Abstract
Background/Objectives: Fouquieria splendens Engelm. is a medicinal plant traditionally used in North America for treating metabolic disorders; however, its antihyperglycemic properties and safety profiles remain poorly studied. We investigated the phytochemical composition, oral acute toxicity, mutagenicity, and antihyperglycemic activity of the foliar ethanolic extract of F. splendens (EFS). Methods: Phytochemical analysis was performed using ultra-performance liquid chromatography–mass spectrometry. Acute toxicity and mutagenicity were evaluated following the OECD guidelines. Antihyperglycemic activity was assessed in streptozotocin-induced diabetic rats treated with EFS (200 mg/kg) alone or in combination with metformin for 30 days. Results: Eleven phenolic compounds were identified in the EFS, including ellagic acid, morin, apigenin, and luteolin 7-Oglucoside. EFS was non-mutagenic and had an LD50 of >2000 mg/kg. This treatment significantly reduced blood glucose levels and enhanced the effect of metformin in diabetic rats. Histopathological analysis showed preserved morphology in the pancreatic, hepatic, and renal tissues of the treated animals. Conclusions: EFS exhibited significant antihyperglycemic activity and a favorable safety profile, supporting its potential as a complementary phytotherapeutic agent for diabetes management. These results highlight the pharmacological value of F. splendens and promote the exploration of native plants as adjuncts for chronic disease therapy.
1. Introduction
1.1. Background on Diabetes Mellitus
Diabetes mellitus (DM) is an endocrine metabolic disorder characterized by chronic hyperglycemia resulting from inadequate insulin secretion, impaired insulin action, or a combination of both [1]. Primary categories of diabetes mellitus (DM) include type 1 diabetes (T1DM), type 2 diabetes (T2DM), gestational diabetes (GDM), and monogenic diabetes. T2DM is the most prevalent form of diabetes, representing approximately 90% of all cases worldwide [2].
In 2024, an estimated 588.7 million adults aged 20–79 were living with diabetes worldwide. In North America and the Caribbean, the total number of diabetes cases was 56.2 million. The global number of people with diabetes is projected to rise to 853 million by 2050 [3]. Persistent hyperglycemia associated with T2DM induces multisystemic organ damage, resulting in a spectrum of complications. These include eye disorders, kidney problems, nerve damage, heart and blood vessel diseases, and changes in the skin and mouth. Collectively, these complications significantly reduce the quality of life [4].
1.2. Public Health Impact and Socioeconomic Burden
Numerous complications associated with T2DM classify it as a critical health problem, with health expenditures increasing by 316% over the last 15 years [5]. In 2021, the global healthcare expenditure for T2DM was estimated to be USD 966 billion. Among the seven International Diabetes Federation (IDF) regions, North America and the Caribbean (NAC) region exhibit the highest expenditure of USD 415 billion [6]. As a member of the NAC region, Mexico allocated approximately USD 19.2 billion for healthcare expenditures in 2021. The future projection for Mexico is alarming, with 14.1 million people diagnosed with diabetes in 2021, and this number is expected to rise to 21.2 million by 2045 [5,7].
1.3. Limitations of Current Therapies and Rationale for Natural Alternatives
The management of T2DM encompasses lifestyle modifications, including dietary adjustments, increased physical activity, and pharmacotherapy [8,9]. Pharmacological interventions exert their effects through diverse mechanisms of action; however, all are associated with adverse side effects [10,11]. The combination of these adverse reactions, along with high costs and limited availability, contributes to treatment non-adherence and subsequent complications, thereby increasing morbidity [12,13]. Therefore, the development of affordable treatments with fewer adverse effects is essential.
Natural remedies containing bioactive components offer a feasible option for preventing and controlling chronic conditions such as DM [14]. Plant compounds, such as flavonoids, anthocyanins, saponins, tannins, and polyphenols, play critical roles in diabetes management [15,16]. The mechanisms of action of these drugs include enhanced insulin sensitivity or production, the inhibition of carbohydrate-digesting enzymes, and reduced hepatic glucose production [17]. These compounds promote gastrointestinal health, stimulate glucose-regulating and appetite-suppressing hormones, reduce inflammation, and counteract oxidative stress, thereby contributing to improved metabolic function and diabetes prevention [15,18,19].
1.4. Fouquieria splendens as a Potential Therapeutic Candidate
Fouquieria splendens Engelm, a member of the Fouquieriaceae family (ocotillo) within the order Ericales, is a tree or shrub found in arid and semi-arid regions of the Southwestern United States and Northern Mexico, particularly in the Sonoran and Chihuahuan deserts [20]. Research on this species has revealed that its foliar tissues are rich in phenolic compounds, such as ellagic acid and flavonols, including derivatives of myricetin, rutin, and quercetin-3-O-glycoside [20,21]. These compounds exhibit antihyperglycemic, antioxidant, anti-inflammatory, hepatoprotective, and nephroprotective properties [22]. The presence of phenols, flavones, and flavonols on the surfaces of leaves and stems, as well as ocotillol in the cortex, indicates that this species may be a promising candidate for the treatment of chronic diseases, such as diabetes.
Although several studies have shown positive responses to plant extracts, it is necessary to investigate their effects and mechanisms of action in preclinical models to determine the association between their composition and biological and toxicological effects. Therefore, this study aimed to assess the phytochemical profile of the foliar ethanolic extract of F. splendens, along with its acute oral toxicological effects and antihyperglycemic properties, in streptozotocin-induced diabetic rats. This investigation sought to explore its potential application in therapeutic interventions for diabetes, serving as an adjunctive herbal medication to enhance the quality of life and treatment adherence of individuals living with diabetes.
2. Materials and Methods
2.1. Chemicals and Reagents
Aroclor 1254 (New England Nuclear, North Haven, CT, USA), Streptozotocin, sodium citrate dihydrate, citric acid monohydrate and dimethyl sulfoxide (DMSO) ≥ 99.9% were used for this study, purchased from Sigma-Aldrich (Sigma, Saint Louis, MO, USA)0.9% NaCl saline solution; and heparin sodium injection solution 5000 IU/mL from PiSA Farmacéutica (PiSA, Ciudad de México, México). A rat insulin ELISA kit was obtained from Merck Millipore (Darmstadt, Germany; Cat. # EZRMI-13K). Analytical grade ethanol and ether were used in this study.
2.2. Plant Material and Preparation of the Extract
Plant specimens were collected from a relict population of F. splendens (curatorial number 49021) in the locality of Acatita (23°39′42.6″, −104°22′17.1″) within the municipality of Mezquital in the state of Durango during October and November 2022. No permission is required for extraction, and the area is not designated as a protected area by the federal or local authorities. The specimen was deposited in the Herbario CIIDIR facility, registered in the Index Herbariorum “https://sweetgum.nybg.org/science/ih/ (accessed on 1 February 2025” under the acronym “CIIDIR,” and holds the national register DGO-FLO-174-0405. The plant material was identified as Fouquieria splendens Engelm. with the curatorial number 49021, and its identification was verified at “http://www.theplantlist.org/tpl1.1/search (accessed on 27 June 2025)”. F. splendens leaves were dried at room temperature, pulverized, and stored in an airtight container for later use. The extract was prepared using a previously described method [21]. The powdered material was combined with 80% ethanol (1:10 w/v), sonicated for 1 h, and macerated using an electric stirrer for 24 h. The resultant mixture was centrifuged at 8000 rpm for 10 min, and the supernatant was collected and concentrated using a Rotavapor R-114 (Büchi Labortechnik AG, Flawil, Switzerland). The extraction yield per gram of powdered plant material was determined based on the differential weight of the container before and after the addition of the extract. Fouquieria splendens extract (EFS) was stored at 4 °C until further use.
2.3. Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) Analysis
Phytochemical analysis of the EFS was performed using Acquity UPLC I-class equipment with a diode array detector coupled to a mass spectrometer with an ESI ionization source and Waters VION IMS time-of-flight (Waters, Milford, MA, USA). The sample was diluted in absolute methanol to a concentration of 1 mg/mL. The column used was BEH C18 2.1 × 100 mm, 1.7 μm in size. The mobile phases used were acetonitrile (ACN) and water, both of which were acidified with 0.1% formic acid. The gradients used at 0, 2, 22, 25, 27, and 30 s were 95, 95, 5, 5, 95, and 95% of phase A, respectively, and 5, 5, 95, 95, 5, and 5% of phase B, respectively, with a flow rate of 0.4 mL/min, column temperature of 35 °C, sample temperature of 10 °C, and injection volume of 6 μL. Ionization was performed in both positive and negative modes. The analysis mode was MSE with a low collision energy of 6 eV and a ramp from 15 to 45 eV at high energy. The mass range was 50–1800 m/z. The capillary voltages used were 2 and 3.5 kV for negative and positive ions, respectively, with a source temperature of 120 °C, desolvation temperature of 450 °C, argon as the collision and desolvation gas with a f120 °Cf 50 L/h and 800 L/h, respectively, and a cone voltage of 40 V. Leucine Enkephalin was used as a reference for mass correction at a concentration of 200 pg/μL with a flow of 10 μL/min.
