The Role of Nuclear and Mitochondrial DNA in Myalgic Encephalomyelitis: Molecular Insights into Susceptibility and Dysfunction
Abstract
1. Introduction
2. Genetic Predispositions and Susceptibility
2.1. Familial Clustering and Heritability
2.2. Candidate Gene Studies
2.3. Genome-Wide Association Studies (GWAS) and Advanced Genomic Approaches
2.4. Structural Variants and Genotype–Phenotype Correlations
2.5. Genetic Overlap with Other Conditions
3. Mitochondrial DNA (mtDNA) Dysfunction
3.1. Mitochondria as Energy Hubs
3.2. Evidence of Mitochondrial Abnormalities in ME

3.3. mtDNA Variants and Haplogroups
3.4. Oxidative Stress and mtDNA Damage
3.5. Mitochondrial Epigenetics
4. Epigenetic Modifications
4.1. DNA Methylation in ME
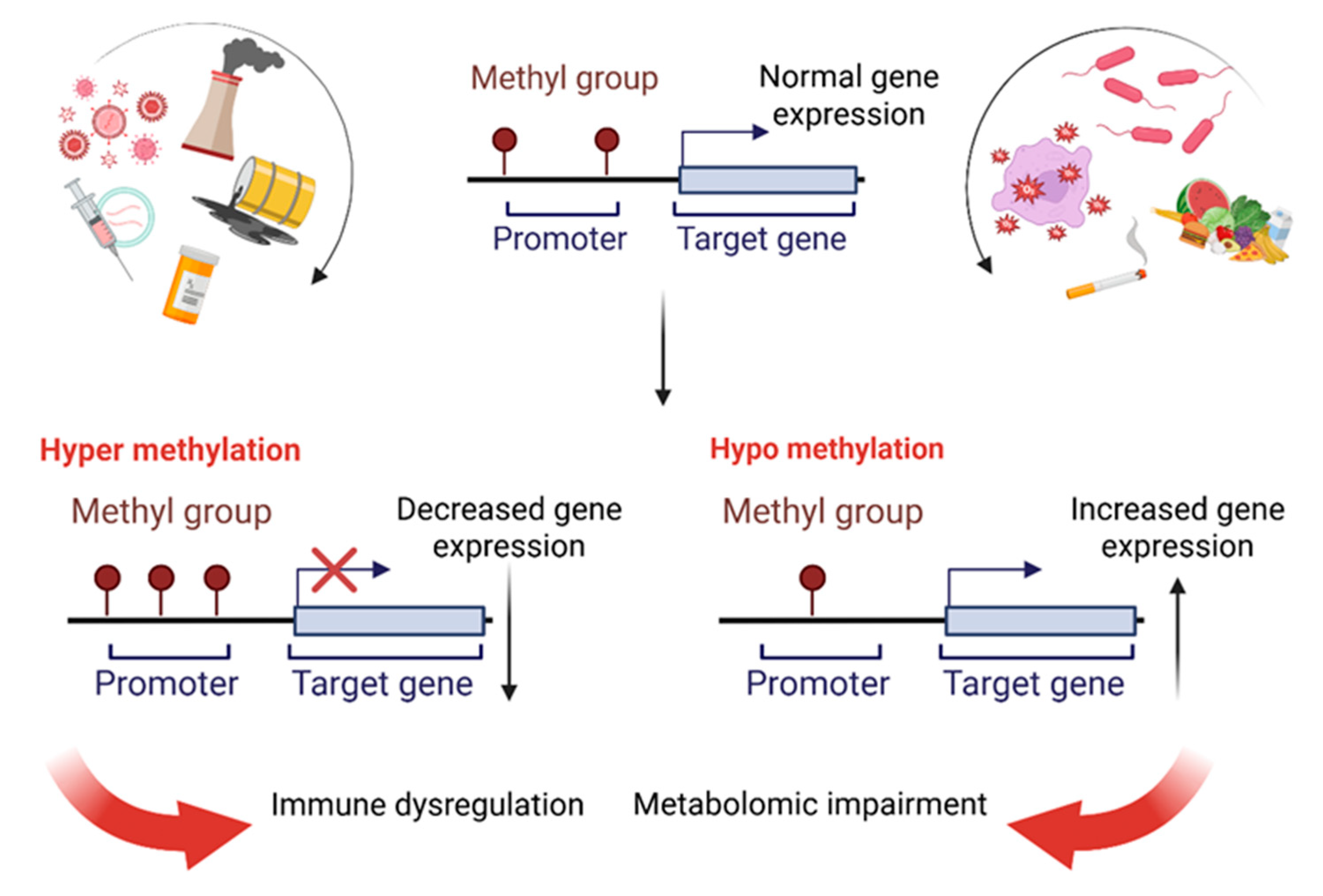
4.2. Dynamic Epigenetic Changes and Relapses
4.3. Transposable Element Activation
5. DNA’s Clinical Frontier in ME: Biomarkers, Therapies, and Challenges
5.1. DNA-Focused Integrative Omics Approaches in ME
5.2. DNA-Based Biomarkers for Diagnosis and Prognosis in ME
5.3. Targeting DNA-Associated Mechanisms for Therapeutic Intervention in ME
5.4. Challenges in Translating DNA Research to Clinical Applications in ME
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-deoxyguanosine |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| CNS | Central nervous system |
| CoQ10 | Coenzyme Q10 |
| CpG | Cytosine-phosphate-Guanine |
| CYP2D6 | Cytochrome P450 enzyme |
| dsRNA | Double-stranded RNA |
| ERVs | Endogenous retroviruses |
| HERV-W | Human endogenous retrovirus W |
| GRIK2 | Glutamate ionotropic receptor kainate type subunit 2 |
| GSH | Reduced glutathione |
| GWAS | Genome-Wide Association Studies |
| HP | Haptoglobin |
| HLA | Human Leukocyte Antigen |
| iVMFs | Intra-individual variably methylated fragments |
| LINEs | Long interspersed nuclear elements |
| ME/CFS | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome |
| MDA | Malondialdehyde |
| mtDNA | Mitochondrial DNA |
| MTHFR | Methylenetetrahydrofolate reductase |
| NK | Natural killer |
| NPAS2 | Neuronal PAS domain protein 2 |
| OXPHOS | Oxidative phosphorylation |
| PBMCs | Peripheral blood mononuclear cells |
| PEM | Post-exertional malaise |
| ROS | Reactive Oxygen Species |
| RRBS | Reduced Representation Bisulfite Sequencing |
| SINEs | Short interspersed nuclear elements |
| SMPDL3B | Sphingomyelin phosphodiesterase acid-like 3B |
| SNPs | Single Nucleotide Polymorphisms |
| TCA | Tricarboxylic Acid |
| TEs | Transposable elements |
| TSPO | Translocator Protein |
| WASF3 | Wiskott–Aldrich Syndrome Protein Family Member 3 |
| WES | Whole-exome sequencing |
| WGS | Whole-genome sequencing |
References
- Rivera, M.C.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef]
- Nacul, L.; O’BOyle, S.; Palla, L.; Nacul, F.E.; Mudie, K.; Kingdon, C.C.; Cliff, J.M.; Clark, T.G.; Dockrell, H.M.; Lacerda, E.M. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Progresses: The Natural History of ME/CFS. Front. Neurol. 2020, 11, 826. [Google Scholar] [CrossRef]
- Ardestani, S.K.; Karkhaneh, M.; Stein, E.; Punja, S.; Junqueira, D.R.; Kuzmyn, T.; Pearson, M.; Smith, L.; Olson, K.; Vohra, S. Systematic Review of Mind-Body Interventions to Treat Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2021, 57, 652. [Google Scholar] [CrossRef]
- Stussman, B.; Williams, A.; Snow, J.; Gavin, A.; Scott, R.; Nath, A.; Walitt, B. Characterization of Post–exertional Malaise in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Neurol. 2020, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Wormgoor, M.E.A.; Rodenburg, S.C. Focus on post-exertional malaise when approaching ME/CFS in specialist healthcare improves satisfaction and reduces deterioration. Front. Neurol. 2023, 14, 1247698. [Google Scholar] [CrossRef]
- Słomko, J.; Newton, J.L.; Kujawski, S.; Tafil-Klawe, M.; Klawe, J.; Staines, D.; Marshall-Gradisnik, S.; Zalewski, P. Prevalence and characteristics of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in Poland: A cross-sectional study. BMJ Open 2019, 9, e023955. [Google Scholar] [CrossRef]
- Johnston, S.; Brenu, E.W.; Staines, D.; Gradisnik, M. The prevalence of chronic fatigue syndrome/myalgic encephalomyelitis: A meta-analysis. Clin. Epidemiol. 2013, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; De Maria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2019, 6, 412. [Google Scholar] [CrossRef]
