Abstract
Due to the importance of microbes in soil health and crop production, manipulation of microbiomes provides a new strategy for improving crop growth and agricultural ecosystems. Current understanding is limited regarding the responses of soil and crop endophytic microbiomes to field management and microbiome programming. In this study, we investigated soil and tea root bacterial communities under conventional and organic cropping systems using 16S rRNA gene sequencing. A significant difference in soil and root bacterial community structure was observed under different field managements, leading to 43% and 35% variance, respectively. We also identified field management-sensitive species both in soils and tea roots that have great potential as bioindicators for bacterial microbiome manipulation. Moreover, through functional profile predictions of microbiomes, xenobiotics degradation in soil bacterial communities is enriched in organic farms, suggesting that biodegradation capabilities are enhanced under organic cropping systems. Our results demonstrate the effects of field management on both soil and tea root bacterial microbiomes and provide new insights into the reprogramming of microbial structures.
1. Introduction
Conventional cropping systems, which rely heavily on chemical fertilizers and control agents, have been utilized for decades, leading to a doubling of crop production and increasing food demand among the global population [1]. However, this agricultural practice has many negative effects on ecological diversity, soil structure and human health [1,2,3]. In contrast, organic cropping systems, which have been practiced for thousands of years, conserve natural sources and maintain environmental health by using organic materials and low intensive tillage, making agriculture sustainable [3]. Field management makes a significant difference and has a great impact on biological diversity, including microorganism community structures and compositions.
Microorganisms in soils and plants affect soil physical and chemical characteristics, affect soil nutrient availability and distribution and are crucial for soil and plant health, aiding in resilience to environmental stresses. Previous reports have shown the effects of agricultural practices on soil and rhizosphere microbiomes. For example, soil bacterial richness and diversity was significantly increased in organic farms, compared to conventional farms [4]. Zhang et al. [5] found that the abundance of bacteria which obtain acid phosphatase activity was altered in rice when the irrigation regimes were changed. In semiarid vineyards, cover crop management not only increased soil organic carbon but also enhanced bacterial diversity compared to conventional tillage. In contrast, fungal diversity was higher under conventional tillage than under cover crop management [6]. In addition, evidence also suggested that soil fungal communities are more sensitive to crop rotation and cropping systems than bacterial communities are [7,8]. There are also reports that show the effects of field management on root endophytic bacterial microbiomes [9,10]. However, there are only a few reports that examine the impacts of field management on both soils and root microbiomes. Hartman et al. [11] reported that field management is a crucial factor in determining soil fungal structure, but in root endosphere it is the main driving force for bacteria rather than fungi. The responses of bacterial and fungal communities to agricultural practices vary based on environmental conditions and crop species, suggesting that more investigation is required to elucidate the effects of agriculture practices on soil, rhizosphere and endosphere microbiomes.
Tea (Camellia sinensis L.) is one of the most important economic crops in the world. It is a perennial crop that is grown for the harvesting of young leaves. Thus, nutrient replenishment, especially nitrogen, is crucial for tea production. However, the long-term application of chemicals is harmful to soil health and the ecosystem at large. Several studies showed that applying organic fertilizers to tea farms increases soil pH, organic carbon and total N content [12,13]. The width and length of tea leaves are also greater under organic farming systems, although yield might be reduced compared to conventional farming systems [12,14]. Moreover, leaves harvested on organic farms also enhance tea’s antioxidant properties [14,15,16]. In addition, Qiu et al. [17] examined soil microbial diversity through temperature gradient gel electrophoresis and found a lower Shannon index when applying chemical fertilizers instead of organic manure, suggesting that field management affects microbial biodiversity in tea farms. However, little is known about the microbial community structures and species that are sensitive to field management in the tea farms.
In this study, we investigated soil and root bacterial microbiomes and evaluated the effects of cropping systems on microbiomes with the use of 16S rRNA gene sequencing. We aimed to identify the potential bioindicators of cropping systems, both in soil and tea roots and to compare the functions of bacterial communities under different field management strategies. These strategies, we suggest, might provide new insight into bacterial microbiome management.
2. Materials and Methods
2.1. Experimental Sites
The organic and conventional tea farms investigated in this study are all located in the Wenshan Branch of Tea Research and Extension Station (24.95° N, 121.63° E) at an altitude of 350–460 m in Shiding District, New Taipei City, Taiwan. The three organic farms and three conventional farms were included for investigation and are separated by the road (Figure S1). The soil texture is yellow loam and the soil pH is 4.9–5.3. In 2018, the monthly temperature ranged from 13.5 to 26.4 °C and the annual precipitation was 2742 mm (Figure 1). In four out of the six total farms, we grew cultivar TTES No. 12. In the other two farms, we have been growing cultivar Wuyi since 1987. Since 2006, three of the farms studied have been using an organic cropping system. All of the farms are fertilized three times per year. On the conventional farms, fertilizers contain 20% N, 5% P2O5 and 10% K2O while, on the organic farms, the fertilizers contain 88% organic matter derived from plant residues. Tea trees are pruned twice a year: at the beginning and the middle of the year.
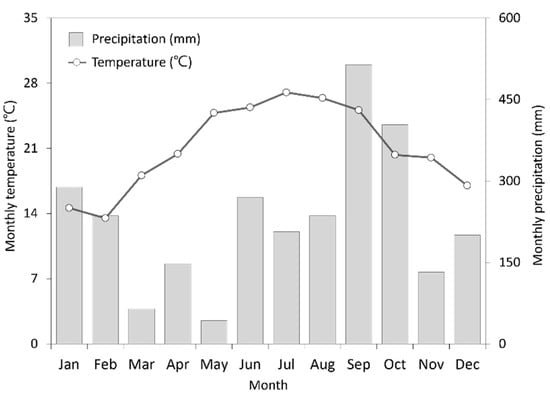
Figure 1.
