casomorphins and related peptides. EFSA J. 2009, 7, 231r. [Google Scholar] [CrossRef]
- De Noni, I.; Cattaneo, S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chem. 2010, 119, 560–566. [Google Scholar] [CrossRef]
- Cieślińska, A.; Kostyra, E.; Kostyra, H.; Oleński, K.; Fiedorowicz, E.; Kamiński, S. Milk from cows of different β-casein genotypes as a source of β-casomorphin-7. Int. J. Food Sci. Nutr. 2012, 63, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Asledottir, T.; Le, T.T.; Poulsen, N.A.; Devold, T.G.; Larsen, L.B.; Vegarud, G.E. Release of β-casomorphin-7 from bovine milk of different β-casein variants after ex vivo gastrointestinal digestion. Int. Dairy J. 2018, 81, 8–11. [Google Scholar] [CrossRef]
- Ul Haq, M.R.; Haq, M.R.U. Structure and Production of Casomorphins. In Opioid Food Peptides: Significant Exorphins from Food Sources; Spring: Berlin/Heidelberg, Germany, 2020; pp. 21–38. [Google Scholar]
- Lambers, T.T.; Broeren, S.; Heck, J.; Bragt, M.; Huppertz, T. Processing affects beta-casomorphin peptide formation during simulated gastrointestinal digestion in both A1 and A2 milk. Int. Dairy J. 2021, 121, 105099. [Google Scholar] [CrossRef]
- Cattaneo, S.; Pica, V.; Stuknytė, M.; Masotti, F.; Mallardi, D.; Tabasso, C.; Roggero, P.; De Noni, I. Effect of protein fortification on heat damage and occurrence of β-casomorphins in (un) digested donor human milk intended for nutrition of preterm infants. Food Chem. 2020, 314, 126176. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, S.; Masotti, F.; Stuknytė, M.; De Noni, I. Impact of in vitro static digestion method on the release of β-casomorphin-7 from bovine milk and cheeses with A1 or A2 β-casein phenotypes. Food Chem. 2023, 404, 134617. [Google Scholar] [CrossRef] [PubMed]
- Meltretter, J.; Schmidt, A.; Humeny, A.; Becker, C.M.; Pischetsrieder, M. Analysis of the peptide profile of milk and its changes during thermal treatment and storage. J. Agric. Food Chem. 2008, 56, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Cieślińska, A.; Kamiński, S.; Kostyra, E.; Sienkiewicz-Szłapka, E. Beta-casomorphin 7 in raw and hydrolyzed milk derived from cows of alternative β-casein genotypes. Milchwissenschaft 2007, 62, 125–127. [Google Scholar]
- Mintel. Dairy and Non-dairy Drinks, Milk and Cream—UK—2021; Mintel Group Ltd.: London, UK, 2021. [Google Scholar]
- Givens, I.; Aikman, P.; Gibson, T.; Brown, R. Proportions of A1, A2, B and C β-casein protein variants in retail milk in the UK. Food Chem. 2013, 139, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Grigoletto, L.; Cecchinato, A.; Gallo, L.; Carnier, P. Validation of a new reversed-phase high-performance liquid chromatography method for separation and quantification of bovine milk protein genetic variants. J. Chromatogr. A 2008, 1195, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.; Ye, A.; Singh, H. Structural changes of bovine milk fat globules during in vitro digestion. J. Dairy Sci. 2012, 95, 3579–3592. [Google Scholar] [CrossRef] [PubMed]
- Fuerer, C.; Jenni, R.; Cardinaux, L.; Andetsion, F.; Wagnière, S.; Moulin, J.; Affolter, M. Protein fingerprinting and quantification of β-casein variants by ultra-performance liquid chromatography–high-resolution mass spectrometry. J. Dairy Sci. 2020, 103, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.M.; Graham, E.B.; Ponzoni, R.W.; McKenzie, H.A. Effects of milk protein genetic variants on milk yield and composition. J. Dairy Res. 1984, 51, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Busetti, F.; Johnson, S.K.; Solah, V.A. Identification and quantification of native beta-casomorphins in Australian milk by LC–MS/MS and LC–HRMS. J. Food Compos. Anal. 2015, 44, 102–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).