Abstract
Luffa cylindrica (called loofah sponge) is a plant from the cucurbitaceae family, popular in Asian countries. The fruit of this plant has a specific fibrous structure and after drying it adopts a three-dimensional mesh structure. The aim of this study was to determine the potential ability of the loofah sponge to bind microorganisms on its surface. Research conducted with the participation of Yarrowia lipolytica yeast has shown that loofah sponge has good sorption capacity and can be an excellent biomaterial for binding whole cells of microorganisms. One gram of Luffa cylindrica is able to adsorb about 0.64 g of Yarrowia lipolytica yeast cells, calculated on the basis of dry weight. Binding on the surface of the biopolymer is most effective when the sponge is introduced into the microbial media with the inoculum.
1. Introduction
Luffa cylindrica, popularly known as a vegetable sponge or a sponge calabash, is an annual climber with a rich history of cultivation, mainly focused on tropical and subtropical regions [1]. It belongs to the vegetables of the Cucurbitaceae family. The fruits of the loofah sponge are characterized by a fibrous vascular system which, when dried, resembles a three-dimensional, natural mesh with an open structure. Moreover, they have struts with a characteristic microcell architecture, with continuous, hollow microchannels (macropore diameter 10–20 µm), which form bundles of vessels and represent a multimodal, hierarchical pore system [2,3]. The highly porous structure is very light (low density) and stiff. It demonstrates excellent mechanical strength and deformation ability [4]. The introduction should briefly place the study in a broad context and define the purpose of the work and its significance.
The chemical composition of the sponge depends on several factors. These include, among others, the origin of the plant, type of soil, weather conditions for growth, pre-treatment, etc. [5]. Luffa cylindrica fibers consist on average of about 65% cellulose, 17% hemicellulose, and 15% lignin [6].
Luffa cylindrica, despite its low popularity on the European continent, enjoys great fame in countries where it is grown on a large scale. It is used in many areas, including such as: materials science, medical sciences, pharmaceutical industry, biotechnology, and bioprocess engineering. The fibers of ripe loofah fruit are used in the production of caps, scarves, flip-flops, table mats, pot handles, and the production of car filters [7]. The lightness of the dried fruit of Luffa cylindrica makes it act as a foam material and therefore it can be potentially used for the production of plastics, which, thanks to the use of a sponge, become more durable, lighter, and partially biodegradable [5,8]. Loofah sponge is a raw material for the production of biofibers and biocomposites, used in the production of packaging, water-absorbing materials, or filters [3,9]. It is also a good adsorbent for removing heavy metals, including copper, lead, chromium, and nickel [10]. In biotechnology, the loofah sponge is mainly used as a carrier for the immobilization of plant cells, algae, bacteria, and yeast, and as the so-called “scaffolding” in the broadly understood field of tissue engineering [11,12].
This article presents the results of research on the possibility of using Luffa cylindrica in the immobilization of Yarrowia lipolytica yeast. The immobilization ability of loofah sponge was assessed on the basis of the measurement of the optical density of the medium, the yield of yeast dry matter, and extracellular catalytic activity.
2. Materials and Methods
2.1. Microorganisms and Reagents
Yarrowia lipolytica KKP379 yeast strain was obtained from the Collection of Industrial Microorganisms, Prof. Wacław Dąbrowski Institute of Agricultural and Food Biotechnology—State Research Institute (Warsaw, Poland). The culture media components: yeast extract, peptone, glucose, and agar were obtained from BTL Sp. Z o.o. (Łódź, Poland). p-Nitrophenyl laurate was synthesized in our laboratory [13] from lauryl chloride (Sigma-Aldrich, Saint Louis, MO, USA) and p-nitrophenol (Avantor Performance Materials, Gliwice, Poland).
