Abstract
Meloxicam is widely prescribed as an analgesic and anti-inflammatory drug in human therapeutics. Owing the very low aqueous solubility of meloxicam, this property has been studied in dimethyl sulfoxide (DMSO)-aqueous solvent systems at several temperatures from 273.15 to 313.15 K to expand the solubility database about analgesic drugs in mixed solvents. The flask shake method followed by ultraviolet-visible (UV-vis) spectrophotometry analysis were used for meloxicam solubility determinations. A number of cosolvency models, including the Jouyban–Acree model, were challenged for solubility correlation/prediction of this drug in these mixtures. The van’t Hoff and Gibbs equations were employed to calculate the apparent standard thermodynamic quantities relative to dissolution and mixing processes. The inverse Kirkwood–Buff integral method was employed for calculating the preferential solvation parameters of meloxicam by DMSO in the mixtures. Meloxicam solubility increases with increasing temperature and maximum solubilities are observed in neat DMSO at all temperatures studied. Dissolution processes were endothermic in all cases and entropy-driven in the composition interval of 0.40 ≤ x1 ≤ 1.00. A nonlinear enthalpy–entropy relationship was observed in the plot of enthalpy vs. Gibbs energy for drug transfer processes. Meloxicam is preferentially solvated by water in water-rich mixtures but preferentially solvated by DMSO in the composition interval of 0.21 < x1 < 1.00.
1. Introduction
Meloxicam (molecular structure shown in Figure 1, IUPAC name: 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide, molar mass 351.40 g·mol−1, CAS number: 71125-38-7, PubChem CID: 54677470) is a non-steroidal anti-inflammatory drug used commonly for pain and inflammatory treatments [1,2,3,4,5]. From a physicochemical point of view, meloxicam exhibits very low aqueous solubility, which influences negatively in vivo dissolution rates, affecting its biological performance. Otherwise, because of the very low aqueous solubility of this drug, all the duties relative to research and development of homogeneous liquid dosage forms, such as peroral or injectable products, based on this drug, are very long and hard at an industrial pharmaceutical level. In order to overcome the drawbacks mentioned above, some investigations have been intended to increase the aqueous equilibrium solubility of meloxicam. These investigations were mainly based in the evaluation of the solubilizing effect of some common pharmaceutical cosolvents, as has recently been summarized in a previous communication of our research group [6]. More recently, some other aqueous mixtures involving different cosolvents, including choline-based deep eutectic solvents, have also been studied and reported [7,8,9,10,11]. It is noteworthy that very good meloxicam solubility-increasing has been reported, reaching more than 1000-fold in some cases, as follows: 1144 with Carbitol® [9], 1399 with N-methylformamide [6], and 11,233 with N,N-dimethylformamide [6].
Figure 1.
Molecular structure of meloxicam.
Dimethyl sulfoxide (molar mass: 78.13 g·mol−1, CAS number: 67-68-5, PubChem CID 679) is a polar, aprotic solvent, miscible with water and with a wide range of organic solvents in all possible compositions, which makes it adequate for dissolving substances of both polar and nonpolar nature [1,12]. It exhibits less toxicity than N-methylformamide and N-methyl-2-pyrrolidone and has been reported about its good power of penetrating the skin and other membranes without damaging them. This is the reason why it has a high potential to increase the penetration of less soluble active ingredients. Otherwise, DMSO exhibits by itself some analgesic and anti-inflammatory properties [13]. Indeed, there are some references in the literature that suggest the potential use of DMSO as a possible pharmacotherapeutic agent in the management of pain and other conditions [14]. Moreover, owing its high solubilizing power for different active pharmaceutical ingredients, it has been used as an excipient in topical and parenteral medications for human and veterinary use [15]. On the other hand, DMSO has also been considered as solvent model in medicinal chemistry for studying intermolecular effects of different drugs [16]. In this way, aqueous mixtures of DMSO have been studied for solubilizing several drugs, drug-alike compounds, and other organic chemicals, including bergenin [17], N-guanylurea dinitramide [18], naringin [19], sinapic acid [20], p-nitrobenzamide [21], d-histidine [22], micoflavin [23], phenformin [24], baricitinib [25], a pyridazinone derivative [26], and nicotinamide [27], among others.
As it is well-described in the specialized chemical and pharmaceutical literature, all the physicochemical data about the equilibrium solubility of drugs or drug-alike compounds in aqueous cosolvent mixtures, as well, as the deep understanding of the respective dissolution mechanisms, are very important for both theoretical and practical points of view in pharmaceutical and chemical sciences. This is because the measured, reported, and analyzed solubility values expand the respective solubility databases, which is very useful for theoretical and practical purposes in both the pharmaceutical and chemical industries, as indicated above [28,29,30,31].
Therefore, the main aims of this research were as follows: (i) to determine and analyze the effects of both mixtures’ composition and temperature on the solubility of meloxicam in {DMSO (1) + water (2)} mixtures; (ii) to correlate equilibrium solubility data with several well-known thermodynamic models; (iii) to calculate the apparent standard dissolution and mixing thermodynamic parameters; and (iv) to study the preferential solvation parameters of meloxicam in binary mixtures conformed by DMSO and water. Therefore, this research is a continuation of some other similar ones reported earlier in the literature [6,7,8,9,11,32] about the meloxicam equilibrium solubility in other aqueous cosolvent systems of pharmaceutical interest for design of dosage forms and its quality control analysis, but using another commonly used cosolvent because of its high solubilizing power.
2. Materials and Methods
2.1. Materials and Reagents
Meloxicam (Technodrugs & Intermediates PVT LTD, component 3, purity > 0.995 in mass fraction), DMSO (Panreac, component 1, purity > 0.995 in mass fraction), and distilled water with conductivity < 2 μS·cm−1 (component 2), were used. Purities of meloxicam and DMSO were reported by the suppliers, as determined by high-performance liquid chromatography and gas chromatography, respectively.
2.2. Preparation of Solvent Mixtures
All the {DMSO (1) + water (2)} binary solvent mixtures were prepared gravimetrically by using an Ohaus Pioneer TM PA214 analytical balance (USA, sensitivity ± 0.1 mg), in quantities of 50.00 g. The mole fractions of DMSO of the nine mixtures prepared, varied by 0.10 in steps from x1 = 0.10 to x1 = 0.90.
2.3. Solubility Determinations
Equilibrium meloxicam solubilities were determined by using the shake-flask method [33], followed by UV-spectrophotometric analysis, as follows: an excess amount of meloxicam was added to 50.00 g of each binary solvent mixture or neat DMSO in dark glass pharmaceutical flasks. The stoppered flasks were putted in an ultrasonic bath (Elma® E60H Elmasonic, Fremont, CA, USA) during 15 min and were later transferred to thermostatic mechanical shakers (Julabo SW23, Seelbach, Germany) or re-circulating thermostatic baths (Neslab RTE 10 Digital One Thermo Electron Company, Waltham, MA, USA) and kept at 313.15 K for at least four days to ensure that the drug saturation had been achieved. After that, the supernatant solutions were isothermally filtered (Millipore Corp. Swinnex®-13, Burlington, MA, USA) to remove undissolved solid particles before sampling. Meloxicam concentrations were determined after appropriate gravimetric dilution with a 0.10 mol·dm−3 NaOH solution, by measuring the UV light absorbance at the maximum absorbance wavelength, λmax = 361 nm (UV/VIS BioMate 3 Thermo Electron Company spectrophotometer, USA), followed by interpolation from a previously validated UV spectrophotometric gravimetric calibration curve prepared in NaOH 0.10 mol·dm−3. The respective equation was: Absorbance = 0.0073 + 52.508·C, with C expressed as μg·g−1. Later, the thermostatic baths temperature was decreased from 313.15 K to 308.15 K allowing the meloxicam excess precipitation during two days, following with the same procedures mentioned above to determine the new meloxicam concentrations at saturation. All these procedures were performed successively until solid-liquid equilibrium was achieved at 293.15 K. All the solubility experiments were performed at least three times and the respective results were averaged. The density of the saturated solutions was measured by using a digital density meter (DMA 45 Anton Paar, Graz, Austria) connected to a re-circulating thermostatic bath (Neslab RTE 10 Digital One Thermo Electron Company, USA) in order to transform the obtained gravimetric solubility values into volumetric concentration scales. The density meter was calibrated at every temperature by using air and water as standards as indicated in the respective instruction manuals [34].
