Abstract
The dynamic relationship between microplastics (MPs) in the air and on the Earth’s surface involves both natural and anthropogenic forces. MPs are transported from the ocean to the air by bubble scavenging and sea spray formation and are released from land sources by air movements and human activities. Up to 8.6 megatons of MPs per year have been estimated to be in air above the oceans. They are distributed by wind, water and fomites and returned to the Earth’s surface via rainfall and passive deposition, but can escape to the stratosphere, where they may exist for months. Anthropogenic sprays, such as paints, agrochemicals, personal care and cosmetic products, and domestic and industrial procedures (e.g., air conditioning, vacuuming and washing, waste disposal, manufacture of plastic-containing objects) add directly to the airborne MP load, which is higher in internal than external air. Atmospheric MPs are less researched than those on land and in water, but, in spite of the major problem of a lack of standard methods for determining MP levels, the clothing industry is commonly considered the main contributor to the external air pool, while furnishing fabrics, artificial ventilation devices and the presence and movement of human beings are the main source of indoor MPs. The majority of airborne plastic particles are fibers and fragments; air currents enable them to reach remote environments, potentially traveling thousands of kilometers through the air, before being deposited in various forms of precipitation (rain, snow or “dust”). The increasing preoccupation of the populace and greater attention being paid to industrial ecology may help to reduce the concentration and spread of MPs and nanoparticles (plastic particles of less than 100 nm) from domestic and industrial activities in the future.
1. Introduction
Studies on microplastics (MPs) in air are recent and few. Possibly the first was published by Dris et al., [1] discussing microplastic contamination in Parisian air; in 2016, they reported that 29% of atmospheric fibers in the air around Paris were petrochemical-based and were more prevalent at urban than non-urban sites. The distribution and movement of these particles through the air is influenced by climatic, meteorological, and human factors [2]. Atmospheric processes involved in MP transport include wind speed and direction, convection, turbulence and vertical drafts. These, as well as transport to and retention in the stratosphere, are under-studied aspects of atmospheric MPs and NPs [3]. In this review, we are particularly interested in the dynamic relationship that exists between MPs and air and water, initially examining the removal of MPs from the atmosphere by rain events and their addition to the air by aerosols, and then considering artificial, human activities that reduce or increase atmospheric MPs in industrial and domestic situations. Throughout, we emphasize the problems created by the lack of standard methods for MP enumeration and analysis. Sampling, arguably the most important step, is fraught with difficulties, and the characterization of MPs in environmental samples is still a challenge. Analytical techniques currently include pyrolysis–gas chromatography–mass spectrometry; matrix-assisted laser desorption/ionization–mass spectrometry; fluorescence, infrared, Raman, and UV–vis spectrometries; X-ray photoelectron spectroscopy; energy-dispersive X-ray spectroscopy and atomic force microscopy; scanning electron microscopy; and transmission electron microscopy.
For this review, we selected the relevant literature using traditional search engines such as Google Scholar and Scopus, with keywords that included “microplastics” together with one or more of the words “aerial, atmosphere, air, transport, clouds, aerosols, rain, wet deposition, air conditioning, condensates, agricultural, industrial”, as well as serendipitous encounters.
2. Atmospheric Circulation of MPs
Compared to the availability of MP research in marine and terrestrial environments, studies on atmospheric MPs have only gained focus during the last few years [4,5,6,7,8]. The air is not a final destination for MPs, but a transport mechanism; nevertheless, they may spend a considerable amount of time as aerial particles, especially under appropriate ventilation or wind conditions. It has been calculated that the smallest particles (less than 10 µm), of moderate hydrophobicity, remain in still air for an approximate average of 8.3 ± 1.0 days; air movements can prolong this, as well as potentially moving MPs to another location. For instance, a marine wind farm could be responsible for moving MPs not only from the air, but also from seawater, sediment and the ground.
The majority of studies on aerial MPs report results of atmospheric deposition measurements. Quantity, size, morphology and components have been recorded and evaluated from urbanized, rural and remote locations [1,9,10,11,12]. Deposition rates in remote areas, for example, vary from 50 to 700 MPs m−2d−1 [2,13,14,15,16,17].
However, the global migration pathways of MPs from their origins to remote areas are still only superficially understood [13,18,19]. Initial studies mostly considered riverine outflows as the main source of MPs to the open ocean [12,19,20,21,22,23], but latterly studies have reported MPs in remote regions lacking local water discharge sources or riverine influences, including mountainous areas [13,16,24], Arctic and Antarctic snow [12,25,26,27], deep-sea polar environments [28,29] and in the surface air layers over the remote Pacific and Indian oceans [30,31]. These records suggest that MPs may move through the atmosphere over long distances. Figure 1 summarizes the flow of MPs over the land. Measurements of MPs in the Western USA, combined with inverse modelling, have suggested that road MPs are mostly deposited in central Europe, S-E Asia and the East USA coast, and that only 1.4% of land MPs are transmitted to the ocean, the latter amount calculated as 0.418 ± 0.201 Tg y−1.
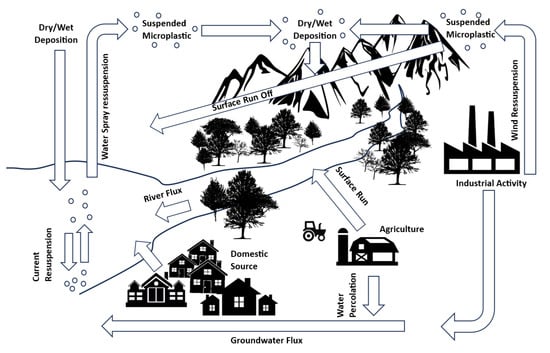
Figure 1.
Microplastic flux over inland areas, showing terrestrial sources of MPs (domestic, industrial and agricultural), their suspension into the air by wind currents and their redeposition onto the ground and into rivers and larger water bodies, whence they can be resuspended in the air. Note that “Industrial activity” includes wastewater treatment plants.
MPs are omnipresent in the atmosphere; their concentrations vary in different areas as a result of altitude, latitude and ecosystem characteristics [30], fluctuating between seasons [31]. Greater numbers of MPs have been recorded at lower heights than at higher altitudes (a vertical concentration gradient) as a result of atmospheric pressure and gravity [30]. However, the temperature variation in the atmospheric column promotes the ascending circulation of MPs, unless the weather conditions have induced temperature inversion [32]. MPs may also be blocked near the ground, preventing their dispersal.
Chemical transformations affect the dynamic of MPs in the atmosphere. UV rays emitted by solar activity contribute to MP photodegradation, changing their surface properties, generally to become more hydrophilic, and thus altering their diffusion dynamics and behavior towards external agents such as contaminating chemical compounds and potential colonizing microorganisms [33]. The aging process induced by solar rays tends to decrease particle size, making it easier for them to be transported through air fluxes. In parallel, atmospheric O2, SO2 and NO2 contribute to the disruption of the polymer chain.
Air flux dynamics around the shoreline of water bodies can influence MP distribution; when the air mass comes from onshore (a land breeze), resulting from temperature differences between the continental and water surfaces, there is, apparently, a higher deposition of MPs compared to a predominantly sea breeze. The deposited particles can, of course, be resuspended in the grasshopping process [34,35], a cyclical exchange between air, water and land; in fact, several authors have suggested that the MP atmospheric pool represents the main MP source for the ocean [32,36,37,38,39,40]. It has been suggested that this atmospheric MP pool comes mainly from textiles [41,42,43,44,45], especially in the case of microfibers, with the wearing and washing of clothes the most important source for indoor air [46,47,48]. Agriculture, road and vehicular emissions and the industrial production of clothing are the major sources of MPs suggested for outdoor air [44,48,49,50]. The release of MPs from industrial processes may become reduced in the future, with the increasing attention to industrial ecology (IE) [51], using sustainable production and distribution, recycling, and end-of-life waste and emission management, particularly if the developing world also accepts the tenets of IE. The situation in the environment is more fragile, with governmental actions required to reduce the use and release of MPs and nanoparticles (NPs).
3. Influence of Rain on Atmospheric MPs
Rainfall is an important transport mechanism for the removal of MPs from the air, carrying them to soil or the Earth’s aqueous resources. Hitchcock [52], for example, found that the levels of atmospheric MPs in Cooks River estuary, Australia, rose from 400 particles/m3 before a 5-day storm event to up to 17,383 particles/m3 after the event. It must, of course, be recognized that MPs may also be washed into rivers from the nearby land by heavy rain. The Cooks River is located in Sydney, a large urban conglomerate, where aerial concentrations of MPs would be expected to be high, and the release of MPs into local water sources, from surrounding soil, for example, would be expected to be under some control. On the other hand, Wei et al. [53], studying atmospheric MPs in the Qing River, Beijing, found that MP concentrations decreased slightly (1164.11/m3 before and 1037.04/m3 after rainfall), but with rain-induced fragmentation of the larger MPs leading to more small-sized particles.