2.2. Yeast Cultivation
Yarrowia lipolytica KKP379 yeast was cultivated on YPG liquid medium, consisting of yeast extract (10 g/L), peptone (20 g/L), and glucose (20 g/L). The media was prepared and sterilized in 500 mL total capacity round bottom flasks (100 mL working volume) inoculated with 1 mL of the 24 h inoculum. The yeast was grown at 28 °C in an IKA Control incubator with shaking at 140 rpm for a period of 72 h.
2.3. Immobilization of Yeast on Loofah Sponge
About 1 g of dried sponge (cut into cubes with a side length of about 1 cm) was introduced into 100 mL of the substrate. Two variants of breeding were carried out. In the first variant, loofah sponge was introduced into the media in which the Yarrowia yeast cells had previously been multiplied within 72 h. In a second variant, an equivalent amount of Luffa cylindrica sponge was introduced into the culture at start-up, simultaneously with the yeast inoculum, and a 72 h cultivation was carried out with it. The results were related to the control culture grown on YPG medium without the addition of loofah sponge.
2.4. Optical Density Measurment
The turbidity level of the media, reflecting the intensity of yeast cell multiplication, was examined spectrophotometrically by measuring the optical density OD600. During the cultivation, 1 mL of the culture medium was collected at appropriate intervals, the cells were centrifuged and washed with distilled water. It was then suspended in 1 mL of distilled water and, after appropriate dilution, the absorbance of the suspension was measured at 600 nm. Samples were taken from control flasks (without sponge) and from flasks with loofah sponge. Each measurement was performed in triplicate.
2.5. Determination of Biomass
Yeast biomass was characterized by the cell dry mass measured by the thermogravimetric method. Fifteen milliliters of the medium was centrifuged at the speed of 8000 rpm for 10 min (Centrifuge MPW-223). The supernatant was eliminated, and the biomass was washed with distilled water. The biomass was oven-dried at 105 °C until constant weight. The results of biomass yield were given with respect to 1 L of medium (g d.w. of cells/L).
2.6. The Level of Cell Yeast Adsorption on the Loofah Sponge
Determination of the level of yeast cell adsorption on Luffa cylindrica sponge was performed 72 h after inoculating the media. For this purpose, the sponges were taken out of the culture medium, washed with distilled water, and dried (drying time 24 h, temperature 100 °C). The difference in the mass of dry sponges removed from the culture and sponges before their introduction into the medium allowed to determine the level of adsorption of Yarrowia lipolytica cells.
2.7. Measurement of Lipase Activity
The measurement of lipolytic activity was carried out using a spectrophotometric method based on the hydrolysis of p-nitrophenyl laurate. One hundred microliters of supernatant was added to 0.02 mol of p-nitrophenyl laurate dissolved in 25 µL of heptane. Reactions were carried out in Eppendorf for 5 min at 37 °C. One unit of enzyme activity was defined as the enzyme quantity that liberated 1 µmol of p-nitrophenol per minute under the assay conditions.
3. Results and Discussion
The evaluation of the effectiveness and purposefulness of Yarrowia lipolytica yeast cell immobilization on Luffa cylindrica sponge was carried out on the basis of the measurement of the optical density of the media, the yield of yeast dry cell mass, the mass of cells multiplied in the medium and adsorbed on the Luffa sponge and their extracellular catalytic activity.
The optical density of the medium was measured for three days at 24 h intervals. In parallel, the value of OD600 in the control medium YPG (without sponge) and the medium into which loofah sponge was introduced with the inoculum was analyzed. The results of this experiment are shown in Figure 1.
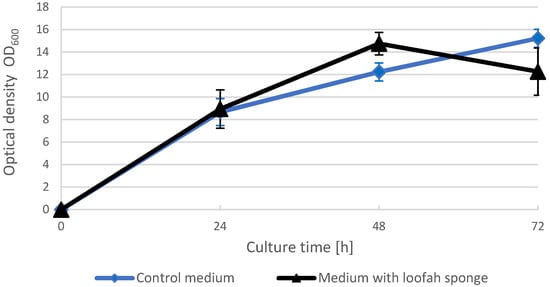
Figure 1.
