Anemia Is a Predictor of Withdrawal from Peritoneal Dialysis in Stable Peritoneal Dialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
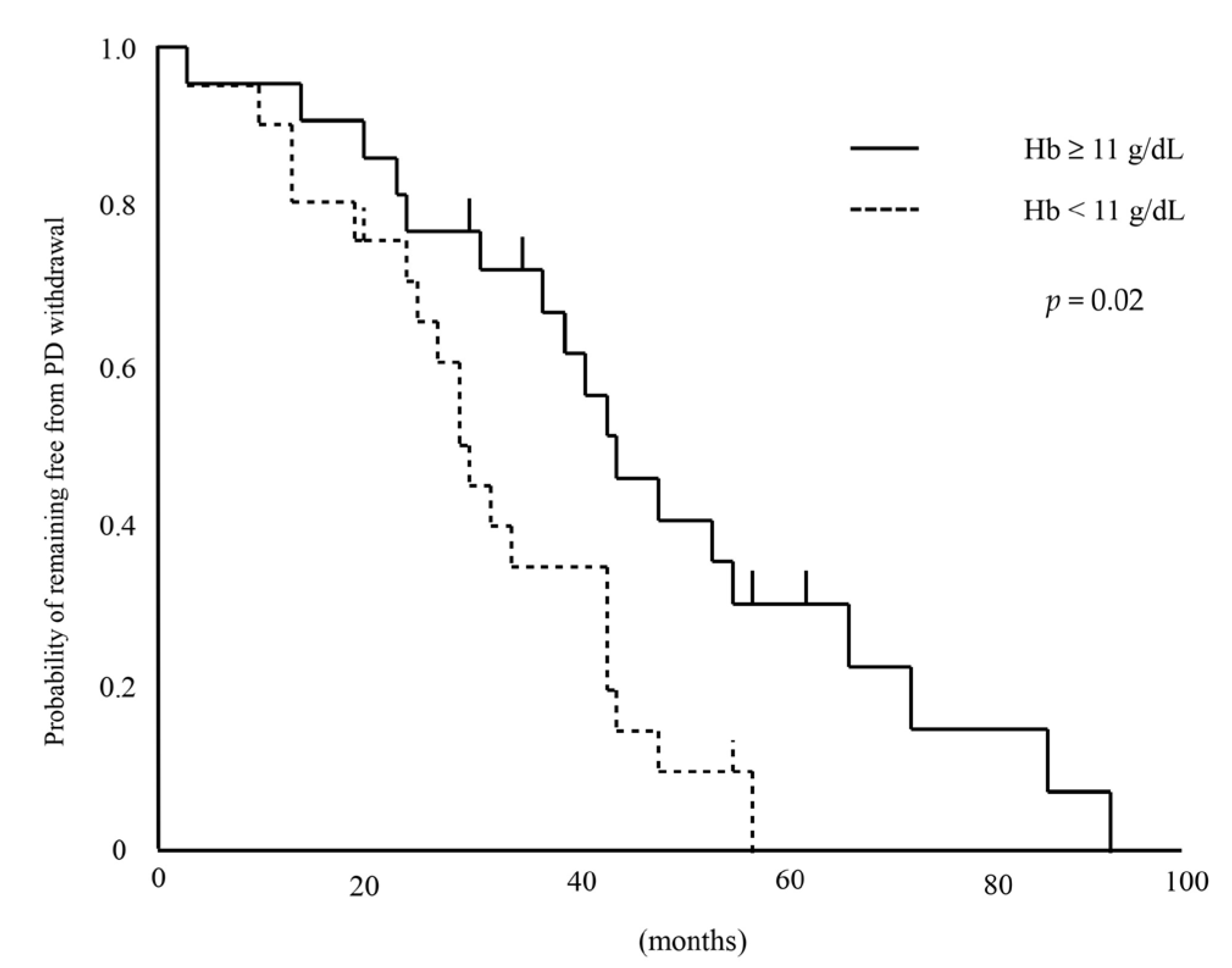
3.2. Survival Analysis by Hemoglobin
3.3. Cause of PD Withdrawal
3.4. Association Between Anemia and Residual Renal Function Decline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Peritoneal dialysis |
| PET | Peritoneal equilibration test |
| Hb | Hemoglobin |
| ESA | Erythropoiesis-stimulating agent |
| ESRD | End-stage renal disease |
| HD | Hemodialysis |
| CCI | Charlson Comorbidity Index |
| CI | Confidence interval |
| HR | Hazard ratio |
References
- Tokgoz, B. Clinical advantages of peritoneal dialysis. Perit. Dial. Int. 2009, 29, S59–S61. [Google Scholar] [CrossRef] [PubMed]
- Banno, T.; Shima, H.; Kawahara, K.; Okada, K.; Minakuchi, J. Risk factors for peritoneal dialysis withdrawal due to peritoneal dialysis-related peritonitis. Nephrol. Ther. 2021, 17, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Ishizaki, T.; Imada, A.; Oohira, S.; Kuriyama, S.; Nakamoto, H.; Nakamoto, M.; Hiramatu, M.; Maeda, K.; Ota, K.; et al. Searching for the reasons for drop-out from peritoneal dialysis: A nationwide survey in Japan. Perit. Dial. Int. 2003, 23, S175–S177. [Google Scholar] [PubMed]
- Nagasaka, T.; Washida, N.; Uchiyama, K.; Hama, E.Y.; Kusahana, E.; Nakayama, T.; Yasuda, I.; Morimoto, K.; Itoh, H. Health-Related quality of life sleep score predicts transfer to hemodialysis among patients on peritoneal dialysis. Healthcare 2022, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Mehrotra, R.; Duong, U.; Kovesdy, C.P.; Kalantar-Zadeh, K. Association of hemoglobin and survival in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.; Li, N.C.; Maddux, F.; Hakim, R.; Finkelstein, F.O.; Lacson, E., Jr. First-year outcomes of incident peritoneal dialysis patients in the United States. Am. J. Kidney Dis. 2014, 64, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Xia, X.; Lin, Z.; Lin, J.; Yang, X.; Huang, F.; Yu, X. Very early withdrawal from treatment in patients starting peritoneal dialysis. Ren. Fail. 2018, 40, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I.; Saito, K.; Nangaku, M.; Hattori, M.; Suzuki, T.; Morita, S.; Ashida, A.; et al. 2015 Japanese Society for Dialysis Therapy: Guidelines for Renal Anemia in Chronic Kidney Disease. Ren. Replace Ther. 2017, 3, 36. [Google Scholar] [CrossRef]