Monthly temperature and precipitation in Shiding District, in 2018.
2.2. Sample Collection and Microbial DNA Isolation
Young tea roots and bulk soils were sampled in December 2018 for bacterial microbiome analysis. Three spots were chosen per farm and the location of sampling spots is shown in Figure S1. However, one of root samples in organic farm 2 was too little for further analysis. In total, we harvested 18 soil samples and 17 root samples. The root samples were surface sterilized by 1% sodium hydrochloride for 1 min, followed by washing with sterile distilled water five times. The root and bulk soil samples were stored at −80 °C prior to DNA isolation.
Microbial DNA was isolated from samples weighing around 200 mg using the DNeasy® Powersoil® Kit (Qiagen, Hilden, Germany), based on the manufacturer’s instructions. The DNA samples were stored at −80 °C prior to further investigation.
2.3. 16S rRNA Gene Library Preparation and Sequencing
The V3–V4 highly variable region of 16S rRNA genes was amplified by primer set 319F (5′-CCTACGGGNGGCWGCAG-3′) and 819R (5′-GACTACHVGGGTATCTAATCC-3′). The amplicons were attached by Illumina sequencing adaptors and dual indices using Nextera XT Index Kit (Illumina, San Diego, CA, USA), and the libraries were further cleaned up using AMPure XP beads (Beckman Coulter, Brea, CA, USA). The libraries were sequenced on an Illumina Miseq platform (Illumina) to generate pair-ended reads. Low quality reads were filtered out in the QIIME pipeline before analysis [18].
2.4. Data Analysis
Pair-ended reads were assembled and sequenced with 97% identity. They were then clustered into operational taxonomic units (OTUs) using the EasyMap platform with default settings [19]. OTUs were assigned to the SILVA database [20]. In root samples, the OTUs classified as mitochondria and chloroplast were filtered out. OTU abundance was normalized by rarefying the minimum sequence depth before further analysis.
Alpha diversity (α-diversity) indices including the phylogenetic diversity (PD_whole_tree), observed species, Shannon and evenness were analyzed using EasyMap. Beta diversity (β-diversity) analysis was performed with Bray–Curtis distance matrices using vegan and microbiome packages in R software [21,22]. Analysis of similarity (ANOSIM) and the variation between datasets were evaluated using the anosim and adonis functions in R package “vegan”, respectively. Principle co-ordinates analysis (PCoA) was conducted using “ggplot2” package [23].
To analyze the functional composition of the metagenome dataset, a phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) [24] was applied based on Greengenes phylogeny [25] and the function was classified using Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology [26]. The abundance of predicted gene function was shown by heatmaps generated using the “Complexheatmap” package.
To display the effects of field management on OTU abundance, linear discrimination analysis effect size (LEfSe) was performed. The threshold score was set to 4.0. To identify the OTUs sensitive to different sample types or field management strategies, the “multipatt” function in the “indicspecies” package [27] was used to demonstrate the correlation between OTU abundance and variables. The correlation was displayed by a bipartite network using the “igraph” package [28].
2.5. Statistical Analysis
The analysis was performed using R stats. Statistical significance of α-diversity indices, species abundance and the abundance of predicted function among datasets was tested by a Kruskal–Wallis test along with a Mann–Whitney U post hoc test (p < 0.05). The difference in relative abundance among species, as well as the abundance variance of predicted functions between datasets, were assessed by Welch’s t test using the “STAMP” package [29].
3. Results
3.1. Summary of 16S rRNA Amplicon Sequencing
We analyzed the bacterial profiles of young roots of tea trees and bulk soils surrounding trees under conventional and organic cropping systems. A total of 785,719 and 1,141,190 high quality reads were yielded in the soil and root samples, respectively. Among 35 samples, a total of 1680 OTUs was obtained at 97% identity. Of these, 1347 OTUs were identified in soil samples and 442 OTUs were identified in root samples. A total of 370 OTUs was shared among the soil samples, while 380 and 516 OTUs were unique in the soil samples collected from conventional and organic farms, respectively. Among root samples, 80 OTUs were shared, while 194 and 63 OTUs were unique in the root samples harvested from conventional and organic farms, respectively (Figure 2).
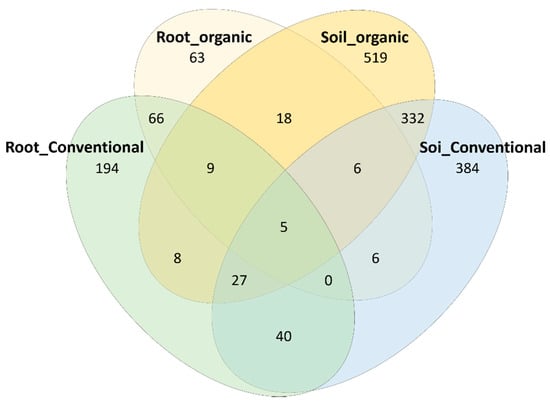
Figure 2.
Venn diagram of soil and root bacterial OTUs under conventional and organic cropping systems.
3.2. The Effects of Cropping System on Soil and Root Bacterial Diversity
To elucidate the impacts of field management on the α-diversity of soil and tea root bacteria, four indices including the Shannon index, observed OTU, evenness and PD_whole_tree were calculated to evaluate the richness, abundance and biodiversity of the samples. The results showed that all the indices of the soil bacteria samples were significantly higher than the root endophytic bacteria (Figure 3). When comparing bacterial diversity under different field management strategies, we only observed a higher evenness in soil bacteria from organic farms (Figure 3A). This difference in evenness indicates that the environmental distinction between soils and roots is a crucial factor in determining the α-diversity of bacteria, while field management only has marginal effects.