Change in the optical density of the media during the cultivation of Yarrowia lipolytica on YPG (control medium) and YPG medium with loofah sponge.
The collected data indicated that the optical density of the mediums differed only on the second day of cultivation. After 48 h, the optical density of the medium with the sponge was slightly higher compared to the control substrate—OD600 at the level of 14.75 ± 1.01 and 12.23 ± 0.80, respectively. Increased cell multiplication may have contributed to shortening the duration of the logarithmic growth phase. At 72 h of cultivation in loofah sponge medium, the OD value decreased by approx. 2.5 units (from OD 14.75 to 12.26), while continuous cell growth was observed in the control medium, indicating an ongoing log phase of yeast growth. A decrease in the OD value in the loofah sponge medium may also indicate that cells were adsorbed on the sponge surface.
In order to confirm the difference in the level of turbidity of the media, the yield of yeast dry mass in the control and loofah sponge cultures was also determined. The results of this experiment are shown in Figure 2.
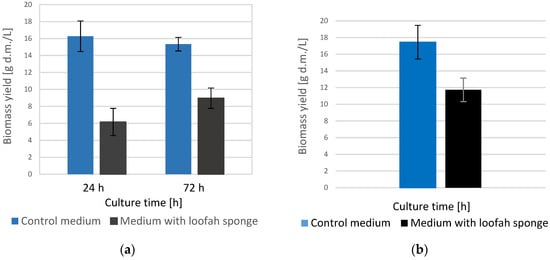
Figure 2.
Yarrowia lipolytica yeast dry mass yield grown on YPG (control medium) and YPG media with loofah sponge: (a) loofah sponge introduced into the medium along with the yeast inoculum; (b) loofah sponge introduced into the medium after 72 h of cultivation.
The presented data confirmed that a significant part of Yarrowia lipolytica yeast cells was immobilized on the loofah sponge, regardless of the moment at which the sponge was inserted. Both on the first and the third day of cultivation, the dry cell mass yield in the control media was, respectively, about 2.6 and 1.7 times higher than in the media with loofah sponge (Figure 2a). A slightly smaller difference in the yield of yeast dry cell mass was visible in the culture in which the sponge was introduced only after the cells had multiplied (Figure 2b). In this experiment, the yield from the control medium was about 1.5 times higher than in the medium with a Luffa cylindrica.
The team of Prakasham et al. [14] and Iqbal and Saeed [15] mentioned the immobilization of yeast or fungal cells on loofah sponge. The authors indicated that the fibrous network of the Luffa cylindrica is an ideal matrix for adsorption due to its large, porous surface. These physical properties facilitated the contact of the biomass with the biosorbent. Scientists indicated that open space additionally allows cells to grow.
In order to verify the level of yeast cell adsorption in the porous space Luffa cylindrica, an additional experiment was performed in which the mass of yeast cells adsorbed on the loofah sponge was determined. For this purpose, the sponge was dried to constant weight and weighed before it was introduced into the medium and after it was removed from the culture. From the difference in mass, the weight of the cells adsorbed in the porous space of the sponge was determined. The results of this experimental part are shown in Table 1. The above data are consistent with the results of the determined yeast dry matter yield (Figure 2) and indicate that regardless of the moment at which the Luff sponge was introduced into the medium, Yarrowia yeast cells adsorbed on its porous surface. With a comparable dry mass of the sponge introduced into the substrates (approx. 1 g), it can be seen, however, that the variant in which the sponge is inserted at the moment of inoculation of the substrates is more advantageous. Back then, a larger mass of cells is bound on the surface of the sponge—approx. 0.642 g/1 g of the sponge, while in the second variant, the mass of adsorbed cells per 1 sponge is approx. 0.511 g. In the perspective of further examination of immobilization of cells on Luff’s sponge, it is worth trying to increase the efficiency of binding of cells to the surface of the sponge. Probably it can be achieved by the usage of higher initial concentrations of yeast cells, extension of the immobilization time, or better crush of the sponge.

