Abstract
Microbial oil (SCO) is lipids accumulated in the cells of oleaginous microorganisms, including yeast, in amounts exceeding 20% of dry mass. These are a valuable source of fatty acids in the human diet. In order to facilitate the extraction of storage lipids from cells, methods of physical and chemical pre-treatment of biomass are used to break the barrier of the cell wall and membrane of these microorganisms to the action of organic solvents, which are used during traditional extraction. The aim of this study was to evaluate the effectiveness of unconventional methods of extracting microbial oil from Yarrowia lipolytica yeast cells. Pulsed electric field (PEF), cell disintegration by ultrasonic waves and high-pressure homogenization (HPH) were used. The use of unconventional methods turned out to be ineffective in the extraction of intracellular lipids of the yeast compared to methods involving organic solvents such as chloroform, methanol and hexane. Nevertheless, the use of a pulsed electric field with a field strength of 200 J/g or high-pressure homogenization (1100 bar) proved to be effective as pre-treatment techniques of Y. lipolytica yeast cells (cell permeabilization) for the high yield extraction of intracellular lipids using the extraction method with organic solvents.
1. Introduction
Y. lipolytica yeast belongs to the oleaginous yeasts, which means that they have the ability to produce and store intracellular lipids (microbial oil) in an amount exceeding 20% dry cell mass [1]. Microbial oil is considered a valuable alternative to vegetable and fish oils. Its extraction is an important issue, because this process is usually not very efficient due to the presence of the cell wall of the microorganisms [2].
There are many methods for the pre-treatment of biological material, including methods of mechanical pre-treatment e.g., shear forces (high-speed and high-pressure homogenization, microfluidization) and direct energy transfer to cells (laser, ultrasound and microwave) [3]. Physical methods of disintegrating cell walls include decompression, osmotic shock, microwave, pulsed electric field and freeze-drying [4]. Cell permeabilization or disruption of cells can also be achieved with the use of various types of chemical compounds [4,5]. Most extraction methods for total microbial lipids involve extraction with an organic solvent such as used in the Soxhlet, Bligh and Dyer and Folch methods [6]. In this study, an attempt has been made to evaluate the impact of unconventional methods for extracting lipids from Y. lipolytica yeast cells. The methods included: pulsed electric field (PEF), ultrasounds (US) and high-pressure homogenization (HPH).
2. Materials and Methods
2.1. Yeast Strain and Culture Conditions
The Y. lipolytica strain KKP 379 from the Collection of Industrial Microorganisms at the Prof. Wacław Dąbrowski Institute of Agricultural and Food Biotechnology in Warsaw (Poland) was used in the study. An inoculum culture was provided for 24 h in a YPG medium (yeast extract 1%, peptone 2%, glucose 2%) on a rotary shaker at 28 °C. The appropriate cultures were produced in a BioFlo 3000 laboratory bioreactor at 28 °C for 66 h, without pH adjustment, under minimum 30% oxygenation conditions for the medium with respect to its initial concentration; agitator speed was in the range of 200–600 RPM in a mineral medium with waste post-frying oil according to the method of Fabiszewska et al. [7]. The biomass obtained after culture in the bioreactor was centrifuged (10 min, 8000 RPM), washed with 0.9% NaCl solution and frozen for further experiments.
2.2. Lipids Extraction
The dry biomass was ground, weighed and transferred to a falcon-type tube. For every 1 g of dry biomass, 10 cm3 of a chloroform:methanol mixture in 2:1 (v/v) ratio was added, carrying out a two-fold extraction of lipids according to the Folch method. The samples were centrifuged (10 min, 8000 RPM). After centrifugation, the obtained liquid was filtered on a filter paper strainer into a weighed round-bottom flask. This procedure was repeated four times. The solvent was evaporated. For the determination of lipid content in solutions after treating cells with ultrasound, PEF or HPH, the procedure was identical, with the addition of 10 cm3 of the solvent mixture for every 20 cm3 of solution. To evaluate the effect of permeabilization on Folch-extracted lipids, yeast biomass was shaken with hexane for 60 min at room temperature, the solvent evaporated, and the biomass subjected to a Folch extraction.
2.3. Non-Conventional Methods for Yeast Cell Treatment
The unfrozen yeast cells were suspended in saline. The resulting solutions were sonicated using a Hielscher UP400S ultrasonic homogenizer (Hielscher, Teltow, Germany) for 10 min under the conditions shown in Table 1.

Table 1.
Parameters of sonication.
Yeast cells were also treated with PEF according to the conditions shown in Table 2. For this purpose, the conductivity of the solutions were measured, and then the cells were transferred to the chamber of the Elea GmbH (Quakenbrück, Germany) pulsed electric field application system.

Table 2.
Conditions for the application of a pulsed electric field.
A 25% (m/v) suspension of yeast cells was passed through a Niro Soavi NS 1001 L2 PANDA high-pressure homogenizer (GEA, Parma, Italy) under the variable pre-treatment conditions shown in Table 3.