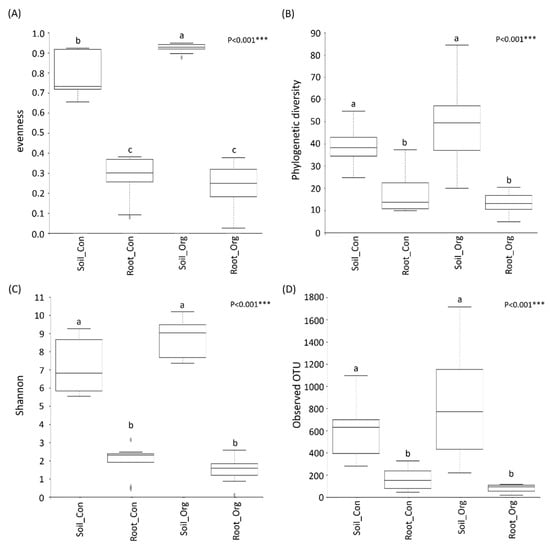
Figure 3.
Effects of cropping systems on alpha diversity of soil and root bacteria. The boxplots displayed the evenness (A), phylogenetic diversity (B), Shannon index (C) and Observed OTU (D) of four different datasets, respectively. The lines within the boxes mark the medians and the bottom and upper boundaries mark the 25th and 75th percentiles. The significance of cropping systems is based on the Kruskal–Wallis pairwise test. ***, p < 0.001. Different letters indicate the significant difference between datasets. Soil_Con and Root_Con are soil and root bacteria from conventional farms, respectively. Soil_Org and Root_Org are soil and root bacteria from organic farms, respectively.
3.3. The Difference between Soil and Tea Root Bacterial Community Structure
We analyzed bacterial community composition in soils and tea roots under conventional and organic cropping systems to examine the effects of field management on their respective bacterial community profiles. Among all the samples, Proteobacteria, Acidobacteriota, Chloroflexi and Actinobacteriota were the most abundant phyla, but their relative abundance was different between soil and root bacterial communities. In soils, the relative abundance of Proteobacteria and Acidobacteriota ranged from 63% to 81% while, in root endophytic profiles, Proteobacteria and Actinobacteriota accounted for 75–89% abundance (Figure 4A). Comparing the relative abundance of phyla in different samples, Proteobacteria, Actinobacteriota and Bacteroidota were significantly higher in roots than in soils while Acidobacteriota, Gemmatimonadota and Planctomycetota were more abundant in soils than in roots (Figure 4B). In addition, we further analyzed the effects of field management on soil and root bacterial community profiles, respectively. The relative abundance of Verrucomicrobiota, Planctomycetota, Gemmatimonadota and Latescibacterota was significantly higher in soils from the organic farms than in those from the conventional farms (Figure 4C). In root endophytic bacterial community profiles, the abundance of Verrucomicrobiota, Acidobacteriota and Bdellovibrionota were significantly higher under conventional cropping systems, while Bacteroidota was more abundant under organic cropping systems (Figure 4D). Intriguingly, the impacts of field management on the relative abundance of Verrucomicrobiota in soils and roots were inverted, suggesting that sensitivity to field management might be affected by the growing environment of certain bacteria.
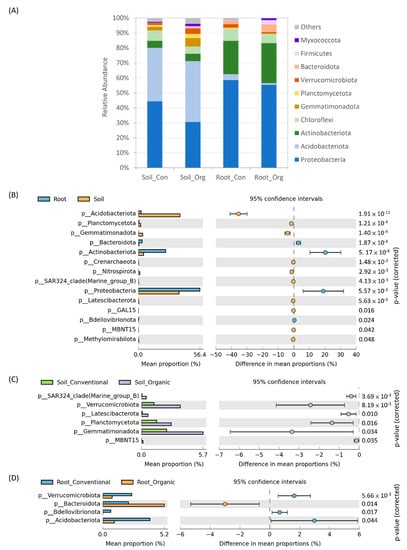
Figure 4.
The effects of cropping systems on bacterial community composition at the phylum level. (A) The top 10 bacterial phyla in soils and tea roots under conventional (Con) and organic (Org) cropping systems. (B) A comparison of relative abundance of bacterial phyla between soil and root samples. (C,D) A comparison of relative abundance among bacterial phyla in soils and roots under different cropping systems, respectively. The left and right boundaries of each circle show the lower and upper limits of the 95% confidential interval.
We further evaluated the heterogeneity of bacterial profiles based on Bray–Curtis distance matrices. First, ANOSIM was performed to evaluate the variation within datasets. The result indicated that the variation between different sample types or management was dominant, compared to the variation within datasets (Table S1). The results of PERMANOVA further showed that sample types, field management and the interaction between two variants had significant impacts on bacterial composition (Table 1). The variance of bacterial structure was further demonstrated by PCoA based on Bray–Curtis distance. All the samples were clustered by sample type, and variances of 15.23% and 13.14% were explained by PC1 and PC2, respectively (Figure 5A). Moreover, when we investigated the impacts of field management on bacterial community structures in both roots and soils, the bacterial commnuity profiles derived from different field management strategies could be clearly separated along PC1. In soils, both PC1 and 2 explained a 42.78% variance while, in roots, both PC1 and 2 explained a variance of 35.82% (Figure 5B,C).

Table 1.