Table 1.
Yarrowia lipolytica yeast cell mass adsorbed on Luffa cylindrica sponge.
The loofah sponge particles were used as efficient biomass support particles, in the form of whole-cell biocatalysts, among others to immobilize Rhizopus oryzae LY6 for biodiesel production [16]. Luffa cylindrica fruits were also used to immobilize Acetobacter aceti cells for use in the production of vinegar. The sponge was cut into small pieces, washed with water, and sterilized by immersion in 4% acetic acid for 24 h before use as a carrier. The sterilized sponge was a very good carrier of cells due to the high diffusion capacity of oxygen, which gives good growth conditions for bacterial cells [17]. The open network of fibrous support provides sufficient and instant contact of immobilized cells to the surrounding aqueous medium [13].
Wanting to have a full picture of the sorption abilities of loofah sponge, the extracellular lipolytic activity of the immobilized yeast cells was also assessed based on the model p-nitrophenyl laurate hydrolysis reaction.
The results of this experiment, presented in Figure 3, show that in the initial cultivation period (24 h), the microorganisms immobilized on the surface of the plant sponge are characterized by a more dynamic, approximately 3.5-fold increase in hydrolytic activity, compared to free cells. This tendency is reversed in the stationary growth phase, adsorbed Yarrowia lipolytica cells show catalytic activity, but at a much lower level compared to free microorganisms. This may result from a specific deformation and blocking of the enzyme’s active sites as a result of the immobilization process [18].
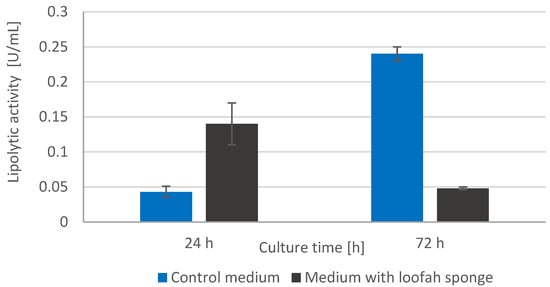
Figure 3.
Extracellular lipolytic activity of Yarrowia lipolytica yeast after 24 and 72 h of cultivation with and without loofah sponge (control medium).
4. Conclusions
Luffa cylindrica, a lignocellulosic biopolymer, can be an excellent carrier in the process of binding/immobilizing microorganisms, as demonstrated by the adsorption of Yarrowia lipolytica yeast cells. The open, three-dimensional, fibrous structure of the loofah sponge with a large number of reactive functional groups allows the adsorption of a large number of cells. One gram of dried sponge is able to adsorb approx. 0.642 g of yeast dry cell mass, while the binding efficiency of microorganisms is much higher (by approx. 25%) when the biopolymer is introduced into the medium at the start of cultivation.