- Thomas, N.; Gurvich, C.; Huang, K.; Gooley, P.R.; Armstrong, C.W. The underlying sex differences in neuroendocrine adaptations relevant to Myalgic Encephalomyelitis Chronic Fatigue Syndrome. Front. Neuroendocr. 2022, 66, 100995. [Google Scholar] [CrossRef]
- Natelson, B.H. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: Definitions, Similarities, and Differences. Clin. Ther. 2019, 41, 612–618. [Google Scholar] [CrossRef]
- Maksoud, R.; Magawa, C.; Eaton-Fitch, N.; Thapaliya, K.; Marshall-Gradisnik, S. Biomarkers for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. BMC Med. 2023, 21, 189. [Google Scholar] [CrossRef]
- Azimi, G.; Elremaly, W.; Elbakry, M.; Franco, A.; Godbout, C.; Moreau, A. Circulating FGF-21 as a Disease-Modifying Factor Associated with Distinct Symptoms and Cognitive Profiles in Myalgic Encephalomyelitis and Fibromyalgia. Int. J. Mol. Sci. 2025, 26, 7670. [Google Scholar] [CrossRef]
- Friedman, K.J. Advances in ME/CFS: Past, Present, and Future. Front. Pediatr. 2019, 7, 131. [Google Scholar] [CrossRef]
- Oter-Quintana, C.; Alameda-Cuesta, A.; Brito-Brito, P.R.; Parro-Moreno, A.I.; Alcolea-Cosín, M.T.; González-Gil, T.; Hernández-Barrera, V.; Esteban-Hernández, J. Psychosocial Nursing Diagnoses of Individuals with Myalgic Encephalomyelitis-chronic fatigue syndrome: A Descriptive Study. Nurs. Open 2025, 12, e70212. [Google Scholar] [CrossRef]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina 2021, 57, 510. [Google Scholar] [CrossRef] [PubMed]
- Bested, A.C.; Marshall, L.M. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev. Environ. Health 2015, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Arron, H.E.; Marsh, B.D.; Kell, D.B.; Khan, M.A.; Jaeger, B.R.; Pretorius, E. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The biology of a neglected disease. Front. Immunol. 2024, 15, 1386607. [Google Scholar] [CrossRef] [PubMed]
- Capelli, E.; Zola, R.; Lorusso, L.; Venturini, L.; Sardi, F.; Ricevuti, G. Chronic fatigue syndrome/myalgic encephalomyelitis: An update. Int. J. Immunopathol. Pharmacol. 2010, 23, 981–989. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Lipkin, W.I. ME/CFS and Long COVID share similar symptoms and biological abnormalities: Road map to the literature. Front. Med. 2023, 10, 1187163. [Google Scholar] [CrossRef]
- Jason, L.A.; Dorri, J.A. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol. Int. 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Albright, F.; Light, K.; Light, A.; Bateman, L.; A Cannon-Albright, L. Evidence for a heritable predisposition to Chronic Fatigue Syndrome. BMC Neurol. 2011, 11, 62. [Google Scholar] [CrossRef]
- Dibble, J.J.; McGrath, S.J.; Ponting, C.P. Genetic risk factors of ME/CFS: A critical review. Hum. Mol. Genet. 2020, 29, R117–R124. [Google Scholar] [CrossRef]
- Crawley, E.; Smith, G.D. Is chronic fatigue syndrome (CFS/ME) heritable in children, and if so, why does it matter? Arch. Dis. Child. 2007, 92, 1058–1061. [Google Scholar] [CrossRef][Green Version]
- Hickie, I.B.; Bansal, A.S.; Kirk, K.M.; Lloyd, A.R.; Martin, N.G. A twin study of the etiology of prolonged fatigue and immune activation. Twin Res. 2001, 4, 94–102. [Google Scholar] [CrossRef][Green Version]
- Hickie, I.; Kirk, K.; Martin, N. Unique genetic and environmental determinants of prolonged fatigue: A twin study. Psychol. Med. 1999, 29, 259–268. [Google Scholar] [CrossRef][Green Version]
- Buchwald, D.; Herrell, R.; Ashton, S.; Belcourt, M.; Schmaling, K.; Sullivan, P.; Neale, M.; Goldberg, J. A twin study of chronic fatigue. Psychosom. Med. 2001, 63, 936–943. [Google Scholar] [CrossRef]
- A Schlauch, K.; Khaiboullina, S.F.; De Meirleir, K.L.; Rawat, S.; Petereit, J.; Rizvanov, A.A.; Blatt, N.; Mijatovic, T.; Kulick, D.; Palotás, A.; et al. Genome-wide association analysis identifies genetic variations in subjects with myalgic encephalomyelitis/chronic fatigue syndrome. Transl. Psychiatry 2016, 6, e730. [Google Scholar] [CrossRef]
