Abstract
Background: Chronic kidney disease-associated pruritus (CKD-aP) is a common condition in dialysis patients, and is associated with lower quality of life, depression, and sleep problems. CKD-aP is under-recognized and undertreated. While question 20 of the KDQOL is used for CKD-aP assessment in the clinical setting, recent studies testing novel drugs for CKD-aP have used the WI-NRS. Therefore, evaluating the correlation between KDQOL-Q20 and the WI-NRS may enable the identification of patients who could potentially benefit from these treatments. Methods: This was an observational cohort study of patients receiving in-center hemodialysis from the Mount Sinai Kidney Center. Patients completed a baseline survey on CKD-aP (KDQOL-Q20 and WI-NRS), depression, and sleep quality. A repeat survey was conducted at 4 weeks, with the order of the CKD-aP surveys reversed. We defined moderate/severe CKD-aP as a KDQOL-Q20 score ≥2 and a WI-NRS score ≥ 4. Our outcomes of interest were the correlations of KDQOL-Q20 with the WI-NRS, missed HD treatments, depression, and sleep quality. Correlation analysis was performed with Spearman correlation analysis. Association testing between CKD-aP and outcomes was conducted by relative risk estimation with robust error variance. Results: A total of 112 patients completed the study. According to the WI-NRS, 42% of patients reported itching (score of ≥4) while according to KDQOL-Q20, 57% of patients reported itching (score of ≥2). KDQOL-Q20 and the WI-NRS were strongly correlated (r = 0.7; p < 0.001). Patients who had moderate/severe CKD-aP according to KDQOL-Q20 had a non-statistically significant trend towards a lower risk of missed HD treatments and a higher risk of depression, and a statistically significantly higher risk of sleep-related problems, compared to those with no or mild CKD-aP. Conclusions: CKD-aP is a common condition, and is associated with various clinical outcomes. We found a strong correlation between two CKD-aP measures. These results can help to identify patients for CKD-aP treatment.
1. Introduction
Chronic kidney disease-associated pruritus (CKD-aP) is common, and affects approximately 40% of patients on dialysis [1]. CKD-aP is associated with lower quality of life, depression, and sleep problems [1,2]. The pathophysiology of CKD-aP is multifactorial and complex. Its potential pathways include the deposition of toxins into the skin (e.g., calcium and phosphorous deposition), immune system dysregulation related to inflammation in the skin or even systemic inflammation (e.g., histamine release), and neuropathic pathways (e.g., peripheral neuropathy or opioid imbalance) [3]. New research indicates a significant role of kappa opioid receptor (KOR) dysregulation [4]. Multiple comorbidities, such as cardiovascular disease and liver disease, have been associated with an increased risk of severe itching [5]. Diabetes has also been shown to be associated with itchy skin and a higher severity of CKD-aP [2,6,7]. Various non-FDA-approved treatments are used for the treatment of CKD-aP, including topical treatments, antihistamines, and gabapentin, which have varying efficacy [8]. In August 2021, the FDA approved a novel treatment for CKD-aP, difelikefalin [9].
Despite the high prevalence of CKD-aP and the availability of treatments, it is under-recognized and undertreated [8,10]. A lack of standardized assessments contributes to a lack of provider awareness and treatment of CKD-aP. Currently, there are four scales that are commonly used to assess the presence and severity of CKD-aP: the visual analog scale (VAS), the numeric rating scale, the verbal rating scale, and question 20 from the Kidney Disease Quality of Life—Short Form (KDQOL-Q20) [3]. While some studies use a VAS called the Worst Itch Numeric Rating Scale (WI-NRS), this is not a scale that is commonly used in nephrology patient care. The largest study evaluating CKD-aP in hemodialysis (HD) patients, using data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), used KDQOL-Q20 [2]. Additionally, the KDQOL is commonly administered yearly as part of the Centers for Medicare and Medicaid quality of life assessment mandate [11]. The aim of this study was to evaluate the correlation between the WI-NRS and KDQOL-Q20, and examine the association of CKD-aP with clinical outcomes.
- Key Learning Points [3]:
What was known: Chronic kidney disease-associated pruritus (CKD-aP) is a common condition in dialysis patients, and is associated with lower quality of life, depression, and sleep problems. There are many different potential measures for CKD-aP, but how they correlate to each other has not been previously explored.
- A high prevalence of CKD-aP was found in a diverse urban cohort.
- There was high correlation between two CKD-aP measures, the WI-NRS and KDQOL Q20.
- Moderate-to-severe CKD-aP was associated with a higher risk of sleep-related problems.
- Potential impact: CKD-aP is a common condition, and is associated with various clinical outcomes. We found a strong correlation between two CKD-aP measures. These results can help to identify patients for CKD-aP treatment.