- Mizuno, M.; Ito, Y.; Tanaka, A.; Suzuki, Y.; Hiramatsu, H.; Watanabe, M.; Tsuruta, Y.; Matsuoka, T.; Ito, I.; Tamai, H.; et al. Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin. Exp. Nephrol. 2011, 15, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Tomonari, H.; Yoshida, H.; Hashimoto, T.; Kawaguchi, Y.; Sakai, O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 1997, 77, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Buliga-Finis, O.N.; Ouatu, A.; Tanase, D.M.; Gosav, E.M.; Seritean Isac, P.N.; Richter, P.; Rezus, C. Managing anemia: Point of convergence for heart failure and chronic kidney disease? Life 2023, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Hasegawa, T.; Kosugi, T.; Nishiwaki, H.; Abe, M.; Hanafusa, N.; Honda, H.; Tsuruya, K.; Ito, Y.; Kuragano, T. Renal anemia and hyporesponsiveness to ESA for preservation of residual kidney function in patients undergoing peritoneal dialysis. Sci. Rep. 2025, 15, 2689. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Bašić-Jukić, N.; Vujičić, B.; Klarić, D.; Radulović, G.; Jakić, M.; Jurić, K.; Altabas, K.; Grđan, Z.; Kovačević-Vojtušek, I.; et al. Anemia is correlated with malnutrition and inflammation in croatian peritoneal dialysis patients: A multicenter nationwide study. Perit. Dial. Int. 2017, 37, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Flores, I.; Coronel, F.; Cigarrán, S.; Herrero, J.A.; Calvo, N. Relationship between residual renal function, inflammation, and anemia in peritoneal dialysis. Adv. Perit. Dial. 2007, 23, 140–143. [Google Scholar] [PubMed]
- Zhang, C.; Wang, J.; Xie, X.; Sun, D. Low serum vitamin D concentration is correlated with anemia, microinflammation, and oxidative stress in patients with peritoneal dialysis. J. Transl. Med. 2021, 19, 411. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Chen, Y.; Hao, C.; Ge, X.; Xie, Q.; Shang, D.; Zhu, T. Addition of roxadustat to erythropoiesis-stimulating agent (ESA) effectively corrects ESA-hyporesponsive anaemia in patients on peritoneal dialysis. J. Clin. Pharm. Ther. 2022, 47, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liang, W.; Ye, T.; Chen, Z.; Zuo, X.; Du, X.; Qian, K.; Zhang, C.; Hu, X.; Li, J.; et al. The association between nutritional markers and biochemical parameters and residual renal function in peritoneal dialysis patients. PLoS ONE 2016, 11, e0156423. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Heimbürger, O.; Stenvinkel, P.; Bergström, J.; Lindholm, B. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol. Dial. Transplant. 2001, 16, 2240–2245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, A.Y.; Woo, J.; Wang, M.; Sea, M.M.; Sanderson, J.E.; Lui, S.F.; Li, P.K. Important differentiation of factors that predict outcome in peritoneal dialysis patients with different degrees of residual renal function. Nephrol. Dial. Transplant. 2005, 20, 396–403. [Google Scholar] [CrossRef] [PubMed]