The effects of field management, sample type and the interaction between two variants on soil and tea root bacterial community compositions; testing was conducted by PERMANOVA.
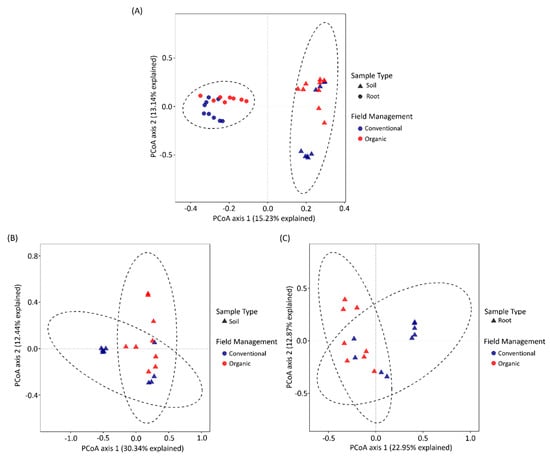
Figure 5.
PCoA of bacterial community composition based on Bray–Curtis distance. (A) PCoA of soil and root bacterial community composition. (B,C) PCoA of soil and root bacterial community composition under different cropping systems, respectively.
We tried to identify the taxonomic groups that were affected by field management. At the family level, JG30-KF-AS9, Acetobacteraceae, Azospirillaceae, Subgroup2 and Subgrop13 were enriched in the soils of organic farms, while Solibacteraceae, Gemmatimonadaceae, Nitrosomonadaceae, Pedosphaeraceae and other five families were relatively abundant in the soils of conventional farms (Figure S2A). In roots, only Saprospiraceae was enriched under organic cropping systems, while Mycobacteriaceae, Acidothermaceae, Rhodonobacteraceae, Acetobacteraceae, Acidobacteriaceae (Subgroup1), Alcaligenaceae, Simkaniaceae, Sphingobacteriaceae and other two families were enriched in the roots under conventional cropping systems (Figure S2B). At the genus level, 10 and four genera were enriched in the soils of organic and conventional farms, respectively (Figure S3A). In roots, Phaeodactylibacter was the only genus enriched under organic cropping systems, and it was present in roots from organic farms, occupying 2% abundance. Under conventional cropping systems, sevens genera including Mycobacterium, Acidothermus, Rhodanobacter, Granulicella, Mucilaginibacter, Chujaibacter and 0319-6G20 were relatively abundant in roots. Among these genera, Acidothermus and Rhodanobacter showed around 11% of abundance in roots from conventional farms, making them 10- and 2.5-fold more abundant there than in roots from organic farms, respectively (Figure S3B).
3.4. Identification of OTUs That Are Sensitive to Field Management and Growth Environments
The distinction of bacterial community composition among samples was demonstrated by PERMANOVA and PCoA. Next, we performed the LEfSe procedure to display how taxonomic abundance was affected by field management strategy. At all the levels, samples derived from organic farms were relatively abundant compared to those from conventional farms. One phylum (Latescibacterota), four classes, six orders and six families (LDA score > 4) were enriched in the samples from organic farms. In contrast, only one class, one order and one family were enriched in the samples from conventional farms (Figure S4).
We further identified the OTUs that were sensitive to field management strategies by performing an indicator species analysis. Among OTUs identified in the pool of soil bacteria, 11 and 26 OTUs were potential indicator species of conventional and organic farms, respectively, and five OTUs were co-occurred in both types of cropping systems, meaning that they might have been indicators of soil bacteria. Among root endophytic OTUs, 21 and 16 OTUs were identified as indicator species of root endophytes under conventional and organic cropping systems, respectively, and only two OTUs, assigned to Phenylobacterium and Thermosporothrix, were co-occurred under both cropping systems; these are the bioindicators of root bacteria. Interestingly, four OTUs were co-occurred in the roots and soils derived from conventional farms, while only one OUT, assigned to Sphingomonas, was co-occurred in the soil and root samples from organic farms, suggesting that these OTUs might be the indicator species of cropping systems in either soils or roots (Figure 6; Table S2). In summary, we identified the indicator species of different sample types under different cropping systems. Further validation will be required to establish a strong association between bacterial groups and field management.
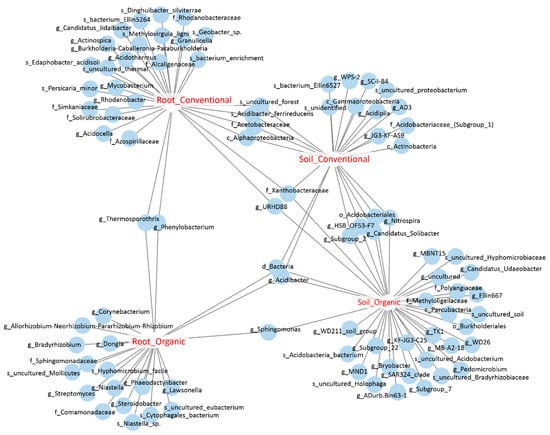
Figure 6.
The bipartite network shows soil and root OTUs that were sensitive to cropping systems. Each circle represents an individual OTU that was significantly associated with the corresponding sample groups (p < 0.05).