2. Methods
2.1. Study Design
This was a prospective study of patients on in-center HD. The primary outcome evaluated was the correlation between KDQOL-Q20 and the WI-NRS. Additionally, we aimed to evaluate which KDQOL-Q20 score best identifies moderate/severe CKD-aP, as defined by a WI-NRS score ≥ 4. The secondary outcomes evaluated included the association of moderate/severe CKD-aP with missed HD treatments, depression, and sleep problems.
2.2. Study Population
This study recruited patients from the Mount Sinai Kidney Center (MSKC) from March 2022 to June 2022. We used a convenience sampling approach and approached all eligible patients for study inclusion. The dialysis unit serves an ethnically and racially diverse population as a result of its location in East Harlem, New York. Patients’ treating physicians or nurses performed the initial screening process, and eligible patients were those who could provide informed consent and were able to answer surveys without assistance. Patients were included in the study if they were older than 18 years old, undergoing in-center HD for more than 30 days, and receiving HD thrice weekly. Patients were excluded if they had been on HD for less than 30 days, to exclude patients who were on HD due to acute kidney injury, and to allow for changes in medications which could potentially impact patients’ symptoms and thus would cause the results not to be reflective of patients’ steady-state CKD-aP burden. Patients who originally consented, but were hospitalized, and therefore did not complete the surveys, were excluded from the final analysis.
Patient data came from surveys and the Mount Sinai Health System (MSHS) electronic health records (EHRs). Written informed consent was obtained from all patients. This study protocol was reviewed and approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai, under protocol number STUDY-21-01485.
2.3. Measures
We assessed CKD-aP using two scales: the WI-NRS score and the KDQOL-Q20 score. A trained bilingual research coordinator administered the surveys in English and Spanish while the patients were receiving their HD treatment. The coordinator read through the survey and the patients stated their degree of severity of itching based on the scale. For the WI-NRS, patients were asked to rate the severity of their worst itching over the past 24 h, with answers ranging from 0 (no itching) to 10 (worst itching imaginable). KDQOL-Q20 asked patients to what extent over the past 4 weeks they had been bothered by itchy skin, with a range from not bothered at all, as 1, to extremely bothered, as 5. Patients were stratified by race/ethnicity and gender at baseline and randomized to answer the WI-NRS first or KDQOL-Q20 first, to account for potential priming. Surveys were repeated with the reversed order at 30 days to ensure survey reliability (Supplementary Figure S1). We further defined moderate/severe CKD-aP as a WI-NRS score of ≥4. According to the KDQOL-Q20 answer options, a KDQOL of ≥3 is equivalent to moderate/severe CKD-aP. However, since there have been no previous studies identifying which score of KDQOL-Q20 correlates with a WI-NRS score ≥4, we defined moderate/severe CKD-aP based on the results of our correlation analysis, as detailed in the Section 3.
Additionally, we conducted surveys of patients to determine their gender, race, ethnicity, medications, and medical history, which were verified with the EHRs. We extracted laboratory values from the EHRs for the month in which patients were surveyed.
2.4. Outcomes
From the EHRs, we determined the number of missed HD sessions during the 4 weeks before the first survey. We categorized the outcome as no missed HD sessions versus ≥1 missed HD session.
We assessed depression using the Patient Health Questionnaire (PHQ-9), a multipurpose instrument for screening, diagnosing, monitoring, and measuring the severity of depression. The survey was administered at the same time as the baseline CDK-aP assessment. Patients were asked how often they had been bothered by 9 depression-related problems over the last 2 weeks [12]. A combined score of ≤4, by adding the scores from each PHQ-9 question, indicated no-to-mild depression, whereas a score of >4 indicated moderate-to-severe depression-related problems.
We assessed the quality of sleep using the questions asked by the DOPPS II study [2]. The patients were asked to what degree they were bothered by problems with achieving the amount of sleep needed, trouble staying awake during the day, and problems with being awake at night and falling asleep again [2]. Scores ranged from not bothered at all (0) to extremely bothered (4). For any sleep-related problems, we combined the scores from each of the three questions, with a combined score of 0 meaning no sleep problems, and a score of ≥1 meaning any sleep-related problem. The survey was administered at the same time as the baseline CDK-aP assessment.
2.5. Statistical Analysis
We have presented the patients’ baseline characteristics by moderate/severe CKD-aP status based on the WI-NRS and KDQOL-Q20 survey scores. The categorical variables are presented as number (%), and were compared by a X2. test or Fisher’s test, as appropriate. The continuous variables are presented as the mean (SD) when normally distributed, or the median (IQR) for skewed distributions. We used Student’s t-test for normally distributed variables, and the Wilcoxon Mann–Whitney test for skewed variables, for the comparison between groups. We generated stacked bar charts displaying the distribution of the scale of the KDQOL-Q20 baseline survey compared to that of the WI-NRS baseline survey by various categories, and similar charts were generated for the follow-up surveys.