Author Contributions
Conceptualization, J.M.; methodology, J.M., A.K. and A.G.; validation, J.M., A.K. and A.G.; formal analysis, J.M.; investigation, J.M.; resources, J.M.; writing—original draft preparation, J.M.; writing—review and editing, J.M.; visualization, J.M., A.K. and A.G.; supervision, J.M.; project administration, J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded from the funds of the Student Scientific Club of Biotechnologists—KNBiotech, as part of the financing program of scientific clubs’ projects in the grant system as part of the Main Competition.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, X.; Tan, T.; Xu, C.; Huang, S.; Tan, J.; Zhang, M.; Wang, C.; Xie, C. Genome-wide transcriptome profiling reveals novel insights into Luffa cylindrica browning. Biochem. Biophys. Res. Commun. 2015, 463, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, A.; Mabande, G.T.P.; Selvam, T.; Schwieger, W.; Rudolph, A.; Hermann, R.; Sieber, H.; Greil, P. Biotemplating of Luffa cylindrica sponges to self-supporting hierarchical zeolite macrostructures for bio-inspired structured catalytic reactors. Mater. Sci. Eng. C 2006, 26, 130–135. [Google Scholar] [CrossRef]
- Oboh, I.O.; Aluyor, E.O. Luffa cylindrica—An emerging cash crop. Afr. J. Agric. Res. 2009, 4, 684–688. [Google Scholar] [CrossRef]
- An, X.; Sui, Q.; Sun, F.; Ma, Z.; Jiang, S.; Ji, B.; Fan, H. Compression responses of bio-cellular Luffa sponges. Bioresources 2015, 10, 8426–8436. [Google Scholar] [CrossRef]
- Shen, J.; Xie, Y.M.; Huang, X.; Zhou, S.; Ruan, D. Mechanical properties of Luffa sponge. J. Mech. Behav. Biomed. Mater. 2012, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Smole, M.S.; Hribernik, S.; Kurečič, M.; Krajnc, A.U.; Kreže, T.; Kleinschek, K.S. Non-conventional plant fibres. In Surface Properties of Non-Conventional Cellulose Fibres, 1st ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Guimaraes, J.L.; Wypych, F. Studies on lignocellulosic fibers of Brazil. Part I: Source, production, morphology, properties and application. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1694–1709. [Google Scholar] [CrossRef]
- Labeeba, K.; Ramya, D.D.; Vedha, H.B.N. Comprehensive review on potential applications of natural Luffa cylindrica fibers. In Phytopharmaceuticals and Drug Delivery Approaches; University Thanjavur: Thanjavur, India, 2019; pp. 2–17. [Google Scholar] [CrossRef]
- Bal, K.J.; Hari, B.K.C.; Radha, K.T.; Madhusudan, G.; Buhwan, R.S.; Madhusudan, P.U. Descriptors for Sponge Gourd [Luffa cylindrica (L.) Roem.]; NARC, LIBIRD and IPGRI: Kathmandu, Nepal, 2004. [Google Scholar]
- Boynard, C.A.; D’Almeida, J.R.M. Morphological characterization and mechanical behavior of sponge gourd (Luffa cylindrica)—Polyster composite materials. Polym.-Plast. Technol. Eng. 2000, 39, 489–499. [Google Scholar] [CrossRef]
- Chen, J.P.; Lin, T.C. Loofa sponge as a scaffold for culture of rat hepatocytes. Biotechnol. Prog. 2005, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Saudagar, P.S.; Shaligram, N.S.; Singhal, R.S. Immobilization of Streptomyces clavuligerus on loofah sponge for the production of clavulanic acid. Bioresours Technol. 2008, 99, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.I.; Furniss, B.S.; Tatchell, A.R.; Hannaford, A.J.; Smith, P.W.G. (Eds.) Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Prakasham, R.S.; Merrie, J.S.; Sheela, R.; Saswathi, N.; Ramakrishna, S.V. Biosorption of chromium VI by free and immobilized Rhizopus arrhizus. Environ. Pollut. 1999, 104, 421–427. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A. Biosorption of reactive dye by loofa sponge—Immobilized fungal biomass of Phanerochaete chrysosporium. Process. Biochem. 2007, 42, 1160–1164. [Google Scholar] [CrossRef]
- He, Q.; Shi, H.; Gu, H.; Naka, G.; Ding, H.; Li, X.; Zhang, Y.; Hu, B.; Wang, F. Immobilization of Rhizopus oryzae LY6 onto loofah sponge as a whole-cell biocatalyst for biodiesel production. BioResources 2016, 11, 850–860. [Google Scholar] [CrossRef]
- Hutchinson, U.F.; Jolly, N.P.; Chidi, B.D.; Ngongang, M.; Ntwampe, S.K.O. Vinegar engineering a bioprocess perspective. Food Eng. Rev. 2019, 11, 290–305. [Google Scholar] [CrossRef]
- Zdarta, J.Ł. Immobilizacja Enzymów na Wybranych Nośnikach Organicznych i Nieorganicznych; Rozprawa Doktorska: Poznań, Poland, 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).