3.5. Functional Prediction of Bacterial Communities
To understand the functions of bacterial communities in different environments, we performed functional prediction using the PISCRUSt algorithm. Only the KEGG functional groups of bacterial communities, whose relative abundance was higher than 0.1%, were considered for further analysis (Table S3). We identified 28 third-level KEGG categories enriched in soils from either cropping system, including 20 functional groups assigned to “metabolism”, 2 assigned to “cellular processes”, 1 assigned to “environmental information processing”, 2 assigned to “genetic information processing” and 3 assigned to “unclassified” functions. Among these 28 functional groups, “two component system” was the most abundant that was enriched in samples from organic farms (Figure 7A). On the other hand, 38 third-level KEGG categories in root bacterial communities that were sensitive to field management were identified, including 19 assigned to “metabolism”, 14 assigned to “genetic information processing”, 2 assigned to “environmental information processing”, and 3 assigned to “unclassified” functions. The most abundant functional groups, “transporters” and “ABC transporters”, were enriched in the root bacterial communities from organic farms (Figure 7B). Among all the functional groups assigned to “metabolism” and affected by field management, there were more functional groups associated with “amino acid biosynthesis and metabolism” functions identified in roots, while, in soils, they were associated with the degradation of other secondary metabolites, especially xenobiotics, such as benzoate, aminobenzoate and naphthalene. Interestingly, only one group assigned to “lipid metabolism” was affected by field management in both root and soil bacterial communities, and it was more abundant under organic cropping systems than under conventional cropping systems; this was true for both root and soil bacterial communities.
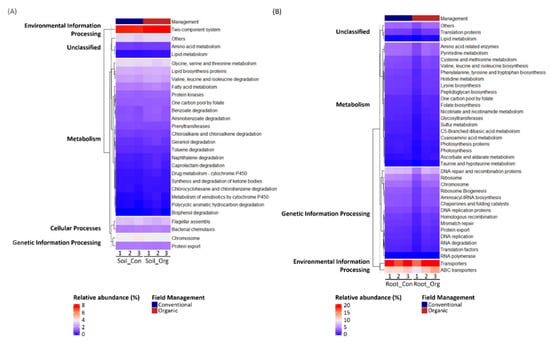
Figure 7.
Third-level KEGG functional groups of (A) soil and (B) root bacterial communities that were varied by field management. Only those with relative abundance greater than 0.1% are shown.
4. Discussion
There is growing evidence to support the impacts of agriculture practices on soils, rhizosphere and root endosphere biota, including microbiota, and to suggest that the effects may be varied by crop species [4,7,9,11,30,31]. Being different from most reports that study microbial community structure and diversity in soil or root-associated compartments, our investigation examined the bacterial microbiome in both soils and tea root endosphere from three organic and conventional farms, respectively. A total of 18 soil samples and 17 root samples were harvested for analysis. Through this study, we demonstrated the impacts of field management on bacteria communities in both soils and roots.
Previous studies showed that soil organic carbon (C) and total N content was higher in organic tea farms than in conventional farms, which might lead to an increase in soil microbial biomass C, N, phosphorus content and the Shannon index [13,17]. Our work was different from previous studies that predominantly used chemicals or temperature gradient gel electrophoresis to analyze the properties of soil microbes. As such, we performed 16S rRNA amplicon-based sequencing to provide a global view of both soil and root endophytic bacterial microbiomes. First, we found that all of the α-diversity indices of soil bacterial microbiomes were higher than root endophytic bacteria. When comparing the effects of field management on α-diversity, only the evenness of soil bacteria in organic tea farms was significantly higher than in conventional farms, while no difference was observed for root endophytic bacteria. This is consistent with previous studies that suggest that species richness in soil bacterial communities is higher than in root communities; clearly, organic cropping systems enhance the α-diversity of soil bacterial communities while field management has no or marginal effects on root bacterial communities [4,9,10,11].
Root endophytes are transmitted from seeds or recruited from soils [32,33]. The strength of physical barrier in roots is one of the selection forces for soil bacteria to pass. Only a limited number of bacteria species can successfully colonize root cells [34]. Thus, it is not surprising to see a lower α-diversity in root bacterial populations than in soil, as well as a difference in soil and root bacterial community structure. When examining the variation of bacterial community composition using PCoA and PERMANOVA, field management, sample type and the interaction between the two variants significantly affected bacterial community structures. The difference between soil bacterial communities under different cropping systems might affect rhizosphere bacterial composition, which is the pool of root endophytes. At the phylum level, Proteobacteria, Actinobacteriota and Chloroflexi were the three major phyla in roots, and they occupied more than 80% abundance, while, in soils, Proteobacteria, Acidobacteriota and Chloroflexi were the three dominant phyla. Although Proteobacteria was abundant both in soil and in root communities, its relative abundance in soils was significantly lower than in roots. In addition, Actinobacteriota and Bacteroidota were also enriched in roots rather than in soils, while Acidobacteriota, Gemmatinonadota and Nitrospirota were more abundant in soils than in roots; this finding is partly consistent with a study on winter wheat [11]. Next, we investigated the effects of field management on soil and root bacterial communities, respectively. We observed the enrichment of Verrucomicrobia, Plantomycetota and Gemmatinonadota in soil bacterial communities derived from organic farms. In contrast, Verrucomicrobia and Acidobacteriota were abundant in roots under conventional cropping systems and Bacterioidota was enriched in roots under organic cropping systems. Verrucomicrobia is a ubiquitous bacterial phylum in soils and other studies even identified Verrucomicrobia communities in the rhizosphere and root endosphere [35,36], suggesting that this phylum might interact with plants. A large-scale study of tallgrass prairie soils showed that the abundance of Verrucomicrobia is affected by climate conditions. In addition, it is positively correlated with carbon metabolism function but negatively correlated with nitrogen metabolism [37]. Another study showed that Verrucomicrobia communities decrease when soil fertility increases [38], supporting the notion that this phylum may be involved in complex carbohydrate degradation and could have a high affinity for nutrients. Some studies showed that the relative abundance of Verrucomicrobia was not affected by cropping systems or even lower in organic-farmed soils [39,40], which is different to what we observed. Comparisons between climate conditions regarding this and previous studies, the latter of which were conducted in temperate climates, might suggest that the difference in results could be attributed to the carbon sources, nutrient availability and consumption. Moreover, the difference in nutrient demand and utility between vegetable crops and tea might also lead to a different response to a novel farming system, thus affecting the microbial community structure. At the genus level, Acidothermus and Rhodanobacter were enriched in roots from conventional fields. Previous studies showed that Acidothermus and Rhodanobacter preferred saline and acidic environments, respectively [35,41]. It is known that the long-term application of chemical fertilizers affects soil pH and salt content, and has a great impact on microbial community composition in the rhizosphere [42,43]. Further studies will be required to examine the seasonal change in soil fertility, plant nutrition status and microbiome to reveal the association between microbiomes and other environmental factors.
