Abstract
Background/Objectives: Janus kinase 2 (JAK2) is a pivotal signaling protein implicated in various hematological malignancies and inflammatory disorders, making it a compelling target for therapeutic intervention. Methods: In this study, we employed an integrative computational approach combining ligand-based screening, pharmacophore modeling, molecular docking, molecular dynamics (MD) simulations, and MM/PBSA free energy calculations to identify JAK2 inhibitors from the ChEMBL database. A comprehensive virtual screening of over 1,900,000 compounds was conducted using Tanimoto similarity and a validated pharmacophore model, resulting in the identification of 39 structurally promising candidates. Docking analyses prioritized compounds with favorable interaction energies, while MD simulations over 100 ns assessed the dynamic behavior and binding stability of top hits. Results: Four compounds, CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033, exhibited consistently superior performance, forming stable hydrogen bonds, favorable RMSD profiles (≤0.5 nm), and strong binding interactions, including salt bridges. Notably, the binding free energies revealed ΔG values as low as −29.91 kcal/mol, surpassing that of the reference inhibitor, momelotinib (−24.17 kcal/mol). Conclusions: Among these, CHEMBL4169802 emerged as the most promising candidate due to its synergistic electrostatic and hydrophobic interactions. Collectively, our results highlight these compounds as probable, JAK2-selective inhibitors with strong potential for further biological validation and optimization.
1. Introduction
Janus kinase 2 (JAK2) is a non-receptor tyrosine kinase and a critical component of the JAK family, which includes JAK1, JAK2, JAK3, and TYK2 [,,]. These kinases are essential mediators of the JAK-STAT (Signal Transducer and Activator of Transcription) signaling pathway, a key mechanism through which extracellular cytokines and growth factors regulate gene expression and cellular function [,,,]. JAK2, in particular, plays a central role in mediating the effects of several hematopoietic growth factors such as erythropoietin (EPO), thrombopoietin (TPO), granulocyte colony-stimulating factor (G-CSF), and various interleukins [,,]. Upon ligand binding to their respective cell surface receptors, JAK2 becomes auto-phosphorylated and subsequently phosphorylates specific tyrosine residues on the receptor itself. This creates docking sites for STAT proteins, which are then phosphorylated by JAK2, dimerize, translocate to the nucleus, and regulate the transcription of genes involved in cell proliferation, differentiation, survival, and immune regulation [,,].
The biological importance of JAK2 is underscored by its tight regulation; however, dysregulation of JAK2 signaling has been implicated in a wide spectrum of diseases [,]. The most notable pathogenic alteration is the JAK2 V617F mutation, a somatic point mutation in exon 14 that results in a valine-to-phenylalanine substitution [,]. This mutation leads to constitutive activation of JAK2 kinase activity, independent of cytokine stimulation, and is a hallmark of myeloproliferative neoplasms (MPNs) such as polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) [,,]. Constitutive JAK2 activity drives uncontrolled hematopoiesis, inflammatory cytokine production, and resistance to apoptosis, contributing to disease progression and therapeutic resistance. Beyond hematologic malignancies, aberrant JAK2 signaling is also implicated in autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, and psoriasis, where it mediates the chronic inflammatory response through dysregulated cytokine signaling [,,].
Given its pivotal role in multiple pathological states, JAK2 has emerged as a validated and high-value therapeutic target, with several small-molecule inhibitors developed to modulate its activity. Approved JAK inhibitors such as ruxolitinib and momelotinib have shown efficacy in treating MPNs and other immune-related conditions [,,]. In addition to ruxolitinib and momelotinib, several other JAK-family inhibitors, such as tofacitinib, baricitinib, upadacitinib, fedratinib, peficitinib, abrocitinib, delgocitinib, filgotinib, pacritinib, ritlecitinib, and the newer approvals such as deuruxolitinib, have gained global attention. These agents differ in isoform selectivity (JAK1, JAK2, JAK3, TYK2), route and formulation (oral versus topical), and are implicated for rheumatologic, dermatologic, and hematologic disorders [,,,,,]. However, many of these inhibitors suffer from a lack of isoform selectivity, often targeting multiple JAK family members and leading to dose-limiting toxicities such as anemia, thrombocytopenia, and immunosuppression [,,]. Additionally, the emergence of drug resistance and incomplete disease remission necessitates the development of new JAK2-specific inhibitors with improved safety, selectivity, and therapeutic index.
In recent years, computational drug discovery techniques have played a pivotal role in identifying and optimizing lead compounds targeting disease-relevant proteins like JAK2 [,]. Pharmacophore modeling, molecular docking, and Molecular Dynamics (MD) simulations have proven to be powerful tools for characterizing ligand–receptor interactions at the molecular level, enabling the prediction of binding affinities, interaction networks, and dynamic stability of protein-ligand complexes [,,]. Furthermore, MM/PBSA-based free energy calculations provide quantitative insights into binding thermodynamics, reinforcing the prioritization of hit compounds [].
In this study, we employed an strategy integrated in silico to identify novel JAK2 inhibitors from the ChEMBL compound library. Our pipeline combined ligand-based virtual screening, validated pharmacophore filtering, molecular docking, 100 ns MD simulations, and MM/PBSA free energy estimation to comprehensively evaluate the binding efficacy, stability, and interaction profiles of selected candidates. From over 1,900,000 compounds, four promising hits, CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033, were identified that demonstrated superior performance compared to the reference compound momelotinib across multiple computational metrics. These findings provide a strong foundation for the development of new, selective JAK2 inhibitors and offer valuable insights into future experimental validation and structure-based drug design efforts targeting JAK2-mediated diseases.
2. Methodology
2.1. Chembl Library Accession and Screening
To initiate the virtual screening of potential JAK2 inhibitors from the ChEMBL database, a ligand-based similarity approach was employed using the Tanimoto coefficient []. Approximately 1,900,000 compounds from the ChEMBL database were downloaded in the form of six libraries (Lib1, Lib2, Lib3, Lib4, Lib5, and Lib6) with the accession link https://www.ebi.ac.uk/chembl/explore/compounds/STATE_ID:H0G-XSIEQn93qa0ndtL4sw%3D%3D (accessed on 25 April 2025). All the libraries were saved in SDF format. Furthermore, all the accessed libraries were merged into one comprehensive file, and the CSV file was generated, having the SMILES and molecular ID of the accessed compounds.
Furthermore, the library of compounds was screened using the Tanimoto similarity with the reference compounds momelotinib and ruxolitinib. The compounds with more than 50% similarity to reference compounds were chosen: 161 compounds from momelotinib and 16 compounds from ruxolitinib. The Morgan fingerprints (radius = 2, nBits = 1024) were generated for both the reference compound and each of the ChEMBL entries. The Tanimoto similarity score between each compound in the database and momelotinib and ruxolitinib was computed using RDKit’s built-in TanimotoSimilarity function. To filter for meaningful structural resemblance, a threshold similarity score of ≥0.5 was applied. Only those compounds with a similarity score meeting or exceeding this cutoff were retained for further analysis. The filtered results were sorted in descending order of similarity and exported to a .csv file for subsequent stages of screening.
2.2. Receptor 3D Model Accession and Pharmacophore Modeling
Pharmacophore models are essential for identifying key molecular features that drive binding affinity and specificity, aiding in the design of targeted therapeutics [,]. The crystal structure of the JAK2 protein was retrieved from the Protein Data Bank (PDB ID: 8BXH) complexed with momelotinib [] to serve as the structural foundation of the pharmacophore analysis.
The 8BXH and its co-crystallized ligand were therefore utilized to generate receptor-ligand interaction pharmacophore features using the RLIPG (Receptor-Ligand Interaction Pharmacophore Generation) module in Discovery Studio. The RLIPG protocol in Discovery Studio is a sophisticated tool designed to construct pharmacophore hypotheses [].
2.3. Validation of Pharmacophore Model
The pharmacophore model’s performance was assessed using the Günther-Henry (GH) score, which is a statistical parameter widely used to evaluate the quality and effectiveness of pharmacophore models in distinguishing active compounds from inactive ones or decoys [,,]. Therefore, the 300 Decoy molecules were generated using DUDe (https://dude.docking.org/generate (accessed on 12 June 2025)), an online tool, with 15 known active compounds. The Pharmacophore model was tested against this dataset to evaluate its screening efficiency. Therefore, all the active and decoys were screened against the pharmacophore hypothesis 10 with the highest selectivity of 3.7137. Furthermore, the GH score was calculated based on the number of correctly identified active compounds, which quantitatively measures the model’s reliability in virtual screening, using the following formula:
where
GH = (1 − (A − Ht)/(A + D − Ht)) (Ht/A) (1 − Hf/N)
- A is the total number of active compounds in the dataset.
- D is the total number of decoy compounds in the dataset.
- Ht is the number of true positives (correctly identified active compounds).
- Hf is the number of false positives (decoys incorrectly identified as active).
- N is the total number of compounds in the dataset.
