Abstract
The bioprocessing strategy is an effective approach to improve bioavailability and stability of bioactive compounds for designing functional foods and ingredients. In this study, food barley was bio-transformed to improve functional bioactives by sprouting, coupled with beneficial lactic acid bacteria (LAB)-based fermentation. Dairy Kefir culture with mixed beneficial LAB strains was targeted to ferment aqueous slurries of sprouted hulless food barley flour (unpigmented, purple, and black barley) for 72 h, and modulation of phenolic-linked antioxidant and anti-hyperglycemic functionalities were evaluated using in vitro assay models. The biochemical parameters analyzed were total soluble phenolic (TSP) content, profile of phenolic compounds, total antioxidant activity, and anti-hyperglycemic property-relevant α-amylase and α-glucosidase enzyme inhibitory activities. Furthermore, human gut health benefits of relevant properties of fermented slurries of barley flour were also evaluated based on growth of Kefir culture and subsequent determination of anti-bacterial potential against pathogenic human ulcer causing bacteria Helicobacter pylori. Kefir culture-mediated fermentation of 48-h sprouted barley flours improved the TSP content and associated antioxidant and anti-hyperglycemic functionalities. Additionally, anti-bacterial potential against H. pylori and sustaining active growth of viable LAB cells above the minimum level required for probiotic activity were also observed in fermented food barley flour slurries.
Keywords:
antioxidant; anti-hyperglycemic; food barley; fermentation; Kefir culture; phenolics; sprouting 1. Introduction
Consumer awareness of healthy diets is growing rapidly, resulting in increased demand for “functional” foods and beverages that may potentially impart enhanced health benefits. Among such foods, probiotics are particularly prominent, and they consist of formulations containing sufficient numbers of selected live microorganisms that beneficially modify the intestinal microbiota of the host, thereby capable of imparting wider health benefits [1,2,3]. Currently, the most commonly available forms of probiotics are dairy-based [4,5]. However, cereal grains can be a viable alternative for developing novel non-diary probiotic rich foods to mitigate disadvantages associated with fermented dairy products in some consumers such as lactose intolerance, allergy, and the adverse impact on body cholesterol levels [5,6,7,8].
Barley grains are a particularly amenable substrate source for the development of grain-based probiotic and prebiotic products [9]. Previously published studies have reported that barley is rich in health-promoting functional compounds, including phenolic bioactives [10,11,12,13,14,15]. Phenolic bioactives from barley have potential antiproliferative, anti-carcinogenic, and anti-inflammatory properties due to their high antioxidant potentials [12,13,14,15]. Furthermore, high antioxidant and anti-hyperglycemic functionality was observed in malting barley cultivars following sprouting [16,17,18].
Phenolic bioactives from whole grains such as barley could play a complimentary role in augmenting current pharmacological treatment strategies for preventing type 2 diabetes (T2D)-linked chronic hyperglycemia, specifically due to their carbohydrate metabolism relevant enzyme inhibitory potential and other functional benefits [18,19,20]. Bioprocessing strategies can play a key role in effectively enhancing such human health protective bioactive functional benefits in barley grains. Sprouting is one such method wherein insoluble, cell wall bound phenolic compounds are converted into biologically available and active soluble forms during grain modification [21,22,23]. Beyond its antioxidant and glucose metabolism modulating functions, the solubilized phenolic compounds also exert anti-bacterial effects, by virtue of the inhibitory effects of the phenolic groups and by complementing the activity of short chain fatty acids produced by beneficial gut bacteria against pathogenic bacteria [24,25]. Additionally, the products derived during sprouting, such as hemicellulose and oligosaccharide fractions and glutamine-rich peptides, can aid the growth and proliferation of beneficial bacteria in the human gut, resulting in potential probiotic and gastro-protective benefits [26,27,28]. Therefore, sprouted food barley potentially may serve as a phenolic-rich functional food ingredient source with several human health protective benefits, such as antioxidant, anti-hyperglycemic and gut health improvements [14,15].
Current published evidence in this area has mostly focused on the sprouting/germination-linked mobilization of phenolic compounds in barley in the context of malting and brewing applications [29,30]. However, there is limited published literature on phenolic mobilization and solubilization in food barley, and their potential impact on improving human health-related functional properties of barley-based food ingredients. Similarly, fermentation of whole grain cereals with beneficial lactic acid bacteria (LAB) is also gaining increased interest from the food industry and consumers. During fermentation, the grain constituents are modified by the action of both endogenous and bacterial enzymes, including esterases, xylanases, and phenol oxidases, thereby affecting their structure, bioactivity, and bioavailability [21,31]. Cereal-based LAB fermentation has been shown to increase the levels of nutrients including folates, soluble dietary fiber, and total phenolic content in cereals [32] and to improve the protein digestibility and short chain fatty acid (SCFA) production in vitro [33,34]. Most cereal-based fermentation studies have focused on the fermentation of rye and wheat for the baking industry, although barley has been shown to be an appropriate substrate source for LAB fermentation. Interestingly, barley-based fermented products have been reported to decrease total cholesterol and increase fecal concentration of probiotic bacteria such as Bifidobacterium spp. in healthy individuals [35]. However, the effect of LAB fermentation on the content of free and bound phenolic compounds, and their bioavailability and human health relevant functionalities such as antioxidant and anti-hyperglycemic properties of food barley varieties, needs to be explored.
Therefore, the broad objective of this study was to investigate the combined effect of sprouting and fermentation with beneficial mixed LAB culture from dairy-based Kefir on the mobilization and solubilization of phenolic compounds in food barley. Such a strategy can potentially support diets and ingredient design to counter chronic oxidative stress and chronic hyperglycemia commonly associated with early stages of type 2 diabetes. Specifically, the changes in the total phenolic content, antioxidant activity, anti-hyperglycemic property, and beneficial gut health benefits-relevant anti-bacterial activity (H. pylori) in sprouted barley flour slurries during 72 h of fermentation with beneficial mixed culture bacteria obtained from commercial dairy-based Kefir product were investigated. The selection of commercial Kefir-based mixed culture for fermentation of sprouted barley flour was based on the previous promising findings of phenolic mobilization and improved antioxidant and anti-diabetic functionalities in Kefir culture-mediated fermentation of soymilk [36,37]. Therefore, in this current study, sprouting and Kefir-mediated fermentation were combined and integrated as a bioprocessing strategy to improve the content and bioactivity of phenolic compounds and associated antioxidant, anti-hyperglycemic, and gut health benefits of food barley for managing/preventing chronic oxidative stress and hyperglycemia, two major health risks of type 2 diabetes.
2. Materials and Methods
2.1. Preparation of Sprouted Barley Flour and Aqueous Slurries
Three types of hulless food barley with varying pigmentation (unpigmented/colorless—UB; black—BB; purple—PB) were obtained from a local grocery store (Walmart, Fargo, ND, USA). Barley grains were steeped in distilled water (1:4, w/v) for 24 h under constant agitation on a rotary shaker (150 rpm) at 20 °C. Steeped barley kernels were transferred to sanitized glass sprouting containers (VWR International, Radanor, PA, USA) (equipped with perforated lids to allow for draining of steep water and aeration) and placed in a controlled environment incubator (VWR International, Radanor, PA, USA) (20 °C; >90% relative humidity) to initiate sprouting. The sprouts were moistened and turned periodically to provide aeration and prevent the matting of rootlets.
Sprouted grains were removed from the containers at 24 h and 48 h, the rate of germination (%) was recorded, and then they were dried in an incubator (40 °C; 48 h) until the dry weight of the kernels remained constant. The rootlets of the desiccated barley sprouts were removed manually and discarded, while the grains were milled using the fine flour setting on a disc-mill (WonderMill, Pocatello, ID, USA). Milled barley flour samples were combined with cold water (1:5, w/v) and homogenized using a benchtop blender (Waring Products Co., CT, USA) for 5 min. The homogenate/slurry was used for the second bioprocessing step, i.e., fermentation.
