Abstract
The gall wasp Aulacidea hieracii L., 1758 (Hymenoptera: Cynipidae) forms a stem gall on the hawkweed Hieracium × robustum Fries, 1848 (Asteraceae), a weedy herb that grows in the steppe biotopes of Eurasia. In its turn, gall former serves as food for a large number of parasitoids and predators, which also live and develop inside and outside the gall. Moreover, the inhabitants of the galls are consumed by birds. In addition, a specific microbiota develops inside the gall, which includes representatives of Gram-negative bacteria Pseudomonas rhizosphaerae, Curtobacterium flaccumfaciens, Pantoea agglomerans and fungi Alternaria alternata. The authors found out the composition of parasitoids, predators, inquilines and microorganisms for a gall on the hawkweed. For the first time, studies were carried out on the development of the moth caterpillars Oxyptilus chrysodactyla (Denis & Schiffermüller, 1775) on the hawkweed H. robustum. We found that the caterpillars of the moths cohabit on their fodder plant on the surface of the gall together with the larvae of gall wasps. Notably, the gall wasp larvae are the first to inhabit the plant. Thus, gall on the plant is a complex ecosystem, which balances and increases diversity of living organisms.
1. Introduction
Gall wasps Aulacidea hieracii L., 1758 (Hymenoptera: Cynipidae) form stem galls on the hawkweed Hieracium × robustum Fries, 1848 (Asteraceae). In spring, the female A. hieracii lays eggs into the shoot apical meristem, after which a gall is formed on the plant, which increases in size as the larvae of the gall former grow [1]. The gall serves not only as a source of food but also performs protective functions, namely it defends its inhabitants from temperature changes and unspecialized parasites [2]. Each larva feeds on the gall tissues, gradually forming a small capsule around itself. In this capsule, the larva winters. There are up to 30 such larvae in the average gall. At the same time, other insect larvae may be present in the gall: inquilines, parasitoids, and predators [3,4]. Therefore, the gall may be of interest as a microecosystem that includes many organisms. However, the most complete list of living organisms capable of inhabiting galls on H. robustum is still unknown. The aim of the work was to study the species composition and ecology of the community of organisms in the gall formed by the gall wasp A. hieracii on the hawkweed H. robustum.
2. Methods
The collection of material on gall formers was carried out in 2016–2021. The galls were collected annually on the hawkweed H. robustum in the steppe biotope. The galls were placed in Petri dishes to breed insects. The emerging insects were fixed and identified. Some emerging insects after detection were immediately transferred to separate Petri dishes to study the life span and behavioral characteristics of particular species. Insects were offered food and water provided daily, and insect hosts were added to Petri dishes with parasitoids. Petri dishes were kept in the laboratory for 2–3 months, during which photos and videos of insect behavior were taken until their natural death. A stereoscopic microscope Mikromed MC2 ZOOM (Micromed, Saint-Petersburg, Russia) with a video camera CANON S100 (Canon Inc., Ohta-ku, Tokyo, Japan) was used for observation.
To study the microbiological composition, the larvae and galls were treated for 5 min in 70% alcohol and then washed twice in saline. Each object was ground in a mortar with saline. Next, 0.1 mL of the suspension was sown on Petri dishes with GRM medium and PDA. Next, a quantitative account of fungi was carried out and the index of occurrence of individual species was determined [5,6]. The identification of the species of microorganisms was confirmed by sequencing bacterial strains for 16S rRNA and fungal strains for 18S rRNA (CJSC Sintol, Moscow, Russia).
3. Results and Discussion
As a result of the study, we have identified one species of gall former on the hawkweed Hieracium robustum Fries, 1848 (Asteraceae):
- –
- Aulacidea hieracii (Bouché, 1834) (Cynipidae).
Although the juvenile development of the gall former occurs inside a closed space, its larvae are constantly attacked by parasitoids and predators. Among parasitoids, 11 species of Hymenoptera were identified [7,8]: Eurytoma cynipsea (Boheman, 1836) (Eurytomidae); E. hybrida Zerova, 1978 (Eurytomidae); E. pr. strigifrons Thomson, 1876 (Eurytomidae); Torymus chloromerus (Walker, 1833) (Torymidae); Ormyrus discolor Zerova, 2005 (Ormyridae); Pteromalus vibulenus (Walker, 1839) (Pteromalidae); Pteromalus sp. (Pteromalidae); Exeristes roborator (Fabricius, 1793) (Ichneumonidae); Sycophila submutica (Thomson, 1876) (Eurytomidae); Eupelmus (Macroneura) messene Walker, 1839 (Eupelmidae); E. (Eupelmus) microzonus Förster, 1860 (Eupelmidae).