2.4. Solid Phase Analyses
2.4.1. X-ray Diffraction (XRD) Analysis
To determine the crystal nature of the solid meloxicam samples both before and after the saturation in neat water, in the mixture of x1 = 0.50, and in neat DMSO, the respective X-ray powder diffraction analyses were performed by using a PANalytical Xpert Pro X-ray diffractometer. The equipment is provided with CuKα radiation λ = 1.5418 Å. Generator setting: 40 kV and 40 mA and Bragg–Brentano geometry. Data were collected at 2θ from 5° to 70° and angle variation of 0.02° with detector data acquisition time of 9.46 min operating under room temperature.
2.4.2. Fourier Transform Infrared (FTIR) Analysis
In additional to XRD analyses, in order to confirm the nature of the solid meloxicam samples, both before and after the saturation in neat water, in the mixture of x1 = 0.50, and in neat DMSO, FTIR analyses were also performed. The meloxicam solid samples were ground with quantities from 10 to 100 times its bulk of pure potassium bromide and the resulting mixtures were pressed into discs by using a special mold and a manual hydraulic press (Specac®, Fort Washington, PA, USA). The respective spectra were obtained in an FTIR spectrophotometer (IRAffinity-1, Shimadzu, Kyoto, Japan).
3. Results and Discussion
3.1. Experimental Mole Fraction and Molarity Solubility
Table 1 and Table 2 summarize the experimental equilibrium solubilities of meloxicam in all {DMSO (1) + water (2)} solvent systems at 293.15 ≤ T/K ≤ 313.15, as expressed in mole fraction and molarity (mol·dm−3), respectively. The studied temperature interval includes what is commonly known as “room temperature” for products storage, as well as, the normal human body temperature. It is worth mentioning that all solubility values in neat water were taken from reference [32]. The average relative uncertainty obtained in the reported solubility values was 2.4%, which is in agreement with that commonly observed in this kind of experiments. If the mole fraction scale is considered, at T = 298.15 K, Table 1 shows that the meloxicam solubility increased 6956 times from x3 = 1.137 × 10−6 in neat water to x3 = 7.909 × 10−3 in neat DMSO, where maximum solubility is obtained. A deep comparison about the meloxicam solubility in neat water has been reported and discussed earlier in one of our previous communications [6]. Regarding the meloxicam mole fraction equilibrium solubility in neat DMSO Sathesh-Babu et al. reported a value of x3 = 5.496 × 10−3 at T = 298.15 K [35], which is in good agreement with the one obtained in this research (i.e., x3 = 7.909 × 10−2, Table 1). Moreover, when considering the molarity scale, a value of C = 1.516 × 10−2 mol·dm−3 was reported by Castro et al. at T = 298.15 K [36], which differs in almost one order of magnitude regarding our value (i.e., C = 0.1089 mol·dm−3, Table 2). The observed differences in solubility values could be attributed to several reasons such as different polymorphic states, different saturation times, or different analytical procedures, among others, as described earlier [31]. Up to the best of our knowledge, no solubility values of meloxicam in aqueous mixtures of DMSO have been reported and no more comparisons are possible.

Table 1.
Experimental mole fraction solubility (x3) of meloxicam in {DMSO (1) + water (2)} mixtures at several temperatures and p = 96 kPa a,b.

Table 2.
Experimental molar solubility (C, mol·dm–3) of meloxicam in {DMSO (1) + water (2)} mixtures at several temperatures and p = 96 kPa. a,b
Figure 2 depicts the meloxicam solubility profiles as function of the Hildebrand solubility parameters (δ1+2) of {DMSO (1) + water (2)} mixtures at T = 298.15 K. As widely described, δ1+2 is a very important polarity descriptor of cosolvent mixtures [28,29,30,31]. This descriptor was calculated considering the Hildebrand solubility parameter of both pure solvents (δ1 = 26.6 MPa1/2 for DMSO and δ2 = 47.8 MPa1/2 for water [37,38]), and the volume fraction (fi) of each solvent, as [29,39]:
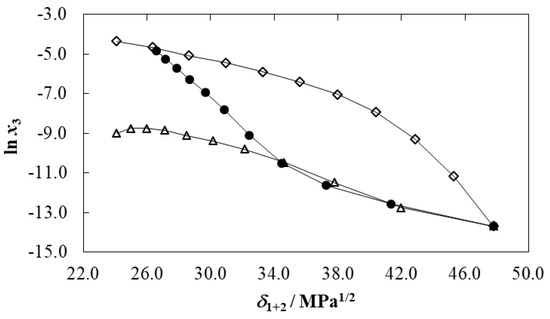
Figure 2.
Logarithmic mole fraction solubility of meloxicam (ln x3) as function of the Hildebrand solubility parameter in some {cosolvent (1) + water (2)} mixtures at 298.15 K. ●: DMSO (1) + water (2), ◊: Dimethylformamide (1) + water (2) [6], Δ: acetonitrile (1) + water (2) [7].
As observed, the solubility curve exhibited maximum in neat DMSO, where δ1 is 26.6 MPa1/2. Because solutes normally reach their maximum solubilities in solvent systems exhibiting similar polarity [28,29], it is expected that the meloxicam δ3 value would be lower than 26.6 MPa1/2 at 298.15 K. However, this δ3 value is lower compared with the one reported earlier (δ3 = 32.1 MPa1/2) [6] calculated by means of the Fedors method [40]. This high discrepancy could be mainly attributed to specific drug solvation processes by DMSO or water, which are not considered in Fedors’ calculations, in particular, if considering the structural effects described for aqueous mixtures of DMSO [41,42]. Otherwise, Figure 2 also compares the logarithmic solubility of meloxicam as function of the Hildebrand solubility parameter in some aqueous-aprotic cosolvent mixtures, namely, {DMSO (1) + water (2)}, {dimethyl formamide (1) + water (2)} [6], and {acetonitrile (1) + water (2)} [7], mixtures at 298.15 K. It is noteworthy that meloxicam solubilities are highest in {dimethyl formamide (1) + water (2)} mixtures followed by {DMSO (1) + water (2)} mixtures of δ1+2 < 34.0 MPa1/2 and lowest in {acetonitrile (1) + water (2)} mixtures of δ1+2 < 34.0 MPa1/2. Otherwise, in mixtures of δ1+2 > 34.0 MPa1/2, the solubilities in aqueous mixtures of DMSO and acetonitrile are similar. This result shows that meloxicam solubility depends not only on the polarity but also on some other physicochemical properties of solute and solvent systems.
3.2. Solid Phases’ Analyses
X-ray diffraction (XRD) spectra for meloxicam of the original untreated sample and after its saturation in neat water, neat DMSO, and the aqueous-DMSO mixture of x1 = 0.50 are shown in Figure 3. Because of the high similarity among all obtained spectra, it could be concluded that changes of the crystalline form of meloxicam are not observed after its dissolution and saturation in these four solvent systems. Moreover, Table 3 summarizes the position, 2q spacing, peak height (in counts), and the relative intensity of peaks exhibiting values higher than 10% for the original untreated meloxicam sample. These values are in good coincidence with those reported by Wu et al. [43]. Moreover, all the obtained XRD spectra of this research are very similar to that reported earlier for the polymorph I of meloxicam [32,44,45,46,47]. Finally, the FTIR spectra of all solid meloxicam samples shown in Figure 4 are also coincident with those reported in the literature, which allows to indicate that the three bottom-solid phases, obtained after drug saturation, have the same nature as the original untreated sample [47,48,49]. Therefore, meloxicam did not suffer crystal polymorphic transitions or solvates formation after saturation in these dissolution experiments.