2.2. Kefir Culture Preparation, Inoculation, and Fermentation
The method used in Kefir culture preparation was adapted from previous studies [37,38]. Commercially available non-fat milk Kefir (100 µL; Lifeway Foods Inc., Morton Grove, IL, USA) was aseptically inoculated into MRS broth (10 mL; Difco, Becton, Dickinson and Co., Franklin Lakes, NJ, USA) for 24 h at 37 °C. Kefir culture contained a mixture of the following probiotic microorganisms—Lactobacillus lactis, Lacticaseibacillus rhamnosus, Streptococcus diacetylactis, Lactiplantibacillus plantarum, Lacticaseibacillus casei, Saccharomyces florentinus, Leuconostoc cremoris, Bifidobacterium longum, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium lactis and Limosilactobacillus reuteri. The same steps were repeated with an aliquot (100 µL) of this culture, and the second set of cultures were used to inoculate the fermented barley flour slurries. Sample slurries were divided (25 mL) into sterile polypropylene centrifuge tubes, sealed and pasteurized by immersion in a water bath (80 °C, 10 min) and immediately transferred into an ice bath for 10 min. Samples were aseptically inoculated with the Kefir cultures (2.5 mL; initial inoculum density of approximately 1010 CFU/mL). Corresponding controls, to which 2.5 mL of sterile distilled water was added instead of inoculum, were also included for each sample × culture combination. All tubes were placed in a closed incubator (VWR International, Radanor, PA, USA) maintained at 37 °C and samples along with a corresponding control tube were removed at 0, 24, 48 and 72 h post-inoculation for analysis. At each time point, half of the sample was transferred to a separate tube and the pH was adjusted to the level of the corresponding control to evaluate any potential effect of acidification from lactic acid production on the functional biochemical parameters, which were investigated in this study. After measuring viable cell counts of LAB, samples were centrifuged at 15,000× g for 15 min prior to carrying out in vitro assays.
2.3. Total Soluble Phenolic (TSP) Content
Total soluble phenolic (TSP) content of the fermented barley flour samples was determined using the Folin–Ciocalteu (FC) method [39]. The fermented sample extract (1 mL) was combined with 95% ethanol (1 mL), distilled water (5 mL), FC reagent (0.5 mL; 50% v/v), and sodium carbonate (1 mL; 5% v/v) in a test tube, vortexed, and incubated in the dark for 60 min. Absorbance values were measured at 725 nm using a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). From this absorbance data, TSP content of the samples was calculated using a gallic acid standard curve (10–300 μg/mL; dissolved in 95% ethanol) and expressed as milligram equivalents of gallic acid per gram dry weight (mg GAE/g DW) of the samples.
2.4. Quantification of Major Phenolic Compounds
The major free phenolic compounds in the fermented barley samples were quantified by using reverse-phase high performance liquid chromatography (RP-HPLC) analysis. A portion of all fermented samples was frozen at −20 °C at each experimental time point. Prior to HPLC (Agilent Technologies, Santa Clara, CA, USA) analysis, the frozen samples were thawed and centrifuged for 10 min at 13,000 rpm. Five microliters of the supernatant were injected into an Agilent 1260 Infinity Series HPLC system for qualitative and quantitative analysis. The phenolic compounds were separated using a Supelco SB-C18 (5 µm; 250 mm × 4.6 mm) analytical column (Agilent Technologies, Palo Alto, CA, USA), maintained at room temperature. The compounds were detected using a DAD 1100 diode array detector (DAD) (Agilent Technologies, Palo Alto, CA, USA). The sample injection volume was 5 µL. A gradient elution program, comprising eluent A (10 mM phosphoric acid; pH 2.5) and eluent B (100% methanol, HPLC grade), with a constant flow rate of 0.7 mL/min, was used. The methanol concentration was maintained at 60% for the first 8 min, increased to 100% over the next 7 min, then decreased to 0% for the next 3 min and was maintained for 7 min with a total run time of 25 min per injected sample. The absorbance of the eluted compounds was recorded at the following wavelengths: 214 nm, 230 nm, 260 nm, and 306 nm. The recorded signals were integrated using Agilent Chemstation enhanced integrator software. Pure standards of gallic acid, protocatechuic acid, catechin, chlorogenic acid, caffeic acid, quercetin, cinnamic acid, dihydroxybenzoic acid, benzoic acid and p-coumaric acid in 100% methanol were used to prepare respective calibration curves and library. Each sample was run in duplicate, and the results were expressed in microgram per gram dry weight (µg/g DW) of the fermented barley flour sample.
2.5. Total Antioxidant Activity Assays
The total antioxidant activity of the fermented sample was measured by their ability to scavenge the following free radicals: 2,2-diphenyl-1-picrylhydrazyl (DPPH) (D9132-5G, Sigma-Aldrich, St. Louis, MO, USA) and 2,2-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (A1888-5G, Sigma-Aldrich). The DPPH radical scavenging assay was based on a protocol described by Cervato et al. [40] and Kwon et al. [41], wherein 0.25 mL of sample extracts (corresponding sample controls contained 95% ethanol instead of samples) were combined with 60 mM DPPH working stock solution (adjusted to an absorbance range of 1.8–2.0 at 517 nm). After 5 min of incubation the extracts and their corresponding controls were centrifuged at 13,000 rpm for 1 min and the absorbance values of the supernatants was measured at 517 nm using a UV-visible spectrophotometer (Genesys 10S UV-VIS spectrophotometer, Thermo Scientific, New York, NY, USA). The ABTS scavenging assay was based on a protocol as described earlier by Re et al. [42], in which 0.05 mL of the sample extracts was combined with 1 mL of ABTS prepared in 95% ethanol (corresponding controls contained 0.05 mL of 95% ethanol instead of samples). After 2.5 min of incubation, sample extracts and their controls were centrifuged at 13,000 rpm for 1 min and the absorbance values of the supernatant was measured at 734 nm with a UV-visible spectrophotometer (Genesys 10S UV-VIS spectrophotometer, Thermo Scientific, New York, NY, USA). For both assays, Trolox standards were prepared in 95% ethanol (10, 5, 2.5, 1.25, 0.625 and 0.3125 mg/mL) and used as the positive control. The absorbance values from the DPPH and ABTS radical scavenging assays were used to calculate the percentage of antioxidant activity for each extract using the following formula:
2.6. α-Amylase Inhibitory Activity
The α-amylase enzyme inhibitory activity was determined in a dose-dependent manner using undiluted, half and one-fifth diluted fermented samples by an assay described in a previous study [43]. Samples (0.5 mL) were combined with 0.5 mL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing porcine α-amylase (0.5 mg/mL) (EC 3.2.1.1, Sigma Chemical Co., St. Louis, MO, USA) and incubated at 25 °C for 10 min. Further, 500 μL of substrate (1% starch solution in buffer) was added to each tube. The reaction mixtures were then incubated at 25 °C for 10 min. After this, 1 mL of 3,5-dinitrosalicylic (DNS) acid was added and the reaction was stopped by incubating the mixtures in a boiling water bath (90–100 °C) for 10 min to halt the enzyme hydrolysis and cooled to room temperature. The mixtures were then diluted with distilled water to obtain control absorbance reading between 0.8 and 1.0 units at 540 nm using a UV-visible spectrophotometer (Genesys 10S UV-VIS spectrophotometer, Thermo Scientific, New York, NY, USA). A positive control with Acarbose was prepared in distilled water and serially diluted to give different concentrations (10, 5, 2.5, 1.25, 0.625 and 0.3125 mg/mL), which was included in the study for comparison. The absorbance of sample blanks (enzyme solution replaced with buffer) and controls (sample extract replaced with buffer) were also recorded. The final extract absorbance was obtained by subtracting its corresponding sample blank reading. The α-amylase enzyme inhibitory activity was calculated as percentage (%) inhibition as per the following equation:
2.7. α-Glucosidase Inhibitory Activity
The α-glucosidase enzyme inhibitory activity was determined in a dose-dependent manner using undiluted, half and one-fifth diluted samples using an assay based on a method described in an earlier study [43]. Sample solutions (50, 25, and 10 µL) were loaded into a 96-well microtiter plate, and the total volume of the sample was made up to 50 µL (for diluted samples), by making appropriate dilutions using 0.1 M potassium phosphate buffer (pH 6.9). Each sample had a corresponding control containing the same amount of buffer instead of the sample. The volume in all wells was made up to 100 µL using the buffer, to which 100 µL of α-glucosidase solution (1 unit/mL) in buffer was added. The plate was incubated at 25 °C for 10 min. Following this, 50 µL of 5 mM p-nitrophenyl-α-D-glucopyranoside (pNPG) solution in buffer was added to each well at timed intervals and reaction mixtures were further incubated at 25 °C for 5 min. Absorbance values were recorded before and after incubation at 405 nm using a microplate reader (Thermomax, Molecular Device Co., Sunnyvale, CA, USA). The α-glucosidase enzyme inhibitory activity was expressed as percentage (%) inhibition and was calculated per the following equation:
Acarbose was used as a positive control, and a calibration curve was prepared using standard solutions of Acarbose in distilled water (10, 5, 2.5, 1.25, 0.625 and 0.3125 mg/mL).