We further identified the indicator species in soils and roots that were sensitive to field management. In soils, most OTUs that were enriched in conventional farms with more than 1% relative abundance belonged to Gemmaproteobacteria, while three out of four organic farm-enriched OTUs, with more than 1% abundance, belonged to Acidobacteriae. Previous studies showed that the long-term application of chemical fertilizers increases the abundance of Gemmaproteobacteria and Acidobacteriae in the 0–10 cm soil compared to no fertilizer control, and applying organic-inorganic mixed fertilizers further boosts their abundance [44,45]. Enebe and Babalola [46] also observed an increase in Gemmaproteobacteria when applying higher amounts of inorganic fertilizer. These pieces of evidence support the notion that an abundance of Gemmaproteobacteria is highly associated with chemical fertilizers. Similarly, we also found that most OTUs enriched in roots from conventional farms with more than 1% abundance belonged to Gemmaproteobacteria, while others belonged to Alphaproteobacteria, Actinobacteria and other classes. Interestingly, in the roots from organic farms, we found an increased abundance of Bradyrhizobium, Streptomyces, Burkholderia-Caballeronia-Paraburkholderia, and Sphingomonas, all of which are genera enriched by well-known plant growth-promoting bacteria [47,48,49,50]. This result is consistent with a recent report by Reid et al. [43] showing a reduction in the population of plant growth-promoting bacteria in the rhizosphere after applying chemical fertilizers. Through both previous studies and this present study, we can see the benefit of organic cropping systems on microbial composition, which might increase crop growth and resilience to environmental stresses.
In order to understand the function of bacterial communities, functional analysis was performed, and 28 and 38 level-three KEGG categories in soil and root microbiota, which were significantly affected by field management, were identified. In soils, it is worth noting that a few pathways related to xenobiotics degradation, including “aminobenzoate degradation”, “benzoate degradation” and “naphthalene degradation” were more abundant in organic farms than in conventional farms. Xenobiotics are chemicals that accumulate in the environment that are harmful to human and environmental health. The biodegradation of xenobiotics is one effective way of removing the toxins [51]. The increase in xenobiotics’ degradation capability in organic farms suggests that soil bacteria might be capable of degrading xenobiotics and removing toxic chemicals. Further analysis is required to validate the presence of xenobiotics degradation-related genes and enzyme activity in organic soils. In roots, we noticed that two KEGG functional groups, “ABC transporters” and “transporters” were enriched in samples from organic fields, which are vital for bacterial growth and survival [52,53]. A study in Allium root bacterial endophytes showed that “ABC transporters”, “transporters” and “secretion system” were the richest pathway [54]. Here we further found that these functions are more abundant in roots under organic cropping systems. More research is needed to ascertain whether these functions benefit the nutrient uptake and stress tolerance of plants.
In summary, we investigated the effects of field management on soil and root microbiome and identified the sensitive OTUs that have great potential to be indicator species. We also observed the benefits of organic cropping systems including the enrichment of plant growth promoting bacteria and the increase in the abundance of xenobiotics’ degradation and transporter functions. Further studies will be performed to examine the association between bacterial community composition and crop production and stress tolerance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/applmicrobiol1020025/s1, Table S1. The analysis of similarity between different datasets and within datasets. Table S2. The taxonomy level and relative abundance of field management-sensitive OTUs. Table S3. The relative abundance of KEGG level 3 categories in all sample groups. Figure S1. The location and area of experimental sites. Figure S2. Comparison of the relative abundance of (A) soil and (B) root bacterial families between different cropping systems. The leaf and right boundaries of each circle show the lower and upper limits of 95% confidential interval. Figure S3. Comparison of the relative abundance of (A) soil and (B) root bacterial genera between different cropping systems. The leaf and right boundaries of each circle show the lower and upper limits of a 95% confidential interval. Figure S4. Linear discrimination analysis effect size (LEfSe) of soil and root microbiomes under different cropping systems.
Author Contributions
Conceptualization, W.-Y.L., B.-J.C. and C.-Y.H.; methodology, G.-Y.L. and W.-Y.L.; validation, C.-Y.H. and W.-Y.L.; formal analysis, G.-Y.L.; investigation, G.-Y.L. and B.-J.C.; resources, B.-J.C. and C.-Y.H.; writing—original draft preparation, W.-Y.L.; writing—review and editing, W.-Y.L.; visualization, G.-Y.L. and W.-Y.L.; supervision, W.-Y.L.; project administration, W.-Y.L.; funding acquisition, W.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology, Taiwan (MOST 106-2313-B-002 -058 -MY2).