For data analysis, Unifi 1.9 SR 4 software was used with libraries from the Laboratory Specialized in Food Analysis of the Faculty of Chemistry of the Autonomous University of Querétaro, Mexico. The target-matching tolerance was set to 5 ppm. Fragment identification was compared with fragmentation patterns reported in PubChem (https://pubchem.ncbi.nlm.nih.gov, accessed on 20 October 2025), FooDB version 1.0 (https://www.foodb.ca, accessed on 20 October 2025), HMDB (version 5.0; https://hmdb.ca, accessed on 20 October 2025), and MassBank of North America “https://www.massbank.jp/MassBank/ (accessed on 1 December 2024)”. as well as with previous analyses performed in the laboratory. For compounds without a reported fragmentation pattern, the in silico fragmentation pattern generated by the software provided a tolerance of 10 mDa. Compounds without reported fragmentation patterns were generated in silico using software with a tolerance of 10 mDa (mass deviation).
2.4. Experimental Animals
Wistar rats aged 6–8 weeks and Balb/c mice aged 8–10 weeks, with approximate weights of 250 ± 50 g and 19 ± 2 g, respectively, were obtained from the Bioterium of Facultad de Estudios Superiores Iztacala, Mexico City. The animals were maintained in polypropylene bedding under controlled environmental conditions (12 h light/12 h dark cycle; 25 ± 3 °C; 25–60% humidity) with ad libitum access to food and water. All procedures were performed in accordance with the official Mexican Standard for Laboratory Animals (NOM-062-ZOO-1999) [23]. This study was approved by the Bioethics Committee of the National School of Medicine and Homeopathy of the National Polytechnic Institute, Mexico City (registration number CBE/001/2022), which complies with International Standards and Policies. Healthy animals were used for the acute toxicity study, and animals with induced hyperglycemia were used for the antihyperglycemic activity study.
2.5. Toxicological Studies
2.5.1. Bacterial Reverse Mutation Assay (Ames Test)
The Salmonella mutagenicity test was conducted using the incorporation method previously described [24], employing histidine auxotrophic Salmonella typhimurium strains TA98, TA100, and TA102 in the presence or absence of the S9 fraction obtained from the liver of a male Wistar rat induced with Aroclor 1254 solution as a metabolic activation system. S. typhimurium strains were grown for 16 h at 37 °C with shaking. In a sterile tube with 2 mL of soft agar at 45 °C, 100 µL of the bacterial cultures were added and exposed to concentrations of 3, 30, and 300 µg/mL of EFS or 10 µL of dimethyl sulfoxide (0.1% DMSO as a negative control). The suspension mixture was then placed in a Petri dish containing Vogel–Bonner minimal medium. After 48 h of incubation at 37 °C, revertant colonies (His+) were quantified. The positive mutagenesis controls used were picrolonic acid, 2-amino-anthracene, methyl-N-nitro-N-nitrosoguanidine and 4-NitroQuinoline 1-Oxide. A two-fold or greater increase in the number of spontaneous revertant colonies was considered a positive result.
2.5.2. Micronucleus Test
Groups of three female Balb/c mice weighing 19 ± 2 g fasted for 8 h were randomly selected and administered EFS (2000 mg/kg) or cyclophosphamide (50 mg/kg) as a positive control, or no treatment as a negative control (healthy), via intragastric and intraperitoneal routes, respectively. After drug administration, the animals were fasted for 2 h and maintained under normal conditions. After 48 and 72 h, 120 µL of blood was drawn by tail puncture and processed according to the instructions of the MicroFlow Kit purchased from Litron Laboratories (Rochester, NY, USA), which utilizes flow cytometry to determine the percentages of reticulocytes (RET), mature normochromatic erythrocytes (NCE), and micronuclear cells (MN) [25]. At the end of the test, the animals were sacrificed in a CO2 chamber.
2.5.3. Acute Oral Toxicity in Mice and Rats
An acute oral toxicity study was conducted according to the guidelines of the Organization for Economic Co-operation and Development (OECD): guidelines for testing chemicals number 423 [26]. Six healthy female mice and 12 healthy male and female rats were used in this study. Animals were segregated by sex and housed in separate enclosures. They were subsequently randomized into two groups: control and treatment. The control group received a single dose of 1 mL/kg body weight of vehicle (1:3 DMSO: sterile water solution), whereas the treated group received EFS (2000 mg/kg body weight) via an intragastric cannula. The animals were fasted prior to drug administration and for 3 h following treatment. After dosing, they were observed at 30 min intervals during the first 4 h and periodically thereafter for up to 14 days to monitor clinical signs or mortality. Behavioral and physiological parameters, including tremors, convulsions, lethargy, somnolence, salivation, diarrhea, and changes in coat condition, eyes, mucous membranes, and respiratory patterns, were recorded to determine the median lethal dose (LD50). The spleen, pancreas, stomach, liver, kidneys, heart, and brain were weighed and examined macroscopically for pathological alterations indicative of toxicity. The female mice used in this study were also employed in the micronucleus assay, allowing for the concurrent assessment of acute toxicity and genotoxic effects.
2.6. Hyperglycemic Rat Model
2.6.1. Induction of Hyperglycemia
After the acclimatization period, the rats were fasted for 12 h. Diabetes was induced by an intraperitoneal injection of streptozotocin (STZ; 50 mg/kg body weight) (Sigma-Aldrich, USA) prepared in freshly cold 0.1 M sodium citrate buffer (pH 4.5), following previously established protocols for experimental diabetes induction [27,28]. To confirm hyperglycemic conditions, fasting blood glucose levels of the rats were evaluated 48 h after the injection. Blood samples were obtained from the tail vein using test strips (FreeStyle Optium, Abbott Diabetes Care, Alameda, CA, USA) and analyzed using a glucometer (Optium Xceed, Abbott Diabetes Care, Alameda, CA, USA). Rats with fasting blood glucose concentrations exceeding 150 mg/dl were considered diabetic and were subsequently included in the investigation of the antihyperglycemic activity of the test samples.
2.6.2. Animal Experimental Design
For the antihyperglycemic study, a total of 56 male Wistar rats were used, which were randomly assigned to seven groups with eight rats each, as follows: group 1: normoglycemic control (NC), administered 1 mL/kg body weight sterile water; group 2: hyperglycemic control (HC), administered 1:3 dimethyl sulfoxide (DMSO):NaCl 0.9% solution, 1 mL/kg; group 3: hyperglycemic treated with sitagliptin (H + S), 10 mg/kg; group 4: hyperglycemic treated with metformin (H + M) 100 mg/kg; group 5: hyperglycemic treated with the extract (H + EFS), 200 mg/kg; group 6: hyperglycemic treated with the extract and sitagliptin (H + EFS + S), 200 mg/kg + 10 mg/kg; and group 7: hyperglycemic treated with the extract and metformin (H + EFS + M) 200 mg/kg + 100 mg/kg. The EFS dose was determined based on an acute oral toxicity study, where an equivalent of 10% of the tested dose was used, and the volume administered was 1 mL/100 g of body weight, as indicated by the OECD guidelines [26]. Additionally, the selected dose was within the range commonly used in experimental rat models and demonstrated significant biological activity [29,30]. Treatment was initiated 48 h after diagnosis. The drugs were dissolved in 0.9% NaCl saline solution, and the extract was dissolved in a 1:3 DMSO: sterile water solution (1 mL/kg body weight). All treatments were administered orally once daily for 30 days using an intragastric cannula.
2.6.3. Body Weight and Peripheral Glucose
The body weight and peripheral glucose levels of each animal were monitored weekly during the 30-day experimental period. Body weight was also recorded prior to the administration of the treatment to adjust the doses and peripheral glucose 4 h later after 7–8 h of fasting.