- Perez, M.; Jaundoo, R.; Hilton, K.; Del Alamo, A.; Gemayel, K.; Klimas, N.G.; Craddock, T.J.A.; Nathanson, L. Genetic Predisposition for Immune System, Hormone, and Metabolic Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Pilot Study. Front. Pediatr. 2019, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Qi, J.-G.; Yan, H.; Zhang, Q.-Y.; Ji, T.-Y.; Chang, X.-Z.; Yang, H.-P.; Jin, H.-F.; Du, J.-B. Comorbidity of chronic fatigue syndrome, postural tachycardia syndrome, and narcolepsy with 5,10-methylenetetrahydrofolate reductase (MTHFR) mutation in an adolescent: A case report. Chin. Med. J. 2021, 134, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P. Long Covid, POTS, and ME/CFS: Lifting the Fog. J. Neurol. Neurophysiol. 2023, 14, 1–8. [Google Scholar] [CrossRef]
- Devereux-Cooke, A.; Leary, S.; McGrath, S.J.; Northwood, E.; Redshaw, A.; Shepherd, C.; Stacey, P.; Tripp, C.; Wilson, J.; Mar, M.; et al. DecodeME: Community recruitment for a large genetics study of myalgic encephalomyelitis/chronic fatigue syndrome. BMC Neurol. 2022, 22, 269. [Google Scholar] [CrossRef]
- Bretherick, A.D.; McGrath, S.J.; Devereux-Cooke, A.; Leary, S.; Northwood, E.; Redshaw, A.; Stacey, P.; Tripp, C.; Wilson, J.; Chowdhury, S.; et al. Typing myalgic encephalomyelitis by infection at onset: A DecodeME study. NIHR Open Res. 2023, 3, 20. [Google Scholar] [CrossRef]
- Das, S.; Taylor, K.; Kozubek, J.; Sardell, J.; Gardner, S. Genetic risk factors for ME/CFS identified using combinatorial analysis. J. Transl. Med. 2022, 20, 598. [Google Scholar] [CrossRef]
- Zhang, S.; Jahanbani, F.; Chander, V.; Kjellberg, M.; Liu, M.; Glass, K.A.; Iu, D.S.; Ahmed, F.; Li, H.; Maynard, R.D.; et al. Dissecting the genetic complexity of myalgic encephalomyelitis/chronic fatigue syndrome via deep learn-ing-powered genome analysis. medRxiv 2025. [Google Scholar] [CrossRef]
- Moezzi, A.; Ushenkina, A.; Widgren, A.; Bergquist, J.; Li, P.; Xiao, W.; Rostami-Afshari, B.; Leveau, C.; Elremaly, W.; Caraus, I.; et al. Haptoglobin phenotypes and structural variants associate with post-exertional malaise and cognitive dysfunction in myalgic encephalomyelitis. J. Transl. Med. 2025, 23, 970. [Google Scholar] [CrossRef]
- Taylor, K.; Pearson, M.; Das, S.; Sardell, J.; Chocian, K.; Gardner, S. Genetic risk factors for severe and fatigue dominant long COVID and commonalities with ME/CFS identified by combinatorial analysis. J. Transl. Med. 2023, 21, 775. [Google Scholar] [CrossRef]
- Eaton-Fitch, N.; Rudd, P.; Er, T.; Hool, L.; Herrero, L.; Marshall-Gradisnik, S. Immune exhaustion in ME/CFS and long COVID. J. Clin. Investig. 2024, 9, e183810. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Hartle, M.; Sullivan, K.; Bell, J.; Abbaszadeh, S.; Unutmaz, D.; Bateman, L. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work 2023, 74, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; de Vega, W.C.; Ashbrook, D.; Vernon, S.D.; McGowan, P.O. Genome-epigenome interactions associated with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Epigenetics 2018, 13, 1174–1190. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Lidbury, B.A.; Thomas, N.; Gooley, P.R.; Armstrong, C.W. Machine learning and multi-omics in precision medicine for ME/CFS. J. Transl. Med. 2025, 23, 68. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondria. Cold Spring Harb. Perspect. Biol. 2021, 13, a040543. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immu-no-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Missailidis, D.; Annesley, S.J.; Fisher, P.R. Pathological Mechanisms Underlying Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2019, 9, 80. [Google Scholar] [CrossRef]
- Wood, E.; Hall, K.H.; Tate, W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 ‘long-haulers’? Chronic Dis. Transl. Med. 2021, 7, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef] [PubMed]
- Scheibenbogen, C.; Wirth, K.J. Key Pathophysiological Role of Skeletal Muscle Disturbance in Post COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Accumulated Evidence. J. Cachex-Sarcopenia Muscle 2025, 16, e13669. [Google Scholar] [CrossRef]