To evaluate the correlation between the two surveys, we conducted Spearman correlation analysis and compared the rates of CKD-aP distribution between the WI-NRS and KDQOL-Q20. To determine what KDQOL-Q20 score would best identify patients with a WI-NRS score ≥ 4, we calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, using a WI-NRS score ≥ 4 as the reference standard. The association between CKD-aP status (determined by the KDQOL-Q20 and WI-NRS scoring methods) and the outcomes were determined by relative risk estimation with robust error variance, adjusted for age, gender, and race/ethnicity. Multivariable logistic regression was conducted to examine the patient characteristics associated with the odds of reporting moderate/severe CKD-aP, adjusted for age, gender, race/ethnicity, comorbidities, and various laboratory values. All analysis was performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). A significance level of 0.05 and 2-sided testing were used throughout, and 95% confidence intervals were reported.
3. Results
3.1. Patient Characteristics
Of the 184 patients who received HD at Mount Sinai Kidney Center, 113 consented to participate, and, ultimately, 112 patients (61%) completed the study (Supplementary Figure S2). Patients had a high comorbidity burden, with 82% having high blood pressure and 38% having diabetes. The mean age of patients was 57 years; 57% were women, 54% were Black, and 39% completed the survey in Spanish (Table 1). The distribution of survey responses by KDQOL-Q20 and the WI-NRS are presented in Supplementary Figure S3.

Table 1.
Baseline characteristics.
3.2. Correlation Between KDQOL and WINRS
As measured by the WI-NRS, 42% of patients reported itching (score of ≥4), while as measured by KDQOL-Q20, 57% of patients reported itching (score of ≥2) (Supplementary Figure S3A,B). The results were similar for the 4-week follow-up surveys (Supplementary Figure S3C,D), and were similar between the surveys that were administered in English and Spanish (Supplementary Figure S4). Of the 64 (57%) patients who reported a score of ≥2 in KDQOL-Q20 survey 1, 50 (78%) of them also reported having a KDQOL-Q20 score ≥ 2 in survey 2 (Supplementary Table S1A). Of the 47 (42%) patients who reported moderate/severe CKD-aP (score of ≥4) in WI-NRS survey 1, 33 (70%) patients also reported similar conditions in WI-NRS survey 2 (Supplementary Table S1B). Patients who had moderate/severe CKD-aP according to KDQOL-Q20 or the WI-NRS had numerically non-significant differences in mean age, cancer, depression, and serum intact PTH (Table 1).
The WI-NRS and KDQOL-Q20 were strongly correlated (r = 0.7; p < 0.001), and the results were similar for the 4-week follow-up survey (r = 0.6, p < 0.001) (Supplementary Table S2). Of the 42% of patients who reported a WI-NRS score of ≥4, 96% of them had a KDQOL-Q20 score of ≥2 (Figure 1A), and the results were similar for the follow-up surveys (Figure 1B). Using results from the baseline survey, a KDQOL-Q20 value of ≥2 had a sensitivity of 96% and a positive predictive value of 70% for a WI-NRS score ≥ 4. Using a KDQOL-Q20 value of ≥3 resulted in a lower sensitivity (55%) and a comparable PPV (79%) for a WI-NRS score ≥ 4 (Supplementary Table S3). Therefore, to ensure capture of patients with moderate/severe CKD-aP as defined by a WI-NRS score of ≥4, we used a KDQOL-Q20 score of ≥2 for further analyses.
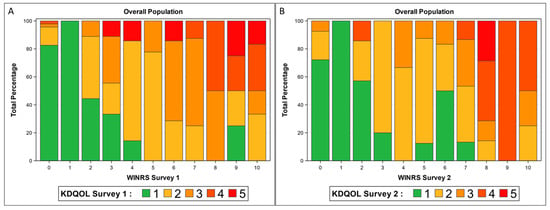
Figure 1.
Distribution of KDQOL survey responses by WI-NRS scores in (A) baseline survey and (B) follow-up survey.
Regarding subgroup analysis, there were no notable differences according to whether patients answered the survey in Spanish or English. (Supplementary Figure S4). The results were also not substantially different by sex (Supplementary Table S4A and Figure S5). Interestingly, analysis by age found notable differences in test parameters when using a KDQOL-Q20 score of ≥3 or ≥2 in patients ≥ 57 years old, but not in those <57 years old (Supplementary Table S4B and Figure S6). In patients ≥ 57 years old, using a KDQOL-Q20 score ≥ 3 resulted in lower sensitivity, specificity, and PPV than in those <57 years old. The subgroup analysis results confirm that the use of a KDQOL-Q20 score of ≥2 would be best for identifying more patients with moderate/severe CKD-aP, but would result in a higher number of false positives.