2.4. Molecular Docking
Molecular docking is a key computational method used to study the interactions between ligands and target receptors []. This method provides valuable insights into the stability and strength of binding interactions. The 3D structure of JAK2 was prepared for docking by removing co-crystallized ligands and water molecules, followed by the addition of Polar hydrogens, and the protonation states of ionizable residues were assigned at physiological pH (7.4) to ensure correct hydrogen bonding and electrostatics. Missing side chains or loops were corrected, and the structure was energy-minimized using the CHARMm force field to relieve steric clashes and optimize geometry using the Receptor Preparation tools in Discovery Studio Client v22 []. The binding site was defined as a sphere encompassing key active-site residues Phe154, Asp153, Leu142, Val70, Leu91, Glu57, Met88, Gly15, Val22, Leu14, Tyr90, and Ala39, which are critical for ligand recognition and catalysis.
Ligand preparation was carried out using the Ligand Preparation module in Discovery Studio, which included generating possible tautomers, adjusting ionization states at physiological pH, and correcting valence issues. Molecular docking was then performed using the CDcoker module [], which employs a CHARMm-based semi-flexible docking protocol, keeping the receptor rigid while allowing full ligand flexibility through molecular dynamics–based simulated annealing. The resulting poses were refined and scored based on CDocker energy and CDocker interaction energy values. The top-ranked protein–ligand complexes were then selected based on their lowest docking energy (kcal/mol), ensuring a reliable evaluation of their binding interactions.
2.5. Molecular Dynamics Simulations
The top ten complexes with the lowest overall docking energy scores underwent a 100 ns MD simulation to analyze their dynamic stability and interactions with the target protein. Simulations were set up using CHARMM36 force field [] parameters generated through the CHARMM-GUI server (https://www.charmm-gui.org/?doc=input/solution) (accessed on 2 July 2025) [], which also provided input files for GROMACS. The system was solvated in a cubic box with the TIP3P water model, neutralized with counter ions, and prepared with periodic boundary conditions.
Key interactions, including electrostatics and van der Waals forces, were calculated using the Verlet method with a 10 Å cutoff radius, while the Particle Mesh Ewald (PME) method ensured precise electrostatic calculations. Bond lengths were constrained using the LINCS algorithm. The systems underwent energy minimization via the steepest descent method and were equilibrated under NVT and NPT conditions before the production run.
Simulations were executed in GROMACS 2019.3 with a 2 fs time step for accurate trajectory sampling []. This workflow, supported by CHARMM-GUI Python 3.9.13 scripts for format conversion, enabled detailed structural and interaction analysis of the protein-ligand complexes, revealing critical insights into their dynamic stability and binding behavior.
2.6. Free Energy Calculation
The gmx_MMPBSA v1.6.3 tool is designed to compute the end-state free energies of protein-ligand complexes using MD trajectory data generated in GROMACS []. We employed the MM/PBSA (Mechanics/Poisson–Boltzmann Surface Area) method to estimate binding free energies by individually analyzing the protein-ligand complex, the isolated receptor, and the ligand within an explicit solvent environment. The binding free energy (ΔGbinding) of the lead compounds to the target protein was determined using the following equation:
ΔGbinding = Gcomplex − (Gprotein + Gligand)
In this equation, Gcomplex refers to the total energy of the protein-ligand complex, while Gprotein and Gligand represent the energies of the isolated protein and ligand in a solvated state, respectively.
3. Results
This flow diagram (Figure 1) summarizes a multi-step computational workflow used to identify selective kinase inhibitors from a large compound library. The process involves initial molecular similarity screening, pharmacophore screening, molecular docking, MD simulations, free energy calculations, and subsequent selectivity analysis to refine and characterize the most promising candidates.
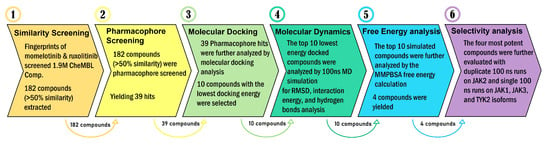
Figure 1.
Workflow for computational identification and evaluation of selective kinase inhibitors. Fingerprint-based similarity screening using momelotinib and ruxolitinib identified 182 CheMBL compounds, followed by pharmacophore screening to yield 39 hits. These were further narrowed down by molecular docking to 10 candidates, which were subjected to 100 ns molecular MD simulations. Free energy analysis was also performed on these top 10 compounds, resulting in four most potent candidates, which then underwent selectivity analysis via additional duplicate MD simulations and across JAK isoforms inhibitory analysis.
3.1. Chembl Library Accession
The ChEMBL library, comprising approx. 1,900,000 compounds having the SMILES and molecular ID of the accessed compounds were subjected to similarity screening with reference compounds momelotinib and ruxolitinib. Therefore, the RDKit-based Morgan fingerprint comparison identified 166 compounds from momelotinib and 16 compounds from ruxolitinib total of 182 compounds (Figure 2), which were accessed with more than 50% similarity to both of the reference compounds. These compounds exhibited varying degrees of structural similarity, with several scoring above 0.6, indicating a high likelihood of retaining key pharmacophoric and bioactive features shared with momelotinib and ruxolitinib. The script successfully filtered and ranked the hits by similarity, providing a data-driven shortlisting for downstream structure-based drug design methods.
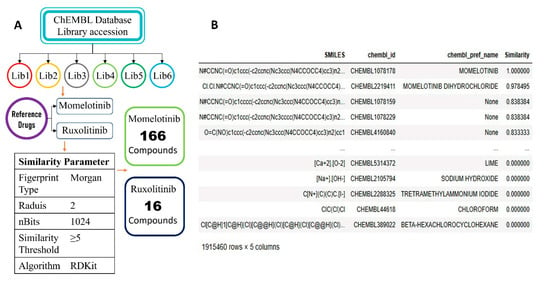
Figure 2.
The overall workflow of the similarity screening (A). The similarity hits of the ChEMBL compounds in descending order (B).
3.2. Receptor 3D Model Accession
The 3D crystal structure of the JAK2 was accessed from the PDB with the Accession ID of 8BXH with 1.30 Å resolution []. The Crystal structure comprised 316 amino acids and co-crystallized with the ligand (momelotinib). VADAR (http://vadar.wishartlab.com/ (accessed on 10 October 2025)) [] statistical analysis of the protein revealed that the protein consists of 37% α-helix, 20% β-Sheets, 41% coils, and 28% turns. Furthermore, Ramachandran Plot analysis showed that 96.9% of all residues were in the favored region, while 99.4% of all the residues were in the allowed region, and there were 2 outliers, Gln872 and Glu1012.
3.3. Pharmacophore Model Generation
Addressing the side effects caused by the non-selective action of drugs, which can result in unintended interactions with off-target proteins, remains a significant challenge in drug discovery [,]. Pharmacophore modeling offers an effective approach to mitigate this issue by identifying and focusing on the essential features required for selective binding. This strategy facilitates the screening and selection of compounds that align closely with the active binding site of the target protein, thereby enhancing specificity and minimizing off-target effects [,,].
In this study, a pharmacophore model was developed for JAK2 to optimize binding efficacy and selectivity. Using the RLIPG approach, ten pharmacophore hypotheses were ranked (Supplementary Data Table S1), and model 10 with the highest selectivity (3.7137) was selected for further pharmacophore model validation. This model incorporated two hydrogen-bond acceptors (green), one hydrogen-bond donor (magenta), and two hydrophobic aromatic regions, corresponding to functional groups on ligands capable of forming strong and specific interactions with complementary regions of JAK2. Additionally, the model included excluded volume features (gray), representing sterically restricted regions that prevent the binding of non-specific compounds (Figure 3). The hydrogen-bond acceptors and donors were critical for stabilizing ligand binding within the active site by forming electrostatic interactions with complementary residues on JAK2. These interactions were further supported by hydrophobic regions in the model, which enhanced binding affinity through interactions with nonpolar residues in the protein’s active site. The excluded volume features ensured that only compounds with optimal size and shape matched the pharmacophore, further enhancing binding selectivity [,,].
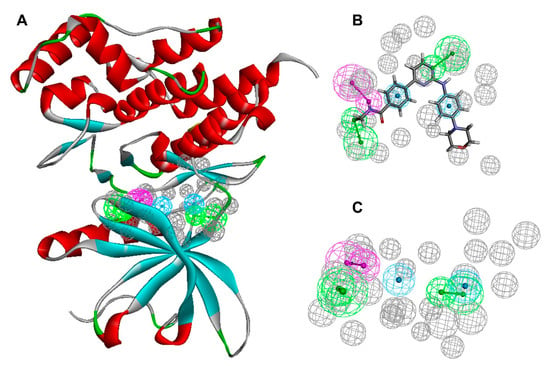
Figure 3.
The pharmacophore features of the receptor (A), Ligand (B), and complex (C). The pharmacophore features of hydrogen-bond acceptors (green), hydrogen-bond donors (magenta), hydrophobic aromatic regions (cyan), and excluded volume features (gray) are colored, respectively.
Compounds aligning with the pharmacophore’s features are expected to exhibit high specificity for JAK2, reducing potential off-target effects and improving drug development outcomes.
