Abstract
Background/Objective: Early hemodynamic instability in sepsis arises from endothelial dysfunction and vasoplegia before capillary leakage and organ failure occur. Albumin administration guided by serum concentration or shock criteria has not improved outcomes. This review synthesized evidence supporting an early, physiology-guided framework for albumin and norepinephrine use in pre-δ vasoplegic sepsis. Methods: A narrative synthesis of experimental and clinical studies examined endothelial injury, sepsis phenotypes, hemodynamic monitoring, biochemical markers, and intravascular albumin mass. Evidence from phenotype cohorts was integrated to construct a physiology-based therapeutic framework. Results: The δ phenotype consistently emerged as a vasoplegic, hyperinflammatory endotype with hypoalbuminemia, elevated lactate, and the highest mortality. Studies showed 20–25% of patients with community-acquired sepsis exhibit early vasoplegia, with low systemic vascular resistance and high cardiac output. Mass-balance analyses showed intravascular albumin mass declines early in sepsis, correlate inversely with fluid balance, and predict mortality. These findings suggest early low-dose norepinephrine may stabilize perfusion pressure, while albumin use should follow intravascular albumin mass trajectories. A dynamic exclusion concept proposes withholding albumin during capillary leak and reintroducing it when intravascular albumin mass stabilizes. Conclusions: Albumin therapy in sepsis should shift from late concentration-based to early physiology-guided endothelial protection. Monitoring intravascular albumin mass, lactate, and fluid balance may guide targeted norepinephrine and albumin use before δ-type endothelial failure occurs. This framework needs phenotype-stratified validation.
1. Introduction
Despite decades of clinical use, the role, timing, and indications for albumin therapy in sepsis remain insufficiently defined. Current albumin-guided fluid strategies face three persistent challenges identified in recent studies. Firstly, routine administration of albumin does not significantly reduce overall mortality in unselected adult sepsis, as demonstrated by large randomized controlled trials (RCTs) and meta-analyses comparing albumin with crystalloids [1,2,3]. Any observed survival benefit appears restricted to specific subgroups, particularly those with septic shock, and even within these groups, the effect is modest and not consistently observed across analyses [2,3,4]. Second, although albumin can enhance hemodynamic endpoints and reduce net fluid balance early in resuscitation, these benefits do not consistently translate into improved hard outcomes [1,5,6]. Third, relying on a single reduced serum albumin concentration to initiate therapy is biologically imprecise; while hypoalbuminemia is a strong prognostic marker, it is not a reliable standalone guide because of confounding factors such as inflammation, capillary leak, and dilution. Furthermore, dynamic trajectories (declining or persistently low albumin levels) are better predictors of risk than isolated values and support the use of composite indices, such as the lactate-to-albumin ratio [7,8,9,10,11]. Together, these observations indicate that the principal limitation of current albumin strategies is not the molecule itself, but the lack of a physiologically grounded framework guiding its clinical use. Together, these limitations indicate the need to move beyond static concentration thresholds toward physiology-informed decision making.
Traditional reliance on serum albumin concentration fails to account for the physiological factors that determine the effective circulating pool of albumin in sepsis. Serum levels are influenced by capillary leakage, dilution due to resuscitation, altered synthesis, and catabolism; thus, they do not accurately reflect the intravascular albumin available to maintain oncotic pressure and transport functions [12,13]. In sepsis, the redistribution of albumin from plasma to the interstitium is significant, driven by endothelial barrier failure and impaired lymphatic return, resulting in a decrease in the measured concentration even when the total body albumin remains unchanged, thereby supporting a mass-based assessment [14,15]. Direct kinetic data indicate that the transcapillary escape rate of albumin increases in sepsis and critical illness, with a portion of the infused albumin exiting the vascular space within hours, thus limiting sustained plasma expansion [16,17]. Consequently, adjusting therapy based on the intravascular albumin mass (IVAM), calculated as albumin concentration multiplied by plasma volume, more accurately captures the interaction between plasma volume, permeability, and albumin kinetics than static concentration alone [18]. Clinically, dynamic trajectories of albumin (decreasing or persistently low) are more prognostic than single values, underscoring the necessity for a quantitative, trajectory-aware IVAM approach rather than threshold-based dosing by concentration [7,8].
The δ sepsis phenotype has been identified as a consistent, high-risk clinical cluster characterized by shock, elevated lactate levels, and the poorest short-term outcomes among the four commonly observed phenotypes (α/β/γ/δ) identified in extensive derivation and validation cohorts [19,20,21,22]. Biologically, the δ phenotype is associated with microvascular and endothelial dysfunction, exhibiting features such as capillary leakage, glycocalyx degradation, impaired vasoreactivity, and microthrombosis, which are linked to organ failure and mortality in sepsis [23,24,25,26]. This endotheliopathy is reflected by elevations in endothelial injury biomarkers, such as angiopoietin-2 and syndecan-1, which correlate with vasoplegia, shock, and mortality [27,28,29,30,31,32,33]. Clinically, the δ phenotype features profound hypoalbuminemia and hyperlactatemia, which are markers of capillary leakage and microcirculatory failure, providing a biologically coherent model for endothelial-targeted therapeutic strategies. However, most albumin trials have intervened after this δ-type phenotype is established, when endothelial failure and capillary leak are already advanced, potentially explaining the limited and inconsistent therapeutic benefit observed in unselected populations. Hemodynamic monitoring studies in intermediate-care and emergency sepsis cohorts indicate that vasoplegia, defined by reduced systemic vascular resistance and increased cardiac output, often arises early, before overt shock or capillary leaks develop. Identifying these pre-δ vasoplegic states through noninvasive impedance cardiography and biochemical markers, such as albumin, lactate, and IVAM, may offer a physiological window for endothelial protection and prevention of δ-type progression.
Accordingly, the central scientific gap addressed by this review is the absence of a physiology-based framework that links early hemodynamic vasoplegia, endothelial integrity, and albumin kinetics to guide the timing and indication of albumin therapy in sepsis. Building on evidence that endothelial dysfunction and vasoplegia precede overt capillary leakage and δ-type progression, this narrative review aims to establish a conceptual and diagnostic framework for early, physiology-guided albumin therapy in sepsis. We propose integrating hemodynamic phenotyping, including noninvasive hemodynamic monitoring data and Sequential Organ Failure Assessment cardiovascular scoring (SOFA-CV), with biochemical and mass-based indicators such as serum albumin, lactate, and IVAM. IVAM provides a quantitative estimate of the effective circulating albumin pool and serves as a marker of vascular integrity and fluid distribution in the body. By linking early vasoplegic states to measurable hemodynamic and biochemical trajectories, this framework seeks to bridge molecular pathophysiology with bedside fluid management, supporting the development of precision-guided, endothelial-protective albumin therapy for sepsis.
This review progresses from sepsis phenotypes and endothelial pathophysiology to their biochemical and hemodynamic clinical expression, with particular emphasis on early vasoplegia preceding overt shock and capillary leak. These elements are then integrated with IVAM and noninvasive hemodynamic monitoring to propose a physiology-guided framework for the timing and indication of norepinephrine (NE) and albumin therapy.
2. Methods
This narrative review aims to synthesize contemporary mechanistic, translational, and clinical evidence regarding albumin dynamics in sepsis and propose diagnostic criteria and a conceptual framework for early, physiology-guided albumin therapy. A literature review was conducted using PubMed (National Library of Medicine, National Institutes of Health (NIH), Bethesda, MD, USA) without temporal restrictions. The search terms included combinations of “sepsis,” “albumin,” “endothelial dysfunction,” “capillary leak,” “intravascular albumin mass,” “transcapillary escape,” “phenotype*,” and “lactate/albumin ratio.” Priority was given to systematic reviews, randomized controlled trials, cohort studies, and key mechanistic investigations.
The findings were synthesized according to the principles of integrative narrative analysis, emphasizing the coherence among clinical phenotypes (α–δ clusters), endothelial pathophysiology, and quantitative albumin kinetics. Recent hemodynamic data from intermediate care and emergency sepsis cohorts were also considered to contextualize the early vasoplegic states detected by noninvasive impedance cardiography. Diagnostic propositions for the δ phenotype, early vasoplegic profiles, and IVAM were formulated through an iterative synthesis of empirical data, physiological modeling studies, and expert consensus publications.
The methodological approach adhered to the Scale for the Assessment of Narrative Review Articles (SANRA) checklist for transparency in aims, the literature search, referencing, evidence synthesis, and logical presentation [34].
3. Results
3.1. Reproducibility and Clinical Characteristics of the Sepsis Phenotypes
Latent class analyses of large sepsis cohorts have consistently identified four reproducible host-response phenotypes, designated α, β, γ, and δ, each characterized by distinct clinical and biochemical profiles. These phenotypes have demonstrated robust internal and external validity across independent datasets, geographic regions, and analytical approaches, including machine learning–based clustering methods [20,22,35]. Collectively, they describe a graded spectrum of disease severity and host response heterogeneity in adult sepsis.