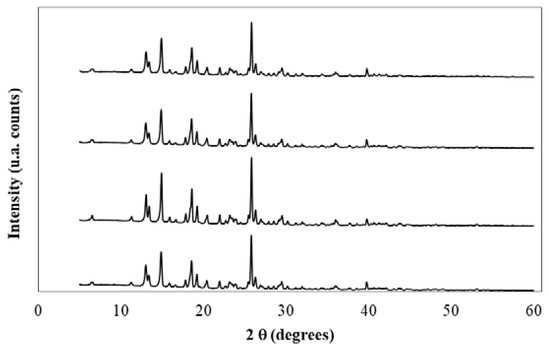
Figure 3.
X-ray diffraction spectra of meloxicam. From top to bottom: crystallized in water, crystallized in {DMSO (1) + water (2)} (x1 = 0.50) mixture, crystallized in DMSO, original sample.

Table 3.
X-ray diffraction analysis of the original untreated meloxicam sample: position, 2q spacing, peak height (in counts) and relative intensity of peaks with values higher than 10%.
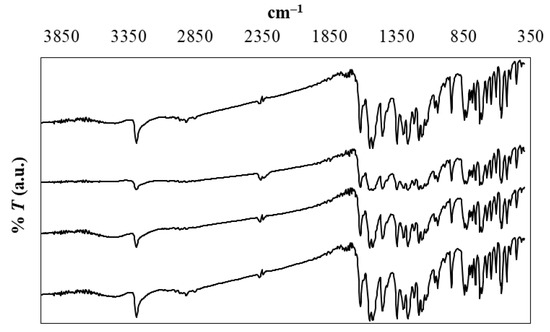
Figure 4.
FTIR spectra of meloxicam. From top to bottom: original sample, crystallized in DMSO, crystallized in {DMSO (1) + water (2)} (x1 = 0.50) mixture, crystallized in water.
3.3. Activity Coefficients in Mixed Solvents
Table 4 summarizes the asymmetrical activity coefficients (γ3) of meloxicam in all the {DMSO (1) + water (2)} solvent systems. These values were calculated as the quotient from the ideal () and experimental solubilities of meloxicam summarized in Table 1. It is noteworthy that ideal solubilities were taken from the literature [32]. As observed, the γ3 values vary from 2708 in neat water (where the lower drug solubilities are observed) to 0.389 in neat DMSO at T = 298.15 K, where the maximum drug solubility is observed at this temperature. At all temperatures, meloxicam exhibits γ3 values higher than unity in neat water and the mixtures of x1 ≤ 0.70, but lower than unity in DMSO-rich mixtures and neat DMSO. On the other hand, in neat water and the mixtures of x1 = 0.10 and 0.20, the γ3 values increase with an increase in temperature. This implies some distancing from the ideal dissolution behavior with increasing temperature. In mixtures of 0.30 ≤ x1 ≤ 0.50, the γ3 values are almost independent on temperature. On the contrary, in mixtures of x1 ≥ 0.60 and neat DMSO, the γ3 values decrease with the increase in temperature.

Table 4.
Activity coefficients of meloxicam in {DMSO (1) + water (2)} mixtures at several temperatures and p = 96 kPa a,b.
Moreover, Equation (2) allows a rough estimate of the magnitudes of solute–solvent intermolecular interactions from γ3 values [50].
where subscript 1 stands for the solvent system that here corresponds to neat solvents or aqueous DMSO binary mixtures, ess, e33, and es3 represent the magnitudes of solvent–solvent, solute–solute, and solvent–solute interaction energies, respectively. However, it is important to keep in mind that in the case of ternary systems, such as DMSO-water-meloxicam studied here, some water–cosolvent interactions are present, which could also play an important role in drug solubilities and dissolution rates. V3 denotes the molar volume of the super-cooled liquid meloxicam, whereas, φs denotes the volume fraction of every solvent system. For low x3 solubility values, V3φs2/RT may be considered as constant despite of the solvent system. Hence, γ3 values would depend mainly on ess, e33, and es3 [50]. As is well-known, ess and e33 are unfavorable for drug solubility and dissolution, whereas es3 favors the respective drug solubility and dissolution rate increasing. The contribution of e33 could be considered as constant in the different solvent systems studied. Thus, from a qualitative viewpoint, based on the energetic quantities described in Equation (2), the following analysis could be established: because ess is highest in neat water (δ2 = 47.8 MPa1/2) and lowest in neat DMSO (δ1 = 26.6 MPa1/2) [37,38], neat water and water-rich mixtures (exhibiting γ3 values higher than 2300) would imply high ess and low es3 values, whereas, in DMSO-rich mixtures (exhibiting γ3 values near the unity), the ess values are relatively low, and therefore, the es3 values would be high. In this way, a higher solvation of meloxicam by DMSO in DMSO-rich mixtures is expected.
3.4. Solubility Modeling
Among the available cosolvency models presented for calculation of drug solubilities in mixed solvents at isothermal condition or at various temperatures [51,52], the Yalkowsky model is the simplest one [53] and requires only two experimental solubility determinations (in neat solvents) to predict the solubility at other solvent compositions. The Yalkowsky model is commonly represented as:
where x3−(1+2) denotes the mole fraction solubility of meloxicam in the aqueous-cosolvent mixtures, x3(1) denotes the mole fraction solubility in neat DMSO (component 1), x3(2) denotes the mole fraction solubility in neat water (component 2), and x1 and x2 are the mole fractions of DMSO (1) and water (2) in the cosolvent mixtures in the absence of meloxicam (3). Thus, the obtained mean percentage deviation (MPD) values after calculation of the solubility of meloxicam in {DMSO (1) + water (2)} mixtures at T = (293.15, 298.15, 303.15, 308.15, and 313.15) K by means of this model were (41.3, 42.9, 44.8, 45.6, and 46.7)%, respectively, with the overall MPD of 44.2%. The numerical values of the MPD were computed using:
where N is the number of experimental data points.
ln x3−(1+2) = x1 ln x3(1) + x2 ln x3(2)
As mentioned above, Equation (3) is capable of estimating drug solubility in cosolvent mixtures at individual T using only the drug solubility data in the mono-solvents at this T. However, it could be extended to obtain:
to be applied at various temperatures () using a single equation. In Equation (5), A and B terms are the model constants [54]. The trained model for solubility of meloxicam in {DMSO (1) + water (2)} mixtures is:
which resulted in the MPD of 44.4%.
The main limitation of the Yalkowsky model is that it does not consider any more interaction term after mixing the solutions and considers the mixing behavior as an ideal one. The Jouyban–Acree model includes as many as required interaction terms (Ji terms) to describe the non-ideality of the mixing and produced the most accurate results in correlating drug solubility data in binary solvent mixtures at various temperatures. The model is presented as [51]:
where Ji terms are the respective model constants that are computed by using a non-intercept least square analysis [31]. Accordingly, the generated solubility values of meloxicam in {DMSO (1) + water (2)} were fitted to Equation (7) and the obtained trained model was:
The F value of Equation (8) was 791 and the adjusted correlation coefficient (R) was 0.979, whereas the correlation and the model constants were significant with p < 0.0005. Equation (8) is valid for calculating the solubility of meloxicam in different {DMSO (1) + water (2)} mixtures at various temperatures or mixtures-composition of interest, by employing the solubility data of meloxicam in neat DMSO and neat water at each temperature. The obtained MPD for the back-calculated solubility data of meloxicam when using Equation (8) was 9.6%.