2.6.4. Biochemical Profile
Blood samples were extracted by cardiac puncture, collected in tubes without anticoagulants, and stored at room temperature for 20 min before centrifugation. Subsequently, the samples were centrifuged at 570× g for 10 min at 4 °C to obtain serum. Serum samples were analyzed by automated photometry using an automated biochemical analyzer (AutoKem II, KontroLab, Istanbul, Turkey). The following biochemical parameters were quantified: serum glucose, renal function markers (urea and creatinine), lipid profile components (total cholesterol, VLDL cholesterol, and triglycerides), and liver function indicators, including total bilirubin and the enzymes aspartate aminotransferase (AST, TGO) and alanine aminotransferase (ALT, TGP).
2.6.5. Glycosylated Hemoglobin (HbA1c)
To quantify HbA1c levels, freshly drawn blood samples were collected in tubes and homogenized with heparin. The analyses were performed using low-pressure liquid chromatography. Levels > 5.5% were considered abnormal.
2.6.6. Insulin Quantification
For insulin quantification, sera previously collected and stored in aliquots at −20 °C were used. Sera were analyzed using the rat/mouse insulin ELISA kit according to the manufacturer’s instructions, and activity was measured spectrophotometrically using a UV/Vis microplate reader (Multiskan GO, Thermo Scientific™, Waltham, MA, USA).
2.7. Euthanasia and Necropsy of the Animals
For both acute toxicity and antihyperglycemic activity studies, animals were fasted for 12 h before euthanasia, anesthetized using an overdose of ether, and sacrificed by cardiac puncture, as indicated by the official standard Mexican (NOM-033-ZOO-1995) [31]. The organs of each animal (brain, heart, lungs, spleen, liver, kidney, pancreas, stomach, ovaries, and testes) were subjected to macroscopic necropsy. Abnormalities in color, size, and morphology were also noted. Finally, the removed organs were weighed using a digital scale and stored in 10% formaldehyde. The relative weight of each organ was calculated using the following formula:
Relative organ weight (%) = (organ weight/body weight) × 100
2.8. Histopathological Analysis
Tissues, including the pancreas, liver, kidney, and intestine, from various experimental groups were immersed and preserved in 10% formaldehyde solution in phosphate-buffered saline (PBS). Following standard procedures for dehydration, clearance, and infiltration, the organs were embedded in paraffin blocks. Subsequently, tissue sections were cut to a thickness of 5 µm, stained with hematoxylin and eosin, and prepared on slides for histopathological analysis. Microscopic examination (20×, 40×) was performed to assess potential alterations in the morphology and architecture of the tissue. Histopathological analysis was conducted only on animals from the hyperglycemic rat model used in the antihyperglycemic study.
2.9. Statistical Analysis
Results are expressed as the mean ± standard error of the mean (SEM). Statistical significance was analyzed using one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test for multiple comparisons using GraphPad Prism Version 9.0.2 (GraphPad Software Inc., La Jolla, CA, USA). The threshold for statistical significance was set at p < 0.05.
3. Results
3.1. UPLC-MS Identification of Phenolic Compounds
Phytochemical analysis using UPLC-MS identified 11 major phenolic compounds in the EFS. Among these, four flavonoid glycosides, four flavonols, two tannins, and one quinic acid derivative were identified (Table 1). The presence of ellagic acid (5), morin (4), apigenin (9), and luteolin 7-O-glucoside (8) suggests that EFS may be a significant antihyperglycemic agent, given its documented biological activity in numerous studies. Hyperoside was the most abundant phenolic compound, whereas the least abundant was kaempferol 3-O-rutinoside.

Table 1.
Compounds identified in the EFS using UPLC-MS.
3.2. Outcomes of the Bacterial Reverse Mutation Assay (Ames Test)
Ames test results demonstrated that EFS did not induce an increase in the number of revertant colonies in any of the strains, both in the absence and presence of metabolic activation in the S9 fraction. No changes were observed with respect to the three tested EFS doses (Figure 1).
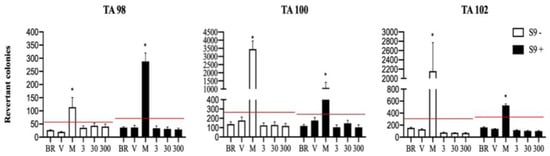
Figure 1.
Effect of 3, 30, and 300 μg/plate of EFS on S. typhimurium quantification of revertants in strains TA98 and TA100 at 48 h and TA102 at 72 h, with and without the S9 fraction. The red line represents two-fold basal revertants. BR: basal revertants. V: vehicle DMSO (0.1%). M: mutagens TA98 Picrolonic acid (S9−) and 2-Amino-anthracene (S9+), TA100 methyl-N-nitrosoguanidine (S9−) and 2-Amino-anthracene (S9+), TA102 4-Nitroquinone oxide (S9−) and 2-Amino-anthracene (S9+). p < 0.05 versus twice the baseline revertants; * Statistically significant difference by one-way ANOVA and Dunnett’s post hoc test. The graph depicts the mean ± standard deviation of two independent experiments performed in triplicate.
3.3. Micronucleus Test Findings
The results showed that the percentages of Micronucleated Normochromatic Erythrocytes, Reticulocytes and Micronucleated Reticulocytes in female mice administered EFS (2000 mg/kg) at 48 h, as well as Micronucleated Normochromatic Erythrocytes and Reticulocytes at 72 h, were similar to those in the healthy negative control (Table 2). However, there was a significant difference in the percentage of Micronucleated Reticulocytes in mice administered EFS at 72 h compared to the healthy negative control. These results indicate that EFS did not cause genotoxic damage in the peripheral blood of Balb/c mice after 48 h.

Table 2.
Effects on peripheral blood of Balb/c mice treated with EFS in the micronucleus assay.
3.4. Acute Oral Toxicity
3.4.1. Behavior Patterns and Body Weight of Animals
Administration of a single dose of EFS (2000 mg/kg) to female BALB/c mice and Wistar rats did not elicit any behavioral effects or alterations in food or water consumption during the 14-day experimental period. Moreover, no signs or symptoms of toxicity (piloerection, mucosal irritation, or changes in motor activity) or mortality were observed during the 14 days following the administration of the acute dose of EFS. An increase in the body weight of the animals was noted compared to their initial weight, and no significant difference was observed between the treated and control groups (Table 3).

Table 3.
Acute effects of oral administration of EFS on body weight of mice and rats compared to controls.
3.4.2. Relative Organ Weight of Balb/c Mice and Wistar Rats
Administration of a single dose of EFS resulted in a statistically significant increase in the relative weights of the liver and kidneys in male Wistar rats and in the liver weight in female rats compared to their respective control groups (Table 4). These observations were made through macroscopic analysis of the organs.

Table 4.
Acute effects of oral administration of EFS on organ weight in female Balb/c mice, female Wistar rats, and male Wistar rats during the evaluation of acute oral toxicity of EFS.
3.5. Antihyperglycemic Activity
3.5.1. Body Weight of Rats
Changes in the body weight of the rats were recorded throughout the experiment for 28 days (Figure 2). On the seventh day of treatment, all hyperglycemia-induced animals exhibited weight loss between 4.6% and 18.7%, whereas healthy controls (normoglycemic) demonstrated a gain of 20%. The percentage variation in body weight indicated that all hyperglycemic animals induced with STZ did not gain weight at the same rate as healthy animals, with a significant weight variation observed between the treatment groups on day 28 compared with healthy controls.
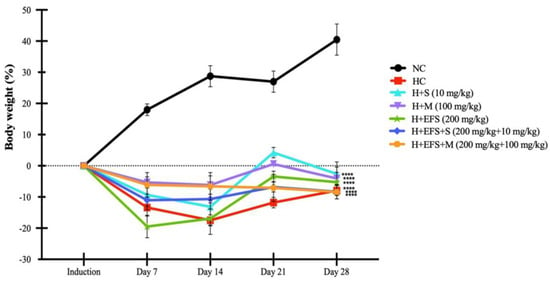
Figure 2.
Percentage variation in body weight with respect to weight prior to induction in the antihyperglycemic evaluation of EFS. EFS: Ethanolic extract of F. splendens. Normoglycemic control (NC), hyperglycemic control (HC), hyperglycemic treated with sitagliptin (H + S), hyperglycemic treated with metformin (H + M), hyperglycemic treated with extract (H + EFS), hyperglycemic treated with extract and sitagliptin (H + EFS + S), and hyperglycemic treated with extract and metformin (H + EFS + M). Statistical significance was set at p < 0.0001. **** Statistically significant difference compared to the normoglycemic control group. Dunnett’s post hoc test was used to compare each hyperglycemic experimental group with the normoglycemic control group. Data are presented as the mean ± SEM. n = 6.