The α, β, and γ phenotypes represent progressively increasing burdens of comorbidity, inflammation, and organ dysfunction. In contrast, the δ phenotype occupies the extreme end of this spectrum and is characterized by severe systemic derangement, including pronounced hepatic, renal, and hematologic dysfunction, heightened inflammatory activation, elevated lactate levels, and a high requirement for vasopressor support. Accordingly, patients classified within the δ phenotype consistently exhibit the highest short-term mortality, with 28-day mortality rates exceeding 30%, even after adjustment for age, comorbidities, and illness severity [20,22]. The defining clinical features and outcome gradients of the four phenotypes are summarized in Table 1.

Table 1.
Clinical and outcome features of the four α/β/γ/δ sepsis phenotypes (adapted from Seymour et al. [36], Zhao et al. [22], and Boussina et al. [20].
Importantly, although transitions between phenotypes may occur early in the course of sepsis, longitudinal and externally validated dynamic phenotyping studies indicate that the δ phenotype represents a largely terminal trajectory, with limited reversibility once established [20,22]. β and γ phenotypes frequently precede δ-type deterioration, suggesting a progression rather than discrete, unrelated categories. This observation supports the concept of a biological continuum in which escalating inflammatory activation and circulatory dysfunction culminate in the δ endotype.
At the biological level, δ-type sepsis is consistently associated with markers of endothelial injury and capillary leakage, including elevated angiopoietin-2 (Ang-2) and syndecan-1 concentrations, alongside clinical vasoplegia and increased vasopressor dependence [27,28,30,37,38,39,40,41,42,43,44]. These features distinguish δ not merely as a severe clinical phenotype, but as a vasoplegic, microvascular failure–dominated endotype. Figure 1 schematically illustrates this continuum, linking progressive inflammatory activation to endothelial breakdown, capillary leakage, and circulatory collapse. Together, these findings position the δ phenotype as the final common pathway of endothelial failure in sepsis and provide a clinically relevant framework for subsequent mechanistic and therapeutic considerations.
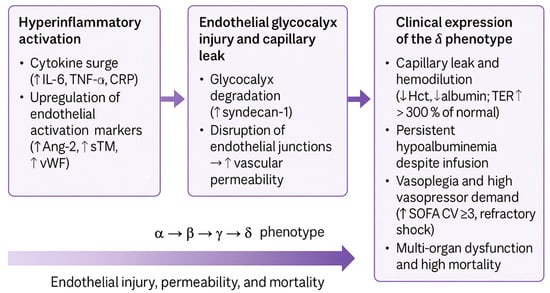
Figure 1.
Mechanistic continuum of endothelial and microvascular injury in δ sepsis phenotype. Abbreviations: Ang-2, angiopoietin-2; Hct, hematocrit; IL-6, interleukin-6; SOFA-CV, Sequential Organ Failure Assessment cardiovascular; sTM, soluble thrombomodulin; TER, transcapillary escape rate; vWF, von Willebrand factor; TNF-α, tumor necrosis factor-α; ↑ denotes an increase, and ↓ denotes a decrease in the indicated parameter.
Across studies, the most consistent finding is the reproducible α/β/γ/δ phenotypic structure and its associated mortality gradient, with δ emerging as a robust vasoplegic endotype across cohorts and analytical methods. In contrast, therapeutic trials in sepsis, including randomized studies of albumin and fluid strategies, have produced heterogeneous and often neutral results when applied to unselected populations. This discrepancy indicates that, although sepsis phenotypes robustly capture underlying biology, they have not yet been systematically incorporated into therapeutic decision-making or trial design. As a result, δ-type sepsis currently serves primarily as a prognostic construct rather than a treatment-defining category, underscoring a translational gap that motivates the mechanistic analyses that follow.
3.2. Mechanistic Continuum of Endothelial and Microvascular Injury
Following the clinical and phenotypic definition of δ-type sepsis, accumulating evidence delineates a mechanistic continuum of endothelial and microvascular injury that explains its characteristic vasoplegia, capillary leak, and poor outcomes. Comparative biomarker studies have demonstrated that δ- or hyperinflammatory phenotypes exhibit the highest levels of endothelial damage markers, such as angiopoietin-2 (Ang-2), syndecan-1, soluble thrombomodulin (sTM), and von Willebrand factor (vWF), relative to the α–γ phenotypes [28,37,38,39]. These biomarkers reflect progressive glycocalyx degradation and disruption of endothelial junctions, leading to increased vascular permeability and capillary leakage.
Across studies, endothelial biomarkers converge on a common pathophysiological signal rather than representing isolated mechanisms. Angiopoietin-2, syndecan-1, soluble thrombomodulin, and von Willebrand factor are variably elevated across cohorts, yet consistently associate δ-type sepsis with endothelial activation, glycocalyx disruption, vasoplegia, and adverse outcomes. Despite differences in molecular origin and kinetics, these markers reflect a shared process of endothelial barrier failure, supporting the interpretation of δ-type sepsis as a unified microvascular dysfunction endotype rather than a collection of distinct biomarker-defined entities.
Mechanistically, this evolution follows a triphasic sequence (Figure 1).
- Hyperinflammatory activation: cytokine surge (increases in IL-6, TNF-α, and CRP levels) and upregulation of endothelial activation markers (increases in Ang-2, sTM, and vWF levels);
- Endothelial glycocalyx injury and capillary leak: elevated syndecan-1 and junctional disruption causing plasma extravasation
- Clinical expression of the δ phenotype: Hemodilution (decrease in Hct) and albumin (TER > 300% of normal), persistent hypoalbuminemia despite infusion, vasoplegia (SOFA-CV ≥ 3), and multiorgan dysfunction.
Elevated Ang-2 and syndecan-1 levels correlate with vasopressor requirements and mortality [27,30,40,45], confirming that endothelial activation contributes directly to hemodynamic instability. Observational data further demonstrate that rapid fluid resuscitation accentuates hemodilution, whereas a positive fluid balance correlates with decreased hematocrit and worse outcomes [46,47,48,49,50]. Together, these findings delineate the mechanistic transition from hyperinflammatory endothelial activation to vascular barrier failure and refractory vasoplegia, which are the defining features of δ-type sepsis.
However, comparison across biomarker studies reveals important limitations. Cut-off values and sampling time points vary widely, most analyses rely on single measurements rather than trajectories, and none of the markers has demonstrated sufficient specificity or feasibility to guide real-time therapeutic decisions. Consequently, endothelial biomarkers have remained primarily prognostic rather than actionable, underscoring the need for integrative strategies that combine biological signals with physiological and hemodynamic context.
3.3. Biochemical and Hemodynamic Correlates of δ-Type Sepsis
The δ phenotype translates this pathophysiology into a distinct biochemical and circulatory profile characterized by hypoalbuminemia, hyperlactatemia, elevated lactate-to-albumin (L/A) ratio, and vasoplegic shock.
Profound hypoalbuminemia (<2.5 g/dL) is a hallmark of δ-type sepsis and an independent predictor of short- and long-term mortality, even after adjustment for confounders [8,51,52,53,54,55,56]. Persistent hypoalbuminemia during supplementation indicates ongoing transcapillary albumin escape rather than nutritional deficit [47,57,58].
In a prospective cohort of 254 septic patients classified by host-response phenotype, Turcato et al. [59] found the lowest albumin concentrations (mean 2.2 g/dL) and highest 30-day mortality (45%) in δ patients, whereas α-type patients exhibited higher albumin levels (2.8 g/dL) and markedly lower mortality (3.6%). Each 1 g/dL increase in albumin concentration corresponded to a 63% reduction in mortality (hazard ratio 0.37; 95% CI 0.25–0.56; p < 0.001).
Lactate >3 mmol/L independently predicts shock, multiorgan failure, and mortality [60,61,62,63,64]. The L/A ratio (often >1.0–1.5 in δ-like patients) integrates metabolic stress and vascular leakage, outperforming lactate alone in terms of prognostic accuracy [10,65,66,67,68,69,70]. Thus, this ratio captures both microcirculatory failure and endothelial permeability within a single pragmatic index.
δ-type patients typically display severe vasodilation, hypotension, and high NE requirements despite adequate preload. Elevated Ang-2 and syndecan-1 levels correlate with SOFA-CV ≥ 3 and refractory shock [30,40].
Collectively, these biochemical and hemodynamic markers delineate δ-type sepsis as a syndrome characterized by vascular leakage and microcirculatory collapse in the host. Persistent hypoalbuminemia and elevated lactate levels reflect capillary hyperpermeability and impaired perfusion, whereas the L/A ratio integrates these processes. Combined with vasoplegic profiles and SOFA-CV elevation, this composite signature provides a reproducible, bedside representation of advanced endothelial failure. Therefore, the δ phenotype represents the final stage of a continuum in which progressive endothelial dysfunction is linked to inflammation, vascular permeability, and mortality.
