Effect of Creep Feeding Supplementation on Growth Performance and Metabolic Characteristics of Nellore Heifers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Treatments
2.2. Experimental Procedures and Sampling
2.3. Laboratory Analyses and Calculations
2.4. Statistical Analyses
3. Results
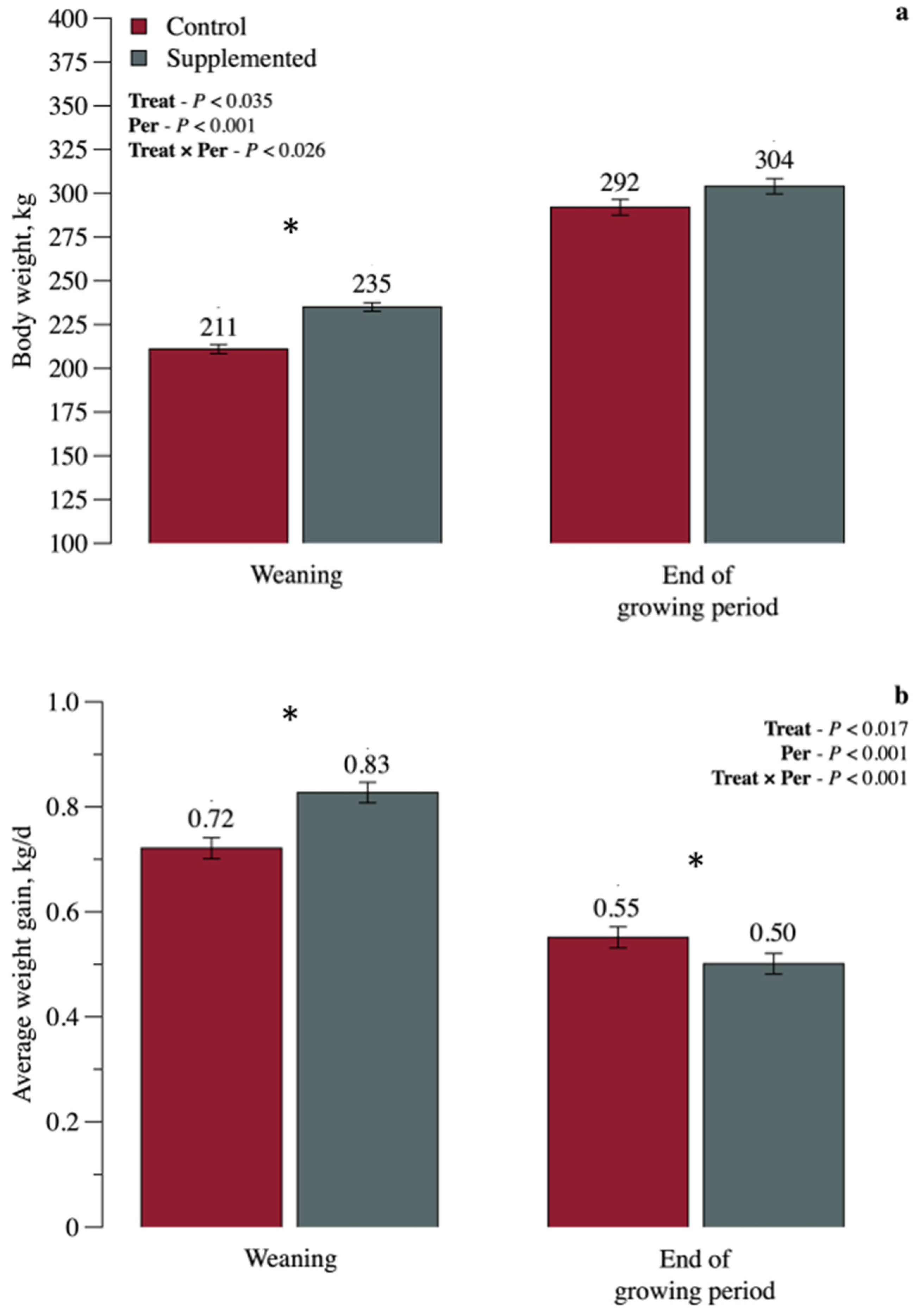
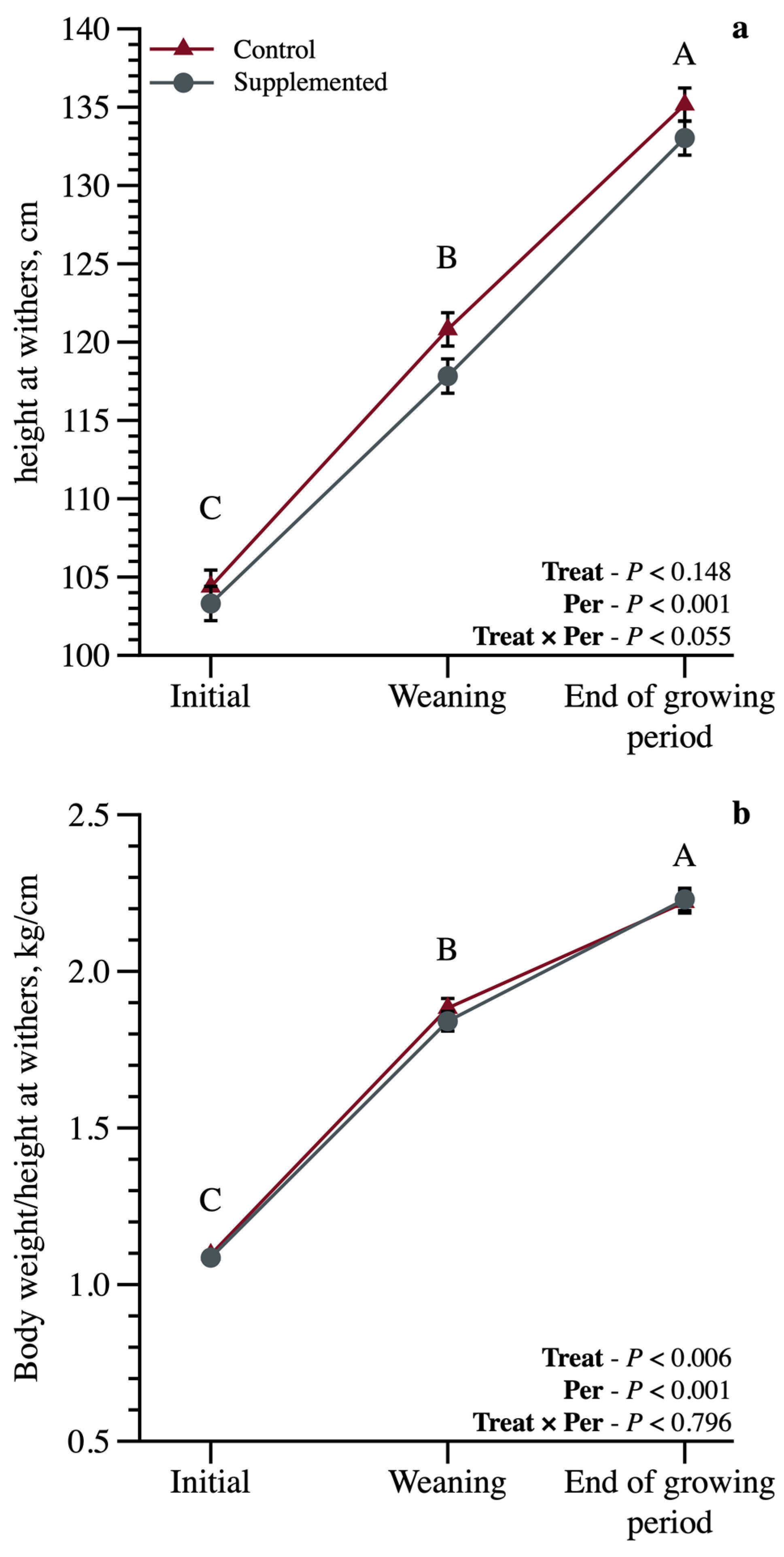
3.1. Growth Performance and Body Measurements
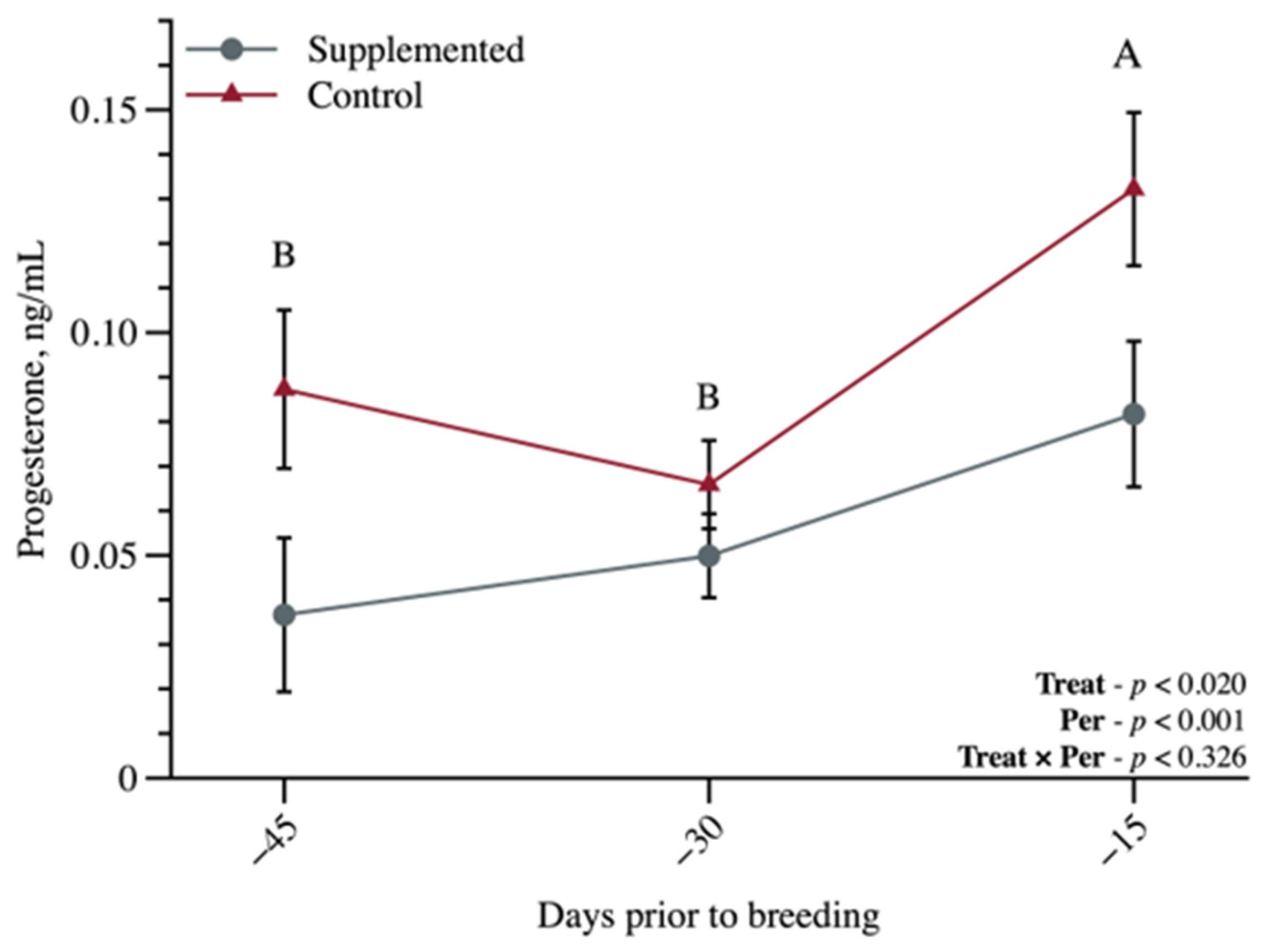
3.2. Metabolic and Reproductive Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedreira, M.S.; Primavesi, O.; Lima, M.A.; Frighetto, R.; de Oliveira, S.G.; Berchielli, T.T. Ruminal methane emission by dairy cattle in Southeast Brazil. Sci. Agric. 2009, 66, 742–750. [Google Scholar] [CrossRef]
- Moreira, H.L.; Buzanskas, M.E.; Munari, D.P.; Canova, É.B.; Lôbo, R.B.; de Paz, C.C.P. Reproductive Traits Selection in Nelore Beef Cattle. Ciência Agrotecnologia 2015, 39, 355–362. [Google Scholar] [CrossRef]
- Cardoso, R.C.; Alves, B.R.C.; Prezotto, L.D.; Thorson, J.F.; Tedeschi, L.O.; Keisler, D.H.; Park, C.S.; Amstalden, M.; Williams, G.L. Use of a stair-step compensatory gain nutritional regimen to program the onset of puberty in beef heifers. J. Anim. Sci. 2014, 92, 2942–2949. [Google Scholar] [CrossRef]
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal 2014, 8, 91–104. [Google Scholar] [CrossRef]
- Gasser, C.L. Considerations on puberty in replacement beef heifers. J. Anim. Sci. 2013, 91, 1336–1340. [Google Scholar] [CrossRef]
- Gasser, C.L.; Behlke, E.J.; Grum, D.E.; Day, M.L. Effect of timing of feeding a high-concentrate diet on growth and attainment of puberty in early-weaned heifers. J. Anim. Sci. 2006, 84, 3118–3122. [Google Scholar] [CrossRef]