- Franco, A.; Walton, C.E.; Dang, X. Mitochondria Clumping vs. Mitochondria Fusion in CMT2A Diseases. Life 2022, 12, 2110. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, L.; Franco, A.; Li, J.; Rocha, A.G.; Devanathan, S.; Dolle, R.E.; Bernstein, P.R.; Dorn, G.W., II. Correction to “Discovery of 6-Phenylhexanamide Derivatives as Potent Stereoselective Mitofusin Activators for the Treatment of Mitochondrial Diseases”. J. Med. Chem. 2020, 63, 10086, Erratum in J. Med. Chem. 2020, 63, 7033–7051. https://doi.org/10.1021/acs.jmedchem.0c00366. [Google Scholar] [CrossRef]
- Booth, N.E.; Myhill, S.; McLaren-Howard, J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyeli-tis/Chronic Fatigue Syndrome (ME/CFS). Int. J. Clin. Exp. Med. 2012, 5, 208–220. [Google Scholar] [PubMed]
- Tomas, C.; Elson, J.L.; Strassheim, V.; Newton, J.L.; Walker, M. The effect of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) severity on cellular bioenergetic function. PLoS ONE 2020, 15, e0231136. [Google Scholar] [CrossRef]
- Tomas, C.; Elson, J.L. The role of mitochondria in ME/CFS: A perspective. Fatigue Biomed. Health Behav. 2019, 7, 52–58. [Google Scholar] [CrossRef]
- Missailidis, D.; Annesley, S.J.; Allan, C.Y.; Sanislav, O.; Lidbury, B.A.; Lewis, D.P.; Fisher, P.R. An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients. Int. J. Mol. Sci. 2020, 21, 1074. [Google Scholar] [CrossRef]
- Missailidis, D.; Sanislav, O.; Allan, C.Y.; Smith, P.K.; Annesley, S.J.; Fisher, P.R. Dysregulated Provision of Oxidisable Substrates to the Mitochondria in ME/CFS Lymphoblasts. Int. J. Mol. Sci. 2021, 22, 2046. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Mitochondria and immunity in chronic fatigue syndrome. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 103, 109976. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Ohmayer, B.; Buhl, J.L.; Schneider, E.M.; Walther, P.; Calzia, E.; Jerg, A.; Matits, L.; Steinacker, J.M. Functional and Morphological Differences of Muscle Mitochondria in Chronic Fatigue Syndrome and Post-COVID Syndrome. Int. J. Mol. Sci. 2024, 25, 1675. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, F.; Maynard, R.D.; Sing, J.C.; Jahanbani, S.; Perrino, J.J.; Spacek, D.V.; Davis, R.W.; Snyder, M.P. Phenotypic characteristics of peripheral immune cells of Myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: A pilot study. PLoS ONE 2022, 17, e0272703. [Google Scholar] [CrossRef]
- Syed, A.M.; Karius, A.K.; Ma, J.; Wang, P.-Y.; Hwang, P.M. Mitochondrial Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Physiology 2025, 40, 319–328. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Ma, J.; Kim, Y.-C.; Son, A.Y.; Syed, A.M.; Liu, C.; Mori, M.P.; Huffstutler, R.D.; Stolinski, J.L.; Talagala, S.L.; et al. WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2023, 120, e2302738120. [Google Scholar] [CrossRef]
- Glass, K.A.; Giloteaux, L.; Zhang, S.; Hanson, M.R. Extracellular vesicle proteomics uncovers energy metabolism, complement system, and endoplasmic reticulum stress response dysregulation postexercise in males with myalgic encephalomyelitis/chronic fatigue syndrome. Clin. Transl. Med. 2025, 15, e70346. [Google Scholar] [CrossRef]
- McCommis, K.S.; Finck, B.N. The Hepatic Mitochondrial Pyruvate Carrier as a Regulator of Systemic Metabolism and a Therapeutic Target for Treating Metabolic Disease. Biomolecules 2023, 13, 261. [Google Scholar] [CrossRef]
- Morin, D.; Musman, J.; Pons, S.; Berdeaux, A.; Ghaleh, B. Mitochondrial translocator protein (TSPO): From physiology to cardioprotection. Biochem. Pharmacol. 2016, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med. Sci. Monit. 2011, 17, SC11–SC15. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Malucelli, E.; Iotti, S. Increase of free Mg2+in the skeletal muscle of chronic fatigue syndrome patients. Dyn. Med. 2006, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Domingo, J.C.; Cordobilla, B.; Ferrer, R.; Giralt, M.; Sanmartín-Sentañes, R.; Alegre-Martín, J. Does Coenzyme Q10 Plus Selenium Supplementation Ameliorate Clinical Outcomes by Modulating Oxidative Stress and In-flammation in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Antioxid. Redox Signal. 2022, 36, 729–739. [Google Scholar] [CrossRef]
- Schon, E.A. The mitochondrial genome. In Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease; Elsevier: Amsterdam, The Netherlands, 2025; pp. 491–503. [Google Scholar] [CrossRef]