Across studies, serum lactate and albumin provide complementary but incomplete information when considered in isolation. Lactate consistently reflects metabolic stress and impaired perfusion, whereas hypoalbuminemia captures illness severity and vascular permeability, but both are influenced by timing, fluid resuscitation, and comorbid conditions. Comparative analyses show that the lactate/albumin (L/A) ratio integrates these dimensions and more consistently stratifies risk across emergency, intermediate-care, and intensive-care settings, often outperforming lactate alone [65,66,67,68]. However, despite its reproducible prognostic value, the lactate/albumin ratio reflects a composite biochemical state and lacks sufficient physiological specificity to distinguish reversible vasoplegia from established capillary leak, even when assessed serially. This limits its utility for guiding dynamic therapeutic decisions.
3.4. Vasoplegia and Intravascular Albumin Mass (IVAM) in Sepsis
Recent findings from a series of prospective studies conducted in the Medical Intermediate Care Unit at Santorso Hospital underscore the diagnostic and pathophysiological significance of IVAM as an integrated marker of vascular integrity, endothelial permeability, and fluid distribution in sepsis. Unlike static serum albumin concentrations, IVAM represents the total quantity of albumin circulating within the plasma compartment and, therefore, captures the effective oncotic capacity of the intravascular space and the degree of redistribution caused by capillary leakage [71].
Early hemodynamic profiling of community-acquired sepsis at Santorso demonstrated that a substantial subgroup of patients (≈20–25%) already presented on admission with vasoplegic hemodynamics, characterized by low systemic vascular resistance and preserved or increased cardiac output, despite mean arterial pressure >65 mm Hg [72]. This pre-shock vasoplegic state, detectable by noninvasive hemodynamic monitoring, frequently precedes overt hypotension and defines a potential window for endothelial protective interventions. In parallel, hypoalbuminemia was identified as an early, independent mortality driver in the same setting: decision tree analysis in 254 consecutive sepsis cases identified admission serum albumin ≤2.3 g/dL as the root node for 30-day mortality [73]. Moreover, a separate study showed that each 500–1000 mL crystalloid bolus produced a mean decrease of 0.28 g/dL in serum albumin and that the magnitude of this post-bolus albumin fall independently predicted death [74]. These findings indicate that early vasoplegia and fluid-induced hemodilution accelerate the decline of the circulating albumin pool, predisposing patients to subsequent endothelial leaks and δ-type progression.
Building upon these results, IVAM was quantified in 247 patients with community-acquired sepsis admitted to the Santorso IMCU using the following equation:
Plasma volume was estimated using validated anthropometric equations corrected for hematocrit (×0.91) [71]. Sequential measurements of albumin, hemoglobin, and hematocrit over five days allowed the calculation of IVAM trajectories and net albumin loss (NAL), defined as the daily change in IVAM, representing the balance between extravasation and intravascular refill.
3.4.1. Temporal Changes in IVAM and Association with Fluid Balance and Clinical Severity
At admission, the mean IVAM was 83 g (SD 21) and declined to 75 g (SD 20) by day 5, corresponding to a mean NAL of 8.9 g (SD 14.5) in the absence of colloid administration. The steepest decline occurs within the first 72 h, coinciding with the phase of maximal capillary leakage and fluid redistribution [71]. IVAM was inversely correlated with cumulative fluid balance (Spearman ρ = −0.29 to −0.17, p < 0.01 across days 1–5). In a repeated-measures generalized estimating equation (GEE) model adjusted for age, comorbidities, body mass index, and severity scores (SOFA, APACHE II), each 1% increase in cumulative fluid balance predicted a 1.08 g decrease in IVAM (95% CI −1.33 to −0.84; p < 0.001), independent of baseline severity. These data confirm that IVAM serves as a mass-based indicator of fluid effectiveness, distinguishing true intravascular repletion from third-space sequestration and interstitial fluid overload.
3.4.2. Prognostic Significance and Physiologic Interpretation
Non-survivors exhibited significantly lower IVAM values both at baseline (72.6 ± 15.4 g) and throughout the follow-up period than survivors (85.1 ± 20.8 g; p < 0.001). Each 1 g increase in IVAM reduced 30-day mortality by 2.1% (OR 0.98; 95% CI 0.97–0.99; p < 0.001). A higher cumulative NAL (≥10 g) was also associated with excess mortality and a greater positive fluid balance [71].
The temporal decline in IVAM reflects three interdependent mechanisms:
- Albumin extravasation across the damaged glycocalyx [41,47],
- plasma volume expansion from fluid therapy, causing hemodilution [46], and
- impaired lymphatic refill, limiting the recovery of circulating albumin [75,76,77].
A persistent IVAM decline indicates capillary barrier failure, whereas stabilization or recovery indicates endothelial repair. Because IVAM can be calculated from routine laboratory variables, it provides a practical, noninvasive measure of vascular competence and fluid distribution, suitable even in non-ICU environments.
3.4.3. Integration with Early Hemodynamics and Therapeutic Implications
Across the Santorso sepsis studies [71,78], declining IVAM paralleled the development of vasoplegia and positive fluid balance, confirming that endothelial failure is a dynamic process rather than a static phenotype (Figure 2). Early noninvasive hemodynamic monitoring-identified vasoplegic patients with moderate IVAM reduction but without overt shock may represent an actionable stage for endothelial protective therapy. In this context, cautious albumin supplementation to maintain oncotic stability and low-dose NE to restore effective capillary pressure may prevent progression toward δ-type capillary leak and multiorgan dysfunction.
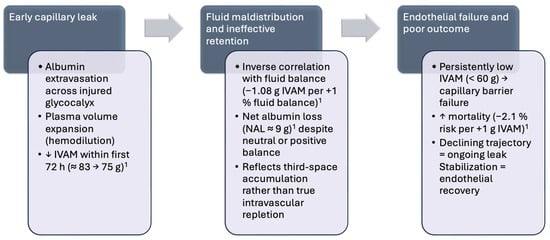
Figure 2.
Temporal dynamics and prognostic implications of intravascular albumin mass (IVAM) decline in sepsis: Transition from reversible endothelial dysfunction to decompensated capillary leak. Early hemodynamic heterogeneity and vasoplegia observed in intermediate-care sepsis cohorts [72,74] mark the pre-δ phase preceding this decline. Abbreviations: IVAM, intravascular albumin mass; NAL, net albumin loss; h, hours; ↑ denotes an increase, and ↓ denotes a decrease in the indicated parameter. 1 IVAM data were derived from Wiedermann et al. [71].
This translational approach reframes IVAM not only as a diagnostic and prognostic biomarker but also as a quantitative tool for the timing and titration of endothelial-targeted therapy in sepsis.
4. Discussion
4.1. The δ Phenotype: Endothelial Failure as the Terminal Expression of Sepsis
A key inconsistency exists between neutral randomized trials of albumin in sepsis and evidence linking albumin kinetics to outcomes. Trials used albumin late or targeted static serum levels, without considering endothelial permeability [1,3,79]. Observational studies show hypoalbuminemia and vasoplegia reflect endothelial failure rather than volume deficit [27,30,59,71]. This suggests timing, patient selection, and vascular state drive trial heterogeneity rather than colloid choice.
This study supports the notion that δ-type sepsis is a distinct vascular-leak/vasoplegic endotype characterized by endothelial injury, microvascular dysfunction, and adverse outcomes. Across independent adult cohorts, the α/β/γ/δ classification is reproducible and biologically coherent, with δ-type patients showing the most severe hepatic, renal, and hematologic impairment, the highest inflammatory activation, and the greatest need for vasopressors. At the bedside, endothelial barrier failure is indicated by hypoalbuminemia [8,51,52,53,54,55,56,59], hyperlactatemia [60,61,62,63,64], an elevated lactate/albumin ratio [10,65,66,67,68,69,70,80], and higher SOFA-CV subscores [30,33,40,43,44,51].
Consistent with this, prospective impedance-cardiography work demonstrates substantial hemodynamic heterogeneity in community-acquired sepsis: distributive (vasoplegic) profiles with low systemic vascular resistance and high cardiac output are present in >20% of cases, often despite preserved mean arterial pressure, underscoring the need for phenotype-based, endothelial-guided assessment beyond conventional vital sign strata [72].
Recent prospective studies have reinforced the pathophysiological axis linking endotheliopathy to outcomes. Turcato et al. showed lower albumin concentrations and higher mortality in δ-type patients (~2.2 g/dL; 30-day mortality 45%), with serial albumin levels independently predicting survival [59]. Complementarily, Wiedermann et al. quantified IVAM, demonstrating a decline from 83 g to 75 g over five days and an inverse association with cumulative fluid balance, connecting albumin extravasation to fluid maldistribution and mortality [71]. Together, these data justify moving “upstream” from established δ-type leaks to the early identification of vasoplegia and preemptive endothelial stewardship, which motivates the following rationale for early NE paired with targeted albumin.