3.5.2. Relative Organ Weight of Male Wistar Rats During the Antihyperglycemic Evaluation of EFS
Hyperglycemic animals exhibited a significant increase in the relative weights of organs, such as the kidney and liver, compared to those in the healthy control group (Table 5). Although the relative liver weight decreased in hyperglycemic animals treated solely with EFS, this reduction was not statistically significant compared to that in the control group. Kidney weights were lower in the group that received combined treatment with EFS and Metformin (EFS + M).

Table 5.
Variation in organ weight in relation to body weight of male Wistar rats in the antihyperglycemic evaluation of EFS.
3.5.3. Biochemical Parameters
The effect of EFS treatment on fasting serum glucose levels at the end of the 30-day period demonstrated a significant decrease in all treated groups compared to that in the normoglycemic control group. However, the combination of EFS + M resulted in greater antihyperglycemic activity. The HbA1c levels in the treated hyperglycemic group were lower than those in the untreated hyperglycemic group. Nevertheless, under the different types of treatment, serum insulin levels were significantly decreased compared to those in the normoglycemic control group. The lipid profile indicated that EFS reduced cholesterol, VLDL, and triglyceride levels, with a significant decrease in the renal profile. EFS alone and in combination with sitagliptin significantly decreased the serum urea levels. The liver profile revealed an increase in transaminases in all hyperglycemic control animals and those treated with EFS and its combination with drugs compared to the normoglycemic control group (Table 6).

Table 6.
Biochemical profile in blood of hyperglycemic rats after 30 days of treatment.
3.5.4. Histopathological Analysis
Histopathological changes in the pancreas, liver, kidneys, and intestines were analyzed to evaluate the effects of EFS (200 mg/kg) alone and in combination with sitagliptin (10 mg/kg) and metformin (100 mg/kg) in hyperglycemia-induced rats. The results demonstrated that the tissues of animals treated with EFS and the EFS + S and EFS + M combinations maintained the morphology and architecture of the analyzed structures, comparable to those observed in the normoglycemic control (Figure 3).
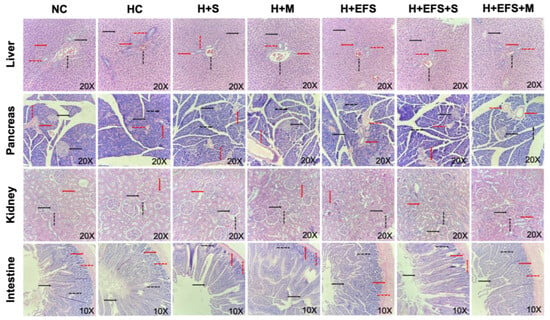
Figure 3.
Microscopic views of tissue samples stained with hematoxylin and eosin. Liver: hepatocyte (black continuous arrow), portal vein (black dashed arrow), hepatic artery (red continuous arrow), bile duct (red dashed arrow); Pancreas: Islet of Langerhans (black continuous arrow), pancreatic acinus (black dashed arrow), interlobular duct (red continuous arrow), vein (red dashed arrow); Kidney: glomerulus (black continuous arrow), Bowman’s capsule (black dashed arrow), tubule (red continuous arrow); Intestine: villi (black continuous arrow), crypts of Lieberkuhn (black dashed arrow), submucosa (red continuous arrow), muscularis (red dashed arrow). Representative images of each tissue from one animal per experimental group with a 20× objective for liver, pancreas, and kidney tissues, and a 10× objective for intestinal tissue.
4. Discussion
Type 2 diabetes mellitus (T2DM) is a chronic disorder characterized by impaired insulin secretion, hyperglycemia, dyslipidemia, and other clinical manifestations, such as decreased body weight, kidney damage, and liver dysfunction [32]. A persistent and severe hyperglycemic state is associated with various diabetic complications through the activation of several pathways, including the hexosamine, advanced glycation end products (AGEs), and diacylglycerol (DAG)-protein kinase C (PKC) pathways [33]. A wide range of pharmacological agents is available for the management of T2DM, each acting through distinct mechanisms of action. For instance, metformin is the preferred first-line medication because of its substantial efficacy in reducing glycated hemoglobin (HbA1c) levels [34]. It belongs to the biguanide class and works by enhancing the activity of protein kinase activated by adenosine monophosphate (AMPK) and reducing hepatic glucose production. Sitagliptin, a dipeptidyl peptidase-IV (DPP-IV) inhibitor, prevents enzymatic degradation of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). This action enhances glucose-dependent insulin secretion while suppressing glucagon release. However, compared to biguanides, its glucose-lowering efficacy is relatively modest [34,35]. Acarbose, an α-glucosidase inhibitor, blocks this enzyme in the intestinal mucosa, thereby delaying the digestion and absorption of carbohydrates. Although its ability to reduce HbA1c levels is moderate, it remains an effective adjunct therapy for glycemic control [35]. Similarly to other antidiabetic agents, its use may be associated with gastrointestinal side effects. Therefore, the search for safer and more effective alternatives has directed increasing attention toward medicinal plants and their bioactive metabolites [36]. It is well established that several classes of plant secondary metabolites possess notable medicinal properties, including antidiabetic effects attributed to polyphenols, glycosides, saponins, stilbenes, tannins, terpenoids, and alkaloids [36,37]. Many of these compounds have served as models for the development of semi-synthetic drugs. A well-known example is metformin, derived from Galega officinalis L. (Fabaceae), a plant rich in guanidine and guanidine derivatives, such as galegine [38]. Therefore, identification of new natural agents capable of improving glucose and lipid metabolism and enhancing insulin sensitivity remains an important goal in diabetes management. In this regard, plant-derived phytochemicals—particularly phenolic compounds—show promise in reducing blood glucose levels and protecting against hyperglycemic complications due to their antioxidant activity and their ability to inhibit starch digestion [39,40]. Numerous studies have demonstrated that many plant species exert hypoglycemic effects in both in vitro and in vivo models of diabetes, particularly in streptozotocin (STZ)-induced diabetic rats. These effects were attributed to the action of the extracts or isolated compounds, including apigenin, ellagic acid, hyperoside, luteolin-7-O-glucoside, quercitrin, and kaempferol. Several mechanisms of action have been proposed, some of which are already well characterized, such as the inhibition of key metabolic enzymes protein tyrosine phosphatase 1 B (PTP-1B) [30], dipeptidyl peptidase-IV (DPP-IV) [41], α-amylase [42] or aldose reductase enzymes [43] as well as stimulating insulin secretion [44] and the enhancement of insulin sensitivity [45]. This study provides, for the first time, phytochemical characterization, acute toxicity assessment, genotoxicity, mutagenic assays, and in vivo information on the antihyperglycemic effects of EFS, a plant species belonging to the genus Fouquieria, family Fouquieriaceae, and order Ericales. Currently, there is no evidence for the antihyperglycemic potential of F. splendens. The lack of previous studies on the antihyperglycemic activity of F. splendens underscores the relevance of the present findings and contributes to expanding the current understanding of the biological properties of this pharmacologically unexplored species. The ethanolic foliar extract of F. splendens (EFS) contained several bioactive compounds previously associated with antihyperglycemic activity in other plant species. Although ellagic acid was identified in EFS, its antihyperglycemic potential has been reported in Syzygium cumini, where it exhibited inhibitory activity against protein tyrosine phosphatase 1B (PTP-1B) in in vitro enzymatic assays, supporting its possible contribution to the effects observed for EFS [46]. Hyperoside, a flavonoid present in Psidium guajava L. and Artemisia capillaris, demonstrated potent DPP-IV and α-glucosidase inhibitory activity in in vitro assays, showing higher efficacy than acarbose used as a positive control [47,48]. Quercitrin and kaempferol, isolated from Embelia ribes leaves, also displayed concentration-dependent α-glucosidase inhibition, with greater potency than acarbose [49]. Similar inhibitory effects were observed for luteolin 7-O-glucoside isolated from Salvia chloroleuca [50] and for kaempferol 3-O-rutinoside obtained from Impatiens balsamina L. [51]. Kaempferol, also reported from Sophorae flos, was found to stimulate basal glucose uptake in human liver (HepG2) cells [52], while apigenin, detected in fractions of Smilax dominguensis, exhibited dual agonistic activity toward PPARγ and PPARα receptors and promoted GLUT-4 translocation [53]. Furthermore, kaempferol identified in Cortex mori enhanced PPARγ activity [54]. Altogether, these findings suggest that the pharmacological effects of EFS may be attributed to the synergistic action of its phenolic and flavonoid constituents. Beyond its rich phytochemical composition and demonstrated pharmacological potential, the safety profile of EFS was also evaluated to support its potential therapeutic application [55]. The mutagenicity assessment revealed that EFS, at concentrations of 3, 30, and 300 µg/plate, exhibited no mutagenic activity in any of the tested S. typhimurium strains (TA98, TA100, and TA102), both in the presence and absence of the S9 metabolic activation system. These results indicate that EFS does not induce point mutations under the experimental conditions employed, reinforcing its safety for further pharmacological evaluation. The absence of mutagenic effects in F. splendens is consistent with findings reported for other medicinal plants exhibiting antidiabetic properties, which have also demonstrated favorable safety and antimutagenic profiles. Such evidence supports their use in traditional medicine and as potential sources for the development of safe nutraceuticals. In this regard, seabuckthorn (Hippophae rhamnoides L.) berry oil has similarly shown