- Tang-Siegel, G.G.; Maughan, D.W.; Frownfelter, M.B.; Light, A.R. Mitochondrial DNA Missense Mutations ChrMT: 8981A > G and ChrMT: 6268C > T Identified in a Caucasian Female with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Triggered by the Epstein–Barr Virus. Case Rep. Genet. 2024, 2024, 6475425. [Google Scholar] [CrossRef]
- Billing-Ross, P.; Germain, A.; Ye, K.; Keinan, A.; Gu, Z.; Hanson, M.R. Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. J. Transl. Med. 2016, 14, 19. [Google Scholar] [CrossRef]
- Venter, M.; Tomas, C.; Pienaar, I.S.; Strassheim, V.; Erasmus, E.; Ng, W.-F.; Howell, N.; Newton, J.L.; Van der Westhuizen, F.H.; Elson, J.L. MtDNA population variation in Myalgic encephalomyelitis/chronic fatigue syndrome in two populations: A study of mildly deleterious variants. Sci. Rep. 2019, 9, 2914. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Fiorini, A.C.; Scorza, F.A.; Scorza, C.A. mtDNA deletion m.8753_16566 with <10% heteroplasmy in muscle and isolated complex-V dysfunction misinterpreted as chronic fatigue syndrome over 21-years. Clinics 2025, 80, 100604. [Google Scholar] [CrossRef]
- Shankar, V.; Wilhelmy, J.; Curtis, E.J.; Michael, B.; Cervantes, L.; Mallajosyula, V.; Davis, R.W.; Snyder, M.; Younis, S.; Robinson, W.H.; et al. Oxidative stress is a shared characteristic of ME/CFS and Long COVID. Proc. Natl. Acad. Sci. USA 2025, 122, e2426564122. [Google Scholar] [CrossRef]
- Sikder, M.M.; Li, X.; Akumwami, S.; Labony, S.A. Reactive Oxygen Species: Role in Pathophysiology, and Mechanism of Endogenous and Dietary Antioxidants during Oxidative Stress. Chonnam Med. J. 2025, 61, 32–45. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N. Why myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: Disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuro Endocrinol. Lett. 2009, 30, 677–693. [Google Scholar]
- Morris, G.; Maes, M. Oxidative and Nitrosative Stress and Immune-inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The Mitochondrial Response to DNA Damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef]
- Van Campenhout, J.; Buntinx, Y.; Xiong, H.-Y.; Wyns, A.; Polli, A.; Nijs, J.; Aerts, J.L.; Laeremans, T.; Hendrix, J. Unravelling the Connection Between Energy Metabolism and Immune Senescence/Exhaustion in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomolecules 2025, 15, 357. [Google Scholar] [CrossRef] [PubMed]
- Zachayus, A.; Loup-Forest, J.; Cura, V.; Poterszman, A. Nucleotide Excision Repair: Insights into Canonical and Emerging Functions of the Transcription/DNA Repair Factor TFIIH. Genes 2025, 16, 231. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Magalhães, L.; Ribeiro-Dos-Santos, Â.; Vidal, A.F. Mitochondrial Epigenetics: Non-Coding RNAs as a Novel Layer of Complexity. Int. J. Mol. Sci. 2020, 21, 1838. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Waingankar, T.P.; D’SIlva, P. Seahorse assay for the analysis of mitochondrial respiration using Saccharomyces cerevisiae as a model system. Methods Enzymol. 2024, 707, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, D.; D’Aquila, P.; Scafone, T.; Giordano, M.; Riso, V.; Riccio, A.; Passarino, G. The control region of mitochondrial DNA shows an unusual CpG and non-CpG methylation pattern. DNA Res. 2013, 20, 537–547. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.M.; Barabási, A.L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research gaps and opportunities in precision nutrition: An NIH workshop report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, E.; Rosén, A. Epigenetic reprograming in myalgic encephalomyelitis/chronic fatigue syndrome: A narrative of latent viruses. J. Intern. Med. 2024, 296, 93–115. [Google Scholar] [CrossRef]
- Addissouky, T.A.; El Tantawy El Sayed, I.; Wang, Y. Epigenetic factors in posttraumatic stress disorder re-silience and susceptibility. Egypt. J. Med. Hum. Genet. 2025, 26, 50. [Google Scholar] [CrossRef]
- Kubota, T.; Miyake, K.; Hirasawa, T. Epigenetic understanding of gene-environment interactions in psychiatric disorders: A new concept of clinical genetics. Clin. Epigenetics 2012, 4, 1. [Google Scholar] [CrossRef]
- Helliwell, A.M.; Sweetman, E.C.; Stockwell, P.A.; Edgar, C.D.; Chatterjee, A.; Tate, W.P. Changes in DNA methylation profiles of myalgic encephalomyelitis/chronic fatigue syndrome patients reflect systemic dysfunctions. Clin. Epigenetics 2020, 12, 167. [Google Scholar] [CrossRef]