Although Equation (8) provided an accurate correlation for the solubility of meloxicam in these {DMSO (1) + water (2)} mixtures, it requires the experimental solubility data in mono-solvents (i.e., x3(1),T and x3(2),T) at any temperature of interest to calculate the solubility of meloxicam in the required binary solvent mixtures. However, one may combine the trained version of Equation (5) with Equation (7) to provide a full predictive model, obtaining the following:
Equation (9) allows to calculate the solubilities of meloxicam in all these binary mixtures at various temperatures with an MPD of 9.9% and it does not require any experimental input data. The F and R values for Equation (9) were 787 and 0.978, respectively. For practical applications of Equation (9), one may train the model using the minimum number of seven experimental solubility points, and then, predict the rest of required data in any aqueous-DMSO mixture composition and temperature of interest, as has been exemplified in the literature [55]. When the model was trained with the solubility data in DMSO and water at T = (293.15 and 313.15) K (i.e., the lowest and highest temperatures) and in x1 = 0.3, 0.5 and 0.7 at T = 298.15 K (total 7 data points), the rest of the data points were predicted with the MPD of 18.7% (N = 48).
In a previous work [56], generally trained version of the Jouyban–Acree–Abraham and Jouyban–Acree–Hansen models were presented for predicting the solubility of meloxicam in various binary solvent mixtures. These models are:
and
The c, e, s, a, b, and v are Abraham solvent’s coefficients and δd1, δp1, δh1, and δd2, δp2, and δh2 are the Hansen parameters for solvents 1 and 2, respectively [56]. Equations (10) and (11) predicted the solubility of meloxicam in (DMSO + water) mixtures with the MPDs of 74.3 and 35.6%. Although the prediction errors are relatively large, these equations only require the solubility data in the neat solvent.
3.5. Apparent Thermodynamic Functions of Dissolution
All the apparent standard thermodynamic quantities relative to meloxicam dissolution processes were calculated at the mean harmonic temperature, Thm = 303.0 K, which was calculated by using Equation (12) [57].
where n is the number of temperatures under study (i.e., 5 in this case). Hence, all the apparent standard enthalpy changes relative to dissolution processes (∆solnH°) were obtained by means of the modified van’t Hoff equation, as shown in Equation (13) [58]:
The apparent standard Gibbs energy changes relative to all the meloxicam dissolution processes (∆solnG°) were calculated by means of [58,59,60]:
The intercepts used in Equation (14) were those obtained as the result of the linear regressions of ln x3 as function of (1/T − 1/Thm). Therefore, Figure 5 depicts the meloxicam solubility linear van’t Hoff behavior in all the {DMSO (1) + water (2)} mixtures, as well as in both neat solvents. It is noteworthy that linear regressions with r2 > 0.993 were observed in all the solvent systems [61,62,63].
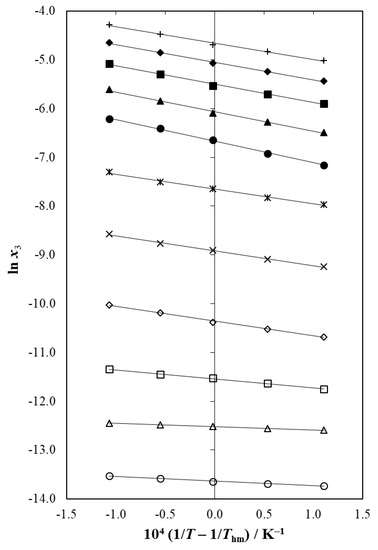
Figure 5.
Van’t Hoff plot of the solubility of meloxicam (3) in {DMSO (1) + water (2)} solvent systems. ○: x1 = 0.00 (neat water), Δ: x1 = 0.10, □: x1 = 0.20, ◊: x1 = 0.30, ×: x1 = 0.40, 🞵: x1 = 0.50, ●: x1 = 0.60, ▲: x1 = 0.70, ■: x1 = 0.80, ♦: x1 = 0.90, +: x1 = 0.10 (neat DMSO).
Finally, the apparent standard changes in entropy, for all the studied meloxicam dissolution processes (∆solnS°) were calculated based on the respective ∆solnH° and ∆solnG° values by using [59,60]:
Table 5 summarizes all the apparent standard thermodynamic quantities for the dissolution processes of meloxicam in all the {DMSO (1) + water (2)} mixtures at Thm = 303.0 K, including those corresponding to dissolution processes in neat water and DMSO. It is notable that all apparent standard dissolution thermodynamic quantities for neat water were taken from previous research results [32]. As expected, all the apparent standard Gibbs energies and apparent enthalpies of dissolution of meloxicam are positive in every case in these DMSO-aqueous systems. Otherwise, the apparent standard entropies of dissolution were negative in neat water, as well as in the mixtures of x1 ≤ 0.30 but positive from the mixture of x1 = 0.40 to neat DMSO. Thus, the global dissolution processes of meloxicam are always endothermic in nature and entropy-driven for those occurring in the composition interval of 0.40 ≤ x1 ≤ 1.00; whereas, in neat water and the mixtures of x1 ≤ 0.30, neither entropy or enthalpy-driving are observed because ∆solnH° > 0 and ∆solnS° < 0). All ΔsolnG° values decrease continuously from neat water (where highest ΔsolnG° value is obtained) to reach the lowest value in neat DMSO. Otherwise, ΔsolnH° decreases from neat water to reach the lowest value in the mixture of x1 = 0.10, and later, it increases with the DMSO proportion to reach a quasi-plateau in the mixtures of x1 = 0.30, 0.40, and 0.50 to increase again, reaching the highest value in the mixture of x1 = 0.60; after, it decreases to reach a new minimum in neat DMSO. The ∆solnS° values increase from a lowest negative value in neat water (−87.99 J·mol−1·K−1) to reach the maximum positive value in the mixture of x1 = 0.60 (66.49 J·mol−1·K−1), and later, they decrease continuously with the DMSO proportion to reach a lower value in neat DMSO (53.35 J·mol−1·K−1). As observed, the lowest ΔsolnH° and ∆solnS° values are observed in neat water or in the mixture of x1 = 0.00. The negative apparent dissolution entropies observed in neat water and the mixtures of composition x1 = 0.10, 0.20, and 0.30 could be a consequence of the possible hydrophobic hydration around the methyl and phenylene groups of meloxicam (Figure 1).

Table 5.
Apparent thermodynamic functions relative to dissolution processes of meloxicam (3) in {DMSO (1) + water (2)} mixtures at Thm = 303.0 K and p = 96 kPa a,b.
Moreover, to calculate the magnitude contributions by enthalpy (ζH) and entropy (ζTS) toward the dissolution processes, the following equations were used [64]:
As observed in Table 5, the higher contribution to the positive apparent standard molar Gibbs energies of meloxicam dissolution is given by the positive enthalpy. This demonstrates that in almost all the mixtures, the main contributor to this positive standard molar Gibbs energy of solution of meloxicam (reflected in the low meloxicam solubility) is the enthalpy except for neat water and the mixture of x1 = 0.10, where ζH = 0.224 and 0.177, respectively, and thus, entropy is the dominant function in these two cases.