3.4. Pharmacophore Model Validation and Ligand Screening
The performance of the pharmacophore model was validated using the GH score, a statistical parameter that evaluates the ability of a model to distinguish between active and inactive compounds [,]. GH score was calculated using a dataset of active and decoys. Therefore, 300 decoys were generated from a set of 15 active compounds (Supplementary Data Table S2). The respective dataset was screened using the pharmacophore model (Model 10) with the highest selectivity, 3.7137, among which all 15 compounds were correctly identified as active, and 91 decoys were misclassified as active. The GH score obtained was 0.711, indicating a good discriminative ability of the pharmacophore model (Figure 4). The detailed GH score calculation has been supplemented in the Supplementary Data Section S1.
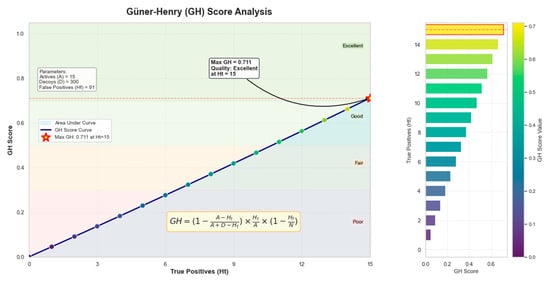
Figure 4.
The Pharmacophore model validation by GH score. 0.711 GH score depicts an excellent quality assessment.
Based on the Tanimoto similarity analysis, a total of 177 compounds showing more than 50% structural similarity to the reference ligand were initially selected. These structurally similar molecules were subsequently evaluated using the validated pharmacophore model to identify candidates that fit the essential chemical features required for JAK2 inhibition. Following this pharmacophore-based screening, 39 compounds were selected for further molecular docking analysis (Supplementary Data Table S3). Interestingly, all the screened compounds manifested highly similar and close fit values compared to each other, ranging from 2.9937 to 2.9764.
3.5. Molecular Docking Analysis
The CDcoker module in Discovery Studio evaluates the binding affinity and interaction between small molecules and a target protein by calculating two key energy parameters: CDcoker Energy and CDocker Interaction Energy. CDocker Energy (measured in kcal/mol) reflects the total internal and binding energy of the ligand-protein complex. A lower CDocker Energy value indicates a more stable and energetically favorable binding conformation, signifying stronger interactions, whereas CDocker Interaction Energy (also in kcal/mol) measures the direct interaction strength between the ligand and the protein [,,]. Highly negative interaction energy values suggest that the compound forms strong interactions with the active site residues of JAK2, as summarized in Table 1. The CDocker energy scores of all 39 compounds and the reference compounds have been manifested in the supplementary Data Table S4.

Table 1.
The docking energy scores of the top 10 docked compounds compared to the reference momelotinib and ruxolitinib compounds, ranked in ascending order.
3.6. Molecular Docking Interactions
The interaction analysis of the top 10 docked compounds, compared to the reference compound, was performed to assess their binding within the active site of the target protein. The findings demonstrated that all compounds effectively docked in the active region, establishing key intermolecular interactions such as hydrogen bonds and salt bridges. Although hydrophobic interactions were identified, the analysis emphasized stronger interactions like hydrogen bonds and salt bridges, which play a crucial role in ensuring binding stability (Table 2).

Table 2.
In the molecular docking interactions of the top compounds, salt bridges are denoted in bold, while hydrogen bonds are represented without any emphasis.
The molecular docking interaction results offer valuable insights into the binding behavior of the screened compounds within the active site of the target protein, with momelotinib serving as the reference inhibitor. Ruxolitinib, another reference compound, manifested very low compatibility in molecular docking analysis and was therefore removed from the dataset. Among all tested compounds, CHEMBL2208033 exhibited an exceptionally robust interaction profile, forming contacts with Pro933, Leu932, Asn981, and Asp994. Importantly, this compound established a salt bridge with Asp994, a potent electrostatic interaction that significantly enhances binding affinity and complex stability. CHEMBL4169802 also demonstrated a highly favorable binding profile, engaging with six critical residues (Lys882, Leu932, Pro933, Leu855, Lys943, and Arg938), forming multiple hydrogen bonds ranging from 2.09 to 2.91 Å. Likewise, CHEMBL4286867 formed close interactions with Asp994, Pro933, and Leu855, including a contact with Asp994, an interaction site also involved with CHEMBL2208033 (Figure 5). The distances (2.12–2.82 Å) confirm a good fit within the active pocket. CHEMBL4162254 also engages Pro933, Leu932, and Lys943 through tightly bound interactions, including one as short as 1.74 Å, indicative of strong hydrogen bonding. This compound’s proximity to these residues, particularly the positively charged Lys943, suggests a stable and possibly selective binding mechanism.
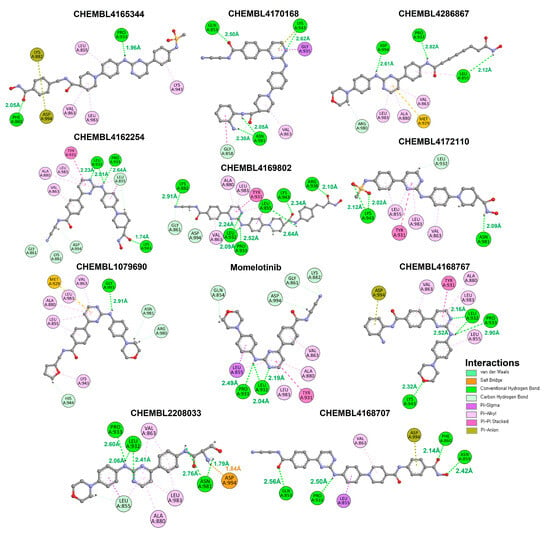
Figure 5.
The 2D interactions of the docked compounds within the active region of the JAK2. All the protein–ligand interactions are illustrated using distinct color codes, with each color representing a specific type of interaction.
Interestingly, CHEMBL4170168 and CHEMBL4172110 also showed close-range interactions with Asn981, an important polar residue that may contribute to ligand specificity. CHEMBL4165344, despite forming close interactions with Pro933 and Phe860 (1.96 and 2.05 Å), appears to have a more limited interaction profile. Compounds such as CHEMBL1079690, interacting only with Gly993, also exhibit limited binding profiles due to less contact with binding pocket residues. In contrast, momelotinib primarily interacted with Pro933 and Leu932, with the bonding distance ranging from 2.04Å to 2.49Å.
Comparative crystallographic and docking studies of inhibitors like ruxolitinib, fedratinib, and momelotinib have identified hydrogen bonding with Asp994 and hydrophobic interactions with Leu932 as key determinants of JAK2 inhibition [,,,,,,,]. The ability of CHEMBL2208033 and CHEMBL4169802 to replicate these interactions supports the reliability of our computational approach and suggests comparable inhibitory potential [,,].
3.7. Molecular Dynamics Simulation
To further evaluate the stability of the top ten screened compounds with JAK2, 100 ns MD simulations were performed using GROMACS. This analysis provided a dynamic assessment to confirm the potential of these compounds as effective inhibitors of JAK2.
3.7.1. Root Mean Square Deviation Analysis
The MD simulation and RMSD-based statistical analyses collectively provide a rigorous assessment of the dynamic behavior and stability of the protein-ligand complexes formed with the screened compounds. This integrative evaluation, encompassing trajectory-based RMSD plots, bar chart comparisons, and quantitative statistical descriptors (mean, standard deviation, and derived stability scores) (Table 3), offers a detailed understanding of each compound’s potential to maintain a stable interaction with the target protein throughout a 100 ns simulation.

Table 3.
Summary of RMSD values, minimal energies, and stability metrics for the simulated compounds.
The RMSD trajectory plots (graphs A and B) in the provided Figure 6 illustrate the temporal evolution of the ligand-protein complex. Graph A highlights a cluster of compounds, CHEMBL2208033, CHEMBL4169802, CHEMBL4286867, and CHEMBL4162254, which exhibit tightly clustered, low RMSD traces over time, indicating excellent conformational stability. Their average RMSD values of 0.22 ± 0.04 nm, 0.50 ± 0.04 nm, 0.53 ± 0.06 nm, and 0.30 ± 0.04 nm, respectively, demonstrate a clear advantage over other candidates. These values are not only comparable to but in some cases even more favorable than the reference inhibitor momelotinib, which displays a mean RMSD of 0.21 ± 0.05 nm. The standard deviations of these top-performing compounds remain low, indicating minimal fluctuations and underscoring the persistence of stable ligand-induced conformations throughout the simulation.
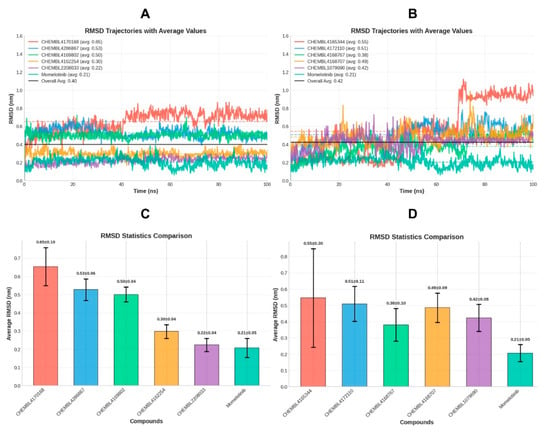
Figure 6.