2.8. Helicobacter Pylori Inhibition Assay
The inhibitory activity of sample slurries towards H. pylori was evaluated using the agar-diffusion disc-assay method adapted from studies performed by McCue et al. [43] and Stevenson et al. [44]. The H. pylori special peptone agar (HPSPA) was chosen due to its longer shelf-life. The growth and size of H. pylori colonies on this medium was found to be greater than those on other non-specific media [44]. The solid medium was prepared with special peptone (10 g/L), granulated agar (15 g/L), sodium chloride (5 g/L), yeast extract (5 g/L) and beef extract (5 g/L) in distilled water. Broth media was prepared with special peptone (10 g/L), sodium chloride (5 g/L), yeast extract (5 g/L), and beef extract (5 g/L) in distilled water. Prepared stock of H. pylori (1 mL) was added to test tubes containing 10 mL of sterile broth media and incubated at 37 °C for 24 h prior to inoculation by spread plate technique. The activated culture was spread evenly into plates containing H. pylori growth agar to prepare bacterial lawn for the agar-diffusion assay. Under aseptic conditions, 100 µL of filter sterilized (0.22 µm pore size) sprouted barley flour slurry with and without fermentation was dispensed on to sterile 12.7-mm diameter paper discs, while sterile distilled water was used as a negative control. The saturated discs were transferred to the surface of agar plates containing H. pylori microbial lawn and incubated at 37 °C for 48 h under anaerobic conditions in GasPak jars (Becton, Dickinson and Co., Sparks, MD, USA) with BD GasPak Campy container system sachets (Becton, Dickinson and Co., Sparks, MD, USA). The diameter of clear zones which indicate the region of H. pylori inhibition surrounding each disc was measured and expressed in millimeters. To determine dose dependence, 50 and 75 µL of the sample were used. The entire procedure was repeated twice, consisting of duplicates for each sample (three discs per sample within each Petri-dish).
2.9. Viable Cell Count of Beneficial LAB Strains
The prebiotic potential of the samples was evaluated by measuring the ability of sprouted food barley samples to promote the proliferation of beneficial LAB strains [45]. At each of the experimental time points during the fermentation process, 1 mL of each sample was collected. From this, 100 µL of sample was aliquoted and using serial dilution with sterile distilled water, solutions of up to 10−6 dilution were obtained. An amount of 100 µL of these solutions was inoculated onto MRS agar plates using the spread plate method and incubated for 48 h at 37 °C within an anaerobic BBL GasPak jar (Becton, Dickinson and Co., Sparks, MD, USA) with BD GasPak EZ anaerobe container system sachets (Becton, Dickinson and Co., Sparks, MD, USA). Plates containing 20–350 colonies were then selected for counting colonies, from which total viable cell count (log CFU/mL) of mixed LAB strains was calculated.
2.10. Statistical Analysis
A completely randomized design (CRD) model was used in this study. The entire fermentation experiment was repeated twice (each run constituting a replicate), and during each repetition, 6 samples were analyzed from each treatment combination. Analysis of variance (ANOVA) was performed using data obtained from the in vitro assays using the Statistical Analysis Software (SAS; version 9.4; SAS Institute, Cary, NC, USA). Statistically significant differences between means in bioactive functionality parameters due to three main effects, i.e., barley type, pH adjustment and duration of sprouting, and their 2-way and 3-way interactions were determined using Tukey’s least mean squares test at a confidence level of 95% (p < 0.05) and the results (mean square with level of statistical significance) are presented (Table 1).

Table 1.
Analysis of variance (ANOVA) table indicating mean square (MS), degree of freedom, and statistically significant differences between pH (P), barley sample type (S) and barley sprouting times (G), with their respective interactions for phenolic content, antioxidant activity and anti-hyperglycemic activity in sprouted flours from three types of food barley samples at 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with Kefir culture.
3. Results and Discussion
3.1. Total Soluble Phenolic Content and Phenolic Compound Profile
Phenolic compounds are the most dominant type of secondary metabolites in cereal grains such as barley, with diverse human health protective functions [12,14]. While barley grains are known to contain a considerable number of various types of phenolic compounds, their composition and content in barley-based food and beverages can vary widely depending on genotype, phenotype and based on growing environment and different food processing strategies [14,15]. In this study, the impact of different types of food barley varying in pigmentation and the effect of integrated bioprocessing strategy combining sprouting and mixed Kefir LAB culture-based fermentation on total soluble phenolic (TSP) content of fermented barley flour slurries were investigated.
Dairy-based Kefir culture with mixed LAB strains-mediated fermentation was observed to considerably impact the TSP content of the fermented barley flour slurries. Statistically significant differences in TSP content due to the 2-way interaction between pH (P) × barley sprouting duration (G) as well as among three main effects, i.e., pH, food barley type and duration of sprouting was observed at all fermentation time points (p < 0.05) (Table 1). Within each sample type, 48-h sprouted barley flour slurries were found to have the highest TSP content with and without fermentation (Figure 1). Overall, as fermentation progressed from 0 to 72 h, the TSP content increased proportionately across all sample combinations. While sprouting coupled with Kefir-mediated fermentation improved the overall TSP content of barley slurries, the mechanisms involved in improved TSP content by these bioprocessing strategies might be different.
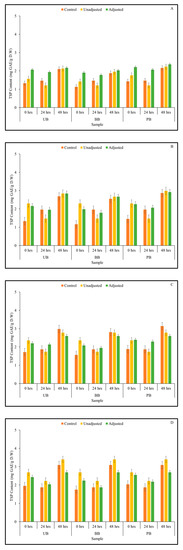
Figure 1.
Total soluble phenolic (TSP) content of flour slurries derived from germinated (0, 24, 48 h) unpigmented barley (UB), black barley (BB) and purple barley (PB) after 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with Kefir culture.
Solubilization of low molecular weight phenolic compounds following LAB fermentation can be attributed to the action of exogenous bacterial enzymes, such as feruloyl esterases, β-glucosidases, decarboxylases, hydrolases, reductases, and xylanases, on grain cell wall bound forms of phenolic compounds [46,47].