Although endothelial injury markers such as angiopoietin-2 and syndecan-1 are consistently associated with disease severity and mortality [27,30,40,45], their clinical translation remains limited. Variability in assay availability, timing, and lack of validated thresholds restrict real-time use, and single biomarkers fail to capture the dynamic interplay between vascular tone, permeability, and fluid responsiveness. These constraints explain why advances in endothelial biology have not yet translated into actionable bedside algorithms.
The directionality between positive fluid balance and declining IVAM remains unresolved. Two mechanisms exist: liberal fluid administration may worsen hemodilution and increase filtration, accelerating IVAM decline; alternatively, early IVAM reduction may indicate endothelial barrier failure, causing fluid maldistribution. The therapeutic approach addresses this through early NE to stabilize vascular tone and limited albumin use. IVAM trajectories reflect the interaction between vascular permeability, fluid administration, and circulatory stability.
4.2. Early Endothelial Protection: Norepinephrine and Albumin as Complementary Preventive Strategies
Early NE initiation aligns with a physiology-guided framework by quickly restoring perfusion pressure, curbing excessive crystalloid exposure, and potentially preventing the transition from pre-δ vasoplegia to overt capillary-leak states. Across randomized trials, meta-analyses, and contemporary reviews, early NE is consistently linked to faster achievement of target mean arterial pressure (MAP), reduced fluid loads, better shock control, and signals of lower short-term mortality [81,82,83,84,85,86].
The direct protection of endothelial or glycocalyx from NE remains unclear. Experimental and translational studies suggest mixed immunomodulatory effects [87] and raise concerns that higher catecholamine exposure may aggravate glycocalyx shedding [88]. Thus, the likely benefit is indirect: earlier vasopressor administration limits positive fluid balance and hemodilution, thereby mitigating the drivers of albumin loss and IVAM decline [82,85,86].
Beyond its oncotic action, albumin exerts anti-inflammatory and endothelial glycocalyx–stabilizing effects that fit the prevention strategy for capillary leak progression. Mechanistic and translational studies have shown reduced cytokine signaling and oxidative nitrosative stress, preservation of the glycocalyx, and inhibition of permeability mediators [89,90,91,92]. Small human studies have reported improved endothelial function with albumin infusion in septic shock [93], and Albumin Italian Outcome Sepsis (ALBIOS) biomarker sub-analyses have linked albumin handling to endothelial injury signatures [41]. A recent proof-of-concept trial in patients with sepsis in the emergency department suggested better peripheral perfusion with albumin than with saline, consistent with microvascular support [5].
Large sepsis RCTs and meta-analyses remain mixed on hard outcomes and consistent reductions in permeability, underscoring context- and timing-dependence [1,2,3]. Within the present framework, albumin’s non-oncotic effects should be leveraged early, paired with low-dose NE in noninvasive hemodynamic monitoring-confirmed vasoplegia, to limit fluid load, support oncotic forces, and potentially blunt endothelial injury, while recognizing that direct prevention of capillary leak and hypoalbuminemia is suggestive but not definitive [5,89,90]. NE is framed as a hemodynamic enabler of endothelial stewardship, initiated early in documented vasoplegia, paired with judicious albumin, and titrated to minimize fluid accumulation and catecholamine burden. In noninvasive hemodynamic monitoring-defined vasoplegia, characterized by low SVR with preserved high cardiac output, early low-dose NE can normalize the transcapillary hydrostatic gradient, while albumin supports oncotic forces, a pairing that is physiologically coherent with our δ-phenotype prevention strategy.
Literature suggests molecular markers and biochemical indices alone cannot guide individualized sepsis therapy. Failure to consider hemodynamic phenotype, endothelial permeability, and albumin kinetics remains a critical gap. Integrating circulatory state with vascular integrity indicators may bridge biological insights and therapeutic targeting, particularly for preventing δ-type endothelial failure.
4.3. Dynamic Endothelial-State Monitoring for Guiding Albumin Therapy
Preventing the progression to δ-type sepsis requires continuous assessment of the endothelial state rather than reactive correction of established leakage. Dynamic monitoring of biochemical, hemodynamic, and albumin-based parameters can distinguish early, potentially reversible vasoplegia from irreversible vasoplegia. This monitoring framework defines the indications for early, physiology-guided albumin and NE use and the exclusion criteria once endothelial failure becomes dominant.
The framework relies on serial assessment of parameters with varying feasibility across settings. Serum lactate and albumin are routinely available and can be reassessed during early sepsis. Non-invasive hemodynamic monitoring enables repeated SVR estimation without invasive instrumentation, but has limitations from motion artifacts and arrhythmias. A monitoring bundle combining non-invasive SVR trends with laboratory markers and clinical indices emphasizes concordant trajectories over single thresholds. This approach balances physiological insight with applicability and needs validation.
4.3.1. Early Recognition of Vasoplegia in Pre-δ Sepsis
Non-invasive hemodynamic monitoring has demonstrated that more than one-fifth of patients with community-acquired sepsis present with distributive hemodynamic profiles despite maintained mean arterial pressure, low systemic vascular resistance (SVR), and preserved or elevated cardiac output [72]. These “pre-δ” vasoplegic states indicate a loss of vascular tone preceding overt shock and offer a physiological window for endothelial protective intervention. Early NE initiation restores perfusion pressure and reduces fluid overload, whereas low-dose albumin supplementation sustains oncotic forces and may attenuate endothelial activation [81,82,83,84,85,86,87,88].
Early NE’s proposed benefit in this framework is functional rather than based on proven endothelial protection. Early vasopressor use restores perfusion pressure and limits fluid administration, reducing hemodilution and endothelial stress. Catecholamines’ direct endothelial effects are complex and may contribute to glycocalyx shedding [94,95]. Thus, endothelial “protection” from early NE results from hemodynamic stabilization and fluid-sparing resuscitation, not direct cellular protection.
4.3.2. Biochemical and Hemodynamic Indicators of Endothelial Dysfunction
As endothelial permeability increases, characteristic biochemical trajectories emerge.
- Serum albumin < 2.5 g/dL [8,51,52,53,54,55,56,59],
- serum lactate > 3 mmol/L [60,61,62,63,64],
- lactate/albumin ratio > 1.0–1.5, integrating metabolic stress and vascular leak [10,65,66,67,68,69,70,80], and
- SOFA-CV subscore ≥ 3, indicating vasoplegic shock and high NE dependence [30,33,40,43,44,51].
In combination, these markers delineate a threshold beyond which albumin administered for volume or oncotic support is likely to redistribute extravascularly, contributing to interstitial edema rather than circulatory stabilization of the patient. These hemodynamic and biochemical features allow a pragmatic, operational distinction between a “pre-δ” vasoplegic state and established δ-type sepsis. The pre-δ state is characterized by low systemic vascular resistance with preserved or elevated cardiac output and limited shock severity, accompanied by early or evolving biochemical risk signals, but without clear evidence of advanced capillary leak or high-dose vasopressor dependence.
In contrast, progression to δ-type sepsis is marked by concordant deterioration across domains, including SOFA-CV ≥ 3, escalating NE requirements, and biochemical thresholds indicating established endothelial barrier failure, beyond which albumin is unlikely to remain intravascular. This operational distinction emphasizes trend concordance rather than single thresholds and is intended as a hypothesis-generating framework for future prospective validation.
4.3.3. Dynamic Exclusion and Reintroduction Framework for Albumin Therapy
A declining IVAM quantifies the circulating albumin pool and reflects endothelial barrier integrity [71]. IVAM < 70 g indicates intravascular depletion, while values < 60 g or negative trajectories indicate active leakage and non-retention of the infused protein. In prevention, downward IVAM trends with rising lactate or positive fluid balance signal δ-type leak physiology, prompting vasopressor stabilization and anti-inflammatory control. Conversely, plateauing or rising IVAM, declining lactate levels, and SVR stabilization suggest endothelial recovery and mark the window for albumin supplementation.
Rather than being a fixed rule, this approach defines the δ-phenotype constellation as a functional exclusion zone for albumin therapy. Infusion merely augments the interstitial protein burden without improving perfusion. Recognizing this transition through serial IVAM, lactate, and noninvasive SVR monitoring allows for the timely withdrawal of albumin therapy and supports physiology-guided, endothelial-protective management. Phenotypic criteria should serve not only as diagnostic descriptors of advanced sepsis but also as boundaries between reversible vasoplegia and irreversible leaks. When integrated with IVAM and noninvasive hemodynamic monitoring, these parameters enable real-time therapy adjustment by initiating albumin administration when the barrier remains responsive and withholding it once capillary permeability dominates (Table 2).

Table 2.
Clinical interpretation of concurrent albumin and intravascular albumin mass (IVAM) trajectories for endothelial-state–adapted therapy.