- KDIGO Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
| All (n = 43) | Hb < 11 g/dL (n = 21) | Hb ≥ 11 g/dL (n = 22) | p-Value | |
|---|---|---|---|---|
| Age (years) | 60.9 ± 12.0 | 59.0 ± 2.6 | 62.7 ± 2.6 | 0.32 |
| Sex (Male—Female) | 26:17 | 11:10 | 15:7 | 0.36 |
| BMI | 22.6 (20.5–25.2) | 22.6 (20.5–25.4) | 22.7 (20.3–25.1) | 0.91 |
| Systolic BP (mmHg) | 130.6 ± 26.3 | 135.2 ± 5.7 | 126.2 ± 5.6 | 0.27 |
| Diastolic BP (mmHg) | 76.3 ± 16.0 | 80.3 ± 3.4 | 72.4 ± 3.4 | 0.11 |
| Duration of PD (months) | 14 (11–24) | 12 (8.5–25) | 14(12–24.5) | 0.13 |
| Diabetes mellitus | 25.6 | 28.6 | 22.7 | 0.74 |
| Charlson Comorbidity Index | 2 (2–4) | 2 (2–4) | 2.5 (2–3.25) | >0.99 |
| Number of peritonitis episodes (n) | ||||
| 0 | 37 | 18 | 19 | 0.71 |
| 1 | 3 | 1 | 2 | |
| 2 | 3 | 2 | 1 | |
| >3 | 0 | 0 | 0 | |
| ARB/ACE-I | 62.8 | 47.6 | 77.3 | 0.06 |
| CCB | 62.8 | 61.9 | 63.6 | >0.99 |
| Diuretic | 83.7 | 85.7 | 81.8 | 0.77 |
| Statin | 27.9 | 23.8 | 31.8 | 0.40 |
| ESA does (μg/4 weeks) | 50 (25–120) | 120 (62.5–150) | 32.5 (0–50) | <0.01 |
| ERI (μg/kg/g/dL/4 weeks) | 0.082 (0.032–0.204) | 0.200 (0.101–0.232) | 0.052 (0–0.083) | <0.01 |
| Iron supplement | 23.3 | 33.3 | 13.6 | 0.16 |
| CAPD—APD | 28:15 | 13:8 | 15:7 | 0.75 |
| Hb (g/dL) | 11 (10.1–11.8) | 10.1 (10.0–10.5) | 11.8 (11.1–12.8) | <0.01 |
| Alb (g/dL) | 3.2 ± 0.4 | 3.1 ± 0.1 | 3.3 ± 0.1 | 0.11 |
| Corrected Ca (mg/dL) | 9.2 ± 0.6 | 9.1 ± 0.1 | 9.3 ± 0.1 | 0.47 |
| P (mg/dL) | 5.3 ± 1.2 | 5.8 ± 0.3 | 4.9 ± 0.2 | 0.01 |
| Intact PTH (pg/mL) | 171.9 (97–260.2) | 153 (104.1–268.6) | 179.2 (91.6–250.3) | 0.95 |
| Ferritin (ng/mL) | 156 (107–252) | 156 (80–252) | 157.5 (111.3–258.3) | >0.99 |
| TSAT (%) | 35.5 ± 17.4 | 31.7 ± 3.9 | 39.1 ± 3.8 | 0.17 |
| CRP (mg/dL) | 0.06 (0.03–0.22) | 0.06 (0.02–0.22) | 0.07 (0.04–0.24) | 0.54 |
| Urinary volume (dL/day) | 14.5 (10.0–17.0) | 12.0 (6.7–16.5) | 15.0 (12.0–17.0) | 0.07 |
| Peritoneal Kt/V | 1.03 ± 0.37 | 1.11 ± 0.08 | 0.96 ± 0.08 | 0.17 |
| Renal Kt/V | 0.79 (0.62–1.23) | 0.64 (0.41–0.82) | 0.96 (0.75–1.52) | <0.01 |
| Total Kt/V | 1.96 ± 0.52 | 1.84 ± 0.11 | 2.08 ± 0.11 | 0.13 |
| 4-h D/P Cr | 0.6 (0.56–0.68) | 0.6 (0.58–0.67) | 0.61 (0.53–0.69) | 0.89 |