RMSD trajectories of the screened top docked compounds and the reference inhibitor momelotinib, (A) includes the top-performing compounds identified by RMSD analysis, while (B) presents the remaining candidates. (C,D) Bar plots comparing the average RMSD values (±standard deviation) of each complex.
Importantly, CHEMBL2208033. achieves the highest stability score (0.965) among all compounds, even exceeding that of momelotinib (0.951), followed closely by CHEMBL4162254 (0.964) and CHEMBL4169802 (0.961). These results are particularly notable because CHEMBL2208033 also forms a salt bridge with Asp994, a crucial electrostatic interaction that likely contributes to its remarkable structural anchoring and overall stability within the active site.
Graph C complements the RMSD trajectory data by providing a bar chart comparison of average RMSD values and standard deviations, visually emphasizing the superior performance of the four lead compounds. Similarly, graph D focuses on the remaining candidates, several of which, such as CHEMBL4170168 (0.65 ± 0.10 nm) and CHEMBL4165344 (0.55 ± 0.30 nm), demonstrate higher mean RMSD values and broader fluctuations, reflected in their lower stability scores. These compounds likely induce more significant perturbations in the protein structure, potentially due to weaker binding or suboptimal fitting within the active site.
Other mid-performing candidates such as CHEMBL4172110, CHEMBL4168767, CHEMBL4168707, and CHEMBL1079690 present moderate RMSD averages (0.38–0.51 nm) and stability scores ranging from 0.903 to 0.923. Although they maintain structural stability within acceptable limits, their performance is clearly outshone by the top four candidates. Collectively, these findings support a strong, evidence-based prioritization of CHEMBL2208033, CHEMBL4169802, CHEMBL4286867, and CHEMBL4162254 as the most promising candidates. These compounds consistently demonstrate superior structural stability, minimal deviation, and tight conformational control when bound to the target protein. Their performance, both in terms of RMSD dynamics and statistical robustness, is comparable to or even better than that of momelotinib, the reference inhibitor used in this study. These results not only validate the docking predictions but also enhance confidence in the reliability of these compounds for downstream lead optimization.
3.7.2. Hydrogen Bonds Plot Analysis
The hydrogen bond (H-bond) dynamics evaluation of the screened compounds in complex with the target protein during a 100 ns MD simulation further emphasizes the RMSD results. Each subplot corresponds to a distinct compound, including the reference drug momelotinib, and illustrates both the actual hydrogen bonds formed (orange line) and potential hydrogen bonds (blue line) over time as manifested in Figure 7. These metrics provide critical insights into the strength, persistence, and frequency of polar interactions, which are essential determinants of binding stability and specificity.
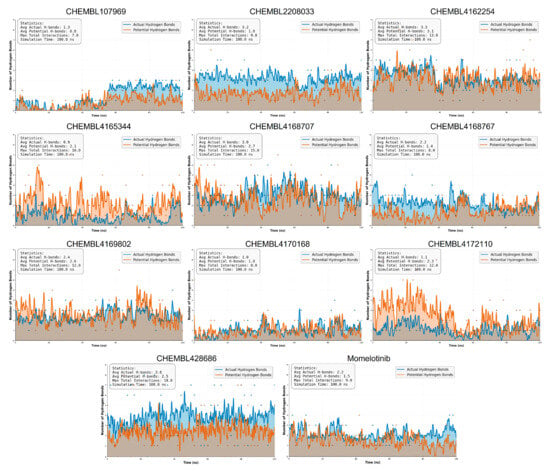
Figure 7.
Hydrogen bond analysis of JAK2-ligand complexes over 100 ns MD simulations. Each graph represents the number of hydrogen bonds formed between the ligand and JAK2 for a specific compound, including the reference inhibitor momelotinib.
Notably, CHEMBL2208033, CHEMBL4169802, CHEMBL4286867, and CHEMBL4162254 again emerge as standout candidates, maintaining a consistently high and stable number of actual hydrogen bonds throughout the simulation. CHEMBL4286867 exhibited the highest average number of hydrogen bonds (3.8), followed closely by CHEMBL4162254 (3.3), suggesting strong and stable polar interactions with JAK2 throughout the simulation. Interestingly, CHEMBL4169802, despite a slightly lower average of 3.1 hydrogen bonds, reached the highest maximum count of 12, indicating its ability to form numerous, though potentially dynamic, hydrogen interactions. CHEMBL2208033 maintained a strong hydrogen bonding profile as well, with an average of 3.02 hydrogen bonds and a maximum of 9, reflecting both stability and consistent binding.
In contrast, compounds like CHEMBL4170168 and CHEMBL4172110 show relatively higher fluctuations and noise in their H-bond profiles. Although they occasionally form a higher number of hydrogen bonds, their averages are lower (1.0 and 1.1), and the temporal instability implies transient interactions. The reference inhibitor momelotinib exhibits a mean of 2.2 hydrogen bonds with a total maximum interaction of 7, reinforcing its credibility as a potent binder. Remarkably, CHEMBL2208033 and CHEMBL4169802 demonstrate comparable or slightly superior hydrogen bonding stability, suggesting their potential as viable alternative inhibitors.
Other compounds such as CHEMBL1079690, CHEMBL4168707, and CHEMBL4168767 show moderate hydrogen bonding behavior, with lower means and greater variability, possibly reflecting weaker or more dynamic interactions.
Collectively, the hydrogen bond analysis supports and complements the RMSD-based findings by offering a molecular rationale for the observed structural stability. CHEMBL2208033, CHEMBL4169802, CHEMBL4286867, and CHEMBL4162254 not only preserve compact structural alignment during MD simulations but also sustain a high frequency of strong, consistent hydrogen bonds with the target protein. These data provide compelling evidence of their potential as lead candidates, capable of engaging the binding pocket through persistent polar interactions, thereby reinforcing their prioritization for further experimental validation.
3.7.3. MD Interaction Energy
The MD interaction energy analysis provides a critical thermodynamic perspective on the binding affinity and stability of the ligand-protein complexes over the course of the simulation. In this context, the interaction energies were dissected into short-range Coulombic (Coul-SR) and Lennard-Jones (LJ-SR) components, which, respectively, represent electrostatic and van der Waals (vdW) contributions [,]. The total interaction energy is the sum of these terms and serves as a direct indicator of the overall favorability of the ligand binding to the target protein (Figure 8).
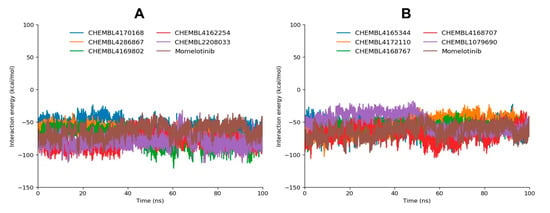
Figure 8.
Interaction energy profiles of JAK2-ligand complexes (A,B). Graph A shows the top-performing candidates, including CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033, along with the reference momelotinib, while graph B displays the remaining ligands.
Among all tested compounds, CHEMBL2208033 exhibits the most favorable total MD interaction energy of −80.01 kcal/mol, primarily driven by a highly negative Coul-SR energy of −47.99 kcal/mol, the most electrostatically favorable among the dataset. This suggests that CHEMBL2208033 forms robust polar/electrostatic interactions with the active site, potentially including salt bridges (e.g., the noted interaction with Asp994), strong hydrogen bonds, and dipole interactions. The lower LJ-SR contribution (−32.02 kcal/mol) implies that while van der Waals forces are relevant, electrostatics are the dominant force stabilizing this complex. Closely following is CHEMBL4162254, with a total MD interaction energy of −79.18 kcal/mol, arising from a highly favorable Coul-SR of −36.31 kcal/mol and LJ-SR of −42.87 kcal/mol (Table 4). Unlike CHEMBL2208033, this compound has a more balanced contribution from both electrostatics and van der Waals interactions, indicating a dual-mode stabilization mechanism. The strong vdW component suggests tight packing into the hydrophobic pockets of the binding site, while still maintaining polar interactions. This duality likely contributes to its excellent RMSD performance and hydrogen bonding consistency, further strengthening its candidacy.

Table 4.
The MD interaction energy of the simulated compounds.
CHEMBL4169802 ranks third with a total MD interaction energy of −73.85 kcal/mol, bolstered by strong electrostatic (−26.96 kcal/mol) and vdW (−46.88 kcal/mol) interactions. The dominance of LJ-SR here emphasizes extensive hydrophobic interactions, which are often essential for enhancing specificity and affinity within a partially nonpolar binding pocket. Its robust hydrogen bonding and high structural stability in MD simulations are consistent with these energetic findings, placing it among the top-tier candidates. CHEMBL4286867 also exhibits a commendable total MD interaction energy of −67.54 kcal/mol, mainly due to strong van der Waals interactions (−51.21 kcal/mol), while the electrostatic component (−16.33 kcal/mol) is moderate. This pattern reflects a ligand that likely relies more on nonpolar packing than polar contacts. Given that it maintains favorable hydrogen bonding patterns and moderate RMSD stability, this compound could be highly effective in occupying the hydrophobic core of the active site.