The importance of rain to the MP air load was demonstrated by Chen et al. [54], who showed that the first rainfall after a long period of dry weather in Beijing contained higher numbers and diversity of morphology and polymer constitution of MPs than the following seven collections; these MPs were highly fragmented. The wet deposition rate was calculated as 146–8629 items·m–2 per rain event. Rain-driven MP deposition rates have been calculated in various places around the world. MP-laden air liberates much of its particulate burden during the first minutes of precipitation, the so-called “first flush effect” [55,56,57,58], and this can lead to mistaken MP deposition rates if rain collections do not include the beginning of the downpour. Bearing in mind this caveat, together with other differences in collection and analysis, as shown in Table 1, we can quote some calculated and published wet deposition rates (Table 1).

Table 1.
Microplastics (MPs) and nanoplastics (NPs) detected in various locations in wet deposition samples.
The “Comments” line of Table 1 shows that the methods used in determining MP deposition are extremely variable. This is discussed further at the end of this section.
Li et al. [65], working in the Quzhou region of China, determined that light rain (less than 2.5 mm/h) has a better MP removal effect than heavy (10–50 mm/h) rain. In spite of this, the MP atmospheric deposition rate was higher in the wet than the dry season (84.00 ± 6.95 items/m2/d compared to 47.88 ± 8.35 items/m2/d), presumably because of greater quantities of rain each day in the wet season. Other workers have also shown higher rain deposition rates in the wet season. For example, in the North China Plain [66], the figure of 892–75,421 particles/m2/day in the wet summer was much higher than the next highest (spring) figure of 735–9428 particles/m2/day. It should also be noted that heavy rain especially tends to reduce suspended particle size, increasing total deposition numbers [53], which would result in higher MP levels later in the rainfall event, gainsaying the “first flush effect”. It should also be borne in mind that rain is often accompanied by wind; the influence of wind velocity on atmospheric MP deposition numbers quoted by various workers [63,69,70,71,72] could be a function of accompanying rainfall, or vice versa. On the other hand, in dry conditions, the wind may play a more important role. High winds may move surface layers of soil that contain MPs used in fertilizers or as carriers of pesticides into the air, where they will remain until the wind drops, along with any other light MPs. Wind can also erode the surfaces of materials such as discarded plastics in landfills [73,74,75] increasing the aerial load. It is important to note that windy conditions may lead to underestimation of the amount of MPs caught by the sampling apparatus because of decreased collection efficiency.
Zhang et al. [68] suggested that MPs enter remote mountainous lakes mainly through atmospheric deposition (rain/snow). Working in the Tibetan Plateau, they found that fibers and films were the major MP morphologies and they could be transported through the atmosphere for up to 800 km. Fibers are the major types of MPs usually reported in the literature on rain washout of atmospheric MPs [68,69,70,76,77]. This may be because their shape, with greater surface area, allows more water collisions. Many publications cite clothing textiles as the major source of atmospheric fibers [13,38,69,77,78,79,80], though not all of these are petrochemical-based. Some of the textile fibers will have been shed during activities of the local population, although clothing manufacturers are also responsible; this process has been estimated, by ultrasonic washing of newly produced clothes, to produce 49% of the total fibers released by the procedure, plus those from simulated wet washing and wearing. It has been calculated that about 0.12 Tg y−1 of synthetic microfibers is released into the environment each year from clothing production (equivalent to one shirt for every 500 manufactured). The recent periods of lockdown imposed on the population during the COVID-19 epidemic led to huge reductions in human activity, one of the factors said to influence MP levels in the air. During the Spring 2020 lockdown in France, MP deposition rates were considerably reduced, with median rates of 5.4 MP m−2.d−1 in 2020 against 29.2 MP m−2.d−1 in 2021 [81]. Wet (and dry) deposition rates of MPs are certainly influenced by the human population, not only because of MPs released from their clothes, but also because of their activity-related air movement.
Sun et al. [64] found that MP deposition in Shanghai was correlated particularly with PM2.5 levels in the air (R2 = 0.76–0.93), and suggested that these small MPs could be used as a marker for deposition rates. Such small particles, which readily enter the human respiratory apparatus, are normally taken as a measure of indoor air quality [81,82], although even in this commonly used determination no standard methods have been published. This is a major problem when comparing MP deposition rates given in the literature (see Table 1). Many factors can influence the results of such measurements, including the height of the collector above the ground (potentially collecting splashback) and the area of the open mouth of the collector, the material of which the collecting vessel is made (with possible high MP adhesion) and, as previously mentioned, timing of collection within the rain event, considering the “first flush effect” [83]. Collections involving a filtration step will differ from those without and Uddin et al. [84] suggest that filters in sampling devices should be avoided. The problems of sample storage are obvious, and include clumping of particles in static liquid and their adhesion to the internal surface of the storage vessel. Care must be taken in choosing the area of collection, avoiding interfering structures such as high buildings, overhangs or even trees. Huang et al. [85] found that trees with 88% coverage and large three-dimensional spaces formed by leaves could intercept about 16.3% of high-density, small-sized MPs. This could mean that soil beneath forests is protected to some extent from MP pollution, and certainly Zhang et al. [86] found that dust MPs were lower in forest soil in the Ebinur Lake Basin in Northwest China than in construction land or farmland. However, the main importance of tree cover is that it should be avoided when taking comparative MP rain deposition rates.
Finally, it must be mentioned that this section concentrates on the removal of atmospheric MPs by rain, but that, in suitable conditions of temperature, hail and snow can also sequester atmospheric particles, with potential future release. This topic is covered in our paper, “Microplastics in the cryosphere—a potential time bomb?” [87].
4. Aerosols and MPS
It is not within the remit of this article to discuss the inhalation of aerosols and the effects of MPs on human beings. There are many papers and reviews available on these aspects of aerobiology; for example, [85,86,87,88,89,90,91]. Rather, we will discuss the formation of aerosols containing MPs in environmental, domestic and industrial situations and their concentration into water or onto the ground.
4.1. Ocean Spray
Breaking ocean waves produce bubbles at the surface; MPs are enriched at the sea surface and even more in these bubbles prior to bursting, due to a process known as bubble scavenging. The bursting of the bubbles produces aerosols that contain inorganic and organic materials. Most particles leaving the sea surface are salt and organic matter, of which 6700–7400 Tg particles (most measuring around 20 µm) are exported per year by aerosols produced by wave and wind activity through convective updrafts [92,93,94,95]. Micro- and nano-sized salt crystals are expelled from the sea when waves break and the resulting bubbles burst; the same scenario can be proposed for MPs (Figure 2), which may have been enriched from the bulk seawater by the bubble scavenging process, especially if they have retained their hydrophobic surfaces. The bursting of the surface bubbles provides nano-sized particles expelled into the adjacent air, able to be carried away by the winds [96,97]. The burst bubble leaves a cavity in the sea surface, which then collapses, generating a second release of particles. This process also exports organic matter that is an acknowledged element for the formation of clouds and rain (particularly in warm air) [97,98] and would lead to the incorporation of MPs into clouds, with subsequent transport through the atmosphere and release in rain (see the Section 2). The ejection capacity of these processes has been proven by tracking bacteria and virus cells as they travel in the wind and as aerosols across continents and oceans, a process similar to what happens with salt crystals and a blueprint for the release of MPs from marine aerosols [99,100,101,102,103,104,105]. Thus, the much-reported ocean “sink” for MPs can become a source of these particles, and evidence for this has been provided by Trainic et al. [104]. Noorimotlagh et al. [105] suggest that “bubble bursting” is one of the major sources of MPs in the external air. Sea spray has been stated to be the dominant source of MPs for long-range transport. Ryan et al. [106], using HYSPLIT modelling, determined that the increased MP levels in the air over Newfoundland after the passing of Hurricane Larry in 2021 likely came from the North Atlantic Gyre Garbage Patch, originating in sea spray [107]. Harb et al. [108] calculated that aerosolization increases with both ocean concentration and decreasing particle size, while Bucci et al. [109] used a Lagrangian model to determine that a major hotspot for such emissions is the tropics and that MPs released and penetrating into the stratosphere may persist for months. The same authors stated that current observations of MP concentrations above the oceans are underestimated by one to two orders of magnitude, though Yang et al., [110] in their review, found the range of published values to vary by four orders of magnitude, from 7.7 × 10–4 to 8.6 megatons per year. In spite of variations in degree, the oceans are certainly important sinks of MPs, contributing to their dispersal around the world.
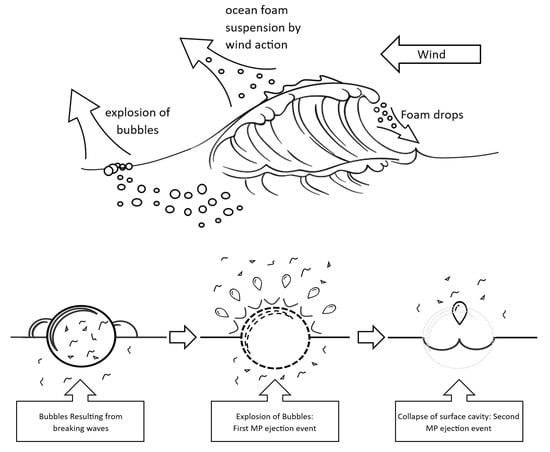
Figure 2.