3.6. Apparent Thermodynamic Quantities of Mixing
The overall dissolution processes of meloxicam in all {DMSO (1) + water (2)} solvent systems may be represented with the following hypothetical stages:
Solute(Solid state) at Thm → Solute(Solid state) at Tfus → Solute(Liquid state) at Tfus → Solute(Liquid state) at Thm → Solute(Solution state) at Thm
Here, the hypothetical stages are considered as follows: (i) the heating and fusion of meloxicam at Tfus = 536.7 K, (ii) the cooling of the liquid fused meloxicam to the considered temperature (i.e., Thm = 303.0 K), and (iii) the subsequent mixing of both the hypothetical super-cooled liquid meloxicam and the liquid aqueous-DMSO solvent system at Thm = 303.0 K [65]. This treatment allowed us to calculate every individual thermodynamic contribution toward the overall meloxicam dissolution processes by means of:
where and represent the thermodynamic quantities relative to meloxicam melting and its cooling at Thm = 303.0 K, which, in turn, are calculated by means of [66]:
where ΔCp denotes the difference of heat capacities of liquid and solid states at the temperature of melting. Owing the difficulties in ΔCp experimental determinations, the entropy of fusion (ΔSf) is used instead [66]. Table 6 summarizes all the apparent standard thermodynamic quantities of mixing of the hypothetical super-cooled liquid meloxicam with all the studied aqueous-DMSO mixtures and the neat solvents, water, and DMSO, as calculated at Thm = 303.0 K. As observed, the Gibbs energies of mixing are positive from neat water to the mixture of x1 = 0.70 because the experimental drug solubilities are lower than ideal solubilities; on the contrary, they are negative from the mixture of x1 = 0.80 to neat DMSO, because the experimental solubilities are higher than the ideal ones. The contributions by the thermodynamic quantities of mixing subprocesses to the overall dissolution processes of meloxicam are variable and depend on the aqueous-DMSO mixtures’ composition. Thus, ΔmixH° values are negative in neat water and the mixtures of x1 = 0.10 and 0.20 but positive in the solvent systems in the interval of 0.30 ≤ x1 ≤ 1.00. Moreover, ΔmixS° values are negative in the interval 0.00 ≤ x1 ≤ 0.50 but positive in the other cases. Thus, the mixing processes in neat water and the mixture of x1 = 0.10 and 0.20 are enthalpy-driven because of the exothermic character exhibited. In the mixtures 0.30 ≤ x1 ≤ 0.50, neither enthalpy nor entropy-driving is observed for mixing. Finally, in the interval of 0.60 ≤ x1 ≤ 1.00, entropy-driven is observed. Furthermore, in order to evaluate the relative contributions by enthalpy (ζH) and entropy (ζTS) to the mixing processes in all these solvent systems, two equations analogous to Equations (16) and (17) were also employed. As observed, in water-rich and DMSO-rich mixtures, the main contributor to Gibbs energies of mixing is the entropy, but in the mixtures’ composition interval of 0.30 ≤ x1 ≤ 0.70, it is the enthalpy.

Table 6.
Apparent thermodynamic functions relative to mixing processes of meloxicam (3) in {DMSO (1) + water (2)} mixtures at Thm = 303.0 K and p = 96 kPa a,b.
As described earlier in the literature, the net variation of ΔmixH° values regarding the aqueous-cosolvent mixtures’ composition depends on the contribution of different kinds of intermolecular interactions. Hence, the cavity formation in the solvent system, required for the solute accommodation, is endothermic because some quantity of energy must be supplied against the respective cohesive forces of the solvent. This contribution diminishes the drug solubility as mentioned above. Oppositely, the solvent–solute interactions, resulting mainly from van der Waals and Lewis acid-base interactions, such as hydrogen bonding, are clearly exothermic in nature. This effect increases the drug solubility and dissolution rate as also indicated before. Even more, the structuring of water molecules around the phenylene ring and the methyl group of meloxicam structure (Figure 1) would be contributing to diminish the net ΔmixH° quantity to small or even negative values in water-rich mixtures [67]. This event is clearly observed with meloxicam in aqueous-DMSO mixtures as indicated in Table 5 for solvent systems from neat water to the mixture of x1 = 0.50.
3.7. Enthalpy–Entropy Compensation Analysis
Classical extra-thermodynamic studies, in particular those based on the enthalpy–entropy compensation analyses, provide a powerful physicochemical tool to find or identify similar mechanisms responsible for some physical and chemical processes involving organic compounds [68,69]. Some well-known literature reports demonstrated the presence of nonlinear-enthalpy–entropy compensation effects in the dissolution processes of many drugs and drug-alike compounds in different aqueous cosolvent binary systems. These extra-thermodynamic studies have usually been performed by different research groups to identify the main mechanisms involved in the cosolvent action for solubility increasing or decreasing, depending on the mixtures’ composition [70,71,72]. As shown in Figure 6, meloxicam exhibits a nonlinear ΔsolnH° vs. ΔsolnG° trend with negative slopes from neat water to the mixture of x1 = 0.10 and from the mixture of x1 = 0.60 to neat DMSO, whereas, in the interval of 0.10 ≤ x1 ≤ 0.30 and from x1 = 0.50 to x1 = 0.60, positive slopes are observed; finally, in the interval of 0.30 ≤ x1 ≤ 0.50, a plateau is observed. In the first cases, the driving mechanism for transferring meloxicam from the most polar solvent systems to less polar solvent systems is the entropy. For the intervals exhibiting positive slopes, the drug transfer is driven by the enthalpy. Otherwise, in the interval of 0.30 ≤ x1 ≤ 0.50, the function driving the transfer is not clear. Nevertheless, it is not easy to identify the molecular effects involved, owing the complexity of aqueous-DMSO mixtures as indicated earlier [41,42].
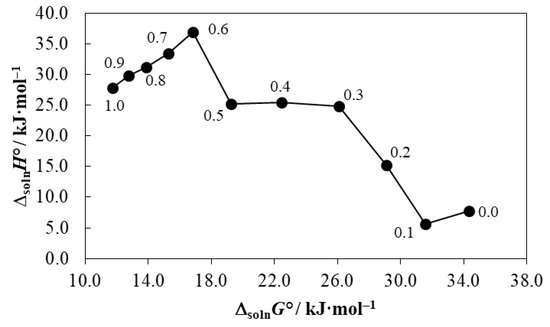
Figure 6.
ΔsolnH° vs. ΔsolnG° enthalpy–entropy compensation plot for the solubility of meloxicam (3) in {DMSO (1) + water (2)} mixtures at Thm = 303.0 K. The points represent the mole fraction of DMSO (1) in the {DMSO (1) + water (2)} mixtures in the absence of meloxicam (3).
3.8. Preferential Solvation Analysis
The preferential solvation parameter of meloxicam (component 3) by DMSO (component 1) in the {DMSO (1) + water (2)} mixtures at saturation is defined as:
where is the local mole fraction of DMSO in the molecular environment around meloxicam and x1 is the bulk mole fraction of DMSO in the initial aqueous-DMSO mixture in the absence of meloxicam. If δx1,3 values were positive, meloxicam would be preferentially solvated by DMSO, but if they were negative, meloxicam would be preferentially solvated by water. Thus, the respective δx1,3 values were obtained by means of the inverse Kirkwood–Buff integrals (IKBI) for the solvent components based on [73,74,75]:
with,
Here, κT represents the isothermal compressibility of every {DMSO (1) + water (2)} mixture. and denote the partial molar volumes of DMSO and water in the aqueous-DMSO mixtures. denotes the partial molar volume of meloxicam. The function D corresponds to the first derivative of the variation of standard molar Gibbs energies of transfer of meloxicam from neat water to {DMSO (1) + water (2)} mixtures regarding the DMSO-proportion in the mixtures free of solute, as shown in Equation (27). The function Q involves the second derivative of the variation of excess molar Gibbs energy of mixing of DMSO and water () regarding the water-proportion in the aqueous-DMSO mixtures, as shown in Equation (28). Vcor is the correlation volume and r3 is the hydrodynamic molecular radius of meloxicam, which is commonly calculated by means of Equation (29), where NAv is the number of Avogadro.
As exposed in the literature, the definitive Vcor values require iteration because they depend on the local mole fractions of DMSO and water around the meloxicam molecules in the equilibrated solutions. Hence, these iterations are performed by substituting δx1,3 and Vcor values in Equations (22), (23), and (26) in order to recalculate the value until almost invariant values of Vcor are obtained.
Figure 7 depicts the apparent Gibbs energies of transfer of meloxicam from neat water to {DMSO (1) + water (2)} mixtures () at T = 298.15 K. These values were calculated from the mole fraction solubilities shown Table 1, by using:
values were correlated according to the regular fourth degree polynomial presented as Equation (31), with adjusted r2 = 0.998, typical error = 0.317, and F value = 1484.