As changes in matrix acidity may affect the phenolic solubilization post-fermentation, the TSP content of pH-adjusted fermented samples (same level as that of the corresponding control) was also measured. At the beginning of fermentation (0 h), most of the pH-unadjusted samples showed lower levels of TSP content compared to the corresponding controls and pH-adjusted samples. However, as fermentation progressed, certain sample combinations indicated a pH-dependent improvement of TSP content, including UB-0 at 24, 48 and 72 h (Figure 1; Supplementary File). Acidification of the barley slurries during fermentation may hydrolyze phenolic acid esters and flavonoid glucosides from cell wall polysaccharides, resulting in the observed trend [46]. Additionally, some strains of LAB are capable of metabolizing phenolic acids by decarboxylation, yielding products such as vinyl catechol, vinyl phenol, vinyl guaiacol and catechol, as well as by reduction, yielding dihydrocaffeic acid and dihydroferulic acid [47,48,49]. Thus, Kefir culture-mediated fermentation may influence TSP content via the combined effect of pH changes and exogenous bacterial enzyme activity, with the former being more dominant, especially in the later stages of fermentation. In this study, grain bioprocessing via sprouting in combination with Kefir culture-mediated fermentation resulted in improved TSP content. Specifically, barley flour with significantly enhanced phenolic content was generated after 48 h of sprouting, which even further improved with Kefir culture-mediated fermentation for 72 h.
In addition to the TSP content, the content of individual phenolic compounds in sprouted barley flour slurries coupled with Kefir culture-mediated fermentation was also determined and quantified using HPLC method. The major phenolic compounds found in Kefir culture-mediated fermented barley samples were protocatechuic acid, gallic acid, cinnamic acid, catechin, and dihydroxybenzoic acid (Table 2). However, benzoic acid was only found in UB and PB flour extracts at 0 h of fermentation time point.

Table 2.
Major phenolic compounds (µg/g DW) in sprouted barley flour slurries at 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with Kefir culture, quantitated by HPLC analysis (C: control; F: fermented).
On the contrary, in UB flour extracts, cinnamic acid was found in 24-, 48-, and 72-h fermented samples, while it was not observed in 0-h fermentation time point. Overall, at 0-, 48-, and 72-h time points 3-way interactions between pH treatment × barley sample type × sprouting duration had a statistically significant effect on all individual phenolic compounds (Table 3) (Supplementary File). However, at the 24-h time point, the effect of the 2-way interaction between pH treatment × barley sample type and barley sample type × sprouting duration on phenolic compounds were statistically significant (Table 3) (Supplementary File). Interestingly, significant improvement in catechin, cinnamic acid, and protocatechuic acid content was observed in fermented barley samples, especially in pigmented barley (BB and PB) flour slurries after 48 and 72 h of fermentation. Previously, other investigators [50] also observed an increase in free phenolic acid (caffeic, p-coumaric, and ferulic acid) content in beneficial LAB-fermented flour slurries of whole grain barley and oat groat. The results of the current study indicated that both sprouting and Kefir culture-mediated (mixed LAB) fermentation are effective food processing strategies to improve health protective phenolic compounds in unpigmented and pigmented food barley. Therefore, this integrated bioprocessing strategy combining sprouting with LAB fermentation can be targeted for developing phenolic-rich barley-based substrates in a simple and cost-effective manner.

Table 3.
Analysis of variance (ANOVA) table indicating mean square (MS), degree of freedom, and statistically significant differences between pH (P), barley sample type (S) and barley sprouting times (G), with their respective interactions for the content of phenolic acids identified in sprouted flours from three types of barley samples at 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with Kefir culture.
Additionally, these bioprocesses may be further optimized to develop phenolic-rich functional foods and beverages with dietary and therapeutic functionalities to target poor quality diet and lifestyle-linked chronic diseases such as type 2 diabetes. The major phenolic compounds, such as gallic acid, cinnamic acid, protocatechuic acid, and catechin found in sprouted and fermented barley flour extracts are also good antioxidants and can be targeted for designing antioxidant enriched functional foods and beverages [51,52]. Therefore, it is also important to investigate the impact of sprouting and LAB fermentation integrated bioprocessing strategy on the health relevant functionalities such as antioxidant and anti-hyperglycemic properties of food barley.
3.2. Total Antioxidant Activity
The presence of phenolic compounds in plant food substrates has been positively correlated with high antioxidant activity and free radical scavenging abilities, as observed in several previous studies [53,54,55,56,57].
In the current study, it was of interest to assess how changes in TSP content observed in the barley samples during Kefir culture-mediated fermentation translated to total antioxidant capacity relevant for managing chronic oxidative stress commonly associated with type 2 diabetes and other chronic diseases. Therefore, total antioxidant activity of sprouted barley flour slurries during fermentation was determined by measuring the ability of the sample to scavenge ABTS and DPPH free radicals using in vitro assays. The results of the ABTS assay indicated that the overall free radical scavenging capacity of fermented barley samples increased consistently during fermentation (Figure 2). In this study, statistically significant differences between 3-way interactions of pH treatment × barley sample type × sprouting duration were observed in DPPH-based antioxidant activity at 0-, 48-, and 72-h fermentation time points (Table 1; Supplementary File). However, for the ABTS-based antioxidant activity result, only the 2-way interaction between pH treatment × sprouting duration was statistically significant at all fermentation time points (Table 1). Additionally, across all fermentation assay time points, the main effects of pH, barley type, and sprouting duration had a statistically significant (except germination duration at 48 h) effect on antioxidant activity. Interestingly, the ABTS free radical scavenging capacity of all fermented (unadjusted pH) barley samples was found to be significantly higher than the corresponding controls and pH-adjusted fermented samples. This is indicative of a pH-dependent improvement in the antioxidant capacity of the samples, rather than being solely due to the phenolic mobilization. However, unlike in the case of the unfermented barley samples, the trend of DPPH-based antioxidant activity differed considerably from the results of the antioxidant activity based on ABTS assay (Figure 3). While UB-0 samples had the highest DPPH scavenging capacity among the food barley types, the pH-unadjusted samples had lower antioxidant activity than the corresponding controls and pH-adjusted solutions.
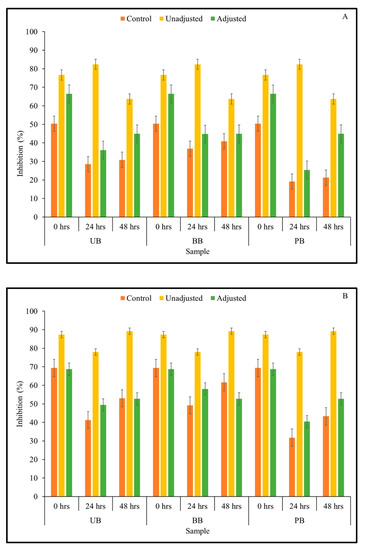
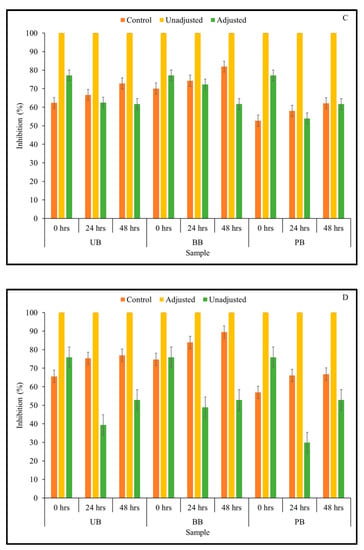
Figure 2.
ABTS free radical scavenging capacity of slurries derived from sprouted (0, 24, 48 h) unpigmented barley (UB), black barley (BB) and purple barley (PB) after 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with mixed Kefir culture.