no genotoxic effects in both S. typhimurium assays and in vivo studies in mice, further supporting the view that plant-derived products with antihyperglycemic activity may possess intrinsic protective effects against genetic damage [56]. Some plant extracts containing phenolic compounds not only lack mutagenic activity but also possess antimutagenic potential, repressing the activity of enzymes associated with the activation of procarcinogens, such as cytochrome P450 [57,58]. Furthermore, EFS at a dose of 2000 mg/kg showed no evidence of genotoxicity 48 h post-administration. Similarly to EFS, Melissa officinalis is rich in a diverse array of phytochemicals, including polyphenolic compounds such as rosmarinic acid, caffeic acid, and protocatechuic acid, as well as essential oils such as citral, monoterpenoid aldehydes, sesquiterpenes, and flavonoids such as luteolin and tannins. The genotoxicity and antigenotoxicity of the ethanolic extract of M. officinalis were assessed using micronucleus assays in CF-1 male mice. The results indicated no genotoxic effects and demonstrated antimutagenic properties at higher doses, which may be attributed to the presence of phytochemicals [59]. The varying results obtained for different plant extracts highlight the importance of conducting thorough genotoxicity evaluations before using these extracts in preclinical studies. Furthermore, ethanol mutagenicity studies of leaf extracts from plants with antidiabetic activity provide evidence supporting their safety and contribute to expanding the list of medicinal plant extracts that do not exhibit mutagenic activity. Therefore, they have a low probability of being carcinogenic and can be safely used as dietary supplements for medicinal purposes. Similarly, the acute toxicity test aims to identify the degree of toxicity of a compound by determining its LD50, which is defined as the statistically derived dose expected to cause death or toxicity in 50% of treated animals in a given period [60]. The results indicated that EFS did not result in the death of the animals, categorizing the extract at level 5 of the Globally Harmonized System (GHS) (>2000–5000 mg/kg) [26]. This suggests that it poses a relatively low acute toxicity hazard; however, under certain circumstances, it may present a danger to vulnerable populations [61]. Therefore, repeated-dose 28-day oral toxicity studies should be conducted to establish its safety. In evaluating the antihyperglycemic potential, EFS exhibited a glucose-lowering effect in rats with STZ-induced diabetes compared to the HC group after a 30-day treatment period. This also resulted in beneficial modifications to VLDL cholesterol and triglyceride levels. STZ-induced diabetic rats typically exhibit significant weight loss, as documented in several studies [62,63]. Similar evidence of a decrease in blood glucose levels in diabetic rats has been reported for the action of compounds such as apigenin, in addition to its contribution to the increase in plasma insulin levels [64] or morin, the latter being able to activate the imidazoline I3 receptor (I-3R) to improve insulin secretion [65]. Weight loss was observed in all animals with hyperglycemia in this study, indicating that EFS did not mitigate this effect and that this reduction may be attributed to both body wear and protein loss. Similar effects have been reported in other studies, which can be associated with STZ induction as a side effect of this drug [66,67,68]. In addition, the decrease in the relative weight of organs, such as the kidneys in the EFS + M group or the liver in the EFS group, was correlated with the reduction in glucose levels in these animals; however, these relative weight values were not similar to those presented by the NC group, as they increased in all diabetic animals, indicating that this increase may be due to the hyperglycemic state itself. The kidney is an organ whose function deteriorates in diabetic conditions; therefore, increased blood urea and creatinine levels indicate alterations in glomerular filtration due to oxidative stress. Serum urea and creatinine levels increase with hyperglycemia in uncontrolled diabetes and are generally correlated with the severity of kidney damage. The results showed that EFS reduced serum urea and creatinine levels in rats. Urea and creatinine levels increase in STZ-induced diabetic rats owing to the action of the compounds present in these extracts. However, antioxidants can protect these tissues, leading to gradual improvement in kidney damage [69]. Diabetes significantly affects liver metabolism and function, leading to hepatic complications. Studies have shown that diabetes induces liver damage, characterized by increased serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which are indicators of liver cell damage [5,70]. The ALT enzyme is cytosolic and found at high concentrations in hepatocytes; therefore, an increase in serum ALT levels may indicate hepatocellular liver damage [71]. The results of this study showed similar AST values in animals administered EFS + S or EFS + M compared to those administered only EFS, and a decrease in ALT levels in all animals treated with EFS compared to the HC group. Currently, no reports have been identified in the literature documenting the elevation of aminotransferases without evident liver damage after treatment with plant extracts in similar animal models. The increase in transaminases (AST and ALT) observed in animals treated with EFS + S and EFS + M indicated that the extract in these combinations, at the evaluated concentrations, may not provide a hepatoprotective effect by reducing these biochemical markers. Instead, it might be involved in a mechanism that leads to the release of these hepatic cytosolic enzymes into the bloodstream as a dose-dependent adverse response, without affecting tissue integrity or the toxic action of STZ on the liver. STZ-induced animals also showed an increase in serum glucose and HbA1c levels compared to the NC group. This elevation was ameliorated by EFS treatment, with the EFS + M combination demonstrating a superior effect in reducing these parameters compared with the untreated HC group. Diabetes is diagnosed by identifying elevated concentrations of glucose in the venous plasma or HbA1c. The latter reflects the long-term average glucose concentration (over 60 and 90 days) [72] and serves as a crucial indicator of glycemic control. It is also a reliable predictor of the lipid profile, as it has been correlated with the risk of developing chronic complications, making it a reliable biomarker for both the diagnosis and prognosis of diabetes [73]. This outcome may be attributed to the phytochemical composition of EFS, which exhibits beneficial antioxidant properties that prevent diseases induced by oxidative stress, such as diabetes. Several studies have demonstrated the beneficial effects of phenolic compounds from various plants and their extracts in the management of T2DM, demonstrating their hypoglycemic, anti-inflammatory, antioxidant, and pancreatic β-cell protective properties. Their mechanisms of action include the inhibition of key enzymes such as α-amylase, α-glucosidase, and angiotensin-converting enzyme (ACE), as well as their antioxidant activity, which contributes to reducing oxidative stress and lipid peroxidation and modulating cell signaling pathways associated with inflammation and carbohydrate metabolism [74]. Many plant extracts exhibit antihyperglycemic effects owing to their antioxidant properties. For example, Ocimum basilicum contains high amounts of flavonoids, which decrease glucose and insulin levels while alleviating oxidative injury in diabetic control rats [75]. In this regard serum insulin levels in all animals subjected to hyperglycemia were markedly decreased, and EFS, as well as its combination with sitagliptin or metformin, did not promote insulin secretion, indicating that EFS does not contribute to the restoration of pancreatic islets, damaged the insulin producers and did not exert a secretory effect on this hormone. Diabetes is associated with multiple metabolic disorders, including alterations in lipid metabolism caused by abnormal metabolism of the lipoprotein lipase enzyme, which leads to an accumulation of triglycerides and total cholesterol and a decrease in HDL cholesterol. This contributes to the development of secondary complications of diabetes and is a risk factor for cerebrovascular disease. Elevated hyperglycemia contributes to cholesterol transport, which increases fatty acid levels and decreases cholesterol, VLDL, and triglyceride levels in hyperglycemic animals treated with EFS, suggesting that the extract could contribute to cholesterol transport by increasing HDL levels. According to a study on C. rubellum, which contains compounds similar to those of F. splendens, blood glucose levels were reduced in the STZ-induced diabetic rat model because its compounds increased insulin signaling activity, decreased PTP-1B protein levels, and inhibited its activity [30]. PTP-1B is involved in insulin sensitivity; therefore, its blockage could provide an effective therapeutic strategy for treating diabetes [76]. Evidence on phenolic compounds, the pathways of which have been mentioned, suggests their great potential to decrease hyperglycemia. In this study, F. splendens demonstrated excellent antihyperglycemic potential. The diversity of natural active compounds found in EFS, known for their antidiabetic effects through various mechanisms and their ability to protect vital organs damaged by diabetes [77], suggests that the beneficial effects observed when EFS is combined with the insulin-sensitizing drug metformin (EFS + M) could be attributed to the compounds in F. splendens. These compounds may individually exert antihyperglycemic effects through their known action pathways or through synergistic biochemical interactions. However, further studies are necessary to confirm this hypothesis.