Regarding the adjusted analyses, we found that older age (aOR 1.07, 95% CI 1.03–1.12) and higher serum phosphorus (aOR 1.79, 95% CI 1.22–2.61) were both significantly associated with higher odds of a KDQOL-Q20 score of ≥2. Similar associations were found for age (aOR 1.06, 95% CI 1.01–1.11) and serum phosphorus (aOR 1.63, 95% 1.08–2.44) for a WI-NRS score of ≥4. There was no association between serum intact parathyroid hormone (iPTH) or Kt/V (Supplementary Table S5) and a KDQOL-Q20 score of ≥2.
3.3. Association with Outcomes
Overall, 27% of patients missed at least one HD treatment, 80% of patients reported depression-related problems, and 67% of patients reported sleep-related problems. Of the patients that had a KDQOL-Q20 score of ≥2, 20% missed at least one HD session, 86% screened positive for depression, and 81% reported sleep disturbance. Of the patients who had a WI-NRS score of ≥4, 23% missed at least one HD session, 85% screened positive for depression, and 81% reported sleep disturbance (Supplementary Table S6).
Regarding the unadjusted analysis, moderate/severe CKD-aP according to KDQOL-Q20 and the WI-NRS were significantly associated with various sleep disturbances, while there was no statistically significant association of moderate/severe CKD-aP with depression or missed HD sessions (Table 2). Multivariable adjustment did not substantially change the results. Sleep-related problems were significantly higher in patients with moderate/severe CKD-aP vs. no or mild CKD-aP as assessed by KDQOL-Q20 (adjusted RR 1.7, 95% CI 1.3–2.4). Those with moderate/severe CKD-aP according to KDQOL-Q20 had a numerically higher prevalence of depression-related problems (RR 1.5, 95% CI 0.9–2.5), and were less likely to miss ≥1 HD session in the prior 4 weeks (RR 0.6, 95% CI 0.3–1.1, p = 0.1), than those with no or mild CKD-aP, although these associations were not statistically significant. The results obtained using the WI-NRS were similar (Figure 2 and Table 2).

Table 2.
Unadjusted and adjusted relative risk for sleep problems, depression, and missing HD for patients with mild vs. moderate/severe CKD-aP.
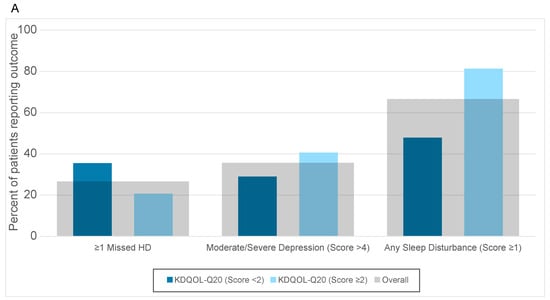
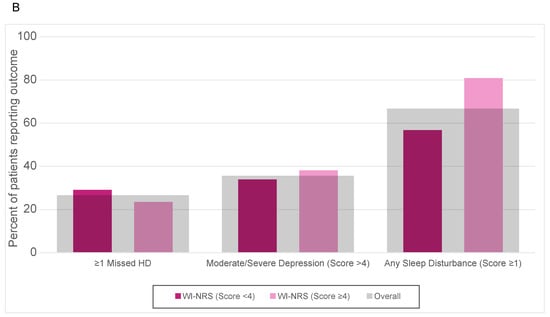
Figure 2.
Outcomes of missed HD, moderate/severe depression, and any sleep disturbance reported by those with CKDaP status according to (A) KDQOL-Q20 and (B) WI-NRS.
4. Discussion
In this study, we demonstrated the high burden of CDKaP in patients who are on maintenance HD. Over half of the patients had moderate/severe CKD-aP as determined by KDQOL-Q20, while over a third of patients had moderate/severe CKD-aP as determined by the WI-NRS. There was a high correlation between the two frequently used CKD-aP assessments, KDQOL-Q20 and the WI-NRS. We also demonstrated that patients who had moderate/severe CKD-aP as determined by KDQOL-Q20 reported higher depression- and sleep-related problems, although this was only statistically significant for sleep-related problems.
The availability of multiple CKD-aP assessment tools makes comparing studies on CKD-aP challenging. Two of the most commonly used tools are the WI-NRS and KDQOL-Q20. The WI-NRS is a single-item visual analog scale used to measure the severity of CKD-aP. KDQOL-Q20 is a survey designed to assess quality of life (QoL) in patients on dialysis. The KDQOL is the most frequently used QoL survey in patients on dialysis, and is obtained yearly, as per CMS mandates. The use of routinely collected CKD-aP surveys can facilitate the recognition and treatment of CKD-aP. In our cohort of HD patients in New York, we found that the WI-NRS and KDQOL-Q20 were highly correlated, and identified a KDQOL-Q20 cutoff value with good positive predictive value for moderate/severe CKD-aP determined by the WI-NRS. These findings can be used to facilitate the conversion of the frequently assessed KDQOL-Q20 scores to WI-NRS scores, to identify individuals who would benefit from treatment.