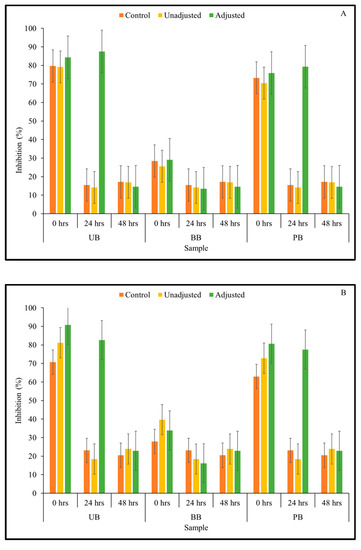
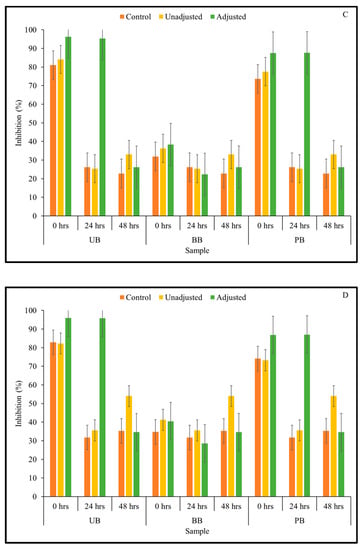
Figure 3.
DPPH free radical scavenging capacity of slurries derived from sprouted (0, 24, 48 h) unpigmented barley (UB), black barley (BB) and purple barley (PB) after 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with mixed Kefir culture.
Overall, fermentation, and more specifically with beneficial LAB-based biotransformation, has been shown to improve the antioxidant activity in various whole grain substrates [58,59]. The production of lower molecular weight phenolic compounds such as phenolic acids and aglycones during fermentation is particularly relevant, as they tend to be the end products of solubilization from the bound forms [58]. However, the pH-dependent improvement of antioxidant activity in fermented food barley slurries may be attributed to the production and combined activity of organic acids [60]. In addition to lactic acid, mixed microbial cultures—such as in Kefir—may produce other organic acids such as acetic, citric, butyric, propionic, and pyruvic acids [61].
These acids may also exert significant antioxidant activity, in combination with or exceeding that of phenolic compounds [60]. Further, differences in trends observed in the two antioxidant assays may arise from the DPPH free radical being more stable in the fermented matrix than ABTS+ cation and the resulting delay in attaining a steady state due to the quenching of DPPH radical by phenolic antioxidants, leading to lower baseline values overall [62].
Improvement of the antioxidant activity is recognized as an important strategy to enhance the overall human health relevant functionalities of foods and beverages [46]. This is especially true for dietary strategies aimed at addressing type 2 diabetes and associated complications, because the underlying pathophysiology fundamentally involves chronic oxidative stress due to the breakdown of cellular redox balance [63]. The current study indicated a considerable increase in total antioxidant activity of barley slurries in response to mixed Kefir culture-mediated fermentation. This trend was sustained throughout the duration of fermentation (72 h), as seen in the DPPH free radical scavenging assay. Therefore, the improvement of total antioxidant activity of food barley flour (with and without germination) by Kefir-mediated fermentation is of relevance to designing functional foods with high antioxidant potential, which is a key dietary target along with anti-hyperglycemic function in the interventions for prevention and long-term management of early stages of type 2 diabetes.
3.3. α-Amylase Enzyme Inhibitory Activity
The ability of a food substrate to modulate the activity of α-amylase, in turn moderating the digestion of starch, is a key factor in determining its ability to effect glycemic control [64,65]. In this context, the ability of Kefir culture-mediated fermentation of food barley flours derived from sprouted and unsprouted grains to inhibit α-amylase was determined using an in vitro assay model.
The fermented samples exhibited a clear dose-dependent relationship, however, only values indicating data from the undiluted samples are presented here (Figure 4). All three main effects on α-amylase enzyme inhibitory were found to be statistically significant at p < 0.05, across all fermentation time points (Table 1).
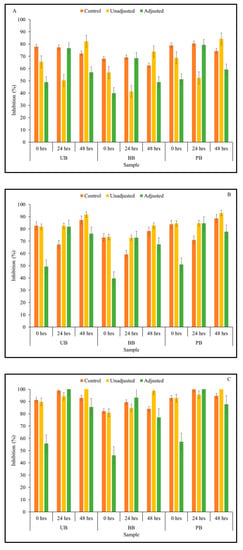
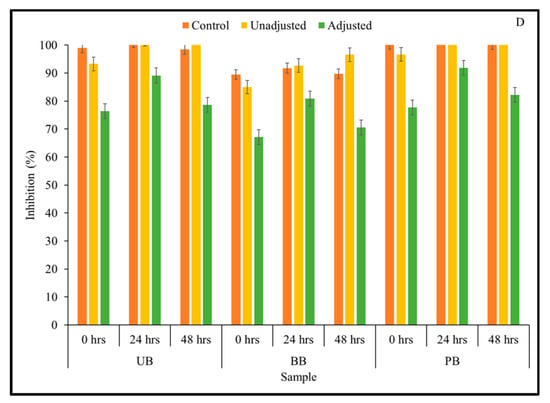
Figure 4.
α-Amylase inhibitory activity of Kefir-fermented slurries derived from sprouted (0, 24, 48 h) unpigmented barley (UB), black barley (BB) and purple barley (PB) after 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation.
The results from Kefir culture-mediated fermentation indicated that the α-amylase inhibitory activity of barley flour slurries improved due to fermentation with mixed dairy-based Kefir LAB culture. The effect of 3-way interactions of pH treatment × barley sample type × sprouting duration on α-amylase enzyme inhibitory activity was statistically significant for undiluted sample at 24- and 72-h fermentation time points (Table 1). At 0 and 48-h fermentation time points, 2-way interactions between pH treatment × sprouting duration had a statistically significant effect on α-amylase enzyme inhibitory activity (Supplementary File). The fermented samples exhibited moderate to high levels of enzyme inhibitory activity. At the beginning of fermentation, the 24-h sprouted flour slurries of all barley sample types had moderate to high levels of α-amylase enzyme inhibitory activity which was pH-independent. However, as fermentation progressed, increase in α-amylase enzyme inhibition in most sample combinations was found to be pH-dependent. This can be observed in Figure 4, where enzyme inhibition of the pH-unadjusted samples was higher than that of the corresponding controls and pH-adjusted samples. Therefore, improvements in α-amylase enzyme inhibitory activity may be attributed to the acidification of the medium due to LAB fermentation to a greater extent, in addition to the mobilization of soluble phenolic compounds in the fermented food matrix.
As fermentation progressed from 0–72 h, α-amylase enzyme inhibitory activity of the fermented barley samples was also found to increase concurrently (Figure 4). Overall, the highest α-amylase enzyme inhibition was observed after 72 h of fermentation. At this fermentation time point, the inhibitory activity of specific sample combinations (UB-24, UB-48, BB-24, BB-48) reached complete saturation in terms of α-amylase enzyme inhibition (i.e., 100% inhibition), while remaining barley samples had 70–90% α-amylase enzyme inhibitory activity.
The linear improvement of α-amylase enzyme inhibitory activity relative to the duration of fermentation is similar to the previous findings in camu-camu substrate fermented with L. plantarum [38]. However, L. acidophilus-fermented pear juice did not show any visible improvements in α-amylase enzyme inhibition in response to fermentation [66]. Therefore, it may be inferred that LAB-fermentation-based improvement of α-amylase enzyme inhibitory activity varies according to the fermentative strains used and their growth dynamics in specific plant-based food substrates.
As in the case of antioxidant activity, α-amylase enzyme inhibitory activity of sprouted food barley flour was found to be improved in response to Kefir-mediated fermentation in this study. Food barley grains sprouted for 24 and 48 h and subsequently fermented for 72 h with Kefir culture can be specifically targeted for this purpose. It is important to note that excessive α-amylase enzyme inhibition may cause detrimental side-effects such as abdominal distension, flatulence, and diarrhea, due to the fermentation of undigested starch in the large intestine [67,68]. However, the dose-dependent response observed in this study suggested the scope for formulating food barley substrates with optimal modulation of starch breakdown. These results further support the relevance of the combined application of these two bioprocessing strategies as an integrated food processing approach to improve α-amylase enzyme inhibitory activity related anti-hyperglycemic functions in cereal grains such as barley.