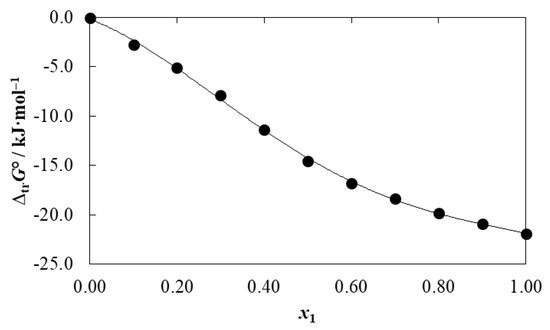
Figure 7.
Gibbs energy of transfer of meloxicam (3) from neat water (2) to {DMSO (1) + water (2)} mixtures at T = 298.15 K.
In this way, the D values shown in Table 7 were calculated from the first derivative of Equation (31) by considering the variation of aqueous-DMSO mixtures composition in incremental x1 = 0.05 steps through all the mixtures’ composition interval. Otherwise, the required Q, RTκT, , and values corresponding to {DMSO (1) + water (2)} mixtures were taken from the literature [76].

Table 7.
Some properties associated to preferential solvation of meloxicam (3) in {DMSO (1) + water (2)} mixtures at T = 298.15 K.
Because values are not available for meloxicam in {DMSO (1) + water (2)} mixtures, these values were considered as the one calculated based on Fedors’ method, i.e., 183.3 cm3·mol−1 [6]. G1,3 and G2,3 shown in Table 7 are negative in all cases, indicating the affinity of meloxicam by DMSO and water. The approximated hydrodynamic radius of meloxicam (r3) was calculated as 0.417 nm by means of Equation (29). In turn, the preferential solvation parameters of meloxicam by DMSO molecules are also summarized in Table 7. According to Figure 8, initially adding of DMSO to water makes the δx1,3 values of meloxicam negative in the interval from neat water to the mixture of x1 = 0.21. The maximum negative value of this parameter is obtained in the mixture of x1 = 0.10, with δx1,3 = −1.36 × 10−2, which is higher than 1.00 × 10−2 if the absolute value is considered; therefore, it could be a consequence of real preferential solvation effects by water on meloxicam, rather than a consequence of the uncertainty propagation in the respective IKBI calculations [77,78]. The cosolvent action of DMSO for increasing the meloxicam solubility in these water-rich mixtures could be associated to the breaking of the ordered structure exhibited by water molecules, such as “icebergs”, around the non-polar moieties of meloxicam, which, in turn, would be increasing the meloxicam solubility and solvation.
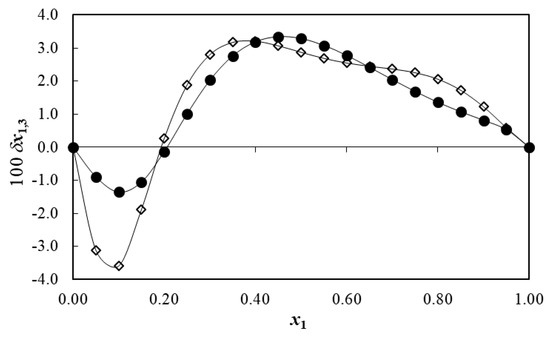
Figure 8.
Preferential solvation parameters of meloxicam (3) in some {cosolvent (1) + water (2)} mixtures at T = 298.15 K. ●: DMSO (1) + water (2), ◊: Dimethylformamide (1) + water (2) [6].
In mixtures of 0.21 < x1 < 1.00, the δx1,3 values are positive indicating preferential solvation of meloxicam by DMSO. Maximum δx1,3 value was obtained in the mixture of x1 = 0.45 (δx1,3 = 3.34 × 10−2). This maximum positive δx1,3 value is also higher than |1.00 × 10−2| being a consequence of real preferential solvation effects by DMSO [77,78]. From a mechanistic viewpoint, in the mixtures’ composition region of 0.19 < x1 < 1.00, it is adequately conjecturable that meloxicam could be acting as a Lewis acid in front of the DMSO molecules owing the unshared electrons of the sulfoxide oxygen atom of this cosolvent. Notably, this cosolvent is more basic than water, as remarkable by the magnitude of their Kamlet–Taft hydrogen bond acceptor parameters, namely β = 0.76 for DMSO and 0.47 for water [38]. Moreover, Figure 8 allows the comparison of preferential solvation of meloxicam by DMSO and N,N-dimethylformamide in their respective aqueous mixtures [6]. As observed, the cosolvent regions of preferential solvation are similar, as well as the magnitudes of preferential solvation by both cosolvents. Nevertheless, preferential hydration of meloxicam is higher with N,N-dimethylformamide, which could be a consequence of its lower polarity (δ1 = 24.1 MPa1/2) regarding DMSO (δ1 = 26.6 MPa1/2) [37,38]. These interesting behaviors could be a consequence of the high water-association effects around the non-polar groups of this drug, which could be favored by the more hydrophobic moieties present in the cosolvents, as they exhibit less polar nature. In turn, the hydrophobic groups present in the cosolvents could also be acting as water-association promotors depending on their respective molecular sizes [11]. Finally, from all the physicochemical analyses reported, it is noteworthy to mention that this investigation expands the equilibrium solubility database about non-steroidal anti-inflammatory drugs in commonly used aqueous cosolvent mixtures [79].
4. Conclusions
Equilibrium molar and mole fraction solubilities of analgesic drug meloxicam in different {DMSO (1) + water (2)} mixtures at five temperatures from 293.15 to 313.15 K were determined by using the shake flask method followed by UV-vis drug quantification, reported and analyzed. Meloxicam mole fraction solubility in these mixtures was adequately correlated with some well-known correlation models obtaining mean percentage deviations (MPDs) of 9.6 to 9.9%. In addition, a number of predictive models which were already trained using published datasets and by employing the minimum number of measured experimental data from this project were produced with MPDs of 35.6 to 74.3%. Apparent standard thermodynamic quantities of dissolution and mixing processes were calculated observing endothermal dissolution processes in all cases and favored in DMSO-rich mixtures. Nonlinear enthalpy-entropy compensation was observed indicating different mechanisms for the cosolvent action. IKBI treatment demonstrated preferential hydration of meloxicam in water-rich mixtures but preferential solvation by DMSO in mixtures of 0.21 < x1 < 1.00.