Through ethological studies, we found that the dominant species of parasitic insects is E. cynipsea (45% of all parasitoids) [7]. A distinctive feature of this parasitoid is that the larvae of E. cynipsea prey inside the gall. While other parasitoids eat their only prey, the E. cynipsea larva actively searches for its prey by burrowing inside the gall. During the season, the predator larva can eat up to three victims—the gall wasp larvae.
In addition to parasitoids and predators, there is a group of bacteria and fungi inside the gall. We found that this specific microbiota is represented by the following types of Gram-negative bacteria [5]: Pseudomonas rhizosphaerae (Peix et al., 2003); Curtobacterium flaccumfaciens (Hedges, 1922); Pantoea agglomerans (Ewing and Fife 1972), as well as fungi: Alternaria alternate (Keissl, 1912).
In addition to the internal inhabitants of the gall, there is a whole community of organisms that live outside the gall, on its surface, and on numerous leaves around the gall [9]. A well-leafed gall formed by A. hieracii attracts various species of insect-phytophages and entomophages from different orders (Figure 1). Among the phytophages that develop annually on H. robustum with galls, the following were noted: leafhopper Lepyronia coleoptrata (Linnaeus, 1758) (Aphrophoridae); miner fly larvae (Agromyzidae); Oxyptilus chrysodactyla (Denis & Schiffermüller, 1775). In its turn, O. chrysodactyla has its own parasitoids from the Pimpilinae family. Special attention should be paid to the fact, established by the authors, that the caterpillars of O. chrysodactyla cohabit together with the A. hieracii on a host plant [10]. Notably, A. hieracii is always the first to inhabit the plant. Perhaps the presence of the gall on the plant is not necessarily for O. chrysodactyla inhabitation. However, according to our data, all plants inhabited by O. chrysodactyla had galls. Among predatory entomophages, bugs Orius niger (Wolf, 1811) (Coccinellidae) and diverse representatives of the order Araneae are regularly found by the authors on the plant.
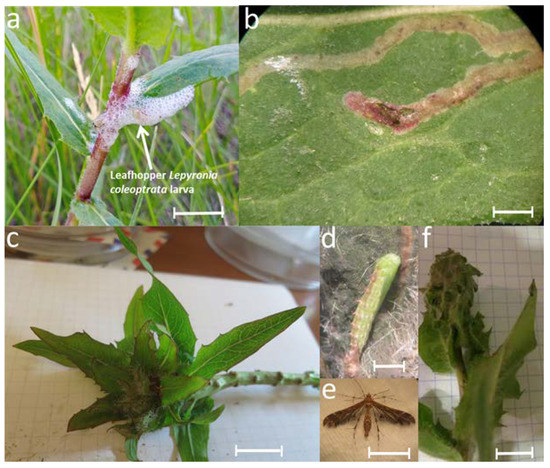
Figure 1.
Insects which develop on the gall surface: (a)—leafhopper larva of Lepyronia coleoptrata; (b)—leaf miner damage by Agromyzidae fly larva; (c)—gall with immature caterpillars of O. chrysodactyla; (d)—pupa of O. chrysodactyla; (e)—imago of O. chrysodactyla; (f)—gall with leaves, folded by mature O. chrysodactyla caterpillars. Scale bar is 1 cm (a,c,e,f) or 0.5 cm (b,d) (M. Nikelshparg’s photo).
4. Conclusions
Galls on the hawkweed are independent microecosystems and an important part of the year-round food chain in nature as part of the macroecosystem—meadow steppe. At least 20 species of organisms from different systematic and ecological groups develop on the hawkweed with gall—insects, bacteria, fungi; phytophages, parasitoids, and inquilines of the gall former, each of which occupies its own ecological niche. Moreover, a plant with a gall successfully blooms and reproduces; therefore, various types of pollinators feed on inflorescences. Additionally, in winter, small birds from the Paridae family feed on wintering insect larvae in the galls. The overwintered galls after the emergence of insects are food for destructors: microorganisms, etc. Thus, using the example of the hawkweed H. robustum, we have shown that the formation of a gall on plants can significantly increase the diversity of living organisms in an ecosystem.