Table 3.
Conditions for yeast treatment with high pressure homogenization.
2.4. Statistical Analysis
Statistical analyses of repeated measurements was performed with the one-way ANOVA, followed by Tukey’s multiple comparison test using STATISTICA 13.3 (Statsoft, Kraków, Poland). Any p-values lower than 0.05 were considered to be statistically significant.
3. Results
Figure 1a shows the average content of microbial oil extracted from the solution after ultrasonic disintegration of Y. lipolytica yeast KKP 379. For the control sample, which was not treated with ultrasound, microbial oil was not detected in the yeast solution. It should be noted that sample 1. had the highest percentage of extracted oil, followed by samples 3, 5 and 7. As in the case of extraction from dried biomass, these results indicate that the sonication process proceeded more efficiently when the concentration of suspended cells was lower (10%). It is worth noting that all sonicated samples were characterized by the presence of microbial oil in the solutions. This demonstrates that the permeabilization of yeast cell structures took place, and intracellular lipids were released into the external environment.
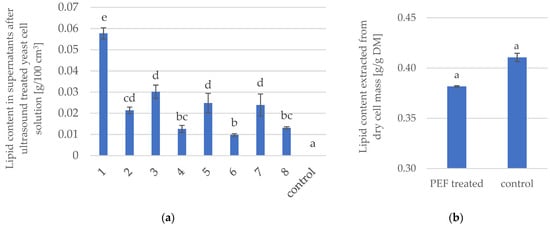
Figure 1.
(a) Lipid content in supernatants after ultrasound treated yeast cells [g oil/100 cm3]. (b) Microbial oil content extracted from dry biomass of PEF-treated Y. lipolytica yeast. Homogeneous groups designated on the basis of Tukey’s test were identified by letters and different posts. Number of treatment refers to individual process parameters according to Table 1 (a) or Table 2 (b). Control is untreated yeast cells.
Preliminary experiments using a pulsed electric field (data not shown) concluded that the higher the applied average field energy the higher the extraction efficiency of microbial oil from yeast biomass and probably the better the permeabilization effect of cell structures. It would have been beneficial to use the highest electric field voltage of 17 kV, but at this value, during the initial experiment, the electric pulse application system was discharged because the conductivity of the solution was too high. In the next experiment, an electrical voltage of 10 kV and an average energy of about 100 J/g of solution was applied (Figure 1b). Non-significant differences were observed in the content of microbial oil extracted from PEF-treated and untreated freeze-dried biomass.
Figure 2 shows the average content of oil extracted from Y. lipolytica yeast biomass after high-pressure homogenization. It should be noted that the average contents of extracted oil from samples 1, 2 and 4 did not show a significant difference from the control sample, while the average content of lipids obtained from sample 3 was even lower compared to the control sample. The effect of extracting microbial oil into solutions from Y. lipolytica yeast cells subjected to high pressure homogenization was also evaluated. A small amount of microbial oil (2%) was extracted, but only from the variant with the highest applied pressure (700 bar, data not shown). Thus, it was confirmed that extraction of lipids from HPH-treated biomass at the assumed high pressure homogenization conditions was most likely insufficient to induce perforation of Y. lipolytica yeast cell structures and lipid leakage.
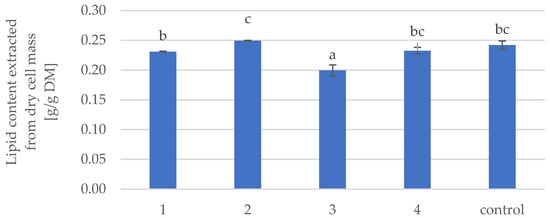
Figure 2.
Average microbial oil content extracted using the Folch method from dried Y. lipolytica yeast biomass after high-pressure homogenization treatment according to parameters in Table 3. Homogeneous groups designated on the basis of Tukey’s test were identified by letters and different posts. Control is untreated yeast cells.
Given the unsatisfactory results of intracellular lipid extraction with the methods described above, their suitability as a form of pre-treatment of yeast cells was evaluated. The degree of permeabilization of the membrane and cell wall structure was assessed by measuring the amount of lipids eluted from cells after treating them with hexane (Table 4). The application of a pulsed electric field with a field strength of 10 kV (200 J/g) (Table 2, parameters for raw biomass) and high-pressure homogenization (Table 3, 10—times application of 1100 bar pressure) proved to be effective methods for the preparation of Y. lipolytica yeast cells (cell permeabilization) which were subsequently used for the proper extraction of intracellular lipids with the Folch method (with a mixture of chloroform and methanol solvents).

Table 4.
Impact of HPH and PEF on yeast permeabilization.