It is important to highlight several key differences between KDQOL-Q20 and the WI-NRS. One significant difference is the timing period that the questions ask about; while KDQOL-Q20 asks about itching over the past 4 months, the WI-NRS asks about itching over a 24 h period. Variability in the chronicity of symptoms experienced by patients may affect how they answer the two questions. For example, a patient who experiences itching every day will likely answer similarly on both surveys, while a patient who has intermittent itching may report a higher severity on the WI-NRS than on KDQOL-Q20 if surveyed on an itching day. Additionally, these two tools cannot be directly compared, since the WI-NRS ranges from 0 to 10 and the KDQOL-Q20 has five potential answer choices. While KDQOL-Q20 is frequently used clinically, the WI-NRS is more frequently used in research settings. Therefore, determining how the two measures are correlated and determining how the answer responses align with each other is important to facilitate in bringing research results to the bedside.
In one of the largest studies to assess the prevalence of CKD-aP, using DOPPS data from over 10,000 patients, moderate-to-severe CKD-aP determined using KDQOL-Q20 was found to range from 42% to 45% across the two DOPPS cycles [2]. In a more recent study using data from phases 4–6 of the DOPPS, 37% of patients reported moderate-to-severe CKD-aP. There are several notable difference between the two studies. While the DOPPS used a cutoff of ≥3 to define moderate/severe CKD-aP, we used a cutoff of ≥2 for KDQOL-Q20 and ≥4 for the WI-NRS. There are also differences in our patient demographics, with this study having a high proportion of Black and Hispanic patients compared to the DOPPS. In another study of predominantly Black and Hispanic patients on in-center HD from a large dialysis organization, it was found that 30% of patients had moderate-to-severe CKD-aP according to KDQOL-Q20 [13]. While others have reported a decreasing trend in CKD-aP over time, we continue to find a high prevalence of CKD-aP in our population [14].
CKD-aP has been found to have a positive association with lower QoL, depression, sleep quality, and mortality in several observational studies [2,13,15,16]. In our study, we ascertained the association between CKD-aP and missed HD sessions, depression, and sleep quality. We did not find a significant association with depression, although the adjusted point estimates were similar to other studies. This may be related to the small sample size in our cohort. While we did not find significant associations of moderate/severe CKD-aP with individual sleep problems, we did find an association between moderate/severe CKD-aP and any sleep problems. Some studies reported that CKD-aP is aggravated during the night-time, and therefore interferes with sleep quality [17]. We found that over 50% of patients responded positively to at least one sleep quality disturbance, and that having moderate/severe CKD-aP was positively associated with having disturbances in sleep quality. Researchers have found that adjustment of their models for sleep quality diminished the association between CKD-aP and both mortality and QoL [2]. As our study was not designed to evaluate the association of CKD-aP with long-term outcomes, we were unable to assess for mortality. The positive association between moderate/severe CKD-aP and sleep quality suggests that a study investigating the effect of CKD-aP treatment on sleep and depression may be warranted.
Despite the high prevalence of CKD-aP, its pathogenesis remains unclear. An initial theory involved the deposition of toxins in the skin and other subcutaneous tissue as a cause of CKD-aP. This was supported by studies which found associations of CKD-aP with higher calcium, phosphorous, and intact parathyroid hormone levels, and prospective studies which found that lower Kt/V and the use of low-flux dialyzers was associated with worsening CKD-aP [3,18]. Patients with CKD have elevated levels of Vitamin A, which can contribute to xerosis and CKD-aP [19,20]. Conflicting evidence came from another study, where CKD-aP was associated with higher Kt/V [21]. General treatment guidance advocates for the optimization of kidney disease and dialysis parameters (e.g., correct calcium and phosphorus levels and ensuring dialysis adequacy) as key steps in the management of CKD-aP [22].
Currently, the two predominant theories are the immune and opioid hypotheses. In the immune hypothesis, CKD-aP is the result of a systemic proinflammatory state. Fallahzadeh et al. previously found that interleukin-2 was significantly higher in patients with CKD-aP compared to those without [23]. While they did not find increases in other inflammatory markers, Kimmel et al. found elevated C-reactive protein and interleukin-6 levels in patients with CKD-aP compared to those without [24]. Other studies have also found elevation of different inflammatory markers in patients with CKD-aP [3]. Improvement of CKD-aP with the use of anti-inflammatory medications, such as ultraviolet-B phototherapy, thalidomide, and linolenic acid, supports the immune hypothesis [25,26,27]. Zinc deficiency has been suggested to activate the histamine pathway and contribute to CKDa-P; however, one case–control study did not identify a correlation between serum zinc concentration and CKDa-P severity [28]. A small randomized controlled clinical trial of 40 adults found that patients treated with zinc sulfate had significantly lower pruritus scores after 12 weeks of treatment than those treated with a placebo [29]. Iron deficiency has been associated with exacerbating CKDa-P through the upregulation of interleukin 6 and the induction of hepcidin, which are also seen in other inflammatory states [30]. One observational study found that patients with severe CKDa-P had higher mean monthly IV iron doses [13]. Therefore, optimization of iron stores may assist in the management of CKDa-P.