3.4. α-Glucosidase Enzyme Inhibitory Activity
The ability of a food or beverage to regulate the formation and uptake of glucose in the small intestine by the controlled modulation of α-glucosidase represents another key target for glycemic control through prevention of post-prandial blood glucose spike [69]. Therefore, in this study, the α-glucosidase enzyme inhibitory activity of aqueous extracts from Kefir-fermented food barley flour was measured using an in vitro assay model.
The α-glucosidase enzyme inhibitory activity of fermented food barley flour extracts was found to be low to moderate (Figure 5). As in the case of the α-amylase assay, a clear dose-dependent response was observed in α-glucosidase enzyme inhibition; data from only the undiluted samples are presented in Figure 5.
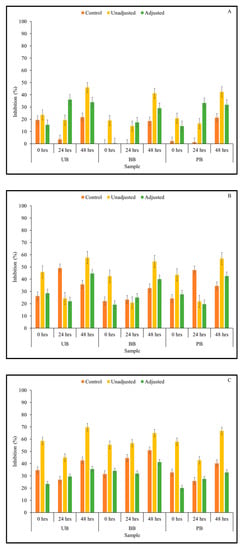
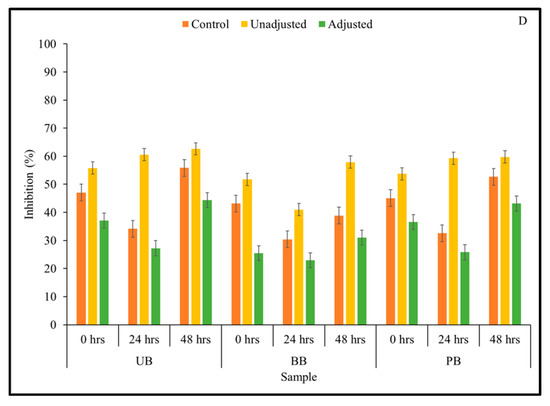
Figure 5.
α-Glucosidase inhibitory activity of Kefir-fermented slurries derived from sprouted (0,24, 48 h) unpigmented barley (UB), black barley (BB) and purple barley (PB) after 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation.
Kefir culture-mediated fermentation was found to improve α-glucosidase inhibitory activity in sprouted and unsprouted barley flour slurries. The 3-way interaction and all 2-way interactions had a statistically significant effect on α-glucosidase enzyme inhibitory activity at 0-h time points (Supplementary File). However, only the 2-way interaction between pH treatment × sprouting duration had a statistically significant effect on α-glucosidase enzyme inhibitory activity at 24-, 48-, and 72-h fermentation time points (Supplementary File). Additionally, all three main effects were found to be statistically significant on α-glucosidase enzyme inhibitory activity at p < 0.05 (Table 1). In most sample combinations, α-glucosidase enzyme inhibition was found to be maintained or improved as fermentation progressed. Particularly, α-glucosidase enzyme inhibitory activity peaked at 48 h of fermentation, and subsequently decreased slightly at 72 h. In terms of the duration of sprouting, 48-h sprouted barley flours of all three food barley types were found to induce the highest level of α-glucosidase enzyme inhibitory activity across all fermentation time points, and this was consistent with other parameters investigated in this study such as TSP content, antioxidant activity, and α-amylase enzyme inhibition.
As seen in the current study, LAB-mediated fermentation was found to improve the α-glucosidase inhibitory activity in other plant-based substrates such as pear juice and camu-camu [38,66]. However, it is important to note that improvements in α-glucosidase inhibition by Kefir-fermented barley slurries was not found to be pH-independent. From Figure 5, it is apparent that the pH-adjusted samples had lower α-glucosidase enzyme inhibitory activity than the corresponding pH-unadjusted samples, which was found consistently across barley sample type and fermentation duration. This trend is comparable to the findings of earlier studies, where the inhibitory activity of L. acidophilus-fermented pear juice was significantly higher at fermented acidic pH than the corresponding pH-adjusted samples at every fermentation time point [38]. Therefore, this anti-hyperglycemic relevant function of fermented plant substrates may be influenced more by the change in acidity of the medium induced by organic acid production than the release of phenolic compounds in fermented matrix.
As with the results of α-amylase enzyme inhibition, the ability of barley flour, especially after sprouting, to inhibit α-glucosidase enzyme in vitro was also positively influenced by Kefir-mediated fermentation. However, in this case, high levels of α-glucosidase inhibition are favored to effectively control post-prandial blood glucose levels [68]. In this context, the study identified Kefir-mediated fermentation of sprouted barley flour for 48 h as an effective integrated bioprocessing strategy for optimal α-glucosidase enzyme inhibitory activity potentially supporting desirable post-prandial blood glucose control. The integrated application of sprouting and Kefir-mediated fermentation to improve these anti-hyperglycemic property-relevant functions in cereal grains such as food barley has not been explored previously. Therefore, the current study delineates an integrated bioprocessing approach to develop functional foods and ingredients from food barley with antioxidant and anti-hyperglycemic properties, which may be further optimized to complement current therapeutic and dietary support strategies to target reduced health risks associated with type 2 diabetes.
3.5. Viable Cell Count of LAB Strains in Fermented Barley Flour and Helicobacter Pylori Inhibition
To achieve higher probiotic function in fermented plant food substrates, it is important to have higher concentration of active and viable cells of beneficial LAB strains. In the current study, the total viable cell counts of the inoculated mixed LAB strains in the pH-unadjusted slurry of barley flour slurries at each fermentation time point were found to increase from the initial level at 0 h. As the duration of the fermentation increased, the cell count was found to either increase in some sample combinations, while in other samples, it remained relatively constant (Figure 6).
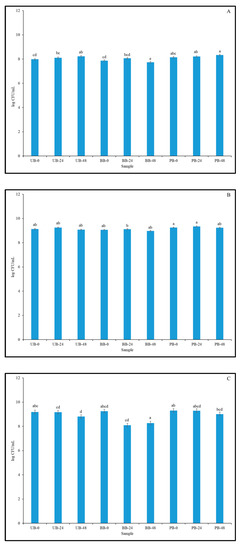
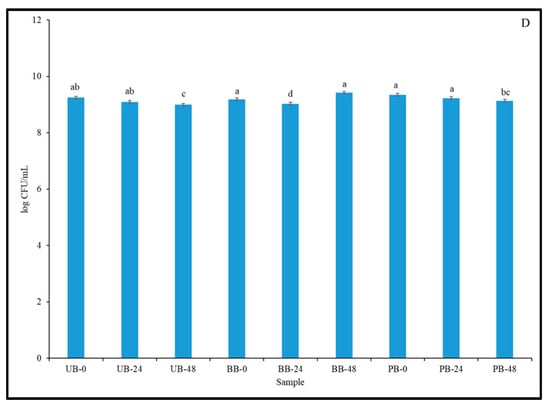
Figure 6.
Viable cell counts of lactic acid bacteria in unpigmented barley (UB), black barley (BB) and purple barley (PB) slurries at 0 h (A), 24 h (B), 48 h (C) and 72 h (D) of fermentation with Kefir culture. Different lower case letters denote statistically significant differences (p < 0.05) in viable cell counts of LAB between different sprouted barley samples separately for each fermentation time point.