5. Conclusions
This is the first report on the antihyperglycemic potential of EFS in an STZ-induced diabetic rat model and constitutes the first experimental evaluation in a species belonging to the genus Fouquieria. We demonstrated that its administration for 30 days reduced the blood glucose levels. In addition, the combined treatment of the extract and metformin had a greater effect on this decrease, as well as on HbA1c values, which could be due to a synergistic interaction between the compounds present in the extract and the insulin-sensitizing drug administered. Similarly, we observed a decrease in VLDL cholesterol and triglyceride levels in the serum of rats after treatment with the extract of M. alba. The bioactive compounds identified in EFS, including hyperoside, kaempferol 3-O-sambubioside, quinic acid, morin, ellagic acid, quercitrin, kaempferol, luteolin 7-O-glucoside, apigenin, 1,2,6 trigalloylglucose, and kaempferol 3-O-rutinoside, may be linked to the observed beneficial effects, as they have been associated with antihyperglycemic activity. Moreover, the predominant presence of several of these compounds suggests their potential involvement in the observed biological activity through various mechanisms of action. Safety studies included the Ames test, which revealed that EFS did not exhibit mutagenic activity in Salmonella typhimurium strains TA98, TA100, and TA102, and an acute oral toxicity study, which revealed that no toxicity was observed in animals and that EFS presented an LD50 value of >2000 mg/kg, corresponding to the range of values for Category 5; this potentially represents a relatively low acute toxicity hazard and could pose a danger to vulnerable populations. This study offers preliminary insights into the potential of F. splendens as a source of bioactive compounds with antihyperglycemic properties and evaluates the safety profile of EFS. These findings support the potential of this plant as a candidate for the development of complementary herbal medicines for future therapeutic interventions for diabetes. Such interventions can enhance treatment adherence, prevent associated complications, and improve the quality of life of individuals with diabetes. Although these preliminary findings are promising, further studies are essential to confirm the long-term safety and efficacy of the EFS. Additionally, it is crucial to investigate the potential mechanisms of action to substantiate its therapeutic application and role as an adjuvant in the treatment of T2DM.
Author Contributions
Conceptualization, K.M.R.-C., J.C.-G. and R.T.-R.; methodology, J.D.B.-C., J.N.U.-S. and M.V.G.-V.; validation, E.R.-B. and E.A.D.-A.; formal analysis, K.M.R.-C., J.D.B.-C., E.R.-B., E.A.D.-A., M.V.G.-V., J.C.-G. and R.T.-R.; resources, J.N.U.-S.; data curation, K.M.R.-C., J.D.B.-C., J.C.-G. and R.T.-R.; writing—original draft preparation, K.M.R.-C., J.C.-G. and R.T.-R.; writing—review and editing, E.R.-B., E.A.D.-A., M.V.G.-V., J.N.U.-S. and R.T.-R.; supervision, J.C.-G. and R.T.-R.; project administration, J.C.-G. and R.T.-R.; funding acquisition, J.C.-G. and R.T.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Politécnico Nacional (National Polytechnic Institute), specifically the Secretary of Research and Postgraduate Studies, under the projects registered as 20250004 and 20250013. This study was also supported by the Programa de Becas de Estímulo Institucional de Formación de Investigadores (BEIFI, Institutional Program for Researcher Training Scholarships) of the Instituto Politécnico Nacional (IPN), Mexico, through the scholarship granted to the first author of this article (registration No. A220309).
Institutional Review Board Statement
The animal study protocol was approved by the Bioethics Committee of the National School of Medicine and Homeopathy of the National Polytechnic Institute (Reg. No. ENMH-CBE/001/2022) on 25 March 2022.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We extend our gratitude to the Secretariat of Sciences, Humanities, Technology, and Innovation (SECIHTI) for their support through the scholarship awarded under CVU No. 934528, to the Instituto Politécnico Nacional (National Polytechnic Institute) and the Programa de Becas de Estímulo Institucional de Formación de Investigadores (BEIFI, Institutional Program for Researcher Training Scholarships) of the Instituto Politécnico Nacional (IPN). We confirm that all authors have reviewed and approved the content of the Acknowledgments Section, and have given their consent for the inclusion of the names and institutions mentioned therein.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Angiotensin-Converting Enzyme |
| ACN | Acetonitrile |
| AGEs | Advanced Glycation End Products |
| ALT | Alanine Aminotransferase |
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| AST | Aspartate Aminotransferase |
| CP | Cyclophosphamide |
| DAG | Diacylglycerol |
| DPP-IV | Dipeptidyl Peptidase-IV |
| DMSO | Dimethyl Sulfoxide |
| DM | Diabetes Mellitus |
| EFS | Ethanolic Extract of Fouquieria splendens |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GDM | Gestational Diabetes Mellitus |
| GHS | Globally Harmonized System (of Classification and Labelling of Chemicals) |
| GIP | Glucose-Dependent Insulinotropic Polypeptide |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLUT-4 | Glucose Transporter Type 4 |
| HbA1c | Glycosylated Hemoglobin |
| HC | Hyperglycemic Control |
| H + EFS | Hyperglycemic + Ethanolic Extract of Fouquieria splendens |
| H + EFS + M | Hyperglycemic + Fouquieria splendens Extract + Metformin |
| H + EFS + S | Hyperglycemic + Fouquieria splendens Extract + Sitagliptin |
| H + M | Hyperglycemic + Metformin |
| H + S | Hyperglycemic + Sitagliptin |
| HMDB | Human Metabolome Database |
| HNC | Healthy Negative Control |
| I-3R | Imidazoline I3 Receptor |
| IDF | International Diabetes Federation |
| LD50 | Lethal Dose 50% |
| MN | Micronuclear Cells |
| MN-NCE | Micronucleus in Normochromatic Erythrocytes |
| MN-RET | Micronucleus in Reticulocytes |
| NAC | North America and the Caribbean |
| NC | Normoglycemic Control |
| NCE | Mature Normochromatic Erythrocytes |
| OECD | Organization for Economic Co-operation and Development |
| PBS | Phosphate-Buffered Saline |
| PKC | Protein Kinase C |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PTP-1B | Protein Tyrosine Phosphatase-1B |
| RET | Reticulocytes |
| RT | Retention Time |
| STZ | Streptozotocin |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| UPLC-MS | Ultra-Performance Liquid Chromatography–Mass Spectrometry |
| VLDL | Very Low-Density Lipoprotein |
References
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Khan, J.T.; Chowdhury, S.; Reberio, A.D.; Kumar, S.; Seidel, V.; Abdel-Wahab, Y.H.A.; Flatt, P.R. Plant-Based Diets and Phytochemicals in the Management of Diabetes Mellitus and Prevention of Its Complications: A Review. Nutrients 2024, 16, 3709. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 11th Edition 2025: Global Prevalence and Projections for 2050. Nephrol. Dial. Transplant. 2025, 0, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, X.; Li, X.; Wang, C.; Lin, L.; Wang, K.; Sun, Y.; Ye, W.; Li, H.; Zhang, Y.; et al. Aerobic Exercise Ameliorates Liver Injury in Db/Db Mice by Attenuating Oxidative Stress, Apoptosis and Inflammation through the Nrf2 and JAK2/STAT3 Signalling Pathways. J. Inflamm. Res. 2023, 16, 4805–4819. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 Diabetes Mellitus in Adults: Pathogenesis, Prevention and Therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Ansari, P.; Hannan, J.M.A.; Azam, S.; Jakaria, M. Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy. Int. J. Transl. Med. 2021, 1, 175–186. [Google Scholar] [CrossRef]