Within this model, albumin is not recommended during active capillary leak phases, characterized by declining IVAM, increasing vasopressor needs, and endothelial barrier failure. During this “dynamic exclusion zone,” infused albumin may redistribute into the interstitial space without improving circulation. Albumin use is limited to pre-leak phases when intravascular retention remains intact and recovery phases showing IVAM stabilization or increase.
The combined assessment of serum albumin and IVAM provides a dynamic means of monitoring endothelial permeability and therapeutic response in sepsis. While albumin concentration reflects both dilutional and inflammatory effects, IVAM captures the effective oncotic reservoir within the circulation [71]. Monitoring these trajectories differentiates active capillary leakage from hemodilution or endothelial recovery, providing clinical guidance.
This interpretation is supported by evidence from kinetic studies. Hahn [96] analyzed 86 infusions of 20% albumin and showed that plasma volume and colloid osmotic pressure remained stable in subjects with intact endothelium but declined rapidly under inflammatory conditions, paralleling the loss of IVAM. These findings confirm that IVAM trends mirror endothelial permeability, which is stable in healthy individuals, transient in early sepsis, and progressively impaired in δ-type physiology.
Serial IVAM monitoring, combined with lactate and noninvasive monitoring-derived systemic vascular resistance, provides a practical physiology-based method for identifying the “window of endothelial opportunity” and preventing the progression toward irreversible capillary leak.
Beyond phenotype classification, individualized hemodynamic trajectories should be interpreted in real time. While phenotype-based approaches (e.g., δ-type vasoplegia) help to conceptualize risk, clinical decisions must increasingly rely on dynamically changing, objectively measured parameters. Stroke volume, cardiac power, and SVR can fluctuate within hours depending on therapy and response, which explains why they do not consistently emerge as static mortality predictors. The essential message is that volume and vasopressor management should never be based on clinical impression alone, but on measurable, reproducible hemodynamic data, even if the available noninvasive tools are not perfect.
4.4. Future Directions and Study Implications
The neutral outcomes of the ARISS trial (Albumin Replacement Therapy in Septic Shock (ARISS) trial (NCT03869385) [79] demonstrated that maintaining static serum albumin targets does not enhance outcomes in unselected septic shock populations. Although concentrations of ≥30 g/L were sustained for up to 28 days, mortality rates at 28, 60, and 90 days did not significantly differ from those of crystalloid-treated controls (31.0% vs. 38.1%, 38.9% vs. 45.2%, and 43.3% vs. 45.9%, respectively) [79]. These findings emphasize the limitations of concentration-based protocols and underscore the necessity of dynamic, physiology-informed approaches that incorporate endothelial permeability, hemodynamic status, and IVAM rather than relying solely on serum levels. The ARISS trial illustrates the distinction between static and physiology-guided strategies. By targeting fixed serum albumin concentrations, ARISS evaluated biochemical correction without regard to timing, hemodynamic state, or endothelial permeability. In contrast, the present framework focuses on identifying a physiological window defined by early vasoplegia and preserved intravascular retention. This contrast emphasizes that the key issue is not albumin use per se, but when, in whom, and under which physiological conditions albumin is administered.
The current synthesis introduces a conceptual framework for phenotype- and IVAM-guided albumin therapy, prioritizing early prevention of endothelial failure over late correction of leakage. By incorporating noninvasive hemodynamic monitoring alongside serial assessments of lactate, albumin, and IVAM, future research may identify patients in reversible vasoplegic states prior to the onset of δ-type leaks. During this initial phase, the combined administration of low-dose NE and guided albumin infusion may stabilize the transcapillary gradient, bolster oncotic forces, and prevent progression to irreversible endothelial failure.
Future clinical trials should therefore move beyond threshold correction and test state-adapted interventions based on measurable endothelial and hemodynamic parameters. Key design features may include:
- Patient stratification by hemodynamic phenotype (noninvasive monitoring-defined vasoplegia) and biochemical markers (lactate, albumin, L/A ratio, IVAM).
- Dynamic inclusion criteria focused on pre-δ or early δ stages rather than fully established capillary leak.
- Serial monitoring of IVAM trajectories and vascular biomarkers as indicators of response.
- Endpoints combining mortality with physiologic recovery metrics (e.g., fluid balance normalization, lactate clearance, SOFA-CV improvement).
From a translational perspective, such studies would clarify whether IVAM-guided, endothelial-state–adapted albumin therapy improves retention, reduces vasoplegia, and enhances recovery compared with static concentration-based dosing.
Several limitations of the present synthesis must be acknowledged. The framework is conceptual, derived from an integrative analysis of prospective and mechanistic data rather than from a unified interventional cohort. IVAM estimation, while physiologically meaningful, relies on anthropometric plasma volume formulas that may introduce variability across patient populations. Furthermore, endothelial biomarkers were not uniformly available in the referenced datasets, and external validation in multicenter cohorts will be required before clinical implementation.
Nevertheless, this model offers a measurable, hypothesis-generating platform for precision fluid and albumin therapy in sepsis. Analogous to findings in cirrhosis, where albumin improves endothelial stability, circulatory volume, and inflammation [97], a physiology-guided approach in sepsis may identify patients who benefit most when albumin is administered at the right moment, in the right dose, and in the right vascular state. Future randomized controlled trials applying these principles are warranted to determine whether early, phenotype-adapted endothelial protection can alter the trajectory of sepsis outcomes.
5. Conclusions
This communication reframes albumin use in sepsis as an early preventive strategy aimed at preserving endothelial integrity rather than correcting late-stage capillary leak. Early recognition of vasoplegia through noninvasive hemodynamics and concurrent monitoring of IVAM, lactate, and fluid balance may help distinguish reversible from irreversible endothelial dysfunction. Within this physiology-guided framework, early low-dose NE can restore perfusion pressure and limit fluid overload, while timely albumin administration may not only stabilize oncotic forces but also exert direct endothelial-protective effects, attenuating inflammation and glycocalyx degradation to prevent progression toward δ-type leak physiology. Applying a dynamic exclusion concept—avoiding albumin during active leakage and reintroducing it when IVAM stabilizes—could optimize retention and therapeutic benefit. Future phenotype- and state-adapted trials integrating noninvasive hemodynamic monitoring, IVAM, and endothelial biomarkers are needed to test whether this precision approach improves vascular integrity and clinical outcomes in sepsis. The proposed framework is hypothesis-generating and requires prospective validation before clinical implementation.
Author Contributions
Conceptualization, C.J.W., A.Z., and G.T.; methodology, C.J.W., A.Z., and G.T.; investigation, C.J.W.; writing—original draft preparation, C.J.W.; writing—review and editing, C.J.W., A.Z., and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
ChatGPT-4 (OpenAI, San Francisco, CA, USA; accessed 24 October 2025) was used to support language editing, clarity improvement, and document structuring. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
G.T. and A.Z. declare no conflicts of interest. C.J.W. has received fees for speaking and/or consulting from Grifols and CSL Behring.