- Garcia, M.R.; Amstalden, M.; Morrison, C.D.; Keisler, D.H.; Williams, G.L. Age at puberty, total fat and conjugated linoleic acid content of carcass, and circulating metabolic hormones in beef heifers fed a diet high in linoleic acid beginning at four months of age. J. Anim. Sci. 2003, 81, 261–268. [Google Scholar] [CrossRef]
- Maciel, M.N.; Zieba, D.A.; Amstalden, M.; Keisler, D.H.; Neves, J.P.; Williams, G.L. Leptin Prevents Fasting-Mediated Reductions in Pulsatile Secretion of Luteinizing Hormone and Enhances Its Gonadotropin-Releasing Hormone-Mediated Release in Heifers. Biol. Reprod. 2004, 70, 229–235. [Google Scholar] [CrossRef]
- Cardoso, R.C.; West, S.M.; Maia, T.S.; Alves, B.R.C.; Williams, G.L. Nutritional control of puberty in the bovine female: Prenatal and early postnatal regulation of the neuroendocrine system. Domest. Anim. Endocrinol. 2020, 73, 106434. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1995, 372, 425–432. [Google Scholar] [CrossRef]
- Carvalho, V.V.; Paulino, M.F.; Detmann, E.; Valadares Filho, S.C.; Lopes, S.A.; Rennó, L.N.; Sampaio, C.B.; Silva, A.G. A meta-analysis of the effects of creep-feeding supplementation on performance and nutritional characteristics by beef calves grazing on tropical pastures. Livest. Sci. 2019, 227, 175–182. [Google Scholar] [CrossRef]
- Silva, A.G.; Paulino, M.F.; Amorim, L.S.; Rennó, L.N.; Detmann, E.; Moura, F.H.; Manso, M.R.; Silva e Paiva, P.H.; Ortega, R.E.M.; Melo, L.P. Performance, endocrine, metabolic, and reproductive responses of Nellore heifers submitted to different supplementation levels pre- and post-weaning. Trop. Anim. Health Prod. 2017, 49, 707–715. [Google Scholar] [CrossRef]