Author Contributions
Conceptualization, D.A.T., F.M., O.A.A., M.Á.P., A.J. and W.E.A.J.; methodology, D.A.T., F.M. and O.A.A.; software, D.A.T., F.M., M.Á.P. and A.J.; validation, D.A.T., F.M., A.J. and W.E.A.J.; formal analysis, D.A.T., F.M., O.A.A., M.Á.P., A.J. and W.E.A.J.; investigation, D.A.T., F.M., O.A.A., M.Á.P., A.J. and W.E.A.J.; resources, D.A.T., F.M. and O.A.A.; data curation, D.A.T. and F.M.; writing—original draft preparation, D.A.T., F.M., M.Á.P. and A.J.; writing—review and editing, D.A.T., F.M., O.A.A., M.Á.P., A.J. and W.E.A.J.; visualization, D.A.T., F.M., O.A.A., M.Á.P., A.J. and W.E.A.J.; supervision, F.M., M.Á.P., A.J. and W.E.A.J.; project administration, D.A.T., F.M., A.J. and W.E.A.J.; funding acquisition, D.A.T., F.M. and O.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Minciencias (formerly Colciencias), grant number 785-2019.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Departments of Pharmacy and Physics of the Universidad Nacional de Colombia for facilitating the equipment and laboratories used. Financial support of Minciencias (formerly Colciencias) is also highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Obenchain, J.R., Jr.; Gallipeau, J.A.R.; DÁrecca, M.A. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Brooks, P.M.; Day, R.O. Non steroidal anti-inflammatory drugs--differences and similarities. N. Engl. J. Med. 1991, 324, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, G.; Homma, D.; Schlegel, K.; Utzmann, R.; Schnitzler, C. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm. Res. 1995, 44, 423–433. [Google Scholar] [CrossRef]
- Türck, D.; Roth, W.; Busch, U. A review of the clinical pharmacokinetics of meloxicam. British J. Rheumatol. 1996, 35, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, S.C. Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility of meloxicam in aqueous binary mixtures of formamide, N-methylformamide and N,N-dimethylformamide: Determination, correlation, thermodynamics and preferential solvation. J. Chem. Thermodyn. 2021, 154, 106332. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Dissolution thermodynamics and preferential solvation of meloxicam in (acetonitrile + water) mixtures. Phys. Chem. Liq. 2021, 59, 733–752. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility, dissolution thermodynamics and preferential solvation of meloxicam in (methanol + water) mixtures. J. Solution Chem. 2021, 50, 667–689. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility of meloxicam in (Carbitol® + water) mixtures: Determination, correlation, dissolution thermodynamics and preferential solvation. J. Mol. Liq. 2021, 324, 114671. [Google Scholar] [CrossRef]
- Golgoun, S.; Mokhtarpour, M.; Shekaari, H. Solubility enhancement of betamethasone, meloxicam and piroxicam by use of choline-based deep eutectic solvents. Pharm. Sci. 2021, 27, 86–101. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility, correlation, dissolution thermodynamics and preferential solvation of meloxicam in aqueous mixtures of 2-propanol. Pharm. Sci. 2022, 28, 130–144. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; American Pharmacists Association and Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Shirley, S.W.; Stewart, B.H.; Mirelman, S. Dimethyl sulfoxide in treatment of inflammatory genitourinary disorders. Urology 1978, 11, 215–220. [Google Scholar] [CrossRef]
- Jacob, S.W.; De la Torre, J.C. Dimethyl Sulfoxide (DMSO) in Trauma and Disease; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Jacob, S.W.; Herschler, R. Introductory remarks: Dimethyl sulfoxide after twenty years. Ann. N. Y. Acad. Sci. 1983, 411, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C. Drug solubility in water and dimethylsulfoxide. In Molecular Drug Properties: Measurement and Prediction; Mannhold, R., Ed.; Wiley: Weinheim, Germany, 2007; pp. 257–282. [Google Scholar]
- Shakeel, F.; Mothana, R.A.; Haq, N.; Siddiqui, N.A.; Al-Oqail, M.M.; Al-Rehaily, A.J. Solubility and thermodynamic function of bergenin in different (DMSO + water) mixtures at different temperatures. J. Mol. Liq. 2016, 220, 823–828. [Google Scholar] [CrossRef]
- Manh, T.N.; Kim, K.-J. Solubility of N-guanylurea dinitramide in binary solvent mixtures. Propellants Explos. Pyrotech. 2016, 41, 709–712. [Google Scholar] [CrossRef]
- Jabbari, M.; Khosravi, N.; Feizabadi, M.; Ajloo, D. Solubility temperature and solvent dependence and preferential solvation of citrus flavonoid naringin in aqueous DMSO mixtures: An experimental and molecular dynamics simulation study. RSC Adv. 2017, 7, 14776–14789. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Salem-Bekhit, M.M.; Raish, M. Solubility and dissolution thermodynamics of sinapic acid in (DMSO + water) binary solvent mixtures at different temperatures. J. Mol. Liq. 2017, 225, 833–839. [Google Scholar] [CrossRef]
- Yuan, Y.; Farajtabar, A.; Kong, L.; Zhao, H. Thermodynamic solubility modelling, solvent effect and preferential solvation of p-nitrobenzamide in aqueous co-solvent mixtures of dimethyl sulfoxide, ethanol, isopropanol and ethylene glycol. J. Chem. Thermodyn. 2019, 136, 123–131. [Google Scholar] [CrossRef]
- Li, W.; Farajtabar, A.; Xing, R.; Zhu, Y.; Zhao, H. Solubility of d-histidine in aqueous cosolvent mixtures of N,N-dimethylformamide, ethanol, dimethyl sulfoxide, and N-methyl-2-pyrrolidone: Determination, preferential solvation, and solvent effect. J. Chem. Eng. Data 2020, 65, 1695–1704. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Zhang, X.; Farajtabar, A.; Zhao, H. Solubility, preferential solvation, and solvent effect of micoflavin in aqueous mixtures of dimethylsulfoxide, isopropanol, propylene glycol, and ethanol. J. Chem. Eng. Data 2020, 65, 1976–1985. [Google Scholar] [CrossRef]
- Zhao, X.; Farajtabar, A.; Han, G.; Zhao, H. Phenformin in aqueous co-solvent mixtures of N,N-dimethylformamide, ethanol, N-methylpyrrolidone and dimethyl sulfoxide: Solubility, solvent effect and preferential solvation. J. Chem. Thermodyn. 2020, 144, 106085. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Shakeel, F. Solubility data and computational modeling of baricitinib in various (DMSO +Water) mixtures. Molecules 2020, 25, 2124. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Alshehri, S.; Imran, M.; Haq, N.; Alanazi, A.; Anwer, M.K. Experimental and computational approaches for solubility measurement of pyridazinone derivative in binary (DMSO +water) systems. Molecules 2020, 25, 171. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Przybyłek, M.; Kowalska, A.; Tymorek, N. Thermodynamics and intermolecular interactions of nicotinamide in neat and binary solutions: Experimental measurements and COSMO-RS concentration dependent reactions investigations. Int. J. Mol. Sci. 2021, 22, 7365. [Google Scholar] [CrossRef] [PubMed]
- Rubino, J.T. Cosolvents and cosolvency. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1988; Volume 3, pp. 375–398. [Google Scholar]
- Martin, A.; Bustamante, P.; Chun, A.H.C. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1993. [Google Scholar]
- Yalkowsky, S.H. Solubility and Solubilization in Aqueous Media; American Chemical Society and Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Jouyban, A. Handbook of Solubility Data for Pharmaceutical; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Delgado, D.R.; Holguin, A.R.; Almanza, O.A.; Martinez, F.; Marcus, Y. Solubility and preferential solvation of meloxicam in ethanol + water mixtures. Fluid Phase Equilib. 2011, 305, 88–95. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Kratky, O.; Leopold, H.; Stabinger, H. DMA45 Calculating Digital Density Meter, Instruction Manual; Anton Paar, K.G.: Graz, Austria, 1980. [Google Scholar]
- Sathesh-Babu, P.R.; Subrahmanyam, C.V.S.; Thimmasetty, J.; Manavalan, R.; Valliappan, K. Extended Hansen’s solubility approach: Meloxicam in individual solvents. Pak. J. Pharm. Sci. 2007, 20, 311–316. [Google Scholar]