Production of sea spray and ejection of MPs by bubble bursting.
4.2. Anthropogenic Aerosols
Can Guven estimates that terrestrial atmospheric MP emissions are much higher than marine concentrations. He calculated a net land-to-sea MP transport of 25 Gg.y−1 (compare with Klein and Fisher’s determination of 0.418 ± 0.201 Tg.y−1 [10]) and suggested that 99% of MPs are emitted as 70 µm particles. There are many sources of terrestrial MPs. Domestic, indoor aerosols containing MPs can result from the wearing and laundering of clothes [46,47,108], including tumble dryers; from artificial turf and other flooring materials [109,110,111]; furnishings; unwrapping of plastic packaging; indoor paints; and enamels [112,113] (for example, nail polishing in beauty salons resulted in an air level of 46 ± 55 MPs/m3 compared to 28 ± 24 MPs/m3 in external air [114]). MP counts in indoor air are routinely higher than in outdoor air [115,116], and the accident of nature provided by the recent COVID-19 epidemic has allowed us to determine that the movement of the populace, both indoors and outdoors, is responsible for much MP prevalence and distribution. In addition, outdoor terrestrial sources include road-related particles, such as tyre-wear MPs and road marking particles [117,118], soil and agriculture-related particles (e.g., from plastic mulch, packaging, micro-encapsulated agrochemicals, landfill sites subject to wind erosion, disposal of wastewater treatment residues/sludge [119,120]), construction materials such as fiber-reinforced concrete, and improperly managed public waste. Even the more recently recognized phenomenon of PPE disposal (especially COVID-protective masks discarded by the public) is a worrying source of atmospheric MP pollution [121,122,123,124,125,126,127,128].
4.3. Air Conditioning
Air conditioners can trap MPs on their filters, initially removing them from the air, but then, when uncleaned for more than 30 days, acting as a source of these particles, as well as of the microorganisms adhering to them [129,130,131,132]. Even clean air conditioners, however, can increase the number of MPs in a room because of an increase in air turbulence, with split air conditioning units increasing MP numbers more than a central AC unit. The latter authors collected and analyzed MP-containing aerosols in domestic and public buildings in Kuwait. In public buildings, the concentrations of MPs ranged from 3.2 to 3.7 m−3 at a time of restricted entry regulations and 8.7 to 15.3 m−3 when the restrictions were removed, showing the importance of building inhabitants and their movements on indoor air MP concentrations. Hospital air, in spite of the use of PPE and presence of air conditioning, contained only 3.9–4.4 MPs.m−3 [133], presumably due to increased care with air contamination. A similarly low level of atmospheric MPs was found in Al-Hussayni et al.’s study [134] in Mosul, Iraq, in 2023, where the lowest concentration of MPs in 90 buildings under various types of use was found in the doctors’ clinics, with the maximum in kindergartens, a clear indication of human activity being a crucial source of MPs in internal air [135]. Torres-Agullo et al. [136] considered that the presence of air conditioning on subway trains in Barcelona was one explanation for lower MP levels here than in buses, suggesting that regular air changes would remove microfibers released by the traveling crowds.
Carpeting, an acknowledged source of microfibers, had no statistically significant effect on MP loading in Kuwait; carpeted offices contained 8.2–11.0 MPs per cubic meter of air [135]. The values in residential, carpeted flats were 10.8–27.1 m−3, while in a mosque, carpeted but also air-conditioned, the value was 14.3 m−3; these numbers were not significantly different from those determined in uncarpeted rooms. Yasin et al. [137], however, considered that polymer carpets concentrate a significant amount of MPs during their use, with smaller MPs accumulating on vacuuming, and the static charge attracting other MPs. Emphasizing the importance of floor coverings, washwaters from carpet-washing shops in Iran were shown to accumulate around 2000–3000 microfibers.m−2 carpet [138], with the majority being smaller sizes (37–300 μm).
The washing of synthetic plastics also releases MPs. It has been calculated that the washing of clothes is responsible for 28% of the MP microfibers generated by clothing-related processes, with 23% produced during their wearing, and the majority (49%) by their manufacture [49].
4.4. Steam
MPs may be liberated into steam used in plastic repair operations, both in the open air and in industrial situations. For instance, the repair of sewage pipes in the open air can generate MP-containing steam; such repairs often take place in highly populated urban areas, where rapid in situ repairs are necessary [139]. The effect of steam on the liberation of MPs and NPs from textiles can be exemplified by the home sterilization of disposable masks, which mainly contain polypropylene (PP). Liang et al. [140] used a home cooker to steam various types of disposable masks that were used during the COVID-19 epidemic for 30 min, followed by overnight drying at room temperature. The masks were then shaken in deionized water for 24 h, after which the water and the mask were examined by microscopy and Raman spectroscopy and for NPs by tracking analysis using ZetaView (Particle Metrix, Germany). Steaming resulted in the release of 554 ± 10 MPs + NPs from each mask, on average, but these particles were detected, not only in the water, but also in the steam, post treatment, at the same level or higher than in the water. Steam treatment resulted in a reduction in the size of the released MPs and a concomitant increase in NPs, suggesting that industrial operations employing steam may result in the aerial release of more dangerous plastic particles that can penetrate more readily into living tissues.
Domestic procedures also show the effects of steam on plastics. The “sterilization” of rubber teats for baby feeding bottles in boiling water, a common alternative to chemical sterilization, has been shown to release MPs and NPs [141]. Submicrometer-resolved steam etching was shown on the teat surfaces. The authors calculated that MP emissions from this source could be as much as 5.2 × 1013 particles per year, globally; a large number, but perhaps less significant than MP release from, for example, road markings and tyres. One of the two common materials used to manufacture teats is silicone, which was not considered an MP-producing material until Hartmann et al.’s 2019 discussion of the classification of MPs and NPs [142]. Indeed, in 2018, Primpke et al. [143] found that silicone interfered with their development of an automated database for FTIR analysis of MPs; removing the silicone cluster from the dendrogram significantly reduced the number of false assignments, confirming that this particular plastic could interfere in MP analysis by FTIR [144]. In 2022, silicone MPs were detected in the dustfall over urban Beijing and in 2023, Fang et al. [145] developed a Raman imaging technique to also reliably detect silicone NPs, with which they successfully demonstrated the potential of silicone sealant in a kitchen to generate both particle sizes. The development of this technique was essential to demonstrate MP production from silicones.
4.5. Paint Sprays
Indoor paints have already been listed as a source of MPs. Spray paints, then, represent a direct source of atmospheric MPs, which will settle not only onto the intended target, but also onto the ground, if not protected; only 50–65% of spray paint finds its intended target, the rest landing on nearby materials [146]. Xu et al. [147], in 2022, found the top layers of soil 2 m from a frequently graffitied wall in Berlin contained a maximum of 2.89 × 107 MPs.kg−1 dry soil; this is the highest soil concentration reported up to the time of their publication. Clearly, any soil disturbance, including by wind, will liberate some of these particles once more into the atmosphere.
Automobiles are also generally subject to spray painting. Even polishing the car, an activity indulged in by many on a Sunday afternoon, can remove and disperse MPs. Sobhani et al. [148] estimated that billions or trillions of MPs and NPs (200 nm to 7 µm) have been produced by the polishing of car hoods over the years. Car washing, either automatically or by hand, will be equally, or more, polluting.
4.6. Body Sprays
Primary MPs are used as microbeads in the production of personal care and cosmetic products (PPCPs), including deodorants, insect repellents, sunscreens, shower gels, shampoos, hair sprays, shaving creams, etc. [148]. It has been estimated that, in India alone, 4.7 × 1010 microbeads are released into the environment through untreated sewage, amounting to 3.8 tonnes of microbeads every year, while the annual per capita emission of MPs from PPCPs in China was estimated to be 2.18 million particles. In a study of body sprays and shower gels, a maximum of 30 different MPs was detected in a single product (a shower gel) [149]. Fibrous particles in both types of product were identified mostly as cellulose-derived, but the two body sprays also contained particles that were identified by µ-FTIR as PP and PET, and others tentatively assigned to PS, PVC, PTFE, and ethylene vinyl acetate (EVA). Sun et al., in their review, state that approximately 1500 tons/year MPs derived from PPCPs escape from wastewater treatment plants to the surrounding air and water, the global emission per year reaching 1.2 × 104 tons. Because of their small size, less than 350 μm in diameter, these MPs can cause considerable damage to marine organisms, as well as being potential carriers of hydrophobic pollutants [150,151], and several countries have banned their use in PPCPs.