Notably, there was no decrease in the cell count below initial levels, even after 72 h of fermentation. Over the course of fermentation, the cell count levels remained above 8 log CFU/mL, which is considered to be the minimum level required to impart probiotic activity and benefits [70]. Barley may serve as a highly suitable substrate for the growth of beneficial microbes, including LAB, as it contains various fermentable substrates required for their growth [71]. Barley also contains various components that are potent prebiotics, such as β-glucans (highest concentration among cereal grains), arabinoxylans, and galactooligosaccharides and fructooligosaccharides, which can help sustain the growth of beneficial LAB [72]. The growth pattern observed in this study, whereby the viable cell count of mixed LAB strains was sustained over the duration of the experiment, may be attributed to the protective and nutritive effects of the aforementioned prebiotic components. However, the increase in cell counts observed here was not as high as that observed in similar studies [73]. This could be attributed to differences in sprouting conditions and grains used, and therefore the extent of grain modification, resulting in variations in the available fermentable substrates in the medium. The growth of individual probiotic LAB strains of L. plantarum, L. reuteri, L. fermentum and L. acidophilus was found to be strongly supported in malt, wheat, and barley flour substrates [8]. Similar observations were made in various other studies, wherein the inclusion of whole and flours of barley and wheat were found to support the growth of various probiotic LAB strains [72,73,74]. The results obtained in this current study are in agreement with previous studies and indicated that both whole and sprouted food barley can be a suitable carrier for beneficial LAB strains, with potential prebiotic properties.
Additionally, certain combinations of sprouted and fermented food barley samples were found to exhibit inhibition against ulcer causing bacteria H. pylori (Table 4). Interestingly, sprouting was observed to positively influence this targeted anti-bacterial parameter, and flours from barley grains sprouted for a longer duration (48 h) tended to have higher anti-bacterial activity. Among the different types of barley used in this study, PB had the highest H. pylori inhibition followed by UB and BB. Further, as fermentation progressed the zone of inhibition produced by these sprouted barley flour slurries also increased (Table 4).

Table 4.
Diameter (mm) of the circular zone of inhibition of H. pylori by unpigmented (UB), black barley (BB) and purple barley (PB) fermented with Kefir culture.
A possible reason for the relative increase in H. pylori inhibition observed over the period of fermentation could be due to the production of lactic acid by the mixed LAB culture. As a result of LAB fermentation, the barley slurries underwent acidification over the duration of the experiment. Sprouting impacted the level of acidification, as the maximum pH level observed in 48 h (5.1, 4.8, 4.7; UB, BB, PB) was lower than that of 24-h sprouted (5.5; 5.5; 5.2) and unsprouted flour samples (5.9; 5.8; 5.6). The germination process results in the hydrolysis of complex carbohydrates into fermentable sugars, which could support the growth of mixed LAB strains, thereby resulting in higher levels of lactic acid accumulation. Previous studies indicated that lactic acid exerts antimicrobial activity and may further enhance the ability of phenolic compounds present in fermented barley samples to inhibit pathogenic bacteria such as H. pylori [46,75,76,77,78]. Lactic acid can disrupt the integrity of the bacterial outer membrane by releasing lipopolysaccharides or other components, and it can enable phenolic antioxidants to scavenge electrons from the electron transport chain along the bacterial membrane [76,77,78]. Thus, sprouted food barley substrates may offer an additional layer of gastrointestinal benefit by inhibiting H. pylori, apart from their potential prebiotic action. The results obtained in this study suggest that further mechanistic and optimization studies targeting barley-based substrates for human gut health improvements, essential for protection against type 2 diabetes and other chronic disease, hold merit.
4. Conclusions
Integrated bioprocessing strategy such as controlled germination (sprouting) and beneficial LAB-mediated fermentation represent viable and cost-effective means of developing grain-based functional food ingredients, with enhanced bioactive profiles and targeted health benefits. In this study, Kefir-mediated fermentation of sprouted barley flour was found to improve the phenolic content, profile of select phenolic compounds, and associated bioactivity such as antioxidant, anti-hyperglycemic and anti-bacterial properties. Sprouted barley flour, specifically the 48-h sprouted sample, was found to support beneficial LAB growth during the fermentation process at levels sufficient to impart probiotic benefits, without supplementation of additional nutrients, indicative of a possible prebiotic effect supporting probiotic bacterial growth. Therefore, this study achieved its objectives of identifying sprouted food barley with 48-h sprouting as a promising ingredient source for functional food development, which can be further enhanced by integrating sprouting with fermentation by mixed a LAB culture such as Kefir culture. Future studies focusing on fermenting food barley substrates with various single and mixed cultures, the long-term survivability of these cultures, and sensory analysis may further help in optimizing sprouted food barley-based functional foods and beverages supporting wider type 2 diabetes health benefits validated by in vivo models.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/applmicrobiol1020026/s1, Supplementary File.
Author Contributions
Conceptualization, R.R., D.S. and K.S.; methodology, R.R. and D.S.; validation, and formal analysis, R.R., D.S. and M.D.; investigation, R.R. and D.S.; resources, K.S.; data curation, R.R. and D.S.; writing—original draft preparation, R.R. and D.S.; writing—review and editing, K.S., M.D., D.S. and R.R.; supervision, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef]
- Havenaar, R.; Brink, B.T.; Veld, J.H.J.H.I. Selection of strains for probiotic use. In Probiotics—The Scientific Basis; Fuller, R., Ed.; Springer: Amsterdam, The Netherlands, 1992; pp. 209–224. [Google Scholar] [CrossRef]
- Alander, M.; De Smet, I.; Nollet, L.; Verstraete, W.; von Wright, A.; Mattila-Sandholm, T. The effect of probiotic strains on the microbiota of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). Int. J. Food Microbiol. 1999, 46, 71–79. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy Based Probiotics: A Healthy Treat for Intestine. Crit. Rev. Food Sci. Nutr. 2014, 56, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Sharma, R.; Mokhtari, S.; Jafari, S.M.; Sharma, S. Barley-based probiotic food mixture: Health effects and future prospects. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Holtekjølen, A.K.; Kinitz, C.; Knutsen, S.H. Flavanol and Bound Phenolic Acid Contents in Different Barley Varieties. J. Agric. Food Chem. 2006, 54, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L. Phenolic Compounds in Cereal Grains and Their Health Benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, R.H. Health Benefits of Whole Grain Phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2014, 56, 25–35. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2016, 25, 148–161. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Shetty, K. Functional Bioactives from Barley for Human Health Benefits. In Functional Foods and Biotechnology; Shetty, K., Sarkar, D., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–85. [Google Scholar] [CrossRef]
- Ha, K.-S.; Jo, S.-H.; Mannam, V.; Kwon, Y.-I.; Apostolidis, E. Stimulation of Phenolics, Antioxidant and α-Glucosidase Inhibitory Activities During Barley (Hordeum vulgare L.) Seed Germination. Plant. Foods Hum. Nutr. 2016, 71, 211–217. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Schwarz, P.; Shetty, K. Phenolic linked anti-hyperglycemic bioactives of barley (Hordeum vulgare L.) cultivars as nutraceuticals targeting type 2 diabetes. Ind. Crop Prod. 2017, 107, 509–517. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Manduri, A.; Iyer, S.G.; Shetty, K. Improving phenolic bioactive-linked anti-hyperglycemic functions of dark germinated barley sprouts (Hordeum vulgare L.) using seed elicitation strategy. J. Food Sci. Technol. 2017, 54, 3666–3678. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, R.B.; Akbar, A.; Ali, M.F.; Yang, Q.; Feng, B. Phytochemical Components of Some Minor Cereals Associated with Diabetes Prevention and Management. J. Biosci. Med. 2018, 06, 9–22. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Donkor, O.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains—Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S. Bioactive components and functional properties of biologically activated cereal grains: A bibliographic review. Crit. Rev. Food Sci. Nutr. 2015, 57, 3051–3071. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Mitsuyama, K.; Andoh, A.; Iwanaga, T. Modulation of intestinal environment by prebiotic germinated barley foodstuff prevents chemo-induced colonic carcinogenesis in rats. Oncol. Rep. 1994, 20, 793–801. [Google Scholar] [CrossRef][Green Version]