| nPCR | 0.86 ± 0.18 | 0.82 ± 0.04 | 0.90 ± 0.04 | 0.12 |
| Urinary protein (g/gCr) | 0.89 (0.54–1.35) | 1.13 (0.75–2.25) | 0.75 (0.43–1.05) | 0.048 |
| Urinary protein (g/day) | 0.51 (0.28–0.78) | 0.51 (0.31–0.77) | 0.51 (0.27–0.83) | 0.92 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (years) | 1.00 (0.97–1.03) | 0.78 | 1.00 (0.96–1.04) | 0.99 |
| Sex (Male) | 1.20 (0.62–2.34) | 0.59 | 1.15 (0.43–3.04) | 0.78 |
| BMI | 0.79 (0.90–1.07) | 0.81 | ||
| Systolic BP (mmHg) | 1.01 (0.99–1.02) | 0.22 | ||
| Diastolic BP (mmHg) | 1.00 (0.98–1.03) | 0.66 | ||
| Duration of PD (months) | 1.00 (0.97–1.03) | 0.99 | 1.00 (0.96–1.03) | 0.81 |
| Diabetes mellitus | 1.53 (0.73–3.21) | 0.27 | ||
| Charlson Comorbidity Index | 1.04 (0.77–1.34) | 0.79 | 0.95 (0.67–1.30) | 0.77 |
| Number of peritonitis episodes | 1.16 (0.62–1.92) | 0.60 | ||
| ARB/ACE-I | 0.94 (0.47–1.86) | 0.85 | ||
| CCB | 1.05 (0.52–2.13) | 0.89 | ||
| Diuretic | 1.76 (0.67–4.58) | 0.22 | ||
| Statin | 0.76 (0.35–1.64) | 0.47 | ||
| ESA does (μg/4 weeks) | 1.00 (1.00–1.01) | 0.11 | ||
| ERI (μg/kg/g/dL/4 weeks) | 13.18 (0.76–149.53) | 0.07 | ||
| Iron supplement | 1.35 (0.63–2.91) | 0.46 | ||
| CAPD—APD | 0.62 (0.30–1.27) | 0.20 | ||
| Hb (g/dL) | 0.71 (0.56–0.92) | 0.009 | 0.73 (0.54–0.99) | 0.04 |
| Alb (g/dL) | 0.14 (0.04–0.46) | 0.001 | ||
| Corrected Ca (mg/dL) | 0.76 (0.45–1.26) | 0.29 | ||
| P (mg/dL) | 2.81 (0.87–1.62) | 0.28 | ||
| Intact PTH (pg/mL) | 1.00 (0.99–1.00) | 0.52 | ||
| Ferritin (ng/mL) | 1.00 (0.99–1.00) | 0.94 | ||
| TSAT (%) | 0.99 (0.97–1.02) | 0.56 | ||
| CRP (mg/dL) | 1.60 (0.42–4.32) | 0.44 | ||
| Urinary volume (dL/day) | 0.90 (0.85–0.97) | 0.006 | 0.93 (0.86–1.01) | 0.09 |
| Peritoneal Kt/V | 1.20 (0.51–2.74) | 0.66 | ||
| Renal Kt/V | 0.51 (0.22–1.04) | 0.06 | ||
| Total Kt/V | 0.56 (0.25–1.15) | 0.12 | ||
| 4-h D/P Cr | 7.09 (0.31–191.09) | 0.23 | ||
| nPCR | 0.27 (0.03–2.60) | 0.26 | ||
| Urinary protein (g/gCr) | 1.24 (1.06–1.41) | 0.009 | ||
| Urinary protein (g/day) | 1.44 (0.87–2.11) | 0.14 | ||
| Hb < 11 g/dL (n = 18) | Hb ≧ 11 g/dL (n = 18) | p-Value | |
|---|---|---|---|
| Inadequate dialysis (uremia or volume overload) | 13 (72.2%) | 9 (50%) | 0.03 |
| Death | 2 (11.1%) | 7 (39.9%) | |
| PD-related infection | 3 (16.7%) | 0 (%) | |
| Others | 0 (0%) | 2 (11.1%) | |
| Total | 18 | 18 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Standard β | p-Value | Standard β | p-Value | |
| Age | −0.37 | 0.01 | −0.22 | 0.18 |
| Sex (Female) | 0.36 | 0.02 | 0.09 | 0.61 |