Secondary MPs can also be present in domestic spray products [152,153,154,155,156,157]. Sprays used to “deodorize” or replace unwanted smells, as well as perfumes for use on the human body or in domestic situations, may contain MPs as materials used to encapsulate, and hence prolong the life of, fragrances. Camerlo et al. [158] used limonene as a test fragrance to evaluate the use of poly(vinyl alcohol) for encapsulation. The PVA fibers held the fragrance for at least 15 days. Many of the MPs are unstable and may be degraded on, or before, release into the environment. The cosmetics industry is attempting to tackle this problem with more “sustainable” cosmetics. More “degradable” plastics are considered options for future products, but truly degradable plastics have yet to be developed. Natural degradable polymers do not have the necessary functionality, mainly strength and toughness [159,160,161]. The preferred solution is to replace petrochemical-based plastics entirely. Goyal and Jerold [162] consider the options for the production of “green cosmetics”, concluding that advanced technologies and industries are necessary to develop natural, sustainable, and less damaging cosmetics for a more demanding market.
4.7. Agricultural Sprays
Agricultural activities resuspend around 0.31 ± 0.13 Tg y−1 of plastics. It used to be considered that the use of waste-treatment effluent (sludge) as a fertilizer was responsible for the presence of most MPs in soil since waste treatment does not remove all MPs [162,163,164]. MP removal efficiencies in wastewater treatment plans around the world have been calculated at 35–99%. When used to improve agricultural soils, this sludge thus becomes a source of atmospheric MPs [163,164,165,166,167,168,169]. However, Radford et al. [170] showed that the difference in MP concentrations between soils could not be explained solely by the use of sludge fertilizer. Other uses of plastics in agricultural practices include plastic mulching, polymer-based fertilizers, and irrigation with untreated wastewater [171,172].
Apart from direct spraying via fertilizers and the use of agricultural mulch, a variety of agrochemicals are applied to soil and as crop sprays; pesticides are perhaps the most obvious. Many of these chemicals are plastic-coated, or encapsulated in plastics [173,174], and are thus primary MPs. Coated fertilizers are used on golf courses and in greening projects throughout the world and on rice fields in Japan. Secondary MPs added to agricultural soil, apart from those present in sewage sludge, include mulching and greenhouse films. All these MPs can be removed by wind from the surface layers (upper 30 cm) of the soil, where they are at their maximum concentrations, entering the atmospheric MP dispersion, either with or without attached soil particles. In the former case, they will be rapidly deposited in other areas because of their increased weight, but free MPs may remain as part of the atmospheric MP load for hours, being spread to faraway places, as detailed in the Section 2.
4.8. Industrial Procedures
Industrial processes used during production of plastic items often involve a cooling phase. Cooling water bleeds and washwaters from various industries (tyre and tube, molded and extruded products, latex industries) can produce MP contamination of the surrounding ground and air. Repair procedures used in the open air (cure-in-place technology) can also give rise to aerial MPs, as demonstrated for sewer pipe repairs [175,176]. Machining and processing of plastic-containing materials in the open air also liberates MPs; examples include carbon fiber-reinforced plastic for automobiles and wind turbine blades.
5. Conclusions and Future Perspectives
The threat of MP pollution around the globe is universally accepted; it is strange that the atmosphere should become the last area to be studied in this respect, considering its direct impact on human health through inhalation. Perhaps this is due to the circulatory dynamics and low stability of suspended particles in the air. The available research methodologies, developed for the study of MPs in earth and water compartments, are less applicable to the air, often producing unreliable data. Universally accepted standard methods must be developed and employed to allow real MP levels to be determined in the air and in their multiple, often complex, sources. The sampling methods currently used are varied, with low correlation between their numerical results. MP characterization remains a challenge, with particular limitations of throughput. Comparative chemical/instrumental analyses and the use of models must be more developed to further knowledge of the aging, transport and distribution in time and space of atmospheric MPs. Currently, several methods are being developed for the detection, enumeration and identification of MPs. Most commonly used in the extant literature are FTIR spectroscopy with microscopy. Other methods include laser-induced breakdown spectroscopy, micro-Fourier I-R spectrometry, hyperspectral imaging and laser direct I-R imaging. A promising high-affinity fluorescent nanoparticle probe for MPs has recently been developed for use in a variety of environmental compartments; this offers rapid but accurate enumeration of MPs.
The atmosphere is the gaseous layer that covers the entire surface of planet Earth, both sea and land, thus constituting a link between all the world’s ecosystems. This leads to the conclusion that the omnipresence of MPs on planet Earth can be attributed principally to atmospheric circulation. Natural phenomena like ocean sprays and snow/rainfall represent the entry and exit ports for MPs between the numerous global pools. Their characteristics define the range and distribution of the particles. The majority of atmospheric plastics are fibers and fragments, which are less dense and thus lighter and more able to resist the downward force of gravity, whilst retaining the durability of traditional plastics. Air currents aid them to reach the most remote environments, potentially traveling thousands of miles through the air, before being deposited in liquid (rain or snow) precipitation, or as “dust”, under wind-free conditions or because of increased weight by co-adhesion, or adhesion to other aerial particles.
MPs can also be dispersed through the atmosphere by anthropogenic phenomena like wearing and washing of clothing containing artificial fibers, vapors from repairs to plastic bodies, paint sprays used in car or wall painting, agricultural sprays, or steam from plastic softening and sterilization procedures. Primary MPs used in personal care and cosmetic preparations add to the atmospheric load, as can manufacturing procedures, especially those in plastics-production industries that involve cooling steps, and in the clothing industry. Finally, MPs can be released from wastewater treatment plants in aerosols, as well as the better-studied liquid effluents and sludge. Improvements in this sector of industry, which implies not only better waste treatment procedures, but also reductions in the amount of processed waste (e.g., recycling MP-containing materials that have reached their useful life) are a real possibility for the future health of the planet.
To reduce the release of MPs into the atmosphere, both personal and industrial changes must be made. We already see the increased preoccupation of the populace with plastic-containing products in the search for environmental sustainability. Industry must look to changes in production and maintenance procedures to reduce the dispersion of MPs in the atmosphere. The increasing importance of industrial ecology, seeking to mimic the circular economy of sustainable production and distribution, recycling, and end-of-life waste and emission management, could take control of industry’s contribution to release of MPs and NPs into the environment, if the necessary regulatory thresholds can be enabled. If this strategy were successful, the job of the world’s governments could be considerably simplified; the situation is urgent.
Author Contributions
All authors made substantial contributions to the conception and design of the study, the writing of the drafts and final manuscript, and the survey of the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The Brazilian authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for ongoing support at UFF.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An overview of analytical methods for detecting microplastics in the atmosphere. Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Fang, C.; Awoyemi, O.S.; Saianand, G.; Xu, L.; Niu, J.; Naidu, R. Characterising microplastics in indoor air: Insights from Raman imaging analysis of air filter samples. J. Hazard. Mater. 2024, 464, 132969. [Google Scholar] [CrossRef]