In the opioid hypothesis, there is an imbalance between the mu and kappa opioid receptors [31]. In a recent trial testing a kappa opioid receptor agonist, difelikefalin, over 50% of patients in the intervention group had a reduction in their WI-NRS score of at least three points [9]. While the WI-NRS is not used in the clinical care of patients on dialysis, the KDQOL is administered yearly. On the WI-NRS, a score of ≥4 is generally considered as moderate CKD-aP, while a value of 3 on KDQOL-Q20 is “Moderately bothered” and a value of 2 is only “Somewhat bothered”. In our analysis, we found that a value of ≥2 on KDQOL-Q20 more closely correlated with a WI-NRS score of ≥4. Therefore, we suggest that patients who report a KDQOL-Q20 ≥ 2 on their annual screening should receive additional testing to determine the severity of their CKD-aP, in order to enhance treatment of moderate/severe CKD-aP. Other potential mechanisms of CKD-aP include toxin deposition and peripheral neuropathy.
Treatment of CKD-aP generally follows a stepwise approach [3,32]. First, dialysis adequacy and management of calcium, phosphorous, and intact parathyroid hormone are ensured, which may improve symptoms [33,34,35]. Topical agents such as emollients and analgesics are next in line. Systemic treatment with antihistamines is common, but has displayed limited efficacy. The most widely studied agents are gabapentin and pregabalin, anticonvulsants which may be useful for the treatment of neuropathically driven CKD-aP [36]. Newer agents such as difelikefalin, a kappa opioid receptor agonist, have also shown promise [9]. There is no consensus or guidelines regarding what severity of CKD-aP necessitates treatment, or how patients with this condition should be treated. We are advocates of shared decision making with patients, and the benefits of treatment should be balanced with the burdens of treatment.
Our study has several limitations. We did not include a long-term follow-up of patients, and therefore we cannot comment on the association of CKD-aP with outcomes such as hospitalization or mortality. We did not obtain blood samples from patients, and so were not able to evaluate inflammatory biomarkers. However, we did review the results of clinically obtained blood tests, which did not find differences in calcium, phosphorus, or KT/V. We did not obtain daily WI-NRS surveys, which may have aided in addressing differences in the WI-NRS and KDQOL-QOL scores related to the duration of symptoms asked about by each survey. However, we did repeat the surveys at 30 days, and found that the majority of patients responded in similar fashion to in their baseline surveys. Patients were recruited from a single center from New York City. Patients predominantly self-identified as Black or Hispanic individuals, which does not reflect the national demographics of the U.S. Our sample size have contributed to the lack of significant associations between CKD-aP and clinical outcomes. Additional studies on larger, more diverse cohorts are needed to assess the generalizability of our results.
5. Conclusion
In conclusion, we found a significant correlation between two CKD-aP measures, and determined a KDQOL-Q20 value with a high positive predictive value for moderate/severe CKD-aP as determined by the WI-NRS. There was a positive association between moderate/severe CKD-aP and problems with sleep quality. Our results suggest that patients who respond with a score of ≥2 on KDQOL-Q20 would benefit from additional testing to identify patients who require treatment for their CKD-aP. Further studies are needed to determine whether treatment of CKD-aP will improve clinical outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/kidneydial5020014/s1, Supplemental Table S1: Proportion of patients with moderate/severe CKDaP on survey 1 and 2 by (A) KDQOL-Q20 and (B) WI-NRS, Supplemental Table S2: Correlation coefficients, Supplemental Table S3: Test parameters for prediction of WI-NRS ≥ 4 using a KDQOL-Q20 value of (A) ≥ 2 or (B) ≥ 3, Supplemental Table S4: Test parameters for prediction of WI-NRS ≥ 4 using a KDQOL-Q20 value of ≥2 or ≥3 by (A) sex and (B) age, Supplemental Table S5: Association of patient factors with the odds of having KDQOL-Q20 ≥ 2 and WI-NRS ≥ 4, Supplemental Table S6: Outcomes by KDQOL-Q20 ≥ 2 and WI-NRS ≥ 4, Supplemental Figure S1: Study design, Supplemental Figure S2: Study flow diagram, Supplemental Figure S3: Distribution of survey responses by (A) KDQOL-Q20, (B) WI-NRS on baseline survey and (C) KDQOL-Q20 and (D) WI-NRS on follow up survey, Supplemental Figure S4: Distribution of KDQOL survey responses by WI-NRS scores on (A) baseline and (B) follow up survey in patients who responded to the Spanish surveys (N = 44) and at (C) baseline and (D) follow up survey in patients who responded to the English surveys, Supplemental Figure S5: Distribution of KDQOL survey responses by WI-NRS scores on (A) baseline and (B) follow up survey in male patients and at (C) baseline and (D) follow up survey in female patients, Supplemental Figure S6: Distribution of KDQOL survey responses by WI-NRS scores on (A) baseline and (B) follow up survey patients younger than 57 years and at (C) baseline and (D) follow up survey patients older or equal to 57 years old.
