The Fucalean Forests of the Island of Lampedusa (Pelagie Islands Marine Protected Area, Central Mediterranean): Past and Present Diversity and Distribution
Abstract
1. Introduction
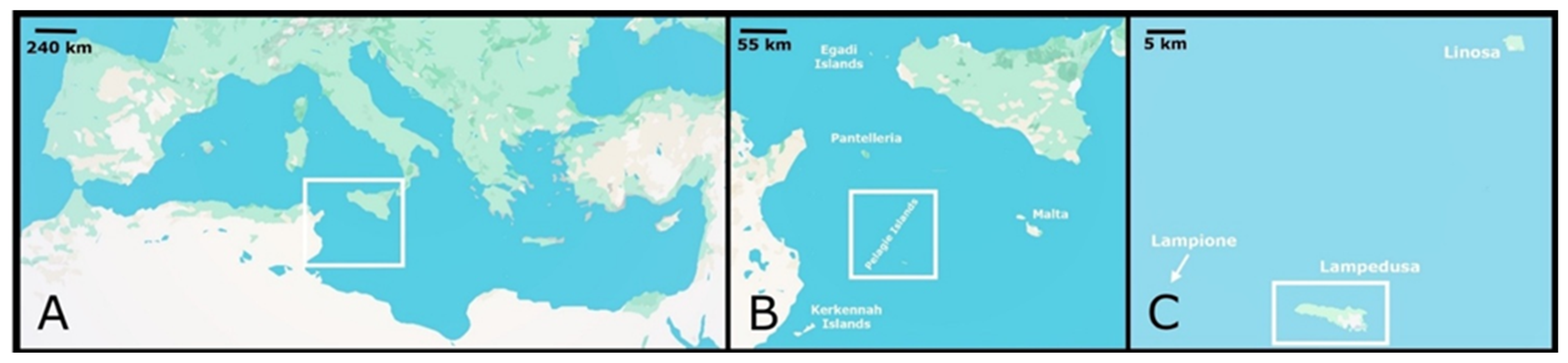

2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data Analysis
3. Results
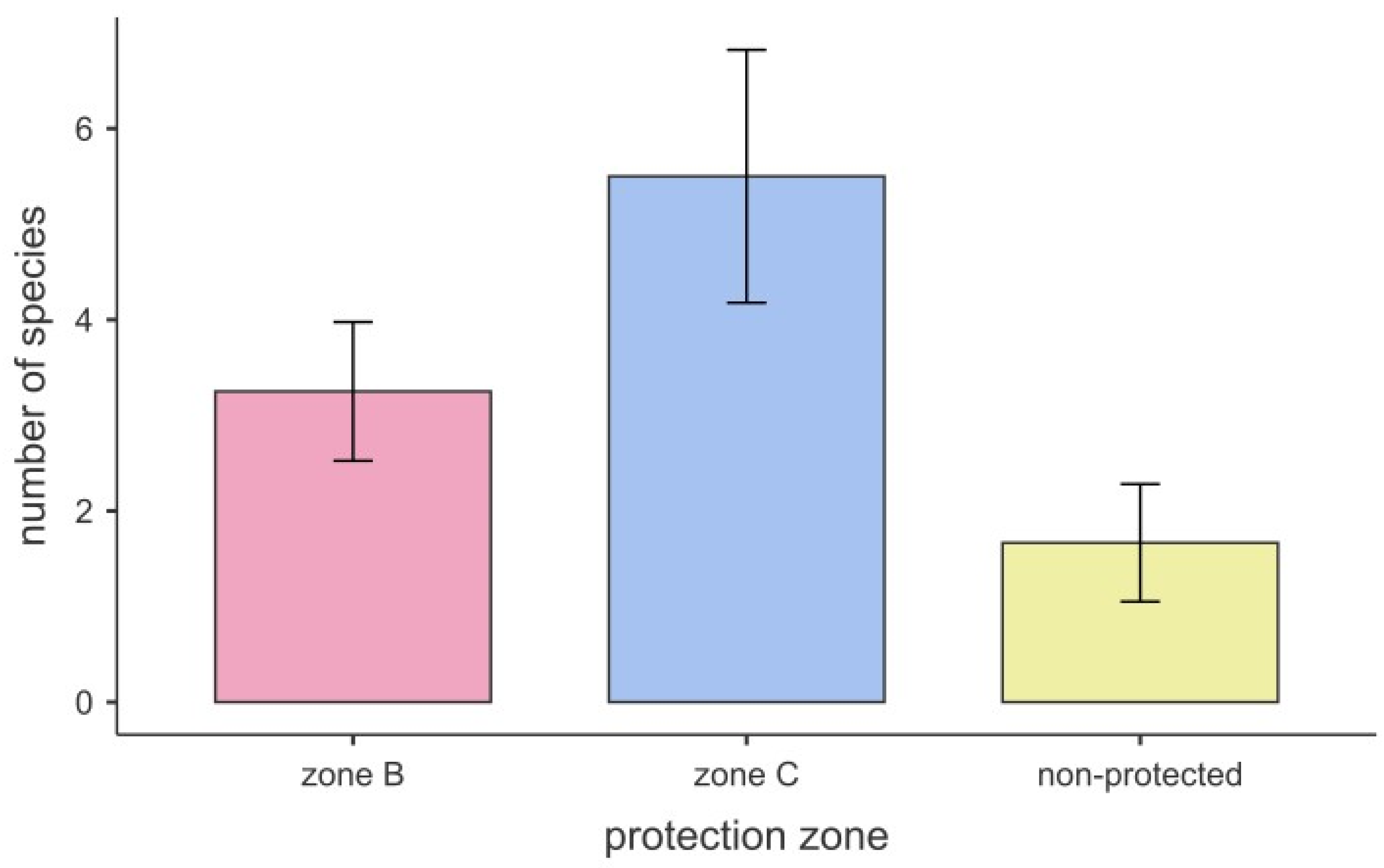
3.1. Current Diversity of Fucales in Lampedusa
3.2. Comparison with Previous Studies
3.3. Species Found During This Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaccone, G.; Scammacca, B.; Cinelli, F.; Sartoni, G.; Furnari, G. Studio preliminare sulla tipologia della vegetazione sommersa del Canale di Sicilia e isole vicine. Giorn. Bot. Ital. 1972, 106, 211–229. [Google Scholar] [CrossRef]
- Giaccone, G.; Colonna, P.; Graziano, C.; Mannino, A.M.; Tornatore, E.; Cormaci, M.; Furnari, G.; Scammacca, B. Revisione della flora marina di Sicilia e isole minori. Boll. Acc. Gioenia Sci. Nat. 1985, 18, 537–781. [Google Scholar]
- Alongi, G.; Dinaro, R.; Furnari, G.; Giaccone, G.; Scammacca, B.; Serio, D. Osservazioni preliminari sulla vegetazione marina dell’Isola di Lampedusa (Isole Pelagie). Biologia Marina Suppl. Notiziario SIBM 1993, 1, 285–286. [Google Scholar]
- Scammacca, B.; Giaccone, G.; Pizzuto, F.; Alongi, G. La vegetazione marina di substrato duro dell’Isola di Lampedusa (Isole Pelagie). Boll. Acc. Gioenia Sci. Nat. 1993, 26, 85–126. [Google Scholar]
- Orellana, S.; Hernández, M.; Sansón, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Molinari-Novoa, E.A.; Guiry, M.D. Reinstatement of the genera Gongolaria Boehmer and Ericaria Stackhouse (Sargassaceae, Phaeophyceae). Notulae Algarum 2020, 172, 456. [Google Scholar]