- INCT. Brazilian National Institute of Science and Technology in Animal Science: INCT-CA, 1st ed.; Detmann, E., Ed.; Federal University of Viçosa: Viçosa, Brazil, 2012; pp. 1–214. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Pereira, G.R.; Barcellos, J.O.J.; Sessim, A.G.; Tarouco, J.U.; Feijó, F.D.; Neto, J.B.; Prates, E.R.; Canozzi, M.E.A. Relationship of post-weaning growth and age at puberty in crossbred beef heifers. Braz. J. Anim. Sci. 2017, 46, 413–420. [Google Scholar] [CrossRef]
- Gasser, C.L.; Grum, D.E.; Mussard, M.L.; Fluharty, F.L.; Kinder, J.E.; Day, M.L. Induction of precocious puberty in heifers I: Enhanced secretion of luteinizing hormone. J. Anim. Sci. 2006, 84, 2035–2041. [Google Scholar] [CrossRef]
- Schubach, K.M.; Cooke, R.F.; Brandão, A.P.; Schumaher, T.F.; Pohler, K.G.; Bohnert, D.W.; Marques, R.S. Impacts of postweaning growth rate of replacement beef heifers on their reproductive development and productivity as primiparous cows. J. Anim. Sci. 2019, 97, 4171–4181. [Google Scholar] [CrossRef]
- Kelly, A.K.; Byrne, C.; McGee, M.; Perry, G.A.; Crowe, M.A.; Sauerwein, H.; Kenny, D.A. Effect of calfhood nutrition on metabolic hormones, gonadotropins, and estradiol concentrations and on reproductive organ development in beef heifer calves. J. Anim. Sci. 2020, 98, skaa310. [Google Scholar] [CrossRef]
- de Almeida, D.M.; Marcondes, M.I.; Rennó, L.N.; Pereira Silva, L.H.; Martins, L.S.; Marquez, D.E.C.; Villadiego, F.A.C.; Saldarriaga, F.V.; Franco, J.D.C.; Moreno, D.P.S.; et al. Nutritional planning for Nellore heifers post-weaning to conception at 15 months of age: Performance and nutritional, metabolic, and reproductive responses. Trop. Anim. Health Prod. 2019, 51, 79–87. [Google Scholar] [CrossRef]
- Miszura, A.A.; Ferraz, M.V.C.; Cardoso, R.C.; Polizel, D.M.; Oliveira, G.B.; Barroso, J.P.R.; Gobato, L.G.M.; Nogueira, G.P.; Biava, J.S.; Ferreira, E.M.; et al. Implications of growth rates and compensatory growth on puberty attainment in Nellore heifers. Domest. Anim. Endocrinol. 2021, 74, 106526. [Google Scholar] [CrossRef]