Abbreviations
The following abbreviations are used in this manuscript:
| Ang-2 | Angiopoietin-2 |
| ALBIOS | Albumin Italian Outcome Sepsis |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| ARISS | Albumin Replacement Therapy in Septic Shock |
| CI | Confidence interval |
| CRP | C-reactive protein |
| GEE | Generalized estimating equation |
| Hct | Hematocrit |
| ICU | Intensive care unit |
| IL-6 | Interleukin-6 |
| IVAM | Intravascular albumin mass |
| L/A ratio | Lactate/albumin ratio |
| MAP | Mean arterial pressure |
| NAL | Net albumin leakage |
| NE | Norepinephrine |
| OR | Odds ratio |
| RCTs | Randomized controlled trials |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SD | Standard deviation |
| SOVA-CV | Sequential Organ Failure Assessment cardiovascular subscore |
| SVR | Systemic vascular resistance |
| sTM | Soluble thrombomodulin |
| TER | Transcapillary escape rate |
| TNF-α | Tumor necrosis factor-α |
| vWF | Von Willebrand factor |
References
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Chen, Q.H.; Xie, J.F.; Pan, C.; Liu, S.Q.; Huang, L.W.; Yang, C.S.; Liu, L.; Huang, Y.Z.; Guo, F.M.; et al. Comparison of the Effects of Albumin and Crystalloid on Mortality in Adult Patients with Severe Sepsis and Septic Shock: A Meta-Analysis of Randomized Clinical Trials. Crit. Care 2014, 18, 702. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Tian, X.; Gao, Z.; Mao, A.; Feng, L.; He, C. Different Concentrations of Albumin Versus Crystalloid in Patients with Sepsis and Septic Shock: A Meta-Analysis of Randomized Clinical Trials. J. Intensive Care Med. 2023, 38, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bannard-Smith, J.; Elrakhawy, M.; Norman, G.; Owen, R.; Felton, T.; Dark, P. The Efficacy, Safety and Effectiveness of Hyperoncotic Albumin Solutions in Patients with Sepsis: A Systematic Review and Meta-Analysis. J. Intensive Care Soc. 2024, 25, 308–318. [Google Scholar] [CrossRef]
- Gabarre, P.; Desnos, C.; Morin, A.; Missri, L.; Urbina, T.; Bonny, V.; Turpin, M.; Baudel, J.-L.; Berard, L.; Montil, M.; et al. Albumin versus Saline Infusion for Sepsis-Related Peripheral Tissue Hypoperfusion: A Proof-of-Concept Prospective Study. Crit. Care 2024, 28, 43. [Google Scholar] [CrossRef]
- Williams, J.M.; Greenslade, J.H.; Hills, A.Z.; Ray, M.T. Intervention With Concentrated Albumin for Undifferentiated Sepsis in the Emergency Department (ICARUS-ED): A Pilot Randomized Controlled Trial. Ann. Emerg. Med. 2025, 86, 59–69. [Google Scholar] [CrossRef]
- Tie, X.; Zhao, Y.; Sun, T.; Zhou, R.; Li, J.; Su, J.; Yin, W. Associations between Serum Albumin Level Trajectories and Clinical Outcomes in Sepsis Patients in ICU: Insights from Longitudinal Group Trajectory Modeling. Front. Nutr. 2024, 11, 1433544. [Google Scholar] [CrossRef]
- Yin, M.; Si, L.; Qin, W.; Li, C.; Zhang, J.; Yang, H.; Han, H.; Zhang, F.; Ding, S.; Zhou, M.; et al. Predictive Value of Serum Albumin Level for the Prognosis of Severe Sepsis Without Exogenous Human Albumin Administration: A Prospective Cohort Study. J. Intensive Care Med. 2018, 33, 687–694. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Sibilio, S.; Fanni Canelles, M.; Rella, E.; Giudiceandrea, A.; Pfeifer, N.; Brigo, F. Prognostic Role of Serum Albumin in Predicting 30-Day Mortality in Patients with Infections in Emergency Department: A Prospective Study. J. Clin. Med. 2023, 12, 3447. [Google Scholar] [CrossRef]
- Hu, J.; Jin, Q.; Fang, H.; Zhang, W. Evaluating the Predictive Value of Initial Lactate/Albumin Ratios in Determining Prognosis of Sepsis Patients. Medicine 2024, 103, e37535. [Google Scholar] [CrossRef]
- Kim, K.; Seo, H.; Chin, J.H.; Son, H.J.; Hwang, J.H.; Kim, Y.K. Preoperative Hypoalbuminemia and Anemia as Predictors of Transfusion in Radical Nephrectomy for Renal Cell Carcinoma: A Retrospective Study. BMC Anesthesiol. 2015, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Levitt, M.D. Human Serum Albumin Homeostasis: A New Look at the Roles of Synthesis, Catabolism, Renal and Gastrointestinal Excretion, and the Clinical Value of Serum Albumin Measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, J.; Sun, L.; Zhang, J.; Gao, Y.; Li, R.; Ren, J.; Hou, Y.; Su, D.; Liu, J.; et al. Prognostic Value of Serum Albumin Level in Critically Ill Patients: Observational Data From Large Intensive Care Unit Databases. Front. Nutr. 2022, 9, 770674. [Google Scholar] [CrossRef]
- Artigas, A.; Wernerman, J.; Arroyo, V.; Vincent, J.-L.; Levy, M. Role of Albumin in Diseases Associated with Severe Systemic Inflammation: Pathophysiologic and Clinical Evidence in Sepsis and in Decompensated Cirrhosis. J. Crit. Care 2016, 33, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Dull, R.O.; Hahn, R.G. Hypovolemia with Peripheral Edema: What Is Wrong? Crit. Care 2023, 27, 206. [Google Scholar] [CrossRef]
- Margarson, M.P.; Soni, N.C. Changes in Serum Albumin Concentration and Volume Expanding Effects Following a Bolus of Albumin 20% in Septic Patients. Br. J. Anaesth. 2004, 92, 821–826. [Google Scholar] [CrossRef]
- Komáromi, A.; Estenberg, U.; Hammarqvist, F.; Rooyackers, O.; Wernerman, J.; Norberg, Å. Simultaneous Assessment of the Synthesis Rate and Transcapillary Escape Rate of Albumin in Inflammation and Surgery. Crit. Care 2016, 20, 370. [Google Scholar] [CrossRef]
- Vincent, J.L.; De Backer, D.; Wiedermann, C.J. Fluid Management in Sepsis: The Potential Beneficial Effects of Albumin. J. Crit. Care 2016, 35, 161–167. [Google Scholar] [CrossRef]
- Li, H.; Markal, A.; Balch, J.A.; Loftus, T.J.; Efron, P.A.; Ozrazgat-Baslanti, T.; Bihorac, A. Methods for Phenotyping Adult Patients in Sepsis and Septic Shock: A Scoping Review. Crit. Care Explor. 2022, 4, e0672. [Google Scholar] [CrossRef]
- Boussina, A.; Wardi, G.; Shashikumar, S.P.; Malhotra, A.; Zheng, K.; Nemati, S. Representation Learning and Spectral Clustering for the Development and External Validation of Dynamic Sepsis Phenotypes: Observational Cohort Study. J. Med. Internet Res. 2023, 25, e45614. [Google Scholar] [CrossRef]
- DeMerle, K.M.; Kennedy, J.N.; Chang, C.-C.H.; Delucchi, K.; Huang, D.T.; Kravitz, M.S.; Shapiro, N.I.; Yealy, D.M.; Angus, D.C.; Calfee, C.S.; et al. Identification of a Hyperinflammatory Sepsis Phenotype Using Protein Biomarker and Clinical Data in the ProCESS Randomized Trial. Sci. Rep. 2024, 14, 6234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kennedy, J.N.; Wang, S.; Brant, E.B.; Bernard, G.R.; DeMerle, K.; Chang, C.-C.H.; Angus, D.C.; Seymour, C.W. Revising Host Phenotypes of Sepsis Using Microbiology. Front. Med. 2021, 8, 775511. [Google Scholar] [CrossRef] [PubMed]
- McMullan, R.R.; McAuley, D.F.; O’Kane, C.M.; Silversides, J.A. Vascular Leak in Sepsis: Physiological Basis and Potential Therapeutic Advances. Crit. Care 2024, 28, 97. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef]
- Raia, L.; Zafrani, L. Endothelial Activation and Microcirculatory Disorders in Sepsis. Front. Med. 2022, 9, 907992. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Fisher, J.; Douglas, J.J.; Linder, A.; Boyd, J.H.; Walley, K.R.; Russell, J.A. Elevated Plasma Angiopoietin-2 Levels Are Associated With Fluid Overload, Organ Dysfunction, and Mortality in Human Septic Shock. Crit. Care Med. 2016, 44, 2018–2027. [Google Scholar] [CrossRef]
- Yu, W.-K.; McNeil, J.B.; Wickersham, N.E.; Shaver, C.M.; Bastarache, J.A.; Ware, L.B. Angiopoietin-2 Outperforms Other Endothelial Biomarkers Associated with Severe Acute Kidney Injury in Patients with Severe Sepsis and Respiratory Failure. Crit. Care 2021, 25, 48. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Molina, C.F.; Salazar-Pelaez, L.M.; Flórez, S.; Alarcón-Forero, L.C.; Sarta, M.; Hernández-Sarmiento, R.; Villar, J.C. Biomarkers of Glycocalyx Injury and Endothelial Activation Are Associated with Clinical Outcomes in Patients with Sepsis: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2023, 38, 95–105. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Wick, K.D.; Zhuo, H.; Wu, N.; Chen, Y.; Kapadia, S.B.; Guimaraes, A.; Chang, D.; Choy, D.F.; Chen, H.; et al. Early Plasma Angiopoietin-2 Is Prognostic for ARDS and Mortality among Critically Ill Patients with Sepsis. Crit. Care 2023, 27, 234. [Google Scholar] [CrossRef]