| BMI | −0.02 | 0.88 | ||
| Systolic BP (mmHg) | 0.26 | 0.09 | ||
| Diastolic BP (mmHg) | 0.28 | 0.07 | ||
| Duration of PD (months) | 0.08 | 0.63 | −0.02 | 0.92 |
| Diabetes mellitus | −0.09 | 0.55 | ||
| Charlson Comorbidity Index | −0.23 | 0.13 | −0.13 | 0.39 |
| Number of peritonitis episodes | 0.15 | 0.95 | ||
| ARB/ACE-I | −0.20 | 0.19 | ||
| CCB | 0.03 | 0.84 | ||
| Diuretic | −0.22 | 0.16 | ||
| Statin | 0.11 | 0.48 | ||
| ESA does (μg/4 weeks) | 0.27 | 0.08 | ||
| ERI (μg/kg/g/dL/4 weeks) | 0.24 | 0.12 | ||
| Iron supplement | 0.41 | 0.01 | ||
| CAPD—APD | 0.13 | 0.41 | ||
| Hb (g/dL) | −0.34 | 0.02 | −0.35 | 0.03 |
| Alb (g/dL) | −0.16 | 0.31 | ||
| Corrected Ca (mg/dL) | −0.13 | 0.41 | ||
| P (mg/dL) | 0.05 | 0.74 | ||
| Intact PTH (pg/mL) | −0.11 | 0.50 | ||
| Ferritin (ng/mL) | −0.10 | 0.57 | ||
| TSAT | −0.09 | 0.58 | ||
| CRP (mg/dL) | 0.06 | 0.70 | ||
| Urinary volume (dL/day) | 0.22 | 0.17 | 0.22 | 0.16 |
| Peritoneal Kt/V | −0.13 | 0.41 | ||
| Renal Kt/V | 0.05 | 0.77 | ||
| Total Kt/V | −0.04 | 0.78 | ||
| 4-h D/P Cr | −0.10 | 0.55 | ||
| nPCR | −0.17 | 0.28 | ||
| Urinary protein (g/gCr) | 0.27 | 0.08 | ||
| Urinary protein (g/day) | 0.32 | 0.04 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torigoe, K.; Otsuka, E.; Tsuji, K.; Yamashita, A.; Kitamura, M.; Takazono, T.; Sakamoto, N.; Muta, K.; Mukae, H.; Nishino, T. Anemia Is a Predictor of Withdrawal from Peritoneal Dialysis in Stable Peritoneal Dialysis Patients. Kidney Dial. 2025, 5, 15. https://doi.org/10.3390/kidneydial5020015
Torigoe K, Otsuka E, Tsuji K, Yamashita A, Kitamura M, Takazono T, Sakamoto N, Muta K, Mukae H, Nishino T. Anemia Is a Predictor of Withdrawal from Peritoneal Dialysis in Stable Peritoneal Dialysis Patients. Kidney and Dialysis. 2025; 5(2):15. https://doi.org/10.3390/kidneydial5020015
Chicago/Turabian StyleTorigoe, Kenta, Emiko Otsuka, Kiyokazu Tsuji, Ayuko Yamashita, Mineaki Kitamura, Takahiro Takazono, Noriho Sakamoto, Kumiko Muta, Hiroshi Mukae, and Tomoya Nishino. 2025. "Anemia Is a Predictor of Withdrawal from Peritoneal Dialysis in Stable Peritoneal Dialysis Patients" Kidney and Dialysis 5, no. 2: 15. https://doi.org/10.3390/kidneydial5020015
APA StyleTorigoe, K., Otsuka, E., Tsuji, K., Yamashita, A., Kitamura, M., Takazono, T., Sakamoto, N., Muta, K., Mukae, H., & Nishino, T. (2025). Anemia Is a Predictor of Withdrawal from Peritoneal Dialysis in Stable Peritoneal Dialysis Patients. Kidney and Dialysis, 5(2), 15. https://doi.org/10.3390/kidneydial5020015