The reference drug momelotinib shows a total MD interaction energy of −63.51 kcal/mol, with balanced Coul-SR (−26.73 kcal/mol) and LJ-SR (−36.78 kcal/mol) contributions. While this affirms its known efficacy and binding stability, several tested compounds, particularly CHEMBL2208033, CHEMBL4162254, and CHEMBL4169802, exhibit superior MD interaction energies, suggesting they may outperform momelotinib in terms of binding strength and potential therapeutic effectiveness. Other compounds such as CHEMBL4170168, CHEMBL4172110, and CHEMBL1079690 exhibit weaker MD interaction energies (ranging from −50.10 to −56.21 kcal/mol), characterized by relatively modest electrostatic and vdW interactions. Their less favorable energetic profiles indicate that they may possess weaker or more transient binding as compared to the top compounds. Interestingly, CHEMBL4168707 stands out with a strong Coul-SR of −27.73 kcal/mol, but its total energy (−66.18 kcal/mol) remains lower than the top contenders due to a less potent LJ-SR term (−38.45 kcal/mol). This suggests it may interact well with polar residues but lacks sufficient hydrophobic stabilization.
Overall, these energy interaction results align well with structural and dynamic analyses, reinforcing the identification of CHEMBL2208033, CHEMBL4162254, CHEMBL4169802, and CHEMBL4286867 as the most potent inhibitors. Their binding is not only stable and structurally favorable but also energetically optimized through synergistic electrostatic and van der Waals interactions.
3.8. gmxMMPBSA Free Energy Calculation
The gmxMMPBSA analysis is a crucial tool in MD simulation studies, offering detailed insights into the binding free energies between ligands and target proteins []. It evaluates the energetics of molecular interactions within a biologically relevant environment, aiding in the assessment of the thermodynamic stability of ligand-receptor complexes. By enabling more accurate predictions of binding affinities, gmxMMPBSA facilitates the identification of effective inhibitors and supports the optimization of lead compounds. The binding free energy (ΔG_total) calculations provide a vital thermodynamic insight into the affinity between the ligands and the target protein, with more negative values indicating stronger and more favorable binding interactions. This analysis, in conjunction with interaction energies, RMSD trends, and hydrogen bonding stability, allows for a comprehensive evaluation of ligand performance during the 100 ns MD simulations. The graphical interpretation manifests the relative strengths and potential of each compound, highlighting candidates with superior binding propensities (Figure 9).
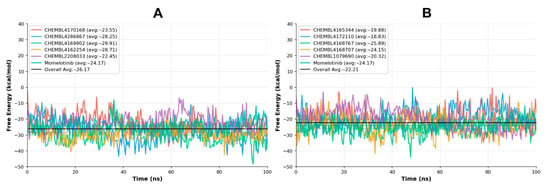
Figure 9.
The free energy graphs of the screened compounds with their average values in comparison with the reference compound. The free energy of CHEMBL4170168, CHEMBL4286867, CHEMBL4169802, CHEMBL4162254 and CHEMBL2208033 in comparison with the reference compound has been manifested in Figure (A), while the free energy of the rest has been depicted in the Figure (B).
Among the evaluated compounds, CHEMBL4169802 exhibited the most favorable binding free energy of −29.91 kcal/mol, with a standard deviation of 5.28, indicating consistent and stable interaction throughout the simulation. This highly negative ΔG suggests a very strong thermodynamic preference for the bound state, potentially reflecting a well-optimized fit within the binding pocket, involving synergistic contributions from hydrophobic, electrostatic, and hydrogen bonding interactions. Given its strong interaction energy profile and stable RMSD trajectory, this compound clearly stands out as a top-tier inhibitor.
Following closely, CHEMBL4162254 and CHEMBL4286867 displayed ΔG values of −28.71 kcal/mol and −28.25 kcal/mol, respectively. CHEMBL4162254, with the lowest standard deviation (4.81), demonstrates not only strong binding but also high energetic stability, indicating that its interactions with the protein are persistent and not significantly fluctuating across the simulation. This stability aligns with its excellent RMSD profile and interaction energies, suggesting that this compound engages the target through both robust van der Waals and electrostatic contacts. On the other hand, CHEMBL4286867, while energetically comparable, showed a slightly higher standard deviation (6.66), indicating a slightly more dynamic binding mode, though still energetically favorable.
Interestingly, while CHEMBL2208033 showed the most favorable total interaction energy in the earlier analysis (−80.01 kcal/mol), its ΔG was −26.45 kcal/mol, which, while still strong, is less favorable than that of the top three candidates in this specific free energy ranking. Still, CHEMBL2208033 remains a promising candidate, especially with its superior ΔG values than the other screened compounds; it maintained the top tier.
CHEMBL4168767 and CHEMBL4168707 yielded moderately strong ΔG values of −25.89 kcal/mol and −24.15 kcal/mol, respectively. These compounds demonstrate a fair binding affinity, with somewhat higher standard deviations (4.98 and 6.49) (Table 5), indicating more variability in their interaction energies during the simulation. Their binding might be influenced by intermittent hydrogen bonding or partial exposure to solvent during trajectory sampling.

Table 5.
The free energy of the screened compounds was calculated from 0ns to 100ns.
Surprisingly, Momelotinib, the reference inhibitor, recorded a ΔG of −24.17 kcal/mol, placing it in the mid-range compared to the tested compounds. Its relatively low standard deviation (3.99) points to a stable but not exceptionally strong binding affinity. This benchmark supports the notion that several repurposed candidates, including CHEMBL4169802, CHEMBL4162254, and CHEMBL4286867, may possess enhanced inhibitory potential relative to the reference drug.
Other compounds, such as CHEMBL4170168 (−23.55 kcal/mol), CHEMBL4165344 (−19.88 kcal/mol), CHEMBL4172110 (−18.83 kcal/mol), and CHEMBL1079690 (−20.32 kcal/mol), exhibited weaker ΔG values, suggesting lower binding affinities compared to the top compounds. These compounds may lack critical interactions within the binding site, leading to less favorable thermodynamic profiles.
In summary, the MM-PBSA-derived binding free energy data complement and validate the results from interaction energy, RMSD, and hydrogen bonding analyses. Compounds CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033 emerge as the most promising candidates, demonstrating strong, stable, and energetically favorable binding profiles as compared to the reference momelotinib. These findings collectively underscore the potential of these molecules as next-generation therapeutic inhibitors targeting JAK2.
3.9. Additional MD Duplicates Run
To ensure the robustness and reproducibility of the MD and free energy outcomes, additional duplicate simulation runs were conducted for the top-performing compounds, CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033. The results of these independent replicates consistently confirmed the real-time stability of all four complexes. As illustrated in Figure 10, the systems maintained highly compact and converged trajectories, with minimal fluctuations in RMSD values across both primary and duplicate runs.
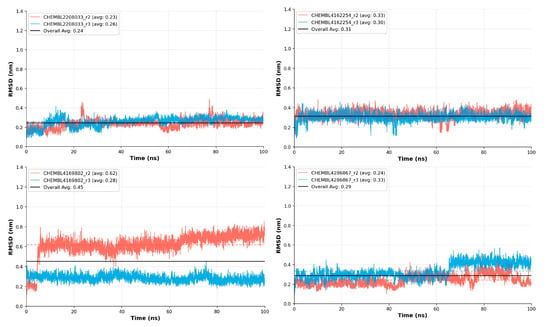
Figure 10.
The top-tier compounds were subjected to additional 100 ns duplicate MD simulations further to validate their dynamic stability and reproducibility of binding behavior. Here, r2 refers to the 2nd run and r3 refers to the 3rd run; however, r1 was considered to be the primary run illustrated in the RMSD analysis.
This reproducibility strongly indicates that the observed conformational stability is not an artifact of a single trajectory but rather an inherent property of these ligand–protein complexes. The compact trajectories suggest that these compounds remain tightly bound within the catalytic pocket, maintaining key stabilizing interactions over extended simulation periods. Such stability often correlates with enhanced binding affinity and lower dissociation probability, which are desirable features for potential inhibitors.
4. Discussion
The present study employed a multi-tiered in silico approach to identify and characterize novel inhibitors targeting JAK2, a key therapeutic target implicated in various hematologic malignancies and inflammatory diseases. Through ligand similarity screening followed by pharmacophore-based virtual screening, molecular docking, MD simulations, and MM/PBSA free energy calculations, we comprehensively evaluated ligand binding behavior, structural stability, and thermodynamic feasibility. Among 1,900,000 screened compounds from the ChEMBL library, four candidates, CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033, consistently outperformed the reference inhibitor momelotinib, thereby presenting themselves as promising leads for future JAK2-targeted drug development.