- Bamba, T.; Kanauchi, O.; Andoh, A.; Fujiyama, Y. A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. J. Gastroenterol. Hepatol. 2002, 17, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Whole Grains and Digestive Health. Cereal Chem. J. 2010, 87, 292–296. [Google Scholar] [CrossRef]
- Ullrich, S.E. Barley: Production, Improvement, and Uses; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Newman, R.K.; Newman, C.W. Barley for Food and Health: Science, Technology, and Products; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Calixto, F.D.S. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [PubMed]
- E El Hag, M.; El Tinay, A.H.; E Yousif, N. Effect of fermentation and dehulling on starch, total polyphenols, phytic acid content and in vitro protein digestibility of pearl millet. Food Chem. 2002, 77, 193–196. [Google Scholar] [CrossRef]
- Anson, N.M.; Havenaar, R.; Vaes, W.; Coulier, L.; Venema, K.; Selinheimo, E.; Bast, A.; Haenen, G.R. Effect of bioprocessing of wheat bran in wholemeal wheat breads on the colonic SCFA production in vitro and postprandial plasma concentrations in men. Food Chem. 2011, 128, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, O.; Öste, R.; Holst, O. The effect of yoghurt culture on the survival of probiotic bacteria in oat-based, non-dairy products. Food Res. Int. 2002, 35, 775–784. [Google Scholar] [CrossRef]
- McCue, P.; Kwon, Y.-I.; Shetty, K. Anti-amylase, anti-glucosidase and anti-angiotensin i-converting enzyme potential of selected foods. J. Food Biochem. 2005, 29, 278–294. [Google Scholar] [CrossRef]
- Kwon MS, Y.I.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar]
- Fujita, A.; Sarkar, D.; Genovese, M.I.; Shetty, K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process. Biochem. 2017, 59, 133–140. [Google Scholar] [CrossRef]
- Shetty, K.; Curtis, O.F.; Levin, R.E.; Witkowsky, R.; Ang, W. Prevention of vitrification associated with in vitro shoot culture of Oregano. (Origanum vulgare) by Pseudomonas spp. J. Plant Physiol. 1995, 147, 447–451. [Google Scholar] [CrossRef]
- Cervato, G.; Carabelli, M.; Gervasio, S.; Cittera, A.; Cazzola, R.; Cestaro, B. Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J. Food Biochem. 2000, 24, 453–465. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Kim, Y.-C.; Shetty, K. Health Benefits of Traditional Corn, Beans, and Pumpkin: In Vitro Studies for Hyperglycemia and Hypertension Management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. Phenolic antioxidant mobilization during yogurt production from soymilk using Kefir cultures. Process. Biochem. 2005, 40, 1791–1797. [Google Scholar] [CrossRef]
- Stevenson, T.H.; Lucia, L.M.; Acuff, G.R. Development of a Selective Medium for Isolation of Helicobacter pylori from Cattle and Beef Samples. Appl. Environ. Microbiol. 2000, 66, 723–727. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Huamán-Alvino, C.; Flores-Báez, O.; Aquino-Méndez, E.M.; Chirinos, R.; Campos, D.; Sevilla, R.; Fuentealba, C.; Pedreschi, R.; Sarkar, D.; et al. Evaluation of phenolic antioxidant-linked in vitro bioactivity of Peruvian corn (Zea mays L.) diversity targeting for potential management of hyperglycemia and obesity. J. Food Sci. Technol. 2019, 56, 2909–2924. [Google Scholar] [CrossRef]
- Sarkar, D.; Ankolekar, C.; Shetty, K. Beneficial Lactic Acid Bacteria (LAB)-Based Biotransformation of Plant and Dairy Substrates to Enhance Type 2 Diabetes-Relevant Health Benefits. In Functional Foods and Biotechnology: Biotransformation and Analysis of Functional Foods and Ingredients; Shetty, K., Sarkar, D., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 345–360. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.; Schieber, A.; Gänzle, M. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Curiel, J.A.; Rodríguez, H.; Landete, J.M.; Rivas, B.D.L.; Muñoz, R. Ability of Lactobacillus brevis strains to degrade food phenolic acids. Food Chem. 2010, 120, 225–229. [Google Scholar] [CrossRef]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Gänzle, M. Phenolic Acids and Flavonoids in Nonfermented and Fermented Red Sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef] [PubMed]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved Bioavailability of Dietary Phenolic Acids in Whole Grain Barley and Oat Groat following Fermentation with Probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Hyun, J.-N.; Kim, J.-A.; Park, J.-C.; Kim, M.-Y.; Kim, J.-G.; Lee, S.-J.; Chun, S.-C.; Chung, I.-M. Relationship between Phenolic Compounds, Anthocyanins Content and Antioxidant Activity in Colored Barley Germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2020, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trend Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, A.T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Sarkar, D.; Shetty, K. Metabolic Stimulation of Plant Phenolics for Food Preservation and Health. Annu. Rev. Food Sci. Technol. 2014, 5, 395–413. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Food 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijevic-Brankovic, S. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Güzel-Seydim, Z.B.; Seydim, A.C.; Greene, A.; Bodine, A. Determination of Organic Acids and Volatile Flavor Substances in Kefir during Fermentation. J. Food Compos. Anal. 2000, 13, 35–43. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Ind. J. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Morón, E.B.; Abad-Jiménez, Z.; De Marañón, A.M.; Iannantuoni, F.; López, E.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- McCue, P.; Shetty, K. Health Benefits of Soy Isoflavonoids and Strategies for Enhancement: A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 361–367. [Google Scholar] [CrossRef]
- Ansari, P.; Afroz, N.; Jalil, S.; Azad, S.B.; Mustakim, M.G.; Anwar, S.; Haque, S.N.; Hossain, S.M.; Tony, R.R.; Hannan, J.M.A. Anti-hyperglycemic activity of Aegle marmelos (L.) Corr. is partly mediated by increased insulin secretion, α-amylase inhibition, and retardation of glucose absorption. J. Pediatr. Endocrinol. Metab. 2017, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ankolekar, C.; Pinto, M.; Greene, D.; Shetty, K. In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of Lactobacillus acidophilus fermented pear juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 221–230. [Google Scholar] [CrossRef]
- Ag, H.B.B. Pharmacology of α-glucosidase inhibition. Eur. J. Clin. Investig. 2010, 24, 3–10. [Google Scholar] [CrossRef]
- Rosenstock, J.; Brown, A.; Fischer, J.; Jain, A.; Littlejohn, T.; Nadeau, D.; Sussman, A.; Taylor, T.; Krol, A.; Magner, J. Efficacy and Safety of Acarbose in Metformin-Treated Patients with Type 2 Diabetes. Diabetes Care 1998, 21, 2050–2055. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; A Koutinas, A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Badejo, A.A. Probiotic potentials of cereal-based beverages. Crit. Rev. Food Sci. Nutr. 2015, 57, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Mridula, D.; Sharma, M. Development of non-dairy probiotic drink utilizing sprouted cereals, legume and soymilk. LWT 2015, 62, 482–487. [Google Scholar] [CrossRef]
- Sharma, M.; Mridula, D.; Gupta, R.K. Development of sprouted wheat based probiotic beverage. J. Food Sci. Technol. 2013, 51, 3926–3933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Midolo, P.D.; Lambert, J.R.; Hull, R.; Luo, F.; Grayson, M.L. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 1995, 79, 475–479. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.-S.; Vattem, D.A.; Lin, Y.-T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process. Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Ankolekar, C.; Johnson, D.; Pinto, M.D.S.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory Potential of Tea Polyphenolics and Influence of Extraction Time Against Helicobacter pylori and Lack of Inhibition of Beneficial Lactic Acid Bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).