Supplementary Materials
The video presentation can be downloaded at: https://www.mdpi.com/article/10.3390/IECD2022-12386/s1.
Author Contributions
Conceptualization, M.I.N. and V.V.A.; methodology, M.I.N., D.L.B. and E.V.G.; investigation, M.I.N. and D.L.B.; writing—original draft preparation, M.I.N. and V.V.A.; supervision, V.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on a reasonable request from the corresponding author.
Acknowledgments
The authors are grateful to unknown reviewer for useful comment on the paper, to V.E. Gokhman (Botanical Garden, Moscow State University, Moscow, Russia) for identifying some species of parasitoids, and to E.I. Nikelshparg (Department of Biophysics, Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia) for fruitful discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sliva, M.D.; Shorthouse, J.D. Comparison of the development of stem galls induced by Aulacidea hieracii (Hymenoptera: Cynipidae) on hawkweed and by Diplolepis spinosa (Hymenoptera: Cynipidae) on rose. Can. J. Bot. 2006, 84, 1052–1074. [Google Scholar] [CrossRef]
- Harris, M.O.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef] [PubMed]
- Askew, R.R. The diversity of insect communities in leaf- mines and plant galls. J. Anim. Ecol. 1980, 49, 817–829. [Google Scholar] [CrossRef]
- Egan, S.P.; Hood, G.R.; Martinson, E.O.; Ott, J.R. Cynipid gall wasps. Curr. Biol. 2018, 28, R1370–R1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikelshparg, M.I.; Glinskaya, E.V. Species composition of fungi in galls formed on Hieracium robustum fr. S. L., 1848 by Aulacidea hieracii bouche, 1834. In Proceedings of the Stavropol Branch of the Russian Entomological Society, Stavropol, Russia, 22 October 2018; pp. 66–69. [Google Scholar]
- Nikelshparg, M.I.; Glinskaya, E.V.; Anikin, V.V.; Nickelshparg, E.I. The species composition of gall bacteria formed on the hawkweed Hieracium robustum Fr. s. L., 1848 gall wasp Aulacidea hieracii Bouche,1834. In Proceedings of the IX Congress of Society Physiologists of Plants of Russia “Plant Physiology Is the Basis for Creating Plants of the Future”; Kazan University Press: Kazan, Russia, 2019; Volume 9, p. 310. [Google Scholar]
- Gokhman, V.E.; Nikelshparg, M.I. Eupelmus messene Walker, 1839 and E. microzonus Förster, 1860 as parasitoids of Aulacidea hieracii (Bouché, 1834) (Hymenoptera: Eupelmidae, Cynipidae). J. Hymenopt. Res. 2021, 84, 87. [Google Scholar] [CrossRef]
- Anikin, V.V.; Nikelshparg, M.I.; Nikelshparg, E.I.; Lavrent’ev, M.V. The number and phenology of Aulacidea hieracii L., 1758 (Hymenoptera: Cynipidae) and its parasitoids on the exit from the galls of mighty hawkweed (Hieracium × robustum fr.). Entomol. Parasitol. Investig. Volga Reg. 2018, 15, 82–88. [Google Scholar]
- Anikin, V.V.; Nikelshparg, M.I. The effect of increasing number of leaves on plants Hieracium × robustum (Asteraceae) at their settling by gall-former Aulacidea hieracii (Hymenoptera: Cynipidae). Bull. Bot. Gard. Saratov State Univ. 2018, 16, 49–54. [Google Scholar] [CrossRef]
- Nikelshparg, M.I.; Anikin, V.V. Development of the plume moth caterpillar Oxyptilus chrysodactyla ([Denis & Schiffermuller], 1775) (Lepidoptera, Pterophoridae) on the hawkweed Hieracium virosum Pall. Entomol. Parasitol. Investig. Volga Reg. 2020, 17, 98–104. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).