- Grech, D. Historical Records and Current Status of Fucales (Cystoseira and Sargassum spp.) in the Gulf of Naples. Ph.D. Thesis, Open University, Buckinghamshire, UK, 2017. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Ballesteros, E.; Torras, X.; Pinedo, S.; Garcia, M.; Mangialajo, L.; De Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfune, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in the French Riviera: The harbinger of future extinctions? Mediterr. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef]
- Buonomo, R.; Chefaoui, R.M.; Lacida, R.B.; Engelen, A.H.; Serrão, E.A.; Airoldi, L. Predicted extinction of unique genetic diversity in marine forests of Cystoseira spp. Mar. Environ. Res. 2018, 138, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.P.; Strain, E.M.A.; Piccioni, E.; De Clerck, O.; Sarà, G.; Airoldi, L. Status of vulnerable Cystoseira populations along the Italian infralittoral fringe, and relationships with environmental and anthropogenic variables. Mar. Pollut. Bull. 2018, 129, 762–771. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A.; Serio, D. Past and present fucalean diversity in the island of Marettimo, Egadi Islands Marine Protected Area (Central Mediterranean, Italy). Bot. Mar. 2025, 68, 221–233. [Google Scholar] [CrossRef]
- Medrano, A. Macroalgal Forests Ecology, Long-Term Monitoring, and Conservation in a Mediterranean Marine Protected Area. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2020. Available online: https://www.tesisenred.net/handle/10803/668804 (accessed on 26 October 2025).