A key aspect of our screening strategy was the use of a validated pharmacophore model built upon the crystal structure of JAK2 complexed with momelotinib (PDB ID: 8BXH). The pharmacophore model, incorporating critical features such as hydrogen bond acceptors, donors, hydrophobic regions, and excluded volumes, achieved a GH score of 0.711, indicating high specificity and reliability. From 177 structurally similar compounds (Tanimoto similarity > 50%), 39 passed pharmacophore filtering and were further evaluated by molecular docking. Notably, all four lead compounds demonstrated favorable docking energies and formed critical interactions with residues central to JAK2 function, such as Pro933, Leu932, Lys943, and Asp994. CHEMBL2208033, in particular, exhibited a salt bridge with Asp994, a strong electrostatic interaction that greatly enhances binding affinity.
The docking results are consistent with prior reports highlighting the importance of the Asp994 and Lys943 interaction in stabilizing inhibitor binding within the JAK2 catalytic domain. For instance, comparative crystallographic and docking analyses of approved inhibitors such as ruxolitinib, fedratinib, and momelotinib have similarly revealed hydrogen bonding with Asp994 and hydrophobic packing near Leu932 as key determinants of potency and selectivity [,,,,,,,]. The ability of CHEMBL2208033 and CHEMBL4169802 to reproduce these critical interactions reinforces the reliability of our computational approach and suggests that these ligands may exhibit comparable inhibitory potential. Moreover, in another study, NSC13626 was reported to form hydrogen bonds with key pharmacological residues Leu932 and Asp994 [].
Therefore, the stability of these protein-ligand complexes was further substantiated by MD simulations over 100 ns. RMSD analysis indicated that CHEMBL2208033, CHEMBL4169802, CHEMBL4162254, and CHEMBL4286867 maintained stable trajectories with low average RMSD values (0.22–0.53 nm), closely comparable or superior to momelotinib (0.21 nm). Additionally, their low standard deviations and high stability scores (0.944–0.965) reflected minimal conformational drift and robust complex formation. This conformational stability is essential for sustained inhibition of JAK2 in physiological conditions. The hydrogen bond analysis further strengthened the case for these top candidates. All four compounds maintained a consistent number of hydrogen bonds with JAK2 throughout the simulation, a key determinant of ligand specificity and durability of interaction. Remarkably, CHEMBL2208033 and CHEMBL4169802 demonstrated average hydrogen bond counts comparable to or exceeding those of momelotinib, highlighting their potential for strong and selective target engagement.
Beyond structural analysis, MD interaction energy calculations provided thermodynamic validation. CHEMBL2208033 demonstrated the most favorable total MD interaction energy (−80.01 kcal/mol), predominantly driven by electrostatic contributions (−47.99 kcal/mol), supporting the critical role of charged and polar interactions in stabilizing this complex. Meanwhile, CHEMBL4162254 showed a balanced MD interaction energy profile (−79.18 kcal/mol), with significant van der Waals (−42.87 kcal/mol) and electrostatic (−36.31 kcal/mol) components, suggesting dual stabilization through both hydrophobic and polar contacts.
Interestingly, while CHEMBL2208033 topped MD interaction energy scores, it ranked slightly lower in MM/PBSA-derived binding free energy (ΔG = −26.45 kcal/mol) compared to CHEMBL4169802 (−29.91 kcal/mol), CHEMBL4162254 (−28.71 kcal/mol), and CHEMBL4286867 (−28.25 kcal/mol). This divergence may reflect entropic contributions or solvation effects not fully captured in MD interaction energies. Nonetheless, the overall ΔG values of these four compounds surpassed momelotinib (−24.17 kcal/mol), confirming their superior binding affinity and validating their therapeutic potential. Moreover, the additional duplicate runs also confirmed the superior and robust binding profile is these compounds by maintaining a highly compact RMSD trajectory.
While several JAK inhibitors have been developed and approved, such as ruxolitinib and momelotinib, their use has been limited by issues related to selectivity, off-target effects, and drug resistance. Many of these inhibitors exhibit pan-JAK activity, inadvertently affecting other isoforms like JAK1, JAK3, and TYK2, leading to adverse events including cytopenia and immunosuppression [,,,,,,]. To assess the binding selectivity of the top four compounds toward JAK2 in comparison to other members of the JAK kinase family, we performed cross-target docking and MD simulation to analyze the compatibility of these ligands against JAK1, JAK3, and TYK2.
The CDocker energy and RMSD analyses together provide a comprehensive understanding of both the binding and dynamic stability of the compounds across JAK isoforms. Among the candidates, CHEMBL4169802 exhibited the strongest preference for JAK2 (−46.08 kcal/mol) compared to JAK3 (−40.81 kcal/mol) and JAK1 (−38.37 kcal/mol), while showing weaker affinity toward TYK2 (−26.51 kcal/mol), suggesting potential JAK2 selectivity. In contrast, CHEMBL4286867 displayed comparable binding energies across JAK isoforms, indicating a likely pan-JAK inhibitory profile. Similarly, CHEMBL4162254 showed moderate and uniform binding across all isoforms, whereas CHEMBL2208033 favored JAK2 and TYK2 over JAK1 and JAK3 (Supplementary Data Table S5). Interestingly, despite some compounds exhibiting favorable docking scores across multiple isoforms, the 100 ns RMSD trajectories revealed that only their complexes with JAK2 maintained highly stable conformations, whereas interactions with JAK1, JAK3, and TYK2 showed loose RMSD trajectories (Supplementary Data Figure S1). This observation suggests that while these ligands may have initial pose stability or fit well in the docking phase, their dynamic stability within the binding pocket is more favorable for JAK2, supporting their role as the most compatible and energetically stable against JAK2. Therefore, these findings imply that these compounds exhibit true structural and energetic selectivity toward JAK2. Moreover, the ADMET and drug likeness properties of these compounds were also predicted and manifested in the supplementary Data Table S6. The physicochemical analysis of the top four JAK2 inhibitors revealed acceptable drug-like properties, with molecular weights between 434.49–616.67 g/mol, 4–5 H-bond donors, and 6–7 acceptors. All compounds showed moderate lipophilicity (logP 1.14–3.02) and suitable TPSA values (125.63–172.37 Å2), while CHEMBL2208033 exhibited the highest QED (0.4448), indicating the most favorable drug-likeness profile.
5. Conclusions
In this study, an integrative computational workflow was employed to identify and characterize potent inhibitors targeting JAK2 from the ChEMBL compound repository. Using a systematic approach combining ligand-based screening, validated pharmacophore modeling, molecular docking, molecular dynamics simulations, interaction energy analyses, and MM/PBSA-based free energy calculations, we were able to comprehensively evaluate the binding behavior, stability, and thermodynamic favorability of candidate molecules.
Out of thousands of compounds, four candidates, CHEMBL4169802, CHEMBL4162254, CHEMBL4286867, and CHEMBL2208033, demonstrated superior performance across multiple evaluation criteria. These compounds consistently exhibited strong binding affinities, stable RMSD profiles, persistent hydrogen bonding patterns, and highly favorable interaction and free energy values. Notably, CHEMBL4169802 emerged as the most promising candidate, showing the lowest binding free energy (−29.91 kcal/mol), stable interaction dynamics, and robust engagement with critical residues within the JAK2 active site. Importantly, some of these candidates outperformed the reference inhibitor momelotinib. ADMET and drug-likeness analyses suggest favorable pharmacokinetic properties and acceptable toxicity profiles, indicating their potential to serve as improved alternatives for therapeutic development. Chemical optimization of these scaffolds to enhance potency and bioavailability, as well as experimental validation through in vitro kinase inhibition assays and cell-based studies to confirm their biological activity. Collectively, these findings reveal promising small-molecule inhibitors with the potential for further optimization and experimental validation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/futurepharmacol5040066/s1, Figure S1: The comparative MD analysis of the screened compounds against the JAK1, JAK3, and TYK2 isoforms of the JAK family; Table S1: The predicted 10 pharmacophore hypothesis; Table S2: The dataset of active and generated Decoys; Table S3: The pharmacophore screening results of all 39 selected compounds; Table S4: The Molecular docking scores of all the docked compounds; Table S5: The Molecular docking energy comparison of the top screened compounds against the isoforms of JAK; Table S6: SwissADME predicted ADME properties and the drug likeness values of the most potent compounds.