4. Discussion
Sonication is a good laboratory method for permeabilizing cell walls and releasing proteins from the cell [8]. This indicates that the use of ultrasound causes a high degree of loosening of the yeast cell wall and membrane. In the experiment, a higher degree of intracellular lipid extraction from yeast cells was achieved relative to the control, confirming that the use of ultrasound has a positive effect on destroying cell structures and improving the efficiency of microbial oil extraction, although the result was far from satisfactory.
Based on the literature, using high-pressure homogenization at 2000 bar, with 15 passes of the sample through the homogenizer, the efficiency of lipid extraction from Saitozyma podzolica DSM 27192 yeast cells after Folch extraction was 37.8% [9]. There are other studies that also used high-pressure homogenization against a 15% solution of Y. lipolytica JMY5578 yeast cells. The biomass solution was passed through a homogenizer 20 times at a pressure of 1500 bar. The conditions used resulted in an oil extraction of 83.9% from the dried biomass compared to a control of 19.8% [10]. This may indicate that the high-pressure homogenization conditions used in the present study were too mild to perforate yeast cell structures and consequently increase the efficiency of intracellular oil extraction with the Folch method. Only the use of high-pressure homogenization (10 × 1100 bar) could be considered as a method for preparing yeast biomass for proper intracellular lipid extraction.
A slightly lower, but also significant difference in microbial oil extraction relative to the control was observed in the sample after application of pulsed electric field action. As reported in the literature, other experiments using pulsed electric field also increased the extraction efficiency of microbial oil from dry biomass, but not as significantly as other techniques. In one study, the average content of intracellular fats extracted from dry biomass after PEF application increased from 19.8% to 29.4% [10]. The use of this method probably allowed permeabilization and/or disintegration of yeast cell walls and membranes, resulting in a slight increase in extraction efficiency relative to the control.
5. Conclusions
The use of unconventional methods under the described parameters (ultrasound treatment, pulsed electric field and high-pressure homogenization) was ineffective in the extraction of intracellular lipids of the yeast compared to methods involving organic solvents such as chloroform, methanol and hexane. Nevertheless, the use of a pulsed electric field with a field strength of 200 J/g or high-pressure homogenization (10 × 1100 bar) proved to be effective as pre-treatment techniques of Y. lipolytica yeast cells (cell permeabilization) for the high yield extraction of intracellular lipids using the extraction method with organic solvents.
Author Contributions
Conceptualization, K.W. and A.F.; methodology, K.W., A.F., D.N. and A.M.; formal analysis, K.W., A.F. and A.M.; investigation, K.W., A.M. and D.N.; resources, A.F.; writing—original draft preparation, K.W.; writing—review and editing, K.W., A.F., B.Z.; visualization, A.M.; supervision, A.F., B.Z. and K.J. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by sources of the Ministry of Education and Science within funds of the Institute of Food Sciences of Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge Ewa Domian and Artur Wiktor (Institute of Food Sciences, Warsaw University of Life Sciences) for kindly lending the scientific equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I. Drożdże jak o potencjalne źródło tłuszczu mikrobiologicznego. Postępy Mikrobiologii (Adv. Microbiol.) 2015, 54, 364–373. [Google Scholar]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef] [PubMed]
- Geciova, J.; Bury, D.; Jelen, P. Methods for disruption of microbial cells for potential use in the dairy industry—A review. Int. Dairy J. 2002, 12, 541–553. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Fai, C.K.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Current Pretreatment/Cell Disruption and Extraction Methods Used to Improve Intracellular Lipid Recovery from Oleaginous Yeasts. Microorganisms 2021, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Fabiszewska, A.U.; Zieniuk, B.; Kozłowska, M.; Mazurczak-Zieniuk, P.M.; Wołoszynowska, M.; Misiukiewicz-Stępień, P.; Nowak, D. Studies on Upgradation of Waste Fish Oil to Lipid-Rich Yeast Biomass in Yarrowia lipolytica Batch Cultures. Foods 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Kapturowska, A.; Stolarzewicz, I.; Chmielewska, I.; Białecka-Florjańczyk, E. Ultradźwięki—narzędzie do inaktywacji komórek drożdży oraz izolacji białek wewnątrzkomórkowych. Żywność. Nauka. Technologia. Jakość 2011, 4, 160–171. (In Polish) [Google Scholar]
- Gorte, O.; Hollenbach, R.; Papachristou, I.; Steinweg, C.; Silve, A.; Frey, W.; Syldatk, C.; Ochsenreither, K. Evaluation of Downstream Processing, Extraction, and Quantification Strategies for Single Cell Oil Produced by the Oleaginous Yeasts Saitozyma podzolica DSM 27192 and Apiotrichum porosum DSM 27194. Front. Bioeng. Biotechnol. 2020, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Drévillon, L.; Koubaa, M.; Nicaud, J.-M.; Vorobiev, E. Cell distruption pre-treatments towards an effective recovery of oil from Yarrowia lipolytica oleaginous yeast. Biomass Bioeng. 2019, 128, 105320. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).