- Bartolo, G.; Brullo, S.; Minissale, P.; Spampinato, G. Flora e vegetazione dell’Isola di Lampedusa. Boll. Acc. Gioenia Sci. Nat. 1988, 21, 119–255. [Google Scholar]
- Segre, A. Geologia. In Biogeografia Delle Isole Pelagie; Zavattari, E., Ed.; Rendiconti Acc. Nazionale dei XL Serie 4; Accademia Nazionale dei XL: Verona, Italy, 1961; pp. 115–162. [Google Scholar]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Chauvet, C.; Duval, C.; Galzin, R.; Lejeune, P.; Barnabé, G.; Blanc, F.; Chevalier, R.; Duclerc, J.; et al. The underwater observation of fish communities and fish populations: Methods and problems. Rev. Ecol. Terre Vie 1985, 40, 467–540. [Google Scholar]
- La Mesa, G.; Salvati, E.; Agnesi, S.; Tunesi, L. Assessment of coastal fish assemblages before the establishment of a new marine protected area in central Mediterranean: Its role in formulating zoning proposal. Mediterr. Mar. Sci. 2017, 18, 11–21. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A. Assessment of grazing impact on deep canopy-forming species in the western Ionian Sea, Central Mediterranean. Int. J. Aquat. Biol. 2020, 8, 365–376. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A. The Fucales (Ochrophyta, Phaeophyceae) of the island of Pantelleria (Sicily Channel, Mediterranean Sea): A new contribution. Ital. Botanist. 2023, 15, 137–163. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A.; Serio, D.; Bianchelli, S. Diversity of Fucales (Ochrophyta, Phaeophyceae) along the Coasts of Lipari and Vulcano (Aeolian Archipelago), Tyrrhenian Sea (Central Mediterranean Sea). J. Mar. Sci. Eng. 2023, 11, 2222. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Cottalorda, J.M.; Hereu, B.; Susini, M.L.; Verlaque, M. Unexpected temporal stability of Cystoseira and Sargassum forests in Port-Cros, one of the oldest Mediterranean marine National Parks. Cryptogam. Algol. 2016, 37, 61–90. [Google Scholar] [CrossRef]
- Gómez-Garreta, A.; Barceló-Martí, M.C.; Ribera-Siguan, M.A.; Rull-Lluch, J. Cystoseira C. Agardh. In Flora Phycologica Iberica, 1st ed.; Gómez-Garreta, A., Ed.; Universidade de Murcia: Múrcia, Spain, 2001; Volume 1, pp. 99–166. [Google Scholar]
- Cormaci, M.; Furnari, G.; Catra, M.; Alongi, G.; Giaccone, G. Flora marina bentonica del Mediterraneo: Phaeophyceae. Boll. Accad. Gioenia Sci. Nat. Catania 2012, 45, 375. [Google Scholar]
- Rodríguez-Prieto, C.; Ballesteros, E.; Boisset, F.; Afonso Carrilo, J. Guía de Las Macroalgas y Fanerógamas Marinas del Mediterráneo Occidental; Ediciones Omega: Barcelona, Barcelona, Spain, 2013; pp. 1–656. [Google Scholar]
- Blanfuné, A.; Verlaque, M.; Boudouresque, C.F.; Rozis, E.; Thibaut, T. Les Forêts Marines de France et de Méditerranée. Guide de Détermination des Espèces-Ingénieurs. Sargassaceae, Fucales, Phaeophyceae; Presses Universitaries de Provence: Marseille, France, 2022; pp. 1–207. [Google Scholar]
- AlgaeBase. Available online: https://www.algaebase.org (accessed on 16 September 2025).