Acknowledgments
We thank Mong-Hsun Tsai, Hang-Kai Shiu and colleagues at the Center of Biotechnology, National Taiwan University for technical support with the sequencing and data analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Pimentel, D.; Harvey, C.; Resosudarmo, P.; Sinclair, K.; Kurz, D.; McNair, M.; Crist, S.; Shpritz, L.; Fitton, L.; Saffouri, R.; et al. Environmental and Economic Costs of Soil Erosion and Conservation Benefits. Science 1995, 267, 1117–1123. [Google Scholar] [CrossRef]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, Energetic, and Economic Comparisons of Organic and Conventional Farming Systems. Bioscience 2005, 55, 573–582. [Google Scholar] [CrossRef]
- Ishaq, S.; Johnson, S.P.; Miller, Z.J.; Lehnhoff, E.A.; Olivo, S.; Yeoman, C.J.; Menalled, F.D. Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure. Microb. Ecol. 2016, 73, 417–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Xu, F.; Song, T.; Du, H.; Gui, Y.; Xu, M.; Cao, Y.; Dang, X.; Rensing, C.; et al. Combining Irrigation Scheme and Phosphorous Application Levels for Grain Yield and Their Impacts on Rhizosphere Microbial Communities of Two Rice Varieties in a Field Trial. J. Agric. Food Chem. 2019, 67, 10577–10586. [Google Scholar] [CrossRef]
- Novara, A.; Catania, V.; Tolone, M.; Gristina, L.; Laudicina, V.A.; Quatrini, P. Cover Crop Impact on Soil Organic Carbon, Nitrogen Dynamics and Microbial Diversity in a Mediterranean Semiarid Vineyard. Sustainability 2020, 12, 3256. [Google Scholar] [CrossRef]
- Benitez, M.-S.; Osborne, S.L.; Lehman, R.M. Previous Crop and Rotation History Effects On Maize Seedling Health and Associated Rhizosphere Microbiome. Sci. Rep. 2017, 7, 15709. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Lee, S.A.; Goddard, M.R. Fungal Communities Are Differentially Affected by Conventional and Biodynamic Agricultural Management Approaches in Vineyard Ecosystems. Agric. Ecosyst. Environ. 2017, 246, 306–313. [Google Scholar] [CrossRef]
- Wemheuer, F.; Kaiser, K.; Karlovsky, P.; Daniel, R.; Vidal, S.; Wemheuer, B. Bacterial Endophyte Communities of Three Agricultural Important Grass Species Differ in Their Response Towards Management Regimes. Sci. Rep. 2017, 7, 40914. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-Y.; Lin, C.-Y.; Chang, S.-J.; Lin, W.-Y. The Dynamics of Endophytic Bacterial Community Structure in Rice Roots under Different Field Management Systems. Agronomy 2020, 10, 1623. [Google Scholar] [CrossRef]
- Hartman, K.; Van Der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping Practices Manipulate Abundance Patterns of Root and Soil Microbiome Members Paving The Way to Smart Farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Chong, K.P.; Ho, T.Y.; Jalloh, M.B. Soil Nitrogen Phosphorus and Tea Leaf Growth in Organic and Conventional Farming of Selected Fields at Sabah Tea Plantation Slope. J. Sustain. Dev. 2009, 1, 117–122. [Google Scholar] [CrossRef][Green Version]
- Han, W.-Y.; Xu, J.-M.; Wei, K.; Shi, R.-Z.; Ma, L.-F. Soil Carbon Sequestration, Plant Nutrients and Biological Activities Affected by Organic Farming System in Tea (Camellia sinensis (L.) O. Kuntze) fields. Soil Sci. Plant Nutr. 2013, 59, 727–739. [Google Scholar] [CrossRef]
- Das, S.; Borua, P.K.; Bhagat, R.M. Soil Nitrogen and Tea Leaf Properties in Organic and Conventional Farming Systems Under Humid Sub-Tropical Conditions. Org. Agric. 2016, 6, 119–132. [Google Scholar] [CrossRef]
- Ghosh, B.C.; Palit, S.; Gupta, S.D.; Swain, D.K. Studies on Tea Quality Grown Through Conventional and Organic Management Practices: Its Impact on Antioxidant and Antidiarrhoeal Activity. Trans. ASABE 2008, 51, 2227–2238. [Google Scholar] [CrossRef]
- Bagchi, A.; Ch, B.; Ghosh, R.; Swain, D.K.; Bera, N. Organic Farming Practice for Quality Improvement of Tea and Its Anti Parkinsonism Effect on Health Defense. J. Phys. Chem. Biophys. 2015, 5, 178. [Google Scholar] [CrossRef]
- Qiu, S.-L.; Wang, L.-M.; Huang, D.-F.; Lin, X.-J. Effects of Fertilization Regimes on Tea Yields, Soil Fertility, and Soil Microbial Diversity. Chil. J. Agric. Res. 2014, 74, 333–339. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Hung, Y.-M.; Lu, T.-P.; Tsai, M.-H.; Lai, L.-C.; Chuang, E.Y. EasyMAP: A User-Friendly Online Platform for Analyzing 16S Ribosomal DNA Sequencing Data. New Biotechnol. 2021, 63, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S.A. Tools for Microbiome Analysis in R. Available online: https://microbiome.github.io/tutorials/ (accessed on 15 December 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for Integration and Interpretation of Large-Scale Molecular Data Sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving Indicator Species Analysis by Combining Groups of Sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph Software Package for Complex Network Research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, A.; Sicardi, M.; Frioni, L. Plant Genotype and Nitrogen Fertilization Effects on Abundance and Diversity of Diazotrophic Bacteria Associated with Maize (Zea mays L.). Biol. Fertil. Soils 2015, 51, 391–402. [Google Scholar] [CrossRef]