Author Contributions
M.Y. and J.P. were involved in the experimental operation and data analysis. J.C., J.-H.H., W.S.P., and E.-T.H. were involved in data curation and the methodology. W.C. was involved in the conceptualization and writing of the manuscript. M.Y. and W.C. confirmed the authenticity of all the raw data. W.C. was involved in reviewing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2025-23323186, W.C.) and by the Regional Innovation System & Education (RISE) program through the Gangwon RISE Center, funded by the Ministry of Education (MOE) and the Gangwon State (G.S.), Republic of Korea (2025-RISE-10-002, W.C.), and by Korea Basic Science Institute (National Research Facilities and Equipment Center) grants funded by the Ministry of Education (RS-2020-NF000330, J.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Hubbard, S.R. Mechanistic insights into regulation of JAK2 tyrosine kinase. Front. Endocrinol. 2018, 8, 361. [Google Scholar] [CrossRef]
- Ferrao, R.; Lupardus, P.J. The Janus kinase (JAK) FERM and SH2 domains: Bringing specificity to JAK–receptor interactions. Front. Endocrinol. 2017, 8, 71. [Google Scholar] [CrossRef]
- Virtanen, A.T.; Haikarainen, T.; Sampathkumar, P.; Palmroth, M.; Liukkonen, S.; Liu, J.; Nekhotiaeva, N.; Hubbard, S.R.; Silvennoinen, O. Identification of novel small molecule ligands for JAK2 pseudokinase domain. Pharmaceuticals 2023, 16, 75. [Google Scholar] [CrossRef]
- Purohit, M.; Gupta, G.; Afzal, O.; Altamimi, A.S.A.; Alzarea, S.I.; Kazmi, I.; Almalki, W.H.; Gulati, M.; Kaur, I.P.; Singh, S.K. Janus kinase/signal transducers and activator of transcription (JAK/STAT) and its role in Lung inflammatory disease. Chem. Biol. Interact. 2023, 371, 110334. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal. Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Kiu, H.; Nicholson, S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 2012, 30, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Choe, J.; Hassan, M.; Kloczkowski, A.; Chun, W. Recent advances and future perspectives in small molecule JAK2 inhibitors. Future Med. Chem. 2025, 17, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.G.; Paes, J.; da Costa, A.G.; Malheiro, A.; Silva, G.V.; Mourão, L.P.d.S.; Tarragô, A.M. JAK2 variant signaling: Genetic, hematologic and immune implication in chronic myeloproliferative neoplasms. Biomolecules 2022, 12, 291. [Google Scholar] [CrossRef]
- Bertsias, G. Therapeutic targeting of JAKs: From hematology to rheumatology and from the first to the second generation of JAK inhibitors. Mediterr. J. Rheumatol. 2020, 31, 105–111. [Google Scholar] [CrossRef]
- Behrens, K.; Alexander, W.S. Cytokine control of megakaryopoiesis. Growth Factors 2018, 36, 89–103. [Google Scholar] [CrossRef]
- Pasquier, F.; Cabagnols, X.; Secardin, L.; Plo, I.; Vainchenker, W. Myeloproliferative neoplasms: JAK2 signaling pathway as a central target for therapy. Clin. Lymphoma. Myeloma. Leuk. 2014, 14, S23–S35. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal. Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the neuroinflammation and its association with neurological disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef]
- Gou, P.; Zhang, W.; Giraudier, S. Insights into the potential mechanisms of JAK2V617F somatic mutation contributing distinct phenotypes in myeloproliferative neoplasms. Int. J. Mol. Sci. 2022, 23, 1013. [Google Scholar] [CrossRef]
- Oh, S.T.; Gotlib, J. JAK2 V617F and beyond: Role of genetics and aberrant signaling in the pathogenesis of myeloproliferative neoplasms. Expert. Rev. Hematol. 2010, 3, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, G.; McMullin, M.F.; Mills, K. Molecular pathogenesis of the myeloproliferative neoplasms. J. Hematol. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Firwana, B.; Zarzour, A.; Morad, M.; Rana, V.; Tiu, R.V. Comprehensive review of JAK inhibitors in myeloproliferative neoplasms. Ther. Adv. Hematol. 2013, 4, 15–35. [Google Scholar] [CrossRef]
- Perner, F.; Perner, C.; Ernst, T.; Heidel, F.H. Roles of JAK2 in aging, inflammation, hematopoiesis and malignant transformation. Cells 2019, 8, 854. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Li, S.; Ma, J.; Dai, X.; Lu, J. Deciphering JAK/STAT signaling pathway: A multifaceted approach to tumorigenesis, progression and therapeutic interventions. Int. Immunopharmacol. 2024, 131, 111846. [Google Scholar] [CrossRef]
- Obeagu, E.I. JAK2 in pediatric leukemia: Mechanisms of pathogenesis and drug development–a narrative review. Ann. Med. Surg. 2025, 87, 3410–3423. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Verstovsek, S. The rationale for immunotherapy in myeloproliferative neoplasms. Curr. Hematol. Malig. Rep. 2019, 14, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, B.; Liu, Y.; Yu, Y.; Wan, Y.; Wu, J.; Wang, Y. JAK2 inhibitors for the treatment of Philadelphia-negative myeloproliferative neoplasms: Current status and future directions. Mol. Divers. 2023, 28, 3445–3456. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Meylan, F.; O’Shea, J.J.; Gadina, M. JAK inhibitors: Ten years after. Eur. J. Immunol. 2021, 51, 1615–1627. [Google Scholar] [CrossRef]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef]
- Tokareva, K.; Reid, P.; Yang, V.; Liew, D.; Peterson, A.C.; Baraff, A.; Giles, J.; Singh, N. JAK inhibitors and black box warnings: What is the future for JAK inhibitors? Expert. Rev. Clin. Immunol. 2023, 19, 1385–1397. [Google Scholar] [CrossRef]
- Sardana, K.; Bathula, S.; Khurana, A. Which is the Ideal JAK Inhibitor for Alopecia Areata—Baricitinib, Tofacitinib, Ritlecitinib or Ifidancitinib—Revisiting the Immunomechanisms of the JAK Pathway. Indian Dermatol. Online J. 2023, 14, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Ritlecitinib: First Approval. Drugs 2023, 83, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Deucravacitinib: First Approval. Drugs 2022, 82, 1671–1679. [Google Scholar] [CrossRef]
- Cai, W.; Tong, R.; Sun, Y.; Yao, Y.; Zhang, J. Comparative efficacy of five approved Janus kinase inhibitors as monotherapy and combination therapy in patients with moderate-to-severe active rheumatoid arthritis: A systematic review and network meta-analysis of randomized controlled trials. Front. Pharmacol. 2024, 15, 1387585. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. JAK2 inhibitors for myeloproliferative neoplasms: What is next? Am. Society. Hematol. 2017, 130, 115–125. [Google Scholar] [CrossRef]
- Pardanani, A.; Tefferi, A. The Journal of the American Society of Hematology, How I treat myelofibrosis after failure of JAK inhibitors. Blood 2018, 132, 492–500. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.T.; Park, W.S.; Han, J.H.; Chun, W. Drug Repositioning via Graph Neural Networks: Identifying Novel JAK2 Inhibitors from FDA-Approved Drugs through Molecular Docking and Biological Validation. Molecules 2024, 29, 1363. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.T.; Park, W.S.; Han, J.H.; Kwon, Y.S.; Lee, H.J.; Chun, W. Machine Learning-Based Drug Repositioning of Novel Janus Kinase 2 Inhibitors Utilizing Molecular Docking and Molecular Dynamic Simulation. J. Chem. Inf. Model. 2023, 63, 6487–6500. [Google Scholar] [CrossRef]
- Schaller, D.; Šribar, D.; Noonan, T.; Deng, L.; Nguyen, T.N.; Pach, S.; Machalz, D.; Bermudez, M.; Wolber, G. Next generation 3D pharmacophore modeling. WIREs Comp. Mol. Sci. 2020, 10, e1468. [Google Scholar] [CrossRef]
- Naqvi, A.A.; Mohammad, T.; Hasan, G.M.; Hassan, M.I. Advancements in docking and molecular dynamics simulations towards ligand-receptor interactions and structure-function relationships. Curr. Top. Med. Chem. 2018, 18, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhong, A.; Wang, Q.; Zheng, T. Structure-based pharmacophore modeling, virtual screening, molecular docking, ADMET, and molecular dynamics (MD) simulation of potential inhibitors of PD-L1 from the library of marine natural products. Mar. Drugs 2021, 20, 29. [Google Scholar] [CrossRef]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory. Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Rácz, A.; Bajusz, D.; Héberger, K. Life beyond the Tanimoto coefficient: Similarity measures for interaction fingerprints. J. Cheminform. 2018, 10, 48. [Google Scholar] [CrossRef]
- Horvath, D. Pharmacophore-based virtual screening. Methods Mol. Biol. 2011, 672, 261–298. [Google Scholar] [PubMed]
- Qing, X.; Yin Lee, X.; De Raeymaeker, J.; RH Tame, J.; YJ Zhang, K.; De Maeyer, M.; RD Voet, A. Pharmacophore modeling: Advances, limitations, and current utility in drug discovery. J. Rec. Lig. Chann. Res. 2014, 2014, 81–92. [Google Scholar]