- Neiva, J.; Bermejo, R.; Medrano, A.; Capdevila, P.; MillaFigueras, D.; Afonso, P.; Ballesteros, E.; Sabour, B.; Serio, D.; Nóbrega, E.; et al. DNA barcoding reveals cryptic diversity, taxonomic conflicts and novel biogeographical insights in Cystoseiras. L. (Phaeophyceae). Eur. J. Phycol. 2022, 58, 351–375. [Google Scholar] [CrossRef]
- Bouafif, C.; Verlaque, M.; Langar, H. Cystoseira taxa new for the marine flora of Tunisia. Cryptogam. Algol. 2014, 35, 269–283. [Google Scholar] [CrossRef]
- Giaccone, G. Una nuova specie mediterranea del genere Cystoseira C. Agardh (Phaeophyta, Fucales): C. hyblaea G. Giaccone, con osservazioni critiche su alcune entità tassonomiche poco note o imperfettamente descritte. Boll. Acc. Gioenia Sci. Nat. 1985, 18, 429–442. [Google Scholar]
- Serio, D.; Furnari, G. Ericaria giacconei sp. nov. (Sargassaceae, Fucophyceae), the species to which the invalidly published Cystoseira hyblaea Giaccone should be referred. Cryptogam. Algol. 2021, 42, 141–151. [Google Scholar] [CrossRef]
- Flores-Moya, A.; Conde, F. Fragmentos taxonómicos, corológicos, nomenclaturales y fitosociológicos (67–74). Acta Bot. Malacit. 1998, 23, 197–228. [Google Scholar] [CrossRef][Green Version]
- Marletta, G.; Lombardo, A.; Serio, D. Ecology and Phenology of the Subtidal Brown Alga Sargassum furcatum (Ochrophyta, Fucales), a Likely Non-Indigenous Species from the Mediterranean Sea. J. Mar. Sci. Eng. 2024, 12, 640. [Google Scholar] [CrossRef]
- Pizzuto, F. On the structure, typology and periodism of a Cystoseira brachycarpa J. Agardh emend. Giaccone community and of a Cystoseira crinita Duby community from the eastern coast of Sicily (Mediterranean Sea). Plant Biosyst. 1999, 133, 15–35. [Google Scholar] [CrossRef]
- Bouafif, C.; Verlaque, M.; Langar, H. New contribution to the knowledge of the genus Cystoseira C. Agardh in the Mediterranen Sea, with the reinstatement of species rank for C. Schiffneri Hamel. Cryptogam. Algol. 2016, 37, 133–154. [Google Scholar] [CrossRef]
- Verdura, J.; Rehues, L.; Mangialajo, L.; Fraschetti, S.; Belattmania, Z.; Bianchelli, S.; Blanfune, A.; Sabour, B.; Chiarore, A.; Danovaro, R.; et al. Distribution, health and threats to Mediterranean macroalgal forests: Defining the baselines for their conservation and restoration. Front. Mar. Sci. 2023, 10, 1258842. [Google Scholar] [CrossRef]
- Capdevila, P.; Hereu, B.; Salguero-Gómez, R.; Rovira, G.; Medrano, A.; Cebrian, E.; Garrabou, J.; Kersting, D.K.; Linares, C. Warming impacts on early life stages increase the vulnerability and delay the population recovery of a long-lived habitat-forming macroalga. J. Ecol. 2019, 107, 1129–1140. [Google Scholar] [CrossRef]
- Verdura, J.; Santamaría, J.; Ballesteros, E.; Smale, D.A.; Cefalì, M.E.; Golo, R.; de Caralt, S.; Vergés, A.; Cebrian, E. Local-scale climatic refugia offer sanctuary for a habitat-forming species during a marine heatwave. J. Ecol. 2021, 109, 1758–1773. [Google Scholar] [CrossRef]
- Mulas, M.; Silverman, J.; Guy-Haim, T.; Noè, S.; Rilov, G. Thermal vulnerability of the Levantine endemic and endangered habitat-forming macroalga, Gongolaria rayssiae: Implications for reef carbon. Front. Mar. Sci. 2022, 9, 862332. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L.; Bitar, G.; Harmelin, J.-G.; Monestiez, P. The littoral fish community of the Lebanese rocky coast (eastern Mediterranean Sea) with emphasis on Red Sea immigrants. Biol. Invasions 2005, 7, 625–637. [Google Scholar] [CrossRef]