- Fernandes Júnior, G.A.; Silva, D.A.; Mota, L.F.M.; de Melo, T.P.; Fonseca, L.F.S.; Silva, D.B.d.S.; Carvalheiro, R.; Albuquerque, L.G. Sustainable Intensification of Beef Production in the Tropics: The Role of Genetically Improving Sexual Precocity of Heifers. Animals 2022, 12, 174. [Google Scholar] [CrossRef]
- Johnston, D.J.; Barwick, S.A.; Corbet, N.J.; Fordyce, G.; Holroyd, R.G.; Williams, P.J.; Burrow, H.M. Genetics of heifer puberty in two tropical beef genotypes in northern Australia and associations with heifer- and steer-production traits. Anim. Prod. Sci. 2009, 49, 399–412. [Google Scholar] [CrossRef]
- Khan, M.A.; Weary, D.M.; von Keyserlingk, M.A.G. Hay intake improves performance and rumen development of calves fed higher quantities of milk. J. Dairy Sci. 2011, 94, 3547–3553. [Google Scholar] [CrossRef]
- Zieba, D.A.; Amstalden, M.; Williams, G.L. Regulatory roles of leptin in reproduction and metabolism: A comparative review. Domest. Anim. Endocrinol. 2005, 29, 166–185. [Google Scholar] [CrossRef]
- Amstalden, M.; Cardoso, R.C.; Alves, B.R.C.; Williams, G.L. Reproduction Symposium: Hypothalamic neuropeptides and the nutritional programming of puberty in heifers. J. Anim. Sci. 2014, 92, 3211–3222. [Google Scholar] [CrossRef]
- Roa, J.; García-Galiano, D.; Castellano, J.M.; Gaytan, F.; Pinilla, L.; Tena-Sempere, M. Metabolic control of puberty onset: New players, new mechanisms. Mol. Cell. Endocrinol. 2010, 324, 87–94. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, J.A.; Sanz, A.; Tamanini, C. Metabolic, endocrine, and reproductive responses of beef heifers submitted to different growth strategies during the lactation and rearing periods. J. Anim. Sci. 2015, 93, 3871–3885. [Google Scholar] [CrossRef]