- Rovas, A.; Sackarnd, J.; Rossaint, J.; Kampmeier, S.; Pavenstädt, H.; Vink, H.; Kümpers, P. Identification of Novel Sublingual Parameters to Analyze and Diagnose Microvascular Dysfunction in Sepsis: The NOSTRADAMUS Study. Crit. Care 2021, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Fu, S.; Chi, Y.; Li, X.; Li, S.; Ma, X.; Li, X. Angiopoietin-2 as a Prognostic Biomarker in Septic Adult Patients: A Systemic Review and Meta-Analysis. Ann. Intensive Care 2024, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Matsumoto, H.; Ogura, H.; Hirose, T.; Shimizu, K.; Yamamoto, K.; Maruyama, I.; Shimazu, T. Circulating Syndecan-1 Predicts the Development of Disseminated Intravascular Coagulation in Patients with Sepsis. J. Crit. Care 2018, 43, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Wang, F.; Peng, Z. Application of Machine Learning for Clinical Subphenotype Identification in Sepsis. Infect. Dis. Ther. 2022, 11, 1949–1964. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Hendrickson, C.M.; Matthay, M.A. Endothelial Biomarkers in Human Sepsis: Pathogenesis and Prognosis for ARDS. Pulm. Circ. 2018, 8, 2045894018769876. [Google Scholar] [CrossRef]
- Mikacenic, C.; Hahn, W.O.; Price, B.L.; Harju-Baker, S.; Katz, R.; Kain, K.C.; Himmelfarb, J.; Liles, W.C.; Wurfel, M.M. Biomarkers of Endothelial Activation Are Associated with Poor Outcome in Critical Illness. PLoS ONE 2015, 10, e0141251. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, J.; Zhang, H.; Wang, X.; Liu, D. Elevated Endothelial Dysfunction-Related Biomarker Levels Indicate the Severity and Predict Sepsis Incidence. Sci. Rep. 2022, 12, 21935. [Google Scholar] [CrossRef]
- Saoraya, J.; Wongsamita, L.; Srisawat, N.; Musikatavorn, K. Plasma Syndecan-1 Is Associated with Fluid Requirements and Clinical Outcomes in Emergency Department Patients with Sepsis. Am. J. Emerg. Med. 2021, 42, 83–89. [Google Scholar] [CrossRef]
- Piotti, A.; Novelli, D.; Meessen, J.M.T.A.; Ferlicca, D.; Coppolecchia, S.; Marino, A.; Salati, G.; Savioli, M.; Grasselli, G.; Bellani, G.; et al. Endothelial Damage in Septic Shock Patients as Evidenced by Circulating Syndecan-1, Sphingosine-1-Phosphate and Soluble VE-Cadherin: A Substudy of ALBIOS. Crit. Care 2021, 25, 113. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Vasopressor Therapy in Critically Ill Patients with Shock. Intensive Care Med. 2019, 45, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Barlow, B.; Bissell, B.D. Evaluation of Evidence, Pharmacology, and Interplay of Fluid Resuscitation and Vasoactive Therapy in Sepsis and Septic Shock. Shock 2021, 56, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Guinot, P.-G.; Martin, A.; Berthoud, V.; Voizeux, P.; Bartamian, L.; Santangelo, E.; Bouhemad, B.; Nguyen, M. Vasopressor-Sparing Strategies in Patients with Shock: A Scoping-Review and an Evidence-Based Strategy Proposition. J. Clin. Med. 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Aragão, N.L.; de Sousa Zaranza, M.; Meneses, G.C.; Lázaro, A.P.P.; Guimarães, Á.R.; Martins, A.M.C.; Aragão, N.L.P.; Beliero, A.M.; da Silva Júnior, G.B.; Mota, S.M.B.; et al. Syndecan-1 Levels Predict Septic Shock in Critically Ill Patients with COVID-19. Trans. R. Soc. Trop. Med. Hyg. 2024, 118, 160–169. [Google Scholar] [CrossRef]
- Quispe-Cornejo, A.A.; Alves da Cunha, A.L.; Njimi, H.; Mongkolpun, W.; Valle-Martins, A.L.; Arébalo-López, M.; Creteur, J.; Vincent, J.-L. Effects of Rapid Fluid Infusion on Hemoglobin Concentration: A Systematic Review and Meta-Analysis. Crit. Care 2022, 26, 324. [Google Scholar] [CrossRef]
- Seldén, D.; Tardif, N.; Wernerman, J.; Rooyackers, O.; Norberg, Å. Net Albumin Leakage in Patients in the ICU with Suspected Sepsis. A Prospective Analysis Using Mass Balance Calculations. Crit. Care 2025, 29, 106. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Salazar-Peláez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300. [Google Scholar] [CrossRef]
- Adekolu, O.; Manthous, C.A. Hemodilution Then Hemoconcentration after Resuscitation of Patients with Severe Sepsis or Septic Shock. Conn. Med. 2017, 81, 169–171. [Google Scholar]
- Chandra, J.; Armengol de la Hoz, M.A.; Lee, G.; Lee, A.; Thoral, P.; Elbers, P.; Lee, H.-C.; Munger, J.S.; Celi, L.A.; Kaufman, D.A. A Novel Vascular Leak Index Identifies Sepsis Patients with a Higher Risk for In-Hospital Death and Fluid Accumulation. Crit. Care 2022, 26, 103. [Google Scholar] [CrossRef]
- Lee, S.-M.; Jo, Y.H.; Lee, J.H.; Hwang, J.E.; Park, I.; Baek, S.; Jeong, H.; Um, Y.W.; Kim, H.E. Associations of the Serum Albumin Concentration and Sequential Organ Failure Assessment Score at Discharge with 1-Year Mortality in Sepsis Survivors: A Retrospective Cohort Study. Shock 2023, 59, 547–552. [Google Scholar] [CrossRef]
- Arnau-Barrés, I.; Güerri-Fernández, R.; Luque, S.; Sorli, L.; Vázquez, O.; Miralles, R. Serum Albumin Is a Strong Predictor of Sepsis Outcome in Elderly Patients. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Kendall, H.; Abreu, E.; Cheng, A.-L. Serum Albumin Trend Is a Predictor of Mortality in ICU Patients With Sepsis. Biol. Res. Nurs. 2019, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.G.; Kanakaraju, K.; Manikandan, V.A.C.; Patel, V.; Pranay, C. The Relationship Between Serum Albumin Levels and Sepsis in Patients Admitted to a Tertiary Care Center in India. Cureus 2024, 16, e59424. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, Y.; Guo, C.; He, L.; Ding, N. Albumin Level Is Associated with Short-Term and Long-Term Outcomes in Sepsis Patients Admitted in the ICU: A Large Public Database Retrospective Research. Clin. Epidemiol. 2023, 15, 263–273. [Google Scholar] [CrossRef]
- Ren, D.; Dang, X.; Ni, T.; Zhou, J.; Zhang, Z.; Fu, S.; Zhang, W.; Yan, T.; Zhao, Y.; Liu, J. On-Treatment Serum Albumin Levels Can Predict 28-Day Mortality and Guide Albumin Infusion in Sepsis Patients. Front. Med. 2025, 12, 1490838. [Google Scholar] [CrossRef]
- Rhee, H.; Jang, G.S.; Kim, S.; Lee, W.; Jeon, H.; Kim, D.W.; Ye, B.-M.; Kim, H.J.; Kim, M.J.; Kim, S.R.; et al. Worsening or Improving Hypoalbuminemia during Continuous Renal Replacement Therapy Is Predictive of Patient Outcome: A Single-Center Retrospective Study. J. Intensive Care 2022, 10, 25. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Filippi, L.; Ferretto, P.; Milazzo, D.; Maggi, M.; Cipriano, A.; Marchetti, M.; Ghiadoni, L.; Wiedermann, C.J. Phenotype-Specific Dynamics of Serum Albumin and Their Impact on Sepsis Mortality. Biomark. Med. 2025, 19, 529–537. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Miltiades, A.N.; Gaieski, D.F.; Goyal, M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Serum Lactate Is Associated with Mortality in Severe Sepsis Independent of Organ Failure and Shock. Crit. Care Med. 2009, 37, 1670–1677. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, Z.; Li, Y.; Zhao, J.; Wu, S.; Gou, S.; Wu, H. Prognostic Accuracy of the Serum Lactate Level, the SOFA Score and the qSOFA Score for Mortality among Adults with Sepsis. Scand. J. Trauma. Resusc. Emerg. Med. 2019, 27, 51. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Fröderberg Schooner, K.; Karlsson Werther, V.; Karlsson, T.; De Geer, L.; Wilhelms, D.B.; Holmbom, M.; Fredrikson, M.; Östholm, Å.; Berg, S.; et al. Prehospital Lactate Analysis in Suspected Sepsis Improves Detection of Patients with Increased Mortality Risk: An Observational Study. Crit. Care 2025, 29, 38. [Google Scholar] [CrossRef] [PubMed]