- Sala, E.; Kizilkaya, Z.; Yildirim, D.; Ballesteros, E. Alien marine fishes deplete algal biomass in the eastern Mediterranean. PLoS ONE 2011, 6, e17356. [Google Scholar] [CrossRef] [PubMed]
- Azzurro, E.; Andaloro, F. A new settled population of the Lessepsian migrant Siganus luridus (Pisces: Siganidae) in Linosa Island—Sicily Strait. J. Mar. Biol. Assoc. UK 2004, 84, 819–821. [Google Scholar] [CrossRef]
- Stamouli, C.; Akel, E.H.K.; Azzurro, E.; Bakiu, R.; Bas, A.A.; Bitar, G.; Boyaci, Y.; Cakalli, M.; Corsinifoka, M.; Crocetta, F.; et al. New Mediterranean Biodiversity Records (December 2017). Mediterr. Mar. Sci. 2018, 18, 534–556. [Google Scholar] [CrossRef]
- Azzurro, E.; Franzitta, G.; Milazzo, M.; Bariche, M.; Fanelli, E. Abundance patterns at the invasion front: The case of Siganus luridus in Linosa (Sicily-Strait, Central Mediterranean Sea). Mar. Freshw. Res. 2016, 68, 697–702. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Mouillot, D. Increasing southern invasion enhances congruence between endemic and exotic Mediterranean fish fauna. Biol. Invasions 2009, 11, 697–711. [Google Scholar] [CrossRef]
- ISPRA, Istituto Superiore per la Protezione e la Ricerca Ambientale. Available online: https://www.mareografico.it/ (accessed on 26 October 2025).
- Mannino, A.M.; Mancuso, F.P.; Toccaceli, M. Efficacia delle AMP nella conservazione della biodiversità: I popolamenti a Cystoseira nell’AMP “Capo Gallo-Isola delle Femmine”(PA). Biogeographia 2011, 30, 241–250. [Google Scholar] [CrossRef]
- Bianchi, F.; Acri, F. The Island of Pantelleria (Sicily Strait, Italy): Towards the establishment of a marine protected area. First oceanographic investigations. Boll. Geofis. Teor. Appl. 2003, 44, 3–9. [Google Scholar]




| Site | Site Number | Protection Zone | Side | GPS Coordinates | Date | Activity |
|---|---|---|---|---|---|---|
| Grotta dell’Acqua | 1 | Zone B | north-east | 35°31′20.0″ N 12°37′06.7″ E | 7 July 2025 | Scuba dive |
| Grotta Santa | 2 | Zone B | north | 35°31′19.7″ N 12°35′39.6″ E | 7 July 2025 | Scuba dive |
| Baia di Taccio Vecchio | 3 | Zone B | north | from 35°31′18.6″ N 12°35′41.6″ E to 35°31′18.1″ N 12°35′46.8″ E | 7 July 2025 | Snorkeling |
| Muro Vecchio | 4 | Zone C | north | 35°31′33.9″ N 12°33′35.2″ E | 8 July 2025 | Scuba dive |
| Scoglio Pignata | 5 | Zone C | north-west | 35°31′37.9″ N 12°31′24.3″ E | 8 July 2025 | Scuba dive |
| Baia di Capo Ponente | 6 | Zone C | west | from 35°31′22.5″ N 12°31′13.0″ E to 35°31′18.3″ N 12°31′07.2″ E | 8 July 2025 | Snorkeling |
| Capo Grecale | 7 | Zone B | east | 35°30′55.6″ N 12°37′58.6″ E | 9 July 2025 | Scuba dive |
| Fortino | 8 | Zone B | east | 35°30′50.3″ N 12°37′44.3″ E | 9 July 2025 | Scuba dive |
| Costa Sud di Capo Grecale | 9 | Zone B | east | from 35°30′55.5″ N 12°37′55.0″ E to 35°30′53.8″ N 12°37′43.8″ E | 9 July 2025 | Snorkeling |
| Panettone | 10 | Zone B | south | 35°30′26.0″ N 12°33′33.2″ E | 10 July 2025 | Scuba dive |
| Madonnina | 11 | Zone B | south | 35°30′28.1″ N 12°33′24.2″ E | 10 July 2025 | Scuba dive |