- Seguí Díaz, M. Uso de Inhibidores de la Dipeptidil Peptidasa 4 en Pacientes con Diabetes en Situaciones Especiales. Semergen 2018, 44 (Suppl. S1), 18–25. [Google Scholar] [CrossRef]
- Aylwin, C.G. Nuevos Fármacos en Diabetes Mellitus. Rev. Méd. Clín. Condes 2016, 27, 235–256. [Google Scholar] [CrossRef]
- Hanefeld, M. The Role of Acarbose in the Treatment of Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Its Complicat. 1998, 12, 228–237. [Google Scholar] [CrossRef]
- Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Gutiérrez-Meza, J.M. Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves. Plants 2022, 11, 3224. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Q.; Vitry, A.; Nguyen, T.A. Availability, Price, and Affordability of Selected Essential Medicines for Chronic Diseases in 11 Countries of the Asia Pacific Region: A Secondary Analysis. Asia Pac. J. Public Health 2017, 29, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Sagbo, I.J.; Hussein, A.A. Antidiabetic Medicinal Plants Used in the Eastern Cape Province of South Africa: An Updated Review. Processes 2022, 10, 1817. [Google Scholar] [CrossRef]
- Aramjoo, H.; Kiani, Z.; Eghbali, S. Antihyperglycemic and hepatoprotective effects of Salvia tebesana Bunge in diabetic rats. Res. Pharm. Sci. 2022, 17, 410–416. [Google Scholar] [CrossRef]
- Bouadid, I.; Akdad, M.; Eddouks, M. Antihyperglycemic Effect of Aqueous Extract of Tetraclinis articulata in Streptozotocin-Induced Diabetic Rats and Acute Toxicity Analysis. Cardiovasc. Hematol. Disord. Drug Targets 2022, 22, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef]
- Qabouche, A.; Amssayef, A.; Bouadid, I.; Lahrach, N.; El-Haidani, A.; Eddouks, M. Antidiabetic and Antidyslipidemic Effects of Artemisia mesatlantica, an Endemic Plant from Morocco. Cardiovasc. Hematol. Disord. Drug Targets 2023, 23, 50–63. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory Effect of Phenolic Compounds and Plant Extracts on the Formation of Advanced Glycation End Products: A Comprehensive Review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Torres-Moreno, H.; Rodríguez-Martínez, K.L.; Ramírez-Audelo, V.; Vidal-Gutiérrez, M.; Hernández, J.; Robles-Zepeda, R.E.; Ayala-Zavala, J.F.; González-Ríos, H.; Valenzuela-Melendres, M.; et al. Fouquieria splendens: A Source of Phenolic Compounds with Antioxidant and Antiproliferative Potential. Eur. J. Integr. Med. 2022, 49, 102084. [Google Scholar] [CrossRef]
- Monreal-García, H.M.; Almaraz-Abarca, N.; Ávila-Reyes, J.A.; Torres-Ricario, R.; González-Elizondo, M.S.; Herrera-Arrieta, Y.; Gutiérrez-Velázquez, M.V. Phytochemical Variation among Populations of Fouquieria splendens (Fouquieriaceae). Bot. Sci. 2019, 97, 398–412. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA). NOM-062-ZOO-1999. Technical Specifications for the Production, Care, and Use of Laboratory Animals. Official Journal of the Federation (Diario Oficial de la Federación). 1999. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 2 October 2024).
- Maron, D.M.; Ames, B.N. Revised Methods for the Salmonella Mutagenicity Test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Schmid, W. The Micronucleus Test for Cytogenetic Analysis. In Chemical Mutagens; Hollaender, A., Ed.; Springer: Boston, MA, USA, 1976; Volume 4, pp. 31–53. [Google Scholar] [CrossRef]
- Gothe, S.R.; Pawade, U.V.; Nikam, A.V.; Anjankar, M.P. OECD Guidelines for Acute Oral Toxicity Studies: An Overview. Int. J. Res. Ayurveda Pharm. 2023, 14, 137–140. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Jayachandran, M.; Vinayagam, R.; Ambati, R.R.; Xu, B.; Chung, S.S.M. Guava Leaf Extract Diminishes Hyperglycemia and Oxidative Stress, Prevents β-Cell Death, Inhibits Inflammation, and Regulates NF-kB Signaling Pathway in STZ Induced Diabetic Rats. BioMed Res. Int. 2018, 2018, 4601649. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Rodrigues, S.O.; Marques, L.A.; Oliveira, C.M.; Salles, B.C.C.; Zanatta, A.C.; Rocha, F.D.; Vilegas, W.; Pagnossa, J.P.; Paula, F.B.d.A.; et al. Eugenia sonderiana O. Berg Leaves: Phytochemical Characterization, Evaluation of In Vitro and In Vivo Antidiabetic Effects, and Structure—Activity Correlation. Biomed. Pharmacother. 2023, 165, 115126. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.; Sahoo, N.; Sahoo, S.K. Antidiabetic Effect of Standardized Chrysanthemum rubellum Hydroethanolic Extract by Targeting α-Glucosidase and the PTP-1B Signaling Pathway for Alleviating Diabetes in Experimental Model. J. Pharmacopunct. 2023, 26, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Secretariat of Agriculture, Livestock, and Rural Development (SAGAR). NOM-033-ZOO-1995. Humane Slaughter of Domestic and Wild Animals. Official Journal of the Federation (Diario Oficial de la Federación). 1995. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4892391&fecha=16/07/1996 (accessed on 2 October 2024).
- Choubey, D.; Paul, S. Classification Techniques for Diagnosis of Diabetes: A Review. Int. J. Biomed. Eng. Technol. 2016, 21, 15. [Google Scholar] [CrossRef]
- Ge, T.; Zhou, S.; Yang, J.; Tong, X.; Wang, Y.; Li, Y. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022: A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach—Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Sukhikh, S.; Babich, O.; Prosekov, A.; Kalashnikova, O.; Noskova, S.; Bakhtiyarova, A.; Krol, O.; Tsvetkova, E.; Ivanova, S. Antidiabetic Properties of Plant Secondary Metabolites. Metabolites 2023, 13, 513. [Google Scholar] [CrossRef]
- Anwar, N.; Teo, Y.K.; Tan, J.B.L. The Role of Plant Metabolites in Drug Discovery: Current Challenges and Future Perspectives. In Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Swamy, M.K., Akhtar, M.S., Eds.; Springer: Singapore, 2019; Volume 2, pp. 25–51. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Ahmad, I.; Alamri, A.; Anwar, S.; Alamri, A.S.; Locatelli, M.; Tartaglia, A.; Mahomoodally, M.F.; et al. Investigation into the Biological Properties, Secondary Metabolites Composition, and Toxicity of Aerial and Root Parts of Capparis spinosa L.: An Important Medicinal Food Plant. Food Chem. Toxicol. 2021, 155, 112404. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Choudhury, A.; Dey, B.K. An Overview on Plant Derived Phenolic Compounds and Their Role in Treatment and Management of Diabetes. J. Pharmacopunct. 2022, 25, 199–208. [Google Scholar] [CrossRef]
- Mohanty, I.R.; Borde, M.; Kumar, C.S.; Maheshwari, U. Dipeptidyl Peptidase IV Inhibitory Activity of Terminalia arjuna Attributes to Its Cardioprotective Effects in Experimental Diabetes: In Silico, In Vitro and In Vivo Analyses. Phytomedicine 2019, 57, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Verma, R.; Kumar, D.; Sivakumar, M.; Malik, T. Investigation into the Impact of Solvents on the Phytochemical Composition, Antioxidant Capacities, and Antihyperglycemic Activities of Erigeron annuus (L.) Pers. BioMed Res. Int. 2025, 2025, 6650124. [Google Scholar] [CrossRef] [PubMed]