- Luo, Y.; Awoyemi, O.; Liu, S.; Niu, J.; Naidu, R.; Fang, C. From celebration to contamination: Analysing microplastics released by burst balloons. J. Hazard. Mater. 2024, 464, 133021. [Google Scholar] [CrossRef]
- Liu, Z.; Nowack, B. Probabilistic material flow analysis and emissions modeling for five commodity plastics (PUR, ABS, PA, PC, and PMMA) as macroplastics and microplastics. Resour. Conserv. Recycl. 2022, 179, 106071. [Google Scholar] [CrossRef]
- Evangeliou, N.; Tichý, O.; Eckhardt, S.; Zwaaftink, C.G.; Brahney, J. Sources and fate of atmospheric microplastics revealed from inverse and dispersion modelling: From global emissions to deposition. J. Hazard. Mater. 2022, 432, 128585. [Google Scholar] [CrossRef]
- Wang, T.; Zou, X.; Li, B.; Yao, Y.; Li, J.; Hui, H.; Yu, W.; Wang, C. Microplastics in a wind farm area: A case study at the Rudong Offshore Wind Farm, Yellow Sea, China. Mar. Pollut. Bull. 2018, 128, 466–474. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; p. 43. [Google Scholar]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Gimenez, B.C.G. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-De-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Zarfl, C.; Fleet, D.; Fries, E.; Galgani, F.; Gerdts, G.; Hanke, G.; Matthies, M. Microplastics in oceans. Mar. Pollut. Bull. 2011, 62, 1589–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hamidian, A.H.; Tubi’c, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching new heights in plastic pollution: Preliminary findings of microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First evidence of microplastics in Antarctic snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Adams, J.K.; Dean, B.Y.; Athey, S.N.; Jantunen, L.M.; Bernstein, S.; Stern, G.; Diamond, M.L.; Finkelstein, S.A. Anthropogenic particles (including microfibers and microplastics) in marine sediments of the Canadian Arctic. Sci. Total Environ. 2021, 784, 147155. [Google Scholar] [CrossRef]
- Huntington, A.; Corcoran, P.L.; Jantunen, L.; Thaysen, C.; Bernstein, S.; Stern, G.A.; Rochman, C.M. A first assessment of microplastics and other anthropogenic particles in Hudson Bay and the surrounding eastern Canadian Arctic waters of Nunavut. Facets 2020, 5, 615–616. [Google Scholar] [CrossRef]
- Liu, K.; Wu, T.; Wang, X.; Song, Z.; Zong, C.; Wei, N.; Li, D. Consistent transport of terrestrial microplastics to the ocean through atmosphere. Environ. Sci. Technol. 2019, 53, 10612–10619. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric microplastic over the South China Sea and East Indian Ocean: Abundance, distribution and source. J. Hazard. Mater. 2020, 389, 121846. [Google Scholar] [CrossRef]
- Yuan, Z.; Pei, C.L.; Li, H.X.; Lin, L.; Hou, R.; Liu, S.; Zhang, K.; Cai, M.G.; Xu, X.R. Vertical distribution and transport of microplastics in the urban atmosphere: New insights from field observations. Sci. Total Environ. 2023, 895, 165190. [Google Scholar] [CrossRef]
- Prajapati, A.; Jadhao, P.; Kumar, A.R. Atmospheric microplastics deposition in a central Indian city: Distribution, characteristics and seasonal variations. Environ. Pollut. 2025, 374, 126183. [Google Scholar] [CrossRef]
- Huang, Y.; He, T.; Yan, M.; Yang, L.; Gong, H.; Wang, W.; Qing, X.; Wang, J. Atmospheric transport and deposition of microplastics in a subtropical urban environment. J. Hazard. Mater. 2021, 416, 126168. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, Y.; Yan, Y.; Junaid, M.; Wang, J. Characteristics, toxic effects, and analytical methods of microplastics in the atmosphere. Nanomaterials 2021, 11, 2747. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, L.; Wang, W.; Zhang, M.; Feng, X.; Li, W.; Zhang, D. Airborne fiber particles: Types, size and concentration observed in Beijing. Sci. Total Environ. 2020, 705, 135967. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, A.; Law, K.L.; Muñoz, S.E.; Bianchi, T.S.; Zhu, L. Plastics in the Earth system. Science 2021, 373, 51–55. [Google Scholar] [CrossRef]
- Szewc, K.; Graca, B.; Dołęga, A. Atmospheric deposition of microplastics in the coastal zone: Characteristics and relationship with meteorological factors. Sci. Total Environ. 2021, 761, 143272. [Google Scholar] [CrossRef]
- Gouin, T. Addressing the importance of microplastic particles as vectors for long-range transport of chemical contaminants: Perspective in relation to prioritizing research and regulatory actions. Microplast. Nanoplast. 2021, 1, 14. [Google Scholar] [CrossRef]
- Ferrero, L.; Scibetta, L.; Markuszewski, P.; Mazurkiewicz, M.; Drozdowska, V.; Makuch, P.; Jutrzenka-Trzebiatowska, P.; Zaleska-Medynska, A.; Andò, S.; Saliu, F.; et al. Airborne and marine microplastics from an oceanographic survey at the Baltic Sea: An emerging role of air-sea interaction? Sci. Total Environ. 2022, 824, 153709. [Google Scholar] [CrossRef]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef]
- Ding, Y.; Zou, X.; Wang, C.; Feng, Z.; Wang, Y.; Fan, Q.; Chen, H. The abundance and characteristics of atmospheric microplastic deposition in the northwestern South China Sea in the fall. Atmos. Environ. 2021, 253, 118389. [Google Scholar] [CrossRef]
- Fu, Y.; Pang, Q.; Ga, S.L.Z.; Wu, P.; Wang, Y.; Mao, M.; Yuan, Z.; Xu, X.; Liu, K.; Wang, X.; et al. Modeling atmospheric microplastic cycle by GEOS-Chem: An optimized estimation by a global dataset suggests likely 50 times lower ocean emissions. One Earth 2023, 6, 705–714. [Google Scholar] [CrossRef]
- Can-Güven, E. Microplastics as emerging atmospheric pollutants: A review and bibliometric analysis. Air Qual. Atmos. Health 2021, 14, 203–215. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Zhu, F.; Zhou, S. Airborne microplastics: A review on the occurrence, migration and risks to humans. Bull. Environ. Contam. Toxicol. 2021, 107, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, M.; Dris, R.; Gasperi, J.; Tassin, B. Microplastics in the atmospheric compartment: A comprehensive review on methods, results on their occurrence and determining factors. Curr. Opin. Food Sci. 2021, 41, 159–168. [Google Scholar] [CrossRef]
- Yao, X.; Luo, X.S.; Fan, J.; Zhang, T.; Li, H.; Wei, Y. Ecological and human health risks of atmospheric microplastics (MPs): A review. Environ. Sci. Atmos. 2022, 2, 921–942. [Google Scholar] [CrossRef]
- Jahandari, A. Microplastics in the urban atmosphere: Sources, occurrences, distribution, and potential health implications. J. Hazard. Mater. Adv. 2023, 12, 100346. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Brimblecombe, P. Clothing as a source of fibres within museums. J. Cult. Herit. 2000, 1, 445–454. [Google Scholar] [CrossRef]
- Gaylarde, C.; Baptista-Neto, J.A.; da Fonseca, E.M. Plastic microfibre pollution: How important is clothes’ laundering? Heliyon 2021, 7, e07105. [Google Scholar] [CrossRef]
- Lim, J.; Choi, J.; Won, A.; Kim, M.; Kim, S.; Yun, C. Cause of microfibers found in the domestic washing process of clothing; focusing on the manufacturing, wearing, and washing processes. Fash. Text 2022, 9, 24. [Google Scholar] [CrossRef]
- Jenner, L.C.; Sadofsky, L.R.; Danopoulos, E.; Chapman, E.; White, D.; Jenkins, R.L.; Rotchell, J.M. Outdoor Atmospheric Microplastics within the Humber Region (United Kingdom): Quantification and Chemical Characterisation of Deposited Particles Present. Atmosphere 2022, 13, 265. [Google Scholar] [CrossRef]
- Vujanović, A.; Puhar, J.; Čolnik, M.; Plohl, O.; Vidovič, T.; Valh, J.V.; Škerget, M.; Čuček, L. Sustainable industrial ecology and environmental analysis: A case of melamine etherified resin fibres. J. Clean. Prod. 2022, 369, 133301. [Google Scholar] [CrossRef]
- Hitchcock, J.N. Storm events as key moments of microplastic contamination in aquatic ecosystems. Sci. Total Environ. 2020, 734, 139436. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dou, P.; Xu, D.; Zhang, Y.; Gao, B. Microplastic reorganization in urban river before and after rainfall. Environ. Pollut. 2022, 314, 120326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Niu, J.; Xu, D.; Zhang, M.; Sun, K.; Gao, B. Wet Deposition of Globally Transportable Microplastics (<25 μm) Hovering over the Megacity of Beijing. Environ. Sci. Technol. 2023, 57, 11152–11162. [Google Scholar] [CrossRef]
- Do, T.; Park, Y.; Lim, B.; Kim, S.; Chae, M.Y.; Chun, C.-H. Effect of the first-flush phenomenon on the quantification of microplastics in rainwater. Mar. Pollut. Bull. 2023, 187, 114559. [Google Scholar] [CrossRef]
- Imbulana, S.; Tanaka, S.; Moriya, A.; Oluwoye, I. Inter-event and intra-event dynamics of microplastic emissions in an urban river during rainfall episodes. Environ. Res. 2024, 243, 117882. [Google Scholar] [CrossRef]