| Tabaccara | 12 | Zone C | south | from 35°30′39.8″ N 12°34′07.3″ E to 35°30′27.6″ N 12°34′11.5″ E | 10 July 2025 | Snorkeling |
| Punta Parrino | 13 | Non-protected zone | east | 35°30′02.6″ N 12°38′04.5″ E | 11 July 2025 | Scuba dive |
| Punta Iavuta | 14 | Non-protected zone | east | 35°29′49.0″ N 12°38′02.2″ E | 11 July 2025 | Scuba dive |
| Baia di Punta Sottile | 15 | Non-protected zone | east | from 35°29′50.0″ N 12°37′58.2″ E to 35°29′46.2″ N 12°37′51.6″ E | 11 July 2025 | Snorkeling |
| Cala Spugne | 16 | Non-protected zone | south-east | from 35°29′35.3″ N 12°36′29.9″ E to 35°29′43.5″ N 12°36′38.3″ E | 12 July 2025 | Snorkeling |
| Cala Maluk | 17 | Non-protected zone | south-east | from 35°29′41.2″ N 12°36′42.5″ E to 35°29′36.1″ N 12°36′52.0″ E | 12 July 2025 | Snorkeling |
| Cala Francese | 18 | Non-protected zone | south-east | from 35°29′40.6″ N 12°37′26.6″ E to 35°29′43.6″ N 12°37′30.7″ E | 12 July 2025 | Snorkeling |
| Site Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | N. Sites for Each Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Side of the Island | North-East | North | North | North | North-West | West | East | East | East | South | South | South | East | East | East | South-East | South-East | South-East | |
| Protection Zone | B | B | B | C | C | C | B | B | B | B | B | C | Non-Protected | Non-Protected | Non-Protected | Non-Protected | Non-Protected | Non-Protected | |
| C. compressa | 0.1–0.3 m (4) * | 0.3–15 m (3) * | 0.1–0.3 m (4) | 0.1–0.3 m (3) | 0.1–0.2 m (4) | 0.1–0.3 m (3) * | 8 m (3) | 7 | |||||||||||
| C. compressa f. rosetta | 0.1–0.3 m (2) * | 0.1–0.3 m (2) * | 0.1–0.3 m (2) * | 0.1–0.3 m (2) * | 4 | ||||||||||||||
| C. pustulata | 14 m (2) | 11–15 m (2) | 6 m (2) | 0.3 m (2) * | 7–9 m (3) | 0.3–0.5 m (3) * | 6 | ||||||||||||
| E. amentacea | 0–0.1 m (3) * | 0–0.1 m (4) * | 0–0.1 m (4) * | 0–0.1 m (4)* | 0–0.1 m (4) | 0–0.1 m (4) | 0–0.1 m (4) | 0–0.1 m (4) | 8 | ||||||||||
| E. brachycarpa | 0.1–0.2 m (3) | 1 | |||||||||||||||||
| E. dubia | 23–24 m (3) | 35 m (3) | 2 | ||||||||||||||||
| E. funkii | 26–28 m (2) | 13–20 m (2) | 19–27 m (3) | 16–24 m (3) | 4 | ||||||||||||||
| E. giacconei | 0.1–0.3 m (3) | 0.1–0.3 m (3) | 0.1–0.3 m (2) | 3 | |||||||||||||||
| G. montagnei | 12–16 m (2) | 14–19 m (2) | 16–22 m (3) | 17 m (2) | 4 | ||||||||||||||
| G. montagnei var. compressa | 25 m (2) | 14–17 m (3) | 16–19 m (2) | 16–20 m (3) | 16 m (2) | 5 | |||||||||||||
| S. cf. acinarium | 16 m (2) | 23–27 m (3) | 2 | ||||||||||||||||
| S. cf. furcatum | 12–13 m (2) | 18 m (2) | 14 m (2) | 3 | |||||||||||||||
| S. vulgare | 0.1–15 m (3) * | 15 m (2) | 0.1–0.5 m (4) * | 13–20 m (2) | 0.1–0.3 m (4) * | 0.1–0.3 m (3) * | 0.2–0.4 m (3) * | 0.1–0.3 m (3) * | 0.1–0.3 m (3) * | 9 | |||||||||
| Number of species x site | 4 | 6 | 5 | 9 | 6 | 3 | 1 | 1 | 3 | 5 | 1 | 4 | 3 | 0 | 3 | 3 | 0 | 1 |
| Taxa | Giaccone et al. [1] | Giaccone et al. [2] | Alongi et al. [3] | Scammacca et al. [4] | Present Study |
|---|---|---|---|---|---|
| Ericaria amentacea (C. Agardh) Molinari & Guiry | x (as Cystoseira stricta) | x (as Cystoseira stricta v. stricta) | x (as Cystoseira amentacea) | x (as Cystoseira amentacea v. amentacea) | x |