- de Vega, W.C.; Herrera, S.; Vernon, S.D.; McGowan, P.O. Epigenetic modifications and glucocorticoid sensitivity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). BMC Med. Genom. 2017, 10, 11. [Google Scholar] [CrossRef]
- Nepotchatykh, E.; Elremaly, W.; Caraus, I.; Godbout, C.; Leveau, C.; Chalder, L.; Beaudin, C.; Kanamaru, E.; Kosovskaia, R.; Lauzon, S.; et al. Profile of circulating microRNAs in myalgic encephalomyelitis and their relation to symptom severity, and disease pathophysiology. Sci. Rep. 2020, 10, 19620. [Google Scholar] [CrossRef]
- de Vega, W.C.; McGowan, P.O. The epigenetic landscape of myalgic encephalomyelitis/chronic fatigue syndrome: Deciphering complex phenotypes. Epigenomics 2017, 9, 1337–1340. [Google Scholar] [CrossRef]
- Soffritti, I.; Gravelsina, S.; D’accolti, M.; Bini, F.; Mazziga, E.; Vilmane, A.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z.; Krumina, A.; Murovska, M.; et al. Circulating miRNAs Expression in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2023, 24, 10582. [Google Scholar] [CrossRef] [PubMed]
- Nepotchatykh, E.; Caraus, I.; Elremaly, W.; Leveau, C.; Elbakry, M.; Godbout, C.; Rostami-Afshari, B.; Petre, D.; Khatami, N.; Franco, A.; et al. Circulating microRNA expression signatures accurately discriminate myalgic encephalomyelitis from fibromyalgia and comorbid conditions. Sci. Rep. 2023, 13, 1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Zhong, X.; Wang, G.; Shi, Z.; Mei, C.; Chen, L.; Zhan, J.; Cheng, J. The emerging role of exosomal LncRNAs in chronic fatigue syndrome: From intercellular communication to disease biomarkers. Front. Mol. Biosci. 2025, 12, 1653627. [Google Scholar] [CrossRef]
- Almenar-Pérez, E.; Ovejero, T.; Sánchez-Fito, T.; Espejo, J.A.; Nathanson, L.; Oltra, E. Epigenetic Components of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Uncover Potential Transposable Element Activation. Clin. Ther. 2019, 41, 675–698. [Google Scholar] [CrossRef]
- Yang, C.-A.; Bauer, S.; Ho, Y.-C.; Sotzny, F.; Chang, J.-G.; Scheibenbogen, C. The expression signature of very long non-coding RNA in myalgic encephalomyelitis/chronic fatigue syndrome. J. Transl. Med. 2018, 16, 231. [Google Scholar] [CrossRef]
- Ljungström, M.; Nathanson, L.; Oltra, E. MicroRNA Profiling of Blood Extracellular Vesicles in ME/CFS. Methods Mol. Biol. 2025, 2920, 39–55. [Google Scholar] [CrossRef] [PubMed]
- González-Cebrián, A.; Almenar-Pérez, E.; Xu, J.; Yu, T.; Huang, W.E.; Giménez-Orenga, K.; Hutchinson, S.; Lodge, T.; Nathanson, L.; Morten, K.J.; et al. Diagnosis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome With Partial Least Squares Discriminant Analysis: Relevance of Blood Extracellular Vesicles. Front. Med. 2022, 9, 842991. [Google Scholar] [CrossRef]
- Almenar-Pérez, E.; Sarría, L.; Nathanson, L.; Oltra, E. Assessing diagnostic value of microRNAs from peripheral blood mononuclear cells and extracellular vesicles in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Sci. Rep. 2020, 10, 2064. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Oltra, E.; Sarria, L.; Rose, N.; Beljanski, V.; Fletcher, M.A.; Klimas, N.G.; Nathanson, L. Identification of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-associated DNA methylation patterns. PLoS ONE 2018, 13, e0201066. [Google Scholar] [CrossRef]
- Hadidchi, R.; Patel, B.; Madan, J.; Liu, A.; Henry, S.; Duong, T.Q. Elevated risk of new-onset chronic fatigue syndrome/myalgic encephalomyelitis up to four years after SARS-CoV-2 infection. J. Transl. Med. 2025, 23, 815. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, A.; Dalla Riva, G.V. A Cartography of Differential Gene Methylation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Different Network Roles in the Protein-Protein Interactions Network Play Different, Biologically Relevant, Roles. bioRxiv 2021. [Google Scholar] [CrossRef]