- Allen, S.; Materić, D.; Allen, D.; Macdonald, A.; Holzinger, R.; Le Roux, G.; Phoenix, V.R. An early comparison of nano to microplastic mass in a remote catchment’s atmospheric deposition. J. Hazard. Mater. Adv. 2022, 7, 100104. [Google Scholar] [CrossRef]
- Napper, I.E.; Parker-Jurd, F.N.; Wright, S.L.; Thompson, R.C. Examining the release of synthetic microfibres to the environment via two major pathways: Atmospheric deposition and treated wastewater effluent. Sci. Total Environ. 2023, 857, 159317. [Google Scholar] [CrossRef]
- Kernchen, S.; Schmalz, H.; Löder, M.G.; Georgi, C.; Einhorn, A.; Greiner, A.; Nölscher, A.C.; Laforsch, C.; Held, A. Atmospheric deposition studies of microplastics in Central Germany. Air Qual. Atmos. Health 2024, 17, 2247–2261. [Google Scholar] [CrossRef]
- Kernchen, S.; Löder, M.G.; Fischer, F.; Fischer, D.; Moses, S.R.; Georgi, C.; Nölscher, A.C.; Held, A.; Laforsch, C. Airborne microplastic concentrations and deposition across the Weser River catchment. Sci. Total Environ. 2022, 818, 151812. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Piñas, F.; Leganes, F.; Gómez, M.; Martínez, I.; Herrera, A.; Hernández-Sánchez, C.; González-Sálamo, J.; Borges, J.H.; López-Castellanos, J.; et al. A nationwide monitoring of atmospheric microplastic deposition. Sci. Total Environ. 2023, 905, 166923. [Google Scholar] [CrossRef]
- Welsh, B.; Aherne, J.; Paterson, A.M.; Yao, H.; McConnell, C. Atmospheric deposition of anthropogenic particles and microplastics in south-central Ontario, Canada. Sci. Total Environ. 2022, 835, 155426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, Y.; Ma, T.; Liu, X.; Wei, H.; Meng, H.; Fu, Y.; Ma, Z.; Zhang, L.; Zhao, J. Distribution and possible sources of atmospheric microplastic deposition in a valley basin city (Lanzhou, China). Ecotoxicol. Environ. Safe 2022, 233, 113353. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhu, Z.R.; Fu, W.; Dai, X.; Ni, B.-J. The atmospheric microplastics deposition contributes to microplastic pollution in urban waters. Water Res. 2022, 225, 119116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Ren, S.; Huang, D.; Liu, F.; Li, Z.; Zhang, H.; Zhao, M.; Cao, Y.; Mofolo, S.; et al. Atmospheric deposition of microplastics in a rural region of North China Plain. Sci. Total Environ. 2023, 877, 162947. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, X.; Wang, K.; Lu, L.; Li, F.; Li, J.; Xu, L. Characteristics, sources and influencing factors of atmospheric deposition of microplastics in three different ecosystems of Beijing, China. Sci. Total Environ. 2023, 20, 883.163567. [Google Scholar] [CrossRef]
- Purwiyanto, A.I.S.; Prartono, T.; Riani, E.; Naulita, Y.; Cordova, M.R.; Koropitan, A.F. The deposition of atmospheric microplastics in Jakarta-Indonesia: The coastal urban area. Mar. Pollut. Bull. 2022, 174, 113195. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2020, 758, 143634. [Google Scholar] [CrossRef]
- Strady, E.; Kieu-Le, T.C.; Tran, Q.V.; Thuong, Q.T. Microplastic in atmospheric fallouts of a developing Southeast Asian megacity under tropical climate. Chemosphere 2021, 272, 129874. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, R.; Niu, T.; Liu, Y. Using street view images and a geographical detector to understand how street-level built environment is associated with urban poverty: A case study in Guangzhou. Appl. Geogr. 2023, 156, 102980. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; dos Santos Galvão, L.; Wiebeck, H.; Carvalho-Oliveira, R.; Mauad, T. Atmospheric microplastic fallout in outdoor and indoor environments in São Paulo megacity. Sci. Total Environ. 2022, 821, 153450. [Google Scholar] [CrossRef]
- Jia, Q.; Duan, Y.; Han, X.; Sun, X.; Munyaneza, J.; Ma, J.; Xiu, G. Atmospheric deposition of microplastics in the megalopolis (Shanghai) during rainy season: Characteristics, influence factors, and source. Sci. Total Environ. 2022, 847, 157609. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.C.; Tong, X.; Li, J.; Xu, Z.; Chng, S.H.Q.; He, Y.; Gin, K.Y.-H. Microplastics in equatorial coasts: Pollution hotspots and spatiotemporal variations associated with tropical monsoons. J. Hazard. Mater. 2022, 424, 127626. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Riksen, M.J.; Sirjani, E.; Sameni, A.; Geissen, V. Wind erosion as a driver for transport of light density microplastics. Sci. Total Environ. 2019, 669, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, P.; Yang, Z.; Wang, W.; Zhang, H.; Shao, L.; Lü, F. Emission of airborne microplastics from municipal solid waste transfer stations in downtown. Sci. Total Environ. 2022, 828, 154400. [Google Scholar] [CrossRef]
- Dong, H.; Wang, L.; Wang, X.; Xu, L.; Chen, M.; Gong, P.; Wang, C. Microplastics in a remote lake basin of the Tibetan Plateau: Impacts of atmospheric transport and glacial melting. Environ. Sci. Technol. 2021, 55, 12951–12960. [Google Scholar] [CrossRef]
- Xia, W.; Rao, Q.; Deng, X.; Chen, J.; Xie, P. Rainfall is a significant environmental factor of microplastic pollution in inland waters. Sci. Total Environ. 2020, 732, 139065. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, X.; Zhang, Y.; Gao, W.; Jiang, J.; Mo, A.; He, D. Size/shape-dependent migration of microplastics in agricultural soil under simulative and natural rainfall. Sci. Total Environ. 2022, 815, 152507. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Q.; Chen, G.; Wang, J. Microplastics in rainwater/stormwater environments: Influencing factors, sources, transport, fate, and removal techniques. TrAC Trends Anal. Chem. 2023, 165, 117147. [Google Scholar] [CrossRef]
- Abbasi, S.; Jaafarzadeh, N.; Zahedi, A.; Ravanbakhsh, M.; Abbaszadeh, S.; Turner, A. Microplastics in the atmosphere of Ahvaz City, Iran. J. Environ. Sci. 2023, 126, 95–102. [Google Scholar] [CrossRef]
- Perera, K.; Ziajahromi, S.; Nash, S.B.; Leusch, F.D. Microplastics in Australian indoor air: Abundance, characteristics, and implications for human exposure. Sci. Total Environ. 2023, 889, 164292. [Google Scholar] [CrossRef]
- Beaurepaire, M.; Gasperi, J.; Tassin, B.; Dris, R. COVID lockdown significantly impacted microplastic bulk atmospheric deposition rates. Environ. Pollut. 2024, 344, 123354. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Gouin, T.; Koelmans, A.A.; Scheuermann, L. Development of screening criteria for microplastic particles in air and atmospheric deposition: Critical review and applicability towards assessing human exposure. Microplast. Nanoplast. 2021, 1, 6. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. A preliminary assessment of size-fractionated microplastics in indoor aerosol—Kuwait’s baseline. Toxics 2022, 10, 71. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Meng, Y.; Liu, G.; Yang, M. Are we ignoring the role of urban forests in intercepting atmospheric microplastics? J. Hazard. Mater. 2022, 436, 129096. [Google Scholar] [CrossRef]
- Zhang, Z.; Zulpiya, M.; Wang, P. Occurrence and sources of microplastics in dust of the Ebinur lake Basin, northwest China. Environ. Geochem. Health 2023, 45, 1461–1474. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.; da Fonseca, E.M. Microplastics in the cryosphere-a potential time bomb? Water Emerg. Contam. Nanoplast. 2023, 2, 20. [Google Scholar] [CrossRef]
- Lombardi, G.; Di Russo, M.; Zjalic, D.; Lanza, T.; Simmons, M.; Moscato, U.; Ricciardi, W.; Chiara, C. Microplastics inhalation and their effects on human health: A systematic review. Eur. J. Public Health 2022, 32 (Suppl. 3), ckac131.152. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Z.; Zhou, W.; Shao, X.; Li, Z.; Zhou, Y. Individual exposure to microplastics through the inhalation route: Comparison of microplastics in inhaled indoor aerosol and exhaled breath air. Environ. Sci. Technol. Lett. 2023, 10, 464–470. [Google Scholar] [CrossRef]
- Borgatta, M.; Breider, F. Inhalation of Microplastics—A Toxicological Complexity. Toxics 2024, 12, 358. [Google Scholar] [CrossRef]
- Ageel, H.K.; Harrad, S.; Abdallah, M.A.E. Microplastics in indoor air from Birmingham, UK: Implications for inhalation exposure. Environ. Pollut. 2024, 362, 124960. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Moss, K.; Le Roux, G.; Phoenix, V.R.; Sonke, J.E. Examination of the ocean as a source for atmospheric microplastics. PLoS ONE 2020, 15, e0232746. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Górecka, E.; McCartney, K.; Borkowski, W. Rising bubbles as mechanism for scavenging and aerosolization of diatoms. J. Aerosol Sci. 2019, 128, 79–88. [Google Scholar] [CrossRef]
- Sofiev, M.; Soares, J.; Prank, M.; De Leeuw, G.; Kukkonen, J. A regional-to-global model of emission and transport of sea salt particles in the atmosphere. J. Geophys. Res. Atmos. 2011, 116, D21302. [Google Scholar] [CrossRef]