Author Contributions
Conceptualization, L.C., S.C., J.O., T.D. and K.H.; methodology, K.C., S.C. and L.C.; formal analysis, K.C. and L.C.; data curation, H.H.W.; writing—original draft preparation, L.C.; writing—review and editing, K.C., H.H.W., S.C., J.O., T.D., K.H. and L.C.; funding acquisition, J.O., T.D. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from CSL Vifor. The sponsor reviewed and approved the final study design. The sponsor was not involved in the execution or analysis of the study. The sponsor edited the manuscript, but did not have any role in the publishing decisions. LC is supported in part by K23DK124645.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (STUDY-21-01485 approved 20 October 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are not publicly available, due to the fact that they contain information that could compromise the privacy of the participants. They can be potentially made available by contacting the corresponding author, L.C., with appropriate regulatory documentation.
Conflicts of Interest
L.C. has received consulting fees from Vifor Pharma, and is supported in part by K23DK124645. S.G.C. has received consulting fees from Renalytix, Takeda, Nuwellis, Vifor, Bayer, Boehringer-Ingelheim, Reprieve Cardiovascular, Axon, and 3ive; has received financial compensation as a scientific board member and advisor to RenalytixAI; and owns equity in RenalytixAI. J.O., T.D., K.H are former or current employees of Vifor Pharma.
References
- Curtin, R.B.; Bultman, D.C.; Thomas-Hawkins, C.; Walters, B.A.; Schatell, D. Hemodialysis patients’ symptom experiences: Effects on physical and mental functioning. Nephrol. Nurs. J. 2002, 29, 562–574, discussion 75, 98. [Google Scholar] [PubMed]
- Pisoni, R.L.; Wikstrom, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transpl. 2006, 21, 3495–3505. [Google Scholar] [CrossRef]
- Verduzco, H.A.; Shirazian, S. CKD-Associated Pruritus: New Insights Into Diagnosis, Pathogenesis, and Management. Kidney Int. Rep. 2020, 5, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Tey, H.L.; Yosipovitch, G. Targeted treatment of pruritus: A look into the future. Br. J. Dermatol. 2011, 165, 5–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Pollock, C. Epidemiology and burden of chronic kidney disease-associated pruritus. Clin. Kidney J. 2021, 14 (Suppl. 3), i1–i7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afsar, B.; Elsurer Afsar, R. HbA1c is related with uremic pruritus in diabetic and nondiabetic hemodialysis patients. Ren. Fail. 2012, 34, 1264–1269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weisshaar, E.; Weiss, M.; Passlick-Deetjen, J.; Tschulena, U.; Maleki, K.; Mettang, T. Laboratory and dialysis characteristics in hemodialysis patients suffering from chronic itch--results from a representative cross-sectional study. BMC Nephrol. 2015, 16, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rayner, H.C.; Larkina, M.; Wang, M.; Graham-Brown, M.; van der Veer, S.N.; Ecder, T.; Hasegawa, T.; Kleophas, W.; Bieber, B.A.; Tentori, F.; et al. International Comparisons of Prevalence, Awareness, and Treatment of Pruritus in People on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 2000–2007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fishbane, S.; Jamal, A.; Munera, C.; Wen, W.; Menzaghi, F.; Investigators, K.-T. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N. Engl. J. Med. 2020, 382, 222–232. [Google Scholar] [CrossRef]
- Weisshaar, E.; Matterne, U.; Mettang, T. How do nephrologists in haemodialysis units consider the symptom of itch? Results of a survey in Germany. Nephrol. Dial. Transplant. 2009, 24, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.E.; Lee, A.; Sibbel, S.; Benner, D.; Brunelli, S.M.; Tentori, F. Use of the KDQOL-36 for assessment of health-related quality of life among dialysis patients in the United States. BMC Nephrol. 2019, 20, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramakrishnan, K.; Bond, T.C.; Claxton, A.; Sood, V.C.; Kootsikas, M.; Agnese, W.; Sibbel, S. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int. J. Nephrol. Renov. Dis. 2013, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathur, V.S.; Lindberg, J.; Germain, M.; Block, G.; Tumlin, J.; Smith, M.; Grewal, M.; McGuire, D.; Investigators, I.N.R. A longitudinal study of uremic pruritus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1410–1419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weng, C.H.; Hu, C.C.; Yen, T.H.; Hsu, C.W.; Huang, W.H. Uremic Pruritus is Associated with Two-Year Cardiovascular Mortality in Long Term Hemodialysis Patients. Kidney Blood Press. R. 2018, 43, 1000–1009. [Google Scholar] [CrossRef]