- Consentini, C.E.C.; Alves, R.L.O.R.; Silva, M.A.; Galindez, J.P.A.; Madureira, G.; Lima, L.G.; Gonçalves, J.R.S.; Wiltbank, M.L.; Sartori, R. What are the factors associated with pregnancy loss after timed artificial insemination in Bos indicus cattle? Theriogenology 2023, 196, 264–269. [Google Scholar] [CrossRef]
- Harvey, K.M.; Cooke, R.F.; and Moriel, P. Impacts of Nutritional Management During Early Postnatal Life on Long-Term Physiological and Productive Responses of Beef Cattle. Front. Anim. Sci. 2021, 2, 730356. [Google Scholar] [CrossRef]
- Reis, M.; Cooke, R.F.; Cappellozza, B.I.; Marques, R.S.; Guarnieri Filho, T.A.; Rodrigues, M.C.; Bradley, J.S.; Mueller, C.J.; Keisler, D.H.; Johnson, S.E.; et al. Creep-feeding to stimulate metabolic imprinting in nursing beef heifers: Impacts on heifer growth, reproductive and physiological variables. Animal 2015, 9, 1500–1508. [Google Scholar] [CrossRef]
- Nepomuceno, D.D.; Pires, A.V.; Ferraz, M.V.C.; Biehl, M.V.; Gonçalves, J.R.S.; Moreira, E.M.; Day, M.L. Effect of pre-partum dam supplementation, creep-feeding and post-weaning feedlot on age at puberty in Nellore heifers. Livest. Sci. 2017, 195, 58–62. [Google Scholar] [CrossRef]

| Preweaning | Postweaning | ||||
|---|---|---|---|---|---|
| Forage 5 | Treatment | Forage 5 | Treatment | ||
| Control | Supplemented | Supplemented | |||
| Ingredients (% dry matter) | |||||
| Ground corn | - | - | 70.3 | - | 20.47 |
| Soybean meal | - | - | 24.8 | - | 46.47 |
| Wheat bran | - | - | 3.08 | - | 29.71 |
| Mineral mix 1 | - | 100 | - | - | - |
| Mineral mix 2 | - | - | 1.81 | - | - |
| Mineral mix 3 | - | - | - | - | 3.34 |
| Chemical composition (% dry matter) | |||||
| Dry matter | 60.6 | 100 | 88.3 | 39.9 | 87.9 |
| Organic matter | 89.0 | - | 95.0 | 91.5 | 91.8 |