- Polli, A.; Godderis, L.; Martens, D.S.; Patil, M.S.; Hendrix, J.; Wyns, A.; Van Campenhout, J.; Richter, E.; Fanning, L.; Vandekerckhove, O.; et al. Exploring DNA methylation, telomere length, mitochondrial DNA, and immune function in patients with Long-COVID. BMC Med. 2025, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Peppercorn, K.; Sharma, S.; Edgar, C.D.; Stockwell, P.A.; Rodger, E.J.; Chatterjee, A.; Tate, W.P. Comparing DNA Methylation Landscapes in Peripheral Blood from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Long COVID Patients. Int. J. Mol. Sci. 2025, 26, 6631. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Helliwell, A.M.; Stockwell, P.A.; Edgar, C.D.; Chatterjee, A.; Tate, W.P. Dynamic Epigenetic Changes during a Relapse and Recovery Cycle in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2022, 23, 11852. [Google Scholar] [CrossRef]
- Tate, W.P.; Walker, M.O.M.; Peppercorn, K.; Blair, A.L.H.; Edgar, C.D. Towards a Better Understanding of the Complexities of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Long COVID. Int. J. Mol. Sci. 2023, 24, 5124. [Google Scholar] [CrossRef]
- Goodier, J.L. Restricting retrotransposons: A review. Mob. DNA 2016, 7, 16. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef]
- Ovejero, T.; Sadones, O.; Sánchez-Fito, T.; Almenar-Pérez, E.; Espejo, J.A.; Martín-Martínez, E.; Nathanson, L.; Oltra, E. Activation of Transposable Elements in Immune Cells of Fibromyalgia Patients. Int. J. Mol. Sci. 2020, 21, 1366. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Orenga, K.; Pierquin, J.; Brunel, J.; Charvet, B.; Martín-Martínez, E.; Lemarinier, M.; Fried, S.; Lucas, A.; Perron, H.; Oltra, E. Blood parameters differentiate post COVID-19 condition from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia. Brain Behav. Immun.—Heal. 2025, 48, 101058. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Orenga, K.; Pierquin, J.; Brunel, J.; Charvet, B.; Martín-Martínez, E.; Perron, H.; Oltra, E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front. Immunol. 2022, 13, 1020064. [Google Scholar] [CrossRef]
- Giménez-Orenga, K.; Martín-Martínez, E.; Nathanson, L.; Oltra, E. HERV activation segregates ME/CFS from fibromyalgia while defining a novel nosologic entity. eLife 2025, 14, RP104441. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Chau, T.; Tantanis, D.; Huang, K.; Scheinberg, A.; Gooley, P.R.; Josev, E.K.; Knight, S.J.; Armstrong, C.W. Serial Paediatrics Omics Tracking in Myalgic Encephalomyelitis (SPOT-ME): Protocol paper for a multidisciplinary, observational study of clinical and biological markers of paediatric myalgic encephalomyelitis/chronic fatigue syndrome in Australian adolescents aged 12–19 years. BMJ Open 2024, 14, e089038. [Google Scholar] [CrossRef]
- Xiong, R.; Gunter, C.; Fleming, E.; Vernon, S.D.; Bateman, L.; Unutmaz, D.; Oh, J. Multi-‘omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microbe 2023, 31, 273–287.e5. [Google Scholar] [CrossRef]
- Xiong, R.; Aiken, E.; Caldwell, R.; Vernon, S.D.; Kozhaya, L.; Gunter, C.; Bateman, L.; Unutmaz, D.; Oh, J. AI-driven multi-omics modeling of myalgic encephalomyelitis/chronic fatigue syndrome. Nat. Med. 2025, 31, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, I.; Shadiack, E.; Ganetzky, R.D.; Falk, M.J. Mitochondrial medicine therapies: Rationale, evidence, and dosing guidelines. Curr. Opin. Pediatr. 2020, 32, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Koekkoek, W.; Grefte, S.; Witkamp, R.; van Zanten, A. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Neergheen, V.; Chalasani, A.; Wainwright, L.; Yubero, D.; Montero, R.; Artuch, R.; Hargreaves, I. Coenzyme Q10 in the treatment of mitochondrial disease. J. Inborn Errors Metab. Screen. 2019, 5, e160063. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Rajan, S.S.; Abrahamse, H. Photodynamic Therapy and Dietary Antioxidants: A Dual Strategy for Genome Stability and DNA Damage Repair. Cancer Med. 2025, 14, e71032. [Google Scholar] [CrossRef]
- Rostami-Afshari, B.; Elremaly, W.; Franco, A.; Elbakry, M.; Akoume, M.-Y.; Boufaied, I.; Moezzi, A.; Leveau, C.; Rompré, P.; Godbout, C.; et al. SMPDL3B a novel biomarker and therapeutic target in myalgic encephalomyelitis. J. Transl. Med. 2025, 23, 748, Erratum in J. Transl. Med. 2025, 23, 911. https://doi.org/10.1186/s12967-025-06900-w. [Google Scholar] [CrossRef]


| Study | Year | Sample Size (Cases, Controls) | Methods Applied | Main Findings | Cohort/Other Relevant Information |