- Erinin, M.A.; Wang, S.D.; Liu, R.; Towle, D.; Liu, X.; Duncan, J.H. Spray Generation by a Plunging Breaker. Geophys. Res. Lett. 2019, 46, 8244–8251. [Google Scholar] [CrossRef]
- Lehmann, M.; Häusl, F.P.; Gekle, S. Modeling of vertical microplastic transport by rising bubbles. Microplast. Nanoplast. 2023, 3, 4. [Google Scholar] [CrossRef]
- Lewis, E.; Schwartz, S. Sea Salt Aerosol Production: Mechanisms, Methods, Measurements and Models; American Geophysical Union: Washington, DC, USA, 2004; ISBN 087590-417-3. [Google Scholar]
- Richer, D.; Veron, F. Ocean Spray: An outsized influence on weather and climate. Phys. Today 2016, 69, 35–39. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; de Leeuw, G. Marine aerosol production: A review of the current knowledge. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 1753–1774. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.A.; Steinbrook, R.A.; Anderson, J.L. Breaking bubbles and the water-to-air transport of particulate matter. Chem. Eng. Sci. 1975, 30, 1177–1184. [Google Scholar] [CrossRef]
- Ganguly, M.; Ariya, P.A. Ice nucleation of model nanoplastics and microplastics: A novel synthetic protocol and the influence of particle capping at diverse atmospheric environments. ACS Earth Space Chem. 2019, 3, 1729–1739. [Google Scholar] [CrossRef]
- Pósfai, M.; Li, J.; Anderson, J.R.; Buseck, P.R. Aerosol bacteria over the Southern Ocean during ACE-1. Atmos. Res. 2003, 66, 231–240. [Google Scholar] [CrossRef]
- Reche, I.; D’Orta, G.; Mladenov, N.; Winget, D.M.; Suttle, C.A. Deposition rates of viruses and bacteria above the atmospheric boundary layer. ISME J. 2018, 12, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Trainic, M.; Flores, J.M.; Pinkas, I.; Pedrotti, M.L.; Lombard, F.; Bourdin, G.; Gorsky, G.; Boss, E.; Rudich, Y.; Vardi, A.; et al. Airborne microplastic particles detected in the remote marine atmosphere. Commun. Earth Environ. 2020, 1, 64. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Hopke, P.K.; Mirzaee, S.A. A systematic review of airborne microplastics emissions as emerging contaminants in outdoor and indoor air environments. Emerg. Contam. 2024, 10, 100372. [Google Scholar] [CrossRef]
- Ryan, A.C.; Allen, D.; Allen, S.; Maselli, V.; LeBlanc, A.; Kelleher, L.; Krause, S.; Walker, T.R.; Cohen, M. Transport and deposition of ocean-sourced microplastic particles by a North Atlantic hurricane. Commun. Earth Environ. 2023, 4, 442. [Google Scholar] [CrossRef]
- Aves, A.; Ruffell, H.; Evangeliou, N.; Gaw, S.; Revell, L.E. Modelled sources of airborne microplastics collected at a remote Southern Hemisphere site. Atmos. Environ. 2024, 325, 120437. [Google Scholar] [CrossRef]
- Harb, C.; Pokhrel, N.; Foroutan, H. Quantification of the emission of atmospheric microplastics and nanoplastics via sea spray. Environ. Sci. Technol. Letts. 2023, 10, 513–519. [Google Scholar] [CrossRef]
- Bucci, S.; Richon, C.; Bakels, L. Exploring the transport path of oceanic microplastics in the atmosphere. Environ. Sci. Technol. 2024, 58, 14338–14347. [Google Scholar] [CrossRef]
- Yang, S.; Lu, X.; Wang, X. A Perspective on the Controversy over Global Emission Fluxes of Microplastics from Ocean into the Atmosphere. Environ. Sci. Technol. 2024, 58, 12304–12312. [Google Scholar] [CrossRef]
- Pedrotti, M.L.; Petit, S.; Eyheraguibel, B.; Kerros, M.E.; Elineau, A.; Ghiglione, J.; Loret, J.; Rostan, A.; Gorsky, G. Pollution by anthropogenic microfibers in North-West Mediterranean Sea and efficiency of microfiber removal by a wastewater treatment plant. Sci. Total Environ. 2021, 758, 144195. [Google Scholar] [CrossRef]
- Tao, D.; Zhang, K.; Xu, S.; Lin, H.; Liu, Y.; Kang, J.; Yim, T.; Giesy, J.P.; Leung, K.M.Y.; Tao, D.; et al. Microfibers released into the air from a household tumble dryer. Environ. Sci. Technol. Lett. 2022, 9, 120–126. [Google Scholar] [CrossRef]
- Salthammer, T. Microplastics and their additives in the indoor environment. Angew. Chem. Int. 2022, 61, e202205713. [Google Scholar] [CrossRef] [PubMed]
- Aini, S.A.; Syafiuddin, A.; Bent, G.A. The presence of microplastics in air environment and their potential impacts on health. Environ. Toxicol. Manag. 2022, 2, 31–39. [Google Scholar] [CrossRef]
- Choi, H.; Lee, I.; Kim, H.; Park, J.; Cho, S.; Oh, S.; Lee, M.; Kim, H. Comparison of microplastic characteristics in the indoor and outdoor air of urban areas of South Korea. Water Air Soil Pollut. 2022, 233, 169. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Ferdous, W.; Kamchoom, V. Microplastics in construction and built environment. Dev. Built Environ. 2023, 15, 100188. [Google Scholar] [CrossRef]
- Chen, E.Y.; Lin, K.T.; Jung, C.C.; Chang, C.L.; Chen, C.Y. Characteristics and influencing factors of airborne microplastics in nail salons. Sci. Total Environ. 2022, 806, 151472. [Google Scholar] [CrossRef]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Jenner, L.C.; Sadofsky, L.R.; Danopoulos, E.; Rotchell, J.M. Household indoor microplastics within the Humber region (United Kingdom): Quantification and chemical characterisation of particles present. Atmos. Environ. 2021, 259, 118512. [Google Scholar] [CrossRef]
- Boakes, L.C.; Patmore, I.R.; Bancone, C.E.; Rose, N.L. High temporal resolution records of outdoor and indoor airborne microplastics. Environ. Sci. Pollut. Res. 2023, 30, 39246–39257. [Google Scholar] [CrossRef]
- Rasmussen, L.A.; Lykkemark, J.; Andersen, T.R.; Vollertsen, J. Permeable pavements: A possible sink for tyre wear particles and other microplastics? Sci. Total Environ. 2023, 869, 161770. [Google Scholar] [CrossRef]
- Burghardt, T.E.; Pashkevich, A.; Babić, D.; Mosböck, H.; Babić, D.; Żakowska, L. Microplastics and road markings: The role of glass beads and loss estimation. Transp. Res. D Transp. Environ. 2022, 102, 103123. [Google Scholar] [CrossRef]
- Kallenbach, E.M.; Rødland, E.S.; Buenaventura, N.T.; Hurley, R. Microplastics in terrestrial and freshwater environments. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; p. 87. ISBN 978-3-030-78627-4. [Google Scholar] [CrossRef]
- Miino, M.C.; Galafassi, S.; Zullo, R.; Torretta, V.; Rada, E.C. Microplastics removal in wastewater treatment plants: A review of the different approaches to limit their release in the environment. Sci. Total Environ. 2024, 930, 172675. [Google Scholar] [CrossRef] [PubMed]
- Ormaniec, P. Occurrence and analysis of microplastics in municipal wastewater, Poland. Environ. Sci. Pollut. Res. 2024, 31, 49646–49655. [Google Scholar] [CrossRef]
- Haave, M.; Henriksen, T. Sources and Fate of Microplastics in Urban Systems. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M.F., Mouneyrac, C., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Zhao, C.; Ting, Z.; You, Z.; Kim, H.; Shah, K.J. Uncontrolled Disposal of Used Masks Resulting in Release of Microplastics and Co-Pollutants into Environment. Water 2022, 14, 2403. [Google Scholar] [CrossRef]
- Le, V.G.; Nguyen, M.K.; Lin, C.; Nguyen, H.L.; Nguyen, T.Q.H.; Hue, N.K.; Truong, Q.M.; Chang, S.W.; Nguyen, X.H.; Nguyen, D.D. Review on personal protective equipment: Emerging concerns in micro (nano) plastic pollution and strategies for addressing environmental challenges. Environ. Res. 2024, 257, 119345. [Google Scholar] [CrossRef]
- Soo, J.C.; Wei, C.H.; Chen, J.K.; Dong, G.C.; Liu, Z.S.; Chou, H.C.; Perez, R.L.; Adhikari, A.; Chen, Y.C. Assessment of inhalation exposure to microplastic particles when disposable masks are repeatedly used. Sci. Total Environ. 2024, 912, 169428. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, X.; Zhang, Y.; Gao, W.; Wang, R.; He, D. Air conditioner filters become sinks and sources of indoor microplastics fibers. Environ. Pollut. 2022, 292, 118465. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; He, D. Microfiber-loaded bacterial community in indoor fallout and air-conditioner filter dust. Sci. Total Environ. 2023, 856, 159211. [Google Scholar] [CrossRef]
- Zhai, X.; Zheng, H.; Xu, Y.; Zhao, R.; Wang, W.; Guo, H. Characterization and quantification of microplastics in indoor environments. Heliyon 2023, 9, e15901. [Google Scholar] [CrossRef]
- Al-Hussayni, R.S.; Al-Ahmady, K.K.; Mhemid, R.K.S. Assessment of Indoor Microplastic Particles Pollution in Selected Sites of Mosul City. J. Ecol. Eng. 2023, 24, 322–332. [Google Scholar] [CrossRef]