| Ericaria barbatula (Kützing) Molinari & Guiry | x (as Cystoseira barbatula) | x (as Cystoseira barbatula) | |||
| Ericaria brachycarpa (J. Agardh) Molinari & Guiry | x | ||||
| Cystoseira compressa (Esper) Gerloff & Nizamuddin | x (as Cystoseira compressa) | x | |||
| Cystoseira compressa f. rosetta (Ercegović) Cormaci, G. Furnari, Giaccone, B. Scammacca & Serio | x (as Cystoseira compressa f. rosetta) | x | |||
| Ericaria crinita (Duby) Molinari & Guiry | x (as Cystoseira crinita) | x (as Cystoseira crinita) | |||
| Ericaria dubia (Valiante) Neiva & Serrão | x (as Cystoseira dubia) | x | |||
| Cystoseira foeniculacea (Linnaeus) Greville | x (as Cystoseira ercegovićii f. ercegovićii) | ||||
| Cystoseira foeniculacea f. latiramosa (Ercegović) A. Gómez Garreta, M. C. Barceló, M. A. Ribera & J. R. Lluch | x (as Cystoseira ercegovićii f. latiramosa) | ||||
| Cystoseira foeniculacea f. tenuiramosa (Ercegović) A. Gómez Garreta, M. C. Barceló, M. A. Ribera & J. R. Lluch | x (as Cystoseira ercegovićii f. tenuiramosa) | ||||
| Ericaria funkii (Gerloff & Nizamuddin) Molinari & Guiry | x | ||||
| Ericaria giacconei D. Serio & G. Furnari | x | ||||
| Cystoseira pustulata (Ercegović) Neiva & Serrão | x (as Cystoseira humilis) | x | |||
| Cystoseira platyclada Sauvageau (taxon inquirendum) | x | ||||
| Gongolaria montagnei (J. Agardh) Kuntze | x (as Cystoseira spinosa) | x (as Cystoseira spinosa v. spinosa) | x (as Cystoseira spinosa v. spinosa) | x (as Cystoseira spinosa v. spinosa) | x |
| Gongolaria montagnei var. compressa (Ercegović) Verlaque, Blanfuné, Boudouresque & Thibaut | x (as Cystoseira spinosa v. compressa) | x | |||
| Gongolaria montagnei var. tenuior (Ercegović) Molinari & Guiry | x (as Cystoseira spinosa v. tenuior) | x (as Cystoseira spinosa v. tenuior) | |||
| Sargassum acinarium (Linnaeus) Setchell | x | x | |||
| Sargassum hornschuchii C. Agardh | x | x | |||
| Sargassum trichocarpum J. Agardh | x | x | |||
| Sargassum cf furcatum Kützing | x | ||||
| Sargassum vulgare C. Agardh | x | x | x | x | |
| Total number of species | 10 | 7 | 4 | 10 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marletta, G.; Lombardo, A.; Serio, D.; Mannino, A.M. The Fucalean Forests of the Island of Lampedusa (Pelagie Islands Marine Protected Area, Central Mediterranean): Past and Present Diversity and Distribution. Coasts 2025, 5, 43. https://doi.org/10.3390/coasts5040043
Marletta G, Lombardo A, Serio D, Mannino AM. The Fucalean Forests of the Island of Lampedusa (Pelagie Islands Marine Protected Area, Central Mediterranean): Past and Present Diversity and Distribution. Coasts. 2025; 5(4):43. https://doi.org/10.3390/coasts5040043
Chicago/Turabian StyleMarletta, Giuliana, Andrea Lombardo, Donatella Serio, and Anna Maria Mannino. 2025. "The Fucalean Forests of the Island of Lampedusa (Pelagie Islands Marine Protected Area, Central Mediterranean): Past and Present Diversity and Distribution" Coasts 5, no. 4: 43. https://doi.org/10.3390/coasts5040043
APA StyleMarletta, G., Lombardo, A., Serio, D., & Mannino, A. M. (2025). The Fucalean Forests of the Island of Lampedusa (Pelagie Islands Marine Protected Area, Central Mediterranean): Past and Present Diversity and Distribution. Coasts, 5(4), 43. https://doi.org/10.3390/coasts5040043