- Castro, G.T.; Filippa, M.A.; Sancho, M.I.; Gasull, E.I.; Almandoz, M.C. Solvent effect on the solubility and absorption spectra of meloxicam: Experimental and theoretical calculations. Phys. Chem. Liq. 2020, 58, 337–348. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Marcus, Y. The Properties of Solvents; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- Connors, K.A. Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy; Wiley–Interscience: Hoboken, NJ, USA, 2002. [Google Scholar]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Kirchner, B.; Reiher, M. The secret of dimethyl sulfoxide−water mixtures. A quantum chemical study of 1DMSO−n-water clusters. J. Am. Chem. Soc. 2002, 124, 6206–6215. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Manias, E.; Macdonald, D.D.; Lanagan, M. Dielectric relaxation in dimethyl sulfoxide/water mixtures studied by microwave dielectric relaxation spectroscopy. J. Phys. Chem. A 2009, 113, 12207–12214. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Tang, P.X.; Li, S.S.; Zhang, L.L.; Li, H. X-ray powder diffraction data for meloxicam, C14H13N3O4S2. Powder Diffr. 2014, 29, 196–198. [Google Scholar] [CrossRef]
- Freitas, J.T.J.; Santos-Viana, O.M.M.; Bonfilio, R.; Doriguetto, A.C.; Benjamim de Araújo, M. Analysis of polymorphic contamination in meloxicam raw materials and its effects on the physicochemical quality of drug product. Eur. J. Pharm. Sci. 2017, 109, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Sarkar, M. Spectroscopic studies of microenvironment dictated structural forms of piroxicam and meloxicam. J. Lumin. 2002, 99, 255–263. [Google Scholar] [CrossRef]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Noolkar, S.B.; Jadhav, N.R.; Bhende, S.A.; Killedar, S.G. Solid-state characterization and dissolution properties of meloxicam–moringa coagulant–PVP ternary solid dispersions. AAPS PharmSciTech 2013, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Sirisolla, J. Solubility enhancement of meloxicam by liquisolid technique and its characterization. Int. J. Pharm. Sci. Res. 2015, 6, 835–840. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Loading and evaluation of meloxicam and atorvastatin in carrageenan microspherical aerogels particles. J. Appl. Pharm. Sci. 2019, 9, 83–88. [Google Scholar] [CrossRef]
- Kristl, A.; Vesnaver, G. Thermodynamic investigation of the effect of octanol–water mutual miscibility on the partitioning and solubility of some guanine derivatives. J. Chem. Soc. Faraday Trans. 1995, 91, 995–998. [Google Scholar] [CrossRef]
- Jouyban, A.; Acree, W.E., Jr. Mathematical derivation of the Jouyban-Acree model to represent solute solubility data in mixed solvents at various temperatures. J. Mol. Liq. 2018, 256, 541–547. [Google Scholar] [CrossRef]
- Jouyban-Gharamaleki, A.; Valaee, L.; Barzegar-Jalali, M.; Clark, B.J.; Acree, W.E., Jr. Comparison of various cosolvency models for calculating solute solubility in water-cosolvent mixtures. Int. J. Pharm. 1999, 177, 93–101. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Roseman, T.J. Solubilization of drugs by cosolvents. In Techniques of Solubilization of Drugs; Yalkowsky, S.H., Ed.; Marcel Dekker: New York, NY, USA, 1981; pp. 91–134. [Google Scholar]
- Jouyban, A.; Romero, S.; Chan, H.K.; Clark, B.J.; Bustamante, P. A cosolvency model to predict solubility of drugs at several temperatures from a limited number of solubility measurements. Chem. Pharm. Bull. 2002, 50, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Dadmand, S.; Kamari, F.; Acree, W.E., Jr.; Jouyban, A. Solubility prediction of drugs in binary solvent mixtures at various temperatures using a minimum number of experimental data points. AAPS PharmSciTech 2019, 20, 10. [Google Scholar] [CrossRef]
- Rahimpour, E.; Jouyban, A. Utilizing Abraham and Hansen solvation parameters for solubility prediction of meloxicam in cosolvency systems. J. Mol. Liq. 2021, 328, 115400. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data. J. Phys. Chem. 1976, 80, 2335–2341. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect. J. Phys. Chem. 1976, 80, 2341–2351. [Google Scholar] [CrossRef]
- Ruidiaz, M.A.; Delgado, D.R.; Martínez, F.; Marcus, Y. Solubility and preferential solvation of indomethacin in 1,4-dioxane + water solvent mixtures. Fluid Phase Equilib. 2010, 299, 259–265. [Google Scholar] [CrossRef]
- Aydi, A.; Dali, I.; Ghachem, K.; Al-Khazaal, A.Z.; Delgado, D.R.; Kolsi, L. Solubility of Hydroxytyrosol in binary mixture of ethanol + water from (293.15 to 318.15) K: Measurement, correlation, dissolution thermodynamics and preferential solvation. Alex. Eng. J. 2021, 60, 905–914. [Google Scholar] [CrossRef]
- Bevington, P.R. Data Reduction and Error Analysis for the Physical Sciences; McGraw-Hill Book, Co.: New York, NY, USA, 1969; pp. 56–65. [Google Scholar]
- Carstensen, J.T. Modeling and Data Treatment in the Pharmaceutical Sciences; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1996; pp. 127–159. [Google Scholar]
- Barrante, J.R. Applied Mathematics for Physical Chemistry, 2nd ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1998; 227p. [Google Scholar]
- Perlovich, G.L.; Kurkov, S.V.; Kinchin, A.N.; Bauer-Brandl, A. Thermodynamics of solutions III: Comparison of the solvation of (+)-naproxen with other NSAIDs. Eur. J. Pharm. Biopharm. 2004, 57, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.R.; Almanza, O.A.; Martínez, F.; Peña, M.A.; Jouyban, A.; Acree, W.E., Jr. Solution thermodynamics and preferential solvation of sulfamethazine in (methanol + water) mixtures. J. Chem. Thermodyn. 2016, 97, 264–276. [Google Scholar] [CrossRef]
- Jouyban, K.; Agha, E.M.H.; Hemmati, S.; Martinez, F.; Kuentz, M.; Jouyban, A. Solubility of 5-aminosalicylic acid in N-methyl-2-pyrrolidone + water mixtures at various temperatures. J. Mol. Liq. 2020, 310, 113143. [Google Scholar] [CrossRef]
- Romero, S.; Reillo, A.; Escalera, B.; Bustamante, P. The behaviour of paracetamol in mixtures of aprotic and amphiprotic-aprotic solvents. Relationship of solubility curves to specific and nonspecific interactions. Chem. Pharm. Bull. 1996, 44, 1061–1066. [Google Scholar] [CrossRef]
- Tomlinson, E. Enthalpy-entropy compensation analysis of pharmaceutical, biochemical and biological systems. Int. J. Pharm. 1983, 13, 115–144. [Google Scholar] [CrossRef]
- Leffler, J.E.; Grunwald, E. Rates and Equilibria of Organic Reactions: As Treated by Statistical, Thermodynamic and Extrathermodynamic Methods; Dover Publications Inc.: New York, NY, USA, 1989. [Google Scholar]
- Bustamante, P.; Romero, S.; Reillo, A. Thermodynamics of paracetamol in amphiprotic and amphiprotic-aprotic solvent mixtures. Pharm. Pharmacol. Commun. 1995, 1, 505–507. [Google Scholar] [CrossRef]
- Bustamante, P.; Romero, S.; Peña, A.; Escalera, B.; Reillo, A. Nonlinear enthalpy-entropy compensation for the solubility of drugs in solvent mixtures: Paracetamol, acetanilide and nalidixic acid in dioxane-water. J. Pharm. Sci. 1998, 87, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Peña, M.A.; Bustamante, P. Thermodynamic analysis and enthalpy-entropy compensation for the solubility of indomethacin in aqueous and non-aqueous mixtures. Fluid Phase Equilib. 2011, 308, 98–106. [Google Scholar] [CrossRef]
- Marcus, Y. Solvent Mixtures: Properties and Selective Solvation; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Marcus, Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 2008, 140, 61–67. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential solvation of drugs in binary solvent mixtures. Pharm. Anal. Acta 2017, 8, 1000537. [Google Scholar] [CrossRef]
- Martínez, F.; Jouyban, A.; Acree, W.E., Jr. Modelling the solubility and preferential solvation of bergenin in DMSO + water mixtures. Lat. Am. J. Pharm. 2016, 35, 2185–2190. [Google Scholar]
- Ben–Naim, A. Preferential solvation in two- and in three-component systems. Pure Appl. Chem. 1990, 62, 25–34. [Google Scholar] [CrossRef]
- Marcus, Y. Solubility and solvation in mixed solvent systems. Pure Appl. Chem. 1990, 62, 2069–2076. [Google Scholar] [CrossRef]
- Acree, W.E., Jr. IUPAC-NIST Solubility Data Series. 102. Solubility of nonsteroidal anti-inflammatory drugs (NSAIDs) in neat organic solvents and organic solvent mixtures. J. Phys. Chem. Ref. Data 2014, 43, 023102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).