- Haque, M.R.; Ahmed, W.; Islam Rayhan, M.R.; Rahman, M.M. Microplastics in indoor dust at Dhaka city: Unveiling the unseen contaminants within our homes. Front. Environ. Sci. 2024, 12, 1437866. [Google Scholar] [CrossRef]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Airborne microplastic particle concentrations and characterization in indoor urban microenvironments. Environ. Pollut. 2022, 308, 119707. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Hussain, M.; Uddin, A.; Zheng, Q.; Shi, J.; Song, Y. Recycling of binary polymer (PET/SBR) carpet into microfibrillar composites: A life cycle perspective with microplastics quantification. Sustain. Mater. Technol. 2024, 40, e00988. [Google Scholar] [CrossRef]
- Alipour, S.; Hashemi, S.H.; Alavian Petroody, S.S. Release of microplastic fibers from carpet-washing workshops wastewater. J. Water Wastewater 2021, 31, 27–33. [Google Scholar] [CrossRef]
- Morales, A.C.; Tomlin, J.M.; West, C.P.; Rivera-Adorno, F.A.; Peterson, B.N.; Sharpe, S.A.; Noh, Y.; Sendesi, S.M.T.; Boor, B.E.; Howarter, J.A.; et al. Atmospheric emission of nanoplastics from sewer pipe repairs. Nat. Nanotechnol. 2022, 17, 1171–1177. [Google Scholar] [CrossRef]
- Liang, H.; Wang, N.; Liu, D.; Ge, W.; Song, N.; Wang, F.; Chai, C. Release of microplastics and nanoplastics in water from disposable surgical masks after disinfection. Mar. Pollut. Bull. 2022, 184, 114184. [Google Scholar] [CrossRef]
- Su, Y.; Hu, X.; Tang, H.; Lu, K.; Li, H.; Liu, S.; Xing, B.; Ji, R. Steam disinfection releases micro (nano) plastics from silicone-rubber baby teats as examined by optical photothermal infrared microspectroscopy. Nat. Nanotechnol. 2022, 17, 76–85. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.M.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Primpke, S.; Wirth, M.; Lorenz, C.; Gerdts, G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal. Bioanal. Chem. 2018, 410, 5131–5141. [Google Scholar] [CrossRef]
- Liu, P.; Shao, L.; Li, Y.; Jones, T.; Cao, Y.; Yang, C.-X.; Zhang, M.; Santosh, M.; Feng, X.; Bérubé, K. Microplastic atmospheric dustfall pollution in urban environment: Evidence from the types, distribution, and probable sources in Beijing, China. Sci. Total Environ. 2022, 838, 155989. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Raman imaging for the analysis of silicone microplastics and nanoplastics released from a kitchen sealant. Front. Chem. 2023, 11, 1165523. [Google Scholar] [CrossRef] [PubMed]
- Heitbrink, W.A.; Verb, R.H.; Fischbach, T.J.; Wallace, M.E. A comparison of conventional and high volume-low pressure spray-painting guns. Am. Ind. Hyg. Assoc. J. 1996, 57, 304–310. [Google Scholar] [CrossRef]
- Xu, Y.; Rillig, M.C.; Waldman, W.R. New separation protocol reveals spray painting as a neglected source of microplastics in soils. Environ. Chem. Lett. 2022, 20, 3363–3369. [Google Scholar] [CrossRef]
- Sobhani, Z.; Zhang, X.; Gibson, C.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm. Water Res. 2020, 174, 115658. [Google Scholar] [CrossRef]
- Pal, K.C. Environmental pain with human beauty: Emerging environmental hazards attributed to cosmetic ingredients and packaging. In Cognitive Data Models for Sustainable Environment; Academic Press: Cambridge, MA, USA, 2022; pp. 231–252. [Google Scholar] [CrossRef]
- Joseph, A.; Goel, S. Microbead nuisance: Estimation of microplastic release into water bodies through personal care and cosmetic products. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023. EGU23-10819. [Google Scholar] [CrossRef]
- Qi, H.; Zeng, S.; Wang, Y.; Dong, X. Exploring the discharge characteristics of personal care behaviors for high precision estimation of microplastic emission. J. Environ. Manag. 2022, 312, 114917. [Google Scholar] [CrossRef]
- Banica, A.L.; Bucur, R.M.; Daniela, I.; Dulama, I.A.B.; Stirbescu, R.M.; Radulescu, C. Assessment of microplastics in personal care products by microscopic methods and vibrational spectroscopy. Sci. Study Research. Chem. Chem. Eng. Biotechnol. Food Ind. 2023, 24, 155–171. [Google Scholar]
- Sun, Q.; Ren, S.Y.; Ni, H.G. Incidence of microplastics in personal care products: An appreciable part of plastic pollution. Sci. Total Environ. 2020, 742, 140218. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Mielańczuk, M.; Syczewski, M. The Raman spectroscopy and SEM/EDS investigation of the primary sources of microplastics from cosmetics available in Poland. Chemosphere 2022, 308, 136407. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, L.; Li, Z.; Hu, X.; Yin, D. Effects of nanoplastics and microplastics on the availability of pharmaceuticals and personal care products in aqueous environment. J. Hazard. Mater. 2023, 458, 131999. [Google Scholar] [CrossRef]
- Habib, R.Z.; Aldhanhani, J.A.; Ali, A.H.; Ghebremedhin, F.; Elkashlan, M.; Mesfun, M.; Kittaneh, W.; Al Kindi, R.; Thiemann, T. Trends of microplastic abundance in personal care products in the United Arab Emirates over the period of 3 years (2018–2020). Environ. Sci. Pollut. Res. 2022, 29, 89614–89624. [Google Scholar] [CrossRef]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.H. Deep A Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Camerlo, A.; Vebert-Nardin, C.; Rossi, R.M.; Popa, A.M. Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur. Polym. J. 2013, 49, 3806–3813. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Bianchet, R.T.; Reis, I.M.A.S.D.; Gouveia, I.C. Plastics and microplastic in the cosmetic industry: Aggregating sustainable actions aimed at alignment and interaction with UN sustainable development goals. Polymers 2022, 14, 4576. [Google Scholar] [CrossRef] [PubMed]
- Agumba, D.O.; Kumar, B.; Kim, J. Advanced hydrostable, recyclable and degradable cellulose hybrid films as renewable alternatives to synthetic plastics. Int. J. Biol. Macromol. 2024, 260, 129370. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Chen, C. Biobased, biodegradable and compostable plastics: Chemical nature, biodegradation pathways and environmental strategy. Environ. Sci. Pollut. Res. 2024, 31, 8387–8399. [Google Scholar] [CrossRef]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 25148–25169. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Liu, J.; Zhong, S.; Qian, Y.; Gao, P. An overlooked entry pathway of microplastics into agricultural soils from application of sludge-based fertilizers. Environ. Sci. Technol. 2020, 54, 4248–4255. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Leusch, F.D. Wastewater treatment plant effluent as a source of microplastics: Review of the fate, chemical interactions and potential risks to aquatic organisms. Water Sci. Technol. 2016, 74, 2253–2269. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef]
- Hooge, A.; Hauggaard-Nielsen, H.; Heinze, W.M.; Lyngsie, G.; Ramos, T.M.; Sandgaard, M.H.; Vollertsen, J.; Syberg, K. Fate of microplastics in sewage sludge and in agricultural soils. TrAC Trends Anal. Chem. 2023, 166, 117184. [Google Scholar] [CrossRef]
- Ou, H.; Liu, R.; Liao, Z.; Zeng, E.Y. Occurrence and fate of microplastics in urban water management systems. In Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2024; pp. 181–228. [Google Scholar] [CrossRef]
- Hechmi, S.; Bhat, M.A.; Kallel, A.; Khiari, O.; Louati, Z.; Khelil, M.N.; Zoghlami, R.I.; Cherni, Y.; Melki, S.; Trabelsi, I.; et al. Soil contamination with microplastics (MPs) from treated wastewater and sewage sludge: Risks and sustainable mitigation strategies. Discov. Environ. 2024, 2, 95. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, W.; Chen, Y.; Wu, Y.; Gan, W.; She, N.; Ma, Y. A bibliometric perspective on the occurrence and migration of microplastics in soils amended with sewage sludge. Water Environ. Res. 2024, 96, e11054. [Google Scholar] [CrossRef] [PubMed]
- Radford, F.; Horton, A.; Hudson, M.; Shaw, P.; Williams, I. Agricultural soils and microplastics: Are biosolids the problem? Front. Soil Sci. 2023, 2, 941837. [Google Scholar] [CrossRef]
- Hoang, V.H.; Nguyen, M.K.; Hoang, T.D.; Ha, M.C.; Huyen, N.T.T.; Bui, V.K.H.; Pham, M.T.; Nguyen, C.M.; Chang, S.W.; Nguyen, D.D. Sources, environmental fate, and impacts of microplastic contamination in agricultural soils: A comprehensive review. Sci. Total Environ. 2024, 950, 175276. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef]
- Jariwala, H.; Santos, R.M.; Lauzon, J.D.; Dutta, A.; Wai Chiang, Y. Controlled release fertilizers (CRFs) for climate-smart agriculture practices: A comprehensive review on release mechanism, materials, methods of preparation, and effect on environmental parameters. Environ. Sci. Pollut. Res. 2022, 29, 53967–53995. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Shier, W.T.; Vicari, A.; Little, N.S.; Aloise, M.R.; Giacomini, S. Degradation of microplastic seed film-coating fragments in soil. Chemosphere 2019, 226, 645–650. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of microcapsules derived from coated fertilizer in paddy fields. Chemosphere 2021, 267, 129185. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