- Sabiu, S.; Balogun, F.O.; Amoo, S.O. Phenolics Profiling of Carpobrotus edulis (L.) N.E.Br. and Insights into Molecular Dynamics of Their Significance in Type 2 Diabetes Therapy and Its Retinopathy Complication. Molecules 2021, 26, 4867. [Google Scholar] [CrossRef]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic Acid in Emblica officinalis Exerts Anti-Diabetic Activity through the Action on β-Cells of Pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef]
- Abdel-Megeed, R.M.; El Newary, S.A.; Kadry, M.O.; Ghanem, H.Z.; El-Shesheny, R.A.; Said-Al Ahl, H.A.H.; Abdel-Hamid, A.Z. Hyssopus officinalis Exerts Hypoglycemic Effects on Streptozotocin-Induced Diabetic Rats via Modulating GSK-3β, C-fos, NF-κB, ABCA1 and ABGA1 Gene Expression. J. Diabetes Metab. Disord. 2020, 19, 483–491. [Google Scholar] [CrossRef]
- Sawant, L.; Singh, V.K.; Dethe, S.; Bhaskar, A.; Balachandran, J.; Mundkinajeddu, D.; Agarwal, A. Aldose Reductase and Protein Tyrosine Phosphatase 1B Inhibitory Active Compounds from Syzygium cumini Seeds. Pharm. Biol. 2015, 53, 1176–1182. [Google Scholar] [CrossRef]
- Eidenberger, T.; Selg, M.; Krennhuber, K. Inhibition of Dipeptidyl Peptidase Activity by Flavonol Glycosides of Guava (Psidium guajava L.): A Key to the Beneficial Effects of Guava in Type II Diabetes Mellitus. Fitoterapia 2013, 89, 74–79. [Google Scholar] [CrossRef]
- Islam, M.N.; Jung, H.A.; Sohn, H.S.; Kim, H.M.; Choi, J.S. Potent α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors from Artemisia capillaris. Arch. Pharm. Res. 2013, 36, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.H.; Nguyen, H.X.; Nguyen, N.T.; Le, H.N.; Nguyen, M.T. α-Glucosidase Inhibitors from the Stems of Embelia ribes. Phytother. Res. 2014, 28, 1632–1636. [Google Scholar] [CrossRef]
- Asghari, B.; Salehi, P.; Sonboli, A.; Nejad Ebrahimi, S. Flavonoids from Salvia chloroleuca with α-Amylase and α-Glucosidase Inhibitory Effect. Iran. J. Pharm. Res. 2015, 14, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Cao, J.; Guo, Z.; Lou, Y.; Ding, M.; Zhao, Y. Depside Derivatives with Anti-Hepatic Fibrosis and Anti-Diabetic Activities from Impatiens balsamina L. Flowers. Fitoterapia 2015, 105, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Zhang, W.Y.; Jin, W.; Lee, I.S.; Min, B.S.; Jung, H.J.; Na, M.; Lee, S.; Bae, K. Flavonoids and Isoflavonoids from Sophorae Flos Improve Glucose Uptake In Vitro. Planta Med. 2010, 76, 79–81. [Google Scholar] [CrossRef]
- Ortiz-Barragán, E.; Estrada-Soto, S.; Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Fortis-Barrera, Á.; Marquina-Rodríguez, H.; Gaona-Tovar, E.; Lazzarini-Lechuga, R.; Suárez-Alonso, A.; Almanza-Pérez, J.C. Antihyperglycemic and Hypolipidemic Activities of Flavonoids Isolated from Smilax dominguensis Mediated by Peroxisome Proliferator-Activated Receptors. Pharmaceuticals 2024, 17, 1451. [Google Scholar] [CrossRef]
- Li, N.; Du, X.; Qu, T.; Ren, H.; Lu, W.; Cui, X.; Hu, J.; Chen, Z.; Tao, H. Pharmacodynamic Material Basis and Pharmacological Mechanisms of Cortex Mori against Diabetes Mellitus. J. Ethnopharmacol. 2024, 324, 117781. [Google Scholar] [CrossRef]
- Ezuruike, U.F.; Prieto, J.M. The Use of Plants in the Traditional Management of Diabetes in Nigeria: Pharmacological and Toxicological Considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef]
- Wen, P.; Zhao, P.; Qin, G.; Tang, S.; Li, B.; Zhang, J.; Peng, L. Genotoxicity and Teratogenicity of Seabuckthorn (Hippophae rhamnoides L.) Berry Oil. Drug Chem. Toxicol. 2020, 43, 391–397. [Google Scholar] [CrossRef]
- Akram, M.; Riaz, M.; Wadood, A.W.C.; Hazrat, A.; Mukhtiar, M.; Ahmad Zakki, S.; Zainab, R. Medicinal plants with anti-mutagenic potential. Biotechnol. Biotechnol. Equip. 2020, 34, 309–318. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Biological functionality of ellagic acid: A review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- de Carvalho, N.C.; Corrêa-Angeloni, M.J.; Leffa, D.D.; Moreira, J.; Nicolau, V.; de Aguiar Amaral, P.; Rossatto, A.E.; de Andrade, V.M. Evaluation of the genotoxic and antigenotoxic potential of Melissa officinalis in mice. Genet. Mol. Biol. 2011, 34, 290–297. [Google Scholar] [CrossRef]
- Walum, E. Acute oral toxicity. Environ. Health Perspect. 1998, 106 (Suppl. S2), 497–503. [Google Scholar] [CrossRef]
- Globally Harmonized System of Classification and Labelling of Chemicals (GHS); United Nations: Geneva, Switzerland, 2021; Available online: https://unece.org/transport/standards/transport/dangerous-goods/ghs-rev9-2021 (accessed on 2 October 2024).
- Lee, S.I.; Kim, J.S.; Oh, S.H.; Park, K.Y.; Lee, H.G.; Kim, S.D. Antihyperglycemic effect of Fomitopsis pinicola extracts in streptozotocin-induced diabetic rats. J. Med. Food 2008, 11, 518–524. [Google Scholar] [CrossRef]
- Sellamuthu, P.; Muniappan, B.; Perumal, S. Antihyperglycemic effect of mangiferin in streptozotocin-induced diabetic rats. J. Health Sci. 2009, 55, 206–214. [Google Scholar] [CrossRef]
- Shahab, F.; Hameed, A.; Ali, A.; Imad, R.; Hafizur, R.M. Apigenin potentiates glucose-stimulated insulin secretion through the PKA-MEK kinase signaling pathway independent of K-ATP channels. Biomed. Pharmacother. 2024, 177, 116986. [Google Scholar] [CrossRef]
- Lin, M.H.; Hsu, C.C.; Lin, J.; Cheng, J.T.; Wu, M.C. Identification of morin as an agonist of imidazoline I-3 receptor for insulin secretion in diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. BioMed Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef]
- Mohan, Y.; Jesuthankaraj, G.N.; Ramasamy Thangavelu, N. Antidiabetic and antioxidant properties of Triticum aestivum in streptozotocin-induced diabetic rats. Adv. Pharmacol. Sci. 2013, 2013, 716073. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sathish, G.; Jayanthi, M.; Muthukumaran, J.; Muruganathan, U.; Ramachandran, V. Ameliorating effect of eugenol on hyperglycemia by attenuating the key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2014, 385, 159–168. [Google Scholar] [CrossRef]
- Chtourou, Y.; Morjen, M.; Ammar, R.; Mhiri, R.; Jemaà, M.; ElBini-Dhouib, I.; Fetoui, H.; Srairi-Abid, N.; Marrakchi, N.; Jebali, J. Investigation of the renal protective effect of combined dietary polyphenols in streptozotocin-induced diabetic aged rats. Nutrients 2022, 14, 2867. [Google Scholar] [CrossRef]
- Zeng, F.; Luo, J.; Han, H.; Xie, W.; Wang, L.; Han, R.; Chen, H.; Cai, Y.; Huang, H.; Xia, Z. Allopurinol ameliorates liver injury in type 1 diabetic rats through activating Nrf2. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211031417. [Google Scholar] [CrossRef] [PubMed]
- Punitha, I.S.; Rajendran, K.; Shirwaikar, A.; Shirwaikar, A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid.-Based Complement. Altern. Med. 2005, 2, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.B.; Bruns, D.E.; Goldstein, D.E.; Maclaren, N.K.; McDonald, J.M.; Parrott, M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 2002, 48, 436–472. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Othman, M.S.; Khaled, A.M.; Al-Bagawi, A.H.; Fareid, M.A.; Ghany, R.A.; Habotta, O.A.; Abdel Moneim, A.E. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother. 2021, 144, 112287. [Google Scholar] [CrossRef]
- Koren, S.; Fantus, I.G. Inhibition of the protein tyrosine phosphatase PTP1B: Potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Hossain, C.M.; Ghosh, M.K.; Satapathy, B.S.; Dey, N.S.; Mukherjee, B. Apigenin causes biochemical modulation, GLUT4 and CD38 alterations to improve diabetes and to protect damages of some vital organs in experimental diabetes. Am. J. Pharmacol. Toxicol. 2014, 9, 39–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).