- Gicheru, B.; Shah, J.; Wachira, B.; Omuse, G.; Maina, D. The Diagnostic Accuracy of an Initial Point-of-Care Lactate at the Emergency Department as a Predictor of in-Hospital Mortality among Adult Patients with Sepsis and Septic Shock. Front. Med. 2023, 10, 1173286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhou, R.; Qin, J.; Li, Y. Hierarchical Capability in Distinguishing Severities of Sepsis via Serum Lactate: A Network Meta-Analysis. Biomedicines 2024, 12, 447. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Wernly, B.; Ohnewein, B.; Franz, M.; Kabisch, B.; Muessig, J.; Masyuk, M.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU. Int. J. Mol. Sci. 2017, 18, 1893. [Google Scholar] [CrossRef]
- Cakir, E.; Turan, I.O. Lactate/Albumin Ratio Is More Effective than Lactate or Albumin Alone in Predicting Clinical Outcomes in Intensive Care Patients with Sepsis. Scand. J. Clin. Lab. Investig. 2021, 81, 225–229. [Google Scholar] [CrossRef]
- Shin, J.; Hwang, S.Y.; Jo, I.J.; Kim, W.Y.; Ryoo, S.M.; Kang, G.H.; Kim, K.; Jo, Y.H.; Chung, S.P.; Joo, Y.S.; et al. Prognostic Value of The Lactate/Albumin Ratio for Predicting 28-Day Mortality in Critically ILL Sepsis Patients. Shock 2018, 50, 545–550. [Google Scholar] [CrossRef]
- Bou Chebl, R.; Geha, M.; Assaf, M.; Kattouf, N.; Haidar, S.; Abdeldaem, K.; Halawi, N.; Khamis, M.; Makki, M.; Tamim, H.; et al. The Prognostic Value of the Lactate/Albumin Ratio for Predicting Mortality in Septic Patients Presenting to the Emergency Department: A Prospective Study. Ann. Med. 2021, 53, 2268–2277. [Google Scholar] [CrossRef]
- Chen, Q.; Zhan, H.; Chen, J.; Mo, J.; Huang, S. Predictive Value of Lactate/Albumin Ratio for Death and Multiple Organ Dysfunction Syndrome in Patients with Sepsis. J. Med. Biochem. 2024, 43, 617–625. [Google Scholar] [CrossRef]
- Yoo, K.H.; Choi, S.-H.; Suh, G.J.; Chung, S.P.; Choi, H.S.; Park, Y.S.; Jo, Y.H.; Shin, T.G.; Lim, T.H.; Kim, W.Y.; et al. The Usefulness of Lactate/Albumin Ratio, C-Reactive Protein/Albumin Ratio, Procalcitonin/Albumin Ratio, SOFA, and qSOFA in Predicting the Prognosis of Patients with Sepsis Who Presented to EDs. Am. J. Emerg. Med. 2024, 78, 1–7. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Zaboli, A.; Lucente, F.; Filippi, L.; Maggi, M.; Ferretto, P.; Cipriano, A.; Voza, A.; Ghiadoni, L.; Turcato, G. Temporal Decline in Intravascular Albumin Mass and Its Association with Fluid Balance and Mortality in Sepsis: A Prospective Observational Study. J. Clin. Med. 2025, 14, 5255. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Zaboli, A.; Filippi, L.; Lucente, F.; Maggi, M.; Cipriano, A.; Marchetti, M.; Milazzo, D.; Wiedermann, C.J.; Ghiadoni, L. Hemodynamic Heterogeneity in Community-Acquired Sepsis at Intermediate Care Admission: A Prospective Pilot Study Using Impedance Cardiography. Healthcare 2025, 13, 2686. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Zaboli, A.; Filippi, L.; Cipriano, A.; Parodi, M.; Sibilio, S.; Ferretto, P.; Milazzo, D.; Marchetti, M.; Ghiadoni, L.; et al. Unveiling Key Predictors of Sepsis Mortality in Intermediate Care Units: A Decision Tree Study. Curr. Med. Res. Opin. 2025, 41, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Filippi, L.; Zaboli, A.; Ferretto, P.; Milazzo, D.; Maggi, M.; Stefani, F.; Parodi, M.; Marchetti, M.; Wiedermann, C.J. Relationship between Fluid Bolus Administration and the Prognostic Role of Serum Albumin in Patients with Sepsis. Am. J. Med. Sci. 2025, 369, 451–459. [Google Scholar] [CrossRef]
- Zawieja, D.C. Contractile Physiology of Lymphatics. Lymphat. Res. Biol. 2009, 7, 87–96. [Google Scholar] [CrossRef]
- Levick, J.R.; Michel, C.C. Microvascular Fluid Exchange and the Revised Starling Principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The Endothelial Glycocalyx: Composition, Functions, and Visualization. Pflugers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Filippi, L.; Cipriano, A.; Ferretto, P.; Maggi, M.; Lucente, F.; Marchetti, M.; Ghiadoni, L.; Wiedermann, C.J. Endothelial Damage in Sepsis: The Interplay of Coagulopathy, Capillary Leak, and Vasoplegia-A Physiopathological Study. Clin. Pract. 2025, 15, 120. [Google Scholar] [CrossRef]
- Sakr, Y. Randomised Controlled Multicentre Study of Albumin Replacement Therapy in Septic Shock. 2024. Available online: https://clinicaltrials.gov (accessed on 24 October 2025).
- Bou Chebl, R.; Jamali, S.; Sabra, M.; Safa, R.; Berbari, I.; Shami, A.; Makki, M.; Tamim, H.; Abou Dagher, G. Lactate/Albumin Ratio as a Predictor of in-Hospital Mortality in Septic Patients Presenting to the Emergency Department. Front. Med. 2020, 7, 550182. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Cavaillon, J.-M. Septic Shock. Lancet 2005, 365, 63–78. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Sebille, V.; Lesieur, O.; Mathieu, B.; Raphael, J.C.; Gajdos, P. Impaired Pressor Sensitivity to Noradrenaline in Septic Shock Patients with and without Impaired Adrenal Function Reserve. Br. J. Clin. Pharmacol. 1998, 46, 589–597. [Google Scholar] [CrossRef]
- Martin, C.; Viviand, X.; Leone, M.; Thirion, X. Effect of Norepinephrine on the Outcome of Septic Shock. Crit. Care Med. 2000, 28, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Aldecoa, C.; Njimi, H.; Vincent, J.-L. Dopamine versus Norepinephrine in the Treatment of Septic Shock: A Meta-Analysis. Crit. Care Med. 2012, 40, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Hamzaoui, O.; De Vita, N.; Monnet, X.; Teboul, J.-L. Vasopressors in Septic Shock: Which, When, and How Much? Ann. Transl. Med. 2020, 8, 794. [Google Scholar] [CrossRef] [PubMed]
- Baby, S.; Reljic, T.; Villalba, N.; Kumar, A.; Yuan, S.Y. Endothelial Glycocalyx-Associated Molecules as Potential Serological Markers for Sepsis-Associated Encephalopathy: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0281941. [Google Scholar] [CrossRef]
- Stolk, R.F.; van der Pasch, E.; Naumann, F.; Schouwstra, J.; Bressers, S.; van Herwaarden, A.E.; Gerretsen, J.; Schambergen, R.; Ruth, M.M.; van der Hoeven, J.G.; et al. Norepinephrine Dysregulates the Immune Response and Compromises Host Defense during Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 830–842. [Google Scholar] [CrossRef]
- Rosengarten, B.; Wolff, S.; Klatt, S.; Schermuly, R.T. Effects of Inducible Nitric Oxide Synthase Inhibition or Norepinephrine on the Neurovascular Coupling in an Endotoxic Rat Shock Model. Crit. Care 2009, 13, R139. [Google Scholar] [CrossRef]
- Ferrer, R.; Mateu, X.; Maseda, E.; Yébenes, J.C.; Aldecoa, C.; De Haro, C.; Ruiz-Rodriguez, J.C.; Garnacho-Montero, J. Non-Oncotic Properties of Albumin. A Multidisciplinary Vision about the Implications for Critically Ill Patients. Expert. Rev. Clin. Pharmacol. 2018, 11, 125–137. [Google Scholar] [CrossRef]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of Albumin in the Preservation of Endothelial Glycocalyx Integrity and the Microcirculation: A Review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The Glycocalyx: A Novel Diagnostic and Therapeutic Target in Sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Fisher, J.; Linder, A.; Bentzer, P.; Boyd, J.; Kong, H.J.; Lee, T.; Walley, K.R.; Russell, J.A. Is Heparin-Binding Protein Inhibition a Mechanism of Albumin’s Efficacy in Human Septic Shock? Crit. Care Med. 2018, 46, e364–e374. [Google Scholar] [CrossRef]
- Hariri, G.; Joffre, J.; Deryckere, S.; Bigé, N.; Dumas, G.; Baudel, J.-L.; Maury, E.; Guidet, B.; Ait-Oufella, H. Albumin Infusion Improves Endothelial Function in Septic Shock Patients: A Pilot Study. Intensive Care Med. 2018, 44, 669–671. [Google Scholar] [CrossRef]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the Endothelial Glycocalyx in Patients Undergoing Major Vascular Surgery with Global and Regional Ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Conzen, P.; Rehm, M. A Rational Approach to Perioperative Fluid Management. Anesthesiology 2008, 109, 723–740. [Google Scholar] [CrossRef]
- Hahn, R.G. Constant Plasma Volume and Colloid Osmotic Pressure after Infusion of Albumin 20%: A Secondary Analysis. Physiol. Rep. 2025, 13, e70623. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Controversies Surrounding Albumin Use in Sepsis: Lessons from Cirrhosis. Int. J. Mol. Sci. 2023, 24, 17606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.