- Narita, I.; Alchi, B.; Omori, K.; Sato, F.; Ajiro, J.; Saga, D.; Kondo, D.; Skatsume, M.; Maruyama, S.; Kazama, J.J.; et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006, 69, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Zucker, I.; Boner, G.; Gafter, U.; Shapira, Y.; David, M. A questionnaire for the assessment of pruritus: Validation in uremic patients. Acta Derm. Venereol. 2001, 81, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Wu, H.Y.; Chen, H.Y.; Chiu, Y.L.; Hsu, S.P.; Pai, M.F.; Ju, Y.; Lai, C.F.; Lu, H.M.; Huang, S.C.; et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: A prospective 5-year cohort study. PLoS ONE 2013, 8, e71404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, P.; Garg, V.; Karagaiah, P.; Szepietowski, J.C.; Grabbe, S.; Goldust, M. Chronic Kidney Disease-Associated Pruritus. Toxins 2021, 13, 527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muth, I. Implications of Hypervitaminosis A in Chronic Renal Failure. J. Ren. Nutr. 1991, 1, 2–8. [Google Scholar] [CrossRef]
- Duque, M.I.; Thevarajah, S.; Chan, Y.H.; Tuttle, A.B.; Freedman, B.I.; Yosipovitch, G. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin. Nephrol. 2006, 66, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.A.; Teixeira, J.P.; Germain, M.J. Pruritus in Kidney Disease. Semin. Nephrol. 2015, 35, 383–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fallahzadeh, M.K.; Roozbeh, J.; Geramizadeh, B.; Namazi, M.R. Interleukin-2 serum levels are elevated in patients with uremic pruritus: A novel finding with practical implications. Nephrol. Dial. Transplant. 2011, 26, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, M.; Alscher, D.M.; Dunst, R.; Braun, N.; Machleidt, C.; Kiefer, T.; Stulten, C.; van der Kuip, H.; Pauli-Magnus, C.; Raub, U.; et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiu, W.T.; Wu, M.S. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am. J. Kidney Dis. 2006, 48, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.R.; Viana, P.C.; Lugon, N.V.; Hoette, M.; Ruzany, F.; Lugon, J.R. Thalidomide for the treatment of uremic pruritus: A crossover randomized double-blind trial. Nephron 1994, 67, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Yang, J.Y.; Wu, H.Y.; Hu, F.C.; Chen, S.I.; Tsai, P.J.; Jee, S.H.; Chiu, H.C. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: A randomized controlled trial. Br. J. Dermatol. 2011, 165, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dashti-Khavidaki, S.; Khalili, H.; Vahedi, S.M.; Lessan-Pezeshki, M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity. Saudi J. Kidney Dis. Transpl. 2010, 21, 641–645. [Google Scholar] [PubMed]
- Najafabadi, M.M.; Faghihi, G.; Emami, A.; Monghad, M.; Moeenzadeh, F.; Sharif, N.; Davarpanah Jazi, A.H. Zinc sulfate for relief of pruritus in patients on maintenance hemodialysis. Ther. Apher. Dial. 2012, 16, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron Balance and the Role of Hepcidin in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 87–93. [Google Scholar] [CrossRef]
- Umeuchi, H.; Togashi, Y.; Honda, T.; Nakao, K.; Okano, K.; Tanaka, T.; Nagase, H. Involvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of kappa-opioid system. Eur. J. Pharmacol. 2003, 477, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lipman, Z.M.; Paramasivam, V.; Yosipovitch, G.; Germain, M.J. Clinical management of chronic kidney disease-associated pruritus: Current treatment options and future approaches. Clin. Kidney J. 2021, 14 (Suppl. 3), i16–i22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hiroshige, K.; Kabashima, N.; Takasugi, M.; Kuroiwa, A. Optimal dialysis improves uremic pruritus. Am. J. Kidney Dis. 1995, 25, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Momose, A.; Kudo, S.; Sato, M.; Saito, H.; Nagai, K.; Katabira, Y.; Funyu, T. Calcium ions are abnormally distributed in the skin of haemodialysis patients with uraemic pruritus. Nephrol. Dial. Transpl. 2004, 19, 2061–2066. [Google Scholar] [CrossRef]
- Blachley, J.D.; Blankenship, D.M.; Menter, A.; Parker, T.F., 3rd; Knochel, J.P. Uremic pruritus: Skin divalent ion content and response to ultraviolet phototherapy. Am. J. Kidney Dis. 1985, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Eusebio-Alpapara, K.M.V.; Castillo, R.L.; Dofitas, B.L. Gabapentin for uremic pruritus: A systematic review of randomized controlled trials. Int. J. Dermatol. 2020, 59, 412–422. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).