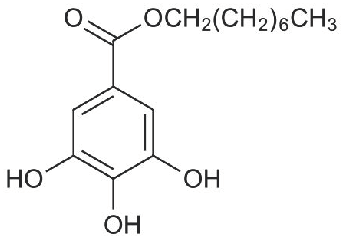
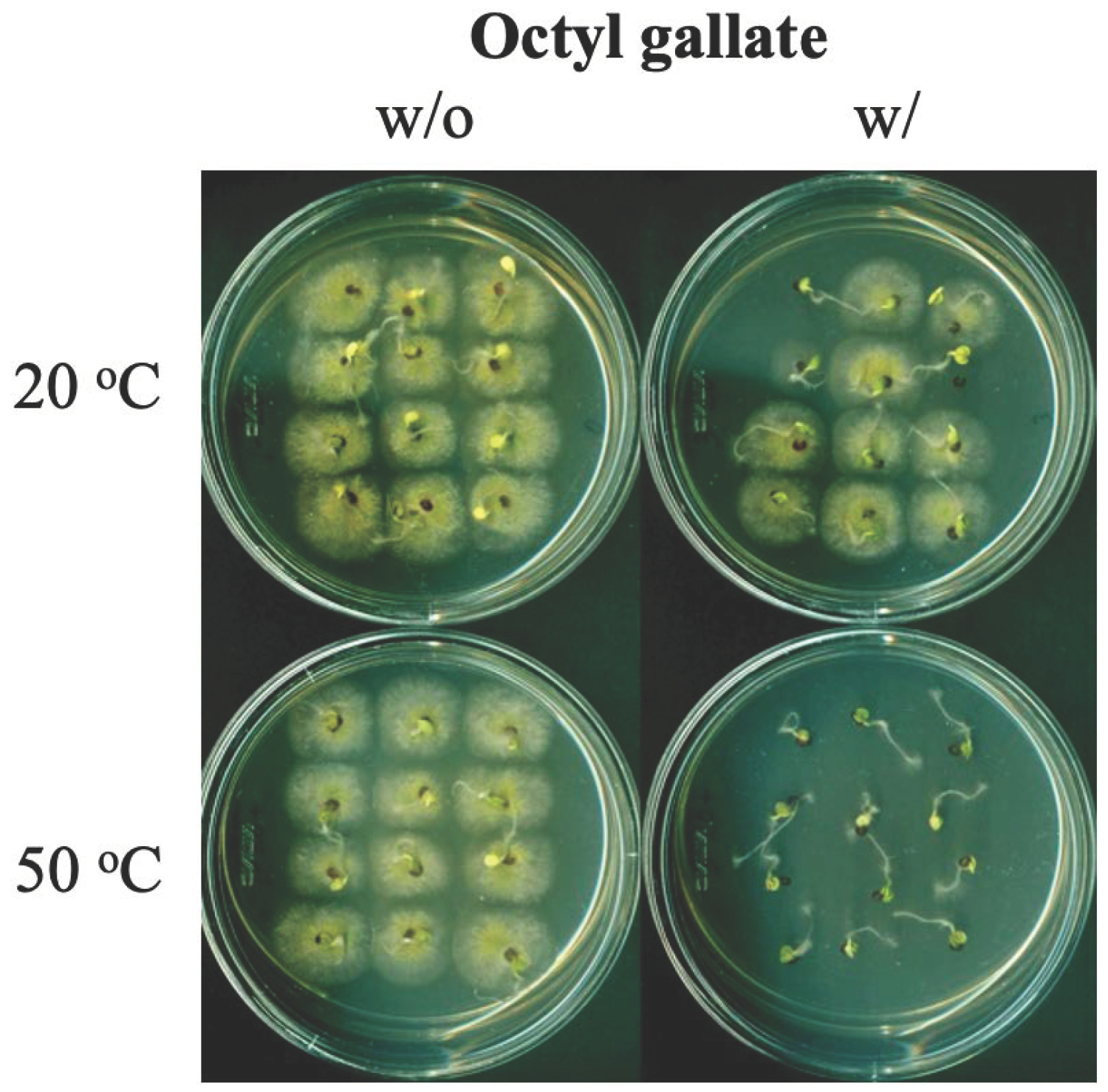
Octyl Gallate Use to Protect Seeds from Foodborne Fungal Pathogens †
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowyer, P.; Denning, D.W. Environmental fungicides and triazole resistance in Aspergillus. Pest Manag. Sci. 2014, 70, 173–178. [Google Scholar] [CrossRef]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A 2016, 33, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.R.; Khodadad, C.L.M.; Hummerick, M.E.; Spern, C.J.; Spencer, L.E.; Fischer, J.A.; Curry, A.B.; Gooden, J.L.; Maldonado Vazquez, G.J.; Wheeler, R.M.; et al. Persistence of Escherichia coli in the microbiomes of red Romaine lettuce (Lactuca sativa cv. ‘Outredgeous’) and mizuna mustard (Brassica rapa var. japonica)—Does seed sanitization matter? BMC Microbiol. 2021, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Dagnas, S.; Membré, J.-M. Predicting and Preventing Mold Spoilage of Food Products. J. Food Prot. 2013, 76, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Stylianou, M.; Kulesskiy, E.; Lopes, J.P.; Granlund, M.; Wennerberg, K.; Urban, C.F. Antifungal Application of Nonantifungal Drugs. Antimicrob. Agents Chemother. 2014, 58, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cheng, L.W.; Chan, K.L.; Tam, C.C.; Mahoney, N.; Friedman, M.; Shilman, M.M.; Land, K.M. Antifungal Drug Repurposing. Antibiotics 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Substances Added to Food (formerly EAFUS). Available online: https://www.fda.gov/food/food-additives-petitions/substances-added-food-formerly-eafus (accessed on 8 August 2022).
- Kirkman, T.W. Statistics to Use. Available online: http://www.physics.csbsju.edu/stats/ (accessed on 5 July 2022).
- Pest Management Regulatory Agency (PMRA) Canada. Re-Evaluation Decision RVD2018-38, Thiram and Its Associated End-use Products. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-decision/2018/thiram.html (accessed on 8 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Chan, K.L. Octyl Gallate Use to Protect Seeds from Foodborne Fungal Pathogens. Biol. Life Sci. Forum 2022, 18, 7. https://doi.org/10.3390/Foods2022-12926
Kim JH, Chan KL. Octyl Gallate Use to Protect Seeds from Foodborne Fungal Pathogens. Biology and Life Sciences Forum. 2022; 18(1):7. https://doi.org/10.3390/Foods2022-12926
Chicago/Turabian StyleKim, Jong H., and Kathleen L. Chan. 2022. "Octyl Gallate Use to Protect Seeds from Foodborne Fungal Pathogens" Biology and Life Sciences Forum 18, no. 1: 7. https://doi.org/10.3390/Foods2022-12926
APA StyleKim, J. H., & Chan, K. L. (2022). Octyl Gallate Use to Protect Seeds from Foodborne Fungal Pathogens. Biology and Life Sciences Forum, 18(1), 7. https://doi.org/10.3390/Foods2022-12926