- Chávez-Romero, Y.; Navarro-Noya, Y.E.; Reynoso-Martínez, S.C.; Sarria-Guzmán, Y.; Govaerts, B.; Verhulst, N.; Dendooven, L.; Luna-Guido, M. 16S Metagenomics Reveals Changes in The Soil Bacterial Community Driven by Soil Organic C, N-Fertilizer and Tillage-Crop Residue Management. Soil Tillage Res. 2016, 159, 1–8. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial Endophytes in Agricultural Crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, P.M.; Johnson, M.T.J. Assembly and Ecological Function of The Root Microbiome Across Angiosperm Plant Species. Proc. Natl. Acad. Sci. USA 2018, 115, E1157–E1165. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Von-Wobeser, E.; Rocha-Estrada, J.; Shapiro, L.R.; De La Torre, M. Enrichment of Verrucomicrobia, Actinobacteria and Burkholderiales drives selection of bacterial community from soil by maize roots in a traditional milpa agroecosystem. PLoS ONE 2018, 13, e0208852. [Google Scholar] [CrossRef] [PubMed]
- Bünger, W.; Jiang, X.; Müller, J.; Hurek, T.; Reinhold-Hurek, B. Novel Cultivated Endophytic Verrucomicrobia Reveal Deep-Rooting Traits of Bacteria to Associate with Plants. Sci. Rep. 2020, 10, 8692. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Ladau, J.; Clemente, J.C.; Leff, J.W.; Owens, S.M.; Pollard, K.S.; Knight, R.; Gilbert, J.A.; McCulley, R.L. Reconstructing the Microbial Diversity and Function of Pre-Agricultural Tallgrass Prairie Soils in the United States. Science 2013, 342, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, A.; Soares, T.; Rossetto, R.; Van Veen, J.A.; Tsai, S.M.; Kuramae, E.E. Verrucomicrobial community structure and abundance as indicators for changes in chemical factors linked to soil fertility. Antonie Leeuwenhoek 2015, 108, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Ercolini, D.; Scala, F. Organic farming induces changes in soil microbiota that affect agro-ecosystem functions. Soil Biol. Biochem. 2016, 103, 327–336. [Google Scholar] [CrossRef]
- Lupatini, M.; Korthals, G.W.; De Hollander, M.; Janssens, T.K.; Kuramae, E.E. Soil Microbiome Is More Heterogeneous in Organic Than in Conventional Farming System. Front. Microbiol. 2017, 7, 2064. [Google Scholar] [CrossRef]
- Green, S.; Prakash, O.; Jasrotia, P.; Overholt, W.A.; Cardenas, E.; Hubbard, D.; Tiedje, J.M.; Watson, D.B.; Schadt, C.W.; Brooks, S.; et al. Denitrifying Bacteria from the Genus Rhodanobacter Dominate Bacterial Communities in the Highly Contaminated Subsurface of a Nuclear Legacy Waste Site. Appl. Environ. Microbiol. 2012, 78, 1039–1047. [Google Scholar] [CrossRef]
- Citak, S.; Sonmez, S. Effects of chemical fertilizer and different organic manures application on soil pH, EC and organic matter content. J. Food Agri. Environ. 2011, 9, 739–741. [Google Scholar]
- Reid, T.E.; Kavamura, V.N.; Abadie, M.; Torres-Ballesteros, A.; Pawlett, M.; Clark, I.M.; Harris, J.; Mauchline, T.H. Inorganic Chemical Fertilizer Application to Wheat Reduces the Abundance of Putative Plant Growth-Promoting Rhizobacteria. Front. Microbiol. 2021, 12, 642587. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Yang, F.; E, Y.; Raza, W.; Huang, Q.; Shen, Q. Application of Bioorganic Fertilizer Significantly Increased Apple Yields and Shaped Bacterial Community Structure in Orchard Soil. Microb. Ecol. 2016, 73, 404–416. [Google Scholar] [CrossRef]
- Ma, B.; Lv, X.; Cai, Y.; Chang, S.X.; Dyck, M. Liming does not counteract the influence of long-term fertilization on soil bacterial community structure and its co-occurrence pattern. Soil Biol. Biochem. 2018, 123, 45–53. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. Effects of inorganic and organic treatments on the microbial community of maize rhizosphere by a shotgun metagenomics approach. Ann. Microbiol. 2020, 70, 49. [Google Scholar] [CrossRef]
- Pan, F.; Meng, Q.; Wang, Q.; Luo, S.; Chen, B.; Khan, K.Y.; Yang, X.; Feng, Y. Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere 2016, 154, 358–366. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, F.; Huang, Y.; Zhou, M.; Gao, J.; Yan, T.; Sheng, H.; An, L. Sphingomonas sp. Cra20 Increases Plant Growth Rate and Alters Rhizosphere Microbial Community Structure of Arabidopsis thaliana Under Drought Stress. Front. Microbiol. 2019, 10, 1221. [Google Scholar] [CrossRef]
- Amaresan, N.; Kumar, K.; Naik, J.H.; Bapatla, K.G.; Mishra, R.K. Streptomyces in plant growth promotion: Mechanisms and role. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent Advanced Technologies for the Characterization of Xenobiotic-Degrading Microorganisms and Microbial Communities. Front. Bioeng. Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef]
- Garai, P.; Chandra, K.; Chakravortty, D. Bacterial peptide transporters: Messengers of nutrition to virulence. Virulence 2016, 8, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Piepenbreier, H.; Fritz, G.; Gebhard, S. Transporters as information processors in bacterial signalling pathways. Mol. Microbiol. 2017, 104, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Illumina-based Analysis of Endophytic Bacterial Diversity of Four Allium Species. Sci. Rep. 2019, 9, 15271. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).