|---|---|---|---|---|---|
| Albright et al. [21] | 2011 | ~1.6 million (population registry) | Genealogical database analysis | Significant familial clustering up to 3rd degree relatives | Utah population, large genealogical study |
| Dibble et al. [22] | 2020 | Review of multiple studies | Systematic review | Familial clustering reported | Diverse populations, different diagnostic criteria |
| Crawley et al. [23] | 2021 | Pediatric patients and relatives | Population epidemiology and registries | High familial risk ratio, strong familial aggregation | Pediatric cohort, national registries |
| Arron et al. [17] | 2024 | Meta-analysis & review | Comprehensive review | Confirms genetic effect with heritability estimates | US health insurance claims, UK Biobank data |
| Gene/Pathway | Proposed Role/Function | Potential Relevance to ME Symptoms |
|---|---|---|
| HLA [26] | Immune system regulation, antigen presentation | Immune dysregulation, chronic inflammation |
| GRIK2 [27] | Glutamate signaling, neuronal excitability | Cognitive dysfunction, neurological symptoms |
| NPAS2 [27] | Circadian rhythm regulation | Sleep disturbances, fatigue |
| CYP2D6 [28] | Drug and toxin metabolism | Impaired detoxification, chemical sensitivities |
| MTHFR [29,30] | Folate metabolism, methylation | Metabolic dysfunction, epigenetic changes |
| Haplogroup | General Characteristics (Europe) | Potential Relevance to ME (Hypothesized) |
|---|---|---|
| H | Most common, generally robust | Potentially protective |
| J | Associated with higher OXPHOS efficiency, but also increased ROS production | May influence neurological symptoms and oxidative stress susceptibility |
| U | Diverse, some sub-haplogroups are linked to specific diseases | Varied impact on energy metabolism and disease risk |
| K | Sub-haplogroup of U, often linked to longevity | Could influence metabolic resilience or vulnerability |
| T | Relatively common | Less clear direct associations, but part of the overall genetic background |
| Epigenetic Mechanism | Description | Potential Impact in ME | Representative Studies |
|---|---|---|---|
| DNA Methylation | Addition of a methyl group to cytosine (CpG sites), often altering transcription factor binding | Altered methylation in immune-related genes (e.g., T-cell activation), metabolic pathways, and neuroendocrine regulation | [39,87,90] |
| Histone Modification | Acetylation, methylation, and phosphorylation of histone proteins | Regulates chromatin accessibility; changes linked to inflammation and impaired energy metabolism | [84,88] |
| MicroRNAs (miRNAs) | Small ncRNAs that post-transcriptionally regulate target mRNAs | Circulating miRNAs (e.g., miR-140-5p, and miR-127-3p) associated with PEM, autonomic dysfunction, and immune dysregulation | [89,91,92] |
| Long Non-coding RNAs (lncRNAs) | Longer ncRNAs modulating chromatin structure and transcription | Suggested roles in chronic inflammation, altered neuronal signaling and oxidative stress | [93,94,95] |
| Extracellular Vesicle (EV)-mediated Epigenetic Regulation | Transfer of miRNAs and other epigenetic regulators via EVs | EV miRNAs modulate neuroimmune pathways | [96,97,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elremaly, W.; Elbakry, M.; Vahdani, Y.; Franco, A.; Moreau, A. The Role of Nuclear and Mitochondrial DNA in Myalgic Encephalomyelitis: Molecular Insights into Susceptibility and Dysfunction. DNA 2025, 5, 53. https://doi.org/10.3390/dna5040053
Elremaly W, Elbakry M, Vahdani Y, Franco A, Moreau A. The Role of Nuclear and Mitochondrial DNA in Myalgic Encephalomyelitis: Molecular Insights into Susceptibility and Dysfunction. DNA. 2025; 5(4):53. https://doi.org/10.3390/dna5040053
Chicago/Turabian StyleElremaly, Wesam, Mohamed Elbakry, Yasaman Vahdani, Anita Franco, and Alain Moreau. 2025. "The Role of Nuclear and Mitochondrial DNA in Myalgic Encephalomyelitis: Molecular Insights into Susceptibility and Dysfunction" DNA 5, no. 4: 53. https://doi.org/10.3390/dna5040053
APA StyleElremaly, W., Elbakry, M., Vahdani, Y., Franco, A., & Moreau, A. (2025). The Role of Nuclear and Mitochondrial DNA in Myalgic Encephalomyelitis: Molecular Insights into Susceptibility and Dysfunction. DNA, 5(4), 53. https://doi.org/10.3390/dna5040053