- Miao, Y.; Virtanen, A.; Zmajkovic, J.; Hilpert, M.; Skoda, R.C.; Silvennoinen, O.; Haikarainen, T. Functional and Structural Characterization of Clinical-Stage Janus Kinase 2 Inhibitors Identifies Determinants for Drug Selectivity. J. Med. Chem. 2024, 67, 10012–10024. [Google Scholar] [CrossRef]
- Banat, R.; Daoud, S.; Taha, M.O. Ligand-based pharmacophore modeling and machine learning for the discovery of potent aurora A kinase inhibitory leads of novel chemotypes. Mol. Divers. 2024, 28, 4241–4257. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Ho, Y.; Liu, H.-L. Structure-Based Pharmacophore Modeling to Discover Novel CCR5 Inhibitors for HIV-1/Cancers Therapy. J. Biom. Sci. Eng. 2019, 12, 10–30. [Google Scholar] [CrossRef]
- Kumar, S.P. Receptor pharmacophore ensemble (REPHARMBLE): A probabilistic pharmacophore modeling approach using multiple protein-ligand complexes. J. Mol. Model. 2018, 24, 282. [Google Scholar] [CrossRef]
- Gupta, N.; Sitwala, N.; Patel, K. Pharmacophore modelling, validation, 3D virtual screening, docking, design and in silico ADMET simulation study of histone deacetylase class-1 inhibitors. Med. Chem. Res. 2014, 23, 4853–4864. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An overview of scoring functions used for protein–ligand interactions in molecular docking. Interdiscip. Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Discovery Studio Modeling Environment, Release 2017; DassaultSystèmes: San Diego, CA, USA, 2016. [Google Scholar]
- Wu, G.; Robertson, D.H.; Brooks, C.L., III; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER—A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Jo, S.; MacKerell, A.D.; Klauda, J.B.; Im, W. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. Biophys. J. 2016, 110, 641a. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Willard, L.; Ranjan, A.; Zhang, H.; Monzavi, H.; Boyko, R.F.; Sykes, B.D.; Wishart, D.S. VADAR: A web server for quantitative evaluation of protein structure quality. Nucleic Acids Res. 2003, 31, 3316–3319. [Google Scholar] [CrossRef]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery: Miniperspective. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Dantas, R.F.; Evangelista, T.C.S.; Neves, B.J.; Senger, M.R.; Andrade, C.H.; Ferreira, S.B.; Silva-Junior, F.P. Dealing with frequent hitters in drug discovery: A multidisciplinary view on the issue of filtering compounds on biological screenings. Expert Opin. Drug Discov. 2019, 14, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Kaserer, T.; Beck, K.R.; Akram, M.; Odermatt, A.; Schuster, D. Pharmacophore models and pharmacophore-based virtual screening: Concepts and applications exemplified on hydroxysteroid dehydrogenases. Molecules 2015, 20, 22799–22832. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Park, J.; Han, E.-T.; Han, J.-H.; Park, W.S.; Chun, W. Identification of Malaria-Selective Proteasome β5 Inhibitors Through Pharmacophore Modeling, Molecular Docking, and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2024, 25, 11881. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A.K. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef]
- Lin, F.-Y.; MacKerell, A.D., Jr. Do halogen–hydrogen bond donor interactions dominate the favorable contribution of halogens to ligand–protein binding? J. Phys. Chem. B 2017, 121, 6813–6821. [Google Scholar] [CrossRef]
- Rafiq, H.; Hu, J.; Hakami, M.A.; Hazazi, A.; Alamri, M.A.; Alkhatabi, H.A.; Mahmood, A.; Alotaibi, B.S.; Wadood, A.; Huang, X. Identification of novel STAT3 inhibitors for liver fibrosis, using pharmacophore-based virtual screening, molecular docking, and biomolecular dynamics simulations. Sci. Rep. 2023, 13, 20147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Di, B.; Niu, M.-M. Structure-based pharmacophore design and virtual screening for novel tubulin inhibitors with potential anticancer activity. Molecules 2019, 24, 3181. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Han, J.-H.; Park, W.S.; Choe, J.; Chun, W. Integration of Deep Learning with Molecular Docking and Molecular Dynamics Simulation for Novel TNF-α-Converting Enzyme Inhibitors. Future Pharmacol. 2025, 5, 55. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Chun, W. Discovery of Novel Aldose Reductase Inhibitors via the Integration of Ligand-Based and Structure-Based Virtual Screening with Experimental Validation. ACS Omega 2024, 9, 20338–20349. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Chun, W. Identification of Potential Tryptase Inhibitors from FDA-Approved Drugs Using Machine Learning, Molecular Docking, and Experimental Validation. ACS Omega 2024, 9, 38820–38831. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.E.; HuangFu, W.-C.; Chao, M.-W.; Sung, T.-Y.; Chang, C.-D.; Chen, Y.-Y.; Hsieh, J.-H.; Tu, H.-J.; Huang, H.-L.; Pan, S.-L. A novel selective JAK2 inhibitor identified using pharmacological interactions. Front. Pharmacol. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Jiménez, L.K.; Rivera, G.; Juárez-Saldivar, A.; Ortega-Balleza, J.L.; Ortiz-Pérez, E.; Jaime-Sánchez, E.; Paz-González, A.; Lara-Ramírez, E.E. Biological Evaluations and Computer-Aided Approaches of Janus Kinases 2 and 3 Inhibitors for Cancer Treatment: A Review. Pharmaceutics 2024, 16, 1165. [Google Scholar] [CrossRef]
- Oliveira, M.P.; Hünenberger, P.H. Influence of the Lennard-Jones Combination Rules on the Simulated Properties of Organic Liquids at Optimal Force-Field Parametrization. J. Chem. Theory Comput. 2023, 19, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Galano-Frutos, J.J.; Sancho, J. Energy, water, and protein folding: A molecular dynamics-based quantitative inventory of molecular interactions and forces that make proteins stable. Protein Sci. 2024, 33, e4905. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, A. Molecular modelling study to discover novel JAK2 signaling pathway inhibitor. J. Biomol. Struct. Dyn. 2023, 41, 5827–5838. [Google Scholar] [CrossRef]
- Baskin, R.; Majumder, A.; Sayeski, P.P. The recent medicinal chemistry development of Jak2 tyrosine kinase small molecule inhibitors. Curr. Med. Chem. 2010, 17, 4551–4558. [Google Scholar] [CrossRef]
- Gorantla, S.P.; Prince, G.; Osius, J.; Dinesh, D.C.; Boddu, V.; Duyster, J.; von Bubnoff, N. Type II mode of JAK2 inhibition and destabilization are potential therapeutic approaches against the ruxolitinib resistance driven myeloproliferative neoplasms. Front. Oncol. 2024, 14, 1430833. [Google Scholar] [CrossRef]
- Davis, R.R.; Li, B.; Yun, S.Y.; Chan, A.; Nareddy, P.; Gunawan, S.; Ayaz, M.; Lawrence, H.R.; Reuther, G.W.; Lawrence, N.J. Structural insights into JAK2 inhibition by ruxolitinib, fedratinib, and derivatives thereof. J. Med. Chem. 2021, 64, 2228–2241. [Google Scholar] [CrossRef]
- Zhao, C.; Khadka, D.B.; Cho, W.-J. Insights into the structural features essential for JAK2 inhibition and selectivity. Curr. Med. Chem. 2016, 23, 1331–1355. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.C.; Piehler, J.; Tvorogov, D.; Ross, D.M.; Lopez, A.F.; Gotlib, J.; Thomas, D. Next-generation JAK2 inhibitors for the treatment of myeloproliferative neoplasms: Lessons from structure-based drug discovery approaches. Blood Cancer Discov. 2023, 4, 352–364. [Google Scholar] [CrossRef]
- Pippis, E.J.; Yacyshyn, B.R. Clinical and mechanistic characteristics of current JAK inhibitors in IBD. Inflamm. Bowel Dis. 2021, 27, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016, 111, 784–803. [Google Scholar] [CrossRef]
- Lv, Y.; Mi, P.; Babon, J.J.; Fan, G.; Qi, J.; Cao, L.; Lang, J.; Zhang, J.; Wang, F.; Kobe, B. Small molecule drug discovery targeting the JAK-STAT pathway. Pharmacol. Res. 2024, 204, 107217. [Google Scholar] [CrossRef]
- Kavanagh, M.E.; Horning, B.D.; Khattri, R.; Roy, N.; Lu, J.P.; Whitby, L.R.; Ye, E.; Brannon, J.C.; Parker, A.; Chick, J.M.; et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. Nat. Chem. Bio. 2022, 18, 1388–1398. [Google Scholar] [CrossRef]
- Gao, Y.; Lan, L.; Wang, C.; Wang, Y.; Shi, L.; Sun, L. Selective JAK1 inhibitors and the therapeutic applications thereof: A patent review (2016–2023). Expert Opin. Ther. Pat. 2025, 35, 181–195. [Google Scholar] [CrossRef]
- Puca, P.; Del Gaudio, A.; Iaccarino, J.; Blasi, V.; Coppola, G.; Laterza, L.; Lopetuso, L.R.; Colantuono, S.; Gasbarrini, A.; Scaldaferri, F. Cancer Risk in IBD Patients Treated with JAK Inhibitors: Reassuring Evidence from Trials and Real-World Data. Cancers 2025, 17, 735. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).