| Crude protein | 8.34 | - | 18.9 | 10.0 | 28.3 |
| apNDF 4 | 73.4 | - | 13.8 | 63.9 | 17.4 |
| Item | Treatment 1 | SEM 2 | p-Value 3 | |||
|---|---|---|---|---|---|---|
| Control | Supplemented | Treat | Period | Treat × Period | ||
| Longissimus dorsi muscle area (cm2) | ||||||
| Weaning | 32.4 | 35.2 | 0.991 | 0.200 | <0.001 | 0.145 |
| End of growing period | 43.2 | 43.6 | ||||
| Rib fat (mm) | ||||||
| Weaning | 0.87 | 1.16 | 0.066 | 0.017 | <0.001 | 0.738 |
| End of growing period | 1.34 | 1.62 | ||||
| Rump fat (mm) | ||||||
| Weaning | 1.70 | 1.74 | 0.092 | 0.988 | <0.001 | 0.423 |
| End of growing period | 2.46 | 2.41 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Paixão, R.T.; Detmann, E.; Marcondes, M.I.; da Silva Júnior, J.M.; Sampaio, C.B. Effect of Creep Feeding Supplementation on Growth Performance and Metabolic Characteristics of Nellore Heifers. Ruminants 2023, 3, 457-467. https://doi.org/10.3390/ruminants3040037
da Paixão RT, Detmann E, Marcondes MI, da Silva Júnior JM, Sampaio CB. Effect of Creep Feeding Supplementation on Growth Performance and Metabolic Characteristics of Nellore Heifers. Ruminants. 2023; 3(4):457-467. https://doi.org/10.3390/ruminants3040037
Chicago/Turabian Styleda Paixão, Robert T., Edenio Detmann, Marcos I. Marcondes, Jarbas M. da Silva Júnior, and Claudia B. Sampaio. 2023. "Effect of Creep Feeding Supplementation on Growth Performance and Metabolic Characteristics of Nellore Heifers" Ruminants 3, no. 4: 457-467. https://doi.org/10.3390/ruminants3040037
APA Styleda Paixão, R. T., Detmann, E., Marcondes, M. I., da Silva Júnior, J. M., & Sampaio, C. B. (2023). Effect of Creep Feeding Supplementation on Growth Performance and Metabolic Characteristics of Nellore Heifers. Ruminants, 3(4), 457-467. https://doi.org/10.3390/ruminants3040037







